Abstract
The use of aqueous film‐forming foam (AFFF) has resulted in the widespread occurrence of per‐ and polyfluoroalkyl substances (PFAS) in groundwater, drinking water, soils, sediments, and receiving waters throughout the United States and other countries. We present the research and development efforts to date by the Strategic Environmental Research and Development Program (SERDP) and the Environmental Security Technology Certification Program (ESTCP) to measure PFAS in the environment, characterize AFFF‐associated sources of PFAS, understand PFAS fate and behavior in the environment, assess the risk to ecological receptors, develop in situ and ex situ treatment technologies for groundwater, treat soils and investigation‐derived wastes, and examine the ecotoxicity of PFAS‐free fire suppression formulations. Environ Toxicol Chem 2021;40:24–36. © 2020 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Keywords: Aqueous film‐forming foam (AFFF), Per‐ and polyfluoroalkyl substances (PFAS), Environmental toxicology, Analytical chemistry, Fate and transport
INTRODUCTION
The use of aqueous film‐forming foam (AFFF) has resulted in the widespread occurrence of per‐ and polyfluoroalkyl substances (PFAS) in groundwater, drinking water, soils, sediments receiving waters, and wildlife throughout the United States and globally. The AFFF formulations have been used since the late 1960s to quickly and effectively extinguish hydrocarbon fires (US Government Accountability Office 2018). All legacy and current‐use AFFFs contain complex mixtures of PFAS, but those mixtures have changed over time. Legacy AFFF mixtures contained long‐chain PFAS, whereas current‐use AFFF is reported by manufacturers to consist exclusively of short‐chain PFAS (see Buck et al. 2011 for a description of long‐ vs short‐chain PFAS). The use of legacy AFFFs for emergency response, firefighter training, and equipment maintenance has resulted in detectable concentrations of PFAS in the environment throughout the United States. The foams have also been widely used outside the United States, in countries such as Australia, New Zealand, Canada, and Europe. Although there are no estimates for global management costs associated with PFAS releases from AFFF to the environment, the scope and cost to clean up airports and defense facilities undoubtedly represents a major liability.
Per‐ and polyfluoroalkyl substances are a class of chemicals of environmental concern and are increasingly regulated by the US Environmental Protection Agency (USEPA) and many state agencies due to their widespread occurrence in the environment and demonstrated bioaccumulation in humans and ecological receptors. Evidence that continued exposure above specific levels to certain PFAS may lead to adverse health effects led the USEPA to publish, in 2009, provisional health advisory levels for drinking water for perfluorooctanoic acid (PFOA) and perfluorooctanoic sulfonate (PFOS; US Environmental Protection Agency 2009). The USEPA has subsequently revised those Lifetime Health Advisories for PFOS and PFOA concentrations to 70 ppt (individually or combined) in drinking water (US Environmental Protection Agency 2016a, 2016b). Since then, several state agencies have published even lower values for drinking water (Interstate Technology and Regulatory Council 2020).
Publication of the Lifetime Health Advisories led to widespread public concern about PFAS fate and transport, human and ecological risk levels and effects, and means of remediation of these recalcitrant compounds. It quickly became evident that there was a paucity of research available to support remedial investigations at AFFF‐impacted sites, much less implement clean‐ups. Analytical methods with rigorous and defensible quality assurance and quality control for low‐level (ng/L) detection of PFAS in drinking water did not exist for compounds other than PFOA and PFOS. Although a number of references in the scientific literature showed that PFAS were present in ecological receptors, there were few reliable publications related to bioaccumulation and individual‐ or population‐level toxic effects. Due to their chemical structure (Figure 1), PFAS (especially perfluoroalkyl acids [PFAAs] such as PFOA and PFOS) are stable in the environment and resistant to treatment by biodegradation, photo‐oxidation, direct photolysis, and hydrolysis. To overcome these significant data gaps, a focused and sustained research effort was needed to develop the tools necessary to identify and clean up PFAS.
Figure 1.
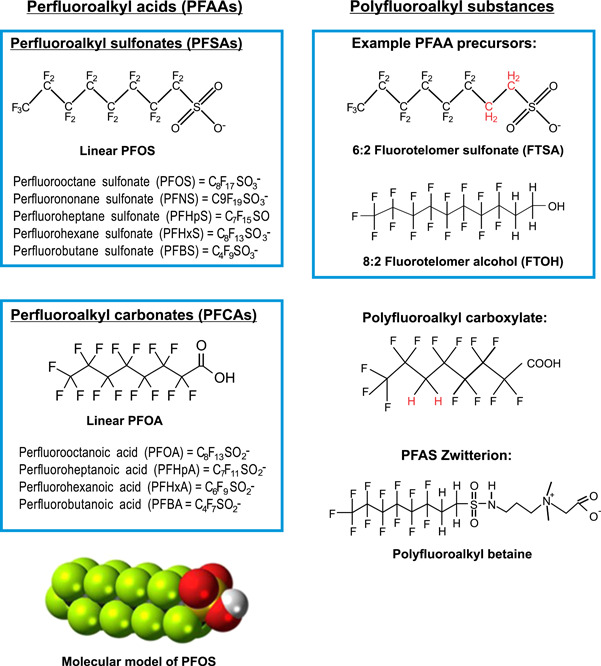
Representative per‐ and polyfluoroalkyl substances (PFAS) structures and formulas (from Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program 2017). For abbreviations, see Table 1.
The present Critical Perspectives provides an overview on the status of research, development, and demonstration efforts related to analytical advancements, fate and transport, ecological risks, and remedial strategies that collectively will assist in the management of AFFF‐impacted sites. Specifically, we discuss efforts to address these issues currently being funded through the Strategic Environmental Research and Development Program (SERDP) and the Environmental Security Technology Certification Program (ESTCP). The US Congress established SERDP in 1990 to address the Department of Defense's (DoD) environmental issues in partnership with the Department of Energy and the USEPA. The ESTCP project is the DoD's environmental technology demonstration and validation program established in 1995 to promote the transfer of innovative technologies that have successfully established proof of concept to field or production use.
Other studies published in this issue cover in more detail research conducted by SERDP and ESTCP on the fate and transport of PFAS (Anderson et al. 2020), and the treatment of PFAS in water, soils, and investigation‐derived wastes (Coyle et al. 2020).
PFAS IN AFFF
The PFAS are a broad classification of several hundred different organic substances whose molecular structures contain one or more carbon (C) atoms with fluorine (F) atoms in the place of hydrogen (H) atoms (Buck et al. 2011). Although the composition of the PFAS in some AFFFs is being documented in the scientific literature, formulations and chemical changes during natural weathering after release of the products are a challenge (e.g., D'Agostino and Mabury 2013; Barzen‐Hanson et al. 2017; Field et al. 2017; US Environmental Protection Agency 2017; Interstate Technology and Regulatory Council 2020).
