Abstract
Isoprenoids, also known as terpenes or terpenoids, represent a large family of natural products composed of five‐carbon isopentenyl diphosphate or its isomer dimethylallyl diphosphate as the building blocks. Isoprenoids are structurally and functionally diverse and include dolichols, steroid hormones, carotenoids, retinoids, aromatic metabolites, the isoprenoid side‐chain of ubiquinone, and isoprenoid attached signaling proteins. Productions of isoprenoids are catalyzed by a group of enzymes known as prenyltransferases, such as farnesyltransferases, geranylgeranyltransferases, terpenoid cyclase, squalene synthase, aromatic prenyltransferase, and cis‐ and trans‐prenyltransferases. Because these enzymes are key in cellular processes and metabolic pathways, they are expected to be potential targets in new drug discovery. In this review, six distinct subsets of characterized prenyltransferases are structurally and mechanistically classified, including (1) head‐to‐tail prenyl synthase, (2) head‐to‐head prenyl synthase, (3) head‐to‐middle prenyl synthase, (4) terpenoid cyclase, (5) aromatic prenyltransferase, and (6) protein prenylation. Inhibitors of those enzymes for potential therapies against several diseases are discussed. Lastly, recent results on the structures of integral membrane enzyme, undecaprenyl pyrophosphate phosphatase, are also discussed.
Keywords: farnesyl diphosphate, isoprenoid, prenyltransferase, terpene, terpenoid
Abbreviations
- ABBA
α‐β‐β‐α barrel
- AtPPPS
polyprenyl pyrophosphate synthase in Arabidopsis
- BacA/UPPP
undecaprenyl pyrophosphate phosphatase
- CLPP
cyclolavandulyl diphosphate
- CLPPS
cyclolavandulyl diphosphate synthase
- DHDDS
dehydrodolichyl diphosphate synthase
- DHS
dehydrosqualene
- DMAPP
dimethylallyl diphosphate
- DMATS
dimethylallyltryptophan synthase
- DolP
dolichol phosphate
- FPG‐trisaccharide
cis‐farnesyl group in the phosphoglycolipid
- FPP
farnesyl diphosphate
- FPPS
farnesyl diphosphate synthase
- FsPP
farnesyl thiopyrophosphate
- FTase
farnesyltransferase
- GPP
geranyl diphosphate
- GGPP
geranylgeranyl diphosphate
- GGPPS
geranylgeranyl diphosphate synthase
- GGTase
geranylgeranyl transferase
- GLPP
geranyl lavandulyl diphosphate
- GPPS
geranyl diphosphate synthase
- HepS and HspT
heptaprenyl diphosphate synthase
- HexPPS
trans‐hexaprenyl diphosphate synthase
- HSQ
hydroxysqualene
- IPP
isopentenyl diphosphate
- ISLPP
isosesquilavandulyl diphosphate
- LPP
lavandulyl diphosphate
- LPPS
lavandulyl diphosphate synthase
- LSU
large subunit
- Mcl22
isosesquilavandulyl diphosphate synthase
- MEP
methylerythritol phosphate
- MVA
mevalonate
- OPP
octaprenyl diphosphate
- OPPS
octaprenyl pyrophosphate synthase
- PSPP
presqualene diphosphate
- SqhC
tetraprenyl‐β‐curcumene cyclase
- SQS
squalene synthase
- SSU
small subunit
- UP
undecaprenyl phosphate
- UPP
undecaprenyl pyrophosphate
- UPPS
undecaprenyl diphosphate synthase
- YtpB
tetraprenyl‐β‐curcumene synthase
- Z,E‐DecPP
decaprenyl diphosphate
- zFPS
Z,Z‐farnesyl diphosphate synthase
1. INTRODUCTION
Isoprenoids, also known as terpenes or terpenoids, are a group of natural products diverse in structure and biological function. To date, more than 80,000 identified members have been characterized structurally and chemically. They are widely distributed in living organisms, including microbes, insects, plants, and marine organisms. 1 For instance, carotenoids and retinoids are involved in light‐sensitive elements that play a role in light absorption in plants. 2 Steroid hormones derived from cholesterol play a central role in lipid physiology in the human body. 3 , 4 Ubiquinone and menaquinone are lipophilic metabolites functioning as electron carriers in the respiratory chain in prokaryotes and eukaryotes. 5 Undecaprenyl pyrophosphate (UPP) serves as a membrane‐associated glycan carrier in the bacterial cell wall synthesis. 6 , 7 Dolichol plays a role in glycoprotein biosynthesis and posttranslational modifications in the endoplasmic reticulum (ER). 8
All these isoprenoid compounds are derived from linear prenyl diphosphate molecules, which are composed of two 5‐carbon intermediates, isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) as the building blocks. Both IPP and DMAPP are synthesized via two distinct pathways: the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways (Scheme 1). The MVA pathway is observed in animals, fungi, archaea, and some bacteria, and the synthesis of isoprene units in liver tissues and yeast through this pathway was first investigated in the 1950s. 9 By contrast, most bacteria synthesize isoprene through the MEP pathway. 10 A group of enzymes called prenyltransferases can subsequently condense with IPP and DMAPP to form various isoprenoid diphosphates with different chain lengths, such as 10‐carbon geranyl diphosphate (GPP), 15‐carbon farnesyl diphosphate (FPP), and 20‐carbon geranylgeranyl diphosphate (GGPP), catalyzed by GPP synthase, FPP synthase and GGPP synthase, respectively (Scheme 1). 11 , 12 , 13 The carbon chain lengths of linear isoprenoids widely range from 10‐carbon GPP to natural rubber (C>10,000). 14
SCHEME 1.
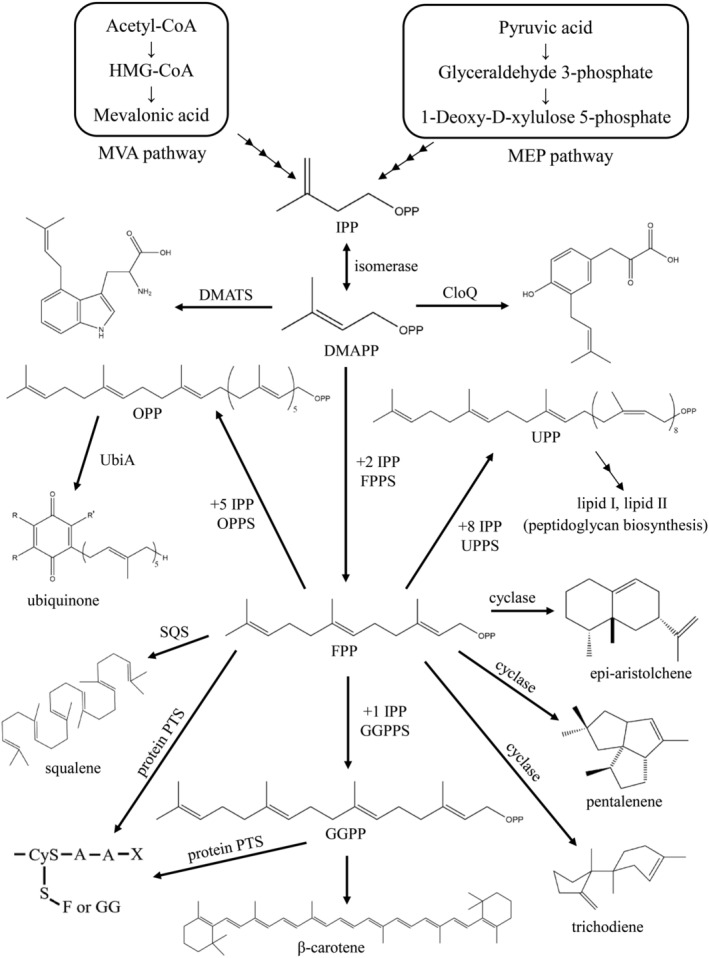
Prenyltransferases can condense with isopentenyl diphosphate and dimethylallyl diphosphate to form various natural isoprenoids with different chain lengths
All carbon skeletons are generated from linear isoprenoids (e.g., GPP, FPP, and GGPP) through consecutive condensation reactions with IPP or DMAPP in the biosynthesis of isoprenoids. These 10–20 carbon linear isoprenoid compounds serve as starting materials of prenyltransferases for the biosynthesis of a wide variety of isoprenoid natural products. For example, FPP can react with IPP through a so‐called head‐to‐tail condensation reaction to form a 20‐carbon GGPP as a building block in plant carotenoid biosynthesis. 15 , 16 Two FPP molecules are catalyzed through two‐step reductive head‐to‐head condensation by squalene synthase (SQS) to form squalene, a precursor in cholesterol and steroid hormone synthesis in plants and animals. 17 , 18 One GPP molecule and the cis‐farnesyl group in phosphoglycolipid (FPG‐ trisaccharide) are catalyzed by a unique head‐to‐middle prenyltransferase, MoeN5, to produce the 25‐carbon moenocinyl side‐chain–containing lipid in moenomycin biosynthesis. 19 , 20 Terpenoid cyclase catalyzes FPP cyclization to form the cyclic sesquiterpene hydrocarbon trichodiene, a precursor in antibiotic and mycotoxin biosynthesis. 21 Two aromatic prenyltransferase, namely CloQ and NphB, can catalyze the transfer of a 5‐carbon DMAPP or 10‐carbon GPP onto electron‐rich aromatic acceptor molecules (Scheme 1). 22 , 23 These natural products are primary and secondary metabolites of living organisms and are important in pharmaceutical research. Either FPP or GGPP can be attached through covalent binding to a conserved cysteine of certain signaling proteins, such as Ras‐family GTPases, via an irreversible posttranslational modification. 24 , 25 Prenylation of Ras‐related GTP‐binding proteins is essential for the proper cellular activity in signal transduction pathways. 26 , 27
In this review, we summarized previous studies on and recent research progresses in enzymatic structures, catalytic mechanisms, and biological functions of prenyltransferase in living organisms. We suggested that a diverse range of prenyltransferase can be classified into six main classes on the basis of their distinct catalytic mechanisms: (1) head‐to‐tail, (2) head‐to‐head, (3) head‐to‐middle condensation, (4) terpenoid cyclase, (5) aromatic prenyltransferase, and (6) protein prenylation. Potential prenyltransferase inhibitors for novel therapies against several diseases are also discussed. A list of enzymes and isoprenoids discussed in this review and their abbreviations is provided in Table S1.
2. CLASS 1: HEAD‐TO‐TAIL PRENYL SYNTHASE
2.1. Z (cis)‐ and E (trans)‐type prenyltransferases
Isoprenoids have diverse structures derived from the coupling of allylic substrates, such as IPP, DMAPP, GPP, FPP, and GGPP. These linear isoprenyl diphosphates are catalyzed through a canonical consecutive head‐to‐tail condensation reaction by prenlytransferases (or prenyl synthase), followed by various modifications, such as transformation, cyclization, and glycosylation, in the biosynthesis of molecules including steroids, carotenoids, 2 , 3 the side chains of respiratory quinones, 5 natural rubber, 14 and glycosyl carrier lipid 6 , 7 (Scheme 1).
On the basis of the stereochemistry of the double bond formation, prenlytransferases are classified as Z and E types, which catalyze the formation of cis and trans double bonds, respectively, through condensation with specific numbers of IPP. 28 , 29 Trans‐prenyltransferases typically share two conserved aspartate‐rich DDXXD (or DXXXD) motifs facing each other on opposite helices of the substrate binding pocket and tend to synthesize short‐ and medium‐chain‐length products ranging from 15 to 50 carbon atoms. 30 , 31 For instance, in octaprenyl pyrophosphate synthase (OPPS), the first aspartate‐rich motif (S1 site) binds with FPP, whereas the second motif (S2 site) binds IPP with Mg2+ ion. The C3–C4 double bond of IPP subsequently attacks FPP at C1 through five consecutive head‐to‐tail condensation reactions to synthesize 40‐carbon long‐chain OPP (Figure 1a,b). 33 This polymer serves as the side‐chain of ubiquinone (or menaquinone) and mediates electron transfer in the respiratory chain. 34 Other well‐known trans‐type enzymes, such as farnesyl diphosphate synthase (FPPS) and geranylgeranyl diphosphate synthase (GGPPS), have been reported to synthesize 15‐carbon FPP and 20‐carbon GGPP, respectively. 12 , 13 Unlike trans‐prenyltransferases, cis‐prenyltransferases do not contain the DDXXD motif and mostly generate ≥C55 long‐chain polymers. A well‐known archetypal example is undecaprenyl diphosphate synthase (UPPS). During the catalysis of head‐to‐tail condensation, FPP binds with Mg2+ ion in the S1 site and with IPP in the S2 site to yield 55‐carbon undecaprenyl diphosphate (UPP), which serves as a lipid carrier in bacterial cell wall biosynthesis (Figure 1d–e). 32 , 35 , 36 , 37 , 38 , 39 , 40 The cis‐ and trans‐prenyltransferases do not share similarity in both primary and tertiary structures, despite the similar catalytic mechanisms.
FIGURE 1.
