INTRODUCTION
This month, September, is Pulmonary Fibrosis Awareness Month, and this year, 2020, will be forever remembered as the year that brought us COVID-19. Pulmonary fibrosis and COVID-19 have more in common than at first seems apparent, and it is incumbent upon us to take what learnings we can from these two dreadful conditions to help us understand the shared mechanisms that will help us improve outcomes for people with progressive scarring conditions of the lung.
Both conditions start with lung injury; in the case of COVID-19 it can be very dramatic, leading to acute respiratory distress syndrome (ARDS), whereas in pulmonary fibrosis it is often insidious, and the originating injury is often unknown until it is too late. Both conditions exhibit their most severe consequences in elderly men, and indeed elderly men with pulmonary fibrosis are at particularly high risk of mortality from COVID-19 (15). Both are diseases that have been associated with the metabolic syndrome, namely, the constellation of hypertension, diabetes, and obesity. Finally, COVID-19 may directly lead to the development of pulmonary fibrosis through the direct consequences of virus-induced acute lung injury (ALI) or indirect consequences of the cytokine-mediated alveolar damage. If we understand the shared mechanisms that exist between these two conditions that characterize the extremes of lung injury and failing repair, we will be better able to deal with both the emerging epidemic of fibrotic lung disease and the very immediate COVID-19 pandemic.
MECHANISMS OF LUNG INJURY
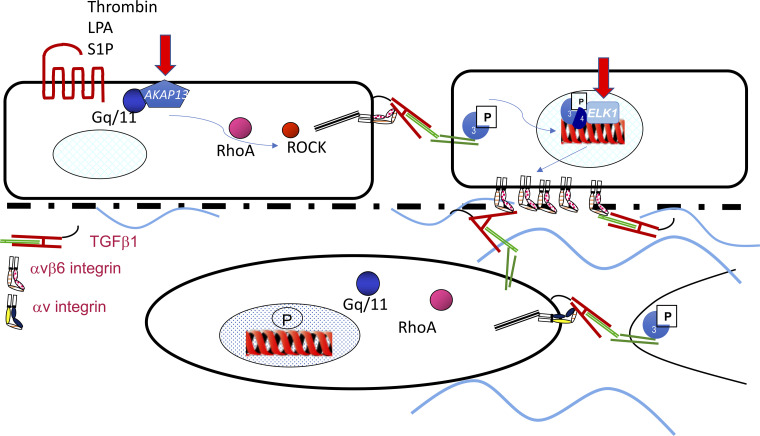
The relationship between lung injury and fibrosis is well described; indeed, much of what is understood about the development of pulmonary fibrosis is based on data obtained from in vivo models of lung injury. In these models of pulmonary fibrosis lung injury is induced, often through inhalation of fibrogenic material such as bleomycin, FITC, or silica, which leads to an inflammatory phase, followed by a fibrotic phase and in many cases a resolving phase (26). Even in longer-term and more “clinically relevant” models such as radiation-induced lung fibrosis, there is an initial inflammatory phase lasting a few weeks with a long latent phase and then a very dramatic, nonresolving, fibrotic phase at between 5 and 6 mo (43). One of the key molecular mechanisms of lung injury and fibrosis triggered by many of these models is activation of epithelial transforming growth factor (TGF)-β1 (56), and indeed models of pulmonary fibrosis where active TGF-β1 is overexpressed in the lung with adenoviral gene transfer or transgenic overexpression can lead to progressive fibrosis without substantial inflammation (30, 52). A number of strategies targeting TGF-β1 activation have been used experimentally to inhibit both acute lung injury (ALI) (42) and fibrosis (22, 43), but global strategies have proved challenging because of the pleiotropic functions of TGF-β1, and its vital roles in inflammation and tumor suppression have led to insurmountable toxicity concerns. However, efforts to selectively inhibit TGF-β1 at sites of epithelial injury or by targeting fibroblast TGF-β1 activation have shown some promise, and indeed inhibitors of galectin-3, which inhibits fibroblast and macrophage TGF-β1 (33), are currently in phase II clinical trials (Clinical.Trials.gov: NCT03832946). However, it is clear that ALI is not by itself sufficient to lead to progressive fibrotic disease, as in many cases even the most severe ALI and ARDS can either resolve to some extent or at least not progress (8, 21), and there either