For legacy and current‐use AFFFs, fluorinated surfactants provide critical performance characteristics for foam creation and fire suppression on application. The AFFF formulations provide a vapor‐sealing film on a hydrocarbon fuel surface to isolate the fuel from the oxygen and provide protection against re‐ignition of the exposed fuel. Due to these unique surface‐active properties, the PFAS were, and continue to be, a key component of AFFFs (Field et al. 2017; Interstate Technology and Regulatory Council 2020). Resiliency to heat, pressure, or oxidation is critical to their success as fire‐fighting agents and ultimately contribute directly to their persistence in the environment.
Information on the commercial formulations approved for use by the DoD was reviewed and compiled into a document, “FAQs regarding PFASs associated with AFFF use at U.S. military sites” (Field et al. 2017). The first AFFFs were created in the 1960s by 3M, and 3M was the sole supplier from the mid‐1960s until 1973. From 1973 onward, several manufacturers created fluorotelomer‐based AFFFs that met the Military Specification criteria (Figure 2). These AFFF formulations contained a broad mixture of both long carbon–fluorine chain and short carbon–fluorine chain PFAS, including dozens to hundreds of other PFAS (Buck et al. 2011; D'Agostino and Mabury 2013). Typically, PFAS represent 3 to 6% by weight of the entire formulation, which also contains other nonfluorinated surfactants, stabilizers, solubilizers, and other chemicals.
Figure 2.
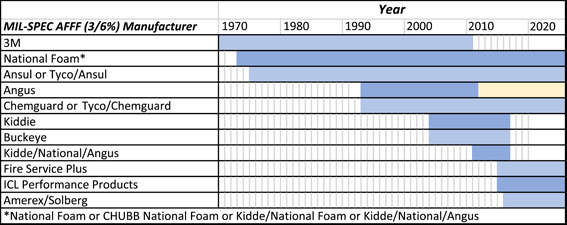
Manufacturers of military specifications (MIL‐SPEC) aqueous fire‐fighting foams (AFFFs) by year. Adapted and updated from Field et al. (2017).
The PFAS composition within historic AFFF formulations contained a significant percentage of PFOS and related perfluoroalkyl sulfonates, such as perfluorohexane sulfonate (PFHxS), as well as other PFAS (Field et al. 2017). Although other AFFFs also included various fluorotelomer‐based formulations, most of the environmental issues with PFAS today probably result from the use of PFOS‐based AFFF formulations (Anderson et al. 2020). The PFAS that are currently monitored, and those on which SERDP and ESTCP have focused research projects, are listed in Table 1. Further PFAS may be added to this list as more research indicates the need.
Table 1.
Focused per‐ and polyfluoroalkyl substances analyte list for projects conducted under SERDP and ESTCP
| Group | Analyte name | Acronym | No. of perfluorinated carbons | Chemical Abstract Services registry no. |
|---|---|---|---|---|
| Perfluoroalkyl carboxylates (PFCAs) | Perfluorobutanoic acid | PFBA | 3 | 375‐22‐4 |
| Perfluoropentanoic acid | PFPeA | 4 | 2706‐90‐3 | |
| Perfluorohexanoic acid | PFHxA | 5 | 307‐24‐4 | |
| Perfluoroheptanoic acid | PFHpA | 6 | 375‐85‐9 | |
| Perfluorooctanoic acid | PFOA | 7 | 335‐67‐1 | |
| Perfluorononanoic acid | PFNA | 8 | 375‐95‐1 | |
| Perfluorodecanoic acid | PFDA | 9 | 335‐76‐2 | |
| Perfluoroundecanoic acid | PFUnA | 10 | 2058‐94‐8 | |
| Perfluorododecanoic acid | PFDoA | 11 | 307‐55‐1 | |
| Perfluorotridecanoic acid | PFTriA | 12 | 72629‐94‐8 | |
| Perfluorotetradecanoic acid | PFTreA | 13 | 376‐06‐7 | |
| Perfluoroalkyl sulfonates (PFSAs) | Perfluorobutanesulfonic acid | PFBS | 4 | 375‐73‐5 |
| Perfluoropentanesulfonic acid | PFPeS | 5 | 3872‐25‐1 | |
| Perfluorohexanesulfonic acid | PFHxS | 6 | 355‐46‐4 | |
| Perfluoroheptanesulfonic acid | PFHpS | 7 | 375‐92‐8 | |
| Perfluorooctanesulfonic acid | PFOS | 8 | 1763‐23‐1 | |
| Perfluorononanesulfonic acid | PFNS | 9 | 68259‐12‐1 | |
| Perfluorodecane sulfonic acid | PFDS | 10 | 335‐77‐3 | |
| Perfluoroalkane sulfonamides (PFASAs) | Perfluorooctane sulfonamide | PFOSA | 8 | 754‐91‐6 |
| Fluorotelomer sulfonates | Flurorotelomer sulfonic acid 4:2 | FtS 4:2 | 4 | 757124‐72‐4 |
| Flurorotelomer sulfonic acid 6:2 | FtS 6:2 | 6 | 27619‐97‐2 | |
| Flurorotelomer sulfonic acid 8:2 | FtS 8:2 | 8 | 39108‐34‐4 | |
| N‐ethyl and N‐methyl perfluoroalkane sulfonamidoacetic acids and salts (EtFASAAs and MeFASAAs) | 2‐(N‐ethyl perfluorooctane sulfonamido) acetic acid | N‐EtFOSAA | 8 | 2991‐50‐6 |
| 2‐(N‐methyl perfluorooctane sulfonamido) acetic acid | N‐MeFOSAA | 8 | 2355‐31‐9 |
PFAS = per‐ and polyfluoroalkyl substances; SERDP = Strategic Environmental Research and Development Program; ESTCP = Environmental Security Technology Certification Program.
Exposure pathways
Conceptual site models (CSMs), developed as part of remedial investigations and risk assessments, are familiar to members of the environmental community. Figure 3 depicts a generic scenario in which AFFF is discharged to the soil surface. Long‐chain PFAS are retained in surface soils, whereas short‐chain PFAS migrate more rapidly into groundwater. The PFAS in surface water runoff are carried into adjacent water bodies, and either further migrate downstream or are retained in sediment. Exposure pathways for humans are thought to be principally through ingestion of impacted water, soil, or food. Short‐chain PFAS in water are also known to be taken up into plants and thus may be found in food (Blaine et al. 2013, 2014). Ecological receptors can be exposed in soil or sediment, through water ingestion and/or ingestion of impacted food/prey.
Figure 3.