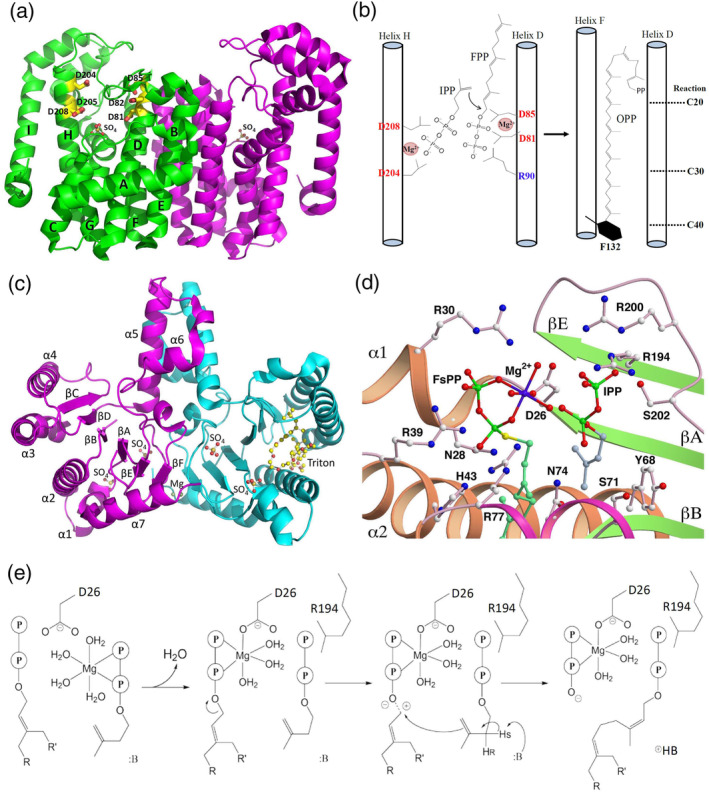
Overall structures of Thermotoga maritima octaprenyl pyrophosphate synthase (OPPS) and Escherichia coli undecaprenyl diphosphate synthase (UPPS). (a) Two identical subunits of OPPS (299 residues) dimerize (green and magenta), and two sulfate ions are in the active site from each subunit. (b) Proposed reaction mechanism and chain length upon OPPS‐mediated catalysis. The bulky hydrophobic residue Phe‐132 located at the bottom of helix F potentially inhibits further chain elongation of OPP and determines the final carbon chain length. (c) UPPS dimerizes (magenta and cyan), and each subunit comprises 253 residues. In the crystal structure, UPPS is in complex with sulfate and magnesium ions and Triton X‐100 molecules, and each monomer has two sulfate groups (termed S1 and S2) bound in the active site. (d) Structural model of UPPS bound with FsPP (FPP analogue) at the S1site and IPP in the S2 site based on the crystal structures of WTF (UPPS + FsPP + IPP) and MTI (D26A mutant + IPP). 32 Reprinted from our previously published data (Reference 32). Originally published in Reference 32. Copyright 2005 American Society for Biochemistry & Molecular Biology. (e) The plausible mechanism of the UPPS reaction
2.2. Homodimeric and heteromeric prenyltransferase
Prenyltransferases are classified as trans‐ and cis‐prenyltransferases. On the basis of the protein composition, trans‐prenyltransferases can be further divided into homodimeric and hetero‐oligomeric enzymes. Over the past decades, the core machinery for trans‐homodimeric enzymes has been identified clearly, and the structural information of several crucial enzyme–substrate complexes has been proposed. In general, two identical subunits are associated to form a dimer with a twofold axis at the interface, and each subunit contains a deep hydrophobic cavity surrounded by helices for the factor and allylic substrate binding, which form longer chain products. The relevant examples include FPPS (15‐carbon), GGPPS (20‐carbon), and OPPS (40‐carbon). 12 , 13 , 34 Notably, Hsieh et al. conducted a series of crystal structures and genetic complementation analyses and proposed that a novel homodimeric polyprenyl pyrophosphate synthase in Arabidopsis (AtPPPS) that can synthesize multiple 25‐carbon (medium‐chain) to 45‐carbon (long‐chain) products. This finding is consistent with the mutagenesis data (I99F/V162F), which concluded that the hydrophobic tunnel can accommodate products >10‐carbon GPP. 41 Therefore, the authors suggested that in Arabidopsis, the precursor GPP for 10‐carbon monoterpene biosynthesis is produced by heteromeric AtPPPS (AtGPPS), 42 but not homomeric AtPPPS. 41
The catalytic mechanism and structural information of hetero‐oligomeric prenyltransferase was not well understood until 2010, when Chang et al. proposed the crystal structure of trans‐heterotetrameric GPPS [(LSU·SSU)2‐type, where LSU = large subunit and SSU = small subunit] extracted from mint (Mentha piperita) and demonstrated that it was involved in menthol biosynthesis in mint glandular trichomes (Figure 2). 43 LSU has high sequence similarity (>50% identity) with homodimeric prenyltransferases as a catalytic subunit, while SSU has less sequence similarity (~15% identity) with other enzymes and lacks the DDXXD (or DXXXD) motif as a noncatalytic subunit. Biochemical and structural analyses have suggested that the LSU serves as a catalytic unit, whereas the SSU may act as a regulatory unit. No activity was detected without the presence of either, probably because the monomer cannot fold a stable structure for enzyme function. 43 Within a similar time frame, Sasaki et al. reported the crystal structure of heterodimeric trans‐hexaprenyl diphosphate synthase (HexPPS) from Micrococcus luteus B‐P 26. This enzyme is composed of an LSU (HexB) and an SSU (HexA) and catalyzes three consecutive condensations of IPP on FPP to produce HexPP (30 carbons). HexB contains two aspartate‐rich motifs responsible for catalysis in substrate condensation, whereas HexA may control the product chain length by using the size of the hydrophobic cavity as a molecular ruler in cooperation with HexB. 44 Another functionally known trans‐heterodimeric enzyme is heptaprenyl diphosphate synthase (HepS and HspT), which catalyzes one FPP and four IPP molecules through a head‐to‐tail condensation reaction to form a 35‐carbon heptaprenyl diphosphate in Bacillus subtilis. 45 However, its structural information remains unclear (see Section 5.2. New subclass: Sesquarterpene cyclase for details).
FIGURE 2.
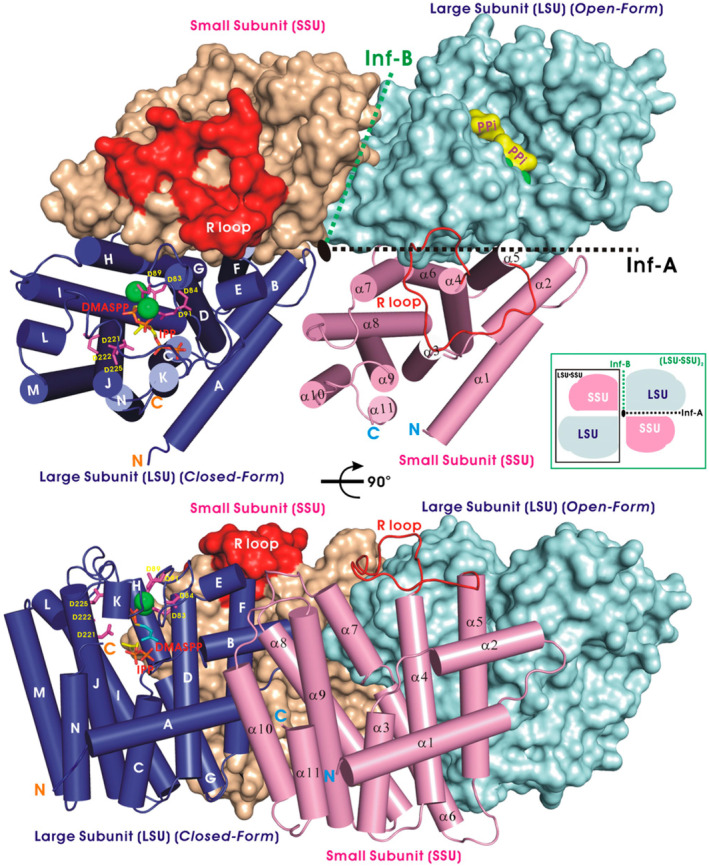
Crystal structure of heterotetrameric geranylgeranyl diphosphate synthase (LSU·SSU)2 from Mentha piperita. LSU subunits are presented as cylinders (blue) and a filled surface (cyan), and SSU subunits are displayed as cylinders (magenta) and a surface model (wheat‐colored). An LSU subunit, a catalytic unit, bound with DMASPP (DMAPP analog) and IPP molecules are shown as sticks and Mg2+ ions (green balls) in the crystal structure. The orientation of the bottom panel is rotated 90° around the horizontal axis relative to the top surface. Reprinted from our previously published data (Reference 43). Originally published in Reference 43. Copyright 2010 American Society of Plant Biologists
Grabinska et al. also classified cis‐prenyltransferase into two subfamilies of homodimeric and heteromeric enzymes. 14 The homodimeric cis‐prenyltransferases are found in bacteria and plants and generally produce short‐ and medium‐chain (10–55‐carbon) prenols, including Z,E‐FPP from Mycobacterium tuberculosis (Rv1086), 46 nerylneryl diphosphate (20‐carbon) from Solanum sp., 47 UPP from various bacteria, 6 , 7 and decaprenyl diphosphate (Z,E‐DecPP, 50‐carbon) from M. tuberculosis. 48 The biological function of Z,E‐DecPP is somewhat similar to that of bacterial UPP, which is essential in bacterial cell wall biosynthesis. By contrast, the heteromeric cis‐prenyltransferases comprise catalytic and noncatalytic subunits and generally synthesize long‐chain (>70‐carbon) prenols. 14 These heteromeric enzymes, such as dehydrodolichyl diphosphate synthase (DHDDS), are typically found in fungi, metazoan, plants, and animals. 14 , 49 Their catalytic subunits have a high similarity with UPPS‐like enzymes, whereas their noncatalytic subunits exhibit sequence similarity with other known cis‐prenyltransferases only in the C‐terminus. In eukaryotic cells, DHDDS plays an essential role in the synthesis of dolichol phosphate (DolP; 55–100‐carbon long), a lipid carrier necessary for protein glycosylation reactions in the ER. 50 Although the physiological functions of several DHDDS enzymes have been investigated for more than a decade, including NgBR/hCIT in mammals, 51 Nus1/Rer2 or Nus1/Srt1 in yeasts, 50 SpNus1/SpRer2 in fungi, 51 SlCPT3/SlCPTBP in tomato plants, 52 and Lew1/At2g17570 in Arabidopsis 53 ; to date, no structure of a heteromeric DHDDS is available. A breakthrough was achieved when Ma et al. recently reported the first crystal structure of Nus1, the noncatalytic subunit of DHDDS from Saccharomyces cerevisiae. 50 The structural modeling of the Nus1/Rer2 heterodimer suggested that the C‐terminus of Nus1 participates in substrate binding through Asn372, a C‐terminal residue. Its equivalent residue in human NgBR and most other cis‐prenyltransferases is arginine in the C‐terminal conserved RXG motif, involved in IPP binding. 50
To date, the core machinery and structural information on several crucial enzyme–substrate complexes of homodimeric cis‐prenyltransferases have been clearly identified, but the molecular machinery responsible for isoprenoid biosynthesis in heteromeric cis‐prenyltransferases is relatively unclear. Therefore, additional studies on the molecular structures, mechanisms, and biological functions of heterodimeric or heteromeric cis‐prenyltransferase are warranted.
2.3. Prenyltransferase inhibition
Isoprenoids comprise a wide variety of natural products that play roles in cell wall synthesis, plasma membrane construction, electron transfer in the respiratory chain, appropriate cellular localization, and signaling activity in bacteria, fungi, and animals, including humans. Therefore, the steps in isoprenoid biosynthesis and metabolism are valuable targets in discovering new inhibitors or drugs. For instance, bacterial UPPS, which generally serves as an antibacterial target, catalyzes consecutive head‐to‐tail condensation reactions to form 55‐carbon UPP, a lipid carrier for bacterial peptidoglycan biosynthesis. 35 , 36 , 37 , 38 , 39 , 40 Chen et al. used farnesyl thiopyrophosphate (FsPP), an FPP analog, as a probe to detect UPPS inhibition on the basis of conformational changes. 54 Guo et al. were the first to propose crystal structures of bacterial UPPS in a complex with certain potent bisphosphonate inhibitors, BPH‐608, −628, −629 and − 676, which exhibited the most active inhibition at an IC50 of 590 nM (Figure 3). 55 Kuo et al. solved the crystal structure of Helicobacter pylori UPPS and found potent inhibition activities against H. pylori UPPS by virtually screening 58,635 compounds, thus indicating the possibility of developing antibiotics specifically targeting pathogenic bacteria rather than other intestinal probiotics. 56 During bacterial cell wall synthesis, UPP is dephosphorylated to undecaprenyl monophosphate as a precursor of lipid‐I and ‐II by membrane integral undecaprenyl pyrophosphate phosphatase (BacA/UPPP). 57 , 58 In a recent breakthrough, the structure and kinetic mechanism of UPP have been determined; thus, it is a promising target for antibiotic development (see Section 8 for details). 59 , 60 , 61 , 62 , 63 For drug discovery and design, however, pharmaceutical scientists should consider that human homologous enzymes, such as DHDDS and dolichyl pyrophosphate phosphatase, have similar catalytic reactions to produce approximately 100‐carbon dolichols for protein glycosylation reactions in the ER. 64
FIGURE 3.
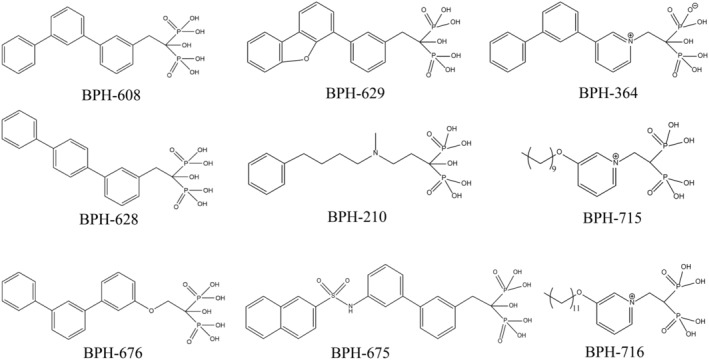
Structures of bisphosphonates investigated as potential head‐to‐tail prenyl synthase inhibitors. BPH‐608, ‐628, ‐629, and ‐676 for bacterial undecaprenyl diphosphate synthase inhibition; BPH‐364, ‐629, ‐675, and ‐210 for yeast geranylgeranyl diphosphate synthase inhibition; BPH‐210 also good for Trypanosoma brucei farnesyl diphosphate synthase inhibition; BPH‐715 and ‐716 for tumor cell growth inhibition
FPPS and GGPPS—the enzymes upstream of farnesyltransferase (FTase) and protein geranylgeranyl transferase (GGTase), respectively—were considered attractive targets for anticancer drugs through the inhibition of subsequent protein Ras or Rab prenylation in cell signaling and survival pathways (see Section 7 for details). Various bisphosphonate inhibitors as cancer chemotherapeutics have been reported to inhibit FPPS and GGPPS. Guo et al. proposed several crystal structures of yeast GGPPS in a complex with potent bisphosphonate inhibitors (BPH‐364, ‐629, and ‐675; Figure 3). Among them, BPH‐364 exhibits the most active inhibition at IC50 = 30 nM and Ki = 10 nM. 55 , 65 Another potent phenylalkyl bisphosphonate, N‐[methyl(4‐phenylbutyl)]‐3‐aminopropyl‐1‐hydroxy‐1,1‐bisphosphonate (BPH‐210), against Trypanosoma brucei FPPS (IC50 of 250 nM and Ki = 21 nM) and yeast GGPPS has been reported. 66 Zhang et al. also designed and modified a series of lipophilic bisphosphonate inhibitors with long‐chain hydrophobic carbon tail (BPH‐715 and ‐716; Figure 3), which exhibited high inhibition activity against FPPS and GGPPS probably due to increased cellular uptake. 67
3. CLASS 2: HEAD‐TO‐HEAD PRENYL SYNTHASE
3.1. Terpenes or isoprenoids synthesis by head‐to‐head prenyl transferases
More than 80,000 identified isoprenoid natural compounds are synthesized using a wide range of prenyl synthases. 1 Among them, one class, so‐called head‐to‐head prenyl transferases, can be found in microbes, fungi, plants, and animals (including humans). These enzymes, including HpnD, 18 CrtM, 68 , 69 and SQS, 70 , 71 mainly catalyze two FPP molecules through a head‐to‐head condensation to form presqualene diphosphate (PSPP), followed by conversion of PSPP to either dehydrosqualene (DHS) or hydroxysqualene (HSQ) and reduction of PSPP to either squalene or botryococcene 18 , 68 , 72 (Figures 4a and S1). These intermediate building blocks are generally involved in the biosynthesis of ergosterol, cholesterol, hopanoids, and staphyloxanthin for maintaining the membrane rigidity and fluidity or act as a virulence factor due to the antioxidant properties in bacteria and eukaryotes (Figure 4a). In plants, two GGPP molecules can be head‐to‐head condensed by phytoene synthases to phytoene, a 40‐carbon intermediate formed in the biosynthesis of plant carotenoids (Figure 4b). 73 Because these enzymes catalyze the committed step of the synthesis of carotenoid or sterol‐like intermediates and are highly conserved across various species, pharmacologists consider their inhibitors as potential therapeutic leads in the drug development against pathogens and for treating hyperlipoproteinemia. 74 , 75 , 76 Over the past few years, the core mechanism of and structural information on several crucial head‐to‐head prenyl transferases have been well established, which provide advanced insights into inhibitor optimization and drug development for further possible therapeutic applications, including cholesterol‐lowering agents and antimicrobial therapies. 77 , 78
FIGURE 4.
