Abstract
While biological invasions have the potential for large negative impacts on local communities and ecological interactions, increasing evidence suggests that species once considered major problems can decline over time. Declines often appear driven by natural enemies, diseases or evolutionary adaptations that selectively reduce populations of naturalised species and their impacts. Using permanent long‐term monitoring locations, we document declines of Alliaria petiolata (garlic mustard) in eastern North America with distinct local and regional dynamics as a function of patch residence time. Projected site‐specific population growth rates initially indicated expanding populations, but projected population growth rates significantly decreased over time and at the majority of sites fell below 1, indicating declining populations. Negative soil feedback provides a potential mechanism for the reported disappearance of ecological dominance of A. petiolata in eastern North America.
Keywords: Alliaria petiolata, Brassicaceae, long‐term monitoring, negative soil feedback, plant invasions, population growth rate, residence time
Using permanent long‐term monitoring locations, we document declines of Alliaria petiolata (garlic mustard) in eastern North America as a function of patch residence time. Projected site‐specific population growth rates initially indicated expanding populations, but projected population growth rates decreased below 1, indicating declining populations.
INTRODUCTION
Biological invasions can reshape local economies and ecological interactions, mutualistic relationships and ecosystem processes such as decomposition, carbon sequestration and energy flow (Keller et al., 2007; Bradley et al., 2010; Clavel et al., 2011; Hof et al., 2011; Powell et al., 2011; Wardle et al., 2011; Hooper et al., 2012; Boyd et al., 2013; Simberloff et al., 2013; Caplan et al., 2015). However, the vast majority of naturalised species are benign with little recognisable impacts. Predicting and distinguishing potential invasive threats for species that have not yet arrived, or just have become established, has occupied scientists and policy makers for decades (Williamson and Fitter, 1996; Lonsdale, 1999; Smith et al., 1999; Sakai et al., 2001; Keller et al., 2007; Hayes and Barry, 2008; Elliott‐Graves, 2016; Hulme and Bernard‐Verdier, 2018; Divíšek et al., 2019; Fourniera et al., 2019; Muthukrishnan et al., 2019). Understanding each species’ demography is at the core of separating major invasive plants that show rapid local population increase, establishment of local dominance or range expansions (Gurevitch et al., 2011) from those that become established but remain minor members of local plant communities.
A phenomenon that has received increased attention is whether introduced species go through boom and bust cycles, ultimately becoming non‐threatening members of local communities. Specifically, whether species that pass from naturalisation, to establishment, to ecological dominance with major impacts, can subsequently decline in dominance. Simberloff and Gibbons (2004) mentioned 17 examples of species exhibiting such patterns, most without documented mechanisms for declines, but cautioned that future potential population declines of invaders should not be a reason to postpone control and management since irreversible impacts may occur. Since this publication, declines have been reported for introduced marine (Benkwitt et al., 2017) and freshwater fishes (Gibson‐Reinemer et al., 2017), a marine crab (Forsstrom et al., 2018), noctuid moths (Elkinton et al., 2006), yellow crazy ants in Australia (Cooling and Hoffmann, 2015), Argentine ants in New Zealand (Cooling et al., 2012), other ant species (Lester and Gruber, 2016; Tartally et al., 2016), the invasive grasses Microstegium vimineum in North America (Flory et al., 2011; Stricker et al., 2016), and Spartina alterniflora in China (Tang et al., 2012), grey squirrels in Ireland (Sheehy and Lawton, 2014), a land snail (Orstan and Cameron, 2015) and birds (Aagaard and Lockwood, 2016; Aagaard et al., 2016). A review of plant invasions in North America (Warren et al., 2019) and New Zealand (Diez et al., 2010) suggests that most species follow a pattern of establishment, increase and spread followed by declines after 100‐300 years of local residence. Mechanisms driving these declines are often unclear, but most appear associated with accumulation of natural enemies (herbivores, parasites, diseases) as a function of residence time and this is well documented for introduced plants (Andow and Imura, 1994; Mitchell and Powers, 2003; Carpenter and Cappuccino, 2005; Diez et al., 2010).
Here we report on work considering local and regional population dynamics of Alliaria petiolata (garlic mustard), a European biennial herb in temperate North America. Range expansion and local abundance increases of A. petiolata in the 20th century co‐occurred with native forest understory plant declines. In response, land managers deployed a range of control treatments, but only achieved temporary suppression, prompting research into biological control (Blossey et al., 2001; Davis et al., 2006; Gerber et al., 2007). We established a regional long‐term monitoring program at 16 sites to establish baseline population dynamics (measuring recruitment as number of rosettes in the first year, survival of rosette cohort to adult plants and fecundity as number of siliques/stem) to enable future assessments in response to a possible introductions of specialised herbivores (Blossey et al., 2001). Particularly rosette survival and seed output are crucial parameters in the demography of A. petiolata (Davis et al., 2006), thus our data cover the most important factors in the life cycle of this biennial plant. Based on experience conveyed to us by land managers we predicted sustained local annual A. petiolata populations and vigour (number of stems, height, cover and reproduction) with some annual fluctuations in recruitment driven by local differences in climate but no sustained declines. Several years into this monitoring program, we observed local A. petiolata declines that lacked an obvious cause (such as presence of above ground natural enemies). We used an exploratory assessment of potential underlying mechanisms at a location where the chronosequence of A. petiolata arrival, spread and decline was well established to test for potential negative soil feedback (van der Putten et al., 2013). We show that (1) widespread A. petiolata declines are a function of local residence time; (2) regional differences in patterns of decline may reflect invasion histories and (3) projected population growth rates decline to < 1 (indicating shrinking populations) after 10–20 years. Our exploratory assessment leads to a new hypothesis that changes in population growth rates are a response to negative soil feedback, but this hypothesis needs confirmation at additional sites and across the range of A. petiolata in North America.
METHODS
Experimental organism
Alliaria petiolata (Brassicaceae) is an obligate biennial first recorded in North America in 1868 on Long Island, New York (Nuzzo, 1993). Seeds germinate in spring, and rosettes form in early summer. Rosettes overwinter, grow rapidly the following spring and bolt. Seeds ripen and drop in early summer forming a seedbank that remains viable > 10 years (Blossey et al., 2017). Phenology of A. petiolata varies with local climates, and after 150 years of residence time in North America, rapid evolutionary changes and phenotypic plasticity resulted in locally adapted populations with striking differences in germination requirements and secondary chemistry (Lankau et al., 2009; Lankau, 2012b; Evans et al., 2016; Blossey et al., 2017). The current A. petiolata distribution ranges from southern Canada to Georgia, and from New York and Quebec west to Oregon, British Columbia and Alaska (USDA NRCS, 2017).
Long‐term population trends
We established a long‐term monitoring program to document local baseline population dynamics that would allow us to separate annual population fluctuations from potential future impacts of specialised biocontrol herbivores (Blossey et al., 2001). Our criteria for selecting study sites included forest size > 2 ha, presence of A. petiolata > 3 years and protection from management (fire, herbicide, weeding or cutting) for at least 10 years. We selected 16 forested sites across northeastern North America (Fig. 1, Table 1) ranging in latitude from 39.4 to 42.7 degrees and in longitude from −74.1 to −89.2 degrees. These locations represent a transect through A. petiolata invasion history in North America (Nuzzo, 1993; Lankau et al., 2009). Depending on location, we established sites between 2000 and 2006, and monitored them regularly for 5 to 15 years following a standardised monitoring protocol (Blossey and Nuzzo, 2003).
Figure 1.

Location of permanent A. petiolata monitoring sites across eastern North America. Numbers refer to locations referenced in Table 1.
Table 1.
