Abstract
The COVID‐19 pandemic has affected most parts of the global society since its emergence, and the scientific community has been challenged with questions urgently demanding answers. One of the early hypotheses on COVID‐19 outcome was that some protection could be offered by the tuberculosis vaccine (BCG), and several clinical studies were initiated along with the emergence of numerous observational studies on the relationship between BCG and COVID‐19 severity. In the present work, I demonstrate a strong correlation between the number of years that countries implemented BCG vaccination plans and age‐standardized mortality rates during the first months of the pandemic in Europe. Further analyses of age groups in two European countries with comparably few confounding factors and easily identifiable groups of BCG‐vaccinated and non‐vaccinated subgroups suggest a population‐level effect of BCG on national outcomes of COVID‐19. This phenomenon of ‘heterologous herd immunity’ deserves further investigation, both in epidemiological and experimental studies.
Keywords: infectious disease, vaccine, virology, immunology

The emergence of the COVID‐19 pandemic
During the first months of 2020, a novel coronavirus causing severe acute respiratory syndrome (SARS) spread to cause a pandemic of historical impact. The disease, termed coronavirus disease‐19 (COVID‐19), emerged in 2019 in Wuhan, China, possibly through a species jump of coronavirus with origin in bats [1]. Most people experience no or mild symptoms [2]; however, for reasons that are not clarified, individuals belonging to certain risk groups such as diabetes patients and patients with cardiovascular disease may develop severe disease [3, 4]. Severe COVID‐19 is associated with a cytokine storm causing acute respiratory distress syndrome associated with cardiovascular effects including an increased risk of thrombosis (reviewed in [5]). Studies are emerging that characterize the immune responses in COVID‐19, and in severe cases, lymphopenia accompanied by increased viral load triggering a cytokine storm seems to precede a rapid deterioration of the condition [6]. At the same time, a robust T‐cell response seems to be associated with mild disease [7]. The genetic, epigenetic, immunological and physiological determinants of individual COVID‐19‐infected subjects’ prognoses remain elusive. Clearly, population‐level differences have been observed during the early phase of the pandemic, with different countries having a highly variable disease burden reflected in COVID‐19‐related hospitalizations and deaths [8]. This observation holds true even when accounting for many confounders such as the countries’ healthcare systems, general health status and life expectancy of the population as well as different pandemic prevention strategies [8].
COVID‐19, BCG and trained immunity
In February 2020, clinical studies were initiated to investigate the potential effect of Bacillus Calmette–Guérin (BCG) vaccination on COVID‐19 [9]. The rationale behind these studies is that the BCG vaccine, along with other live vaccines, has been demonstrated to induce trained immunity, which is based on epigenetic reprogramming of the immune system [10, 11, 12] that results in its fine‐tuning to protect against severe infections that can be unrelated to the immune‐training agent (e.g. BCG, β glucans from Candida albicans) [11, 13]. These studies are underway, and until a possible causal relationship between BCG vaccination and a mild course of COVID‐19 is established, it can only be studied in observational approaches. Such studies have suggested a strong relationship between national BCG vaccination policies and the COVID‐19 disease burden in different countries [9, 14, 15, 16]. However, these findings are still controversial [17, 18, 19].
An early study from the 1940s showed that the all‐cause mortality in children was significantly reduced after introduction of BCG in Sweden [20]. Most of the effect could not be explained by reduction of tuberculosis (TB), against which the vaccine was intended. This finding has been confirmed in several recent international studies [21, 22, 23], and other live vaccines such as measles and yellow fever vaccines have been demonstrated to have similar effects [24]. This heterologous immunity induced through BCG can be viewed as targeted priming of immunological responses, so that a second microbial challenge will result in a more powerful and tailored counterstrike by the immune system [10, 11, 25]. The trained immunity concept has been best characterized in monocytes and macrophages, with enhanced release of cytokines and chemokines in response to a secondary stimulus [11] and an improved antimicrobial defence [10, 11]. Evidence exists that the epigenetic reprogramming also takes place in T cells, B cells and NK cells [10, 25] but the function in these cells is poorly understood. A role for epigenetically rewired T cells in directing and priming the immune memory of macrophages has been suggested [26].