needs to be the development of a self-amplifying (positive feedback) loop generating perpetually high levels of active TGF-β1, which promotes tissue fibrosis rather than repair, or the signals that switch off profibrotic pathways once the injury has resolved are defective. At least two such mechanisms have been identified, although there are likely many more. Recently, a polymorphism near the AKAP13 gene associated with high levels of AKAP13 mRNA expression in the lung has been identified to increase the risk of developing idiopathic pulmonary fibrosis (IPF) (2). A-kinase anchoring protein 13 (AKAP13) is a Rho guanine nucleotide exchange factor (GEF) that leads to RhoA activation, and therefore it is possible that patients with high levels of AKAP13 will have enhanced RhoA activation in response to injury, which in turn could amplify TGF-β activation; studies are currently ongoing to address this hypothesis (see Fig. 1). Similarly, it is well known that TGF-β leads to increased expression of the ITGB6 gene and αvβ6 integrin expression, which in turn activates further TGF-β (54). Under normal circumstances this feedback loop is regulated by a number of transcription factors including the glucocorticoid receptor, progesterone receptors, and the transcriptional repressor ELK1 (55). Loss of ELK1 leads to enhanced ITGB6 expression, increased αvβ6 integrin on the epithelium, and enhanced fibrosis both in response to lung injury (55) and in spontaneous fibrosis in aged Elk1-null mice (9) (see Fig. 1). It is interesting to note that ELK1 is an X-linked gene, and this might explain some of the sex imbalance seen in IPF. There is considerable work to be done to understand the molecular mechanisms that may amplify TGF-β, and other fibrotic mechanisms, after lung injury and whether targeting these amplification pathways will be sufficient to prevent the development of progressive fibrosis without affecting the important homeostatic functions of TGF-β.
Fig. 1.
Injury to the alveolar surface leads to release of thrombin and lysophosphatidic acid (LPA), which act through G protein-coupled receptors PAR1 and LPAr2 to signal via Gq/11, RhoA, and Rho kinase (ROCK) to activate transforming growth factor (TGF)-β via the αvβ6 integrin. This in turn leads to TGF-β receptor activation in neighboring cells and Smad2/3 phosphorylation, which translocates to the nucleus, leading to increased transcription of the ITGB6 gene and increased expression of the αvβ6 integrin on the cell surface. This is able to activate fibroblast TGF-β if the epithelial basement membrane is denuded, leading to activation via fibroblast RhoA signaling and mesenchymal integrin-mediated TGF-β activation (αvβ1 and αvβ5) leading to autonomous fibroblast TGF-β activation. A-kinase anchoring protein 13 (AKAP13) is a Rho guanine nucleotide exchange factor (GEF) that facilitates Gq/11 to RhoA signaling and may act as an amplification signal in epithelial cells, and ELK1 is a transcriptional repressor that is lost in pulmonary fibrosis. S1P, sphingosine-1 phosphate.
An emerging role for TGF-β in COVID-19 has been described (58), and it would be surprising if TGF-β were not involved in the response to COVID-19-induced ARDS, given the known role of TGF-β in response to virus-induced lung injury (36) and the potential for SARS-CoV-2 to engage integrins through the RGD domain in its Spike protein (51). It is interesting to also note the pronounced sex imbalance in severe COVID-19 (17), and therefore understanding the role of its amplification factors including AKAP13 and ELK1 in COVID-19-related complications will be important.