Alliaria petiolata population growth rate per site over the study period (λyear.site = nth root of cumulative growth rate over the study period, estimated from multi‐year matrices B from each site, n = number of years monitored per site
| Site | State | No. years monitored | Population growth rate over the study period | 95% CI |
|---|---|---|---|---|
| West Point | NY | 12 | 1.125 | 0.973–1.365 |
| Thompson | NJ | 9 | 0.977 | 0.967–0.986 |
| Jones | NJ | 12 | 0.977 | 0.966–0.985 |
| Point | NJ | 12 | 1.133 | 0.969–1.402 |
| Union | NJ | 12 | 0.977 | 0.967–0.986 |
| Waterman | NY | 11 | 0.977 | 0.967–0.986 |
| Leopold | NY | 15 | 0.977 | 0.967–0.986 |
| Ives Rd. | MI | 6 | 0.977 | 0.967–0.986 |
| Box Woodlot | MI | 5 | 0.977 | 0.967–0.986 |
| Pokagon | IN | 12 | 0.978 | 0.968–1.019 |
| Merry Lea | IN | 12 | 0.977 | 0.966–0.986 |
| Fernwood | MI | 5 | 1.893 | 1.478–2.298 |
| Nippersink | IL | 8 | 1.262 | 0.979–1.524 |
| Fermilab 1 | IL | 10 | ||
| Marengo Ridge | IL | 7 | 1.511 | 1.188–1.784 |
| Hall Woods 1 | IL | 10 |
Data are means and 95% CI based on 1000 bootstrap samples. Sites are ordered East to West. Population growth rates are estimated only for time span for which demographic data are complete (missing years of data at some sites truncate the data available to estimate the growth rate) and do not necessarily reflect population size at the end of the study. Values above and below one indicate the population is projected to grow or decline respectively. Sites are ordered East to West. State two letter designations are: NY = New York; NJ = New Jersey; MI = Michigan; IN = Indiana; and IL = Illinois.
Fermilab and Hall Woods were not included in the matrix population analysis.
At each site we established 20–26 0.5 m2 (1 m × 0.5 m) permanent quadrats (marking corners with PVC stakes) along two to four randomly established transects. We placed the first quadrat at random in an existing A. petiolata patch and spaced additional quadrats at 10 m intervals. We shifted quadrat locations as necessary to ensure that A. petiolata rosettes or adults were present in every quadrat, except for two sites in Michigan where some quadrats did not contain A. petiolata at the start of the monitoring period.
At each site, we recorded data twice/year. In June or July, we recorded stem densities, stem height (cm), number of fully formed siliques on each stem, estimated percent cover of all A. petiolata, and estimated cover of adults and rosettes (independently, within cover classes). In September or October, we estimated rosette cover (%) and counted or estimated rosette density.
Data analyses
We standardised the time axis as date when monitoring was initiated at each site. Initial analyses indicated a nonlinear relationship between A. petiolata performance measures and time. Therefore, we employed generalised additive mixed models (GAMMs) to evaluate change in A. petiolata performance over time. Generalised additive models are a nonparametric approach that can account for nonlinear relationships through splines (Hastie and Tibshirani, 1990).
We evaluated long‐term changes in adult density, June cover of adults and seedlings, mean adult stem height per quadrat, and mean number of siliques/stem/quadrat (hereafter referred to as silique number) via separate models (with Gaussian errors) applied to log‐transformed (adult density, cover and silique number) or raw (stem height) data. Models included random intercept effects for calendar year and nested effects for site and quadrat within site, as well as a random slope for monitoring year. We ran a separate GAMM model with binomial errors to evaluate changes in proportion of quadrats with A. petiolata presence in June.
In all models, we evaluated a smoothed function of time, fixed effects of latitude and longitude and included initial A. petiolata adult density as covariate. We did not test for interactions between explanatory variables. For height and silique number we also evaluated possible density dependent effects by including adult density per quadrat. For silique number we included stem height as a covariate. We estimated smoothers with cubic regression splines (Wood, 2006). We fitted two sets of models for each performance variable. In the first set, models had a separate time smoother for each geographical region. In the second set, models had a single smoother across all sites. Residual inspection did not indicate violation of normal distribution, constant variance assumptions or auto‐correlation in the data. We selected the best model based on Akaike Information Criterion (AICc) (Burnham and Anderson, 2002). We selected models using the full set of candidate models and a reduced candidate set where we identified and removed uninformative parameters following (Burnham and Anderson, 2002; Arnold, 2010). We fitted all models in R (R Core Team, 2014) using gamm4 function (Wood and Scheipl, 2017).
Matrix population model construction
We developed a linear, deterministic stage‐structured matrix population model: n t +1 = An t, where n t is a vector that quantifies number of individuals in each stage class at time t, and A is a transition matrix (Caswell, 2001). We constructed annual transition matrices A based on three life stages (Fig S1: seed (s), rosette (r) and adults (p) (Davis et al., 2006; Kalisz et al., 2014). We estimated parameter values for matrix from permanent marked quadrats at each site. We estimated seedling survival (sg) and rosette survival (sr) by counting or estimating number of emergent seedlings per quadrat in June and number of surviving rosettes in September. We then counted number of surviving adults the following summer. We counted number of siliques produced per stem in each quadrat and estimated seed production based on average number of seeds per silique (12 seeds/silique, Nuzzo unpublished data). The monitoring protocol did not include measures of seed survival (ss) or seed germination (g1 and g2), therefore we obtained estimates from an experimental seed basket study (Kalisz et al., 2014) and applied those estimates to all sites and years. We averaged data across all quadrats per site to populate one matrix per year and site; therefore, sites were treated as replicates. We eliminated two sites (Fermi and Hall Woods) because of incomplete time series.
We calculated the dominant eigenvalue of each matrix to determine projected growth rates (λ). At λ > 1 populations are expected to grow; at λ < 1 declines are projected; and at λ = 1 populations are expected to remain stable (Caswell, 2000). We evaluated change in λ over the monitoring period with a Linear Mixed Model (LMM) with site as a random factor and a first order autocorrelation structure. Models evaluated second order polynomial effects of year, initial A. petiolata density, continental region (Northeast or Midwest) and their second order interactions. We constructed simplified models by removing one term at a time in a hierarchical fashion and retained lower‐level terms if an interaction or polynomial term was included in the model. We evaluated competing models with AICc (Burnham and Anderson, 2002) and followed the same procedure as described above to remove models that appeared legitimate but were not because they contained uninformative parameters (Arnold, 2010).
We constructed multiyear projection matrices, B, for each study site to capture cumulative demographic trends over the study period. The matrix was estimated as the product of annual transition matrices, such that B site = A site.n A site.n‐1… A site.n=1; where n = years monitored (Caswell, 2001; Kalisz et al., 2014). We estimated the cumulative growth rate over the whole period (λc) as the dominant eigenvalue of matrix B and time‐averaged growth rates per site (λyear.site) over the study period as nth root of λc. We constructed 95% CI based on 1000 bootstrap samples to evaluate whether λyear.site was significantly different from one.
RESULTS
When we established sites, A. petiolata was present in all quadrats (except in three quadrats at Box Woodlot and two at Ives Rd.). By the end of the study, A. petiolata frequency (proportion of quadrats containing A. petiolata) in June declined at 11 of 16 sites by 9% to 53%. Declines were greater at eastern sites (Fig. 2) and longitude had a significant explanatory effect (estimate ± 1SE: −0.93 ± 0.43, P = 0.03). Although two of the top three models indicated regional differences between the Northeast or Midwest, the best model (adj. R 2 = 0.07, AICc weight = 0.28) included a single smoother for both regions and longitude (Table S2). Models that incorporated latitude (models 4, 5, 7 and 8) had AICc values greater than the corresponding models (models 1, 2, 3 and 6) that lacked a latitude effect (Table S2). Removing this uninformative parameter rendered an AICc weight = 0.40 for the best model.