Navigating around confounders to explore a possible BCG‐COVID‐19 relationship
Given the documented ability of BCG to induce trained innate immunity, it would not be surprising if the vaccine also provides some level of protection against COVID‐19. Numerous confounders may influence the validity of studies comparing the COVID‐19 pandemic outcome in different populations and countries, including different management and testing strategies, reporting of confirmed cases, COVID‐19‐related hospitalizations or death rates [27]. Instead, a more reliable measure for early phase pandemic outcomes could be the all‐cause mortality relative to previous years, as, for example, the seasonal flu is reflected in the all‐cause mortality during the winter months in many countries [28, 29, 30]. Also, limiting analyses to regions with comparable national public health policies, societal organization and life expectancy should be considered, and Europe provides a good example for that [16].
The recommendations of neonatal BCG vaccination vary greatly amongst the national vaccine plans (NVP) of European countries. Whilst many countries, especially in Eastern Europe where tuberculosis is still relatively prevalent, have retained BCG in their NVPs (hereafter referred to as BCG‐NVPs), a number of countries stopped the broad use of BCG in the 1980s as TB rates dropped [31]. Some countries never had BCG in the NVPs including Italy, Belgium and the Netherlands.
To investigate a possible relationship between BCG‐NVPs and excess mortality, I extracted data on the all‐cause mortality of 17 European countries (Table 1), which were selected on the availability of BCG‐NVP history and complete records in a recent report by the Office for National Statistics in the UK [32]. This report on age‐standardized mortality rates in Europe at the beginning of 2020 clearly demonstrated an effect of COVID‐19 on excess mortality in many European countries [32].
Table 1.
Usage of BCG in European NVPs for young children and the relative age‐standardized mortality rates (means from calendar weeks 10‐24 in year 2020 vs. the means of 2015‐2019 during the same period)
| Country | Two‐letter code | BCG‐NVP initiation | BCG‐NVP discont. | Years of BCG‐NVP | Relative mortality |
|---|---|---|---|---|---|
| Austria | AT | 1952 | 1990 | 38 | 1.08 |
| Belgium | BE | Never | ‐ | 0 | 1.55 |
| Bulgaria | BG | 1951 | ongoing | 69 | 0.93 |
| Czech Rep. | CZ | 1953 | 2010 | 57 | 0.98 |
| Denmark | DK | 1946 | 1986 | 40 | 1.00 |
| Estonia | EE | 1947 a | ongoing | 73 | 1.01 |
| Finland | FI | 1941 | 2006 | 65 | 1.04 |
| France | FR | 1950 | 2007 | 57 | 1.22 |
| Hungary | HU | 1953 | ongoing | 67 | 1.00 |
| Italy | IT | Never | ‐ | 0 | 1.43 |
| Lithuania | LT | 1947 a | ongoing | 73 | 1.01 |
| Netherlands | NL | Never | ‐ | 0 | 1.37 |
| Portugal | PT | 1965 | ongoing | 55 | 1.11 |
| Spain | ES | 1965 | 1981 | 16 | 1.69 |
| Sweden | SE | 1940 | 1975 | 35 | 1.29 |
| Slovakia | SK | 1953 | 2012 | 59 | 0.94 |
| Switzerland | CH | 1960 | 1987 | 27 | 1.15 |
Unclear starting date but most probably around 1947 after a BCG vaccination promotion campaign in Poland that year that spread to many European countries [41]. Country codes are the internationally accepted two‐letter code.
The data on BCG‐NVP vaccination policies were from the ‘BCG atlas’ 2nd Edition [33] and validated through communication with staff/search at the countries’ public health authorities’ web pages or other available documentation. UK and Norway have never had newborns in the BCG‐NVP [34, 35]. To avoid the parameter of age at BCG administration, which has been demonstrated to affect vaccine efficacy [9], these countries were excluded from the present analysis.
Through inspection of the week‐by‐week mortality in the countries listed in Table 1 during early 2020, calendar week 10 through 24 were selected for the analysis as representative for the first wave of the COVID‐19 pandemic [32]. To compare the mortality during these weeks to that of previous years, I averaged the weekly mortality during these weeks and divided it by the corresponding average of previous years (2015–2019). The emerging relative mortality rates (hereafter referred to as ‘relative mortality’) of 2020 are listed in Table 1.
