Abstract
Epidemiological studies suggest that environmental factors (e.g., air pollution) can influence the spread and infectivity of coronavirus disease 2019 (COVID-19); however, very few papers have investigated or discussed the mechanism behind the phenomenon. Given the fact that pollution will increase as social distancing rules are relaxed, we summarized the current understanding of how air pollution may affect COVID-19 transmission and discussed several possible mechanisms. Air pollution exposure can dysregulate the human immune response and make people more susceptible to infections, and affect infectivity. For example, in response to exposure to air pollution, angiotensin-converting enzyme 2 will increase, which is the receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This may increase the efficiency of viral infection. It is also possible that air pollution can facilitate SARS-CoV-2 spread by increasing the transmission, and potentially, SARS-CoV-2 can also survive longer when attached to a pollutant.
Keywords: ACE2, infection, SARS-CoV-2, transmission
INTRODUCTION
In December 2019, the first confirmed case of coronavirus disease 2019 (COVID-19) was reported in Wuhan, Hubei Province of China (15). Then, it caused a rapid outbreak in the rest of China despite the tough regional lockdown at the end of January 2020. Now, 6 months after the first reported case in China, COVID-19 has emerged in more than 180 countries in six continents (9), with more than 11.5 million people infected and more than 500,000 deaths.
In addition to close personal contact, environmental factors may also play a critical role in accelerating the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, which is responsible for COVID-19. Patients with COVID-19 showed a similar cluster of symptoms to severe acute respiratory syndrome (SARS) caused by SARS-CoV, which initiated in the Guangzhou province of China in 2003. Both viruses are coronaviruses, which are thought to originate in bats. As SARS-CoV-2 is novel, it is understandable that people would refer to SARS-CoV as a reference point for transmission, pathogenesis, and treatments.
EPIDEMIOLOGY
An epidemiological study suggested a positive correlation between the amount of particulate matter (PM) (measured by the air pollution index, API) and the fatality rate of SARS in 2003 (14). The death rate was increased by 100% and 84% in those patients in high and moderate API regions, respectively, compared with those in regions with low API. This finding is echoed by another study that demonstrated that each increment of 10 µg/m3 PM10 over a 5-day period results in a 1.06 (1.00–1.12) increased risk of daily mortality (26).
If air pollution plays an important role during the new coronavirus, i.e., SARS-CoV-2, infection, there should be a positive relationship between the number of COVID-19 cases and measures of pollution, for example, the PM concentration. Globally, the highest population-weighted mean PM2.5 (50–60 µg/m3) is in eastern and southern Asia (52). Hubei is one of the most polluted provinces in the winter in China and the epicenter of COVID-19 (34). According to the European Environment Agency (EEA) reports, the Lombardia and Emilia Romagna are the most polluted areas in Italy and one of the more polluted regions in Europe (10). In fact, Lombardy became the COVID-19 epicenter in Italy, with the highest confirmed cases and death rate, with more than 78,000 confirmed cases and 14,000 deaths when the manuscript was prepared.
The relationship between PM level and the COVID-19 fatality rate was investigated in a recent epidemiological study of a Wuhan cohort. It found a positive correlation between PM10 and PM2.5 concentrations and the COVID-19 fatality rate (56). A further nationwide study in China, including 49 cities outside and 15 cities inside Hubei province, demonstrated that short-term PM10 and PM2.5 exposure have a strong association with COVID-19 death rates (57). The European Public Health Alliance recently reported that people living in polluted cities face greater COVID-19 threat (17), which was confirmed by later research in Italy. Studies from Italy demonstrated high PM10 concentration in northern Italy promoted the spreading of SARS-CoV-2, and another study confirmed the significant correlation between PM10 distribution in 110 Italian provinces and SARS-CoV-2 infection rates (47). Data in those studies showed that there were only three positive cases every 100,000 residents in the less polluted provinces, while in highly polluted provinces, there were around nine times higher positive cases of COVID-19 (23a, 48). A nationwide study from the United States showed that every 1 µg/cm3 increment in PM2.5 exposure in the long term results in 8% increase in COVID-19 death rate (53). A similar observation in the Netherlands was made, which concluded that PM2.5 exposure has a close association with COVID-19 confirmed cases, where a 20% increase in the pollution doubled the COVID-19 case number (3).
