Abstract
Objective
Psychiatric comorbidity is frequent in rheumatoid arthritis (RA) and complicates treatment. The present study was undertaken to describe the impact of psychiatric comorbidity on health care use (utilization) in RA.
Methods
We accessed administrative health data (1984–2016) and identified a prevalent cohort with diagnosed RA. Cases of RA (n = 12,984) were matched for age, sex, and region of residence with 5 controls (CNT) per case (n = 64,510). Within each cohort, we identified psychiatric morbidities (depression, anxiety, bipolar disorder, and schizophrenia [PSYC]), with active PSYC defined as ≥2 visits per year. For the years 2006–2016, annual rates of ambulatory care visits (mean ± SD per person) categorized by provider (family physician [FP], rheumatologist, psychiatrist, other specialist), hospitalization (% of cohort), days of hospitalization (mean ± SD), and dispensed drug types (mean ± SD per person) were compared among 4 groups (CNT, CNT plus PSYC, RA, and RA plus PSYC) using generalized linear models adjusted for age, sex, rural versus urban residence, income quintile, and total comorbidities. Estimated rates are reported with 95% confidence intervals (95% CIs). We tested within‐person and RA‐PSYC interaction effects.
Results
Subjects with RA were mainly female (72%) and urban residents (59%), with a mean ± SD age of 54 ± 16 years. Compared to RA without PSYC, RA with PSYC had more than additive (synergistic) visits (standardized mean difference [SMD] 10.92 [95% CI 10.25, 11.58]), hospitalizations (SMD 13% [95% CI 0.11, 0.14]), and hospital days (SMD 3.63 [95% CI 3.06, 4.19]) and were dispensed 6.85 more medication types (95% CI 6.43, 7.27). Cases of RA plus PSYC had increased visits to FPs (an additional SMD 8.92 [95% CI 8.35, 9.46] visits). PSYC increased utilization in within‐person models.
Conclusion
Managing psychiatric comorbidity effectively may reduce utilization in RA.
Introduction
Rheumatoid arthritis (RA) is a chronic arthropathy that requires regular assessment to ensure optimal management. The prevalence and incidence of psychiatric disorders in RA, particularly depression and anxiety, is high and exceeds that of the general population (1, 2, 3). Psychiatric comorbidity can complicate treatment decisions in RA, leading to increased potential for adverse outcomes, including reduced disease control (4), poor quality of life (5), and synergistically (more than additive) higher mortality rates (6).
Significance & Innovations.
Active psychiatric comorbidities, including anxiety disorder and depression, increase health care use in individuals with diagnosed rheumatoid arthritis with a more than additive (synergistic) interaction effect than either condition alone.
Family physicians provide the majority of additional ambulatory care.
Access to effective mental health supports and interventions in primary care settings has the potential to significantly reduce health care use in these individuals.
Health care use, hereinafter referred to as “utilization,” is elevated in chronic disease. Compared to non‐RA populations, the RA population continues to have substantially higher rates of utilization despite improvements in disease control with contemporary treatment approaches. Multimorbidity, which is often associated with psychiatric disorders (7), further increases utilization particularly when combined with musculoskeletal disorders (8, 9). The effects of psychiatric comorbidity in RA on utilization may be complex, possibly synergistic or more than additive, given the potential for amplification of shared symptomatology and disability. However, while individually, both RA and psychiatric disorders are known to increase utilization (10, 11), limited data exist regarding the combined impact and potential interaction of RA and psychiatric disorders on utilization.
Psychiatric conditions are treatable but frequently remit and relapse, leading to increased resource utilization and health care costs (12, 13, 14). Understanding how psychiatric comorbidity impacts utilization over time within an individual is needed to evaluate the potential impact of effective psychiatric interventions on utilization. Absolute utilization rate differences between periods with or without active psychiatric morbidity provide a readily understandable perspective on the potential benefit of interventions. Such information is not yet available for RA but is essential to evaluate the cost‐effectiveness of investments to improve access to mental health services or new treatment approaches.
We aimed to examine the effects of psychiatric comorbidity on utilization in individuals with RA, focusing on depression, anxiety, bipolar disorder, and schizophrenia. Our first aim was to compare utilization between individuals with and without active psychiatric comorbidity. Our second aim was to compare within individuals how utilization changes over time as psychiatric comorbidity relapses or remits.
Subjects and Methods
Data sources
This retrospective cohort study was conducted in Manitoba, a Canadian province with a population of 1.37 million. Universal health care is publicly funded and provided for medically necessary hospital and physician services. The provincial health department maintains a population registry and databases of health services used. We linked 4 deidentified databases held at the Population Research Data Repository at the Manitoba Centre for Health Policy at the individual level using an encoded unique identifier. These databases included the population registry, the Discharge Abstract Database (DAD), the medical services database, and the Drug Product Information Network (DPIN) database. The population registry captures data on sex, dates of birth, dates of health care coverage, including termination of coverage due to death, and region of residence (by postal code) for all Manitoba residents. The DAD captures data on hospitalizations, including admission and discharge dates, and up to 25 diagnoses. Diagnoses were recorded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes until 2004, and using the ICD‐10 Canadian version (ICD‐10‐CA) codes thereafter. The medical services database captures data on service date, physician provider category, tariff code, and 1 physician‐assigned diagnosis using ICD‐9‐CM codes. Since 1995, DPIN has captured data on prescriptions dispensed in the community, including the drug name, drug identification number (DIN), and date of dispensation; the DIN is connected to the World Health Organization’s Anatomical Therapeutic Chemical (ATC) Classification System (15). Except for the DPIN, we accessed data for the period April 1, 1984 to March 31, 2016. The University of Manitoba Health Research Ethics Board approved the study, and Manitoba’s Health Information Privacy Committee approved data access.
Study populations
Using validated case definitions, we first identified Manitobans with RA (16). For each case, the first health claim with an RA diagnosis constituted the index date. Next, as described elsewhere (17), we identified a general population cohort, which excluded anyone with ICD‐9‐CM/ICD‐10‐CA codes for RA. Because this was part of a complementary study, individuals with codes for multiple sclerosis and inflammatory bowel disease were also excluded. From this cohort, we selected 5 controls for each case, matched for sex, year of birth within ±5 years, and forward sortation area (first 3 digits of the postal code); controls were assigned the index date of their matched cases.