GENETICS, AGING, AND FIBROTIC LUNG DISEASE
Pulmonary fibrosis is an age-related disease caused by epithelial injury to people with appropriate genetic susceptibility. Pulmonary fibrosis can occur without obvious cause, when it is called idiopathic pulmonary fibrosis (IPF), it can be caused by exposure to fibrogenic substances such as asbestos fibers (asbestosis) or bird proteins (hypersensitivity pneumonitis), or it can occur as clusters in families, so-called familial pulmonary fibrosis (FPF). Indeed, it is emerging that FPF is probably much more common than previously thought, with a recent study identifying a family history of pulmonary fibrosis being associated with a 12-fold increased risk of IPF (1), and in ~25% of asymptomatic family members of patients with IPF there was evidence of subclinical interstitial lung abnormalities (24). Although there are a large number of genes that promote the development of pulmonary fibrosis including common variants in the MUC5B promoter, DSP, and AKAP13 and rare variants in genes related to surfactant production and telomere function (RTEL1, PARN, TERT, and TERC), the proportion of the genetic risk explained by these variant remains small and there is considerable further work required to identify causal variants. For example, the best-described, and most commonly found, rare variants are in a number of genes associated with telomere function, which lead to shortened telomeres, but they are responsible for only ~20% of FPF (34). Furthermore, these variants have also been found in people with IPF, where they also lead to shorter telomeres and are associated with a worse prognosis (14, 41), although the proportion of risk explained in IPF is likely to be smaller than in FPF. Interestingly, males with FPF caused by TERT mutations have a lower age of death than females (13). Short telomeres lead to a process of accelerated aging known as cellular senescence, and indeed cellular senescence is particularly associated with pulmonary fibrosis, raising the prospect that senolytics may be useful therapies in IPF (16, 48, 60). Somewhat worryingly in the context of the global COVID pandemic, a number of studies have demonstrated that virus-induced lung injury leads to worse pulmonary fibrosis in aged mice (7, 40, 57), and understanding the relationship between SARS-CoV-2 infection and ARDS in elderly patients, or those with telomere gene mutations, is clearly going to be important to understand the risk of developing post-COVID pulmonary fibrosis.
METABOLIC SYNDROME AND IPF
Metabolic syndrome is the combination of abdominal obesity, insulin resistance, hypertension, and hyperlipidemia (47). Patients with IPF are usually overweight, with an average body mass index (BMI) of >28 kg/m2 (18, 27), and are more likely to have diabetes and hypertension at diagnosis than matched non-IPF control subjects, leading to an increased risk of cardiovascular disease (11). Metabolic syndrome is associated with increased activity of mammalian target of rapamycin (mTOR) and reduced activity of AMP-activated protein kinase (AMPK) (20, 46), and these two key metabolic pathways have been implicated in the pathogenesis of pulmonary fibrosis and, which may afford therapeutic opportunities, mTOR regulates cell growth and proliferation. mTOR forms two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 is regulated by multiple signals such as growth factors, amino acids, and cellular energy, activates numerous essential cellular processes including translation and transcription, and inhibits autophagy and has also been implicated in other pathological processes including cancer (39). There is evidence of increased mTOR signaling in IPF both from experimental (37, 61) and genetic (3) studies. Furthermore, inhibitors of mTOR are well tolerated and able to inhibit cellular metabolism in patients with IPF (31). AMPK is a cellular bioenergetic sensor and metabolic regulator (25). Metformin is an AMPK activator, has been shown to inhibit established fibrosis via activation of AMPK (45), and promotes resolution of fibrosis by altering myofibroblast cell fate (29), although observational data from clinical trials in patients with IPF are not supportive of metformin for use in patients with IPF (53). The increased risk of severe COVID-19 in patients with metabolic syndrome is also well described and is leading to policy changes in the United Kingdom. Similarly, inhibitors of mTOR have been identified as a potential novel therapy for COVID-19 (19), and observational studies have identified that metformin is associated with reduced mortality in COVID-19, particularly in women (6, 32). Understanding the interaction between cellular metabolism, respiratory infection, and fibrosis is likely to lead to important insights.
