Abstract
PM2.5 and formaldehyde (FA) are major outdoor and indoor air pollutants in China, respectively, and both are known to be harmful to human health and to be carcinogenic. Of all the known chronic health effects, leukaemia is one of the most serious health risks associated with these two pollutants. To explore the influence and underlying mechanisms of exposure to formaldehyde and PM2.5 on hematopoietic toxicity, we systematically studied the toxicity induced in hematopoietic organs: bone marrow (BM); spleen; and myeloid progenitor cells (MPCs). Male Balb/c mice were exposed to: PM2.5 (20, 160 μg/kg·d) at a dose of 40 μL per mouse or formaldehyde (0.5, 3.0 mg/m3) for 8 h per day for 2 weeks or co-exposed to formaldehyde and PM2.5 (20 μg/kg·d PM2.5 + 0.5 mg/m3 FA, 20 μ/kg·d PM2.5 + 3 mg/m3 FA, 160 μg/kg·d PM2.5 + 0.5 mg/m3 FA, 160 μg/kg·d PM2.5 + 3 mg/m3 FA) for 2 weeks. Similar toxic effects were found in the formaldehyde-only and PM2.5-only groups, including significant decrease of blood cells and MPCs, along with decreased expression of hematopoietic growth factors. In addition, individual exposure of formaldehyde or PM2.5 increased oxidative stress, DNA damage and immune system disorder by destroying the balance of Th1/Th2, and Treg/Th17. DNA repair was markedly inhibited by deregulating the mammalian target of rapamycin (mTOR) pathway. Combined exposure to PM2.5 and formaldehyde led to more severe effects. Administration of Vitamin E (VE) was shown to attenuate these effects. In conclusion, our findings suggested that PM2.5 and formaldehyde may induce hematopoietic toxicity by reducing the expression of hematopoietic growth factors, increasing oxidative stress and DNA damage, activating the ‘immune imbalance’ pathway and suppressing the DNA-repair related mTOR pathway. The hematopoietic toxicity induced by combined exposure of PM2.5 and formaldehyde might provide further insights into the increased incidence of hematological diseases, including human myeloid leukaemia.
Keywords: PM2.5, Formaldehyde, Combined exposure, Hematopoietic toxicity, Molecular mechanism
1. Introduction
Data from the World Health Organisation (WHO) indicates that air pollution causes millions of deaths every year, creating a huge social burden (WHO., 2014). Leukaemia is a malignant tumour in the hematopoietic system, and it is the primary cause of death from tumour in children and people under 35 years old in China (Chen et al., 2012). Leukaemia is known to be the most serious outcome of hematopoietic toxicity, and it is among three typical consequences including anaemia, myelodysplastic syndrome and hematopoietic malignancies (Xiong, 2012). Several studies have found that PM2.5 and formaldehyde may independently be aetiological factors in leukaemia development (Jin et al., 2016; Wei et al., 2017). Air pollution is a combined exposure system that includes multiple air pollutants present in outdoor and indoor air. The outdoor and indoor pollutants with the most prominent health impacts on urban Chinese residents are PM2.5 and formaldehyde, respectively. The combined exposure to formaldehyde and PM2.5 is common in the living environment of urban residents in China. However, the hematopoietic toxicity resulting from combined exposure to PM2.5 and formaldehyde has not been extensively studied. Furthermore, the underlying molecular mechanism and characteristics of hematopoietic toxicity from combined exposure remains unknown. Therefore, it is of great importance to determine whether there is a causal relationship between the combined exposure and haemopoietic toxicity, whether there is a synergistic effect and what is the main molecular mechanism of PM2.5 and/or formaldehyde induced hematopoietic toxicity.
Formaldehyde is a massively produced industrial chemical widely used in construction, wood processing, furniture, textiles and carpeting. Since formaldehyde-containing products are so ubiquitous, indoor formaldehyde pollution affects nearly 70% of the urban population (Tang et al., 2009). It has been reported that long-term exposure to formaldehyde at concentrations exceeding the national standard exert a variety of toxic effects on the nasopharynx, lung, brain and skin, and also on the hematopoietic organs bone marrow (BM) and the spleen (IARC, 2012). On the basis of epidemiological studies, the International Agency for Research on Cancer (IARC) classified formaldehyde as a human leukaemogen (IARC, 2012), while some studies on humans and animals showed that occupational exposure to formaldehyde can disrupt hematopoietic function and lead to hematopoietic toxicity (Wei et al., 2017; Zhang et al., 2010a). There is much debate regarding formaldehyde-induced leukaemia because the biological evidence is insufficient, and further exploration of the detailed mechanism is needed.
PM2.5 is mainly generated from vehicle exhaust and coal combustion. Hence, northern Chinese cities suffer from serious PM2.5 pollution when residents start heating their homes in winter (Jin et al., 2016). At present, roughly 70% of the total Chinese urban population is affected by PM2.5 pollution (SEPA, 2017), and PM2.5 is ranked the most serious air pollutant in China (Ostro et al., 2018). To date, PM2.5 pollution has received increased attention since once it is inhaled into the respiratory system, it can easily be spread by the circulatory system via microvessels (special capillaries) and then further transported to other organs to induce toxicity in a variety of systems, including the respiratory and cardiovascular systems as well as the immune and hematopoietic systems (Bai et al., 2019; Cai et al., 2019; Kim et al., 2015; Oliveira et al., 2019; Ramgolam et al., 2012). The first evidence for a link between exposure to particulate matter and a higher risk of developing leukaemia came primarily from epidemiological studies. One of these studies found that exposure to a lesser concentration PM10 increases the risk of leukaemia (Vinceti et al., 2012), while another found that polycyclic aromatic hydrocarbons (PAHs), a major component of PM2.5, increased the incidence of childhood leukaemia (Talbott et al., 2011). Cell and animal studies have shown that exposure to PM2.5 induced leukaemia cell (HL-60 and K562 cell lines) growth to a significant extent and accelerated development of leukaemia in leukaemia model mice (Jin et al., 2016). However, results from a Canadian epidemiological study found no linkage between PM2.5 exposure and leukaemia (Winters et al., 2015). More research is needed to confirm whether there is a causal association.
Immunologists have shown that dysfunction of the immune system is a pathogenesis for acute myeloid leukaemia (AML) (Ersvaer et al., 2010; Wu et al., 2009). Animal studies have shown that there is an increase in levels of inflammatory factors in the BM of mice while exposed to formaldehyde (Zhang et al., 2013). Likewise, PM2.5 exposure amplified the release of inflammatory factors in leukaemia model mice (Jin et al., 2016). Based on the above, we looked at the release of inflammatory factors in target organs and cells to explore the role and the immunological mechanism of formaldehyde and/or PM2.5 exposure on hematopoietic toxicity.
DNA damage has a causative role in several human diseases, including infectious diseases, cancers, immune system dysfunction, neurodegeneration, and leukaemia (Rohr and Wyzga, 2012; Thys et al., 2015). DNA damage includes DNA base damage, DNA oxidative damage, DNA-adducts, DNA strand breaks and DNA–protein crosslinks (DPCs) (Li et al., 2017). Studies have shown that once DNA damage occurs, the body initiates DNA repair, in which the mammalian target of rapamycin (mTOR) pathway plays a vital role (Guo et al., 2013; Shen et al., 2013). Whether formaldehyde and/or PM2.5 exposure lead to hematopoietic toxicity by inhibiting DNA repair after DNA damage has occurred, remains unclear.
Vitamin E (VE) is a potent fat-soluble antioxidant that can prevent or minimise free-radical damage (Cerecetto et al., 2007). VE could remove excessive reactive oxygen species (ROS) to protection against ambient PM2.5 or formaldehyde induced inflammatory response and oxidative stress (Liang et al., 2016). VE is also easily absorbed by cells and has the benefit of being readily available from foods and medicines. Thus, it is reasonable to apply VE as an effective and economic protectant for ROS induction.
In this study, we assessed the influence, and the underlying mechanism of exposure to formaldehyde and/or PM2.5 on hematopoietic toxicity of the blood, bone marrow (hematopoietic organ) and spleen (compensatory hematopoietic organ) and target cells of leukaemogenesis myeloid progenitor. We examined whether PM2.5 and/or formaldehyde exposure induced oxidative damage, inflammation and DNA damage in BM, spleen and myeloid progenitor cells (MPCs). We also examined the colony-stimulating factors, including granulocyte–macrophage colony-stimulating factor (GM-CSF) and erythropoietin (EPO), key regulators of hematopoietic stem cells (HSCs) that differentiate into mature blood cells.
2. Materials and methods
2.1. Animal care
Specific pathogen-free class (SPF) male Balb/c mice (about 24 g) were housed in a standard animal centre (temperature 20–25 °C, 12 h day/night, humidity, 50–70%). Mice were acclimatised for seven days before the experiment initiation. The office of Scientific Research Management of Central China Normal University (Wuhan, China) had agreed to all experimental procedures for this research, and approved the certificate of application for the use of animals on May 20, 2018 (approval ID: SCXK2018-0013).
2.2. Reagents and kits
Formalin was purchased from Sigma (St. Louis, MO, USA). Mouse methylcellulose semi-solid medium was purchased from StemCell Technologies (Vancouver, BC, Canada). We bought Trizol, M – MLV, OligodT, dNTP, RRI and SYBR premix Ex TaqTM from TAKARA (Otsu, Japan). Enzyme-linked immunosorbent assay (ELISA) kits of 8-hydroxy-deoxyguanosine (8-OH-dG), nuclear factor kappa-B (NF-κB), EPO, GM-CSF were obtained from Shanghai Blue Gene. ELISA kits of interferon-γ (IFN-γ), interleukin-4 (IL-4), interleukin-10 (IL-10) and interleukin-17A (IL-17A) were obtained from eBioscience (San Diego, CA, USA).