Figure 2.
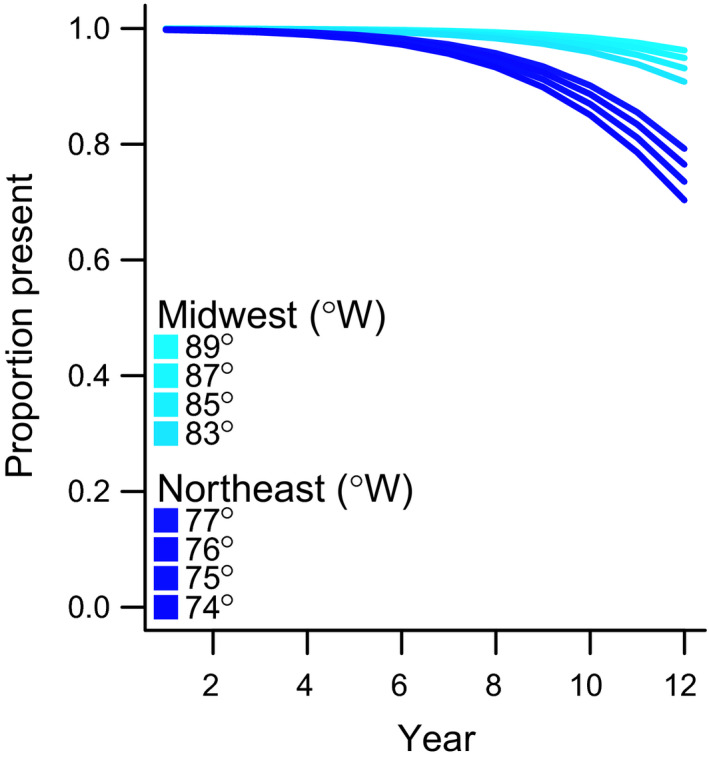
Proportion of quadrats with A. petiolata presence in June. Lines show predictions from a Generalised Additive Mixed Model with binomial errors for 0th, 25th, 50th, 75th and 100th quantiles of longitude. Model included random intercept effects for calendar year and nested effects for site and quadrat within site, as well as a random slope for monitoring year. Declines in the Northeast are more substantial than in areas in the Midwest.
Across all sites, adult density showed large annual fluctuations but declined 48–100% from an initial mean of 13.7 ± 1.9 stems/0.5m2 (mean ± 1SE; N = 16 sites; range 3.62–31.05 per site) to 1.33 ± 0.4 stems/0.5m2 at the end of the study, and this pattern was most obvious for sites with the longest observation periods (Fig. S2). The best performing models included initial A. petiolata adult density as a covariate and indicated a significant decline in adult density over time (Fig. S3) with important differences between the Northeast and Midwest (Fig. 3a and b). Sites in the Northeast showed two phases of decline, whereas at Midwest sites adult density oscillated downwards (Fig. 3a and b). Sites in the Midwest were typically monitored fewer years than sites in the Northeast, increasing model uncertainty in the last years of the study. The best model of adult density (adj. R 2 = 0.16; AICc weight = 0.46) included initial density as a covariate (Estimate ± 1SE: 0.17 ± 0.02, P < 0.001), separate smoothers per region (P < 0.001), but no effect of longitude or latitude (Table S3). Models including latitude and longitude (models 2, 3, 4, 6, 7, 8) had larger AICc values than the corresponding models without these parameters (models 5, 9 and 10; Table S3). Removal of these parameters resulted in a AICc weight of 1 for the best model (Table S3).
Figure 3.
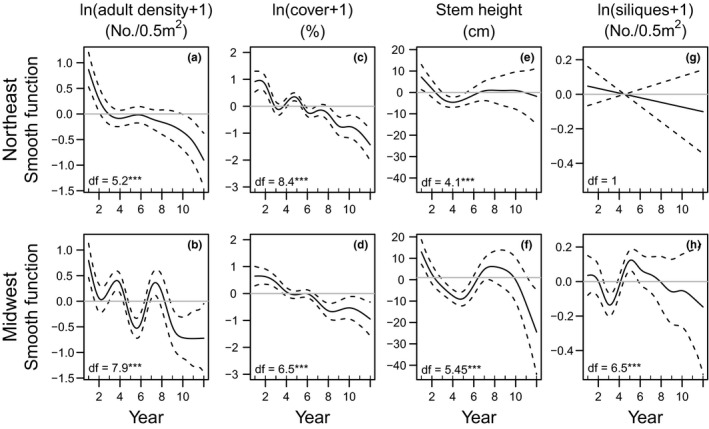
Generalised Additive Mixed Model smooth curves (solid lines) with 95% credible intervals (dashed lines) for A. petiolata adult density (number/0.5 m2, log transformed, a and b), seedling and adult cover in June (%, log transformed, c and d), stem height (cm, e and f) and mean number of siliques per stem per quadrat (number/0.5 m2, log transformed, g and h) in Northeast regions (top panels, N = 7 sites) and Midwest regions (bottom panels N = 9 sites; see Table S1 for site designation). Locations with zero stem density were excluded from analyses of stem height and number of siliques. Models included random intercept effects for calendar year and nested effects for site and quadrat within site, as well as a random slope for monitoring year. Note different y‐axis scales for different performance measures. df = degrees of freedom; ***P < 0.001.
Over time, A. petiolata cover (%; adults and seedlings combined) in June declined 17–98% from 29.5% ± 3.64 (range 7–63% per site) at the beginning of the study to 0.5–41.3% (mean ± 1SE) (Fig. S4). At one site (Nippersink) cover remained stable through the study period. The best model (adj. R 2 = 0.20; AICc weight = 0.40) included initial A. petiolata density (0.21 ± 0.03, P < 0.001; Fig. S5), separate smoothers for Northeast and Midwest regions (P < 0.001) and no latitude or longitude effects (Table S4,). In the Northeast, cover declined with some oscillations, but in the Midwest this pattern was less pronounced (Fig. 3c and d). Models including latitude and/or longitude had larger AICc values than corresponding models without these parameters. Removing these uninformative parameters rendered a weight of 1 for the best model (Table S4).
Average stem height per quadrat at the beginning of the study ranged from 39 to 92 cm and declined at most sites (Fig. S6). The two top models included longitude effects and indicated a decline in height over time (Table S5). Although the rate of decline differed by region in the best model (R 2 = 0.1; AICc weight = 0.24), it was similar for both regions in the second top model (R 2 = 0.08; AICc weight = 0.19) (Table S5). The best performing model indicated a decline in stem height in the Midwest, whereas in the Northeast stem height decreased in the first years of the study and then remained stable (Fig. 3e and f). Stem height significantly decreased with longitude (−6.57 ± 2.96, P = 0.03; with taller stems at western sites, Fig. S7) but was not significantly correlated with initial A. petiolata adult density or latitude (Table S5). Removing models with latitude and/or initial density (uninformative parameters) from the set resulted in AICc weight = 0.46 and 0.35 for the two top models respectively.
Silique production/stem remained relatively stable at most sites throughout the study period (5.7–22.8 siliques/stem at study initiation) (Fig. 3, Fig. S8). All best models included a smoother per region, an interaction between stem height and adult density and longitude and latitude effects. The best model (adj. R 2 = 0.8, AICc weight = 0.53; Table S6), indicated mean silique number per stem per quadrat decreased in the Midwest but did not change in the Northeast. Silique production was positively correlated with stem height (estimate ± 1SE: 0.03 ± 0.0007, P < 0.001) and adult density (0.06 ± 0.02, P = 0.002), with taller stems in quadrats with low stem densities producing more siliques than stems of the same height in quadrats with high stem densities (Fig. 4). Silique production was negatively correlated with latitude (−0.17 ± 0.04, P < 0.001) and longitude (−0.13 ± 0.5, P = 0.007), with stems at northern and eastern sites producing more siliques than stems at southern and western sites (Fig. 3, Fig. S9). Initial A. petiolata density was not included as covariate in the best model and its addition resulted in lower AICc values than the corresponding model without initial density (Table S6). Removing this model from the candidate set resulted in an AICc weight of 0.80 for the best model.