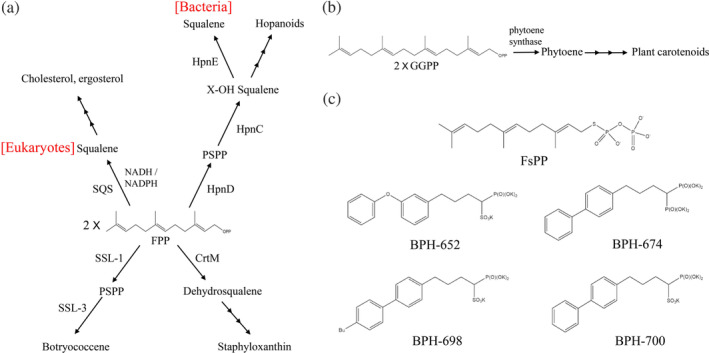
Natural isoprenoids synthesized by head‐to‐head prenyl transferases. (a) Certain crucial head‐to‐head condensation reactions catalyzed by squalene synthase, HpnD, CrtM, and SSL‐1 enzymes in bacteria and eukaryotes. (b) Two geranylgeranyl diphosphate molecules are converted by phytoene synthase to phytoene during plant carotenoid biosynthesis. (c) Structures of bisphosphonates investigated as potential CrtM inhibitors against the virulent pathogen Staphylococcus aureus
3.2. Basic building blocks of PSPP biosynthesis in bacteria and eukaryotes
PSPP, a triterpenyl phosphate, is an essential cyclopropyl intermediate in biosynthetic pathways for eukaryotic botryococcene, sterols, bacterial hopanoids, and the carotenoid pigment staphyloxanthin. PSPP can be simply synthesized through the head‐to‐head condensation of two FPP molecules by various enzymes, such as HpnD (involved in HSQ and hopanoid biosynthesis), CrtM (involved in DHS and staphyloxanthin biosynthesis), SQS (involved in squalene and sterols biosynthesis), and SQS‐like enzymes (SSL‐1, involved in botryococcene biosynthesis). 18 , 68 , 69 , 70 , 71 , 79 , 80 Several elegant studies that conducted crystallographic and biochemical analyses with green algae Botryococcus braunii SSL‐1, 79 S. cerevisiae and human SQS, 70 , 81 , 82 , 83 Staphylococcus aureus CrtM, 68 and Rhodopseudomonas palustris and Zymomonas mobilis HpnD 18 have provided detailed mechanisms underlying the joining of the two FPPs in a head‐to‐head coupling to form PSPP, with subsequent reduction and rearrangement to yield other metabolic products.
Liu et al. 13 determined the crystal structure of CrtM in the virulent pathogen Sta. aureus (SaCrtM; DHS synthase), similar to that of human SQS (HsSQS). 17 Liu et al., therefore, screened numerous SQS inhibitors to inhibit CrtM and thereby block the biosynthesis of staphyloxanthin. Three phosphonosulfonates exhibited a strong inhibition toward CrtM (BPH‐652; Ki = 1.5 nM; BPH‐698, Ki = 135 nM; BPH‐700, Ki = 6 nM), and potent activity against pigment formation of Sta. aureus (median inhibitory concentration = 100–300 nM; Figure 4c). BPH‐674 was a more potent CrtM inhibitor (Ki = 0.2 nM), but with no significant activity in pigment inhibition in vitro, probably because of poor cellular uptake. High‐resolution enzyme structural information in a complex with crucial inhibitors was also proposed. Because staphyloxanthin acts a virulence factor in Sta. aureus, inhibiting its production could result in the production of reactive oxygen species from host neutrophils, significantly hindering bacterial growth. 17 Schwalen et al. also proposed the structural characterization of CrtM and HpnD enzymes from pathogens Enterococcus hirae and Neisseria meningitides, respectively, thus providing valuable information regarding anti‐infective or antimicrobial drug development against pathogenic bacteria. 68 A combination of structural information, mutagenesis, and computational modeling can help elucidate the role of Arg‐15 residue (equivalent to Ser‐19 in CrtM) in stabilizing PSPP formation in HpnD. When CrtM Ser‐19 was substituted with arginine, the rate of DHS production decreased, indicating a distinct mechanistic reactivity between staphyloxanthin and hopanoid biogenesis. 68
3.3. Squalene biosynthesis
Squalene is a metabolic intermediate in the biosynthetic pathway for eukaryotic cholesterol and ergosterol and bacterial hopanoids. This naturally occurring terpenoid hydrocarbon is essential in maintaining membrane rigidity and fluidity. 74 In humans, squalene can be simply synthesized through the head‐to‐head condensation of two FPP molecules by a NADH/NADPH‐dependent SQS. 83 In other eukaryotes, for example, the photosynthetic green algae B. braunii can catalyze two FPPs to form either squalene or botryococcene through 1′−1 or 1′ − 3 linkage by SQS and SSL‐1/‐3, respectively. 79 , 80 Botryococcene is a branched triterpene hydrocarbon and is believed to have potential applications in biofuel production (Figure 5a). By contrast, in bacteria, Z. mobilis and R. palustris have been proposed to produce squalene by using three reactions catalyzed by three enzymes, HpnD, HpnC, and HpnE. This brand new pathway also plays a crucial role in the biosynthesis of hopanoids for enhancing membrane integrity in bacteria. 18 , 84
FIGURE 5.
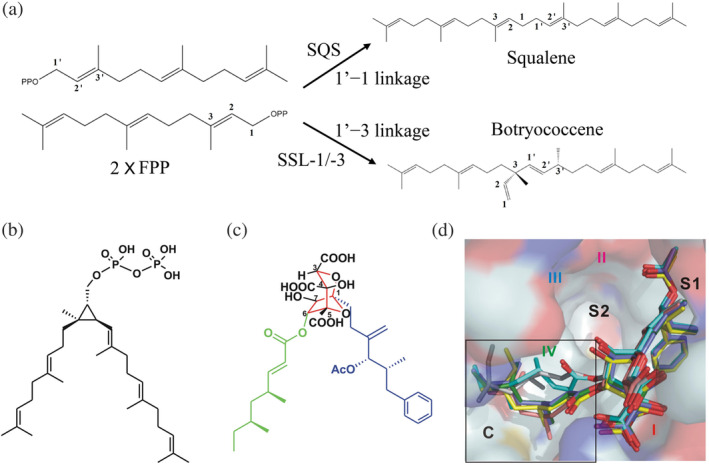
Reactions catalyzed by “head‐to‐head” prenyl transferases. (a) In green algae, photosynthetic Botryococcus braunii can catalyze two farnesyl diphosphate molecules to generate squalene via 1′−1 by squalene synthase (SQS) or form botryococcene via 1′−3 linkage by SSL‐1/‐3 enzymes. Potent inhibition of zaragozic acid A (ZA‐A) on human SQS. Structures of presqualene diphosphate (PSPP) (b) and ZA‐A. ZA (c) is a polyketide natural product produced by fungi and shares partial structural similarity with PSPP. (d) Crystal structures of human SQS in complex with ZA‐A molecule shown as stick models. Reprinted (b–d) from our previously published data (Reference 70). Originally published in Reference 70. Copyright 2012 American Society for Biochemistry & Molecular Biology
Because SQS catalyzes the committed step of sterol synthesis in bacteria and humans, it has attracted considerable attention as a potential target for generating antimicrobial and anticholesterolemic inhibitors. Through a thermodynamics analysis, Liu et al. demonstrated the inhibitory activity of zaragozic acid A (ZA‐A) on human SQS at nano‐ to picomolar concentrations (K d = 18.9 nM). 70 ZA is a polyketide natural product produced by fungi and shares partial structural similarity with PSPP (Figure 5b,c). The molecular mechanism of binding modes of ZA‐A with human SQS suggested that the C‐1 alkyl group of six ZA molecules stays at S1 site, whereas the extensive C site is occupied by the C‐6 acyl group with a distinct binding pattern (Figure 5d). This finding indicates the possibility of developing new drugs and treatments for hypercholesterolemia.
In fungi and some parasitic protozoa, SQS is involved in the ergosterol biosynthesis pathway and was thought to serve as a classic drug target for many infectious diseases. Song et al. recently proposed that ZA (IC50 270 nM), phosphonosulfonate BPH‐652 (IC50 430 nM), and celastrol (IC50 830 nM) have potent inhibition activities against SQS in Aspergillus flavus (AfSQS), the leading cause of human invasive aspergillosis. 71 Celastrol, a natural quinone methide, is a noncompetitive inhibitor of AfSQS and can inhibit the flavin‐dependent monooxygenase siderophore A in the siderophore biosynthesis of the highly virulent A. fumigatus, which could serve as a promising multitarget lead for antifungal medication. 71 In trypanosomatid parasites, SQS has also been proposed as a target for Chagas disease therapeutics because of its essential role in ergosterol biosynthesis. Shang et al. solved the first crystal structure of SQS in Trypanosoma cruzi in a complex with certain potent inhibitors of quinuclidine and lipophilic bisphosphonate and demonstrated potent cell growth inhibition on blocking sterol production. 85
4. CLASS 3: HEAD‐TO‐MIDDLE PRENYL SYNTHASE
4.1. Mechanisms of action of head‐to‐middle condensation
Irregular prenyl synthase, a noncanonical type of UPPS‐fold‐like prenyltransferase, was discovered recently. It catalyzes the head‐to‐middle (i.e., non–head‐to‐tail) condensation reaction of the C2–C3 double bond of the first allylic substrate (located at S2 site) and C1 in a second allylic substrate (located at S1 site), subsequently producing branched mono‐ or sesquiterpenes (Figure 6a). Some well‐known examples are 10‐carbon lavandulyl diphosphate (LPP), catalyzed by lavandulyl diphosphate synthase (LPPS) or Z,Z‐farnesyl diphosphate synthase (zFPS) 86 , 87 ; 10‐carbon cyclolavandulyl diphosphate (CLPP), catalyzed by cyclolavandulyl diphosphate synthase (CLPPS) 88 ; 15‐carbon isosesquilavandulyl diphosphate (ISLPP), catalyzed by isosesquilavandulyl diphosphate synthase (Mcl22) 89 ; and 20‐carbon geranyl lavandulyl diphosphate (GLPP), catalyzed by MA1831, a cis‐prenyltransferase homologue from Methanosarcina acetivorans (Figure 6b–f). 90
FIGURE 6.
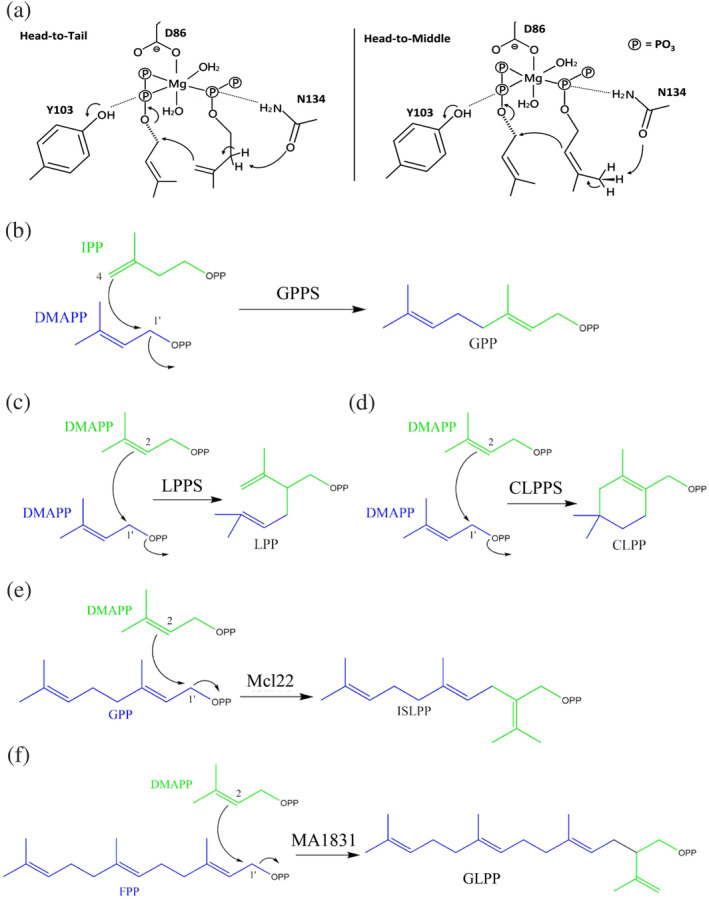
(a) Two proposed mechanisms of the condensation reaction of Z,Z‐farnesyl diphosphate synthase (zFPS) in the tomato species Solanum habrochaites; zFPS can catalyze one dimethylallyl diphosphate (DMAPP) and two isopentenyl diphosphate (IPP) molecules to form a Z,Z‐farnesyl diphosphate (Z,Z‐FPP) via the head‐to‐tail condensation (left panel) and two DMAPP molecules to form an LPP via the head‐to‐middle condensation (right panel). Reprinted from our previously published data (Reference 86). Originally published in Reference 86. Copyright 2017 American Chemical Society. ( https://pubs.acs.org/doi/10.1021/acsomega.6b00562; further permissions related to the material excerpted should be directed to the ACS). Reactions catalyzed by “head‐to‐tail” prenyl transferase as shown in geranylgeranyl diphosphate synthase (GPPS) (b) and by “head‐to‐middle” prenyl transferases, as shown in LPPS, CLPPS, Mcl22, and MA1831 (c–f)
The crystal structure and molecular mechanism of head‐to‐middle LPPS were first reported by Liu et al. in 2016. 87 This irregular prenyl synthase catalyzes the coupling reaction of C2 in the first DMAPP (S2 site) and attacks C1 in the second DMAPP allylic substrate (S1 site) to form the 10‐carbon LPP, a precursor of lavandulol, which is used as a lavender oil fragrance in the perfume industry (Figure 6c). 87 The structural information of its homologous zFPS, with ~39% amino acid sequence identity to LPPS, was proposed by Chan et al. within a similar timeframe. zFPS, discovered from the glandular trichomes of the tomato species Solanum habrochaites, catalyzes two DMAPPs to form a LPP molecule if IPP is absent in the reaction. 86 The overall structure of LPPS is quite similar to those of zFPS and other cis‐prenyl diphosphate synthases, such as UPPS. However, the catalytic residue His‐78 in LPPS, which plays a key role in the head‐to‐middle condensation reaction and facilitates the release of diphosphate from the S1 ligand, is replaced with asparagine in UPPS (Asn‐28) and zFPS (Asn‐88). Replacement of His‐78 with an asparagine residue (H78N) did not result in LPP production in LPPS. 86 However, mutagenesis for the functional analysis of this equivalent residue in either UPPS (Asn‐28) or zFPS (Asn‐88) has not been investigated.