2.3. PM2.5 collection and characterisation
We used high-traffic total suspended particulate (TSP) sampler to collect PM2.5 samples during the winter of 2015 in the Heping District of Tianjin, China, the PM2.5 sample collection covers certain districts. During the period of collecting PM2.5 (November 2015 to March 2016), the pollution level of PM2.5 in Tianjin is moderate and severe, which represents the pollution level in North China. Ion chromatograph (ICS-500, Dionex, Sunnyvale, CA), High-performance liquid chromatography (HPLC, Agilent 1200 Series, Palo Alto, USA) and inductively coupled plasma atomic emission spectroscopy (ICP-AES, 61E Trace and ICP-750, Termo Jarrell-Ash, Franklin, MA) were used to detect inorganic ions concentrations, PAHs and mineral elements in the PM2.5 samples, respectively.
2.4. Preparation of formaldehyde and PM2.5
Gaseous formaldehyde concentration was controlled at 0.5 mg/m3 or 3.0 mg/m3. We used a WH2 small intelligent environment climate chamber (Yuxin Company, Wuhan, China) to monitor parameters. Temperature, humidity and airflow rate were maintained at (22 ± 1)°C, (44 ± 2)% and 3 L/min. During exposure, air formaldehyde concentration in the chamber was monitored every 2 h using a gaseous formaldehyde analyser (4160–2, InterScan, Simi Valley, CA, USA).
Loading PM2.5 samples filters were cut up and ultrasound dispersed PM2.5 samples into deionised water, vacuum freeze-dried PM2.5 samples. PM2.5 was dispersed in 0.9% physiological saline to use. The volume of intranasal instillation PM2.5 physiological saline used was 40 μL per mouse.
2.5. Experimental protocol
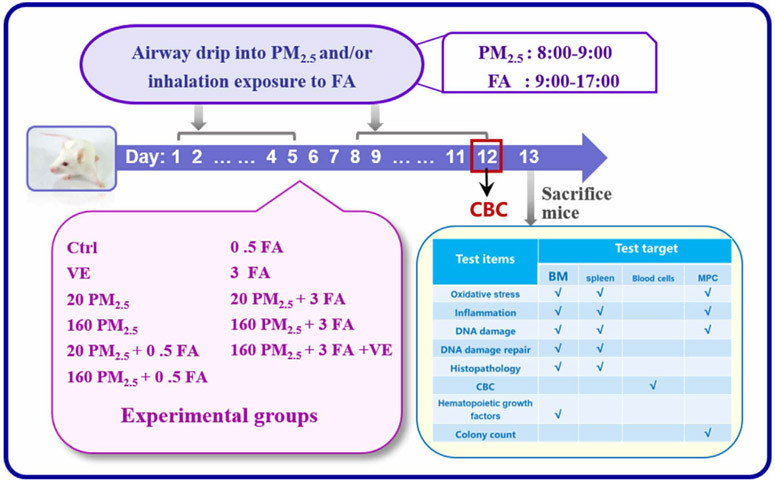
There were 11 experimental groups with six mice randomly assigned to each group, and the specific procedure of this study is given in Fig. 1. The route of formaldehyde exposure simulated the typical occupational exposure patterns of eight-hour working time, and the occupational exposure population included pathologists, anatomy students, nurses and preservatives and manufacturing workers (Zhang et al., 2010a). Mice were exposed to formaldehyde via whole-body inhalation (5 days/week, 8 h/day), PM2.5 exposure via airway drip (3 days/week, 2 weeks). During the airway drip process, mice need to be anaesthetised. To minimise the damage caused by anaesthesia, we took a total of 2 days of PM2.5 dose in once airway drip. Although the airway drip was administered every other day, 3 days/week, the total PM2.5 dose was 5 days a week.
Fig. 1.
The experimental protocol. (PM2.5: 20 μg/kg·d, 160 μg/kg·d; FA: 0.5 mg/m3, 3 mg/m3).
2.6. Sample preparation
After the last treatment on the 12th day, we collected 30 μL of blood samples from the caudal veins for blood cells count. On the 13th day, mice were anaesthetised and then sacrificed; we collected BM cells and spleen for subsequent experiments.
2.7. Complete blood count (CBC)
The number of lymphocytes (LYM), monocytes (MON), neutrophilic granulocytes (GRA), red blood cells (RBC) and platelets (PLT) were counted in a blood cell analyser (Motenu MTN-21, China).
2.8. Colony-forming experiment of myeloid progenitor cells
Colonies originated from committed progenitor cells that differentiate erythrocytes and reticulocytes are named burst-forming unit–erythroids (BFU-E); however, those that differentiate macrophages and granulocytes are named colony-forming unit–granulocyte-macrophages (CFU-GM) (Wei et al., 2017). We used methylcellulose media containing growth factors to culture BM cells, and further differentiated into BFU-E and CFU-GM. 2 × 105 BM cells were plated in a 35-mm culture dish for CFU-GM and BFU-E differentiation. We counted CFU-GM and BFU-E colonies forming and harvested after 12 days.
2.9. Histological assay
Both femurs and the spleen were excised and fixed with a 4% neutral buffered polyformaldehyde overnight at room temperature. Then, haematoxylin and eosin (H&E) staining was used, and the tissues were embedded in paraffin and sectioned into 10-μm-thick slices.
2.10. ROS, glutathione (GSH), DPCs content detection
The ROS and DPCs concentrations in mouse BM, spleen and MPCs were detected by means of previous method (Zhang et al., 2010b). GSH concentration was measured using GSH test kits (Nanjing, China). Methods refer to the kit instructions.
2.11. Quantitative analyses of cytokines (NF-κB, IFN-γ, IL-4, IL-10, IL-17A) and other proteins (8-OH-dG, EPO, GM-CSF) in BM, spleen and MPCs
ELISA kits were used to detect the concentration of 8-OH-dG, EPO, GM-CSF and cytokines of NF-κB, IFN-γ, IL-4, IL-10 and IL-17A in the BM, spleen and myeloid progenitor cells. Specific testing methods refer to the kit instructions.
2.12. Expression of NF-κB, EPO, GM-CSF, mTOR, S6K1, FANCD2, ATM, Chk2, γH2AX mRNA
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was used to measure NF-κB, EPO, GM-CSF, mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (S6K1), Fanconi anaemia complementation group D2 (FANCD2), ataxia telangiectasia mutated gene (ATM), checkpoint kinase (Chk2) and histone H2AX phosphorylation (γH2AX) mRNA expression in BM. The two-step reverse transcription method was first used to extract total RNA from BM cells, and then it was reverse transcribed into complementary DNA (cDNA). RT-qPCR amplification was performed using a fluorescence quantitative PCR instrument (CFX96, Bio-Rad, San Diego, CA, USA). PCR primers are listed in the Supplemental Materials, Table 1. We used β-actin as the reference gene (refer to Supplemental Materials for details).
2.13. Immunohistochemical assay
The 5-μm slice of femur was deparaffinised, rehydrated and microwave retrieved, then incubated in the dark with H202, blocked by albumin from bovine serum. Immunohistochemical measurement of mTOR, FANCD2, Chk2 and γH2AX were conducted using primary antibodies anti-mTOR (1:100, Abcam, Cambridge, UK), anti- FANCD2 (1:100, Abcam, Cambridge, UK), anti-Chk2 (1:50, Santa Cruz, Dallas, USA) and anti-γH2AX (1:50, Millipore, MA, USA). Primary antibody incubation was completed, then the sections were incubated in the corresponding secondary antibody. Sections were visualised using diaminobenzidine tetrahydrochloride (DAB) and counterstained with haematoxylin, dehydrated and mounted in DPX.
2.14. Statistical analysis
GraphPad Prism 7.0 (San Diego, CA, USA) was used to generate statistical graphs. All experimental statistics were shown as mean ± standard deviation. Statistical analyses were conducted by SPSS ver13 (SPSS, Chicago, IL, USA). A one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was applied to analyse the significance between the groups. Based on statistical norms, values of p < 0.05 were considered statistically significant, and p < 0.01 was considered extremely significant.
3. Results and discussion
3.1. PM2.5 chemical composition
Inorganic ions, PAHs, and mineral elements are the main constituents of PM2.5. The analysis of PM2.5 sample composition can be found in the Supplemental Material, Table S2.
3.2. The effect of PM2.5 and/or formaldehyde on blood cells counts, MPCs and hematopoietic growth factors
BM is the most important hematopoietic organ because mature blood cells are derived from the HSCs in BM. Therefore, any change in peripheral blood cell counts can be used to evaluate hematopoiesis after environmental/occupational exposure to PM2.5 and/or formaldehyde (Wei et al., 2017). Our study found that exposure to formaldehyde (3 mg/m3) led to a significant reduction in RBCs, PLT, MON, LYM and GRA, as did combined exposure to PM2.5 and formaldehyde (160 PM2.5 + 3 FA) (Fig. 2a). The number of LYM, MON, GRA, RBC and PLT in the 160 PM2.5 + 3 FA group was significantly lower (p < 0.01) than in the 160 PM2.5 group, but were not significantly different compared to the 3 FA group. The significantly lower counts of LYM, MON, GRA, RBC and PLT after exposure to PM2.5 and formaldehyde closely resembles some clinical manifestations of hematopoietic diseases, such as myelofibrosis, aplastic anaemia and megakaryocytic leukaemia (Oki et al., 2006; Zhang et al., 2013). A decrease in blood cell counts largely indicates that hematopoiesis in the BM has been suppressed. Since exposure to PM2.5 and formaldehyde induced hematopoietic toxicity, further study of hematological diseases, such as human myeloid leukaemia, should be undertaken.
Fig. 2.