VIRUS-INDUCED PULMONARY FIBROSIS
It is not possible to discuss the etiology of pulmonary fibrosis in 2020 without considering the role of viruses. Early analysis from patients hospitalized with COVID-19 suggests a high rate of lung function abnormalities consistent with pulmonary fibrosis, particularly impaired gas transfer (47%), although this may reflect in part the vasculopathy that is emerging as a key feature of COVID-19. In patients hospitalized with SARS abnormalities in gas transfer were observed in 16% and abnormal chest X-rays were observed in 30%, and 62% of patients had computed tomographic (CT) evidence of pulmonary fibrosis soon after hospital discharge (5, 23). In a follow-up study of 36 patients surviving Middle East respiratory syndrome (MERS) coronavirus infection, 33% had radiographic evidence of pulmonary fibrosis (12). The role of other respiratory viruses in promoting pulmonary fibrosis is less clear and is generally limited to acute exacerbations of IPF and in vivo models (10, 28, 44).
However, there is clear evidence for a role of virus-induced fibrosis in the liver. Hepatitis C, which like the coronaviruses, is a positive-sense single-stranded RNA virus, is a potently fibrogenic virus, leads to over 200 million infected people worldwide, and is a major indication for liver transplantation (49). Furthermore, there are some historical data suggesting a high prevalence of hepatitis C in patients with IPF (4, 35, 59). More recent data have suggested that viral infections are also associated with an increased risk of IPF; again these were related primarily to chronic rather than acute viral infections (50) and support data implicating herpesviruses as an etiological factor for IPF. The fact that these viruses establish lifelong latency and may reemerge as cell-mediated immunity wanes with aging makes for a compelling hypothesis (38). Whether COVID-19 can lead to progressive pulmonary fibrosis at the current time remains to be determined, but clearly this is an area in urgent need of study to inform the likely risks of long-term post-COVID disease.
CONCLUSIONS
COVID-19 and pulmonary fibrosis are severe diseases characterized by lung injury and repair. In some people, particularly elderly men with metabolic syndrome, there is dysregulated repair that may be due to a genetic predisposition or prolonged environmental exposures to inhaled matter or ingested calorific foods, coupled with shortening telomeres and waning cell-mediated immunity with advancing years. This failing repair may manifest as an overexuberant immune response exacerbating viral injury in COVID-19 or excessive fibroblast proliferation and matrix synthesis in pulmonary fibrosis. The mechanisms driving the failing repair may, however, be linked, and therefore studying these molecular pathways in detail may reveal evidence to support repurposing drugs such as metformin or senolytics such as danazol or trialing compounds inhibiting mTOR or TGF-β signaling and improve outcomes in both conditions.
GRANTS
G. Jenkins is funded by a National Institute for Health Research, Research Professorship Award (RP-2017-08-ST2-014) and is a founding trustee of the charity Action for Pulmonary Fibrosis.
DISCLOSURES
G. Jenkins reports grants from Astra Zeneca, grants from Biogen, personal fees from Boehringer Ingelheim, personal fees from Daewoong, personal fees from Galapagos, grants from Galecto, grants from GlaxoSmithKline, personal fees from Heptares, nonfinancial support from NuMedii, grants and personal fees from Pliant, personal fees from Promedior, nonfinancial support from Redx, and personal fees from Roche.