POSSIBLE MECHANISMS
There are many plausible reasons why there might be a relationship between air pollution and COVID-19. It is important here to recognize that short-term effects of air pollution are generally proinflammatory, while chronic exposure can cause immune dysregulation and other diseases. Here, we will discuss several possible mechanisms.
Air Pollutants Impair the Immune Function, Making People More Susceptible to the Infection
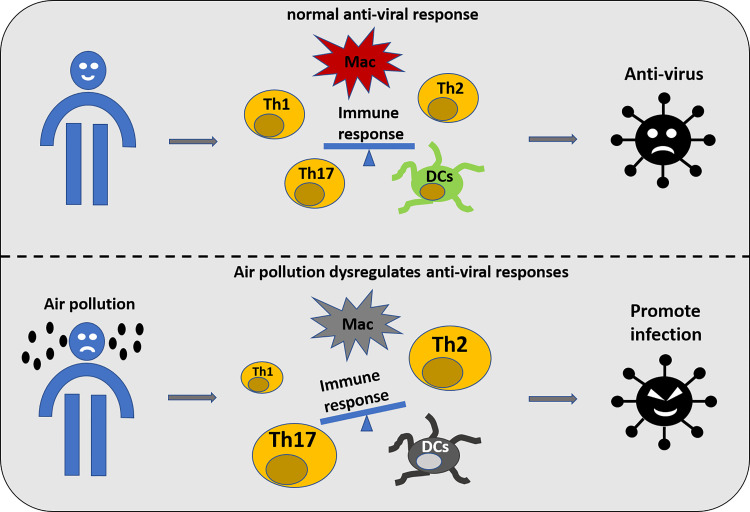
It is well accepted that air pollution is detrimental to health, not only damaging the lung, which is exposed to the air directly, but also inducing pathological changes in other organ systems through excessive mitochondrial-produced oxidative stress. Interestingly, the enzyme that produces reactive oxygen species, NOX2, has been shown to be critical for PM-induced NADPH-oxidase activation and systemic vascular dysfunction (25, 33). In the context of respiratory viral infection, NOX2 oxidase activation suppresses antiviral and humoral signaling networks (51); therefore, PM may increase NOX2 activity through producing a suppressed antiviral response prior to SARS-CoV-2 infection. The respiratory mucosa is the first line of defense against all of the inhaled pollutants and toxicants with the help of alveolar epithelial cells, alveolar macrophages (AM), dendritic cells (DC), and adaptive T and B lymphocytes. Air pollution could affect the function of this mucosal barrier, as depicted in Fig. 1. These changes caused by air pollution could dysregulate the antiviral immune response (19). Both SARS-CoV-2 and PM activate cells via mechanisms, including Toll-like receptors (TLRs) and their downstream signaling cascades (5); however, PM signals via TLRs 2 and 4, which are important in bacterial infections, and SARS-CoV-2 is recognized by the “viral” TLRs 3, 7, and 8. Studies have shown that alveolar macrophages from people living in highly polluted cities have a reduced innate immune capacity, which is proportional to the amount of phagocytosed PM (20). Experimental studies in mice have found that PM exposure reduces activity at the interferon (INF)-β gene promoter, and subsequent INF-β transcription, and enchanted influenza replication (37). As innate immunity, in general, and in particular INF-β, a key antiviral cytokine, is reduced by PM, it is likely that these mechanisms would enhance SARS-CoV-2 pathogenicity. Epidemiological studies demonstrated that even short-term PM2.5 exposure could increase the risk of infectious diseases, such as influenza, pneumonia, and other lower respiratory infections (12, 23). Experiments using mouse models also demonstrated that PM exposure could create an inflammatory environment and promote respiratory syncytial virus infection (28, 29). In fact, PM exposure has been shown to induce or exacerbate respiratory diseases, such as chronic obstructive pulmonary disease (COPD) through dysregulated immune response (16, 30). A recent systematic review of 2,002 COVID-19 cases concluded that COPD patients experienced a four-fold higher infection risk and worse progress and outcome of COVID-19 (61). This suggested an impaired immune response against SARS-CoV-2 infection and dysregulated inflammatory responses after the infection.