A unique irregular prenyl synthase, CLPPS, catalyzes the head‐to‐middle condensation of two DMAPP molecules, followed by a cyclization reaction to form a branched and cyclic 10‐carbon CLPP (Figure 6d). The molecular mechanism and function of CLPPS have been increasingly studied by organic chemists and biologists because of the ability of CLPPS to consecutively catalyze both condensation and cyclization reactions, which are generally accomplished by two independent enzymes, isoprenyl diphosphate synthase and cyclase (see Section 5 for details), in isoprenoid biosynthesis. Tomita et al. recently solved the crystal structure of CLPPS from the soil bacterium Streptomyces sp. CL190 and indicated a high structural similarity to UPPS‐like proteins. Site‐directed mutagenesis (P8I/F173L mutant) combined with GC/MS analyses suggested that a narrower catalytic pocket is suitable for the approach of two dimethyl moieties of DMAPP for cyclization in CLPPS, whereas a wider catalytic pocket is required for maintaining a longer distance between two dimethyl moieties of DMAPP for condensation in LPPS.
Another irregular prenyl synthase, Mcl22, may be involved in the production of the merochlorin, a meroterpenoid antibiotic produced by the marine bacterium Streptomyces sp. strain CNH‐189. 91 This enzyme catalyzes the head‐to‐middle condensation of DMAPP (S2 site) and GPP (S1 site) to generate a branched 15‐carbon ISLPP (Figure 6e). Although the overall polypeptide fold of each domain in Mcl22 is similar to that of cis‐prenyl transferases, the width of the ligand‐binding pocket (between helices α2 and α3) compared with ligand‐binding pockets of other UPPS enzymes is narrow. 89 This spatial restriction allows the binding of the GPP substrate in a horizontal position at the surface of the binding pocket (PBD ID: 5XK9), unlike other UPPS homologs, whereby the substrate binds (such as FPP, citronellyl diphosphate, and tuberculosinyl diphosphate) in a vertical orientation toward the hydrophobic tunnel during catalysis. This unique spatial control for ligand binding and catalysis may prove to be a suitable strategy in engineering new merochlorin‐class antibiotics. Moreover, MA1831, a homologue of Mcl22, was recently demonstrated to produce 20‐carbon GLPP from one DMAPP and FPP in M. acetivorans (Figure 6f). However, its structure and catalytic mechanism remain unclear. 90 Whether MA1831 produces a molecular machinery similar to that in Mcl22 must be determined.
4.2. MoeN5 prenyltransferase in moenomycin biosynthesis
MoeN5 can catalyze one GPP molecule (S1 site) and the cis‐farnesyl group in the phosphoglycolipid (FPG‐trisaccharide; S2 site) through head‐to‐middle condensation to produce the 25‐carbon moenocinyl‐side‐chain‐containing lipid in the biosynthesis of moenomycin (Figure 7). The crystal structure and reaction mechanism of MoeN5 were recently reported by Zhang et al. 20 MoeN5 forms a dimer both in the solution and in the crystal structure. Unlike the previously mentioned head‐to‐middle enzymes, MoeN5 exhibits a structural similarity to head‐to‐tail trans‐prenyl synthases, such as geranyl transferase and GGPPS, which generally contain two canonical aspartate‐rich domains, DDXXD and DXXXD, coordinating Mg2+ ion for the ionization of allylic substrates. Crystal structures of several ligand‐bound complexes of MoeN5 provide a structural basis for a rational catalytic reaction mechanism, in which the long side‐chain of FPG‐trisaccharide exhibits a unique bent conformation (C6–C11) to attack the C1 of GPP, followed by the formation of intermediate of 5‐ and 6‐membered‐ring carbocations and then the 25‐carbon moenocinyl side‐chain. 20
FIGURE 7.
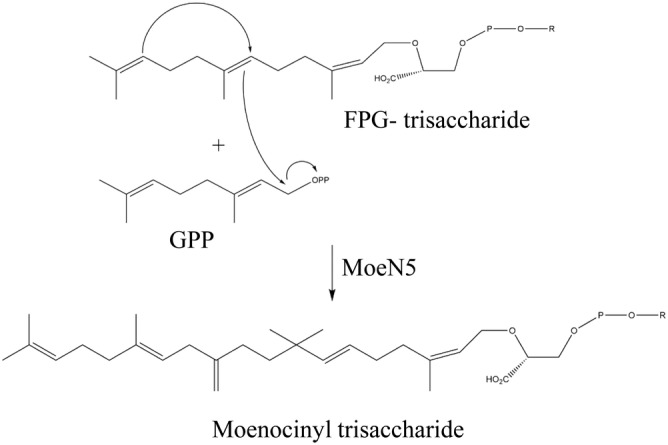
Proposed mechanisms of the head‐to‐middle condensation reaction of MoeN5. R indicates trisaccharide
5. CLASS 4: TERPENOID CYCLASE
5.1. Distinct structures and mechanisms underlying cyclization
Terpene cyclase, a type of prenyltransferase, catalyzes the cyclization of linear isoprenyl pyrophosphates, such as GPP, FPP, and GGPP, into various cyclic isoprenoid compounds. Terpene cyclases include squalene cyclase, pentalenene synthase, 5‐epi‐aristolochene synthase, and trichodiene synthase, responsible for the synthesis of cholesterol, a precursor of the pentalenolactone (a sesquiterpenoid antibiotic), the antifungal phytoalexin capsidiol, and antibiotics and mycotoxins, respectively (Scheme 1). These enzymes are generally divided into two groups, mainly characterized by the distinct α‐helical folds and chemical catalysis mechanisms. Group I terpenoid cyclases are metal‐dependent (Mg2+ or Mn2+) and usually contain two conserved aspartate‐rich DDXX[D/E] and NSE[N/D]DXX[S/T]XX[K/R]E (NSE/DTE) motifs for metal ion binding. These two motifs form a trinuclear metal cluster to initiate the ionization of an isoprenoid diphosphate substrate to an allylic cation, which acts as an electrophile to react with one of the π bonds in the substrate for cyclization. By contrast, group II enzymes contain one DXDD motif in which the central aspartic acid serves as a catalytic general acid for the initiation of protonation of a carbon–carbon double bond in an isoprenoid diphosphate substrate to form a tertiary carbocation for cyclization. 1 , 92 The 3D crystal structures of terpenoid cyclases exhibit considerably different α‐helical folds. The crystal structure typically comprises three domains, namely α, β, and γ, in various combinations. For instance, Taxus brevifolia taxadiene synthase (group I), which synthesizes Taxol for cancer chemotherapy, consists of α, β, and γ domains. 93 5‐epi‐aristolochene synthase (Group I) in tobacco, Nicotiana tabacum, comprises α and β domains. 94 Pentalenene synthase (Group I) in bacterial Streptomyces exfoliatus contains only one α domain. 95 Squalene‐hopene cyclase (group II) in bacterial Alicyclobacillus acidocaldarius comprises β and γ domains. 96 In group I cyclases, the catalytic site is located in the middle of the α domain, whereas in group II cyclases such as squalene‐hopene cyclase, the catalytic site is located in the β–γ domain interface.
Several elegant studies on crystallographic and functional analyses of certain distinct terpene cyclases have provided detailed mechanisms of how a linear prenyl diphosphate molecule undergoes changes in bonding through ionization and rotation during a multistep cyclization reaction to generate various metabolic compounds containing one or more fused rings (Figure 8a–d). In 2016, Chen et al. solved the crystal structure of fusicoccadiene synthase, which can cyclize GGPP to form molecular fusicoccadiene (Figure 8a), the hydrocarbon precursor of fusicoccin‐A applied in cancer chemotherapy. 98 Within a similar time frame, Kries et al. determined the crystal structure of plant iridoid cyclase (Catharanthus roseus), which can catalyze a different substrate, 8‐oxogeranial, into iridodial and nepetalactol (Figure 8b). 99 Morehouse et al. subsequently reported the crystal structure of orange limonene synthase, which catalyzes GPP via a simple cyclization reaction to form molecular limonene, which is used as a fruit oil fragrance (Figure 8c). 100 Finally, Blank et al. also recently solved the crystal structure of cucumene synthase, which can generate a linear triquinane sesquiterpene from molecular FPP, which is useful for its anticancer, antibiotic, and anti‐inflammatory properties (Figure 8d). 101
FIGURE 8.
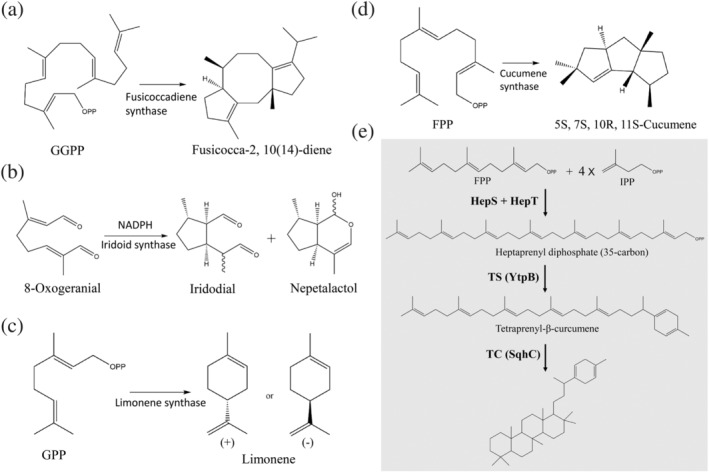
Certain critical cyclization reactions catalyzed by terpene cyclases, as shown in fusicoccadiene synthase (a), iridoid cyclase (b), limonene synthase (c), and cucumene synthase (d). (e) Potential biosynthesis pathway for sesquarterpenes (C35 Terpenes). The heterodimeric heptaprenyl diphosphate synthase (HepS and HepT) converts one farnesyl diphosphate and four isopentenyl diphosphate molecules via a head‐to‐tail condensation reaction to form heptaprenyl diphosphate. This reaction is subsequently followed by cyclization by YtpB and SqhC enzymes, generating one ring at one end of a 35‐carbon chain of heptaprenyl diphosphate and 35‐carbon terpenol with a polycyclic skeleton, respectively. Reproduced from Reference 97. Originally published in Reference 97. Copyright 2011 American Chemical Society
5.2. New subclass: Sesquarterpene cyclase
Despite the rapid progress in understanding the biological function and synthetic mechanism of a large diverse group of terpenoid natural products, little is known regarding the 35‐carbon terpene. Only 19 cyclic and 8 linear 35‐carbon terpenes have been identified to date. Sato et al. suggested a brand new biosynthesis pathway of mono‐ and pentacyclic C35 terpenes by two unique terpene cyclases, namely tetraprenyl‐β‐curcumene synthase (YtpB) and tetraprenyl‐β‐curcumene cyclase (SqhC), in B. subtilis and Mycobacterium species. 97 The mono‐ and pentacyclic terpene compounds are derived from the 35‐carbon heptaprenyl diphosphate, which was synthesized by one FPP and four IPP molecules through head‐to‐tail condensation by heterodimeric enzymes HepS and HepT (Figure 8e). 45 YtpB can catalyze the ionization and cyclization of heptaprenyl diphosphate to form a ring at one end of the 35‐carbon chain, but with no amino acid sequence homology to any known terpenoid synthase. Moreover, YtpB does not contain the canonical DDXX(D,E) or NSE/DTE metal‐binding motifs typically found in group I terpenoid synthases. 97 Although the structural information of YtpB in terpene cyclization is currently unknown, Fujihashi et al. recently proposed the crystal structure of BalTS, an ortholog of YtpB from B. alcalophilus. Interestingly, the overall structure of BalTS is similar to that of group I terpene synthases, but it consists of two novel aspartate‐rich motifs, DYLDNLxD and DY(F,L,W)IDxxED, which forms the cyclic skeleton. 102 More recently, Stepanova et al. also determined the crystal structure of BalTS in a complex with a substrate surrogate and elucidated the catalytic mechanisms in unusual large terpene synthesis and in drug design applications. On the basis of these findings, the authors designated 35‐carbon terpene as a new family named sesquarterpenes. 103
6. CLASS 5: AROMATIC PRENYLTRANSFERASE
6.1. Classification
Aromatic prenyltransferases catalyze the transfer of isoprenoid diphosphates to aromatic compounds, a reaction corresponding to Friedel–Crafts alkylation. This essential prenylation of aromatic compounds in biological systems generates a wide diversity of primary and secondary metabolites, which play critical roles in bacteria, fungi, and plants. This superfamily is generally divided into three groups, characterized by its secondary metabolisms and localization of biological activities 1 : cytosolic ABBA (α‐β‐β‐α barrel) type, 2 dimethylallyltryptophan synthase (DMATS) type, and 3) membrane‐embedded UbiA type. 23 , 104 , 105 , 106
6.2. Cytosolic ABBA prenyltransferase
NphB (formerly named Orf2) was the first proposed ABBA‐type prenyltransferase, involved in the biosynthesis of the antioxidant naphterpin in Streptomyces sp. (strain CL190). 107 NphB has been reported to possess high tolerance to aromatic substrates, including resorcinols, flavonoids, and dihydroxynaphthalenes, that interact with GPP to generate various O‐ or C‐prenylated aromatic products. The 3D crystal structure of NphB exhibits a 10‐stranded antiparallel β‐barrel fold and consists of five repetitive αββα elements. Its catalytic site is located in the middle of the β‐barrel fold and contains an aspartate‐rich metal‐binding motif (NDxxD). NphB catalyzes the isoprenyl diphosphate substrate by using the Mg2+ ion. 107 By contrast, some ABBA‐type enzymes, such as CloQ and DMATS, do not require Mg2+ to initiate the ionization of prenyl diphosphate substrate. 22 CloQ, originally isolated from Str. roseochromogenes, is involved in the biosynthesis of clorobiocin, an aminocoumarin antibacterial that inhibits DNA gyrase. A primary and tertiary structural analysis revealed that CloQ does not contain the NDxxD motif for metal ion binding and remains active in the absence of any divalent cations. The reaction mechanism may be based on the hydrogen bond interactions for substrate ionization. 22 DMATS is essential in the biosynthesis of ergot alkaloids, originally isolated from the ergot fungus Claviceps purpurea, which are secondary metabolites with a high value in regulatory toxicology and pharmacology. 108 DMATS shows no amino acid sequence homology to NphB, CloQ, or orthologs but shares a similar conformation of β‐barrel fold with the ABBA‐type superfamily according to a number of solved crystal structures (PDBID: 3I4X; 3O2K; 6OS3). 22 Recent studies on the crystal structure of the prenyltransferases AmbP1 and AmbP3 have indicated that these prenyltransferases can catalyze the 5‐ or 10‐carbon intermediate prenylation of hapalindoles. 109 , 110 AmbP1 catalyzes GPP to either C‐2 or C‐3 of cis‐indolylvinyl isonitrile in the presence or absence of Mg2+, whereas AmbP3 can transfer DMAPP on C‐2 of the hapalindole compound in a reverse manner. Structural and biochemical data clearly indicated that both enzymes exhibit flexible selectivity in hapalindole biosynthesis, which could expand our understanding of the enzymology for new bioactive compound synthesis. 104
6.3. Membrane‐embedded UbiA prenyltransferase
In contrast to cytosolic ABBA prenyltransferases, enzymes belonging to the UbiA superfamily are integral membrane proteins. They catalyze a key biosynthetic step in the production of lipoquinones, including ubiquinone, menaquinone, vitamin E, heme, and other prenylated aromatic compounds (Scheme 1). These natural products play essential roles in important processes such as oxidative phosphorylation, photosynthesis, and antioxidation in all living organisms. Li reviewed a similarity analysis of the sequence and function of the UbiA prenyltransferase superfamily, including COX10, COQ2, DPPR synthase, Archaeal UibA homologue, DGGGP synthase, ChlorophyII synthase, UbiAD1, and MenA. 105 Studies on the crystal structure of archaeal UbiA homologs, Aeropyrum pernix (ApUbiA) and Achaeoglobus fulgidus (AfUbiA), have clearly demonstrated the conformation of two aspartate‐rich motifs, NDXXDXXXD and DXXXD, in complexes with GPP or DMAPP and Mg2+ ion. The conformation of the catalytic site is similar to the corresponding metal‐binding motifs of farnesyl diphosphate synthase and group I terpenoid cyclases. 111 The structural and biochemical analyses provide new insights into the molecular basis of the enzyme specificity for aromatic substrates in membrane bilayers.