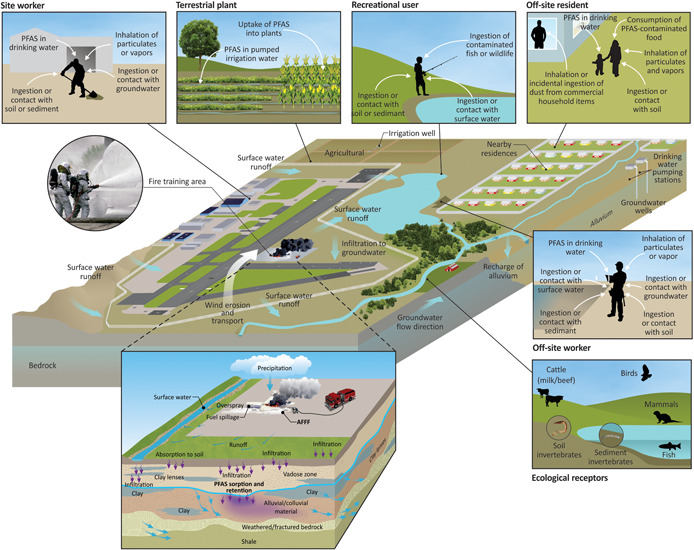
Conceptual site model for aqueous fire‐fighting foam (AFFF) release and per‐ and polyfluoroalkyl substances (PFAS) uptake to environmental receptors.
Although useful for illustration, these generic CSMs are overly simplistic; critical fate and transport properties and processes are crucial in the evolving science (Anderson et al. 2020). Also, even though the USEPA has made progress in developing analytical methods for some PFAS (such as PFOA and PFOS) in drinking water, there remain numerous PFAS for which standardized analytical methods in multiple environmental media (e.g., tissue, sediments) are lacking. There is a large data gap in our knowledge of bioaccumulative processes, exposure pathways in the food chain, and the toxicity of PFAS to those ecological receptors. These focused, needs‐driven research and development knowledge gaps are what SERDP and ESTCP are specifically addressing.
On‐going PFAS research
Initial funding by SERDP and ESTCP specifically focused on PFAS began in 2011 shortly after the USEPA Provisional Health Advisories were released. At that time, the most pressing need facing the management of PFAS‐impacted sites was developing and evaluating technologies and management approaches to mitigate impacts to groundwater (Figure 4). In the ensuing years, it became evident that fundamental science was lacking on the characterization (analytical), ecotoxicity, and remediation of PFAS. The DoD needed to fill these critical scientific data gaps to conduct remedial investigations and feasibility studies, as well as to design and implement site clean‐ups.
Figure 4.
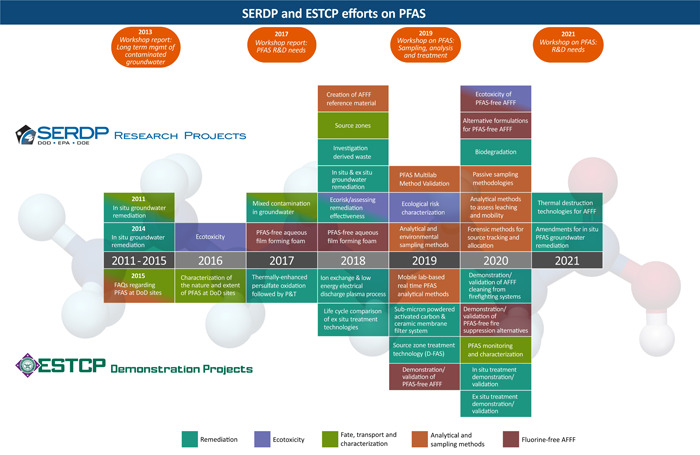
Chronology of SERDP statements of research need and ESTCP demonstration projects on AFFF PFAS in the environment. See Supplemental Data, Table S1, for a complete listing of all projects. AFFF = aqueous film‐forming foam; DoD = Department of Defense; ESTCP = Environmental Security Technology Certification Program; FAQ = frequently asked question; PFAS = per‐ and polyfluoroalkyl substances; P&T = Pump and Treat; SERDP = Strategic Environmental Research and Development Program.
To provide strategic guidance and prioritize future research and demonstrations on the management of AFFF‐impacted sites, SERDP and ESTCP held a workshop in May 2017 in Washington, DC (Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program 2017). Experts on PFAS and representatives from the USEPA, the DoD, the states, academia, and industry were invited to provide 1) a review of the current state of the science regarding sources of PFAS in the environment, particularly AFFF; 2) an evaluation of currently available and developing technologies for characterization and remediation of AFFF‐impacted sites; and 3) an assessment of research and demonstration needs to improve remediation performance and efficiency, and ultimately reduce the cost of managing AFFF‐impacted sites.
Following the recommendations from that meeting, statements of need (SONs) were published to encourage funding of innovative research and demonstrations to characterize and manage PFAS‐impacted areas (Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program 2020). Figure 4 provides an overview of the SONs from 2011 to 2021 in terms of the characterization and management of PFAS. Table 2 provides a list of the portfolio topic areas, and the number of projects currently funded. A complete list of PFAS‐related research projects funded by SERDP and ESTCP is given in the Supplemental Data, Table S1, with links to the projects on the Program website. Summaries of the technical areas of research and demonstrations that have been and are planned are as follows: 1) analytical and environmental sampling methods; 2) fate, transport, and characterization; 3) bioaccumulation, ecotoxicity, and ecological risk assessment; 4) PFAS treatment technologies; and 5) ecotoxicity of PFAS‐free fire suppression formulations.
Table 2.
SERDP and ESTCP portfolio topic areas
| Topic area | No. of projects | Topics |
|---|---|---|
| Analytical and environmental sampling methods | 21 | Development and validation of PFAS analytical methods in environmental media |
| Rapid screening tools including PIGE, NMR, CR, and combustion gas analysis | ||
| Methods for mitigating bias in PFAS levels | ||
| On‐site mobile laboratory | ||
| Forensics | ||
| Passive sampling | ||
| Ecotoxicity and ecological risk assessment | 18 | Toxicity reference values for PFOA and PFOS for birds, amphibians, benthic infauna and fish |
| Methods for ecological risk assessment for PFAS exposure for threatened and endangered species | ||
| Bioaccumulation and biomagnification of PFAS and PFAS‐free formulations in terrestrial and aquatic environments | ||
| Predictive modeling of bioaccumulation and toxicity | ||
| Fate, transport, and characterization | 10 | Nature and extent of PFAS in environmental media |
| Fate and transport of PFAS in saturated and unsaturated zones | ||
| Precursor transport and transformation in groundwater | ||
| Numerical modeling of transport of PFAS | ||
| Groundwater and surface water remediation methods | 42 | In situ barrier and vault treatment systems |
| In situ and ex situ chemical and electrocatalytic treatment | ||
| Chemical oxidation/reduction defluorination | ||
| Plasma, electro‐oxidation, and incineration treatment technologies | ||
| Novel polymers for PFAS sorption | ||
| Ion exchange | ||
| Biotransformation | ||
| Life cycle assessment of PFAS treatment technologies | ||
| BMPs for treatment of PFAS in stormwater | ||
| Biodegradation | ||
| Destruction of PFAS treatment residuals | 26 | Electrochemical membrane reactors |
| Oxidative‐reduction destruction | ||
| Thermal technologies | ||
| Plasma and electron beam technologies | ||
| Photoelectrochemical reductive pathways | ||
| Novel catalytic treatment systems | ||
| Ultrasound | ||
| Development of PFAS‐free AFFF | 22 | Testing of commercially available off‐the‐shelf foams to military specifications |
| Siloxane surfactants | ||
| Oleophobic surfactants | ||
| Organosilicate nanostructures | ||
| Nano‐encapsulated ionic foams |
SERDP = Strategic Environmental Research and Development Program; ESTCP = Environmental Security Technology Certification Program; PFAS = poly‐ and perfluoroalkyl substances; PIGE = particle‐induced gamma ray emission; NMR = nuclear magnetic resonance; CR = complex resistivity; PFOA = polyfluorooctanoic acid; PFOS = polyfluorooctane sulfonic acid; BMP = best management practice; AFFF = aqueous fire‐fighting foam.