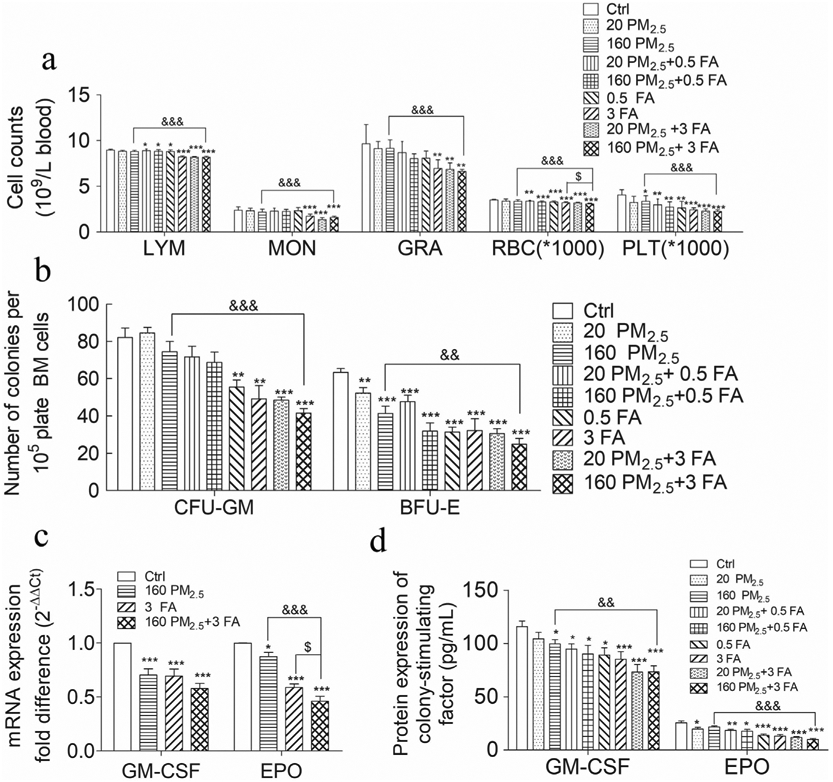
a Complete blood cell counts, lymphocytes (LYM), monocytes (MON), neutrophilic granulocytes (GRA), red blood cells (RBC) and platelets (PLT); b colony-forming unit–granulocyte-macrophages (CFU-GM) and burst-forming unit–erythroids (BFU-E) colonies forming from BM; c granulocyte–macrophage colony-stimulating factor (GM-CSF) and erythropoietin (EPO) mRNA expression in BM; d GM-CSF and EPO protein expression in BM. n = 6 for each group, * means p < 0.05, ** means p < 0.01, *** means p < 0.001, compared with the control group; & means p < 0.05, && means p < 0.01, &&& means p < 0.001, compared with the 160 PM2.5 group; $ means p < 0.05, $$ means p < 0.01, $$$ means p < 0.001, compared with the 3 FA group.
Leukaemia, and other hematopoietic system diseases, such as myelofibrosis and aplastic anaemia, originate from damaged HSCs in BM. Two published studies suggest an inhibitory and/or toxic effect from exposure to formaldehyde on the MPC in humans and mice, respectively (Wei et al., 2017; Zhang et al., 2010a). However, these studies focus only on human exposure to occupationally high levels of formaldehyde, or the effects of low-level environmental formaldehyde exposure on mice, and not on the effects of PM2.5 exposure on BM and BM HSCs or hematopoietic progenitor cells (HPCs). In this study, occupational and/or environmental formaldehyde and/or PM2.5 exposure were considered, and we found that formaldehyde exposure (3 mg/m3) led to a significant reduction in the CFU-GM and BFU-E colonies (p < 0.01), as did combined exposure to PM2.5 and formaldehyde (160 PM2.5 + 3 FA). CFU-GM and BFU-E colonies in the 160 PM2.5 + 3 FA group were also reduced significantly (p < 0.01). However, compared with the 3 FA group, CFU-GM and BFU-E numbers in the 160 PM2.5 + 3 FA group were not significantly altered compared with the 3 FA group (Fig. 2b). MPC formation decreased in the BM of mice exposed to FA and/or PM2.5, occupationally or environmentally. This suggests that both FA and PM2.5 are toxic for BM and MPC.
Hematopoiesis is strictly controlled by many cytokines involved in the process of HSCs differentiating into mature blood cells (Stein and Baldwin, 2013). GM-CSF and EPO play a crucial role in HSCs differentiating into CFU-GM and BFU-E, and GM-CSF could also positively regulate CFU-GM differentiating into GRA and MON (Shieh J H and A, 1989). Therefore, changes induced by exposure to formaldehyde and PM2.5 in the expression of GM-CSF and EPO would lead to changes in the formation of CFU-GM and BFU-E colonies and in the mature blood cell count.
We measured GM-CSF, EPO mRNA and protein expression in BM cells and found that exposure to PM2.5 (160 μg/kg·d) and/or formaldehyde (3 mg/m3) significantly decreased (p < 0.01) levels of GM-CSF, EPO mRNA and protein compared with the control group (Fig. 2c, Fig. 2d). GM-CSF and EPO protein expression decreased significantly in the 160 PM2.5 + 3 FA group (p < 0.01) when compared with the 160 PM2.5 group.
In our study, we found that exposure to formaldehyde or PM2.5 alone could significantly downregulate both mRNA levels and GM-CSF and EPO protein expression. Combined exposure to formaldehyde and PM2.5 further reduced the expression of GM-CSF, EPO, mRNA and protein. The decrease in GM-CSF and EPO levels reduced the formation and differentiation of myeloid progenitor cells and decreased the blood cell counts.
3.3. The effect of PM2.5 and/or formaldehyde on the histology of BM and the spleen
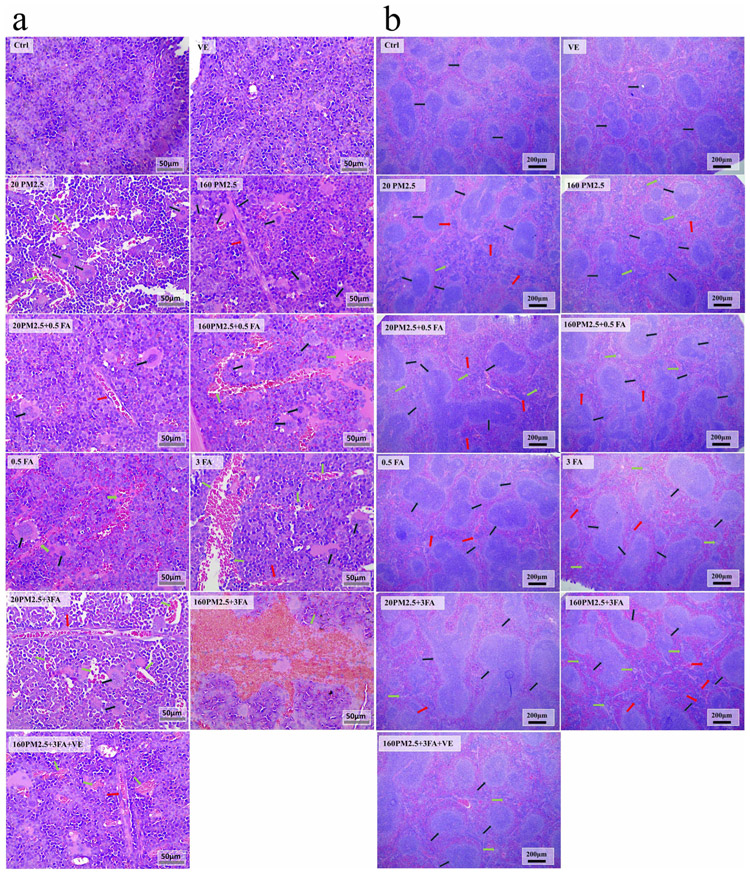
We used H&E staining to evaluate histological changes in the BM (Fig. 3a) and in the compensatory hematopoietic organ, the spleen (Fig. 3b). Exposure to formaldehyde and/or PM2.5 increased myelofibrosis and megakaryocytes, along with some large cavities filled with RBCs because of the reduction of BM cells in BM. This exposure also increased lymphoproliferation, megakaryocytes and expansion of the germinal centre of splenic corpuscles. In addition, as the concentration of formaldehyde and PM2.5 increased, these effects were exacerbated. Administration of VE to the combined exposure group demonstrated a significant protective effect from the adverse effects described above.
Fig. 3.
a Representative images (×400) of H&E stained mouse femurs after formaldehyde and/or PM2.5 exposure, n = 3 for each group. Red arrows: myelofibrosis, black arrows: megakaryocytes, green arrows: the cavity formed by the reduction of BM cells; b representative images (×400) of H&E stained mice spleen after formaldehyde and/or PM2.5 exposure. Red arrows: lymphoproliferation, green arrows: macrophages, black arrows: the germinal centre of splenic corpuscle.
The abnormal histological changes in the BM and spleen provide direct evidence of the toxicity induced by exposure to formaldehyde and/or PM2.5. The histological changes seen in the BM indicate the potential risk of developing hematopoietic diseases, such as megakaryocytic leukaemia and blood cancer, which are characterised by fibrotic BM (Lowery and Schneider, 2013). As a compensatory hematopoietic organ, the spleen provides extramedullary hematopoiesis when the bone marrow hematopoietic function is damaged (Wan, 2009). The histological changes in the spleens of the mice indicated spleen injury, which would reduce its extramedullary hematopoiesis and further increase hematopoietic toxicity.
We examined the change of blood cell counts, the BFU-E and CFU-GM colonies, mRNA and protein expression of both GM-CSF and EPO, respectively. The above results of blood cells counts, HSCs and colony-stimulating factor were all consistent with the results of histology. It was mutually verified from different levels that the combined exposure of PM2.5 and formaldehyde did cause hematopoietic toxicity.
3.4. The effect of PM2.5 and/or formaldehyde on oxidative stress, NF-κB expression and the protective effects of VE
The results of complete blood counts, colony-formation from myeloid progenitors and histopathology suggest that exposure to formaldehyde and PM2.5 induced BM and spleen toxicity, and potentially increased the risk of hematopoietic toxicity. Several studies have shown that benzene exposure induced oxidative stress in the HSC/HPC niche, which is one of the potential mechanisms of leukaemogenesis (Battisti et al., 2008; McHale et al., 2012; Snyder, 2012; Zhou et al., 2010). Excessive ROS, generated by oxidative stress, is widely recognised as one of the initial events resulting in damage to health as a result of exposure to many environmental chemicals (Apel and Hirt, 2004; Finkel and Holbrook, 2000). GSH can remove excessive ROS to restore the body’s oxidant and antioxidant balance (Li et al., 2018). We therefore assessed the levels of the oxidative stress markers (ROS and GSH) in the BM, spleen and MPCs after formaldehyde and/or PM2.5 exposure.