Fig. 1.
Exposure to air pollution may increase the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection through dysregulating immune response. DCs, dendritic cells; Mac, macrophages; Th, T helper cell.
These mechanisms of immune dysregulation are synonymous with chronic diseases, which may also occur as a result of exposure to air pollution. These include, but are not limited to COPD, cardiovascular disease, diabetes, and cancers. As exemplified above, these diseases can independently confer an increased risk of adverse outcomes following SARS-CoV-2 infection.
PM Exposure Could Deteriorate COVID-19 Symptoms Through Damaging Surfactant Homeostasis
Surfactant is synthesized, stored, and secreted by type II pneumocytes. The primary function of surfactant is to reduce surface tension at the air-liquid interface of the lung and prevent alveoli from collapsing at the end of expiration. In adults, abnormal surfactant production can occur after critical insults such as trauma or sepsis, and this lack of surfactant will result in acute respiratory distress syndrome (ARDS) (24). Patients with severe COVID-19 also have ARDS, which histologically manifests as diffuse alveolar damage, including type II pneumocyte hyperplasia and dysfunctional alveolar barrier (18, 60). This suggests that the pulmonary surfactant system may be compromised by SARS-CoV-2 infection. As surfactant covers the entire air-liquid interface of the lung, it is vulnerable to attack from inhaled toxicants (38). In particular, experimental studies have shown that physical interaction between small PM and surfactant occur that can change the biomechanical function of surfactant (27), and in mice, PM can cause alveolar collapse (44). Therefore, it is possible that people exposed to PM have dysfunctional surfactant. When combined with infection of alveolar epithelial cells with SARS-CoV-2, the combined insult to the lungs may accelerate the development of ARDS (23a).
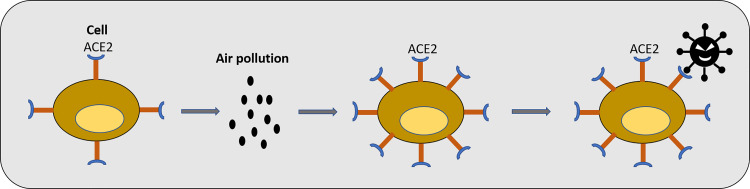
PM Could Increase Angiotensin-Converting Enzyme 2 Expression Level and Facilitate COVID-19 Infection
The entry of COVID-19 into the cell depends on the spike protein (S), which can bind to angiotensin-converting enzyme 2 (ACE2) receptor on the cellular membranes (22). However, whether this means the ACE2 expression level can determine the viral load of SARS-CoV-2 infection has yet to be investigated. There is abundant ACE2 in a transient secretory cell type in subsegmental bronchial branches (49). In a murine model of PM2.5 exposure, ACE2 expression is increased in the lung tissue at 2 and 5 days after installation of PM2.5 (31). This indicates PM may facilitate SARS-CoV-2 infection through upregulating ACE2 expression (Fig. 2).
Fig. 2.
Air pollution could increase angiotensin-converting enzyme 2 (ACE2) level in patients with respiratory diseases.