First, I performed a linear regression analysis to investigate how the relative mortality of the 17 countries and the total number of BCG‐NVP years correlated. Figure 1a shows a negative correlation between the total number of BCG‐NVP years and relative mortality in Europe, which is in line with another study published during the preparation of this manuscript that compared COVID‐19 death tolls with a calculated BCG index in Europe [16]. In a second linear regression analysis, I compared the relative mortality of 14 countries with the year of discontinuation of the BCG‐NVP for newborns or young children (Table 1, Fig. 1b). Since Portugal, Spain and Switzerland started very late (1965, 1965 and 1960, respectively), they were excluded from this second analysis. The other countries all started their BCG‐NVPs for newborns or young children between 1940 and 1953. Both analyses point towards a strong negative correlation of BCG coverage and excess mortality during the early phase of the COVID‐19 pandemic.
Figure 1.
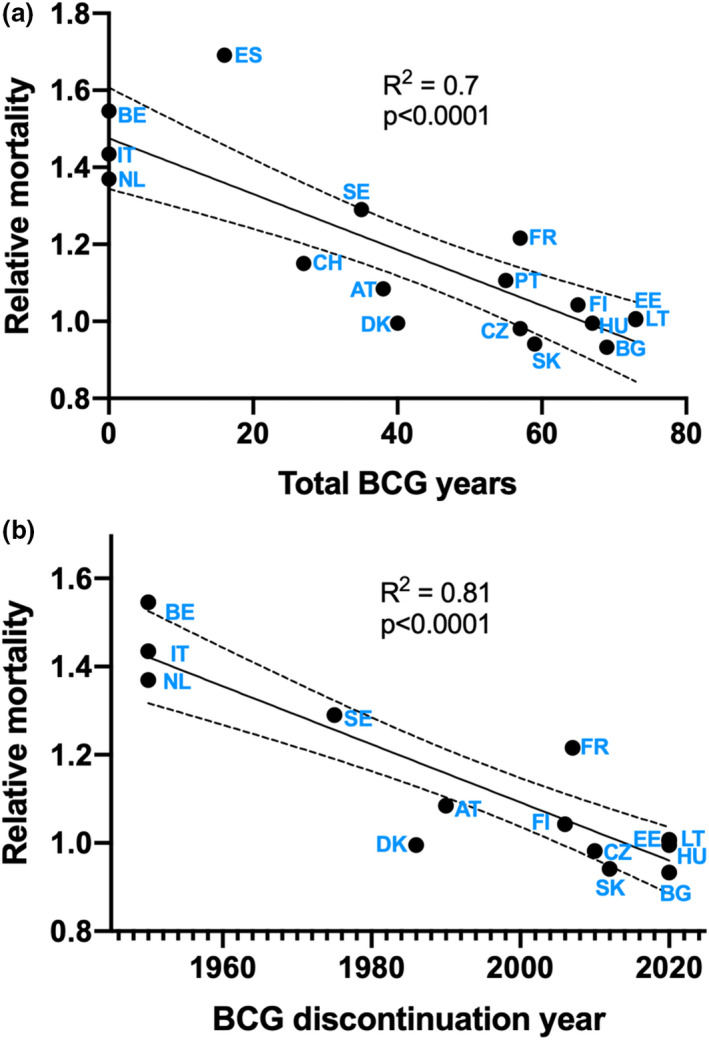
(a) Linear regression analysis of the total number of BCG‐NVP years and relative age‐standardized mortality rates in European countries. Countries that never had BCG‐NVPs for young children were assigned the ‘mock year’ 1950, which is the approximated average year the other countries started. (b) Linear regression analysis of year of discontinuation of neonatal BCG vaccine in the NVP and relative age‐standardized mortality rates in European countries. Spain, Portugal and Switzerland were excluded from this analysis due to late start of BCG‐NVP (1965, 1965 and 1960, respectively).
Getting closer to the point – who is protected and how?