Comorbidity
We applied case definitions validated in Manitoba to identify members of each cohort with a diagnosis for depression, anxiety disorder, bipolar disorder, or schizophrenia (18). For each condition, the date of the first claim was the diagnosis date. For each year, an individual was considered an annual prevalent psychiatric case (hereinafter referred to as “active”) if there were ≥2 physician claims or 1 hospital claim for the psychiatric disorder in that year; for hospital claims, the disorder had to be the diagnosis most responsible for admission, that is, considered most responsible for the greatest portion of length of stay or use of resources (14). Thus, an individual who ever met the case definition for a psychiatric disorder could vary with respect to their status (active or inactive) from one year to another, thereby allowing assessment of utilization over time as their condition relapsed or remitted (12, 13, 14). We assessed the impact of active psychiatric comorbidity on utilization during the following year (14) to ensure that the findings were not confounded by our definition of active psychiatric disorder. We identified physical comorbidity using the John Hopkins Adjusted Clinical Group System Aggregated Diagnosis Groups (ADGs), specifically using major physical ADGs that were not time limited (ADGs 9, 11, 16, 22, and 32), as we wished to account for chronic physical conditions. These were categorized as none, 1, or ≥2 ADGs. The use of ADGs for risk adjustment in regression models was facilitated using the Johns Hopkins Adjusted Clinical Group Case‐Mix System, version 9.
Covariates
We included the following covariates in the regression models: sex (male as reference group), age (categorized as 18–25 years, 25–44 years, 45–64 years, or ≥65 years and updated annually), socioeconomic status (SES) at the index date, region of residence at the index date, and physical comorbidity as defined above (updated annually). To determine SES, we linked postal code to dissemination area–level census data and then calculated the Socioeconomic Factor Index, version 2 (SEFI‐2), which incorporates information regarding average household income, percentage of single‐parent households, unemployment rate, and high school education rate; scores <0 indicate higher SES (19). We categorized SES into quintiles (highest quintile [with highest SES] as reference group). Region of residence was classified as urban (Winnipeg, population >600,000, and Brandon, population >47,000) versus rural.
Health care use (utilization)
Our outcomes of interest were the number of physician visits (mean ± SD) subcategorized by type of provider (family physician, rheumatologist, psychiatrist, and other specialist), all‐cause non‐obstetrical hospitalization (percentage of cohort hospitalized and number of days hospitalized [mean ± SD]), and the number of types of prescription medications used at the fourth level of the ATC system, which represents chemical, pharmacologic, and therapeutic subgroups. To better assess the effects of psychiatric morbidity on non–mental health use, we examined physician visits, after excluding those attributed to mental health, and examined the number of immunomodulating drugs normally used for RA (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract).
Statistical analysis
We restricted the time period for comparative analysis to April 1, 2006 to March 31, 2016 to reduce the effects of temporal trends in utilization (see Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract) (20, 21). We used descriptive statistics to characterize the cohorts, including mean ± SD, median (interquartile range), and frequency (percentage). We report standardized differences for these comparisons, where a standardized difference of ≤0.20 represents a small effect size per Cohen’s d coefficient (22).
We used multivariable negative binomial regression models with generalized estimating equations to test for associations between cohort (RA versus matched controls) and psychiatric morbidity (yes versus no) on utilization outcomes. Specifically, we examined utilization in the year after determining psychiatric status as active or inactive to make certain that our findings were not confounded by the determination of psychiatric status, which relies on measuring physician visits. We accounted for differences in follow‐up time by including the natural logarithm of person‐years as the model offset. We modeled the risk difference for each outcome so we could estimate the absolute (incremental) effects of the independent variables of interest. This approach allows determination of the number needed to treat. Generally, these models provide population averages of within‐person and between‐person effects but can be parameterized to estimate these effects separately by using a person‐mean variable and a within‐person centered variable (23). The within‐person analysis allowed us to examine the effects of changes in psychiatric status within persons over time on utilization while controlling for fixed, measured, and unmeasured characteristics of that person. We also tested for the presence of interactions between cohort and psychiatric morbidity. In the absence of any interaction, we would expect the effects of each exposure (RA and psychiatric disorder) on utilization to be additive on the risk difference scale. A positive (synergistic) interaction would indicate that the effect of cohort and psychiatric disorder together exceeded the sum of the individual effects, whereas a negative interaction would indicate that the joint effect of these exposures was less than the effect of the separate exposures. We report rate differences and 95% confidence intervals (95% CIs).
We repeated our analysis for any psychiatric disorder, depression, and anxiety disorder. Limited sample size prevented separate analysis of bipolar disorder and schizophrenia. In a sensitivity analysis, we further adjusted the regression models for the number of physician visits in the year before RA diagnosis/index date (categorized as 0–4, 5–9, ≥10) to account for baseline differences in health system use. Statistical analyses were conducted using SAS, version 9.4.
Results
We initially identified 16,975 cases of RA and 84,756 matched controls for the period 1984–2016. The sociodemographic characteristics of these cohorts have been reported previously (1). During this period, drug dispensations increased while rates of physician visits, hospitalizations, and days of hospital stay decreased, with relative stabilization in 2006. Throughout this time period, RA cases with psychiatric comorbidity had more utilization compared to RA cases without psychiatric comorbidity (see Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract).
We identified 12,984 RA and 64,510 matched controls for the period 2006–2016. Differences in rates of death before 2006 between the RA and control cohorts reduced the matching ratio to 1:4.31. The cohorts were well matched at the index date and similar to the larger cohorts for the period 1984–2016 (Table 1). Most subjects were female and lived in urban centers. The prevalence of lifetime psychiatric comorbidity and active psychiatric comorbidity at the index date were both higher in the RA cohort than the matched cohort.
Table 1.
Demographic information for rheumatoid arthritis (RA) and matched control cohorts*
| Characteristic |
RA (n = 12,984) |
RA matches (n = 64,510) |
Std. diff. |
|---|---|---|---|
| Female sex | 9,458 (72.8) | 47,102 (73.0) | 0 |
| Age at diagnosis, mean ± SD years | 51.4 ± 15.3 | 51.8 ± 15.2 | |
| Urban region of residence | 7,609 (58.6) | 37,559 (58.2) | 0.01 |
| Socioeconomic status† | 0.04 (1.04) | 0.06 (1.02) | |
| Physical comorbidity status at study start | |||
| No. of ADGs | |||
| 0 | 8,594 (66.2) | 51,284 (79.5) | 0.30 |
| 1 | 3,577 (27.5) | 11,140 (17.3) | 0.20 |
| ≥2 | 813 (6.3) | 2,086 (3.2) | 0.03 |
| Lifetime prevalence psychiatric disorders, at study start | |||
| Any psychiatric disorder | 4,549 (35.0) | 17,313 (26.8) | 0.18 |
| Depression | 3,808 (29.3) | 14,548 (22.6) | 0.15 |
| Anxiety disorder | 5,063 (39.0) | 21,332 (33.1) | 0.12 |
| Bipolar disorder | 575 (4.4) | 2,347 (3.7) | 0.04 |
| Schizophrenia | 119 (0.9) | 790 (1.2) | |
| Active prevalence psychiatric disorders, year before study start | |||
| Any psychiatric disorder | 1,209 (9.3) | 4,776 (7.4) | 0.07 |
| Depression | 1,048 (8.1) | 4,191 (6.5) | 0.06 |
| Anxiety disorder | 705 (5.4) | 2,883 (4.5) | 0.04 |
| Bipolar disorder | 104 (0.8) | 512 (0.8) | 0 |
| Schizophrenia | 31 (0.2) | 251 (0.4) | 0.12 |
| Years followed to 2017, no. (SD) | 14.2 (9.3) | 9.6 (9.6) | 0.01 |
Values are the number (%) unless indicated otherwise. Std. diff. = standardized difference; ADG = Aggregated Diagnosis Group.