In the 160 PM2.5 + 3 FA group, the ROS levels in the BM cells, spleen and MPCs were all significantly increased compared to the control group (Fig. 4a, p < 0.01). In comparison with the 160 PM2.5 + 3 FA group, ROS levels in the BM cells, spleen and MPCs in the 160 PM2.5 + 3 FA + VE group tended to decrease, indicating that VE had a protective effect (Fig. 4c). GSH levels in the 160 PM2.5 + 3 FA group decreased significantly in the BM cells and in the spleen, while levels of GSH in the MPCs decreased dramatically (Fig. 4b, p < 0.05). In comparison with the 160 PM2.5 group or the 3 FA group, the levels of GSH in the BM cells decreased significantly in the 160 PM2.5 + 3 FA group (p < 0.01). Moreover, GSH in the spleen of the 160 PM2.5 + 3 FA group also decreased when compared with the 160 PM2.5 group (p < 0.01). Administration of VE attenuated the decrease in GSH levels in the BM and spleen of the 160 PM2.5 + 3 FA group ((Fig. 4b, p < 0.05). Levels of NF-κB mRNA and protein increased significantly compared with the control group (Fig. 4e, Fig. 4f). Levels of NF-κB protein in the BM of the 160 PM2.5 + 3 FA group were higher than those of the 160 PM2.5 group (p < 0.05). Our study confirmed that formaldehyde and/or PM2.5 exposure induced excessive ROS generation and GSH depletion in the BM, spleen and MPCs. The decline in GSH levels reflects a reduction in antioxidant capacity, suggesting that combined exposure induces oxidative stress. Excessive ROS accumulation, and GSH depletion indicated the imbalance of the body’s oxidants and antioxidants, demonstrating the occurrence of oxidative damage. Shen’s study found that ROS accumulation induced the upregulation of NF-κB, which is consistent with the results described above (Shen et al., 2017).
Fig. 4.
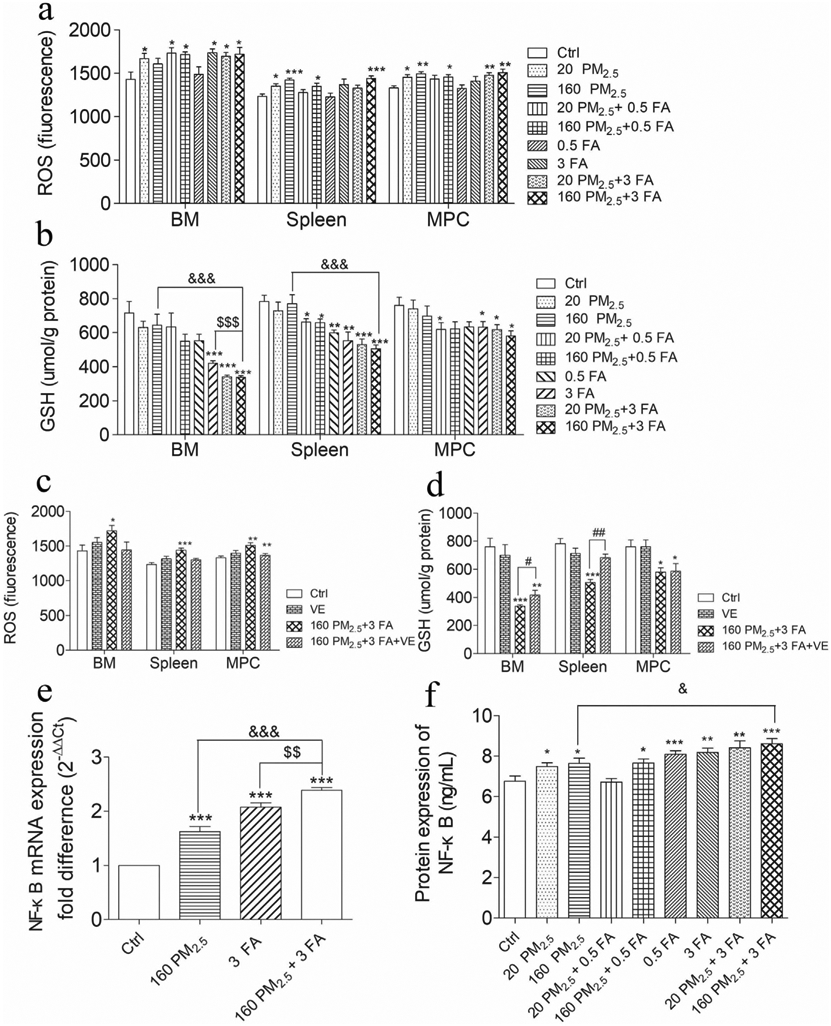
Oxidative stress in bone marrow (BM), spleen and myeloid progenitor cells (MPCs) and the protective effects of VE. a Reactive oxygen species (ROS) level in BM, spleen and MPC; b glutathione (GSH) level in BM, spleen and MPCs; c ROS level in BM, spleen and MPCs after VE administration; d GSH level in BM, spleen and MPC after VE administration; e Nuclear factor kappa-B (NF-κB) mRNA expression, f NF-κB protein expression. n = 6 for each group, * means p < 0.05, ** means p < 0.01, *** means p < 0.001, compared with the control group; & means p < 0.05, && means p < 0.01, &&& means p < 0.001, compared with the 160 PM2.5 group; $ means p < 0.05, $$ means p < 0.01, $$$ means p < 0.001 compared with the 3 FA group.
Several human panel and mouse studies have demonstrated that exposure to air pollutants, such as particulate matter, formaldehyde and ozone, result in adverse health effects. Oxidative stress has been shown to be one of the most important mechanisms involved in such damage. VE has been found to play a protective role in preventing deterioration (Liang et al., 2016). Romieu’s study found that supplementation with VC and VE might modulate the impact of ozone exposure on the small airways of children with moderate to severe asthma, and that the levels of plasma α-tocopherol, an oxidation product of VE, significantly increased after VE supplementation (Romieu et al., 2002). A high-resolution metabolomics study showed that traffic-related air pollution (TRAP) could activate VE metabolism, and these antioxidants decreased with TRAP exposure (Liang et al., 2018). Salonen’s study showed that men who smoked had considerably lowered baseline levels of both plasma a-tocopherol and ascorbate and that the mean plasma a-tocopherol concentration increased after combined supplementation with reasonable doses of VE and VC. In addition, VE and VC can slow the progression of common carotid atherosclerosis in men, this preventive effect may be generalisable to all men (Salonen et al., 2000). Jin’s study found that the effects of PM2.5 on the progression of leukaemia are caused by both inflammation and ROS-mediated signal transducer and activator of transcription 3 (STAT3) activation. The ROS inhibitor, NAC, inhibits the growth of leukaemia cells and could be a potential target for intervention (Jin et al., 2016). Maternal exposure to PM2.5 causes progressive senescence of HSCs via the ROS-p38 mitogen-activated protein kinase (MAPK) pathway and the nuclear factor erythroid-2 related factor 2 (Nrf2) pathway under the exposure-induced preferential impairment of the BM microenvironment with age related phenotypes (Bhattarai et al., 2019). Previous mouse studies have shown that ROS levels were increased in the BM, spleen and BM-derived CFU-GM cells of formaldehyde-exposed mice and that ROS played a role in formaldehyde-induced hematopoietic toxicity (Wei et al., 2017). Results from our study show that ROS levels significantly increased, and GSH levels significantly decreased in the BM, spleen and MPCs after the administration of VE, indicating that VE had an antioxidant effect, results which are generally consistent with the above-mentioned human panel studies. In summary, the mechanism by which VE protects against adverse effects caused by air pollutants is that VE has an effective anti-oxidative and anti-inflammatory effect, hence blocking the biological responses that initiate the pathophysiological process and thus averting downstream adverse health effects.
3.5. The effect of PM2.5 and/or formaldehyde on DNA damage and DNA repair related to the mTOR pathway
It has been found that some environmental chemicals such as organophosphate pesticides, benzene and formaldehyde can cause oxidative stress in isolated cells and in different organisms, leading to DNA damage to cells, including hematopoietic stem cells/progenitor cells (HSPCs) and consequently inducing hematopoietic toxicity (Bedard and Krause, 2007; Kolachana et al., 1993; Ojha and Srivastava, 2014; Thys et al., 2015; Wei et al., 2017). Increased production of DPCs, 8-OH-dG, γH2AX is a marker for DNA damage. The formation of DPCs is thought to be the major mode of DNA damage caused by formaldehyde exposure (Ren et al., 2013), while PM2.5 exposure increased the formation of 8-OH-dG, a biomarker of oxidative DNA damage (Li et al., 2018), and γH2AX is the biomarker of DNA double-strand breaks (Chan et al., 2016). We therefore looked for the DPCs, 8-OH-dG and γH2AX biomarkers, to evaluate the level of DNA damage in the BM, spleen and MPCs after exposure to formaldehyde and/or PM2.5.