As a republic consisting of federal states with similar demographics and health policies today, Germany poses a particularly interesting example for medical research questions for which historical aspects like vaccination policies play a role, since it was separated into the Federal Republic of Germany (West Germany) and the German Democratic Republic (East Germany) from 1949 until 1989. The BCG vaccination policies differed profoundly between East Germany and West Germany, with a rigorous BCG‐NVP for newborns in East Germany until the reunion, and thereafter gradually discontinued [36]. In West Germany, BCG was used from 1953‐1975 as a general recommendation. Thereafter, BCG vaccination was only recommended for targeted groups and the BCG coverage has been estimated to have fallen from 50% to below 10% between the 80s and the 90s [37]. The recommendations may have varied between West Germany’s federal states until the reunion (STIKO group, Robert Koch Institute, personal communication).
I took advantage of the fact that Germany consists of regions with comparable economy, demographics and healthcare systems and with identical implementation of pandemic prevention measures and SARS‐CoV‐2 testing to compare the number of accumulated cases (as identified by positive SARS‐CoV‐2 PCR test, as per 27 August 2020) of 15 Federal States (excluding the three city states Hamburg, Bremen and Berlin, due to the metropolitan character of these regions). The data were available at the Robert Koch Institute (https://corona.rki.de). The available information on case numbers was stratified into the following age categories: 15–34, 35–59 and 60–79. As previously published [38], an overrepresentation of COVID‐19 cases in the Western states was identified across all age groups (Fig. 2a). The distribution of the cases within each part of Germany (Eastern and Western states separated) did not differ profoundly. Remarkably, the BCG coverage in the youngest age categories (<31 years of age) is expected to be very limited in these states, but still the number of COVID‐19 cases is not higher in this fraction than amongst the older citizens of the Eastern states. This observation suggests that the possible effect of BCG is not in preventing infection, since in such case, it should be reflected in the pattern of case – age distribution, with more cases than observed in the younger population of the Eastern states (Fig. 2a).
Figure 2.
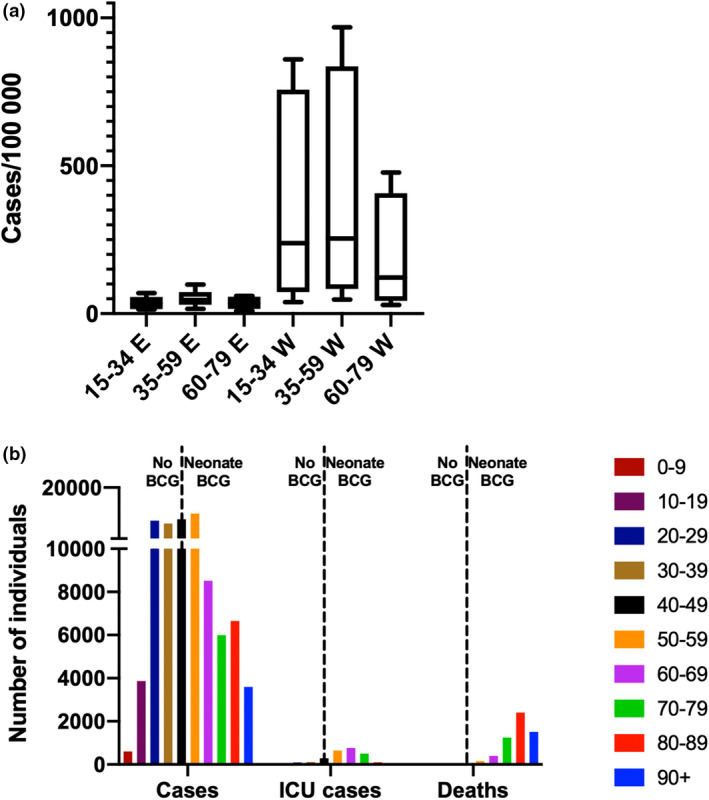
(a) Number of COVID‐19 cases by age group and German federal state (E = Eastern, W = Western) as per 27 August 2020. (b) Number of COVID‐19 cases, ICU cases and deaths in Sweden by age groups (source: the Swedish Public Health Agency, as per September 2, 2020). The dotted lines represent the cut‐off age for individuals who were BCG‐vaccinated as neonates in the Swedish NVP (1940–1975).