Socioeconomic status = Socioeconomic Factor Index scores (values <0 indicate higher socioeconomic status).
In crude analyses adjusting only for psychiatric comorbidity, RA cases had least 4 additional ambulatory physician visits, at least 1 extra hospital day, and used 3.5 more medication types per year than matched controls (Figure 1). As compared to individuals without active psychiatric comorbidity, individuals with active psychiatric comorbidity had ~10 more physician visits, 5 more days in the hospital, and used >6 more medications. Within individuals, rates of utilization were also higher for those who had active psychiatric comorbidity during the previous year as compared to individuals with inactive psychiatric comorbidity, although the magnitude of these effects was less. These effects on utilization were seen for all categories of psychiatric comorbidity and were generally highest for anxiety.
Figure 1.
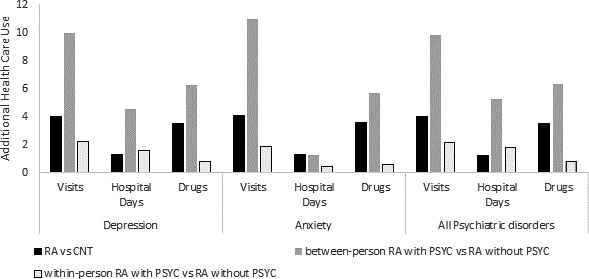
Additional health care utilization related to rheumatoid arthritis and psychiatric disorders, modeled with negative binomial regression. Rates are the number of visits per year, the number of hospital days per year, and the number of drug categories per year. Adjusted for psychiatric comorbidity only. RA = rheumatoid arthritis; CNT = matched control; PSYC = active psychiatric disorder; RA vs. CNT = additional health care use due to RA diagnosis; between‐person RA with PSYC vs. RA without PSYC = additional health care use for RA with active psychiatric disorder versus RA without psychiatric disorder; within‐person RA with PSYC vs. RA without PSYC = within an individual, additional health care use associated with periods of active psychiatric disorder versus periods without active psychiatric disorder.
After adjusting for cohort and covariates and assessing for interactions, RA cases with active psychiatric comorbidity had >11 additional physician visits than individuals without active psychiatric comorbidity (Table 2). There was a more than additive (synergistic) between‐person interaction of ~2 (95% CI 1.29, 2.70) extra visits for RA with psychiatric comorbidity than expected for the sum of each condition. This synergistic effect was greatest for comorbid anxiety, with 2.70 (95% CI 1.68, 3.72) extra visits. We did not observe a synergistic within‐person effect of active psychiatric diagnosis on visits for cases with RA (or matched controls). Similar effects were seen for physician visits after excluding visits for mental health diagnoses, although the magnitudes were smaller (see Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract).
Table 2.
Difference in physician ambulatory visits per year for individuals with psychiatric comorbidity*
| Variable | Depression | Anxiety | All psychiatric disorders |
|---|---|---|---|
| Cohort effect (RA vs. matched control)† | |||
| When between‐person PSYC effect = 0 | 4.05 (3.94, 4.15) | 4.14 (4.04, 4.25) | 4.03 (3.93, 4.14) |
| When between‐person PSYC effect = 1 | 6.02 (5.31, 6.74) | 6.84 (5.85, 7.84) | 6.03 (5.35, 6.71) |
| When within‐person PSYC effect = 0 | 4.05 (3.94, 4.15) | 4.14 (4.04, 4.25) | 4.03 (3.93, 4.14) |
| When within‐person PSYC effect = 1 | 3.98 (3.69, 4.27) | 4.31 (4.01, 4.61) | 3.95 (3.68, 4.20) |
| Among RA cases† | |||
| Between‐person effect PSYC | 11.18 (10.49, 11.87) | 13.25 (12.30, 14.20) | 10.92 (10.25, 11.58) |
| Within‐person effect PSYC | 2.17 (1.92, 2.42) | 1.99 (1.73, 2.25) | 2.08 (1.85, 2.31) |
| Among matches† | |||
| Between‐person effect PSYC | 9.20 (8.93, 9.47) | 10.56 (10.18, 10.93) | 8.92 (8.67, 9.17) |
| Within‐person effect PSYC | 2.23 (2.12, 2.35) | 1.82 (1.71, 1.93) | 2.17 (2.06, 2.27) |
| Interaction between‐person (RA PSYC) | 1.98 (1.24, 2.72) | 2.70 (1.68, 3.72) | 2.00 (1.29, 2.70) |
| Interaction within‐person (RA PSYC) | –0.06 (–0.33, 0.21) | 0.17 (–0.11, 0.45) | –0.09 (–0.34, 0.17) |
| Year of diagnosis | 0.03 (0.03, 0.04) | 0.03 (0.022, 0.033) | 0.04 (0.03, 0.04) |
| Disease duration, per year | –0.16 (–0.16, –0.15) | –0.16 (–0.16, –0.15) | –0.16 (–0.16, –0.15) |
| Sex | |||
| Female (male = reference) | 0.78 (0.72, 0.84) | 0.88 (0.81, 0.95) | 0.80 (0.74, 0.86) |
| Age group | |||
| 18–24 | Ref. | Ref. | Ref. |
| 25–44 | 0.40 (0.26, 0.54) | 0.54 (0.40, 0.69) | 0.39 (0.25, 0.53) |
| 45–64 | 1.68 (1.54, 1.82) | 1.82 (1.68, 1.96) | 1.67 (1.53, 1.81) |
| ≥65 | 3.63 (3.48, 3.79) | 3.80 (3.64, 3.96) | 3.60 (3.44, 3.75) |
| Region | |||
| Urban (rural = reference) | 0.66 (0.60, 0.72) | 0.73 (0.66, 0.79) | 0.64 (0.580, 0.70) |
| SEFI scores | |||
| Quintile 1 (lowest) | 0.34 (0.24, 0.43) | 0.31 (0.20, 0.41) | 0.29 (0.20, 0.39) |
| Quintile 2 | 0.14 (0.048, 0.24) | 0.21 (0.10, 0.31) | 0.13 (0.02, 0.22) |
| Quintile 3 | 0.05 (–0.04, 0.15) | 0.11 (0.001, 0.21) | 0.04 (–0.05, 0.13) |
| Quintile 4 | 0.07 (–0.03, 0.16) | 0.10 (–0.007, 0.20) | 0.06 (–0.05, 0.15) |
| Quintile 5 (highest) | Ref. | Ref. | Ref. |
| Linear trend SEFI scores | –0.075 (–0.096, –0.053) | –0.073 (–0.096, –0.049) | –0.065 (–0.087, –0.044) |
| Count of ADGs (in prior year) | |||
| 0 | Ref. | Ref. | Ref. |
| 1 | 0.84 (0.80, 0.88) | 0.84 (0.81, 0.88) | 0.83 (0.80, 0.87) |
| ≥2 | 1.71 (1.63, 1.78) | 1.73 (1.66, 1.81) | 1.69 (1.62, 1.77) |
Values are the standardized mean difference (95% confidence interval). ADG = Aggregated Diagnosis Group; PSYC = active psychiatric comorbidity; RA = rheumatoid arthritis; Ref. = reference; SEFI = Socioeconomic Factor Index (values <0 indicate higher socioeconomic status).