PM Exposure Could Contribute to the Cytokine Storm Induced by COVID-19
When SARS-CoV-2 infection progresses from pneumonitis to ARDS, patients showed a sustained high level of inflammation with overexpression of proinflammatory cytokines (i.e., TNF-α, IL-6), followed by multiple organ failure, eventually leading to death (36). This highly sustained inflammation is commonly referred to as a “cytokine storm”. Macrophages play an important role in the cytokine storm. During the infection, alveolar macrophages are quickly mobilized by signals released by the SARS-CoV-2-infected cells to defend COVID-19 invasion through the phagocytosis and secreting proinflammatory cytokines to attract more immune cells (including monocytes, macrophages, and T cells) to the infected sites. However, this results in a positive feedback loop leading to hyperinflammation and vascular hyperpermeability (50). In turn, this leads to pulmonary and interstitial tissue damage, excessive fluid in the lungs reducing gas exchange, and unfortunately death (58).
PMs have been regarded as toxic pollutants inducing prolonged inflammation and hyperactivation of the innate immune system, even at a low dose. In our murine model of low-dose PM10 exposure (5 µg/day per mouse), subchronic exposure for 3 weeks significantly increased macrophage number in the bronchoalveolar fluid and IL1-β level in the lung tissue (7). A similar phenomenon was found in another murine model of PM2.5 exposure for 3 months, where TNF-α level and macrophage number were significantly increased (55). A clinical study also confirmed the findings with overexpression of TNF-α and IL-6 in the healthy subjects after PM10 and PM2.5 exposure (42). Those cytokines induced by short-term and long-term PM exposure are the same types in the cytokine storm in patients with COVID-19. This suggests that PM exposure may boost the development of a cytokine storm in a SARS-CoV-2 infection.
PM Could Increase the Transmission Distance of SARS-CoV-2
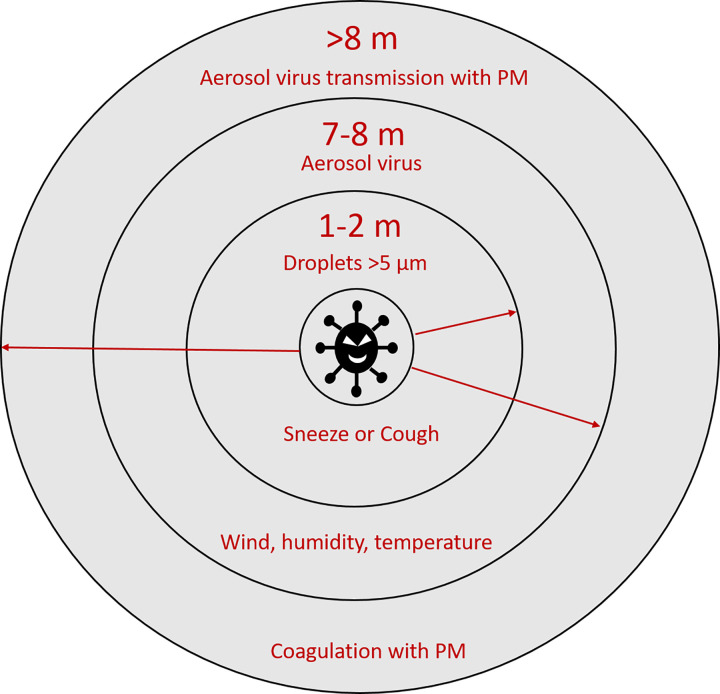
PM fractions (PM10 and PM2.5) can float in the air for a long time and disperse over long distances (35). During their formation, black carbon can absorb various chemical and biological components on the surface. Thus, PMs can act as a carrier to transport viruses over longer distances than that would otherwise occur (48). The SARS-CoV-2 diameter is around 100 nm. Similar to other respiratory viruses, SARS-CoV-2 can travel through the air in droplets known as Flügge droplets (Fig. 3). The large droplets (>5 µm) from a cough or sneeze can only travel <1–1.5 meters because of the resistance of the air and their mass, and then deposit on surfaces. However, smaller virus-laden particles (<5 µm) could remain in the air for hours and spread over longer distances (11). A previous study demonstrated a positive correlation between virus deposition rate and organic aerosols smaller than 0.7 µm, making viruses stay longer in the air (43). According to the data collected in hospitals from Wuhan, aerosolized SARS-CoV-2 had an aerodynamic diameter size ranging from >2.5 µm to 0.25–1 µm (32).