Sweden provides another interesting example of the relationship between BCG vaccination policies and COVID‐19, since the country is rather unique with its sudden discontinuation of a rigorous BCG‐NVP in 1975. Thus, in 2020, most Swedish‐born individuals between the age of 45 and 80 were BCG‐vaccinated as neonates (the BCG‐NVP for newborns was initiated in 1940). In Fig. 2b, data on COVID‐19 cases, COVID‐19‐related intensive care unit (ICU) cases and deaths are plotted (the Public Health Agency of Sweden as per September 2nd, 2020). First of all, given the high coverage of BCG in the Swedish population in individuals between 45 and 80 years of age, the graph reveals that the severe (ICU care‐requiring) COVID‐19 cases, obviously, on an individual level, were not protected by their BCG vaccination that they received as neonates. Second, the majority of the casualties were older than 80 years, and as this age group was not neonatally BCG‐vaccinated but may have been included in BCG mass vaccinations beginning in 1940, it is difficult to draw any conclusions on whether and at what age BCG was administered. Third, the number of COVID‐19 cases seems to be similar amongst the non‐vaccinated young adults (20–45) as amongst the middle‐aged, vaccinated individuals (46–59). Finally, COVID‐19 did not cause severe disease in young and healthy individuals, the majority of which have not been BCG‐vaccinated.
Does BCG induce heterologous herd immunity?
In line with the presented observations, a recent study, which investigated the COVID‐19 mortality in individuals born right before and after the discontinuation of the Swedish BCG‐NVP in mid‐seventies, concluded that there was no difference in number of COVID‐19 cases or COVID‐19‐related hospitalizations depending on BCG vaccination status in middle‐aged individuals [18]. However, the observations from Sweden and Germany presented here indicate that the possible association of BCG‐NVPs and nation‐level mortality during the COVID‐19 pandemic cannot be simply explained by protection against infection (i.e. getting infected or not by SARS‐CoV‐2), severe COVID‐19 disease or death at an individual level, but may involve more complex, population‐level effects. Possibly, BCG could reduce the R0 value (the basic reproduction number, defined as the average number of new infections caused by an infected individual during the early stage of an outbreak in a fully susceptible population [39]), that is by reducing COVID‐19 symptoms (the virus’ mechanism of spread is to induce symptoms that enhances transmission) and/or SARS‐CoV‐2 viral load. Unfortunately, the SARS‐CoV‐2 PCR gives only a qualitative result (positive or negative) and does not provide any information on viral load, which most likely correlates with how infectious a person is. If BCG significantly reduces the R0 value of SARS‐CoV‐2, a critical mass of BCG‐vaccinated individuals in a population could possibly generate (a heterologous) herd immunity that protects individuals vulnerable to COVID‐19 (e.g. with diabetes or cardiovascular disease). It is important to remember that in Sweden, as an example, 0.06% of the total population has succumbed to COVID‐19 (as of 10 September 2020, 5843 casualties, amongst 10.3 million citizens). The majority of the casualties were suffering from comorbidities such as chronic diseases and were of high age or transplant recipients [40]. Thus, for most of these victims, a childhood BCG vaccination that induces trained immunity through reversible mechanisms, and given many decades ago, is not likely to protect. Instead, the findings presented here suggest that these vulnerable individuals are better protected through heterologous herd immunity in countries with rigorous BCG coverage.
Concluding remarks
Whilst the COVID‐19 pandemic death tolls may be comparable to that of TB in 2020, the TB pandemic has been going on for decades and is affecting humans in their most productive age. Although a century has passed since the launch of BCG, the first and only TB vaccine, no vaccine candidate has so far performed superior to BCG in preventing TB. In addition, as discussed above, there is very strong evidence that BCG reduces childhood mortality in countries, where infectious disease remains the number one cause of death. Therefore, a sudden rush for BCG vaccine in countries with strong economy, based on the belief that the vaccine would protect against COVID‐19, could worsen the shortage of BCG, which was alarming already before the pandemic. High‐qualitative studies of BCG’s impact on COVID‐19 like those currently ongoing are needed to determine whether and how BCG can help control the pandemic. Independently of the outcome, it is a responsibility of the world’s strongest economies to scale up and safeguard the manufacturing of BCG so that the truly most vulnerable, newborns in developing economies, have continuous access to this lifesaving ‘cousin’ of M. tuberculosis.