Adjusted for sex, age group, region, year of diagnosis, disease duration, Socioeconomic Factor Index score, and Aggregated Diagnosis Groups. Between‐person and within‐person interaction terms included in the model.
Most of these additional ambulatory care visits were to family physicians (Figure 2 and Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract). After adjusting for age and sex, individuals with RA and active psychiatric comorbidity had ~9 extra family physician visits compared to subjects with RA without psychiatric comorbidity; those with anxiety had 11.3 (95% CI 10.48, 12.12) more visits, while those with depression had 9.10 (95% CI 8.51, 9.70) more visits. Within an individual, active psychiatric comorbidity was associated with ~1.6 extra family physician visits the following year. RA cases with psychiatric comorbidity had fewer rheumatologist visits, but this effect was small. Covariates including age, sex, socioeconomic status (SEFI‐2), region of residence, other comorbidity (ADGs), disease duration, year of index diagnosis, and disease duration all affected total ambulatory physician visit rates (Table 2).
Figure 2.
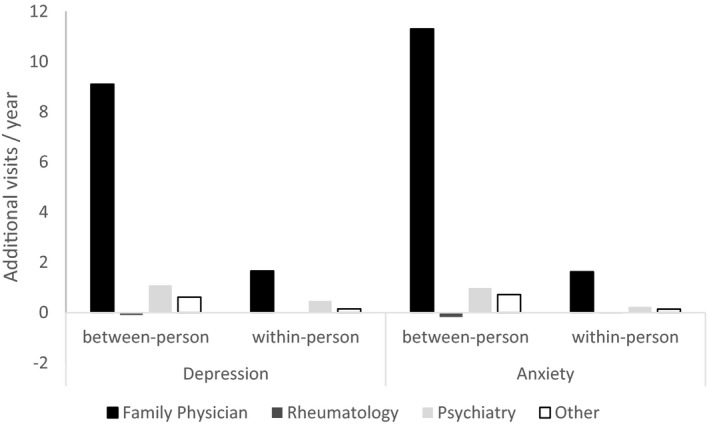
Additional physician visits by provider for rheumatoid arthritis (RA) with or without psychiatric disorder, modeled with negative binomial regression. Adjusted for age and sex. Other = other specialist; between‐person = difference in ambulatory visits per year for RA with active psychiatric disorder versus RA with inactive psychiatric disorder; within‐person = difference in ambulatory visits per year for individuals with RA during periods with active psychiatric disorder versus periods without active psychiatric disorder.
Hospitalization rates were higher and length of hospital stay was longer for RA cases than for matched controls, particularly for individuals with psychiatric comorbidity (Figure 1 and Table 3). After adjusting for cohort and covariates and assessing for interactions, the difference in the proportion of cohort members hospitalized between RA with psychiatric comorbidity and RA without psychiatric comorbidity was ~13%, and there was a more than additive (synergistic) between‐person effect of RA and psychiatric comorbidity across psychiatric disorders of ~4% (95% CI 2%, 6%). This synergistic effect was greatest for anxiety (6% [95% CI 3%, 8%]). RA cases with psychiatric comorbidity also had ~2–4 more days in the hospital compared to RA cases without psychiatric comorbidity, with a more than additive between‐person interaction of ~1 extra day (between‐person interaction for depression 0.88 [95% CI 0.24, 1.51] and 1.36 days [95% CI 0.60, 2.12] for anxiety).
Table 3.
Differences in proportions of the cohort hospitalized and total days of hospital stay for subjects with rheumatoid arthritis (RA) and matched controls with or without psychiatric comorbidity*
| Variable | Depression | Anxiety |
All psychiatric disorders |
|---|---|---|---|
| Proportion hospitalized | |||
| Cohort effect† | |||
| When between‐person PSYC effect = 0 | 0.04 (0.04, 0.05) | 0.04 (0.04,0.05) | 0.04 (0.04, 0.05) |
| When between‐person PSYC effect = 1 | 0.08 (0.06, 0.10) | 0.10 (0.08, 0.12) | 0.08 (0.07, 0.10) |
| When within‐person PSYC effect = 0 | 0.04 (0.04, 0.05) | 0.04 (0.04, 0.05) | 0.04 (0.04, 0.05) |
| When within‐person PSYC effect = 1 | 0.05 (0.03, 0.06) | 0.04 (0.03, 0.06) | 0.05 (0.03, 0.06) |
| Among RA cases† | |||
| Between‐person effect PSYC | 0.11 (0.10, 0.13) | 0.12 (0.10, 0.15) | 0.13 (0.11, 0.14) |
| Within‐person effect PSYC | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03)‡ | 0.03 (0.02, 0.04) |
| Among matches† | |||
| Between‐person effect PSYC | 0.08 (0.07, 0.08) | 0.07 (0.06, 0.08) | 0.09 (0.09, 0.10) |
| Within‐person effect PSYC | 0.02 (0.02, 0.03) | 0.02 (0.01, 0.02) | 0.02 (0.02, 0.03) |
| Interaction between person (RA/PSYC) | 0.04 (0.02, 0.06) | 0.06 (0.03, 0.08) | 0.04 (0.02, 0.06) |
| Interaction within person (RA/PSYC) | 0.00 (–0.01, 0.01) | 0.00 (–0.01, 0.01) | 0.00 (–0.01, 0.01) |
| Days of hospital stay | |||
| Cohort effect† | |||
| When between‐person PSYC effect = 0 | 0.42 (0.34, 0.47) | 0.41 (0.34, 0.48) | 0.41 (0.34, 0.47) |
| When between‐person PSYC effect = 1 | 1.28 (0.66, 1.91) | 1.77 (1.03, 2.51) | 1.38, (0.76, 1.99) |
| When within‐person PSYC effect = 0 | 0.41 (0.34, 0.47) | 0.41 (0.34, 0.48) | 0.41 (0.34, 0.47) |
| When within‐person PSYC effect = 1 | 0.58 (0.09, 1.07) | 0.89 (0.40, 1.19) | 0.41 (–0.07, 0.88) |
| Among RA cases† | |||
| Between‐person effect PSYC | 3.18 (2.61, 3.75) | 2.40 (1.71, 3.08) | 3.63 (3.06, 4.19) |
| Within‐person effect PSYC | 1.04, 0.60, 1.48) | 0.63 (0.26, 1.00)‡ | 1.18 (0.75, 1.62) |
| Among matches† | |||
| Between‐person effect PSYC | 2.30 (2.01, 2.59) | 1.04 (0.71, 1.36) | 2.66 (2.39, 2.92) |
| Within‐person effect PSYC | 0.86 (0.66, 1.06) | 0.25 (0.12, 0.38) | 1.18 (1.00, 1.38) |
| Interaction between person (RA/PSYC) | 0.88 (0.24, 1.51) | 1.36 (0.60, 2.12) | 0.97 (0.35, 1.60) |
| Interaction within person (RA/PSYC) | 0.18 (–0.31, 0.66) | 0.38 (–0.01, 0.77) | 0.00 (–0.47, 0.47) |
Values are the standardized mean difference (95% confidence interval). PSYC = active psychiatric comorbidity.