Fig. 3.
Mechanism of virus transmission through transport on particulate matter (PM).
A number of studies have confirmed that viruses could spread faster through airborne transmission routes beyond close contact with infected people, for example, for viruses such as measles (8, 32, 40, 46). A retrospective cohort study published in 2014 of patients with SARS during the 2003 outbreak in Hong Kong also suggested that the airborne transmission route played an important role in spreading the virus (59).
In a study collecting three types of aerosol samples (total suspended particles, aerodynamic size-segregated particles, and deposited samples) from two hospitals in Wuhan, SARS-CoV-2 was found in the patient’s toilets (19 copies/m3) and Protective Apparel Removal Rooms (PARRs) (18–42 copies/m3). The patients’ breath and excrement may contribute to the high COVID-19 concentration in the toilet. The high COVID-19 concentration in PARRs may be due to the resuspension of the virus-laden aerosol from the personal protective equipment and floor dust aerosol containing virus (32).
Researchers have found that aerosolized PM could facilitate COVID-19 transmission. A study in Italy also showed SARS-CoV-2 RNA in environmental PM samples (3). The high concentration of dust and airflow conditions in northern Italy could promote SARS-CoV-2 viral transmission by forming viral clusters with PMs (48, 49).
Worryingly, studies also found that PM can not only extend transmission distance, but also increase the infectivity in the aerosol. Respiratory syncytial virus was found to be stable for up to 6 months in a solution mixed with PM, and had increased infectivity in vitro (13). Researchers from Italy speculated that PM could induce a “boost effect” on the SARS-CoV-2 viral infectivity after analyzing the PM concentration and death rate in Milan and Rome (48).
PM Exposure May Weaken COVID-19 Treatment Efficiency
PM is an oxidative and proinflammatory stimulus in the lungs. In general, most therapeutics would have altered (typically reduced) efficacy when oxidative or inflammatory conditions coexist. In the case of SARS-CoV-2 infection, it is possible that as we have found with rhinovirus infection (6), pollution enhances virus-induced inflammation, which could reduce the efficacy of therapeutics. One plausible mechanism by which this may occur is sequestration and elimination of inhaled therapeutics in mucous (produced as part of the inflammatory response). Interestingly, PM increases lung neutrophil numbers, and therefore affects neutrophil-to-lymphocyte ratios. This may also influence clinical decisions around when to initiate remdesivir treatment (21, 39). Other potential interactions may also occur, for example, systemic clotting is found in patients with late-stage SARS-CoV-2 infection, leading to multiple organ failure. As such, anticoagulation treatment has been used in patients with COVID-19 to increase their survival rate (4). However, PM exposure has been shown to induce venous thrombosis through platelet overactivation (45), which may compromise the efficiency of anticoagulation treatment.
CONCLUSIONS
Air pollution is a modifiable environmental factor that will affect the pathogenesis of SARS-CoV-2. PM exposure could weaken and dysregulate immune response, resulting in a failure to defend against virus invasion. PM exposure could cause ACE2 overexpression to increase viral load during invasion (54). Airborne PM can also increase transmission distance of SARS-CoV-2. If the cited studies are correct, this pollution may facilitate a second wave of infection by transmitting the virus from one country to another.
GRANTS
B. Wang is supported by a scholarship awarded by the China Scholarship Council. Y. L. Chan is supported by a Peter Doherty Fellowship by National Health & Medical Research Council of Australia. The study is supported by a project grant (APP1158186) by National Health & Medical Research Council of Australia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W. and Y.L.C. prepared figures; B.W., H.C., and B.G.O. drafted manuscript; xxx edited and revised manuscript; H.C. and B.G.O. approved final version of manuscript.