Conflict of interest
No conflict of interest was declared.
Supporting information
Figure S1. Interactive figure on age‐standardized mortality rates in Europé January‐June 2020.
Acknowledgements
This study was funded through a generous grant from the Swedish Heart Lung Foundation (20200319). I owe my thanks to Associate Professors Jakob Paues and Judith Bruchfeld and Professors Olle Stendahl and Camille Locht for careful review of the manuscript.
Lerm M (Faculty of Medicine and Health Sciences, Linköping University, Linköping, Sweden). On the relationship between BCG coverage and national COVID‐19 outcome: could ‘heterologous’ herd immunity explain why some countries are better off? (Perspective). J Intern Med, 2020; 288: 682–688. 10.1111/joim.13198
References
- 1. Boni MF, Lemey P, Jiang X et alEvolutionary origins of the SARS‐CoV‐2 sarbecovirus lineage responsible for the COVID‐19 pandemic. Nat Microbiol 2020;5:1408–1417. [DOI] [PubMed] [Google Scholar]
- 2. Bi Q, Wu YY, Mei S et al Epidemiology and transmission of COVID‐19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karagiannidis C, Mostert C, Hentschker C et al Case characteristics, resource use, and outcomes of 10 021 patients with COVID‐19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 20208:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta A, Madhavan MV, Sehgal K et al Extrapulmonary manifestations of COVID‐19. Nat Med 2020; 26:1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A et al Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. J Exp Med 2020; 217:e20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aibin W, Guiju G, Sa W et al Clinical characteristics and risk factors of acute respiratory distress syndrome (ARDS) in COVID‐19 patients in Beijing, China: A Retrospective Study. Med Sci Monit 2020; 26:e925974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O et al Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell 2020183:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allel K, Tapia‐Muñoz T, Morris W. Country‐level factors associated with the early spread of COVID‐19 cases at 5, 10 and 15 days since the onset. Glob Public Health 2020. [DOI] [PubMed]
- 9. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID‐19. Lancet Publishing Group 2020;395:1545–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verma D, Parasa VRVR, Raffetseder J et al Anti‐mycobacterial activity correlates with altered DNA methylation pattern in immune cells from BCG‐vaccinated subjects. Sci Rep 2017; 7:12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kleinnijenhuis J, Quintin J, Preijers F et al Bacille Calmette‐Guerin induces NOD2‐dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaufmann E, Sanz J, Dunn JL et al BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018; 172:176‐190.e19. [DOI] [PubMed] [Google Scholar]
- 13. Saeed S, Quintin J, Kerstens HHD et al Epigenetic programming of monocyte‐to‐macrophage differentiation and trained innate immunity. Science 2014; 345:1251086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozdemir C, Kucuksezer UC, Tamay ZU. Is BCG vaccination affecting the spread and severity of COVID‐19? Allergy 2020:all.14344.75:1824–1827. [DOI] [PubMed] [Google Scholar]
- 15. Geller A, Yan J. Could the induction of trained immunity by β‐glucan serve as a defense against COVID‐19? Front Immunol 2020; 11:1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Escobar LE, Molina‐Cruz A, Barillas‐Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci U S A 2020; 117:17720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caminati M, Furci F, Senna G et al BCG vaccination and COVID‐19: Much ado about nothing? Med Hypotheses 2020; 144:110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Chaisemartin C, de Chaisemartin L. BCG vaccination in infancy does not protect against COVID‐19. Evidence from a natural experiment in Sweden. Clin Infect Dis 2020. Online ahead of print.1223: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamiel U, Kozer E, Youngster I. SARS‐CoV‐2 Rates in BCG‐Vaccinated and Unvaccinated Young Adults. JAMA ‐ J Am Med Assoc 2020; 323:2340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naeslund C. Experience wiht BCG vaccination in the province of Norrbotten (Sweden). Rev Tuberc 1931; 12:617–36. [Google Scholar]