Figure 4.
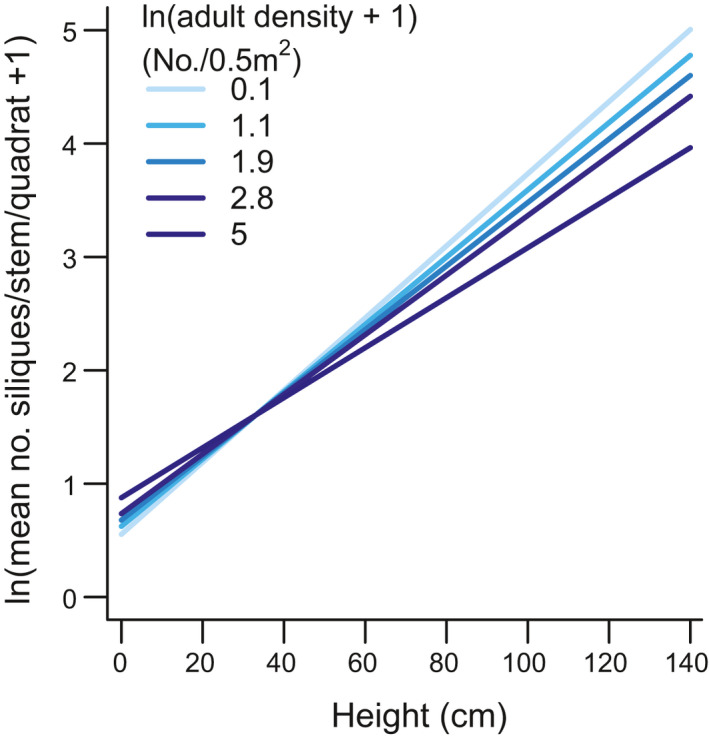
Number of A. petiolata siliques per stem as a function of stem height (cm) and adult density (number/0.5 m2, log transformed). Lines depict model predictions averaged across all study sites and years for mean latitude (41.57) and longitude (−81.58) and for 0th, 25th, 50th, 75th and 100th quantiles of log‐transformed adult density (number/0.5 m2). Model included random intercept effects for calendar year and nested effects for site and quadrat within site, as well as a random slope for monitoring year. Darker colours indicate higher adult density (log‐transformed).
Demographic analysis
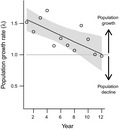
At the beginning of our study, projected site‐specific population growth rates (1.33 ‐ 1.85; mean ± 1SE: 1.56 ± 0.04) indicated expanding populations. However, population growth rates significantly decreased over time (Table S7; Fig 5) at all sites (Fig. S10). Analysed over the whole study period, the time‐averaged growth rate per site (λyear.site) was significantly less than one at eight sites, did not differ from one at four sites and was significantly higher than one at two sites (Table 1). Estimates assume that seed survival and germination are similar across sites and did not vary over time.
Figure 5.
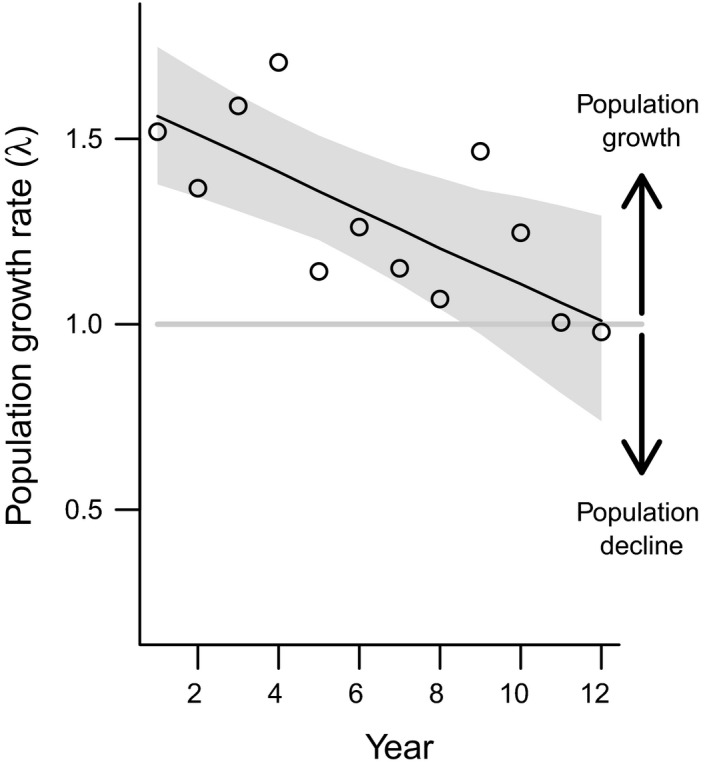
Population growth rate of A. petiolata over time. Points represent mean growth rate across sites, lines and shaded areas represent marginal means and 95% CI based on linear mixed model predictions (N = 1000 bootstrap samples). Population growth rates are estimated only for time spans for which demographic data are complete (missing years of data at some sites truncate the data available to estimate growth rates) and do not necessarily reflect population size at the end of the study. Values above and below one indicate the population is projected to grow or decline respectively. Model included random intercept effects for site.
DISCUSSION
The results of our regional investigation require us to reject our first assumption—A. petiolata is unable to maintain stable or increasing populations over time. While our monitoring protocol was not intended to capture very early stages of arrival at a site, our data suggest an initial local abundance increase, followed by sustained vigour declines. Earlier demographic models developed to aid in selection of potential biocontrol agents for A. petiolata identified transitions from rosettes to flowering plants as most important in A. petiolata demography (Davis et al., 2006; Evans et al., 2012). This is exactly what we document and, despite abundant germination and a fairly long‐lived (10–13 years) seedbank (Blossey et al., 2017), over time projected population growth rates decline to <1. This phenomenon is not restricted to our long‐term monitoring sites and has been noted observationally or through demography investigations by others (Kalisz et al., 2014; Dávalos et al., 2015b; Faison et al., 2019), but we are the first to document and quantify these declines through a chronosequence at many different locations.
We find support for our second prediction that local declines are a function of regional residence time. The species first established in the East and then spread into the Midwest (Nuzzo, 1993) and our data indicate that A. petiolata declines occurred faster and were more pronounced at eastern study locations (Fig. 1). Although detailed mechanisms responsible for these findings remain elusive at present, unique regional climates, soil and vegetation characteristics may be important in determining local site trajectories. However, longer overall residence time in the East may also facilitate and enhance local build‐up of biotic resistance, or other eco‐evolutionary feedbacks. While we did encounter above ground natural enemies (herbivores or diseases), they never appeared abundant enough to strongly affect A. petiolata populations. Instead, the patterns we describe are consistent with expectations of plant‐soil feedback (PSF), where plant species‐specific rapid soil conditioning leads to performance reductions (negative soil feedback) (Klironomos, 2002; Bever et al., 2010; van der Putten et al., 2013). The role of PSF in structuring plant communities (Bennett et al., 2017) and the importance in range expansion or biological invasions is now well established (Koorem et al., 2020; Wolfe and Klironomos, 2005; Reinhart and Callaway, 2006) but how these impacts change over time is far less clear.