REFERENCES
- 1.Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, Nishioka H, Akita H, Sato Y, Kataoka M, Katano H, Tobiume M, Sekizuka T, Itokawa K, Kuroda M, Suzuki T. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerg Infect Dis. In press. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andree BPJ. Incidence of COVID-19 and connections with air pollution exposure: evidence from the Netherlands (Preprint). bioRxiv 2020. doi: 10.1101/2020.04.27.20081562. [DOI]
- 4.Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother 6: 260–261, 2020. doi: 10.1093/ehjcvp/pvaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker S, Fenton MJ, Soukup JM. Involvement of microbial components and Toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol 27: 611–618, 2002. doi: 10.1165/rcmb.4868. [DOI] [PubMed] [Google Scholar]
- 6.Capistrano SJ, Zakarya R, Chen H, Oliver BG. Biomass smoke exposure enhances rhinovirus-induced inflammation in primary lung fibroblasts. Int J Mol Sci 17: 1403, 2016. doi: 10.3390/ijms17091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan YL, Wang B, Chen H, Ho KF, Cao J, Hai G, Jalaludin B, Herbert C, Thomas PS, Saad S, Oliver BGG. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am J Physiol Lung Cell Mol Physiol 317: L424–L430, 2019. doi: 10.1152/ajplung.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Zhang W, Li S, Williams G, Liu C, Morgan GG, Jaakkola JJK, Guo Y. Is short-term exposure to ambient fine particles associated with measles incidence in China? A multi-city study. Environ Res 156: 306–311, 2017. doi: 10.1016/j.envres.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Chughtai A. COVID-19: In charts and maps (Online). https://www.aljazeera.com/indepth/interactive/2020/03/covid-19-charts-maps-200310163714493.html.
- 10.Conticini E, Frediani B, Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in northern Italy? Environ Pollut 261: 114465, 2020. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contini D, Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere 11: 5, 2020. doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- 12.Croft DP, Zhang W, Lin S, Thurston SW, Hopke PK, van Wijngaarden E, Squizzato S, Masiol M, Utell MJ, Rich DQ. Associations between source-specific particulate matter and respiratory infections in New York State adults. Environ Sci Technol, 54: 975–984, 2020. doi: 10.1021/acs.est.9b04295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz-Sanchez TM, Haddrell AE, Hackett TL, Singhera GK, Marchant D, Lekivetz R, Meredith A, Horne D, Knight DA, van Eeden SF, Bai TR, Hegele RG, Dorscheid DR, Agnes GR. Formation of a stable mimic of ambient particulate matter containing viable infectious respiratory syncytial virus and its dry-deposition directly onto cell cultures. Anal Chem 85: 898–906, 2013. doi: 10.1021/ac302174y. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Zhang ZF, Froines J, Zhao J, Wang H, Yu SZ, Detels R. Air pollution and case fatality of SARS in the People’s Republic of China: an ecologic study. Environ Health 2: 15, 2003. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson H. First Covid-19 case happened in November, China government records show–report (Online). https://www.theguardian.com/world/2020/mar/13/first-covid-19-case-happened-in-november-china-government-records-show-report.
- 16.Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, Hansell AL. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J 54: 1802140, 2019. doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
- 17.European Public Health Alliance Coronavirus threat greater for polluted cities. https://epha.org/coronavirus-threat-greater-for-polluted-cities/.
- 18.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 213: 54–56.e1, 2020. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glencross DA, Ho T-R, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med 151: 56–68, 2020. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez Y, Carranza C, Iñiguez M, Torres M, Quintana R, Osornio A, Gardner C, Sarkar S, Schwander S. Effect of inhaled air pollution particulate matter in alveolar macrophages on local pro-inflammatory cytokine and peripheral interferon γ production in response to mycobacterium tuberculosis. Lancet Glob Health 6: S29, 2018. doi: 10.1016/S2214-109X(18)30158-X. [DOI] [Google Scholar]
- 21.Hillaker E, Belfer JJ, Bondici A, Murad H, Dumkow LE. Delayed initiation of remdesivir in a COVID‐19‐positive patient. Pharmacotherapy 40: 592–598, 2020. doi: 10.1002/phar.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne BD, Joy EA, Hofmann MG, Gesteland PH, Cannon JB, Lefler JS, Blagev DP, Korgenski EK, Torosyan N, Hansen GI, Kartchner D, Pope CA III. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med 198: 759–766, 2018. doi: 10.1164/rccm.201709-1883OC. [DOI] [PubMed] [Google Scholar]
- 23a.Istituto Superiore di Sanità Coronavirus Surveillance Bulletin. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_16-aprile-2020.pdf [21 April 2020].