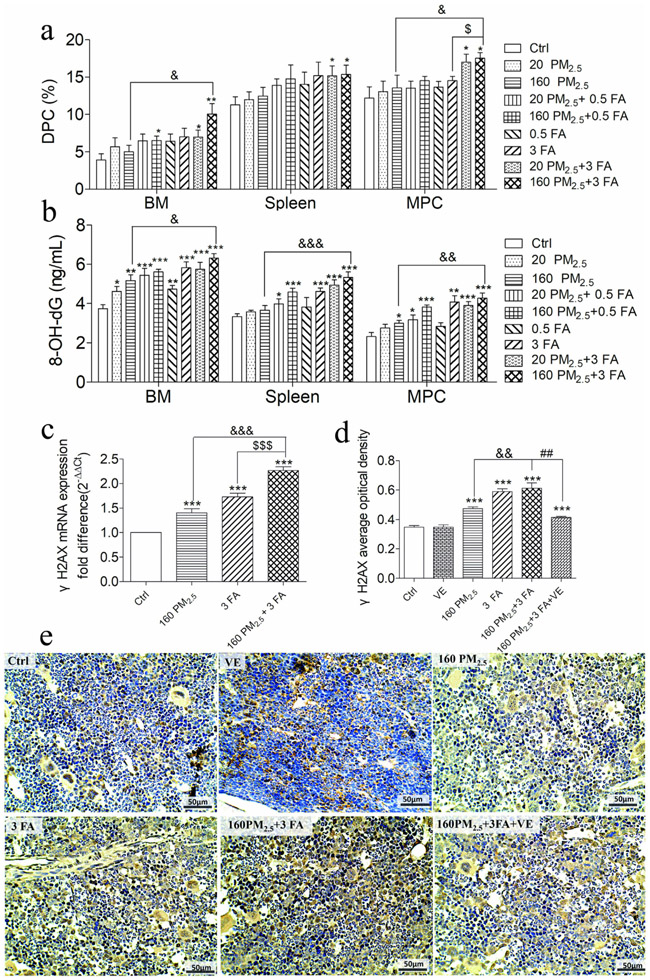
Levels of DPCs in BM cells, spleen and MPCs in the 160 PM2.5 + 3 FA group were significantly increased compared to the control group (Fig. 5a, p < 0.05). In comparison with the 160 PM2.5 group and the 3 FA group, the level of DPCs in the MPCs in the 160 PM2.5 + 3 FA group was significantly increased (Fig. 5a, p < 0.05). Levels of 8-OH-dG in BM cells, spleen MPCs in the 160 PM2.5 + 3 FA group were significantly increased compared to the control group (Fig. 5b, p < 0.01). In addition, 8-OH-dG in BM (p < 0.05), spleen and MPCs (p < 0.01) in the 160 PM2.5 + 3 FA group were much higher than those in the 160 PM2.5 group. The level of γH2AX mRNA and protein in the 160 PM2.5 group, in the 3 FA group, in the 160 PM2.5 + 3 FA group were significantly increased compared to the control group (Fig. 5c, 5d). Increased levels of DPCs, 8-OH-dG and γH2AX in the BM, spleen and MPCs indicate consistently that PM2.5 and/or formaldehyde exposure could lead to DNA damage.
Fig. 5.
DNA damage in the bone marrow (BM), spleen and myeloid progenitor cells (MPCs). a DNA–protein crosslinks (DPCs) level in BM, spleen and MPCs; b 8-hydroxy-deoxyguanosine (8-OH-dG) level in BM, spleen and MPCs; c histone H2AX phosphorylation (γH2AX) mRNA expression; d γH2AX average optical density analyses; e representative images (×400) of the expression of γH2AX by immunohistochemistry (brown colour stain), nuclei were stained by 4′ , 6-diamidino-2-phenylindole (DAPI) reagents (blue). n = 6 for each group, * means p < 0.05, ** means p < 0.01, *** means p < 0.001, compared with the control group; & means p < 0.05, && means p < 0.01, &&& means p < 0.001, compared with the 160 PM2.5 group; $ means p < 0.05, $$ means p < 0.01, $$$ means p < 0.001 compared with the 3 FA group.
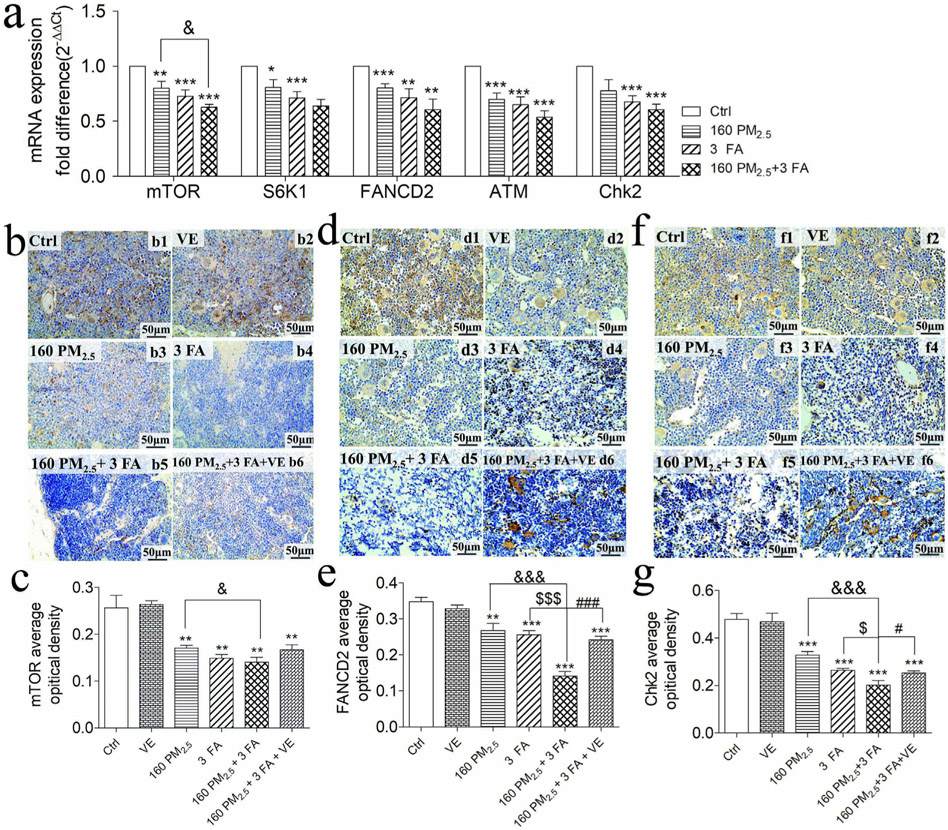
Under normal circumstances, when DNA damage occurs, the body activates the DNA repair pathway, where mTOR activates FANCD2 through mTORC1-S6K1, which in turn activates the ATM-Chk2 test site to repair DNA, thereby reducing DNA damage (Shen et al., 2013). We were therefore interested in whether exposure to PM2.5 and/or formaldehyde activated NF-κB expression, hence suppressing expression of FANCD2 and DNA repair. To examine this, we measured the mRNA levels of mTOR, S6K1, FANCD2, ATM and Chk2 in the mTOR signalling pathway that participates in DNA repair (Fig. 6a). The results indicated that mRNA expression levels of all five of these molecules decreased significantly in the BM when compared with the control group (p < 0.01). The mRNA expression of mTOR in the 160 PM2.5 + 3 FA group was downregulated in comparison with the 160 PM2.5 group (p < 0.05). We examined the protein of these molecules using immunohistochemistry (Fig. 6b, d, f) and found that the protein expression of mTOR, FNACD2 and Chk2 in BM decreased significantly compare to the control group and that all of these protein expressions were upregulated when VE was administered (Fig. 6c, e, g).
Fig. 6.
mRNA expression of molecules in the mTOR signalling pathway and immunohistochemical staining of mouse bone marrow (×400), nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) reagents (blue). a mRNA expression of mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (S6K1), Fanconi anaemia complementation group D2 (FANCD2), ataxia telangiectasia mutated gene (ATM) and checkpoint kinase (Chk2); b representative images of the expression of mTOR by immunohistochemistry (brown colour stain); c mTOR average optical density analyses; d representative images of the expression of FANCD2 by immunohistochemistry (brown colour stain); e FANCD2 average optical density analyses; f representative images of the expression of Chk2 by immunohistochemistry (brown colour stain); g Chk2 average optical density analyses. n = 6 for each group, * means p < 0.05, ** means p < 0.01, *** means p < 0.001, compared with the control group; & means p < 0.05, && means p < 0.01, &&& means p < 0.001, compared with the 160 PM2.5 group.
Our study found that exposure to PM2.5 and/or formaldehyde causes DNA damage in bone marrow, the essential hematopoietic organ. We also observed that the expression of mTOR and FANCD2, a key molecule of the mTOR pathway, were downregulated. Previous studies have also shown that both PM2.5 and formaldehyde can inhibit the mTOR signalling pathway. Zhang et al. reported the downregulation of the mTOR/P70S6K1 signalling pathway after PM2.5 exposure (Zhang et al., 2019), and Fang et al. found that formaldehyde exposure inhibits the expression of mTOR in a dose-dependent manner (Fang et al., 2015). Formaldehyde exposure has been reported to result in DPCs in lymphocytes, which was then shown to be reversed by the FANCD2-participating pathway (Ren et al., 2013). Coincidentally, FANCD2 was shown in another study to protect HSCs from formaldehyde-induced DNA damage (Pontel et al., 2015). Further study confirmed that activation of FANCD2 by monoubiquitination, is an essential step in the DNA damage response, during which the activated FANCD2 is translocated to the DNA repair centre and interacts with DNA repair proteins, such as the breast cancer susceptibility gene1 (BRCA1) or Rad51 (Deng, 2009). Animal experiments have shown that sustained activation of NF-κB can be induced by independent exposure to PM2.5 or formaldehyde (Song et al., 2017b; Zhang et al., 2013). Moreover, Guo's study showed that NF-κB activated in HSCs can bind to the promoter of FANCD2, inhibit the expression of FANCD2, and further lead to the lack of DNA repair. They also found that DNA repair can be restored when exogenous FANCD2 was applied (Guo et al., 2013). Based on our experimental findings, as well as the study findings mentioned above, the order of changes for these molecules could be that mTOR-S6K1 inhibition or NF-κB activation takes place first, followed by FANCD2 inhibition, which then induces the ATM-Chk2 inhibition.
We therefore assumed that the activation of NF-κB downregulated the expression of FANCD2 to inhibit DNA repair, leading to irreversible DNA damage in the BM, spleen and HSCs. The result of increased DNA damage is consistent with the result of DNA repair inhibition, both indicated that DNA damage might be caused by two factors: on one hand, the increase of DNA damage itself caused by the combined exposure of PM2.5 and formaldehyde, and on the other hand, the inhibition of DNA repair.
3.6. The effect of PM2.5 and/or formaldehyde on inflammatory cytokines
An immunological study found that immune system dysfunction is associated with the development of leukaemia, since an imbalance between Th1 and Th2 immune functions was noted in leukaemia patients (Ersvaer et al., 2010). Wrobel's study showed abnormal expression and differentiation of Th17 cells, and the cytokine IL-17 in the peripheral blood of AML patients (Wrobel et al., 2003). Our study confirmed that ROS could increase in bone marrow, spleen, myeloid progenitor cells after formaldehyde and/or PM2.5 exposure. The accumulation of ROS can activate NF-κB, which has a series of effects on its specific binding to DNA transcription, translation and other processes (Lu et al., 2010; Morgan and Liu, 2011). It is widely known that NF-κB can control the expression of immune-system, and inflammatory-related factors such as interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-12 (IL-12), to determine the inflammatory response (Tak and Firestein, 2001). Some recent studies have found that NF-κB has a crucial role in the development of Th2 and Th17 cells, mainly in the regulation of IL-4 and IL-17A secretion (Oh and Ghosh, 2013). We therefore measured NF-κB, mRNA and protein expression, in addition to IL-4, IFN- γ, IL-17A and IL-10 protein expression in the BM, spleen and MPCs after formaldehyde and/or PM2.5 exposure.