Adjusted for sex, age group, region, year of diagnosis, disease duration, Socioeconomic Factor Index score, and Aggregated Diagnosis Groups. Between‐person and within‐person interaction terms included in the model.
P < 0.005.
Psychiatric comorbidity was associated with more medication use (Figure 1). After adjusting for cohort and covariates and including interaction terms, subjects with RA with psychiatric comorbidity used ~7 more medication types than subjects with RA without psychiatric comorbidity (Table 4). There was a synergistic between‐person interaction, with ~1.6 more medications used in RA cases with psychiatric comorbidity (between‐person interaction effect of depression was 1.61 [95% CI 1.13, 2.08], and of anxiety, 1.83 [95% CI 1.17, 2.49]). Subjects with RA with depression or anxiety used slightly fewer RA‐specific medications than those without these comorbidities; however, the effects were quite small (for depression, 0.07 fewer medications [95% CI 0.13, 0.00]; P = 0.04; for anxiety, 0.13 fewer medications [95% CI 0.21, 0.05]; P = 0.003) (Table 4). In the sensitivity analyses that adjusted for number of physician visits preindex date, between‐person effects on visits were slightly attenuated, while within‐person effects were slightly attenuated or unchanged (see Supplementary Tables 3, 4, and 5, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24386/abstract).
Table 4.
Drug types dispensed (total and rheumatoid arthritis [RA]‐specific) by presence of psychiatric comorbidity*
| Variable | Depression | Anxiety |
All psychiatric disorders |
|---|---|---|---|
| Total drug categories | |||
| Cohort effect† | |||
| When between‐person PSYC effect = 0 | 3.40 (3.32, 3.48) | 3.50 (3.42, 3.58) | 3.39 (3.31, 3.48) |
| When between‐person PSYC effect = 1 | 5.00 (4.55, 5.47) | 5.33 (4.69, 5.97) | 4.98 (4.55, 5.42) |
| When within‐person PSYC effect = 0 | 3.40 (3.32, 3.48) | 3.50 (3.42, 3.58) | 3.39 (3.31, 3.48) |
| When within‐person PSYC effect = 1 | 3.47 (3.23, 3.52) | 3.50 (3.35, 3.64) | 3.37 (3.24, 3.51) |
| Among RA cases† | |||
| Between‐person effect PSYC | 6.96 (6.52, 7.40) | 7.01 (6.39, 7.62) | 6.85 (6.43, 7.27) |
| Within‐person effect PSYC | 0.67 (0.56, 0.78) | 0.56 (0.44, 0.67) | 0.71 (0.61, 0.81) |
| Among matches† | |||
| Between‐person effect PSYC | 5.35 (5.17, 5.53) | 5.17 (4.92, 5.43) | 5.26 (5.09, 5.43) |
| Within‐person effect PSYC | 0.70 (0.65, 0.75) | 0.56 (0.51, 0.60) | 0.73 (0.68, 0.78) |
| Interaction between person (RA/PSYC) | 1.61 (1.13, 2.08) | 1.83 (1.17, 2.49) | 1.59 (1.13, 2.05) |
| Interaction within person (RA/PSYC) | –0.03 (–0.15,0.09) | –0.00 (–0.12, 0.12) | –0.02 (–0.13, 0.09) |
| RA immunomodulatory drugs‡ | |||
| Cohort effect† | |||
| When between‐person PSYC effect = 0 | 0.75 (0.74, 0.76) | 0.75 (0.74, 0.76) | 0.75 (0.73, 0.76) |
| When between‐person PSYC effect = 1 | 0.65 (0.60, 0.71) | 0.59 (0.51, 0.67) | 0.67 (0.61, 0.72) |
| When within‐person PSYC effect = 0 | 0.75 (0.74, 0.76) | 0.75 (0.74, 0.76) | 0.75 (0.73, 0.76) |
| When within‐person PSYC effect = 1 | 0.753 (0.73, 0.78) | 0.76 (0.73, 0.78) | 0.75 (0.73, 0.78) |
| Among RA cases† | |||
| Between‐person effect PSYC | –0.07 (–0.13, –0.003) | –0.13 (–0.21, –0.05) | –0.05 (–0.12, 0.01) |
| Within‐person effect PSYC | –0.00 (–0.02, 0.02) | 0.01 (–0.02, 0.03) | 0.001 (–0.02, 0.02) |
| Among matches† | |||
| Between‐person effect PSYC | 0.03 (0.02, 0.04) | 0.04 (0.03, 0.05) | 0.03 (0.02, 0.03) |
| Within‐person effect PSYC | –0.01 (–0.01, –0.001) | –0.00 (–0.004, 0.004) | –0.001 (–0.01, 0.002) |
| Interaction between person (RA/PSYC) | –0.10 (–0.16, –0.03) | –0.16 (–0.25, –0.08) | 0.74 (0.73, 0.75) |
| Interaction within person (RA/PSYC) | 0.00 (–0.02, 0.02) | 0.01 (–0.02, 0.03) | 0.00 (–0.02, 0.02) |
Values are the standardized mean difference (95% confidence interval). PSYC = active psychiatric comorbidity.