Ecological dominance waning over 100–300 years associated with negative soil feedback are described for introduced plants in New Zealand (Diez et al., 2010) and they may play a role in the reduction in abundance of invasive plant species described for eastern North America (Warren et al., 2019). We have some evidence suggesting the importance of negative soil feedback in affecting survival and vigour of A. petiolata from a site in central New York. At this location, which is not part of our long‐term monitoring, we were able to observe a detailed chronosequence of A. petiolata arrival, spread and decline over a 10‐year period (for details see Plant‐Soil Feedback section in Supplementary Material). Our preliminary experiments from this single location leads to a new hypothesis: the decline of A. petiolata as a function of local residence time, is caused by negative soil feedback. PSF may also help explain the regional differences between eastern and western sites. For example, legacy effects of differences in precipitation (Crawford & Hawkes, 2020) or previous land use histories that create persistent different microbial communities can affect plant demography and persist from decades to centuries (Wubs et al., 2019).
We need additional mechanistic work to assess whether similar processes occur in other regions throughout the range of A. petiolata in North America. We were unable to link a chronosequence to population trends at our long‐term sites because we established quadrats after the initial colonisation phase had occurred. In addition, the small‐scale patch dynamics described as advance and retreat (Nuzzo, 1999) and the potential for long‐lasting legacy effects (Bezemer et al., 2019) may lead to erroneous conclusions. But because abundance and vigour declines are a function of local residence times and small‐scale patch dynamics (at the order of a few m), a perplexing local and regional invasion dynamic emerges. At invaded sites A. petiolata abundance typically initially increases with some annual fluctuations and patchy spread through a site. The areas first occupied then go through a prolonged phase of decline until λ is reduced to <1, whereas at the same time A. petiolata populations at the invasion fronts are rapidly expanding. The continued availability of previously unoccupied areas allows A. petiolata to spread regionally and across the continent. This is likely to continue, although only areas already invaded by introduced earthworms appear to allow A. petiolata invasions, (Nuzzo et al., 2009), thus limiting available habitat the species may be able to colonise in North America. The potentially available habitat for expansion of A. petiolata continually shrinks as once occupied patches become inhospitable, at least for establishment and maintenance of large populations. Although we also do not know how long this biotic resistance may last (but see Bezemer et al., 2019). Furthermore, rapid evolutionary changes in the invader and the invaded lead to a reduction in A. petiolata impact as a function of residence time (Lankau et al., 2009; Lankau, 2012a; Lankau et al., 2014).
Our data were collected in areas with the oldest invasion history of A. petiolata in North America (Nuzzo, 1993; Lankau et al., 2009) with unique regional climate, soil and vegetation characteristics. The species has since spread more widely triggering continued widespread manual and chemical removal experiments. We have no information on the long‐term population dynamics in areas outside of our investigation, but we only detected long‐term declines because we required cessation of A. petiolata management for the duration of our study. Removal of A. petiolata may in fact set back negative soil feedback or other processes that collectively lead to population declines. It appears important that local management efforts are cognizant of the possibility that their activities may be detrimental to A. petiolata declines and local plant community recovery. We recommend for local managers to set aside certain locations for observational investigations to assess whether the phenomena we describe occur outside of our region.
Arrival and spread of A. petiolata into local ecosystems that continue to lose their diversity worries land managers, as they assume a cause‐and‐effect relationship. The species certainly has a potent arsenal of secondary chemistry (Callaway et al., 2008; Lankau, 2011a) that may affect many biota; however, effects reported by some (Roberts and Anderson, 2001; Stinson et al., 2006; Wolfe et al., 2008; Barto et al., 2011; Hale et al., 2011; Bialic‐Murphy et al. 2019) do not materialise in other studies (Dávalos and Blossey, 2004; Burke, 2008; Burke and Chan, 2010; Lankau, 2010; Wixted and McGraw, 2010; Ivanov and Keiper, 2011; Koch et al., 2011; Hahn and Dornbush, 2012; Lankau, 2012b). Decadal dynamics we describe further complicate A. petiolata impact assessments, which need to be separated from impacts of other stressors, such as white‐tailed deer (Odocoileus virginianus) and invasive earthworms (Didham et al., 2007; Dávalos et al., 2014, 2015a, b; Frelich et al., 2019). Few such studies exist (Waller and Maas, 2013; Dávalos et al., 2014, 2015a, b, c; Nuzzo et al., 2017; Dávalos et al. 2014, 2015a, b) but future study designs, as well as interpretation of exiting data need to be cognizant of presence and importance of other stressors and the possibility of A. petiolata invasion and decline that occur over a decadal timeframe. This limits the ability of short‐term experiments to capture long‐term impacts in the field and calls for more longitudinal research.
Our work contributes to the increasing body of evidence for declines in invasiveness and therefore potential ecological impacts of introduced species across many different taxa. Rapid evolutionary changes allowed A. petiolata to thrive in North America (Lankau, 2011b) but rapid eco‐evolutionary feedback in native species has reduced ecological and evolutionary novelty and initial negative impacts (Hierro and Callaway, 2003; Callaway et al., 2008; Mangla et al., 2008; Wolfe et al., 2008) and we show widespread declining A. petiolata population growth rates. These phenomena have important implications for how we approach research in and management of biological invasions. If impacts are caused by A. petiolata, how quickly do impacts materialise, do they persist, or do they fade (Lankau, 2012b) once the initial high abundances are reduced? Do affected species or communities recover over time, and how quickly? Does active management of garlic mustard extend local dominance potentially increasing impacts?
Build‐up of native natural enemies (including diseases and parasites) that affect species demography appear common in documented declines (Carlsson et al., 2009; Diez et al., 2010; Ross et al., 2010; Faillace et al., 2017; Gerard et al., 2018). This biotic resistance may be a widespread but unrecognised phenomenon for many species that naturalise but never become invasive, and therefore escape scientific or management focus. We believe it is critically important for scientists to embrace use of better and long‐term assessment protocols to ascertain demography of naturalising or introduced species. We recognise logistical and financial difficulties of such approaches, but also opportunities for advanced collaborative manager‐research scientist partnerships. Using a standardised monitoring approach offers one possible example but there are many others that need to be developed to capture the potential for waxing and waning in invasive species, similar to patterns we describe for A. petiolata.
Where large plant populations exist and eradication is no longer an option, we recommend establishing long‐term ‘research refuges’ to allow evaluation of eco‐evolutionary processes and potential buildup of local biotic resistance or biological control by native organisms to occur over time in absence of management. This switch in scientific focus from potential traits of invaders, to assessing biotic resistance traits of invaded communities may lead to improved understanding of invasion dynamics.
AUTHOR CONTRIBUTIONS
BB and VN conceived the idea and developed the long‐term monitoring protocol. All authors contributed to data collection. VN collated and proofed all data, AD analysed the data, BB led the writing and all authors contributed to the final version.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13649.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate field assistance over the years by many undergraduates and staff and landowners. Early funding (to BB) for this work was provided by the Strategic Environmental Research and Development Program US Department of Defense (CS 1146). DAL acknowledges support from the National Science Foundation Long‐term Ecological Research, Program (DEB 1637653) at the Kellogg Biological Station, and by Michigan State University AgBioResearch. JAE acknowledges support from the Michigan State University Plant Sciences Fellowship and an EPA Science to Achieve Results (STAR) Graduate Fellowship FP‐91650101.
DATA AVAILABILITY STATEMENT
The data are available in Dryad (https://doi.org/10.5061/dryad.mpg4f4qxh).