Analytical and environmental sampling methods
The development and demonstration of standardized, fully validated procedures for the sampling of AFF products and environmental media, including waters (groundwater, surface water, storm water), soils, and sediments, as well as biological tissues for analyses of the individual and total PFAS have been identified as critical priority research needs. The range and type of sampling and analysis methods needed to investigate and delineate the occurrence of PFAS far exceed the range and types currently available. Analytical methods developed with SERDP funding are geared toward meeting the PFAS quality control requirements needed to support decision making (DoD Quality Systems Manual Ver 5.3; Department of Defense 2019). Funding from SERDP for projects to develop standardized analytical and environmental sampling methods for PFAS is briefly discussed in the following sections.
Analytical methods for PFAS
The Programs are actively engaged in promoting analytical methods beyond the current USEPA published procedures for PFAS analyses in water (methods 537.1 [Shoemaker and Tettenhorst 2018] and 533 for drinking water [US Environmental Protection Agency 2019a], and method 8327 for nonpotable water [US Environmental Protection Agency 2019b]). Several commercial laboratories accredited under the DoD Environmental Laboratory Accreditation Program offer analyses of these media using in‐house developed methods that are based on USEPA method 537.1 (US Environmental Protection Agency 2019a). In collaboration with the USEPA and the Naval Seas Systems Command Laboratory Quality and Accreditation Office, SERDP and ESTCP are currently conducting laboratory method validation of a solid phase extraction/isotope dilution method. Matrices in the study include groundwater, surface water, soils, sediment, landfill leachate, municipal wastewater, tissue, and biosolids (i.e., municipal wastewater treatment plant residuals).
In addition, SERDP is funding studies at McGill University (Montreal, QC, Canada; Liu 2019) to develop and validate improved analytical procedures for the comprehensive profiling of PFAS in AFFF‐impacted environmental matrices and biological samples. This research is directed toward analytical compound–specific PFAS analyses encompassing the breadth of anionic, cationic, and zwitterionic PFAS, while minimizing matrix interference.
A development of analytical methods is being undertaken to assess leaching and mobility of PFAS from soils, sediments, and solid wastes (Guelfo 2020). This project aims to modify the USEPA's Leaching Environmental Assessment Framework standard procedures for use with PFAS.
Organofluorine quantification methods
The development of procedures to assess the total organofluorine in environmental media (water, soil, sediment) or investigation‐derived waste was begun by SERDP in 2019. Measures of total organofluorine are needed as rapid field screening tools for PFAS‐impacted sites, to assess the transformation and distribution of PFAS in surface and subsurface transport; these measures are important in conducting fluorine mass balance procedures, to assess the efficacy of various applications of PFAS remediation technologies. Total extractable organofluorine, total oxidizable precursors, and particle‐induced gamma‐ray emission are being evaluated as techniques that can screen groundwater, surface runoff, soils, and sediments for total PFAS constituents (Liu 2019; Peaslee 2019). Rapid field screening methods for determining the extent of a plume over AFFF source areas that could provide semiquantitative screening values include adapting two existing borehole‐deployable geophysical technologies, nuclear magnetic resonance, and complex resistivity as source tools (Slater 2019), and modification of an off‐the‐shelf combustion laser spectroscopy to combust PFAS and produce a measure of total fluorine (Hannigan 2019).
Passive sampling methods for PFAS
New research has recently been funded (Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program 2020) to develop PFAS passive sampling methods. The aim is not only to develop media that could competently quantify a wide range of PFAS in water, but also to aid in understanding partitioning through an evaluation of sorbent/water coefficients, as well as natural solid/water coefficients and molecular diffusivities in water. Results from these efforts are expected to be finalized beginning in 2024.
Forensic techniques
Additional new research in 2020 is exploring forensic methods and tools for source tracking and allocation of PFAS (Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program 2020). Adaptations of conventional, or novel, analytical techniques or methodologies to differentiate the PFAS in AFFF from non‐AFFF sources are being examined in these projects. Efforts are under way to develop spectral libraries of PFAS that will include AFFF‐derived PFAS and PFAS derived from other sources, improved analytical methods, and/or validated models to predict changes to AFFF mixtures over time, including chemical pathways to the most toxic compounds. Final results for some of these efforts are expected as early as 2024.
FATE, TRANSPORT, AND CHARACTERIZATION
Understanding the fate and transport of PFAS in the environment is critical for assessing risks and developing accurate CSMs. Fate and transport evaluations are complicated by the sheer number of PFAS and the diverse chemical structures present in complex AFFF formulations. The chemistry is further complicated by the variations and uncertainties in linear and branched forms (i.e., isomers) of many PFAS. A large number of different isomers, as well as variations in function groups and chain lengths can be found at AFFF‐impacted sites, and these variations in the chemical composition of the formulations can have pronounced impacts on key fate and transport properties, as well as on the bioaccumulation potential and susceptibility to different treatment technologies. The basic physical–chemical properties of most of the PFAS compounds typically found in AFFF formulations that are needed to predict fate and transport behavior are not available, and there are large inherent variabilities in the physical–chemical properties for the PFAS that have been studied.
Complicating the issue is that there are probably significant mixture effects and interactions with co‐occurring chemicals that can alter fate and transport properties and potentially their toxicity and associated risk. In addition, a variety of abiotic and biotic processes can transform certain PFAA precursors under specific environmental conditions into the more problematic PFAAs (e.g., PFOA and PFOS); these processes need to be considered in risk assessments, model predictions, and CSMs. Fate and transport research being undertaken by the Programs is discussed in the Focus article by Anderson et al. in this issue (2020).