Adjusted for sex, age group, region, year of diagnosis, disease duration, Socioeconomic Factor Index score, and Aggregated Diagnosis Groups. Between‐person and within‐person interaction terms included in the model.
RA immunomodulatory drugs included methotrexate, hydroxychloroquine, chloroquine, sulfasalazine, leflunomide, gold salts, cyclosporin, cyclophosphamide, mycophenolate, azathioprine, penicillamine, biologics, and JAK inhibitors.
Discussion
This population‐based study found that individuals with RA on average have more physician visits, more hospitalizations, more days of hospital stay, and used more types of medications than individuals without RA. The presence of active psychiatric comorbidity, either depression or anxiety, substantially increased these rates, as did active psychiatric comorbidity within an individual over time. This excess utilization by individuals with both RA and psychiatric comorbidity was more than additive over either condition alone. The majority of extra physician visits were provided by family physicians, and the effect of psychiatric comorbidity on utilization was present even when visits for mental health disorders were excluded from the analysis. Rates of ambulatory visits and hospitalization for RA declined over the full study period while medication use increased.
Our findings are consistent with recent population‐based work from North America and Europe reporting increased utilization in patients with RA compared to the general population (11, 21, 24, 25) and with the findings of studies from our region on individuals with depression (10). In line with our study, some resource utilization rates, particularly hospitalization rates and days in hospital, are declining over time for RA (26). Ambulatory care visits to generalists and specialists are typically highest around the time of RA diagnosis (11). Similar trends are seen in other chronic inflammatory diseases (27, 28, 29). In contrast, and also in line with our study, medication use, including RA‐specific therapies, has gradually increased over time. Contemporary RA management strategies that lead to improved disease control are likely contributing to these trends (30, 31, 32).
Previous studies that have examined the impact of psychiatric comorbidity on utilization in RA have focused mainly on depression and did not compare rates for individuals with RA to those of the general population. These studies have reported increased rates of hospitalization (25, 33, 34, 35), including hospitalizations for RA (34), as well as longer hospital stays (34, 35), more physician and emergency room visits (33, 34), and more medication use (35) in those with RA and depression compared to those with RA without depression. In RA, the prevalence of anxiety is at least as high as depression and adversely impacts RA outcomes (1, 36), yet data on the impact of comorbid anxiety on utilization are even more limited (37). Our study demonstrated significantly increased utilization for those with RA and comorbid depression as well as for those with RA and comorbid anxiety, bipolar disorder, or schizophrenia. While the impacts of multiple morbidities on utilization, including the combination of musculoskeletal and mental health disorders, has been reported (8), we show that the effects of RA and psychiatric morbidity on utilization are synergistic and that comorbid anxiety had the greatest impact on utilization.
Notable findings of this study are the dramatic excess burden of physician visits and drug types dispensed for RA with comorbid depression, anxiety disorder, bipolar disorder, or schizophrenia. Affected individuals had >10 additional visits annually (>4 after excluding those attributed to a mental health disorder) and were dispensed ~7 additional drug types. This represents an additional 2 visits and 1.5 more drugs than either condition alone and was greatest for individuals with RA and anxiety. Most extra visits were provided by family physicians, with a modest increase in visits to psychiatrists and other specialists but not rheumatologists. The additional medication use was directed to non‐RA management, as there was no clinically relevant difference in the use of immunomodulating agents prescribed for RA. In fact, rheumatology visits and medication use were slightly reduced, suggesting prioritization of mental health care. This highlights the critical role of family physicians in managing mental health both in our region and globally (38, 39, 40), particularly in these complex cases. Our findings also highlight the importance of access to resources to address mental health concerns (41, 42), such as models of care that integrate mental health services into family practice clinics (43).
The effects of psychiatric comorbidity were synergistic, indicating that the 2 conditions together produce additional problems for patients beyond that expected for each individual condition alone. Mental disorders, by definition, are major causes of disability, globally affecting both occupational and social function. In individuals with RA who already experience physical disability, the combined effects may reduce effectiveness of personal coping strategies either through passive avoidance, such as noncompliance with medication and physical activity, or through adopting maladaptive coping strategies, such as self‐medicating. Additionally, the observed synergistic effects may relate to bidirectional interactions between depression or anxiety, with inflammation and pain leading to amplification of each condition (44). This may arise from shared biologic pathways (45).
Psychiatric conditions are treatable but frequently remit and relapse (12, 13, 14), leading to increased resource utilization and cost. Our study design enabled us to estimate visit frequencies for psychiatric disorders in care‐seeking populations that can be assumed to be active and to observe the effect on an individual’s subsequent utilization over time (13). In an individual with RA, active psychiatric disease led to ~2 extra physician visits, 2% more hospital admissions, and ~1 extra hospital day over the following year compared to periods of inactive psychiatric disease. Although, to our knowledge, this has not previously been reported for RA, a prior study using health claims data from 22,236 Americans with major depressive disorder reported that among individuals who relapsed, inpatient hospital days and emergency room visits were >2 times higher postrelapse than prerelapse (14). In another study of 33,893 Americans, small synergistic interactions on cost of care were observed between worse mental health and diabetes mellitus, cancer, or chronic obstructive pulmonary disease but not between worse mental health and stroke, coronary artery disease, or asthma (46). These findings highlight the variability of interactions between psychiatric disorders and chronic medical conditions on health care use and the potential impact that effective treatment of psychiatric disorders in individuals with RA might have on the health care system.
Strengths of this study include the population‐based design, large sample size, use of locally validated case definitions for RA and psychiatric disorders, and the assessment of the within‐person effects of psychiatric disorders on health care utilization. However, limitations of this study should be recognized. Our administrative data captured individuals who sought treatment for psychiatric disorders from physicians and hospitals. Thus, it underestimates the total number of individuals affected by psychiatric disorders by not capturing those who do not seek treatment or those who sought treatment from non‐physician providers, including mental health counselors, psychologists, and social workers. However, these limitations would not be expected to differ between the RA and matched cohorts. Because we lacked an individual level measure of SES, we used an area‐based measure, the SEFI‐2; therefore, residual confounding due to SES may have occurred. However, the SEFI‐2 is strongly associated with health and developmental outcomes (19). Administrative data also lack clinical information; therefore, we could not describe the severity of psychiatric disorders or clinical characteristics of RA such as disability. Our “active” or “inactive” designation may not represent true psychiatric disease activity. Nonetheless, it is a representation of care‐seeking behavior because the most responsible diagnosis attributed to the visit was used to define an active psychiatric disorder, and for within‐person analyses, each person acted as their own control. Many drug types, with the exception of immunomodulatory agents, have multiple indications for use, and we were not able to confidently subcategorize based on treatment indication. In future studies, it may be helpful to explore the drivers of utilization in those with active psychiatric disorders, which we were unable to examine in sufficient detail. Due to the complexity of the analysis, we only examined the potential interactions of psychiatric comorbidity with RA. However, other comorbidities are also common in RA, and we did not examine the potential interactions between RA and these comorbidities on utilization; this warrants future investigation. Our study reflects practice patterns in a system with near universal health coverage and may not be applicable to regions with other models of health care delivery. Finally, our study is descriptive in nature, and we cannot draw causal inferences as to the reasons for the increases in health care utilization observed. Future studies that are psychologically oriented and incorporate elements of theoretical models of health care use would be useful for better understanding the mechanisms underpinning our findings.