7. CLASS 6: PROTEIN PRENYLATION
7.1. Prenylation of farnesyl and geranylgeranyl isoprenoids
Over the past decades, posttranslational modification has been extensively investigated because of its significance in proper cellular processes and expanding the diversity of protein functions. Posttranslational modification processes, including glycosylation, methylation, acetylation, phosphorylation, and sumoylation, play a crucial role in regulating biological processes such as protein trafficking and cell growth and division in all eukaryotic cells. Protein prenylation, a posttranslational modification, forms an irreversible covalent bond between the protein and either a farnesyl or a geranylgeranyl isoprenoid compound. Three prenyltransferase enzymes are involved in this catalytic reaction: (1) FTase, (2) GGTase type 1 (GGTase‐I), and (3) GGTase type 2 (GGTase‐II or Rab GGTase; Figure 9a,b). 24 , 112
FIGURE 9.
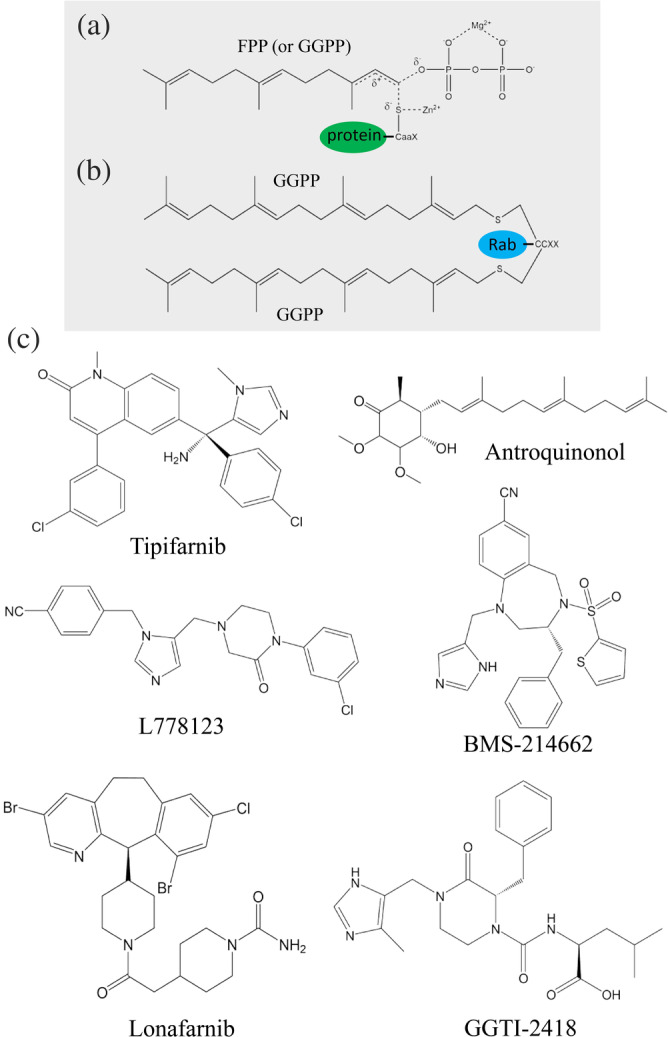
Structures of farnesylated or geranylgeranylated Ras protein and geranylgeranylated Rab protein. (a) FTase or GGTase‐I, a Zn2+‐dependent prenyltransferase, catalyzes the transfer of farnesyl diphosphate or geranylgeranyl diphosphate (GGPP) to a conserved cysteine residue in a CaaX motif of Ras protein. (b) Two GGPP molecules can be transferred by GGTase‐II to two conserved cysteine residues in the consensus CCXX region located at the C‐terminus of Rab protein. (c) Structures of six protein prenylation inhibitors investigated in clinical trials
Prenylation of Ras protein is initiated by the attachment of a 15‐carbon FPP (by FTase) or a 20‐carbon GPP (by GGTase‐I) to a conserved cysteine residue located at a C‐terminal consensus region commonly known as CaaX box. Next, the –aaX tripeptide of protein C‐terminus is removed by an endoprotease termed Ras‐converting CaaX endopeptidase 1, followed by isoprenylcysteine carboxylmethyltransferase, which catalyzes the methylation reaction of the modified cysteine residue (Figure 9a). 112 , 113 However, GGTase‐II is quite different from FTase and GGTase‐I in that it requires an additional Rab escort protein for prenylation activity. GGTase‐II generally catalyzes a single subfamily of Ras‐related small GTPase, which belongs to the Rab protein family involved in the regulation of intracellular vesicular transport in the biosynthetic secretory and exocytic/ endocytic pathways. 114 , 115 , 116 Basically, two GGPP molecules can be transferred by GGTase‐II to two conserved cysteine residues in the consensus region CXC or CCXX located at the C‐terminus of Rab proteins (Figure 9b). Finally, prenylation produces modified proteins with a hydrophobic C‐terminus for further plasma membrane association.
7.2. Structural and functional analyses
FTase and GGTase enzymes make crucial contributions to the posttranslational modification of Ras or Rab proteins. FTase, GGTase‐I, and GGTase‐II are Zn2+‐dependent prenyltransferases and contain one α and one β domain to form a heterodimer. The primary and tertiary structural analyses revealed that FTase and GGTase‐I share an identical α‐subunit, with a sequence identity of approximately 22% with the α‐subunit of GGTase‐II. By contrast, the β‐subunit of FTase shares only approximately 28% and 32% identity with GGTase‐I and GGTase‐II, respectively. 24 , 117 , 118 The predicted reaction mechanism of prenylation is shown in Figure 9a,b. The Zn2+‐activated thiolate of cysteine residue could act as a nucleophile and attack the ionized farnesyl or geranylgeranyl isoprenoid compound. Crystal structures of FTase and GGTase‐I in a complex with FPP, GGPP, or peptide substrates were reported in 2000 and 2003, respectively. 117 , 119 Both α and β domains are largely composed of helices, and a Zn2+ ion and FPP (or GGPP)‐binding site are located at the center of the barrel surrounded by certain hydrophobic amino acids. 25 Notably, although the overall structures of FTase and GGTase‐II are quite similar, GGTase‐II has a deeper hydrophobic cavity than FTase for accommodating a longer GGPP isoprenoid. 120 FPP may serve as a more effective substrate than GGPP even though GGPP binds competitively with FPP to FTase. A rational explanation is that the diphosphate moiety of the 15‐carbon FPP is closer to the catalytic Zn2+ ion than that of the 20‐carbon GGPP, which lies within the hydrophobic funnel of FTase. This conclusion also explains the reason that FPP binds to GGTase‐I with 330‐fold lower affinity than GGPP, but still serves as a moderately effective substrate to GGTase‐I during the catalytic reaction. 121 The Rab prenylation of two GGPP molecules by GGTase‐II is more complex because it involves the participation of the Rab escort protein. The superimposition of the FTase, GGTase‐I, and GGTase‐II structures suggested that GGTase‐II has a more extended binding pocket for the target substrates, and residues in this region are not conserved, reflecting the peptide substrate specificity. 116 , 122
7.3. Biotechnological and therapeutic applications
Studies have suggested that farnesylation and geranylgeranylation of FTase, GGTase‐I, and GGTase‐II are involved in various clinical conditions such as progeria, infectious diseases, and aging. 123 , 124 Among these, cancer remains the leading cause of mortality worldwide. 125 , 126 , 127 , 128 Therefore, the inhibition of prenylation (prenyltransferase) has provided a new alternative for treating cancer or oncogenic activity. Over the past few years, numerous analogs, including natural isoprenoids, have been synthesized or extracted and subsequently used in investigations into various aspects of the prenylation reaction including its mechanisms, protein structures, enzyme kinetics, and prenylated proteomes. Moreover, some of these inhibitors have been assessed in clinical trials. 24 , 128 , 129 , 130 , 131 , 132 Wu et al. extracted natural terpenoid antroquinonols from Antrodia camphorate, 133 which may inhibit the activity of FTase and GGTase in cancer cells; in turn, this may inhibit the function of Ras and Ras‐related GTP‐binding proteins. Thus far, antroquinonol has been assessed in phase II clinical trials investigating its efficacy against several types of cancer (Figure 9c, Tables 1 and 2). 134 Another natural cyclic monoterpenoid, limonene, a major component of fruit‐derived essential oils, is a flavoring agent in food manufacturing. Research on its D‐form isomer, d‐limonene, has progressed to phase one clinical studies for breast, parotid, and submandibular gland cancers (Figure 8c, Tables 1 and 2). 135 Other well‐known FTase and GGTase inhibitors with various skeletons, including imidazoles, thiazoles, polycyclics, and pyridines, have been developed and assessed. For example, tipifarnib, lonafarnib, BMS‐214662, L778123, and GGTI‐2418 (PTX‐100) are presently being clinically investigated to determine their roles in treating on various types of tumors and other human diseases including hepatitis, Hutchinson–Gilford syndrome, and neurodegenerative diseases (Figure 9c, Tables 1 and 2).
TABLE 1.
A summary of clinical trials for protein prenylation inhibitors
| Clinical trials | |||
|---|---|---|---|
| Compound | Inhibition | Phase | Application in medicine |
| Tipifarnib | FTase inhibitor | II | Atypical chronic myeloid leukemia, BCR‐ABL1 |
| II | Chronic myelogenous leukemia, BCR‐ABL1 | ||
| I | Adult glioblastoma | ||
| II | Adult acute Monoblastic leukemia | ||
| II | Adult acute myeloid leukemia | ||
| II | Adult acute Monocytic leukemia | ||
| I/II | Untreated childhood brain stem glioma | ||
| II | Anaplastic large cell lymphoma | ||
| II | Estrogen receptor‐positive breast cancer | ||
| II | Recurrent melanoma | ||
| II | Neurofibroma, plexiform | ||
| II | Non‐small cell lung cancer | ||
| II | Bladder cancer | ||
| II | Pancreatic cancer | ||
| Antroquinonol | FTase inhibitor | II | Chronic hepatitis B |
| I/II | Pancreatic neoplasm | ||
| II | Non‐small cell lung cancer | ||
| II | Atopic dermatitis | ||
| II | Hyperlipidemias | ||
| d‐limonene | FTase inhibitor | I | Breast cancer |
| I | Parotid gland tumor | ||
| I | Submandibular gland tumor | ||
| L‐778123 | FTase inhibitor | I | Head and neck cancer |
| I | Lymphoma | ||
| BMS‐214662 | FTase inhibitor | I | Myelogenous leukemia |
| I | Myelodysplastic syndromes | ||
| Lonafarnib | FTase inhibitor | II | Chronic hepatitis D |
| II | Hutchinson‐Gilford syndrome (progeria) | ||
| I | Chronic myelogenous leukemia | ||
| I | Brain and central nervous system tumors | ||
| II | Epithelial ovarian cancer | ||
| I | Gliosarcoma | ||
| GGTI‐2418 | GGTase I inhibitor | I | Advanced cancer |
| (PTX‐100) | |||
Note: Data obtained from https://clinicaltrials.gov/.