- 21. de Bree LCJ, Koeken VACM, Joosten LAB et al Non‐specific effects of vaccines: Current evidence and potential implications. Semin Immunol 2018; 39:35–43. [DOI] [PubMed] [Google Scholar]
- 22. Thysen SM, Benn CS, Gomes VF et al Neonatal BCG vaccination and child survival in TB‐exposed and TB‐unexposed children: A prospective cohort study. BMJ Open 2020; 10:e035595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benn CS, Roth A, Garly M‐L et al BCG scarring and improved child survival: a combined analysis of studies of BCG scarring. J Intern Med 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 24. Pfeiffer G, Fisker AB, Nebié E et al Non‐specific effects of childhood vaccinations – A case control study nested into a Health and Demographic Surveillance System in rural Burkina Faso. Vaccine 2017; 35:7114–20. [DOI] [PubMed] [Google Scholar]
- 25. Kleinnijenhuis J, Quintin J, Preijers F et al BCG‐induced trained immunity in NK cells: Role for non‐specific protection to infection. Clin Immunol 2014; 155:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yao Y, Jeyanathan M, Haddadi S et al Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 2018; 175:1634‐1650.e17. [DOI] [PubMed] [Google Scholar]
- 27. Lau H, Khosrawipour T, Kocbach P, Ichii H, Bania J, Khosrawipour V. Evaluating the massive underreporting and undertesting of COVID‐19 cases in multiple global epicenters. Pulmonology 2020. Online ahead of print.S2531‐0437(20)30129‐X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosano A, Bella A, Gesualdo F et al Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons). Int J Infect Dis 2019; 88:127–34. [DOI] [PubMed] [Google Scholar]
- 29. Cheng KJG, Rivera AS, Lam HY et al Influenza‐associated excess mortality in the Philippines, 2006–2015. PLoS One 2020; 15:e0234715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paget J, Spreeuwenberg P, Charu V et al Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J Glob Health 2019; 9:020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boman G. The ongoing story of the Bacille Calmette‐Guérin (BCG) vaccination. Acta Paediatr 2016; 105:1417–20. [DOI] [PubMed] [Google Scholar]
- 32. Campbell A, Morgan E. Comparisons of all‐cause mortality between European countries and regions: January to June 2020.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/comparisonsofallcausemortalitybetweeneuropeancountriesandregions/januarytojune2020
- 33. Zwerling A, Pai M. The BCG world atlas: A new, open‐access resource for clinicians and researchers. Expert Rev Anti Infect Ther 2011; 9:559–61. [DOI] [PubMed] [Google Scholar]
- 34. BCG Vaccine (TB vaccine), Vaccine Knowledge . https://vk.ovg.ox.ac.uk/vk/bcg‐vaccine
- 35. History of the Norwegian Institute of Public Health – NIPH . https://www.fhi.no/en/about/this‐is‐the‐norwegian‐institute‐of‐public‐health/history‐of‐the‐norwegian‐institute‐/
- 36. Klein S, Schöneberg I, Krause G. Vom Zwang zur Pockenschutzimpfung zum Nationalen Impfplan Die Entwicklung des Impfwesens vom Deutschen Kaiserreich bis heute. Bundesgesundheitsbl 2012; 55:1512–23. [DOI] [PubMed] [Google Scholar]
- 37. Stich HL, Beblo F. Impfmonitoring bei schulanfangern: Ein regionalbeitrag aus einem landkreis Bayerns. Gesundheitswesen 2000; 62:320–4. [DOI] [PubMed] [Google Scholar]
- 38. Hauer J, Fischer U, Auer F, Borkhardt A. LETTER Acute lymphoblastic leukemia Regional BCG vaccination policy in former East‐and West Germany may impact on both severity of SARS‐CoV‐2 and incidence of childhood leukemia. Leukemia 2020; 34:2217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Britton T, Ball F, Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS‐CoV‐2. Science 2020; 369:846–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uppdatering av tidigare rapport gällande identifiering av riskgrupper som löper störst risk att drabbas av ett särskilt allvarligt sjukdomsförlopp vid insjuknande i covid‐19. www.socialstyrelsen.seagrebneviene G.
- 41. Korabilov P. The historical experience and practice of fight against tuberculosis in country which is one of the high drug resistant‐tuberculosis (DR‐TB) burden countries in European Union (EU). J Prev Med Hyg 2018; 59:E328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interactive figure on age‐standardized mortality rates in Europé January‐June 2020.