We determined IL-4, IFN- γ, IL-17A and IL-10 protein expression in BM, spleen and MPCs (Fig. 7). Compared with the control group, the level of IFN-γ in the 160 PM2.5 + 3 FA group decreased significantly in the BM (p < 0.05), spleen and MPCs (p < 0.01), while IL-4 and IL-17A increased markedly (p < 0.01). In comparison with the 160 PM2.5 group, IL-4, IL-17A in the 160 PM2.5 + 3 FA group increased significantly (p < 0.01), and the level of IL-10 in the 160 PM2.5 + 3 FA group decreased significantly (p < 0.01).
Fig. 7.
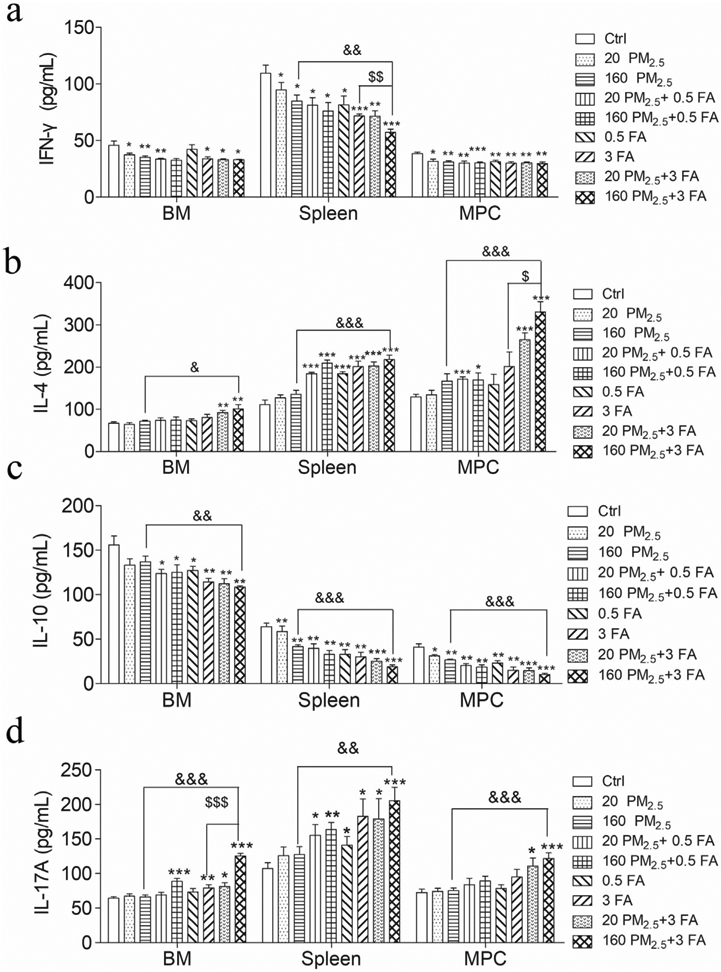
a Interferon-γ (IFN- γ) levels in BM, spleen and MPC; b interleukin-4 (IL-4) levels in BM, spleen and MPC; c interleukin-10 (IL-10) levels in BM, spleen and MPC; d interleukin-17A (IL-17A) levels in BM, spleen and MPCs. n = 6 for each group, * means p < 0.05, ** means p < 0.01, *** means p < 0.001 compared with the control group; & means p < 0.05, && means p < 0.01, &&& means p < 0.001 compared with the 160 PM2.5 group; $ means p < 0.05, $$ means p < 0.01, $$$ means p < 0.001 compared with the 3 FA group.
Results from our previous study that showed that ROS accumulation induced by exposure to phthalates led to the upregulation of NF-κB and promoted the release of IL-4 are consistent with the results described above (Shen et al., 2017). In the current study, activation of NF-κB after PM2.5 and/or FA exposure was seen to promote the release of the inflammatory cytokines, IL-4 and IL-17A, leading to an imbalance in Th1/Th2 and Treg/Th17 immune cells and resulting in an inflammatory reaction in the BM. In addition, emerging evidence confirms that certain inflammatory cytokines significantly affect HSCs in BM because it can induce HSC exhaustion following which hematopoietic malignancies might develop (Schuettpelz and Link, 2013).
Since air pollution comprises a multi-pollutant, combined exposure system, sometimes either synergistic or antagonistic effects between pollutants may lead to unpredictable results. In our study, we therefore investigated the hematopoietic impact of exposure to a combination of PM2.5 and formaldehyde in addition to the impact of exposure to PM2.5 or formaldehyde at low or high concentrations. We examined the level of some key molecules in vitro and in vivo, as well as looking for pathological changes in the BM and spleen in experimental animals. The results confirmed that exposure to PM2.5 or formaldehyde alone had relatively little effect on the BM and spleen of mice; however, the combined exposure to PM2.5 and formaldehyde had a significant synergistic adverse effect. Unfortunately, synergistic effects of the combined exposure may induce the hygienic or safety concerns that were not as great when exposed to either PM2.5 or formaldehyde. Our results are generally consistent with existing findings on combined exposure to formaldehyde and PM2.5. Liu’s study suggested PM2.5 plus formaldehyde combined exposure was more likely to induce AD-like pathologies than exposure to either PM2.5 or formaldehyde alone. Oxidative stress and inflammation may be involved in the toxic mechanisms (Liu et al., 2017). Song et al. showed that combined exposure to PM2.5 and formaldehyde can greatly aggravate allergic asthma in mice, as compared to exposure to PM2.5 or formaldehyde alone, and that the combined exposure indicated a certain synergistic effect (Song et al., 2017a).
3.7. Potential mechanisms of hematopoietic toxicity induced by PM2.5 and/or formaldehyde
Previous studies have shown that occupational exposure to formaldehyde damages hematopoietic function and causes chromosomal change-related leukaemia in cultured MPCs (Zhang et al., 2010a). Our recent studies have found that after formaldehyde exposure, myeloid progenitor and blood cell counts were lower, followed by a further decrease in hematopoietic growth factors, as well as the induction of oxidative stress, inflammation genotoxicity and apoptosis (Wei et al., 2017; Zhang et al., 2013). Jin’s in vitro and animal study showed that PM2.5 led to HL-60 and K562 leukaemia cell growth and increased release of inflammatory cytokines in animal models of leukaemia, suggesting that exposure to PM2.5 promoted the progression of leukaemia (Jin et al., 2016). To date, however, few comprehensive studies have been reported regarding combined exposure to PM2.5 and formaldehyde, and their potential to cause hematopoietic toxicity. This study systematically examines hematopoietic toxicity, including BM, the spleen, and stem/progenitor cells in the BM after PM2.5 and/or formaldehyde exposure. The results indicate that exposure to formaldehyde has a strong inhibitory effect in the BM, spleen and MPCs, which is similar to that for exposure to PM2.5. Moreover, we found that PM2.5 induced a weaker effect compared to formaldehyde.
A review of the literature identified several studies that showed that PM2.5 and formaldehyde might cause hematopoietic toxicity via different mechanisms outlined below. We will explore these in future work.
Zhang et al. proposed that the possible mechanism of formaldehyde causing hematopoietic toxicity was that formaldehyde directly induces DNA damage and chromosome aneuploidy in hematopoietic stem or early progenitor cells in the BM (Zhang et al., 2010a). In addition, considering that formaldehyde cannot reach the BM directly, Zhang suggested that formaldehyde may induce hematopoietic toxicity by damaging hematopoietic stem/progenitor cells circulating in the peripheral blood or by damaging the primitive pluripotent stem cells present within the nasal turbinates and/or olfactory mucosa, and then the damaged stem/progenitor cells return to the BM and become leukaemia stem cells (Zhang et al., 2009).
Other research found that PM2.5 disrupted the proliferation and secretion of cytokines in bone marrow mesenchymal stem cells (BM-MSCs), which destroyed the BM hematopoietic microenvironment (Abu-Elmagd et al., 2017; Méndez-Ferrer et al., 2010) and further induced progressive senescence and functional defects of HSC, resulting in hematopoietic toxicity (Bhattarai et al., 2020). Other studies have found that PAHs and heavy metals in PM2.5 are important components that cause hematopoietic toxicity. Grevenynghe et al. found that BaP markedly impair CD34+ cell expansion, and inhibit CD34+ cell differentiation into various hematological cell lineages through the caspase-and mitochondrion-related apoptosis process (van Grevenynghe et al., 2005). A subsequent study found that BM lymphoid and MPCs were suppressed in DMBA-treated mice and clearly identified the adverse effects of DMBA on BM hematopoiesis (N'Jai et al., 2010). Cadmium (Cd) activates the small GTPase cdc42 of the noncanonical Wnt signalling pathway, and further increases the expression of CCAAT/enhancer binding protein (C/EBPα), and decreases the expression of Hhex to impair HSC function (Zhao et al., 2018). Plumbum (Pb) destroys multiple enzyme systems of heme biosynthesis, and impairs the incorporation of iron into the mitochondrial enzyme, iron chelate enzyme, to inhibit BM hematopoietic function (Beier et al., 2015; Khalid and Abdollahi, 2019). In addition, since heavy metals and PAHs lead to shorter telomeres in HSC and blood, this may be another mechanism of hematopoietic toxicity (Hou et al., 2012; Sanders et al., 2009). From the above, we can conclude that formaldehyde and PM2.5 destroy the proliferation, differentiation of HSCs and hematopoietic microenvironment in BM through different mechanisms, inhibit hematopoietic function and lead to hematopoietic toxicity.