PFAS ecotoxicity, ecological risk assessment, and bioaccumulation
Ecological risk characterization for AFFF‐derived PFAS was identified at the 2017 Workshop by DoD site managers as an immediate research need. Toxicity information for wildlife and aquatic life in the scientific literature has been primarily limited to reporting concentrations present for a few PFAS, particularly PFOA and PFOS. Scientifically defensible toxicity reference values (TRVs) to use in ecological risk assessments have generally been lacking. Moreover, there has been an absence of information on what other PFAS are prevalent at AFFF‐impacted sites, and their relative toxicities compared with PFOA and PFOS. Determinations of whether the different PFAS had synergistic or antagonistic effects on toxicity expression have been needed, as well as whether a relative potency factor similar to those developed for polychlorinated biphenyl (PCB) congeners and for dioxin/furans could be determined and applied to PFAS ecological risk assessments. These ecotoxicity research questions formed the basis for the 2016 ecotoxicity SON. The target species, endpoints, and individual PFAS for which TRVs are being developed are given in Table 3.
Table 3.
Development of toxicity reference values (TRVs) for PFAS from AFFF: test species and exposure endpoints
| Performing investigator | ||||
|---|---|---|---|---|
| Project ID | Quinn (2016) | Sepulveda (2016) | Salice (2016) | Simcik (2016) |
| Species |
Mammals White‐footed mouse (Peromyscus leucopus) Deer mouse (Peromyscus maniculatus) Lab mice (Mus sp.) |
Amphibians Northern leopard frog (Rana pipiens) Eastern tiger salamander (Ambystoma trigrinum) American toad (Anaxyrus americanus) |
Freshwater Invertebrate Midge (Chironomus tentans) Freshwater fish Fathead minnow (Pimephales promelas) Avian Bobwhite quail (Colinus virginianus) Reptile Brown anoles (Anolis sagrei) |
Avian Japanese quail (Coturnix japonica) |
| Exposure and endpoints |
Range‐finding Oral gavage Benchmark dose NOEL/LOEL LE10/EC10 |
Range‐finding LD50 Chronic larvae water EC50 Subchronic larvae sediment EC50 Chronic adult diet EC50 Chronic adult sediment EC50 |
C. tentans 20‐d survival and growth P. promelas 10‐d survival/growth. 7‐d development/teratogenicity C. virginianus 20‐wk survival, growth, reproductive output, hatching success, hatchling survival A. sagrei 14‐ and 60‐d survival, growth, behavior, immunotoxicity |
Dietary 5‐ and 22‐d exposure LC50 and LT50 to 1) PFOA, PFOS, PFOS + PFOA; and 2) 3M AFFF Dietary 18‐wk reproduction, clutch size, hatch, juvenile survival EC50 after exposure to PFOS, PFOA, and AFFF |
| AFFF‐PFAS TRVs |
PFOS PFOA PFHxS PFBS 6:2 FTS PFNA |
PFOS PFOA PFHxS 6:2 FTS |
PFOS PFOA PFNA PFHxS PFOS + PFHxS PFHpA PFBS |
PFOS PFOA PFOS + PFOA 3M AFFF |
PFAS = per‐ and polyfluoralkyl substances; AFFF = aqueous fire‐fighting foam; LD50 = median lethal does; EC50 = median effect concentration; LC50 = median lethal concentration; LT50 = median lethal time; NOEL/LOEL = no‐observed effect level/lowest‐observed effect level; LE10/EC10 = 10% lethal effect/10% effect concentration. For other abbreviations, see Table 1.
An additional set of research needs focused on the immediate need to provide AFFF site managers and ecological risk assessors with information on how PFAS bioaccumulate and biomagnify through the food web, potentially posing risks to threatened and endangered species. It took many years of research and literally thousands of scientific publications to gather similar information for hydrophobic organic contaminants that bioaccumulate (e.g., polycyclic aromatic hydrocarbons [PAHs], PCBs, dioxins), to support the models and assessment tools used at Superfund sites such as the Lower Fox River (WI, USA), the Hudson River (NY, USA), the St. Louis River (MN, USA), the Lower Willamette River (OR, USA), and many others. Management decisions relative to ecological risks at PFAS‐impacted sites do not have the luxury of a large body of information from the scientific literature. Federal, state, and the public are requiring ecological risk assessments at AFFF‐impacted sites to be conducted now, with whatever information is available. The 2018 SON from SERDP focused on developing approaches assessing PFAS risks to threatened and endangered species at AFFF‐impacted sites using what currently available literature and bioaccumulations tools could be identified.
The third kind of supported research is intended to provide information on the data gap identified above: to foster a longer term body of evidence on the bioavailability, bioaccumulation, and biomagnification of PFAS in the environment. Research questions included determining the rate and extent of PFAS uptake from soils, sediment, and water by lower trophic level organisms. Does biotransformation of PFAA precursors occur within these organisms, and could we then learn the relevant mixtures for further study with higher trophic level organisms? Very little is known about the uptake and excretion rates of PFAS by organisms throughout the food web to at least include competitive uptake and/or selective bioaccumulation at different trophic levels. Are we able to assess PFAS bioaccumulation/biomagnification throughout a food web? These questions formed the basis of the 2019 SON.
The most recent ecotoxicity 2020 SON focuses on defining the relative toxicity of candidate formulations to replace the PFAS‐containing legacy AFFFs at DoD sites. The Programs are currently funding research assessing both commercially available AFFF replacements and are also funding development of new surfactant formulations. The ongoing work will first confirm which of these candidate replacement surfactants meet the current DoD fire‐fighting performance requirements. For those formulations meeting the specifications, this initial set of studies will compare the nominal toxicities of the candidate formulations relative to the PFAS‐containing AFFF formulations currently used by the DoD. Dose–response exposures are being explored to establish effective and lethal dose concentrations that are toxic to a wide array of relevant species including sediment and soil invertebrates, pelagic algae and invertebrates, fish, birds, mammals, and plants (Table 4).
Table 4.