TABLE 2.
Therapeutic and industrial applications of well‐known natural terpenoid compounds
| Natural compound | Source | Application | Reference |
|---|---|---|---|
| Antroquinonol | Antrodia camphorata | Chronic hepatitis B, cancer, atopic dermatitis | a ,134 |
| d‐limonene | Plant essential oils | Breast cancer, flavoring agent | a ,135 |
| Xanthohumol | Humulus lupulus | Metabolic syndrome, oxidative stress | a |
| Squalene | Plants and animals | Virus diseases, respiratory tract diseases | a |
| β‐carotene | Carrot, tomato | Vitamin A source, antioxidant activities | a |
| Fusidic acid | Fusidium coccineum | Antibiotic activity | 136 |
| Manoalide | Luffariella variabilis | Antiinflammatory, antibiotic activity | 137 |
| Artemisinin | Artemisia annua | Antimalaria | a |
| Coenzyme Q10 | Legumes | Inflammation, obesity | a |
| Menthol | Mentha piperita | Antibacterial | 138 |
| Ingenol mebutate | Euphorbia peplus | Actinic keratosis (LEO pharma trade name Picato) | 139 |
| Icariin | Epimedium | Osteoporosis, dipolar disorder | a |
| Cannabidiol | Cannabis sativa | Analgesic against spasms and asthma | 131, 140 |
| Taxol | Taxis brevifolia | Cancer therapy | a |
| α‐Santonin | Artemisia maritima | Anthelmintic | 141 |
| Bisabolol | Matricaria chamomilla | Chronic insomnia, cosmetic products | a ,142 |
| Sclareol | Salvia sclarea | Fragrant chemical compound, leukemia | 143 |
| Carveol | Seeds of caraway | Flavor additive in the food industry | 144 |
| Lavandulol | Lavandula | Flavor and fragrance materials | 87 |
| Forskolin | Plectranthus barbatus | Weight loss | 145 |
| Cucurbitacin | Cucurbitaceae sp. | Otitis media with effusion | a |
| Moenomycin A | Streptomyces | Antibiotic activity | 19 |
Data obtained from https://clinicaltrials.gov/.
A recent study on crystal structures of FTase from A. fumigatus, a human pathogen, made great contributions to the fields of human health and agriculture. Mabanglo et al. proposed that A. fumigatus FTase (AfFTase) exhibited some structural differences from the human FTase. 146 AfFTase exhibited a significant wider substrate binding and product exit groove than that of human; thus, AfFTase provides the potential for developing selective antifungal drugs. The IC50 measurement indicated that Tipifarnib (an anticancer agent) preferentially inhibits human FTase, whereas the ethylenediamine scaffold inhibitor ED5 strongly inhibits AfFTase alone. A 3D structural analysis also revealed that AfFTase has a wider binding pocket to accommodate the larger inhibitor (ED5) in the presence of FPP. 146 However, similar results were not deduced from the crystal structures of human FTase.
8. UNDECAPRENYL PYROPHOSPHATE PHOSPHATASE
8.1. Bacterial cell wall synthesis
The lipid 55‐carbon undecaprenyl pyrophosphate (UPP) is synthesized by UPPS through a consecutive head‐to‐tail condensation reaction of eight IPP and one FPP molecules in the cytoplasm. 32 , 35 , 36 , 37 , 38 , 39 , 40 Thereafter, UPP is dephosphorylated to undecaprenyl phosphate (UP) by an integral membrane protein, undecaprenyl pyrophosphate phosphatase (BacA/UPPP), through a de novo synthesis pathway (Figure 10). 57 , 58 During bacterial cell wall synthesis, UP serves as a carrier lipid for the translocation of the oligosaccharide precursors (lipid II) across the cell membranes via identified flippases (MurJ, RodA, and FtsW) to the periplasm for peptidoglycan assembly. 147 , 148 , 149 Therefore, this essential step in UP metabolism is a potentially viable target when screening for new antibiotics (Figure 10). A recent landmark study determined the crystal structures and kinetics of UPPP, providing valuable insights into the mechanism underlying the enzyme–substrate interaction in membrane bilayers. 59 , 60 , 61 , 62 , 63 However, limited information is available regarding the mechanism underlying the flip of UP molecules back to the cytoplasmic site in the recycling pathway. Figure 10 displays the detailed steps involved in polymerization during bacterial peptidoglycan synthesis and summarizes a generally accepted model of carrier lipid biosynthesis in de novo and recycling pathways.
FIGURE 10.
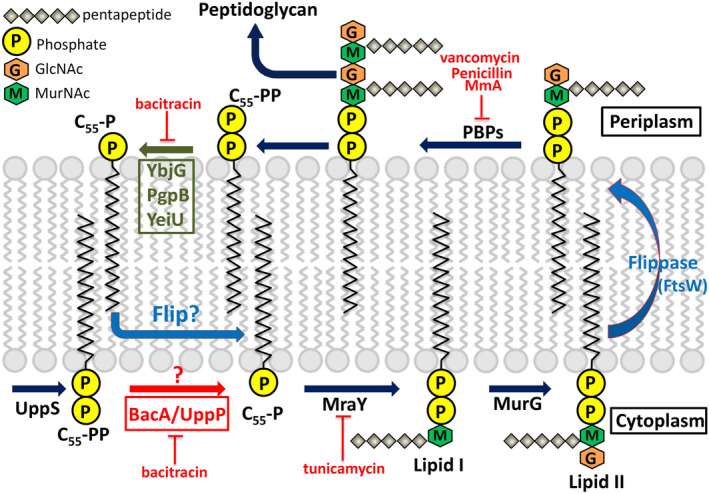
Peptidoglycan biosynthesis in Escherichia coli. The undecaprenyl pyrophosphate synthase (UPPS) synthesizes undecaprenyl pyrophosphate (UPP), which is dephosphorylated to undecaprenyl phosphate (UP) by membrane phosphatase BacA/UPPP. The sugar pentapeptides are then transferred to UP by enzymes MraY and MurG for producing lipid I and lipid II, respectively. Lipid II is then translocated to the periplasm via flippase (MurJ, RodA, and FtsW) for peptidoglycan assembly by the penicillin‐binding proteins (PBPs). Finally, UP is flipped back to the cytoplasmic side via an unknown mechanism in the recycling pathway (blue question mark). Certain cell‐wall‐active antibiotics, including bacitracin, tunicamycin, and vancomycin, are also presented. Reprinted from our previously published data (Reference 59). Originally published in Reference 59. Copyright 2014 American Society for Biochemistry & Molecular Biology
8.2. Structural and functional analyses of UPPP
The X‐ray crystal structure of Escherichia coli UPPP has been recently reported by Ghachi et al. and Workman et al. in the same time frame. 62 , 63 All crystals were obtained through the same method of lipid cubic phase crystallization. UPPP exhibits a dimeric architecture in crystal packing and chemical cross‐linking using disuccinimidyl suberate or succinimidyl succinate. This integral membrane enzyme comprises 10 membrane‐embedded α‐helices, which form two domains with a twofold pseudosymmetrical axis in the plane of the cell membrane (Figure 11a). Six of these are full‐span transmembrane helices (H3–5 and H8–10), and the remaining four form two antiparallel inverted reentrant helices (H1–H2 and H6–H7), which comprise the active site containing two conserved regions (residues 17–30 and residues 170–178) embedded at the membrane midplane (Figure 11b). 62 , 63 A topological comparison between UPPP and flippase MurJ reveals that several structural characteristics are shared, including the unique interlocked inverted repeats. This unexpected finding raises the question whether UPPP has any flippase activity during UP recycling back to the cytoplasm. The mechanism underlying these processes remains obscure. Structural and mutagenesis studies have reported that the catalytic event likely begins by serine (Ser‐27) initiating a nucleophilic attack on the phosphorylated center to form a phosphoserine intermediate. Thereafter, a water ion may initiate a second nucleophilic attack on the phosphate ion of the phosphoserine intermediate. Arg‐174 may form a hydrogen bond with the OH group of the pyrophosphate moiety to stabilize the electrophilic phosphocenter during hydrolysis. Glu‐17 and Glu‐21 residues may stabilize the pyrophosphate moiety of UPP through a magnesium or calcium ion, and His‐30 is likely to play a structural role in maintaining stability between H2 and H10 residues. 59 , 62 , 63 , 148 150
FIGURE 11.
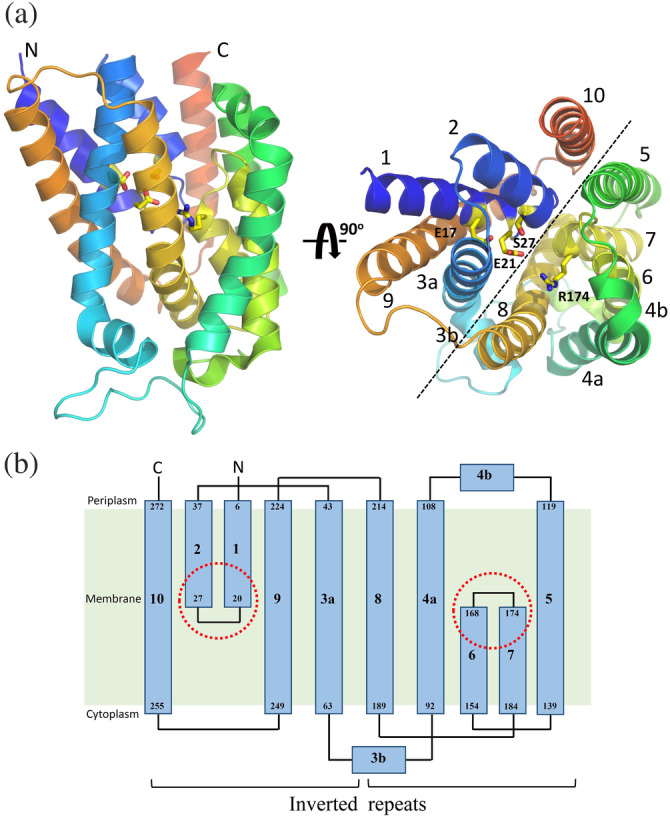
Crystal structure and topology of Escherichia coli undecaprenyl pyrophosphate phosphatase (UPPP). (a) Overall structure of E. coli UPPP. Structural data are available from the PDB file: 5OON or 6CB2. 62 , 63 Ten α‐helices, labeled 1–10, are displayed as a helical cartoon and colored rainbow from the N‐ (blue) to the C‐terminal (red). The orientation of the panel on the right is rotated 90° along the horizontal axis relative to that on the left. The catalytic site residues, Glu‐17, Glu‐21, Ser‐27, and Arg‐174 are presented in yellow and displayed as a stick model. The black dotted line denotes the unique twofold pseudosymmetry axis parallel to the membrane plane. (B) The 10 transmembrane helices are displayed in the two‐dimensional topology of E. coli UPPP to highlight the interlocked inverted repeats. The red dotted circles denote two conserved regions (residues 17–30 and residues 170–178) embedded at the membrane midplane
9. CONCLUSION
In this review, we compared the molecular structures, catalytic mechanisms, and potent inhibitors of six primary classes of prenyltransferases involved in numerous crucial steps of isoprenoid biosynthesis. The critical role of isoprenoid synthases and the requirement of their exact specificity for distinct catalytic processes indicate a clear mechanistic association between the biogenesis of various natural isoprenoids and normal growth, development, and metabolism of all living organisms. More than 80,000 isoprenoid compounds have been structurally and chemically characterized; however, only a few prenyltransferases have been reported. Therefore, future studies are required to identify new enzyme functions and structures and elucidate the synthetic gene clusters involved in isoprenoid biosynthesis pathways. To identify useful natural products, numerous isoprenoids are of significant commercial value owing to their broad‐spectrum clinical and industrial applications (Tables 1 and 2). 19 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 150 At present, the screening, isolation, and identification of new forms of natural compounds with pharmacological properties have gained increasing attention in academia and industry. In recent preclinical and clinical studies, the number of newly identified natural products with therapeutic efficacy in humans against diseases such as cancer, hepatitis, osteoporosis, and respiratory tract diseases has rapidly increased. Moreover, active inhibitors and their derivatives with fewer side effects extracted from natural materials warrant further investigation as a new class of low‐toxicity drugs. These materials may provide novel alternatives for cancer treatment (and treatment of other human diseases) because numerous current therapies for patients with cancers tend to produce strong toxic effects and result in chemotherapeutic resistance. Our results further the current understanding of the formation of these highly diverse carbon‐based materials, which are potentially applicable in the generation of new drugs and food supplements.
CONFLICT OF INTEREST
The authors declare no conflicts of interest with the contents of this article.
AUTHOR CONTRIBUTIONS
Hsin‐Yang Chang and Andrew H.‐J. Wang organized and wrote the manuscript. Hsin‐Yang Chang and Tien‐Hsing Cheng designed the tables and figures.
Supporting information
Data S1: Supporting Information
ACKNOWLEDGMENTS
This work was supported by grants (MOST 108‐2311‐B‐110‐003 and 109‐2311‐B‐110 ‐001 ‐MY3 to Hsin‐Yang Chang) from the Ministry of Science Technology, the Taiwan Protein Project (Grant No. AS‐KPQ‐109‐TPP2), and the Program for Translational Innovation of Biopharmaceutical Development–Technology Supporting Platform Axis (Grant No. AS‐KPQ‐106‐TSPA).
Chang H‐Y, Cheng T‐H, Wang AH‐J. Structure, catalysis, and inhibition mechanism of prenyltransferase. IUBMB Life. 2021;73:40–63. 10.1002/iub.2418
Funding information Ministry of Science and Technology, Grant/Award Number: 109‐2311‐B‐110 ‐001 ‐MY3; Taiwan Protein Project, Grant/Award Number: AS‐KPQ‐109‐TPP2; Translational Innovation of Biopharmaceutical Development‐Technology Supporting Platform Axis, Grant/Award Number: AS‐KPQ‐106‐TSPA
Contributor Information
Hsin‐Yang Chang, Email: hychang7@ym.edu.tw.
Andrew H.‐J. Wang, Email: ahjwang@gate.sinica.edu.tw.