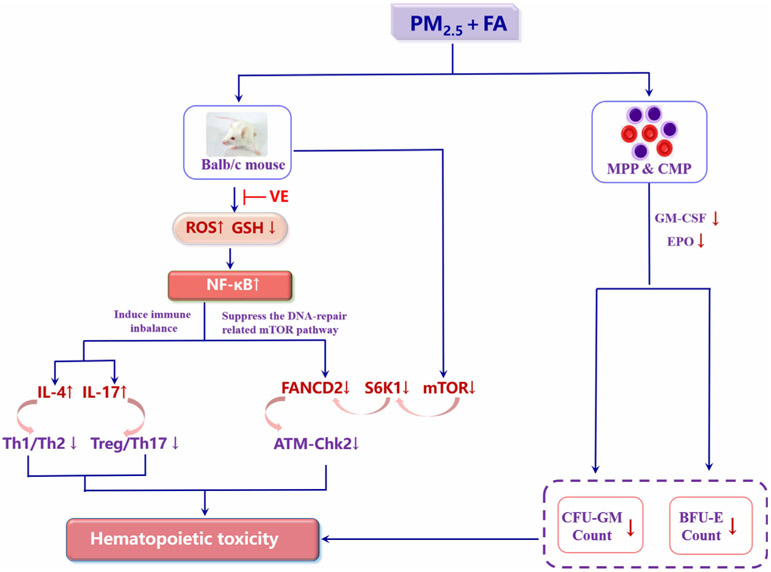
Regarding mechanisms of hematopoietic toxicity, a dual-pathway, mediated by NF-κB and NF-κB, can independently activate the ‘immune imbalance’ pathway (a promotion of the release of IL-4 and IL-17A by NF-κB, leading to an imbalance of both Th1/Th2 and Treg/Th17, resulting in inflammation in the BM, which in turn induces hematopoietic toxicity) and suppress the DNA-repair related mTOR pathway (leading to irreversible DNA damage in both bone marrow and spleen, thus resulting in hematopoietic toxicity) Fig. 8. We propose that these mechanisms underlie the hematopoietic toxicity induced by exposure to formaldehyde and/or PM2.5. Further study is essential to elucidate these mechanisms.
Fig. 8.
Schematic illustration of the potential mechanism of hematopoietic toxicity induced by PM2.5 and/or formaldehyde in mice. (ROS: reactive oxygen species; GSH: glutathione; NF-κB: nuclear factor kappa-B; IL-4: interleukin-4; IL-17A: interleukin-17A; mTOR: mammalian target of rapamycin; S6K1:ribosomal protein S6 kinase; FANCD2: Fanconi anaemia complementation group D2; ATM: ataxia telangiectasia mutated gene; Chk2: checkpoint kinase; HSC: hematopoietic stem cells; MPP: multipotent progenitor; CMP: common myeloid progenitor; GM-CSF: granulocyte–macrophage colony-stimulating factor; EPO: erythropoietin; CFU-GM: colony forming unit–granulocyte-macrophages ; BFU-E: burst-forming unit–erythroids).
4. Conclusion
We demonstrated that inhaled formaldehyde and PM2.5 is toxic to BM (hematopoietic organ), spleen (compensatory hematopoietic organ) and hematopoietic stem/progenitor cells. While formaldehyde and PM2.5 are both toxic to BM, spleen and hematopoietic stem/progenitor cells, we showed that formaldehyde has a stronger effect. Combined exposure to PM2.5 and formaldehyde leads to more severe hematopoietic toxicity in mice. These effects could, however, be largely blocked by concurrent administration of VE. The increase in oxidative stress, dysregulation of hematopoietic growth factors, the Th1/Th2 and Treg/Th17 immune imbalance activated by NF-κB and the suppression of the DNA-repair related mTOR pathway by NF-κB leading to irreversible DNA damage are potential underlying mechanisms of formaldehyde- and PM2.5-induced hematopoietic toxicity on mice.
Supplementary Material
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFC0702700) and the Fundamental Research Funds for the Central Universities (CCNU18JCXK07 and CCNU19TS066).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.106050.
References
- Abu-Elmagd M, Alghamdi MA, Shamy M, Khoder MI, Costa M, Assidi M, Kadam R, Alsehli H, Gari M, Pushparaj PN, Kalamegam G, Al-Qahtani MH, 2017. Evaluation of the Effects of Airborne Particulate Matter on Bone Marrow-Mesenchymal Stem Cells (BM-MSCs): Cellular, Molecular and Systems Biological Approaches. Int. J. Environ. Res. Public Health 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H, 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Goldberg MS, Lavigne E, Copes R, Martin RV, Kopp A, Chen H, 2019. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: A population-based study of 5.1 million Canadian adults living in Ontario. Environ. Int 132, 11. [DOI] [PubMed] [Google Scholar]
- Battisti V, Maders LD, Bagatini MD, Santos KF, Spanevello RM, Maldonado PA, Brule AO, Araujo Mdo C, Schetinger MR, Morsch VM, 2008. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem 41, 511–518. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K-H, 2007. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiolog. Rev 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Beier EE, Sheu T-J, Dang D, Holz JD, Ubayawardena R, Babij P, Puzas JE, 2015. Heavy Metal Ion Regulation of Gene Expression: MECHANISMS BY WHICH LEAD INHIBITS OSTEOBLASTIC BONE-FORMING ACTIVITY THROUGH MODULATION OF THE Wnt/βCATENIN SIGNALING PATHWAY. J. Biol. Chem 290, 18216–18226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai G, Lee JB, Kim M-H, Ham S, So H-S, Oh S, Sim H-J, Lee J-C, Song M, Kook S-H, 2020. Maternal exposure to fine particulate matter during pregnancy induces progressive senescence of hematopoietic stem cells under preferential impairment of the bone marrow microenvironment and aids development of myeloproliferative disease. Leukemia 34, 1481–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai G, Lee JB, Kim MH, Ham S, So HS, Oh S, Sim HJ, Lee JC, Song M, Kook SH, 2019. Maternal exposure to fine particulate matter during pregnancy induces progressive senescence of hematopoietic stem cells under preferential impairment of the bone marrow microenvironment and aids development of myeloproliferative disease. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Huang JY, Lin YP, Miao WL, Chen P, Chen X, Wang JX, Chen ML, 2019. Particulate matter 2.5 induced arrhythmogenesis mediated by TRPC3 in human induced pluripotent stem cell-derived cardiomyocytes. Arch. Toxicol 93, 1009–1020. [DOI] [PubMed] [Google Scholar]
- Cerecetto H, Lopez GV, 2007. Antioxidants Derived from Vitamin E: An Overview. Mini Reviews in Medicinal Chemistry. [DOI] [PubMed] [Google Scholar]
- Chan TK, Loh XY, Peh HY, Tan WNF, Tan WSD, Li N, Tay IJJ, Wong WSF, Engelward BP, 2016. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J. Allergy Clin. Immunol 138, 84-+. [DOI] [PubMed] [Google Scholar]
- Chen W, Shan B, Zheng R, Lin G, 2012. Analysis of incidence and mortality of leukemia in registration areas of China from 2003 to 2007. Tumor 32, 251–255. [Google Scholar]
- Deng N, 2009. Expression of FANCF/FANCD2 in acute myeloid leukemia and myelodysplastic syndrome. China medical university. [Google Scholar]
- Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O, 2010. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Li DH, Yu XQ, Lv MQ, Bai LZ, Du LZ, Zhou DX, 2015. Formaldehyde exposure inhibits the expression of mammalian target of rapamycin in rat testis. Toxicol. Indus. Health 0748233715592992. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ, 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. [DOI] [PubMed] [Google Scholar]
- Guo F, Li J, Du W, Zhang S, O'Connor M, Thomas G, Kozma S, Zingarelli B, Pang Q, Zheng Y, 2013. mTOR regulates DNA damage response through NF-kappaB-mediated FANCD2 pathway in hematopoietic cells. Leukemia 27, 2040–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Wang S, Dou C, Zhang X, Yu Y, Zheng Y, Avula U, Hoxha M, Díaz A, McCracken J, Barretta F, Marinelli B, Bertazzi PA, Schwartz J, Baccarelli AA, 2012. Air pollution exposure and telomere length in highly exposed subjects in Beijing, China: a repeated-measure study. Environ. Int 48, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC, 2012. Monographs on the evaluation of carcinogenic risks to humans -formaldehyde. World Health Organization, International Agency for Research on Cancer, pp. 35. [Google Scholar]
- Jin XT, Chen ML, Li RJ, An Q, Song L, Zhao Y, Xiao H, Cheng L, Li ZY, 2016. Progression and inflammation of human myeloid leukemia induced by ambient PM2.5 exposure. Arch. Toxicol 90, 1929–1938. [DOI] [PubMed] [Google Scholar]
- Khalid M, Abdollahi M, 2019. Epigenetic modifications associated with pathophysiological effects of lead exposure. J. Environ. Sci. Health Part C-Environ. Carcinogenesis Ecotoxicol. Rev 37, 235–287. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kabir E, Kabir S, 2015. A review on the human health impact of airborne particulate matter. Environ. Int 74, 136–143. [DOI] [PubMed] [Google Scholar]
- Kolachana P, Subrahmanyam VV, Meyer KB, Zhang L, Smith MT, 1993. Benzene and its phenolic metabolites produce oxidative DNA damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 53, 1023–1026. [PubMed] [Google Scholar]
- Li B, Guo L, Ku T, Chen M, Li G, Sang N, 2018. PM2.5 exposure stimulates COX-2-mediated excitatory synaptic transmission via ROS-NF-kappa B pathway. Chemosphere 190, 124–134. [DOI] [PubMed] [Google Scholar]
- Li R, Zhao L, Zhang L, Chen M, Shi J, Dong C, Cai Z, 2017. Effects of ambient PM2.5 and 9-nitroanthracene on DNA damage and repair, oxidative stress and metabolic enzymes in the lungs of rats. Toxicol. Res 6, 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Jiang S, Xie Y, Kan H, Song W, Zhao J, Ding W, 2016. Effect of Vitamin E and Omega-3 Fatty Acids on Protecting Ambient PM2.5-Induced Inflammatory Response and Oxidative Stress in Vascular Endothelial Cells. Plos One 11, e0152216-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, Walker DI, Sarnat SE, Chang HH, Greenwald R, Jones DP, Russell AG, Sarnat JA, 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ. Int 120, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Zhang YC, Luo C, Kang J, Li JQ, Wang K, Ma P, Yang X, 2017. At seeming safe concentrations, synergistic effects of PM2.5 and formaldehyde co-exposure induces Alzheimer-like changes in mouse brain. Oncotarget 8, 98567–98579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EW, Schneider SM, 2013. Ruxolitinib: a new treatment for myelofibrosis. Clin. J. Oncol. Nurs 17, 312–318. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, Liu CM, Shan Q, Wang YJ, 2010. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Cereb. Cortex 20, 2540–2548. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS, 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale CM, Zhang L, Smith MT, 2012. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 33, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG, 2011. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 21, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Jai AU, Larsen M, Shi L, Jefcoate CR, Czuprynski CJ, 2010. Bone marrow lymphoid and myeloid progenitor cells are suppressed in 7,12-dimethylbenz(a)anthracene (DMBA) treated mice. Toxicology 271, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Ghosh S, 2013. NF-kappaB: roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev 252, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha A, Srivastava N, 2014. In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen 761, 10–17. [DOI] [PubMed] [Google Scholar]