Standardized comparisons of relative chronic toxicity for whole foam PFAS‐free AFFF formulations: species and endpoints
| Performing investigator | ||||||
|---|---|---|---|---|---|---|
| Wirth (2020) | Quinn (2020) | Suski (2020) | Kuperman (2020) | Hoverman (2020) | Wu (2020) | |
| Species |
Fish Fathead minnow (Pimephales promelas; LC50, EC50) Sheepshead minnow (Cyprindon variegatus; LC50, EC50) Rainbow trout (Oncorhynchus mykiss) Aquatic invertebrates Water flea (Ceriodaphnia dubia; (LC50, EC50) Opossum shrimp (Americamysis bahia; LC50, EC50) Copepod (Pseudodiaptomus pelagicus, or Acartia tonsa; LC50) Amphipod (Hyalella azteca; LC50, EC50) Midge (Chironomus dilutus; LC50) Hard clam (Mercenaria mercenaria), or Eastern oyster (Crassostrea virginica), or blue mussel (Mytilus edulis) Diatoms Marine microalgae (Phaeodactylum tricornutum; LC50, EC50) Green algae (Raphidocelis subcapitata; EC50) |
Mammals White‐footed mouse (Peromyscus leucopus; LD50, EC50) Deer mouse (Peromyscus maniculatus; LD50, EC50) |
Avian Bobwhite quail (Colinus virginianus; LC50/EC50) Reptile Brown anole (Anolis sagrei; LC50/EC50) Fish Fathead minnow (Pimephales promelas; LC50, EC50) Aquatic invertebrate Midge (Chironomus tentans; LC50, EC50) Diatom Green algae (Raphidocelis subcapitata; LC50, EC50) |
Plants Alfalfa (Medicago sativa) Perennial ryegrass (Lolium perenne) Barnyard grass (Enchinochloa crus‐galli) Soil invertebrates Earthworm (Eisenia andrei; EC50) Collembola (Folsomia candida; EC50) Potworm (Enchytraeus crypticus; EC50) |
Fish Fathead minnow (Pimephales promelas) Amphibian Gray treefrog (Hyla versicolor; EC50) Aquatic invertebrates Water flea (Daphnia magna) |
Plant Field mustard (Brassica rapa; EC50) Soil invertebrates Nematode (Caenorhabditis elegans; EC50) Microarthropod (Folsomia candida; EC50) |
PFAS = poly‐ and perfluoroalkyl substances; AFFF = aqueous fire‐fighting foam; LC50 = median lethal concentration; EC50 = median effect concentration; LD50 = median lethal dose.
PFAS treatment technologies
Per‐ and polyfluoroalkyl substances are highly resistant to biological and chemical degradation, particularly the PFAAs such as PFOS. This recalcitrance to degradation results from very strong carbon–fluorine bonds. Currently, the technologies for treating water are limited and often expensive. These technologies largely rely on separation technologies for removing the PFAS from the water (Crone et al. 2019; US Environmental Protection Agency 2019c). In addition to being able to treat the wide range of PFAS present in AFFF formulations, treatment technologies must also be able to remove and/or destroy PFAA precursors such as the fluorotelomer sulfonates. Finally, effective PFAS treatment technologies for water matrices (e.g., drinking water) must be able to achieve the low part/trillion (ng/L) clean‐up criteria required while remaining as sustainable and cost effective as possible.
Initial treatment systems focused on the use of ex situ treatment with sorptive media such as granular activated carbon (GAC) as a relatively low‐cost, off‐the‐shelf technology. However, GAC has limited adsorption capacity for certain PFAS, and breakthrough is relatively rapid. This performance can further be impacted by co‐occurring chemicals present in the groundwater and the fact that performance for shorter chain PFAS is also limited. These types of technologies also generate spent media, regenerate solutions, or reject waters that require further disposal and treatment.
Research is ongoing for treatment systems that focus on in situ and ex situ treatment of PFAS‐impacted soil and waters, as well as on‐site treatment of concentrated PFAS waste streams such as drill cuttings, spent GAC, spent ion‐exchange resins, and regenerant brines. For all research projects under SERDP, whether the treatment involves PFAS transformation, separation, or both, the treatment process must be shown to perform cost effectively under typical field conditions, considering geochemistry, the presence of likely co‐occurring chemicals (with respect to the impacts of treatment on the co‐occurring chemicals, as well as the impact of the co‐occurring chemicals on PFAS treatment), and the relatively low concentrations of PFAS that are present at many sites. Separation technologies may protect human and ecological receptors from further exposure by concentrating PFAS from the media (e.g., GAC sorption, reverse osmosis), and destruction of the PFAS is desired to limit future issues associated with the concentrated media disposal. Mature technologies such as thermal treatment and developing technologies such as plasma and electron‐beam offer attractive options to completely destroy or mineralize the PFAS. However, the efficacy of existing technologies is largely unknown, and performance and costs are still to be evaluated for the developing technologies. Demonstrating the effectiveness and sustainability of thermal destruction technologies is a high priority demonstration need (see Textbox 1). Research being undertaken by the Programs related to PFAS treatment is discussed in the companion article in this issue (Coyle et al. 2020).
FUTURE RESEARCH AND CONCLUSIONS
Many of the critical and priority research needs identified in the Program's 2017 Workshop Report are under investigation. Even with the substantive investment to date (Supplemental Data, Table S1), there remains considerably more work to do.
Data gaps that need to be addressed to support ecological risk assessments include uptake, trophic transfer, and TRVs of PFAS to freshwater and marine pelagic and benthic invertebrates, terrestrial invertebrates, fish, and avian species. Although TRVs have been and are being developed for PFOA and PFOS, values are lacking for compounds that readily bioaccumulate such as perfluorohexane sulfonic acid (PFHxS), perfluorohexanoic acid (PFHXA), and perfluorobutanoic acid (PFBA) in terrestrial and aquatic environments. Little is known regarding the toxicity of PFAS mixtures outside of PFOS and PFOA. Whether other compounds are toxic in themselves, whether they may contribute additive or synergistic toxicity, or whether they do not significantly contribute to toxicity remains largely unknown. How toxicity effects observed in laboratory exposures translate to population effects is completely unknown at this time.
Analytical methods for detecting low levels of PFAS are progressing; more may still be needed. The PFAS standard reference materials (SRMs) for soils, sediments, and fish and plant tissue are considered a data gap. The SRMS for environmental media are available for analyses of PCBs, PAHs, dioxin/furans, semivolatile organic compounds, volatile organic compounds, and metals; there are few SRMs for PFAS, and those typically only include PFOS. The SRMs are a requirement for analytical characterization at Superfund and Resource Conservation and Recovery Act sites. At a minimum, SRMs for environmental media should include PFCAs, PFSAs, PFSAs, and fluorotelomer sulfonates.
Further research to understand PFAS partitioning among media, phase, and kinetics may also be needed. The lack of data on structure–property relationships (ionic state, carbon chain length), physical and chemical properties, solubilities, sorption/desorption, and distribution coefficients in solid, aqueous, and biological phases is an impediment to understanding fate and transport, as well as biological uptake and food web transfer.
Figure 4 highlights the 2020 and 2021 new research topics; the research and demonstration projects have been selected, but work has yet to begin. Thermal and hydrothermal destruction technologies are expected to be a high‐priority research funding area in the next several years, as is the improvement of amendments for in situ treatment of impacted groundwater. In the coming years, the Programs will look to move the remediation technologies developed with SERDP research funding into demonstration and validation projects under ESTCP.
The SONs for research and demonstration projects are published on SERDP and ESTCP's home webpage: SERDP solicitations are typically released in the fall, and ESTCP solicitations are released in the early new year. Both SERDP and ESTCP welcome new and innovative methods for addressing this issue of national and international concern.