AUTHOR CONTRIBUTIONS
G.J. prepared figure; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Abramson MJ, Murambadoro T, Alif SM, Benke GP, Dharmage SC, Glaspole I, Hopkins P, Hoy RF, Klebe S, Moodley Y, Rawson S, Reynolds PN, Wolfe R, Corte TJ, Walters EH; Australian IPF Registry . Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case-control study. Thorax. In press. doi: 10.1136/thoraxjnl-2019-214478. [DOI] [PubMed] [Google Scholar]
- 2.Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM, Guillen-Guio B, Ma SF, Okamoto T, John AE, Obeidat M, Yang IV, Henry A, Hubbard RB, Navaratnam V, Saini G, Thompson N, Booth HL, Hart SP, Hill MR, Hirani N, Maher TM, McAnulty RJ, Millar AB, Molyneaux PL, Parfrey H, Rassl DM, Whyte MK, Fahy WA, Marshall RP, Oballa E, Bossé Y, Nickle DC, Sin DD, Timens W, Shrine N, Sayers I, Hall IP, Noth I, Schwartz DA, Tobin MD, Wain LV, Jenkins RG. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med 5: 869–880, 2017. doi: 10.1016/S2213-2600(17)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen RJ, Guillen-Guio B, Oldham JM, Ma SF, Dressen A, Paynton ML, Kraven LM, Obeidat M, Li X, Ng M, Braybrooke R, Molina-Molina M, Hobbs BD, Putman RK, Sakornsakolpat P, Booth HL, Fahy WA, Hart SP, Hill MR, Hirani N, Hubbard RB, McAnulty RJ, Millar AB, Navaratnam V, Oballa E, Parfrey H, Saini G, Whyte MK, Zhang Y, Kaminski N, Adegunsoye A, Strek ME, Neighbors M, Sheng XR, Gudmundsson G, Gudnason V, Hatabu H, Lederer DJ, Manichaikul A, Newell JD Jr, O’Connor GT, Ortega VE, Xu H, Fingerlin TE, Bossé Y, Hao K, Joubert P, Nickle DC, Sin DD, Timens W, Furniss D, Morris AP, Zondervan KT, Hall IP, Sayers I, Tobin MD, Maher TM, Cho MH, Hunninghake GM, Schwartz DA, Yaspan BL, Molyneaux PL, Flores C, Noth I, Jenkins RG, Wain LV. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 201: 564–574, 2020. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, Saito S, Ikeda K, Kumada H. Hepatitis C virus enhances incidence of idiopathic pulmonary fibrosis. World J Gastroenterol 14: 5880–5886, 2008. doi: 10.3748/wjg.14.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology 228: 810–815, 2003. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 6.Bramante C, Ingraham N, Murray T, Marmor S, Hoversten S, Gronski J, McNeil C, Feng R, Guzman G, Abdelwahab N, King S, Meehan T, Benson B, Pendleton K, Vojta D, Tignanelli CJ. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19 (Preprint). medRxiv 2020. doi: 10.1101/2020.06.19.20135095. [DOI] [PMC free article] [PubMed]
- 7.Bueno M, Lai YC, Romero Y, Brands J, St. Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera S, Selman M, Lonzano-Bolaños A, Konishi K, Richards TJ, Kaminski N, Pardo A. Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L593–L601, 2013. doi: 10.1152/ajplung.00320.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairns JT, Habgood A, Edwards-Pritchard RC, Joseph C, John AE, Wilkinson C, Stewart ID, Leslie J, Blaxall BC, Susztak K, Alberti S, Nordheim A, Oakley F, Jenkins G, Tatler AL. Loss of ELK1 has differential effects on age-dependent organ fibrosis. Int J Biochem Cell Biol 120: 105668, 2020. doi: 10.1016/j.biocel.2019.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 194: 265–275, 2016. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 11.Dalleywater W, Powell HA, Hubbard RB, Navaratnam V. Risk factors for cardiovascular disease in people with idiopathic pulmonary fibrosis: a population-based study. Chest 147: 150–156, 2015. doi: 10.1378/chest.14-0041. [DOI] [PubMed] [Google Scholar]