- 24.Jacobson W, Park GR, Saich T, Holcroft J. Surfactant and adult respiratory distress syndrome. Br J Anaesth 70: 522–526, 1993. doi: 10.1093/bja/70.5.522. [DOI] [PubMed] [Google Scholar]
- 25.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 108: 716–726, 2011. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kan HD, Chen BH, Fu CW, Yu SZ, Mu LN. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed Environ Sci 18: 1–4, 2005. [PubMed] [Google Scholar]
- 27.Kodama AT, Kuo C-C, Boatwright T, Dennin M. Investigating the effect of particle size on pulmonary surfactant phase behavior. Biophys J 107: 1573–1581, 2014. doi: 10.1016/j.bpj.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert AL, Mangum JB, DeLorme MP, Everitt JI. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol Sci 72: 339–346, 2003. doi: 10.1093/toxsci/kfg032. [DOI] [PubMed] [Google Scholar]
- 29.Lambert AL, Trasti FS, Mangum JB, Everitt JI. Effect of preexposure to ultrafine carbon black on respiratory syncytial virus infection in mice. Toxicol Sci 72: 331–338, 2003. doi: 10.1093/toxsci/kfg031. [DOI] [PubMed] [Google Scholar]
- 30.Liao CM, Hsieh NH, Chio CP. Fluctuation analysis-based risk assessment for respiratory virus activity and air pollution associated asthma incidence. Sci Total Environ 409: 3325–3333, 2011. doi: 10.1016/j.scitotenv.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CI, Tsai CH, Sun YL, Hsieh WY, Lin YC, Chen CY, Lin CS. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int J Biol Sci 14: 253–265, 2018. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak (Preprint). bioRxiv 2020. doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
- 33.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol 2011: 487074, 2011. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Z, Hu X, Huang L, Bi J, Liu Y. Estimating ground-level PM2.5 in China using satellite remote sensing. Environ Sci Technol 48: 7436–7444, 2014. doi: 10.1021/es5009399. [DOI] [PubMed] [Google Scholar]
- 35.Martelletti L, Martelletti P. Air pollution and the novel Covid-19 disease: a putative disease risk factor. SN Compr Clin Med. In press. doi: 10.1007/s42399-020-00274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra R, Pandikannan K, Gangamma S, Raut AA, Kumar H. Imperative role of particulate matter in innate immunity during RNA virus infection (Preprint) bioRxiv 2020. doi: 10.1101/2020.03.28.013169. [DOI]
- 38.Müller B, Seifart C, Barth PJ. Effect of air pollutants on the pulmonary surfactant system. Eur J Clin Invest 28: 762–777, 1998. doi: 10.1046/j.1365-2362.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 39.Narasaraju T, Tang BM, Herrmann M, Muller S, Chow VT, Radic M. Neutrophilia and NETopathy as key pathologic drivers of progressive lung impairment in patients with COVID-19. Front Pharmacol 11: 870, 2020. doi: 10.3389/fphar.2020.008702020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA 323: 707–708, 2020. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 42.Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119: 1204–1214, 2016. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reche I, D’Orta G, Mladenov N, Winget DM, Suttle CA. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J 12: 1154–1162, 2018. doi: 10.1038/s41396-017-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riva DR, Magalhães CB, Lopes AA, Lanças T, Mauad T, Malm O, Valença SS, Saldiva PH, Faffe DS, Zin WA. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol 23: 257–267, 2011. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- 45.Robertson S, Miller MR. Ambient air pollution and thrombosis. Part Fibre Toxicol 15: 1–16, 2018. doi: 10.1186/s12989-017-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santarpia JL, Rivera DN, Herrera V, Morwitzer MJ, Creager H, Santarpia GW, Crown KK, Brett-Major D, Schnaubelt E, and Broadhurst MJ. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center (Preprint). medRxiv 2020. doi: 10.1101/2020.03.23.20039446. [DOI]