- Oki Y, Kantarjian HM, Zhou X, Cortes J, Faderl S, Verstovsek S, O'Brien S, Koller C, Beran M, Bekele BN, Pierce S, Thomas D, Ravandi F, Wierda WG, Giles F, Ferrajoli A, Jabbour E, Keating MJ, Bueso-Ramos CE, Estey E, Garcia-Manero G, 2006. Adult acute megakaryocytic leukemia: an analysis of 37 patients treated at MD Anderson Cancer Center. Blood 107, 880–884. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Slezakova K, Delerue-Matos C, Pereira MC, Morais S, 2019. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int 124, 180–204. [DOI] [PubMed] [Google Scholar]
- Ostro B, Spadaro JV, Gumy S, Mudu P, Awe Y, Forastiere F, Peters A, 2018. Assessing the recent estimates of the global burden of disease for ambient air pollution: Methodological changes and implications for low- and middle-income countries. Environ. Res 166, 713–725. [DOI] [PubMed] [Google Scholar]
- Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu LM, Swenberg JA, Crossan GP, Patel KJ, 2015. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 60, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramgolam K, Hamel R, Rumelhard M, Marano F, Baeza-Squiban A, 2012. Autocrine effect of EGFR ligands on the pro-inflammatory response induced by PM2.5 exposure in human bronchial epithelial cells. Arch. Toxicol 86, 1537–1546. [DOI] [PubMed] [Google Scholar]
- Ren X, Ji Z, McHale CM, Yuh J, Bersonda J, Tang M, Smith MT, Zhang L, 2013. The impact of FANCD2 deficiency on formaldehyde-induced toxicity in human lymphoblastoid cell lines. Arch. Toxicol 87, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr AC, Wyzga RE, 2012. Attributing health effects to individual particulate matter constituents. Atmos. Environ 62, 130–152. [Google Scholar]
- Romieu I, Sienra-Monge JJ, Ramfrez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, Rfo-Navarro B.E.d., Ruiz-Navarro MX, Hatch G, Slade R, 2002. Antioxidant Supplementation and Lung Functions among Children with Asthma Exposed to High Levels of Air Pollutants. Am. J. Respir. Crit. Care Med 166, pp. 703–709. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Nyyss?nen K, Salonen R, Lakka H-M, Kaikkonen J, Porkkala E-S, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, 2000. The effect of vitamin E and vitamin C on 3-year progression of carotid atherosclerosis: The antioxidant supplementation in atherosclerosis prevention (ASAP) study. 151, 25. [DOI] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB, 2009. Neurotoxic effects and biomarkers of lead exposure: a review. Rev. Environ. Health 24, 15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettpelz LG, Link DC, 2013. Regulation of hematopoietic stem cell activity by inflammation. Front. Immunol 4, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEPA, S.E.P.A., 2017. 2016 Report on the State of the Environment in China.
- Shen C, Oswald D, Phelps D, Cam H, Pelloski CE, Pang Q, Houghton PJ, 2013. Regulation of FANCD2 by the mTOR pathway contributes to the resistance of cancer cells to DNA double-strand breaks. Cancer Res. 73, 3393–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Li J, You H, Wu Z, Wu Y, Zhao Y, Zhu Y, Guo Q, Li X, Li R, Ma P, Yang X, Chen M, 2017. Oral exposure to diisodecyl phthalate aggravates allergic dermatitis by oxidative stress and enhancement of thymic stromal lymphopoietin. Food Chem. Toxicol 99, 60–69. [DOI] [PubMed] [Google Scholar]
- Shieh JH, A MM, 1989. Hematopoietic growth factor receptors. Cytotechnology 2. [DOI] [PubMed] [Google Scholar]
- Snyder R, 2012. Leukemia and benzene. Int. J. Environ. Res. Public Health 9, 2875–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kang J, Lin B, Li J, Zhu Y, Du J, Yang X, Xi Z, Li R, 2017a. Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci. Rep 7, 11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Li D, Li X, Ma L, Bai X, Wen Z, Zhang X, Chen D, Peng L, 2017b. Exposure to PM2.5 induces aberrant activation of NF-kappa B in human airway epithelial cells by downregulating miR-331 expression. Environ. Toxicol. Pharmacol 50, 192–199. [DOI] [PubMed] [Google Scholar]
- Stein SJ, Baldwin AS, 2013. Deletion of the NF-kappaB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood 121, 5015–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS, 2001. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest 107, 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott EO, Xu XH, Youk AO, Rager JR, Stragand JA, Malek AM, 2011. Risk of leukemia as a result of community exposure to gasoline vapors: A follow-up study. Environ. Res 111, 597–602. [DOI] [PubMed] [Google Scholar]
- Tang X, Bai Y, Duong A, Smith MT, Li L, Zhang L, 2009. Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ. Int 35, 1210–1224. [DOI] [PubMed] [Google Scholar]
- Thys RG, Lehman CE, Pierce LC, Wang YH, 2015. Environmental and chemotherapeutic agents induce breakage at genes involved in leukemia-causing gene rearrangements in human hematopoietic stem/progenitor cells. Mutat. Res 779, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grevenynghe J, Bernard M, Langouet S, Le Berre C, Fest T, Fardel O, 2005. Human CD34-positive hematopoietic stem cells constitute targets for carcinogenic polycyclic aromatic hydrocarbons. J. Pharmacol. Exper. Therapeutics 314, 693–702. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, Maffeis G, Ferretti E, Fabbi S, Teggi S, Consonni D, De Girolamo G, Meggiato A, Palazzi G, Paolucci P, Malagoli C, 2012. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case-control study in an Italian population. Eur. J. Epidemiol 27, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, 2009. Study of combined toxicity of formaldehyde and benzene on DNA damage of bone marrow cells of femal mice. J. Xinjiang Medical University. [Google Scholar]
- Wei C, Wen H, Yuan L, McHale CM, Li H, Wang K, Yuan J, Yang X, Zhang L, 2017. Formaldehyde induces toxicity in mouse bone marrow and hematopoietic stem/progenitor cells and enhances benzene-induced adverse effects. Arch. Toxicol 91, 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO., 2014. Health and the environment: addressing the health impact of air pollution. J. Chem. Phys 19, 1345–1351. [Google Scholar]
- Winters N, Goldberg MS, Hystad P, Villeneuve PJ, Johnson KC, Canadian Canc Registries E, 2015. Exposure to ambient air pollution in Canada and the risk of adult leukemia. Sci. Total Environ 526, 153–176. [DOI] [PubMed] [Google Scholar]
- Wrobel T, Mazur G, Jazwiec B, Kuliczkowski K, 2003. Interleukin-17 in acute myeloid leukemia. J. Cellular Mol. Med 7, 472–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, Qian J, Jin J, Xu H, 2009. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin. Exp. Immunol 158, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, 2012. Study on mechanism of hematopoietic toxicity and cell malignant transformation by benzene, Study on mechanism of hematopoietic toxicity and cell malignant transformation by benzene. South East University. [Google Scholar]
- Zhang L, Steinmaus C, Eastmond DA, Xin XK, Smith MT, 2009. Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms. Mutat. Res./Rev. Mutat. Res 681, 150–168. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tang X, Rothman N, Vermeulen R, Ji Z, Shen M, Qiu C, Guo W, Liu S, Reiss B, Freeman LB, Ge Y, Hubbard AE, Hua M, Blair A, Galvan N, Ruan X, Alter BP, Xin KX, Li S, Moore LE, Kim S, Xie Y, Hayes RB, Azuma M, Hauptmann M, Xiong J, Stewart P, Li L, Rappaport SM, Huang H, Fraumeni JF Jr., Smith MT, Lan Q, 2010a. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol. Biomarkers Prev 19, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yuan F, Liu X, Yang S, Zhang H, 2010b. Genotoxicity of Formaldehyde and Benzene Joint Inhalation on Bone-marrow Cells of Male Mice. J. Environ. Occupat. Med 27, 295–297. [Google Scholar]
- Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y, Ye X, Yang X, Ding S, 2013. Bone Marrow Injury Induced via Oxidative Stress in Mice by Inhalation Exposure to Formaldehyde. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang D, Yang B, Li B, Guo J, Xiao C, 2019. PM2.5 induces cell cycle arrest through regulating mTOR/P70S6K1 signaling pathway. Exper. Therapeutic Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li Q, Yang Z, Shao Y, Xue P, Qu W, Jia X, Cheng L, He M, He R, Zhou Z, Zhang Y, 2018. Cadmium Activates Noncanonical Wnt Signaling to Impair Hematopoietic Stem Cell Function in Mice. Toxicol. Sci 165, 254–266. [DOI] [PubMed] [Google Scholar]
- Zhou FL, Zhang WG, Wei YC, Meng S, Bai GG, Wang BY, Yang HY, Tian W, Meng X, Zhang H, Chen SP, 2010. Involvement of oxidative stress in the relapse of acute myeloid leukemia. J. Biol. Chem 285, 15010–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.