Health care utilization is higher in individuals with RA than in those without RA. As compared to individuals without psychiatric comorbidity, patients with RA who have psychiatric comorbidity attend more physician visits, particularly to family physicians, have more days in the hospital, and use more prescription medication classes. In individuals with RA, the presence of active psychiatric disorder is associated with even more health care utilization over time. These findings highlight the substantial impact of psychiatric comorbidity on the health care system and potential benefits of prevention and treatment of these prevalent conditions for individuals with RA.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Hitchon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Hitchon, Walld, Peschken, Bernstein, Bolton, El‐Gabalawy, Fisk, Katz, Lix, Marriott, Patten, Sareen, Singer, Marrie.
Acquisition of data
Hitchon, Peschken, Bernstein, Katz, Marrie.
Analysis and interpretation of data
Hitchon, Walld, Bernstein, Bolton, El‐Gabalawy, Fisk, Katz, Lix, Marriott, Patten, Sareen, Singer, Marrie.
Supporting information
Supplementary Material
Supplementary Material
Appendix A. The CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease
Members of this CIHR Team are as follows: Ruth Ann Marrie, James M. Bolton, Jitender Sareen, John R. Walker (deceased), Scott B. Patten, Alexander Singer, Lisa M. Lix, Carol A. Hitchon, Renée El‐Gabalawy, Alan Katz, John D. Fisk, Charles N. Bernstein, Lesley Graff, Lindsay Berrigan, Ryan Zarychanski, Christine A. Peschken, and James Marriott.
The conclusions reported herein are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. The authors acknowledge the Manitoba Centre for Health Policy for use of the Manitoba Population Research Data Repository under project number 2014‐030 (HIPC number 2014/2015‐19A).
Supported by the Canadian Institutes of Health Research (CIHR) (grant THC‐135234), Crohn’s and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis (grant to Dr. Marrie). Dr. Bernstein’s work was supported in part by the Bingham Chair in Gastroenterology. Dr. El‐Gabalawy’s work was supported by the University of Manitoba (Start‐Up funding) and the CIHR Chronic Pain Strategy for Patient‐Oriented Research Network. Dr. Lix’s work was supported by a Tier 1 Canada Research Chair. Dr. Patten holds the Cuthbertson & Fischer Chair in Pediatric Mental Health at the University of Calgary. Dr. Sareen’s work was supported the CIHR (grant 333252). Dr. Marrie’s work was supported by a Research Manitoba Chair.
Dr. Hitchon has received research support from Pfizer Canada and UCB Canada. Dr. Peschken has received consulting fees from Astra Zeneca, Celgene, Janssen, GlaxoSmithKlein, and Eli Lilly and Company (less than $10,000 each). Dr. Bernstein has received consulting fees, speaking fees, and/or honoraria from AbbVie Canada, Janssen Canada, Pfizer Canada, Shire Canada, Takeda Canada, Medtronic Canada, Celgene, Roche, and Boehringer Ingelheim (less than $10,000 each) and research support from AbbVie Canada, Janssen Canada, Pfizer Canada, Shire Canada, and Takeda Canada. Dr. Marriott has received consulting fees from Biogen Idec and Roche (less than $10,000 each). No other disclosures relevant to this article were reported.
References
- 1. Marrie RA, Hitchon CA, Walld R, Patten SB, Bolton JM, Sareen J, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2018;70:970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marrie RA, Hitchon CA, Peschken CA, Chen H, Bernstein CN, Garland A. Health care utilisation before and after intensive care unit admission in rheumatoid arthritis. Clin Exp Rheumatol 2017;35:975–82. [PubMed] [Google Scholar]
- 3. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Rising incidence of psychiatric disorders before diagnosis of immune‐mediated inflammatory disease. Epidemiol Psychiatr Sci 2019;28:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hitchon CA, Boire G, Haraoui B, Keystone E, Pope J, Jamal S, et al. Self‐reported comorbidity is common in early inflammatory arthritis and associated with poorer function and worse arthritis disease outcomes: results from the Canadian Early Arthritis Cohort. Rheumatology (Oxford) 2016;55:1751–62. [DOI] [PubMed] [Google Scholar]
- 5. Witney AG, Treharne GJ, Tavakoli M, Lyons AC, Vincent K, Scott DL, et al. The relationship of medical, demographic and psychosocial factors to direct and indirect health utility instruments in rheumatoid arthritis. Rheumatology (Oxford) 2006;45:975–81. [DOI] [PubMed] [Google Scholar]
- 6. Marrie RA, Walld R, Bolton JM, Sareen J, Patten SB, Singer A, et al. Psychiatric comorbidity increases mortality in immune‐mediated inflammatory diseases. Gen Hosp Psychiatry 2018;53:65–72. [DOI] [PubMed] [Google Scholar]
- 7. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 8. Van der Zee‐Neuen A, Putrik P, Ramiro S, Keszei A, de Bie R, Chorus A, et al. Impact of chronic diseases and multimorbidity on health and health care costs: the additional role of musculoskeletal disorders. Arthritis Care Res (Hoboken) 2016;68:1823–31. [DOI] [PubMed] [Google Scholar]
- 9. Han GM, Han XF. Comorbid conditions are associated with healthcare utilization, medical charges and mortality of patients with rheumatoid arthritis. Clin Rheumatol 2016;35:1483–92. [DOI] [PubMed] [Google Scholar]
- 10. Tanner JA, Hensel J, Davies PE, Brown LC, Dechairo BM, Mulsant BH. Economic burden of depression and associated resource use in Manitoba, Canada. Can J Psychiatry 2019;65:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hanly JG, Thompson K, Skedgel C. A longitudinal study of ambulatory physician encounters, emergency room visits, and hospitalizations by patients with rheumatoid arthritis: a 13‐year population health study. J Rheumatol 2017;44:1421–28. [DOI] [PubMed] [Google Scholar]
- 12. Batelaan NM, Bosman RC, Muntingh A, Scholten WD, Huijbregts KM, van Balkom A. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive‐compulsive disorder, and post‐traumatic stress disorder: systematic review and meta‐analysis of relapse prevention trials. BMJ 2017;358:j3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burcusa SL, Lacono WG. Risk for recurrence in depression. Clin Psychol Rev 2007;27:959–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gauthier G, Mucha L, Shi S, Guerin A. Economic burden of relapse/recurrence in patients with major depressive disorder. J Drug Assess 2019;8:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norwegian Institute of Public Health . Anatomic Therapeutic Chemical classification system. 2018. URL: http://www.whocc.no/atc/structure_and_principles/.