TEXTBOX 1. SERDP and ESTCP research and demonstration PFAS projects at former US Air Station Joint Reserve Base Willow Grove, PA (USA).

The US Navy is managing PFAS environmental liabilities at the former Naval Air Station Joint Reserve Base (NASJRB) Willow Grove. Located within 25 miles of Philadelphia (PA, USA) releases of PFOA/PFOS occurred with historical AFFF usage related to firefighting training, fire suppression systems, and emergency response. In 2014, municipal groundwater drinking water wells, adjacent to NASJRB Willow Grove, were found to have exceeded the USEPA's provisional health advisory levels for PFOA/PFOS.
The NASJRB Willow Grove is voluntarily serving as a test or demonstration site to support SERDP and ESTCP projects on PFAS characterization, remediation, and ecological risk assessments. This also includes supporting field tests of emerging groundwater treatment technologies into a pilot groundwater treatment system it is currently testing at an area of the former base.This integration allows researchers to field‐test and evaluate their technologies' ability to treat PFAS, while the Navy evaluates existing commercial technologies with its own pilot treatment systems. Projects to date include the following.
| Principal investigator | Institution | Research topic | |
|---|---|---|---|
| Treatment of PFAS in groundwater | Paul Edmiston | College of Wooster | PFAS Removal from Water Using Molecularly Engineered Coatings on Sand and Silica |
| Michelle Crimi | Clarkson University | Combined In Situ/Ex Situ Treatment Train for Remediation of PFAS Contaminated Groundwater | |
| Douglas Call | Research Triangle | Electrically Assisted Sorption and Desorption of PFAS | |
| Timothy Strathmann | Colorado School Mines | Regenerable Resin Sorbent Technologies with Regenerant Solution Recycling for Sustainable Treatment of PFAS | |
| Destruction of PFAS in investigation‐derived waste | Don Zhao | Auburn University | A Cost‐Effective Technology for Destruction of PFAS from IDW |
| Thomas Boving | University of Rhode Island | Innovative Treatment of IDW containing PFAS and Other Co‐Contaminants | |
| Christopher Sales | Drexel University | Application of Non‐Thermal Plasma Technology for the Removal of PFAS from IDW | |
| Dave Major | Geosyntec | Demonstration of Smoldering Combustion Treatment of PFAS‐impacted IDW | |
| Ezra Cates | Clemson University | Pilot Scale Assessment of a Deployable Photocatalytic Treatment System for PFAS Destruction in IDW | |
| James Hatton | Jacobs Engineering | Demonstration of Infrared Thermal Treatment of PFAS‐contaminated Soils from Subsurface Investigations | |
| Suresh Pillai | Texas A&M University | Ex Situ Remediation of IDW containing PFAS by Electron Beam Technology | |
| Treatment of PFAS in stormwater | Staci Simonich | Oregon State University | BMPs for Optimizing Removal of PAHs, PCBs, PFAS, and Metals from Stormwater at DoD Sites |
| Fate, transport, and ecological risk of PFAS | Marie Kurz | Drexel University | Uptake and Bioaccumulation/Biomagnification of Subsurface‐Derived PFAS in Aquatic Food Webs |
| Chris Salice | Towson University | Environmental Determinants of PFAS Accumulation in Fish: Towards an Improved Bioaccumulation Model |
IDW = investigation‐derived waste; PFAS = per‐ and polyfluoroalkyl substances; BMP = best management practice; PAH = polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyls; DoD = US Department of Defense.
Supplemental Data
The Supplemental Data are available on the Wiley Online Library at https://doi.org/10.1002/etc.4894.
Supporting information
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (leeson.andrea@gmail.com).
REFERENCES
- Anderson RH, Thompson T, Stroo HF, Leeson A. 2020. US Department of Defense‐funded FAE and transport research on per‐ and polyfluoroalkyl substances at aqueous film‐forming foam‐impacted sites. Environ Toxicol Chem 40:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen‐Hanson K, Roberts S, Choyke S, Oetjen K, McAlees A, Riddell N, Higgins C, Field J. 2017. Discovery of 40 classes of per‐ and polyfluoroalkyl substances in historical aqueous film‐forming foams (AFFFs) and AFFF‐impacted groundwater. Environ Sci Technol 51:2047–2057. [DOI] [PubMed] [Google Scholar]
- Blaine A, Rich C, Hundal L, Lau C, Mills M, Harris K, Higgins C. 2013. Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: Field and greenhouse studies. Environ Sci Technol 47:14062–14069. [DOI] [PubMed] [Google Scholar]
- Blaine A, Rich C, Sedlacko E, Hundal L, Kumar K, Lau C, Mills M, Harris K, Higgins C. 2014. Perfluoroalkyl acid distribution in various plant compartments of edible crops grown in biosolids‐amended soils. Environ Sci Technol 48:7858–7865. [DOI] [PubMed] [Google Scholar]
- Buck R, Franklin J, Berger U, Conder J, Cousins I, Voogt Pd, Jensen A, Kannan K, Mabury S, van Leeuwen S. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classifications, and origins. Integr Environ Assess Manag 7:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C, Ghosh R, Leeson A, Thompson T. 2020. US Department of Defense‐funded research on treatment of per‐ and polyfluoroalkyl substance‐laden materials. Environ Toxicol Chem 40:44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone B, Speth T, Wahman D, Smith S, Abulikemu G, Kleiner E, Pressman J. 2019. Occurrence of per‐ and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Environ Sci Technol 49:2359–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino LA, Mabury SA. 2013. Identification of novel fluorinated surfactants in 32 aqueous film forming foams and commercial surfactant concentrates. Environ Sci Technol 48:121–129. [DOI] [PubMed] [Google Scholar]
- Department of Defense . 2019. Quality Services Manual 5.3. Consolidated Quality Systems Manual (QSM) for Environmental Laboratories . US Department of Defense and US Department of Energy, Washington, DC. [cited 2020 November 10]. Available from: https://denix.osd.mil/edqw/documents/manuals/qsm-version-5-3-final/
- Field J, Higgins C, Deeb R, Conder J. 2017. FAQs regarding PFASs associated with 15 AFFF use at U.S. military sites. Catalyzing rapid information transfer among key 16 stakeholders on per‐ and polyfluoroalkyl substances (PFASs) at contaminated military 17 sites. ESTCP project ER‐201574‐T2 Final Report. [cited 2020 November 10]. Available from: https://apps.dtic.mil/dtic/tr/fulltext/u2/1044126.pdf
- Guelfo J. 2020. Development and validation of novel techniques to assess leaching and mobility of per and polyfluoroalkyl substances (PFAS) in impacted media. SERDP ER20‐1126. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER20-1126/ER20-1126/
- Hannigan D. 2019. Rapid site profiling of organofluorine: Quantification of PFASs by combustion gas analysis. SERDP ER19‐1214. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Monitoring/ER19-1214/ER19-1214