REFERENCES
- 1. Christianson DW. Structural and chemical biology of terpenoid cyclases. Chem Rev. 2017;117:11570–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spurgeon S. Biosynthesis of isoprenoid compounds. New York: John Wiley and Sons, 1981. [Google Scholar]
- 3. Poulter C, Rilling HC. Conversion of farnesyl pyrophosphate to squalene In J. W. Porter and S. L. Spurgeon (ed.), Biosynthesis of isoprenoid compounds, New York, NY: 1981; p. 413–441. [Google Scholar]
- 4. Zhou W, Guo D. Chemical biology of sterols, triterpenoids and other natural products: A themed issue in honor of professor W. David Nes on the occasion of his 65th birthday, MDPI; 2019.
- 5. Bamba T, Fukusaki E i, Kajivama S i, Ute K, Kitayama T, Kobayashi A. The occurrence of geometric polyprenol isomers in the rubber‐producing plant, Eucommia ulmoides oliver. Lipids. 2001;36:727–732. [DOI] [PubMed] [Google Scholar]
- 6. Allen CM. Purification and characterization of undecaprenyl pyrophosphate synthetase. Meth Enzymol. 1985;110: 281–299. [DOI] [PubMed] [Google Scholar]
- 7. Robyt JF. Essentials of carbohydrate chemistry. New York, NY: United States Springer Science & Business Media, 2012. [Google Scholar]
- 8. Eichler J, Imperiali B. Stereochemical divergence of polyprenol phosphate glycosyltransferases. Trends Biochem Sci. 2018;43:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloch KJS. Sterol molecule: Structure, biosynthesis, and function. Steroids. 1992;57:378–383. [DOI] [PubMed] [Google Scholar]
- 10. Rohmer M. The discovery of a mevalonate‐independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. [DOI] [PubMed] [Google Scholar]
- 11. Burke CC, Wildung MR, Croteau R. Geranyl diphosphate synthase: Cloning, expression, and characterization of this prenyltransferase as a heterodimer. Proc Natl Acad Sci U S A. 1999;96:13062–13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohnuma S i, Suzuki M, Nishino T. Archaebacterial ether‐linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl‐diphosphate synthase. J Biol Chem. 1994;269:14792–14797. [PubMed] [Google Scholar]
- 13. Tachibana A, Yano Y, Otani S, Nomura N, Sako Y, Taniguchi M. Novel prenyltransferase gene encoding farnesylgeranyl diphosphate synthase from a hyperthermophilic archaeon, Aeropyrum pernix: Molecular evolution with alteration in product specificity. Eur J Biochem. 2000;267:321–328. [DOI] [PubMed] [Google Scholar]
- 14. Grabińska KA, Park EJ, Sessa W. cis‐Prenyltransferase: New insights into protein glycosylation, rubber synthesis, and human diseases. J Biol Chem. 2016;291:18582–18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang T‐H, Guo R‐T, Ko T‐P, Wang AH‐J, Liang P‐H. Crystal structure of type‐III geranylgeranyl pyrophosphate synthase from Saccharomyces cerevisiae and the mechanism of product chain length determination. J Biol Chem. 2006;281:14991–15000. [DOI] [PubMed] [Google Scholar]
- 16. Lo C‐H, Chang Y‐H, Wright JD, et al. Combined experimental and theoretical study of long‐range interactions modulating dimerization and activity of yeast geranylgeranyl diphosphate synthase. J Am Chem Soc. 2009;131:4051–4062. [DOI] [PubMed] [Google Scholar]
- 17. Liu C‐I, Liu GY, Song Y, et al. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319:1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan J‐J, Solbiati JO, Ramamoorthy G, et al. Biosynthesis of squalene from farnesyl diphosphate in bacteria: Three steps catalyzed by three enzymes. ACS Cent Sci. 2015;1:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huber G, Nesemann G. Moenomycin, an inhibitor of cell wall synthesis. Biochem Biophys Res Commun. 1968;30:7–13. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Chen CC, Ko T‐P, et al. Moenomycin biosynthesis: Structure and mechanism of action of the prenyltransferase MoeN5. Angew Chem Int Ed Engl. 2016;128:4794–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cane DE, Swanson S, Murthy PP. Trichodiene biosynthesis and the enzymic cyclization of farnesyl pyrophosphate. J Am Chem Soc. 1981;103:2136–2138. [Google Scholar]
- 22. Metzger U, Keller S, Stevenson CE, Heide L, Lawson DM. Structure and mechanism of the magnesium‐independent aromatic prenyltransferase CloQ from the clorobiocin biosynthetic pathway. J Mol Biol. 2010;404:611–626. [DOI] [PubMed] [Google Scholar]
- 23. Tello M, Kuzuyama T, Heide L, Noel J, Richard S. The ABBA family of aromatic prenyltransferases: Broadening natural product diversity. Cell Mol Life Sci. 2008;65:1459–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palsuledesai CC, Distefano MD. Protein prenylation: Enzymes, therapeutics, and biotechnology applications. ACS Chem Biol. 2015;10:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park HW, Boduluri SR, Moomaw JF, Casey PJ, Beese LS. Crystal structure of protein farnesyltransferase at 2.25 angstrom resolution. Science. 1997;275:1800–1805. [DOI] [PubMed] [Google Scholar]
- 26. Gelb MH, Scholten JD, Sebolt‐Leopold JS. Protein prenylation: From discovery to prospects for cancer treatment. Curr Opin Chem Biol. 1998;2:40–48. [DOI] [PubMed] [Google Scholar]
- 27. Sinensky M. Recent advances in the study of prenylated proteins. Biochim Biophys Acta Mol Cell Biol Lipids. 2000;1484:93–106. [DOI] [PubMed] [Google Scholar]
- 28. Ogura K, Koyama T. Enzymatic aspects of isoprenoid chain elongation. Chem Rev. 1998;98:1263–1276. [DOI] [PubMed] [Google Scholar]
- 29. Wang KC, Ohnuma S‐i. Isoprenyl diphosphate synthases. Biochim Biophys Acta Mol Cell Biol Lipids. 2000;1529:33–48. [DOI] [PubMed] [Google Scholar]
- 30. Ashby MN, Edwards P. Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem. 1990;265:13157–13164. [PubMed] [Google Scholar]
- 31. Ogura T. Dynamic aspects of natural products chemistry. Tokyo, Japan: Kodansha Ltd., 1997. [Google Scholar]
- 32. Guo R‐T, Ko T‐P, Chen AP‐C, Kuo C‐J, Wang AH‐J, Liang P‐H. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate roles of the metal ion and conserved residues in catalysis. J Biol Chem. 2005;280:20762–20774. [DOI] [PubMed] [Google Scholar]
- 33. Guo R‐T, Kuo C‐J, Chou C‐C, et al. Crystal structure of octaprenyl pyrophosphate synthase from hyperthermophilic Thermotoga maritima and mechanism of product chain length determination. J Biol Chem. 2004;279:4903–4912. [DOI] [PubMed] [Google Scholar]
- 34. Han X, Chen CC, Kuo CJ, et al. Crystal structures of ligand‐bound octaprenyl pyrophosphate synthase from Escherichia coli reveal the catalytic and chain‐length determining mechanisms. Proteins. 2015;83:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang S‐Y, Ko T‐P, Chen AP‐C, Wang AH‐J, Liang P‐H. Substrate binding mode and reaction mechanism of undecaprenyl pyrophosphate synthase deduced from crystallographic studies. Protein Sci. 2004;13:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang S‐Y, Ko T‐P, Liang P‐H, Wang AH‐J. Catalytic mechanism revealed by the crystal structure of undecaprenyl pyrophosphate synthase in complex with sulfate, magnesium, and triton. J Biol Chem. 2003;278:29298–29307. [DOI] [PubMed] [Google Scholar]
- 37. Chang S‐Y, Chen Y‐K, Wang AH‐J, Liang P‐H. Identification of the active conformation and the importance of length of the flexible loop 72−83 in regulating the conformational change of undecaprenyl pyrophosphate synthase. Biochemistry. 2003;42:14452–14459. [DOI] [PubMed] [Google Scholar]
- 38. Ko T‐P, Chen Y‐K, Robinson H, et al. Mechanism of product chain length determination and the role of a flexible loop in Escherichia coli undecaprenyl‐pyrophosphate synthase catalysis. J Biol Chem. 2001;276:47474–47482. [DOI] [PubMed] [Google Scholar]
- 39. Kharel Y, Zhang Y‐W, Fujihashi M, Miki K, Koyama T. Identification of significant residues for homoallylic substrate binding of Micrococcus luteus BP 26 undecaprenyl diphosphate synthase. J Biol Chem. 2001;276:28459–28464. [DOI] [PubMed] [Google Scholar]
- 40. Fujihashi M, Zhang YW, Higuchi Y, Li XY, Koyama T, Miki K. Crystal structure of cis‐prenyl chain elongating enzyme, undecaprenyl diphosphate synthase. Proc Natl Acad Sci U S A. 2001;98:4337–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh F‐L, Chang T‐H, Ko T‐P, Wang AH‐J. Structure and mechanism of an Arabidopsis medium/long‐chain‐length prenyl pyrophosphate synthase. Plant Physiol. 2011;155:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang G, Dixon RA. Heterodimeric geranyl (geranyl) diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc Natl Acad Sci U S A. 2009;106:9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang T‐H, Hsieh F‐L, Ko T‐P, Teng K‐H, Liang P‐H, Wang AH‐J. Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. Plant Cell. 2010;22:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sasaki D, Fujihashi M, Okuyama N, et al. Crystal structure of heterodimeric hexaprenyl diphosphate synthase from Micrococcus luteus BP 26 reveals that the small subunit is directly involved in the product chain length regulation. J Biol Chem. 2011;286:3729–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang YW, Koyama T, Marecak DM, Prestwich GD, Maki Y, Ogura K. Two subunits of heptaprenyl diphosphate synthase of Bacillus s ubtilis form a catalytically active complex. Biochemistry. 1998;37:13411–13420. [DOI] [PubMed] [Google Scholar]
- 46. Schulbach MC, Brennan PJ, Crick DC. Identification of a short (C15) chain Z‐isoprenyl diphosphate synthase and a homologous long (C50) chain isoprenyl diphosphate synthase in Mycobacterium tuberculosis . J Biol Chem. 2000;275:22876–22881. [DOI] [PubMed] [Google Scholar]
- 47. Schilmiller AL, Schauvinhold I, Larson M, et al. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci U S A. 2009;106:10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaur D, Brennan PJ, Crick DC. Decaprenyl diphosphate synthesis in Mycobacterium tuberculosis . J Bacteriol. 2004;186:7564–7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harrison KD, Park EJ, Gao N, et al. Nogo‐B receptor is necessary for cellular dolichol biosynthesis and protein N‐glycosylation. EMBO J. 2011;30:2490–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ma J, Ko T‐P, Yu X, et al. Structural insights to heterodimeric cis‐prenyltransferases through yeast dehydrodolichyl diphosphate synthase subunit Nus1. Biochem Biophys Res Commun. 2019;515:621–626. [DOI] [PubMed] [Google Scholar]
- 51. Park EJ, Grabińska KA, Guan Z, et al. Mutation of Nogo‐B receptor, a subunit of cis‐prenyltransferase, causes a congenital disorder of glycosylation. Cell Metab. 2014;20:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brasher MI, Surmacz L, Leong B, et al. A two‐component enzyme complex is required for dolichol biosynthesis in tomato. Plant J. 2015;82:903–914. [DOI] [PubMed] [Google Scholar]
- 53. Kwon M, Kwon E‐J, Ro D. cis‐Prenyltransferase and polymer analysis from a natural rubber perspective. Methods Enzymol. 2016;576:121–145. [DOI] [PubMed] [Google Scholar]
- 54. Chen Y‐H, Chen AP‐C, Chen C‐T, Wang AH‐J, Liang P‐H. Probing the conformational change of Escherichia coli undecaprenyl pyrophosphate synthase during catalysis using an inhibitor and tryptophan mutants. J Biol Chem. 2002;277:7369–7376. [DOI] [PubMed] [Google Scholar]
- 55. Guo R‐T, Cao R, Liang P‐H, et al. Bisphosphonates target multiple sites in both cis‐and trans‐prenyltransferases. Proc Natl Acad Sci U S A. 2007;104:10022–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuo C‐J, Guo R‐T, Lu I‐L, et al. Structure‐based inhibitors exhibit differential activities against Helicobacter pylori and Escherichia coli undecaprenyl pyrophosphate synthases. J Biomed Biotechnol. 2008;2008:1–6. 10.1155/2008/841312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. El Ghachi M, Bouhss A, Blanot D, Mengin‐Lecreulx D. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem. 2004;279:30106–30113. [DOI] [PubMed] [Google Scholar]
- 58. Tatar LD, Marolda CL, Polischuk AN, van Leeuwen D, Valvano MA. An Escherichia coli undecaprenyl‐pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling. Microbiology. 2007;153:2518–2529. [DOI] [PubMed] [Google Scholar]
- 59. Chang H‐Y, Chou C‐C, Hsu M‐F, Wang AHJ. Proposed carrier lipid‐binding site of undecaprenyl pyrophosphate phosphatase from Escherichia coli . J Biol Chem. 2014;289:18719–18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chiang C‐Y, Chou C‐C, Chang H‐Y, et al. Biochemical and molecular dynamics studies of archaeal polyisoprenyl pyrophosphate phosphatase from Saccharolobus solfataricus . Enzyme Microb. Technol. 2020;139:109585 10.1016/j.enzmictec.2020.109585. [DOI] [PubMed] [Google Scholar]
- 61. Chang H‐Y, Chou C‐C, Wu M‐L, Wang AH‐J. Expression, purification and enzymatic characterization of undecaprenyl pyrophosphate phosphatase from Vibrio vulnificus . Protein Expr Purif. 2017;133:121–131. [DOI] [PubMed] [Google Scholar]
- 62. El Ghachi M, Howe N, Huang C‐Y, et al. Crystal structure of undecaprenyl‐pyrophosphate phosphatase and its role in peptidoglycan biosynthesis. Nat Commun. 2018;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Workman SD, Worrall LJ, Strynadka NC. Crystal structure of an intramembranal phosphatase central to bacterial cell‐wall peptidoglycan biosynthesis and lipid recycling. Nat Commun. 2018;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rush JS, Cho SK, Jiang S, Hofmann SL, Waechter CJ. Identification and characterization of a cDNA encoding a dolichyl pyrophosphate phosphatase located in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2002;277:45226–45234. [DOI] [PubMed] [Google Scholar]