- 47.Setti L, Passarini F, De Gennaro G, Barbieri P, Pallavicini A, Ruscio M, Piscitelli P, Colao A, Miani A. Searching for SARS-COV-2 on particulate matter: a possible early indicator of COVID-19 epidemic recurrence. Int J Environ Res Public Health 17: 2986, 2020. doi: 10.3390/ijerph17092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Piazzalunga A, Borelli M, Palmisani J, Di Gilio A, Piscitelli P, Miani A. The potential role of particulate matter in the spreading of COVID-19 in northern Italy: first evidence-based research hypotheses (Preprint). medRxiv 2020. doi: 10.1101/2020.04.11.20061713. [DOI] [PMC free article] [PubMed]
- 49.Setti L, Passarini F, De Gennaro G, Baribieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Pallavicini A. SARS-Cov-2 RNA found on particulate matter of Bergamo in northern Italy: first preliminary evidence (Preprint). medRxiv 2020. doi: 10.1101/2020.04.15.20065995. [DOI] [PMC free article] [PubMed]
- 50.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.To EE, Vlahos R, Luong R, Halls ML, Reading PC, King PT, Chan C, Drummond GR, Sobey CG, Broughton BRS, Starkey MR, van der Sluis R, Lewin SR, Bozinovski S, O’Neill LAJ, Quach T, Porter CJH, Brooks DA, O’Leary JJ, Selemidis S. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat Commun 8: 69, 2017. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, Lyapustin A, Sayer AM, Winker DM. Global estimates of fine particulate matter using a combined geophysical-statistical method with information from satellites, models, and monitors. Environ Sci Technol 50: 3762–3772, 2016. doi: 10.1021/acs.est.5b05833. [DOI] [PubMed] [Google Scholar]
- 53.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States (Preprint). medRxiv 2020. doi: 10.1101/2020.04.05.20054502. [DOI] [PMC free article] [PubMed]
- 54.Xue T, Wei N, Xin Z, Qingyu X. Angiotensin-converting enzyme-2 overexpression attenuates inflammation in rat model of chronic obstructive pulmonary disease. Inhal Toxicol 26: 14–22, 2014. doi: 10.3109/08958378.2013.850563. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Chen Y, Yu Z, Ding H, Ma Z. The influence of PM2.5 on lung injury and cytokines in mice. Exp Ther Med 18: 2503–2511, 2019. doi: 10.3892/etm.2019.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Y, Pan J, Liu Z, Meng X, Wang W, Kan H, Wang W. Temporal association between particulate matter pollution and case fatality rate of COVID-19 in Wuhan, China (Preprint). medRxiv 2020. doi: 10.1101/2020.04.09.20049924. [DOI] [PMC free article] [PubMed]
- 57.Yao Y, Pan J, Wang W, Liu Z, Kan H, Meng X, Wang W. Spatial correlation of particulate matter pollution and death rate of COVID-19 (Preprint). medRxiv 2020. doi: 10.1101/2020.04.07.20052142. [DOI]
- 58.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “cytokine storm” in COVID-19. J Infect 80: 607–613, 2020. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu ITS, Qiu H, Tse LA, Wong TW. Severe acute respiratory syndrome beyond Amoy Gardens: completing the incomplete legacy. Clin Infect Dis 58: 683–686, 2014. doi: 10.1093/cid/cit797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med 172: 629–632, 2020. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Lian N, Deng Y, Lin S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. In press. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]