References
- Aagaard, K. & Lockwood, J.L. (2016). Severe and rapid population declines in exotic birds. Biol. Inv., 18, 1667–1678. [Google Scholar]
- Aagaard, K. , Lockwood, J.L. & Green, E.J. (2016). A Bayesian approach for characterizing uncertainty in declaring a population collapse. Ecol. Model., 328, 78–84. [Google Scholar]
- Andow, D.A. & Imura, O. (1994). Specialization of phytophagous arthropod communities on introduced plants. Ecology, 75, 296–300. [Google Scholar]
- Arnold, T.W. (2010). Uninformative parameters and model selection using Akaike's information criterion. J Wildlife Manage, 74, 1175–1178. [Google Scholar]
- Barto, E.K. , Antunes, P.M. , Stinson, K. , Koch, A.M. , Klironomos, J.N. & Cipollini, D. (2011). Differences in arbuscular mycorrhizal fungal communities associated with sugar maple seedlings in and outside of invaded garlic mustard forest patches. Biol. Inv., 13, 2755–2762. [Google Scholar]
- Benkwitt, C.E. , Albins, M.A. , Buch, K.L. , Ingeman, K.E. , Kindinger, T.L. , Pusack, T.J. et al (2017). Is the lionfish invasion waning? Evidence from The Bahamas. Coral Reefs, 36, 1255–1261. [Google Scholar]
- Bennett, J.A. , Maherali, H. , Reinhart, K.O. , Lekberg, Y. , Hart, M.M. & Klironomos, J. (2017). Plant‐soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science, 355, 181–184. [DOI] [PubMed] [Google Scholar]
- Bever, J.D. , Dickie, I.A. , Facelli, E. , Facelli, J.M. , Klironomos, J. , Moora, M. et al (2010). Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol., 25, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezemer, T.M. , Jin, J. , Bakx‐Schotman, J.M.T. & Bijleveld, E.‐J. (2019). Plant competition alters the temporal dynamics of plant‐soil feedbacks. J. Ecol., 106, 2287–2300. [Google Scholar]
- Bialic‐Murphy, L. , Brouwer, N.L. & Kalisz, S. (2019). Direct effects of a non‐native invader erode native plant fitness in the forest understory. J. Ecol., 108(1), 189–198. 10.1111/1365-2745.13233. [DOI] [Google Scholar]
- Blossey, B. , Nuzzo, V. & Dávalos, A. (2017). Climate and rapid local adaptation as drivers of seed bank dynamics of Alliaria petiolata (garlic mustard) in North America. J. Ecol., 105, 1485–1495. [Google Scholar]
- Blossey, B. , Nuzzo, V. , Hinz, H. & Gerber, E. (2001). Developing biological control of Alliaria petiolata (M. Bieb.) Cavara and Grande (garlic mustard). Nat. Areas J., 21, 357–367. [Google Scholar]
- Blossey, B. & Nuzzo, V.A. (2003). Garlic mustard monitoring protocol. Ecology and Management of Invasive Plant Species Program. Cornell University, www.invasiveplants.net.
- Boyd, I.L. , Freer‐Smith, P.H. , Gilligan, C.A. & Godfray, H.C.J. (2013). The consequence of tree pests and diseases for ecosystem services. Science, 342, 823‐+. [DOI] [PubMed] [Google Scholar]
- Bradley, B.A. , Blumenthal, D.M. , Wilcove, D.S. & Ziska, L.H. (2010). Predicting plant invasions in an era of global change. Trend Ecol. Evol., 25, 310–318. [DOI] [PubMed] [Google Scholar]
- Burke, D.J. (2008). Effects of Alliaria petiolata (garlic mustard; Brassicaceae) on mycorrhizal colonization and community structure in three herbaceous plants in a mixed deciduous forest. Am. J. Bot., 95, 1416–1425. [DOI] [PubMed] [Google Scholar]
- Burke, D.J. & Chan, C.R. (2010). Effects of the invasive plant garlic mustard (Alliaria petiolata) on bacterial communities in a northern hardwood forest soil. Can. J. Microbiol., 56, 81–86. [DOI] [PubMed] [Google Scholar]
- Burnham, K.P. & Anderson, D.R. (2002). Model Selection and Multimodel Inference: A Practical Information‐Theoretic Approach. Springer‐Verlag, New York, NY. [Google Scholar]
- Callaway, R.M. , Cipollini, D. , Barto, K. , Thelen, G.C. , Hallett, S.G. , Prati, D. et al (2008). Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology, 89, 1043–1055. [DOI] [PubMed] [Google Scholar]
- Caplan, J.S. , Hager, R.N. , Megonigal, J.P. & Mozdzer, T.J. (2015). Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environmental Research Letters, 10(11), 115006 [Google Scholar]
- Carlsson, N.O.L. , Sarnelle, O. & Strayer, D.L. (2009). Native predators and exotic prey ‐ an acquired taste? Front. Ecol. Environ., 7, 525–532. [Google Scholar]
- Carpenter, D. & Cappuccino, N. (2005). Herbivory, time since introduction and the invasiveness of exotic plants. J. Ecol., 93, 315–321. [Google Scholar]
- Caswell, H. (2000). Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology, 81, 619–627. [Google Scholar]
- Caswell, H. (2001). Matrix Population Models: Construction, Analysis, and Interpretation, 2nd edn. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Clavel, J. , Julliard, R. & Devictor, V. (2011). Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ., 9, 222–228. [Google Scholar]
- Cooling, M. , Hartley, S. , Sim, D.A. & Lester, P.J. (2012). The widespread collapse of an invasive species: Argentine ants (Linepithema humile) in New Zealand. Biol. Lett., 8, 430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooling, M. & Hoffmann, B.D. (2015). Here today, gone tomorrow: declines and local extinctions of invasive ant populations in the absence of intervention. Biol. Inv., 17, 3351–3357. [Google Scholar]
- Crawford, K.M. & Hawkes, C.V. (2020). Soil precipitation legacies influence intraspecific plant‐soil feedback. Ecology, 101, e03142. [DOI] [PubMed] [Google Scholar]
- Dávalos, A. & Blossey, B. (2004). Influence of the invasive herb garlic mustard (Alliaria petiolata) on ground beetle (Coleoptera : Carabidae) assemblages. Environ. Entomol., 33, 564–576. [Google Scholar]
- Dávalos, A. , Nuzzo, V. & Blossey, B. (2014). Demographic responses of rare forest plants to multiple stressors: the role of deer, invasive species and nutrients. J. Ecol., 102, 1222–1233. [Google Scholar]
- Dávalos, A. , Nuzzo, V. & Blossey, B. (2015a). Interactive effects of deer, earthworms and non‐native plants on rare forest plant recruitment. Biodivers. Conserv., 187, 173–181. [Google Scholar]
- Dávalos, A. , Nuzzo, V. & Blossey, B. (2015b). Single and interactive effects of deer and earthworms on non‐native plants. Forest Ecol. Manag., 351, 28–35. [Google Scholar]
- Dávalos, A. , Simpson, E. , Nuzzo, V. & Blossey, B. (2015c). Non‐consumptive effects of native deer on introduced earthworm abundance. Ecosystems, 18, 1029–1042. [Google Scholar]
- Davis, A.S. , Landis, D.A. , Nuzzo, V. , Blossey, B. , Gerber, E. & Hinz, H.L. (2006). Demographic models inform selection of biocontrol agents for garlic mustard (Alliaria petiolata). Ecol. Appl., 16, 2399–2410. [DOI] [PubMed] [Google Scholar]
- Didham, R.K. , Tylianakis, J.M. , Gemmell, N.J. , Rand, T.A. & Ewers, R.M. (2007). Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol., 22, 489–496. [DOI] [PubMed] [Google Scholar]
- Diez, J.M. , Dickie, I. , Edwards, G. , Hulme, P.E. , Sullivan, J.J. & Duncan, R.P. (2010). Negative soil feedbacks accumulate over time for non‐native plant species. Ecol. Let., 13, 803–809. [DOI] [PubMed] [Google Scholar]
- Divíšek, J. , Chytrý, M. , Beckage, B. , Gotelli, N.J. , Lososová, Z.K. , Pyšek, P. et al (2019). Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nature Comm., 9, 4631. 10.1038/s41467‐018‐06995‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkinton, J.S. , Parry, D. & Boettner, G.H. (2006). Implicating an introduced generalist parasitoid in the invasive browntail moth's enigmatic demise. Ecology, 87, 2664–2672. [DOI] [PubMed] [Google Scholar]
- Elliott‐Graves, A. (2016). The problem of prediction in invasion biology. Biol. Philos., 31, 373–393. [Google Scholar]
- Evans, J.A. , Davis, A.S. , Raghu, S. , Ragavendran, A. , Landis, D.A. & Schemske, D.W. (2012). The importance of space, time, and stochasticity to the demography and management of Alliaria petiolata . Ecol. Appl., 22, 1497–1511. [DOI] [PubMed] [Google Scholar]