- 16. Hitchon CA, Khan S, Elias B, Lix LM, Peschken CA. Prevalence and incidence of rheumatoid arthritis in Canadian first nations and non‐first nations people: a population‐based study. J Clin Rheumatol 2020;26:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Increased incidence of psychiatric disorders in immune‐mediated inflammatory disease. J Psychosom Res 2017;101:17–23. [DOI] [PubMed] [Google Scholar]
- 18. Marrie RA, Fisk JD, Yu BN, Leung S, Elliott L, Caetano P, et al. Mental comorbidity and multiple sclerosis: validating administrative data to support population‐based surveillance. BMC Neurol 2013;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chateau D, Metge C, Prior H, Soodeen RA. Learning from the census: the Socio‐economic Factor Index (SEFI) and health outcomes in Manitoba. Can J Public Health 2012;103 Suppl S:23S–27S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Tennakoon A, et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014;83:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagel S, Petersson IF, Bremander A, Lindqvist E, Bergknut C, Englund M. Trends in the first decade of 21st century healthcare utilisation in a rheumatoid arthritis cohort compared with the general population. Ann Rheum Dis 2013;72:1212–6. [DOI] [PubMed] [Google Scholar]
- 22. Cohen JA. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale (NJ): Erlbaum; 1988. [Google Scholar]
- 23. Curran PJ, Bauer DJ. The disaggregation of within‐person and between‐person effects in longitudinal models of change. Annu Rev Psychol 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CI, Wang L, Wei W, Yuce H, Phillips K. Burden of rheumatoid arthritis among US Medicare population: co‐morbidities, health‐care resource utilization and costs. Rheumatol Adv Pract 2018;2:rky005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michet CJ III, Strobova K, Achenbach S, Crowson CS, Matteson EL. Hospitalization rates and utilization among patients with rheumatoid arthritis: a population‐based study from 1987 to 2012 in Olmsted County, Minnesota. Mayo Clin Proc 2015;90:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rai SK, Avina‐Zubieta JA, McCormick N, De Vera MA, Lacaille D, Sayre EC, et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada From 2000 to 2011. Arthritis Care Res (Hoboken) 2017;69:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Tennakoon A, et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014;83:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Targownik LE, Kaplan GG, Witt J, Bernstein CN, Singh H, Tennakoon A, et al. Longitudinal trends in the direct costs and health care utilization ascribable to inflammatory bowel disease in the biologic era: results from a Canadian population‐based analysis. Am J Gastroenterol 2020;115:128–37. [DOI] [PubMed] [Google Scholar]
- 29. Pelkonen MK, Notkola IK, Laatikainen TK, Jousilahti P. 30‐year trends in asthma and the trends in relation to hospitalization and mortality. Respir Med 2018;142:29–35. [DOI] [PubMed] [Google Scholar]
- 30. Chen DY, Yu F, Tuan LW, Tang CH. Comparison of healthcare utilization and costs between RA patients receiving biological and conventional synthetic DMARDs: a Nationwide Population‐Based Cohort Study in Taiwan. Front Pharmacol 2019;10:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 32. Bansback N, Fu E, Sun H, Guh D, Zhang W, Lacaille D, et al. Do biologic therapies for rheumatoid arthritis offset treatment‐related resource utilization and cost? A review of the literature and an instrumental variable analysis. Curr Rheumatol Rep 2017;19:54. [DOI] [PubMed] [Google Scholar]
- 33. Guelfucci F, Kaneko Y, Mahlich J, Sruamsiri R. Cost of depression in Japanese patients with rheumatoid arthritis: evidence from administrative data. Rheumatol Ther 2018;5:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ 2019;22:372–8. [DOI] [PubMed] [Google Scholar]
- 35. Joyce AT, Smith P, Khandker R, Melin JM, Singh A. Hidden cost of rheumatoid arthritis (RA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol 2009;36:743–52. [DOI] [PubMed] [Google Scholar]
- 36. Bernstein MT, Mackenzie CS, Sareen J, Dufault B, Hitchon C, El‐Gabalawy R. Examining the cross‐sectional and longitudinal effects of anxiety sensitivity on indicators of disease severity among patients with inflammatory arthritis. J Anxiety Disord 2019;67:102117. [DOI] [PubMed] [Google Scholar]
- 37. Pasma A, Schenk C, Timman R, van ‘t Spijker A, Appels C, van der Laan WH, et al. Does non‐adherence to DMARDs influence hospital‐related healthcare costs for early arthritis in the first year of treatment? PLoS One 2017;12:e0171070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang PS, Aguilar‐Gaxiola S, Alonso J, Angermeyer MC, Borges G, Bromet EJ, et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet 2007;370:841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watson DE, Heppner P, Roos NP, Reid RJ, Katz A. Population‐based use of mental health services and patterns of delivery among family physicians, 1992 to 2001. Can J Psychiatry 2005;50:398–406. [PubMed] [Google Scholar]
- 40. Reilly S, Planner C, Hann M, Reeves D, Nazareth I, Lester H. The role of primary care in service provision for people with severe mental illness in the United Kingdom. PLoS One 2012;7:e36468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clatney L, MacDonald H, Shah SM. Mental health care in the primary care setting: family physicians’ perspectives. Can Fam Physician 2008;54:884–9. [PMC free article] [PubMed] [Google Scholar]
- 42. Fleury MJ, Farand L, Aubé D, Imboua A. Management of mental health problems by general practitioners in Quebec. Can Fam Physician 2012;58:e732–8. [PMC free article] [PubMed] [Google Scholar]
- 43. Fleury MJ, Grenier G, Vallee C, Aube D, Farand L, Bamvita JM, et al. Implementation of the Quebec mental health reform (2005–2015). BMC Health Serv Res 2016;16:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emptage NP, Sturm R, Robinson RL. Depression and comorbid pain as predictors of disability, employment, insurance status, and health care costs. Psychiatr Serv 2005;56:468–74. [DOI] [PubMed] [Google Scholar]
- 45. Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry 2019;6:164–73. [DOI] [PubMed] [Google Scholar]
- 46. Kaplan RM, Glassman JR, Milstein A. Effects of mental health on the costs of care for chronic illnesses. Psychiatr Serv 2019;70:1013–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