- 12.Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, Larsson SG, Langer RD. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging 27: 342–349, 2017. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, Rosenblatt RL, Girod CE, Garrity ER, Xing C, Garcia CK. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One 5: e10680, 2010. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhindsa RS, Mattsson J, Nag A, Wang Q, Wain LV, Allen R, Wigmore EM, Ibanez K, Vitsios D, Deevi SV, Wasilewski S, Karlsson M, Lassi G, Olsson H, Muthas D, Mackay A, Murray L, Young S, Haefliger C; FinnGen Consortium, Maher TM, Belvisi MG, Jenkins G, Molyneaux P, Platt A, Petrovski S. Identification of a novel missense variant in SPDL1 associated with idiopathic pulmonary fibrosis (Preprint). bioRxiv 2010. doi: 10.1101/2020.06.29.178079. [DOI] [PMC free article] [PubMed]
- 15.Drake T, Docherty AB, Harrison E, Quint J, Adamali H, Agnew S, Babu S, Barber C, Barratt S, Bendstrup E, Bianchi S, Castillo D, Chaudhuri N, Chua F, Coker R, Chang W, Cranshaw A, Crowley L, Dosanjh D, Fiddler C, Forrest IA, George P, Gibbons M, Groom K, Haney S, Hart S, Heiden E, Henry M, Ho LP, Hoyles R, Hutchinson J, Hurley K, Jones M, Jones S, Kokosi M, Kreuter M, Mackay L, Mahendran S, Margaritopoulos G, Molina-Molina M, Molyneaux P, O'Brien AD, O'Reilly K, Packham A, Parfrey H, Poletti V, Porter J, Renzoni E, Rivera-Ortega P, Russell AM, Saini G, Spencer LG, Stella G, Stone H, Sturney S, Thickett D, Thillai M, Wallis T, Ward K, Wells AU, West A, Wickremasinghe M, Woodhead F, Herson G, Howard L, Openshaw PJM, Baillie JK, Semple MG, Stewart I, Jenkins RG. Outcome of hospitalisation for COVID-19 in patients with interstitial lung disease: an international multicentre study (Preprint). medRxiv 2020. doi: 10.1101/2020.07.15.20152967. [DOI] [PMC free article] [PubMed]
- 16.Dressen A, Abbas AR, Cabanski C, Reeder J, Ramalingam TR, Neighbors M, Bhangale TR, Brauer MJ, Hunkapiller J, Reeder J, Mukhyala K, Cuenco K, Tom J, Cowgill A, Vogel J, Forrest WF, Collard HR, Wolters PJ, Kropski JA, Lancaster LH, Blackwell TS, Arron JR, Yaspan BL. Analysis of protein-altering variants in telomerase genes and their association with MUC5B common variant status in patients with idiopathic pulmonary fibrosis: a candidate gene sequencing study. Lancet Respir Med 6: 603–614, 2018. doi: 10.1016/S2213-2600(18)30135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369: m1985, 2020. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JH, Kolb M, Algamdi M, Morisset J, Johannson KA, Shapera S, Wilcox P, To T, Sadatsafavi M, Manganas H, Khalil N, Hambly N, Halayko AJ, Gershon AS, Fell CD, Cox G, Ryerson CJ. Baseline characteristics and comorbidities in the CAnadian REgistry for Pulmonary Fibrosis. BMC Pulm Med 19: 223, 2019. doi: 10.1186/s12890-019-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med 8: 807–815, 2020. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 220: T1–T23, 2014. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 22.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O’Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177: 56–65, 2008. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 23.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, Ko FW, Chan MC, Chan DP, Tong MW, Rainer TH, Ahuja AT, Cockram CS, Sung JJ. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 60: 401–409, 2005. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frías S, Baumgartner MA, DiGianni L, Gampala-Sagar SN, Leone DA, Gulati S, El-Chemaly S, Goldberg HJ, Putman RK, Hatabu H, Raby BA, Rosas IO. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med 201: 1240–1248, 2020. doi: 10.1164/rccm.201908-1571OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52: 381–400, 2012. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Königshoff M, Kolb M, Laurent GJ, Nanthakumar CB, Olman MA, Pardo A, Selman M, Sheppard D, Sime PJ, Tager AM, Tatler AL, Thannickal VJ, White ES; ATS Assembly on Respiratory Cell and Molecular Biology . An official American Thoracic Society Workshop Report: Use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol 56: 667–679, 2017. doi: 10.1165/rcmb.2017-0096ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A, Hubbard RB, Leeming DJ, Marshall RP, Karsdal MA, Lukey PT, Maher TM. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med 3: 462–472, 2015. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 28.Jolly L, Stavrou A, Vanderstoken G, Meliopoulos VA, Habgood A, Tatler AL, Porte J, Knox A, Weinreb P, Violette S, Hussell T, Kolb M, Stampfli MR, Schultz-Cherry S, Jenkins G. Influenza promotes collagen deposition via αvβ6 integrin-mediated transforming growth factor β activation. J Biol Chem 289: 35246–35263, 2014. doi: 10.1074/jbc.M114.582262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G, Herold S, Schermuly RT, Mari B, Li X, Seeger W, Günther A, Bellusci S, El Agha E. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 10: 2987, 2019. doi: 10.1038/s41467-019-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 200: 377–389, 2004. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukey PT, Harrison SA, Yang S, Man Y, Holman BF, Rashidnasab A, Azzopardi G, Grayer M, Simpson JK, Bareille P, Paul L, Woodcock HV, Toshner R, Saunders P, Molyneaux PL, Thielemans K, Wilson FJ, Mercer PF, Chambers RC, Groves AM, Fahy WA, Marshall RP, Maher TM. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J 53: 1801992, 2019. doi: 10.1183/13993003.01992-2018. [DOI] [PubMed] [Google Scholar]
- 32.Luo P, Qiu L, Liu Y, Liu XL, Zheng JL, Xue HY, Liu WH, Liu D, Li J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg 103: 69–72, 2020. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, Sethi T. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 185: 537–546, 2012. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathai SK, Newton CA, Schwartz DA, Garcia CK. Pulmonary fibrosis in the era of stratified medicine. Thorax 71: 1154–1160, 2016. doi: 10.1136/thoraxjnl-2016-209172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meliconi R, Andreone P, Fasano L, Galli S, Pacilli A, Miniero R, Fabbri M, Solforosi L, Bernardi M. Incidence of hepatitis C virus infection in Italian patients with idiopathic pulmonary fibrosis. Thorax 51: 315–317, 1996. doi: 10.1136/thx.51.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meliopoulos VA, Van de Velde LA, Van de Velde NC, Karlsson EA, Neale G, Vogel P, Guy C, Sharma S, Duan S, Surman SL, Jones BG, Johnson MD, Bosio C, Jolly L, Jenkins RG, Hurwitz JL, Rosch JW, Sheppard D, Thomas PG, Murray PJ, Schultz-Cherry S. An epithelial integrin regulates the amplitude of protective lung interferon responses against multiple respiratory pathogens. PLoS Pathog 12: e1005804, 2016. doi: 10.1371/journal.ppat.1005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer PF, Woodcock HV, Eley JD, Platé M, Sulikowski MG, Durrenberger PF, Franklin L, Nanthakumar CB, Man Y, Genovese F, McAnulty RJ, Yang S, Maher TM, Nicholson AG, Blanchard AD, Marshall RP, Lukey PT, Chambers RC. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 71: 701–711, 2016. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore BB, Moore TA. Viruses in idiopathic pulmonary fibrosis. Etiology and exacerbation. Ann Am Thorac Soc 12, Suppl 2: S186–S192, 2015. doi: 10.1513/AnnalsATS.201502-088AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14: 473–480, 2015. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naik PN, Horowitz JC, Moore TA, Wilke CA, Toews GB, Moore BB. Pulmonary fibrosis induced by γ-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-β. J Gerontol A Biol Sci Med Sci 67: 714–725, 2012. doi: 10.1093/gerona/glr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrovski S, Todd JL, Durheim MT, Wang Q, Chien JW, Kelly FL, Frankel C, Mebane CM, Ren Z, Bridgers J, Urban TJ, Malone CD, Finlen Copeland A, Brinkley C, Allen AS, O’Riordan T, McHutchison JG, Palmer SM, Goldstein DB. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am J Respir Crit Care Med 196: 82–93, 2017. doi: 10.1164/rccm.201610-2088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544, 2001. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177: 82–90, 2008. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao J, Zhang M, Bi J, Wang X, Deng G, He G, Luan Z, Lv N, Xu T, Zhao L. Pulmonary fibrosis induced by H5N1 viral infection in mice. Respir Res 10: 107, 2009. doi: 10.1186/1465-9921-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, Zmijewski JW. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med 24: 1121–1127, 2018. [Erratum in Nat Med 24: 1627, 2018]. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123: 2764–2772, 2013. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep 20: 12, 2018. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol 20: 11033–11053, 2014. doi: 10.3748/wjg.v20.i32.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, Wang Y, Zhang W, Zhang HL. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest 157: 1175–1187, 2020. doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res 177: 104759, 2020. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spagnolo P, Kreuter M, Maher TM, Wuyts W, Bonella F, Corte TJ, Kopf S, Weycker D, Kirchgaessler KU, Ryerson CJ. Metformin does not affect clinically relevant outcomes in patients with idiopathic pulmonary fibrosis. Respiration 96: 314–322, 2018. doi: 10.1159/000489668. [DOI] [PubMed] [Google Scholar]
- 54.Tatler AL, Goodwin AT, Gbolahan O, Saini G, Porte J, John AE, Clifford RL, Violette SM, Weinreb PH, Parfrey H, Wolters PJ, Gauldie J, Kolb M, Jenkins G. Amplification of TGFβ induced ITGB6 gene transcription may promote pulmonary fibrosis. PLoS One 11: e0158047, 2016. doi: 10.1371/journal.pone.0158047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatler AL, Habgood A, Porte J, John AE, Stavrou A, Hodge E, Kerama-Likoko C, Violette SM, Weinreb PH, Knox AJ, Laurent G, Parfrey H, Wolters PJ, Wallace W, Alberti S, Nordheim A, Jenkins G. Reduced Ets domain-containing protein Elk1 promotes pulmonary fibrosis via increased integrin αvβ6 expression. J Biol Chem 291: 9540–9553, 2016. doi: 10.1074/jbc.M115.692368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatler AL, Jenkins G. TGF-β activation and lung fibrosis. Proc Am Thorac Soc 9: 130–136, 2012. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 57.Torres-González E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, Mora AL. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 46: 748–756, 2012. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trieu V. TGF-beta as the target for COVID-19 therapies. BMJ 369: m1610, 2020. doi: 10.1136/bmj.m1610. [DOI] [PubMed] [Google Scholar]
- 59.Ueda T, Ohta K, Suzuki N, Yamaguchi M, Hirai K, Horiuchi T, Watanabe J, Miyamoto T, Ito K. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis 146: 266–268, 1992. doi: 10.1164/ajrccm/146.1.266. [DOI] [PubMed] [Google Scholar]
- 60.Waters DW, Blokland KE, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, Schuliga M, Grainge CL, Knight DA. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 315: L162–L172, 2018. doi: 10.1152/ajplung.00037.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodcock HV, Eley JD, Guillotin D, Platé M, Nanthakumar CB, Martufi M, Peace S, Joberty G, Poeckel D, Good RB, Taylor AR, Zinn N, Redding M, Forty EJ, Hynds RE, Swanton C, Karsdal M, Maher TM, Bergamini G, Marshall RP, Blanchard AD, Mercer PF, Chambers RC. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun 10: 6, 2019. doi: 10.1038/s41467-018-07858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]