- Evans, J.A. , Lankau, R.A. , Davis, A.S. , Raghu, S. & Landis, D.A. (2016). Soil‐mediated eco‐evolutionary feedbacks in the invasive plant Alliaria petiolata . Funct. Ecol., 30, 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faillace, C.A. , Lorusso, N.S. & Duffy, S. (2017). Overlooking the smallest matter: viruses impact biological invasions. Ecol. Let., 20, 524–538. [DOI] [PubMed] [Google Scholar]
- Faison, E.K. , Foster, D.R. , Holle, B.V. , Rapp, J.M. & Moore, S. (2019). Nonnative vegetation dynamics in the understory of a fragmented temperate forest. J. Torrey Bot. Soc., 146, 252–261. [Google Scholar]
- Flory, S.L. , Kleczewski, N. & Clay, K. (2011). Ecological consequences of pathogen accumulation on an invasive grass. Ecosphere, 2, 120. [Google Scholar]
- Forsstrom, T. , Vesakoski, O. , Riipinen, K. & Fowler, A.E. (2018). Post‐invasion demography and persistence of a novel functional species in an estuarine system. Biol. Inv., 20, 3331–3345. [Google Scholar]
- Fourniera, A. , Penoneb, C. , Penninoc, M.G. & Courchampa, F. (2019). Predicting future invaders and future invasions. P. Nat. Acad. Sci. USA, 116, 7905–7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelich, L.E. , Blossey, B. , Cameron, E.K. , Dávalos, A. , Eisenhauer, N. , Fahey, T. et al (2019). Side‐swiped: ecological cascades emanating from earthworm invasions. Front. Ecol. Environ., 17, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard, C. , Herve, M. & Hechinger, R.F. (2018). Long‐term population fluctuations of the exotic New Zealand mudsnail Potamopyrgus antipodarum and its introduced aporocotylid trematode in northwestern France. Hydrobiologia, 817, 253–266. [Google Scholar]
- Gerber, E. , Hinz, H.L. & Blossey, B. (2007). Interaction of specialist root and shoot herbivores of Alliaria petiolata and their impact on plant performance and reproduction. Ecol. Entomol., 32, 357–365. [Google Scholar]
- Gibson‐Reinemer, D.K. , Chick, J.H. , VanMiddlesworth, T.D. , VanMiddlesworth, M. & Casper, A.F. (2017). Widespread and enduring demographic collapse of invasive common carp (Cyprinus carpio) in the Upper Mississippi River System. Biol. Inv., 19, 1905–1916. [Google Scholar]
- Gurevitch, J. , Fox, G.A. , Wardle, G.M. , Inderjit Taub, D. (2011). Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecol. Let., 14, 407–418. [DOI] [PubMed] [Google Scholar]
- Hahn, P.G. & Dornbush, M.E. (2012). Exotic consumers interact with exotic plants to mediate native plant survival in a Midwestern forest herb layer. Biol. Inv., 14, 449–460. [Google Scholar]
- Hale, A.N. , Tonsor, S.J. & Kalisz, S. (2011). Testing the mutualism disruption hypothesis: physiological mechanisms for invasion of intact perennial plant communities. Ecosphere, 2, art110. [Google Scholar]
- Hastie, T. & Tibshirani, R. (1990). Generalized additive models. Chapman and Hall, London. [DOI] [PubMed] [Google Scholar]
- Hayes, K.R. & Barry, S.C. (2008). Are there any consistent predictors of invasion success? Biol. Inv., 10, 483–506. [Google Scholar]
- Hierro, J.L. & Callaway, R.M. (2003). Allelopathy and exotic plant invasion. Plant Soil, 256, 25–39. [Google Scholar]
- Hof, C. , Araujo, M.B. , Jetz, W. & Rahbek, C. (2011). Additive threats from pathogens, climate and land‐use change for global amphibian diversity. Nature, 480, 516–U137. [DOI] [PubMed] [Google Scholar]
- Hooper, D.U. , Adair, E.C. , Cardinale, B.J. , Byrnes, J.E.K. , Hungate, B.A. , Matulich, K.L. et al (2012). A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature, 486, 105–109. [DOI] [PubMed] [Google Scholar]
- Hulme, P.E. & Bernard‐Verdier, M. (2018). Comparing traits of native and alien plants: Can we do better? Funct. Ecol., 32, 117–125. [Google Scholar]
- Ivanov, K. & Keiper, J. (2011). Potential impacts of the invasive herb garlic mustard (Alliaria petiolata) on local ant (Hymenoptera: Formicidae) communities in northern temperate forests. Jeffersoniana, 26, 1–14. [Google Scholar]
- Kalisz, S. , Spigler, R. & Horvitz, C. (2014). In a long‐term experimental demography study, excluding ungulates reversed invader's explosive population growth rate and restored natives. P. Nat. Acad. Sci. USA, 111, 4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, R.P. , Lodge, D.M. & Finnoff, D.C. (2007). Risk assessment for invasive species produces net bioeconomic benefits. P. Nat. Acad. Sci. USA, 104, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos, J.N. (2002). Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature, 417, 67–70. [DOI] [PubMed] [Google Scholar]
- Koch, A.M. , Antunes, P.M. , Barto, E.K. , Cipollini, D. , Mummey, D.L. & Klironomos, J.N. (2011). The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol. Inv., 13, 1627–1639. [Google Scholar]
- Koorem, K. , Snoek, B.L. , Bloem, J. , Geisen, S. , Kostenko, O. , Manrubia, M. , Ramirez, K.S. , Weser, C. , Wilschut, R.A. & van der Putten, W.H. (2020) Community‐level interactions between plants and soil biota during range expansion. J. Ecol. 10.1111/1365-2745.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau, R. (2010). Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biol. Inv., 12, 2059–2068. [Google Scholar]
- Lankau, R.A. (2011a). Intraspecific variation in allelochemistry determines an invasive species' impact on soil microbial communities. Oecologia, 165, 453–463. [DOI] [PubMed] [Google Scholar]
- Lankau, R.A. (2011b). Resistance and recovery of soil microbial communities in the face of Alliaria petiolata invasions. New Phytol, 189, 536–548. [DOI] [PubMed] [Google Scholar]
- Lankau, R.A. (2012a). Coevolution between invasive and native plants driven by chemical competition and soil biota. P. Nat. Acad. Sci. USA, 109, 11240–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau, R.A. (2012b). Interpopulation variation in allelopathic traits informs restoration of invaded landscapes. Evol Appl, 5, 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau, R.A. , Bauer, J.T. , Anderson, M.R. & Anderson, R.C. (2014). Long‐term legacies and partial recovery of mycorrhizal communities after invasive plant removal. Biol. Inv., 16, 1979–1990. [Google Scholar]
- Lankau, R.A. , Nuzzo, V. , Spyreas, G. & Davis, A.S. (2009). Evolutionary limits ameliorate the negative impact of an invasive plant. P. Nat. Acad. Sci. USA, 106, 15362–15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester, P.J. & Gruber, M.A.M. (2016). Booms, busts and population collapses in invasive ants. Biol. Inv., 18, 3091–3101. [Google Scholar]
- Lonsdale, W.M. (1999). Global patterns of plant invasions and the concept of invasibility. Ecology, 80, 1522–1536. [Google Scholar]
- Mangla, S. , Inderjit Callaway, R.M.,(2008). Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J. Ecol., 96, 58–67. [Google Scholar]
- Mitchell, C.E. & Powers, A.G. (2003). Release of invasive plants from fungal and viral pathogens. Nature, 421, 625–627. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan, R. , Jordan, N.R. , Davis, A.S. & Forester, J.D. (2019). Use of simulation‐based statistical models to complement bioclimatic models in predicting continental scale invasion risks. Biol. Inv., 21, 847–859. [Google Scholar]
- Nuzzo, V. , Dávalos, A. & Blossey, B. (2017). Assessing plant community composition fails to capture impacts of white‐tailed deer on native and invasive plant species. AoB PLANTS, 9, plx026 10.1093/aobpla/plx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo, V.A. (1993). Distribution and spread of the invasive biennial Alliaria petiolata (Bieb. [Cavara and Grande]) in North America. In: Biological Pollution: Control and impact of invasive exotic species (ed. McKnight, BL). Indiana Academy of Science Indianapolis, pp. 115–124.