- Hoverman J. 2020. The relative toxicities of current use aqueous film forming foams and next generation alternatives to aquatic species for informing risk assessment. ER20‐1537. Purdue University, Purdue University, Hammond, Indiana, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER20-1537
- Interstate Technology and Regulatory Council . 2020. Per‐ and polyfluoroalkyl substances (PFAS). The Interstate Technology and Regulatory Council (ITRC) Per‐ and Polyfluoroalkyl Substances (PFAS) Team. Washington, DC, USA. [cited 2020 April]. Available from: https://pfas-1.itrcweb.org
- Kuperman R. 2020. Soil ecotoxicity of PFAS‐free surfactant formulations: Soil invertebrates and terrestrial plants. ER20‐1506. US Army Chemical Biological Center, Aberdeen Proving Ground, Maryland. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER20-1506
- Liu J. 2019. Development and validation of analytical methods for comprehensive profiling of perfluoroalkyl and polyfluoroalkyl substances in firefighting foam impacted environmental matrices. SERDP Project ER19‐1157. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Sediments/Characterization/ER19-1157
- Peaslee G. 2019. Developing PIGE into a rapid field‐screening test for PFAS. SERDP ER19‐1142. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER19-1142/ER19-1142
- Quinn M. 2016. Development of toxicity data to support toxicity reference values for perfluorinated compounds. US Army Public Health Command ER‐2625. Aberdeen Proving Ground, Maryland. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-2625/(language)/eng-US
- Quinn M. 2020. Assessing the ecotoxicity of fluorine-free surfactant formulations in wild mice and japanese quail ER20‐1508. US Army Public Health Command, Aberdeen Proving Ground, Maryland. [cited 2020 November 10]. Available from: https://serdp-estcp.org/News-and-Events/News-Announcements/Program-News/SERDP-announces-FY-2020-new-start-project-selections/(language)
- Salice C. 2016. Advancing the understanding of ecological risk of per‐ and polyfluoroalkyl substances. ER‐2627. Towson University, Towson, Maryland, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER-2627/(language)/eng-US
- Sepulveda M. 2016. Development of amphibian per‐ and polyfluoroalkyl substances toxicity reference values for use in ecological risk assessment at aqueous film‐forming foam sites. ER‐2626. Purdue University, Hammond, Indiana, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-2626/(language)/eng-US
- Shoemaker J, Tettenhorst D. 2018. Method 537.1: Determination of per‐ and polyfluoalkyl alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). National Center for Environmental Assessment, Office of Research and Development, US Environmental Protection Agency, Washington, DC. [cited 2020 November 10]. Available from: https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=537290
- Simcik M. 2016. Development of toxicity reference values (TRVs) for birds exposed to PFOS, PFOA, and associated mixtures of fluorinated compounds. ER‐2624. University of Minnesota, Minneapolis St. Paul, Minnesota, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Emerging-Issues/ER-2624/(language)/eng-US
- Slater L. 2019. Bench‐scale assessment of nuclear magnetic resonance (NMR) and complex resistivity (CR) screening technologies for rapid assessment of PFASs in soils and sediments. SERDP ER19‐1128. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER19-1128/ER19-1128
- Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program . 2017. SERDP and ESTCP Workshop on Research and Demonstration Needs for Management of AFFF‐Impacted Sites, May 2–3, 2017, Washington, DC. [cited 2017 September]. Available from: https://www.serdp-estcp.org/content/download/45585/425201/file/PFAS%20Workshop%20Report%20Final%20September%202017.pdf
- Strategic Environmental Research and Development Program and the Environmental Security Technology Certification Program . 2020. Past statements of need for fiscal years 2012–2019. [cited 2020 September 25]. Available from: https://www.serdp-estcp.org/Funding-Opportunities/SERDP-Solicitations/Past-SONs#FY16
- Suski J. 2020. Multi‐taxa ecotoxicity of novel PFAS‐free foam versus new generation short‐chain‐PFAS AFFF products: Aquatic and terrestrial species. ER20‐1531. EA Engineering, Science, and Technology, Hunt Valley, Maryland, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER20-1531
- US Environmental Protection Agency . 2009. Provisional health advisories for drinking water for perfluorooctanoic acid (PFOA) and perfluorooctanoic sulfonate (PFOS). Washington, DC. [cited 2020 November 20]. Available from: https://www.epa.gov/sites/production/files/2015-09/documents/pfoa-pfos-provisional.pdf
- US Environmental Protection Agency . 2016a. Drinking water health advisory for perfluorooctanoic acid (PFOA). EPA 822‐R‐16‐005. United States Environmental Protection USEPA, Office of Water, Washington, DC.
- US Environmental Protection Agency . 2016b. Drinking water health advisory for perfluorooctane sulfonate (PFOS). EPA 822‐16‐004. United States Environmental Protection USEPA, Office of Water, Washington, DC.
- US Environmental Protection Agency . 2017. Per‐ and polyfluoroalkl substances (PFAS). EPA/600/F‐17/022. [cited 2017 March]. Available from: https://www.epa.gov/sites/production/files/2017-04/documents/pfas_methods_tech_brief_09mar17_final_508compliant.pdf
- US Environmental Protection Agency . 2019a. Method 533: Determination of per‐ and polyfluorinated alkyl substances in drinking water by isotope dilution anion exchange solid phase extraction and liquid chromatography/tandem mass spectrometry. US Environmental Protection Agency, Washington, DC.
- US Environmental Protection Agency . 2019b. Method 8327: Per‐ and polyfluoroalkyl substances (PFAS) using external standard calibration and multiple reaction monitoring (MRM) liquid chromatography/tandem mass spectrometry (LC/MS/MS). US Environmental Protection Agency, Washington, DC.
- US Environmental Protection Agency . 2019c. Research on per‐ and polyfluoroalkyl substances (PFAS). [cited 2019 December 16]. Available from: https://www.epa.gov/chemical-research/research-and-polyfluoroalkyl-substances-pfas
- US Government Accountability Office . 2018. Status of DOD efforts to address drinking water contaminants used in firefighting foam. [cited 2018 September 26]. Available from: https://www.gao.gov/assets/700/694759.pdf
- Wirth E. 2020. Ecotoxicity of PFAS‐free fire fighting foams: Fish and aquatic invertebrate species. ER20‐1518. National Oceanic and Atmospheric Administration, Washington, DC, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER20-1518
- Wu X. 2020. Comparative assessment of toxicity and bioaccumulation of PFAS‐free formulations in terrestrial plants and model soil invertebrates. ER20‐1511. Lawrence Berkeley National Laboratory, Berkeley, California, USA. [cited 2020 November 10]. Available from: https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Risk-Assessment/ER20-1511
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (leeson.andrea@gmail.com).