- Nuzzo, V.A. (1999). Invasion pattern of the herb garlic mustard (Alliaria petiolata) in high quality forests. Biol. Inv., 1, 169–179. [Google Scholar]
- Nuzzo, V.A. , Maerz, J.C. & Blossey, B. (2009). Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conserv. Biol., 23, 966–974. [DOI] [PubMed] [Google Scholar]
- Orstan, A. & Cameron, R.A.D. (2015). Cepaea nemoralis in Burlington, New Jersey, usa: Its possible origin and state 157 years after its introduction. J. Conchol., 42, 193–198. [Google Scholar]
- Powell, K.I. , Chase, J.M. & Knight, T.M. (2011). A synthesis of plant invasion effects on biodiversity across spatial scales. Am. J. Bot., 98, 539–548. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. http://www.R‐project.org/.
- Reinhart, K.O. & Callaway, R.M. (2006). Soil biota and invasive plants. New Phytol., 170, 445–457. [DOI] [PubMed] [Google Scholar]
- Roberts, K.J. & Anderson, R.C. (2001). Effect of garlic mustard Alliaria petiolata (Beib. Cavara & Grande) extracts on plants and arbuscular mycorrhizal (AM) fungi. Am. Midl. Nat., 146, 146–152. [Google Scholar]
- Ross, J.L. , Ivanova, E.S. , Severns, P.M. & Wilson, M.J. (2010). The role of parasite release in invasion of the USA by European slugs. Biol. Inv., 12, 603–610. [Google Scholar]
- Sakai, A.K. , Allendorf, F.W. , Holt, J.S. , Lodge, D.M. , Molofsky, J. , With, K.A. et al (2001). The population biology of invasive species. Ann. Rev. Ecol. Syst., 32, 305–332. [Google Scholar]
- Sheehy, E. & Lawton, C. (2014). Population crash in an invasive species following the recovery of a native predator: the case of the American grey squirrel and the European pine marten in Ireland. Biodivers. Conserv., 23, 753–774. [Google Scholar]
- Simberloff, D. & Gibbons, L. (2004). Now you see them, now you don't ‐ population crashes of established introduced species. Biol. Inv., 6, 161–172. [Google Scholar]
- Simberloff, D. , Martin, J.L. , Genovesi, P. , Maris, V. , Wardle, D.A. , Aronson, J. et al (2013). Impacts of biological invasions: what's what and the way forward. Trend Ecol. Evol., 28, 58–66. [DOI] [PubMed] [Google Scholar]
- Smith, C.S. , Lonsdale, W.M. & Fortune, J. (1999). When to ignore advice: invasion predictions and decision theory. Biol. Inv., 1, 89–96. [Google Scholar]
- Stinson, K.A. , Campbell, S.A. , Powell, J.R. , Wolfe, B.E. , Callaway, R.M. , Thelen, G.C. et al (2006). Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol., 4, 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker, K.B. , Harmon, P.F. , Goss, E.M. , Clay, K. & Flory, S.L. (2016). Emergence and accumulation of novel pathogens suppress an invasive species. Ecol. Let., 19, 469–477. [DOI] [PubMed] [Google Scholar]
- Tang, L. , Gao, Y. , Wang, C.H. , Zhao, B. & Li, B. (2012). A plant invader declines through its modification to habitats: A case study of a 16‐year chronosequence of Spartina alterniflora invasion in a salt marsh. Ecol. Eng., 49, 181–185. [Google Scholar]
- Tartally, A. , Antonova, V. , Espadaler, X. , Csosz, S. & Czechowski, W. (2016). Collapse of the invasive garden ant, Lasius neglectus, populations in four European countries. Biol. Inv., 18, 3127–3131. [Google Scholar]
- USDA NRCS (2017). The PLANTS Database (http://plants.usda.gov). National Plant Data Team, Greensboro, North Carolina, USA.
- van der Putten, W.H. , Bardgett, R.D. , Bever, J.D. , Bezemer, T.M. , Casper, B.B. , Fukami, T. et al (2013). Plant‐soil feedbacks: the past, the present and future challenges. J. Ecol., 101, 265–276. [Google Scholar]
- Waller, D.M. & Maas, L.I. (2013). Do white‐tailed deer and the exotic plant garlic mustard interact to affect the growth and persistence of native forest plants? Forest Ecol. Manag., 304, 296–302. [Google Scholar]
- Wardle, D.A. , Bardgett, R.D. , Callaway, R.M. & Van der Putten, W.H. (2011). Terrestrial ecosystem responses to species gains and losses. Science, 332, 1273–1277. [DOI] [PubMed] [Google Scholar]
- Warren, R.J. , Candeias, M. , Labatore, A. , Olejniczak, M. & Yang, L. (2019). Multiple mechanisms in woodland plant species invasion. J. Plant Ecol., 12, 201–209. [Google Scholar]
- Williamson, M.H. & Fitter, A. (1996). The characters of successful invaders. Biol. Conserv., 78, 163–170. [Google Scholar]
- Wixted, K.L. & McGraw, J.B. (2010). Competitive and allelopathic effects of garlic mustard (Alliaria petiolata) on American ginseng (Panax quinquefolius). Plant Ecol., 208, 347–357. [Google Scholar]
- Wolfe, B.E. & Klironomos, J.N. (2005). Breaking new ground: Soil communities and exotic plant invasion. Bioscience, 55, 477–487. [Google Scholar]
- Wolfe, B.E. , Rodgers, V.L. , Stinson, K.A. & Pringle, A. (2008). The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol., 96, 777–783. [Google Scholar]
- Wood, S. (2006). Generalized additive mixed models: an introduction with R. Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- Wood, S. & Scheipl, F. (2017). gamm4: Generalized Additive Mixed Models using 'mgcv' and 'lme4'. R package version 0.2‐5. https://CRAN.R‐project.org/package=gamm4.
- Wubs, E.R.J. , van der Putten, W.H. , Mortimer, S.R. , Korthals, G.W. , Duyts, H. , Wagenaar, R. et al (2019). Single introductions of soil biota and plants generate long‐term legacies in soil and plant community assembly. Ecol. Let., 22, 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data are available in Dryad (https://doi.org/10.5061/dryad.mpg4f4qxh).