- 65. Hudock MP, Zhang Y, Guo R‐T, et al. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates: A crystallographic and computational investigation. J Med Chem. 2008;51:5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao R, Chen CKM, Guo R‐T, Wang AH‐J, Oldfield E. Structures of a potent phenylalkyl bisphosphonate inhibitor bound to farnesyl and geranylgeranyl diphosphate synthases. Proteins. 2008;73:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, Cao R, Yin F, et al. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: An X‐ray and NMR investigation. J Am Chem Soc. 2009;131:5153–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schwalen CJ, Feng X, Liu W, et al. “Head‐to‐head” prenyl synthases in some pathogenic bacteria. Chembiochem. 2017;18:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lin F‐Y, Liu Y‐L, Li K, et al. Head‐to‐head prenyl tranferases: Anti‐infective drug targets. J Med Chem. 2012;55:4367–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu C‐I, Jeng W‐Y, Chang W‐J, Ko T‐P, Wang AH‐J. Binding modes of zaragozic acid A to human squalene synthase and staphylococcal dehydrosqualene synthase. J Biol Chem. 2012;287:18750–18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Song J, Shang N, Baig N, et al. Aspergillus flavus squalene synthase as an antifungal target: Expression, activity, and inhibition. Biochem Biophys Res Commun. 2019;512:517–523. [DOI] [PubMed] [Google Scholar]
- 72. Pan J‐J, Ramamoorthy G, Poulter CD. Absolute configuration of hydroxysqualene. An intermediate in bacterial hopanoid biosynthesis. Org Lett. 2016;18:512–515. [DOI] [PubMed] [Google Scholar]
- 73. Camagna M, Welsch R. Expression, purification, and enzyme activity assay of phytoene synthase in vitro . Methods Mol Biol. 2020;2083:39–52. [DOI] [PubMed] [Google Scholar]
- 74. Do R, Kiss R, Gaudet D, Engert J. Squalene synthase: A critical enzyme in the cholesterol biosynthesis pathway. Clin Genet. 2009;75:19–29. [DOI] [PubMed] [Google Scholar]
- 75. Charlton‐Menys V, Durrington PN. Squalene synthase inhibitors. Br J Pharmacol. 2007;67:11–16. [DOI] [PubMed] [Google Scholar]
- 76. Toutouzas K, Drakopoulou M, Skoumas I, Stefanadis C. Advancing therapy for hypercholesterolemia. Expert Opin Pharmacother. 2010;11:1659–1672. [DOI] [PubMed] [Google Scholar]
- 77. de Souza W. Sterol biosynthesis pathway as target for anti‐trypanosomatid drugs. Interdiscip Perspect Infect Dis. 2009;2009:642502. 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bergstrom JD, Dufresne C, Bills GF, Nallin‐Omstead M, Byrne K. Discovery, biosynthesis, and mechanism of action of the zaragozic acids: Potent inhibitors of squalene synthase. Annu Rev Microbiol. 1995;49:607–639. [DOI] [PubMed] [Google Scholar]
- 79. Bell SA, Niehaus TD, Nybo SE, Chappell J. Structure–function mapping of key determinants for hydrocarbon biosynthesis by squalene and squalene synthase‐like enzymes from the green alga Botryococcus braunii race B. Biochemistry. 2014;53:7570–7581. [DOI] [PubMed] [Google Scholar]
- 80. Niehaus TD, Okada S, Devarenne TP, Watt DS, Sviripa V, Chappell J. Identification of unique mechanisms for triterpene biosynthesis in Botryococcus braunii . Proc Natl Acad Sci U S A. 2011;108:12260–12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Radisky ES, Poulter CD. Squalene synthase: Steady‐state, pre‐steady‐state, and isotope‐trapping studies. Biochemistry. 2000;39:1748–1760. [DOI] [PubMed] [Google Scholar]
- 82. Jarstfer MB, Zhang D‐L, Poulter CD. Recombinant squalene synthase. Synthesis of non‐head‐to‐tail isoprenoids in the absence of NADPH. J Am Chem Soc. 2002;124:8834–8845. [DOI] [PubMed] [Google Scholar]
- 83. Pandit J, Danley DE, Schulte GK, et al. Crystal structure of human squalene synthase A key enzyme in cholesterol biosynthesis. J Biol Chem. 2000;275:30610–30617. [DOI] [PubMed] [Google Scholar]
- 84. Welander PV, Hunter RC, Zhang L, Sessions AL, Summons RE, Newman DK. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE‐1. J Bacteriol. 2009;191:6145–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shang N, Ko T‐P, et al. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog. 2014;10 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chan Y‐T, Ko T‐P, Yao S‐H, Chen Y‐W, Lee C‐C, Wang AH‐J. Crystal structure and potential head‐to‐middle condensation function of a Z, Z‐farnesyl diphosphate synthase. ACS Omega. 2017;2:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu M, Chen CC, Chen L, et al. Structure and function of a “head‐to‐middle” prenyltransferase: Lavandulyl diphosphate synthase. Angew Chem Int Ed Engl. 2016;55:4721–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tomita T, Kobayashi M, Karita Y, et al. Structure and mechanism of the monoterpene cyclolavandulyl diphosphate synthase that catalyzes consecutive condensation and cyclization. Angew Chem Int Ed Engl. 2017;129:15109–15113. [DOI] [PubMed] [Google Scholar]
- 89. Gao J, Ko T‐P, Chen L, et al. “Head‐to‐middle” and “head‐to‐tail” cis‐prenyl transferases: structure of isosesquilavandulyl diphosphate synthase. Angew Chem Int Ed Engl. 2018;130:691–695. [DOI] [PubMed] [Google Scholar]
- 90. Ogawa T, Emi K i, Koga K, Yoshimura T, Hemmi H. A cis‐prenyltransferase from Methanosarcina acetivorans catalyzes both head‐to‐tail and nonhead‐to‐tail prenyl condensation. FEBS J. 2016;283:2369–2383. [DOI] [PubMed] [Google Scholar]
- 91. Kaysser L, Bernhardt P, Nam S‐J, et al. Merochlorins A–D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium‐dependent chloroperoxidases. J Am Chem Soc. 2012;134:11988–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Blank PN, Barrow GH, Christianson DW. Crystal structure of F95Q epi‐isozizaene synthase, an engineered sesquiterpene cyclase that generates biofuel precursors β‐and γ‐curcumene. J Struct Biol. 2019;207:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Köksal M, Jin Y, Coates RM, Croteau R, Christianson DW. Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis. Nature. 2011;469:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Starks CM, Back K, Chappell J, Noel JP. Structural basis for cyclic terpene biosynthesis by tobacco 5‐epi‐aristolochene synthase. Science. 1997;277:1815–1820. [DOI] [PubMed] [Google Scholar]
- 95. Lesburg CA, Zhai G, Cane DE, Christianson DW. Crystal structure of pentalenene synthase: Mechanistic insights on terpenoid cyclization reactions in biology. Science. 1997;277:1820–1824. [DOI] [PubMed] [Google Scholar]
- 96. Wendt KU, Poralla K, Schulz GE. Structure and function of a squalene cyclase. Science. 1997;277:1811–1815. [DOI] [PubMed] [Google Scholar]
- 97. Sato T, Yoshida S, Hoshino H, Tanno M, Nakajima M, Hoshino TJ. Sesquarterpenes (C35 terpenes) biosynthesized via the cyclization of a linear C35 isoprenoid by a tetraprenyl‐β‐curcumene synthase and a tetraprenyl‐β‐curcumene cyclase: Identification of a new terpene cyclase. J Am Chem Soc. 2011;133:9734–9737. [DOI] [PubMed] [Google Scholar]
- 98. Chen M, Chou WK, Toyomasu T, Cane DE, Christianson DW. Structure and function of fusicoccadiene synthase, a hexameric bifunctional diterpene synthase. ACS Chem Biol. 2016;11:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kries H, Caputi L, Stevenson CE, et al. Structural determinants of reductive terpene cyclization in iridoid biosynthesis. Nat Chem Biol. 2016;12:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Morehouse BR, Kumar RP, Matos JO, Olsen SN, Entova S, Oprian DD. Functional and structural characterization of a (+)‐limonene synthase from Citrus sinensis . Biochemistry. 2017;56:1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Blank PN, Pemberton TA, Chow J‐Y, Poulter CD, Christianson DW. Crystal structure of cucumene synthase, a terpenoid cyclase that generates a linear triquinane sesquiterpene. Biochemistry. 2018;57:6326–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fujihashi M, Sato T, Tanaka Y, et al. Crystal structure and functional analysis of large‐terpene synthases belonging to a newly found subclass. Chem Sci. 2018;9:3754–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Stepanova R, Inagi H, Sugawara K, et al. Characterization of class IB terpene synthase: The first crystal structure bound with a substrate surrogate. ACS Chem Biol. 2020;15:1517–1525. [DOI] [PubMed] [Google Scholar]
- 104. Awakawa T, Abe I. Molecular basis for the plasticity of aromatic prenyltransferases in hapalindole biosynthesis. Beilstein J Org Chem. 2019;15:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li W. Bringing bioactive compounds into membranes: The UbiA superfamily of intramembrane aromatic prenyltransferases. Trends Biochem Sci. 2016;41:356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yu X, Li S‐M. Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol. 2012;516: 259–278. [DOI] [PubMed] [Google Scholar]
- 107. Kuzuyama T, Noel JP, Richard SB. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature. 2005;435:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tudzynski P, Hölter K, Correia T, Arntz C, Grammel N, Keller U. Evidence for an ergot alkaloid gene cluster in Claviceps purpurea . Mol Gen Genet. 1999;261:133–141. [DOI] [PubMed] [Google Scholar]
- 109. Awakawa T, Mori T, Nakashima Y, et al. Molecular insight into the Mg2+‐dependent allosteric control of indole prenylation by aromatic prenyltransferase AmbP1. Angew Chem Int Ed Engl. 2018;57:6810–6813. [DOI] [PubMed] [Google Scholar]
- 110. Wong CP, Awakawa T, Nakashima Y, et al. Two distinct substrate binding modes for the normal and reverse prenylation of hapalindoles by the prenyltransferase AmbP3. Angew Chem Int Ed Engl. 2018;57:560–563. [DOI] [PubMed] [Google Scholar]
- 111. Cheng W, Li W. Structural insights into ubiquinone biosynthesis in membranes. Science. 2014;343:878–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. [DOI] [PubMed] [Google Scholar]
- 113. Ghomashchi F, Zhang X, Liu L, Gelb MH. Binding of prenylated and polybasic peptides to membranes: Affinities and intervesicle exchange. Biochemistry. 1995;34:11910–11918. [DOI] [PubMed] [Google Scholar]
- 114. Pfeffer SR. Rab GTPases: Master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. [DOI] [PubMed] [Google Scholar]
- 115. Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. [DOI] [PubMed] [Google Scholar]
- 116. Farnsworth CC, Seabra MC, Ericsson LH, Gelb MH, Glomset JA. Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A, and Rab5A. Proc Natl Acad Sci U S A. 1994;91:11963–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Taylor JS, Reid TS, Terry KL, Casey PJ, Beese LS. Structure of mammalian protein geranylgeranyltransferase type‐I. EMBO J. 2003;22:5963–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang H, Seabra MC, Deisenhofer J. Crystal structure of Rab geranylgeranyltransferase at 2.0 Å resolution. Structure. 2000;8:241–251. [DOI] [PubMed] [Google Scholar]
- 119. Long SB, Casey PJ, Beese LS. The basis for K‐Ras4B binding specificity to protein farnesyl‐transferase revealed by 2 Å resolution ternary complex structures. Structure. 2000;8:209–222. [DOI] [PubMed] [Google Scholar]
- 120. Pylypenko O, Rak A, Reents R, Niculae A, Sidorovitch V, et al. Structure of Rab escort protein‐1 in complex with Rab geranylgeranyltransferase. Mol Cell. 2003;11:483–494. [DOI] [PubMed] [Google Scholar]
- 121. Yokoyama K, Zimmerman K, Scholten J, Gelb MH. Differential prenyl pyrophosphate binding to mammalian protein geranylgeranyltransferase‐I and protein farnesyltransferase and its consequence on the specificity of protein prenylation. J Biol Chem. 1997;272:3944–3952. [DOI] [PubMed] [Google Scholar]
- 122. Liang P‐H, Ko T‐P, Wang AH‐J. Structure, mechanism and function of prenyltransferases. Eur J Biochem. 2002;269:3339–3354. [DOI] [PubMed] [Google Scholar]
- 123. Wang M, Casey PJ. Protein prenylation: Unique fats make their mark on biology. Nat Rev Mol Cell Biol. 2016;17:110–122. [DOI] [PubMed] [Google Scholar]
- 124. Young S, Yang S, Davies B, Jung H, Fong L. Targeting protein prenylation in progeria. Sci Transl Med. 2013;5:171ps3 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ochocki JD, Distefano M. Prenyltransferase inhibitors: Treating human ailments from cancer to parasitic infections. Medchemcomm. 2013;4:476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schafer WR, Kim R, Sterne R, Thorner J, Kim S‐H, Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989;245:379–385. [DOI] [PubMed] [Google Scholar]
- 127. Berndt N, Hamilton AD, Sebti S. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11:775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang J, Yao X, Huang J. New tricks for human farnesyltransferase inhibitor: Cancer and beyond. Medchemcomm. 2017;8:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Klochkov SG, Neganova ME, Yarla NS, et al. Implications of farnesyltransferase and its inhibitors as a promising strategy for cancer therapy. Semin Cancer Biol. 2019;56: 128–134. [DOI] [PubMed] [Google Scholar]
- 130. Shen M, Pan P, Li Y, Li D, Yu H, Hou T. Farnesyltransferase and geranylgeranyltransferase I: Structures, mechanism, inhibitors and molecular modeling. Drug Discov Today. 2015;20:267–276. [DOI] [PubMed] [Google Scholar]
- 131. Jaeger R, Cuny E. Terpenoids with special pharmacological significance: A review. Nat Prod Commun. 2016;9:1373–1390. [PubMed] [Google Scholar]
- 132. Dai X, Sun Y, Zhang T, Ming Y, Gao H. An overview on natural farnesyltransferase inhibitors for efficient cancer therapy. J Enzyme Inhib Med Chem. 2020;35:1027–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wu S‐H, Ryvarden L, Chang T‐T. Antrodia camphorata (“niuchang‐ chih”), new combination of a medicinal fungus in Taiwan. Bot Bull Acad Sin (Taipei). 1996;38:273–275. [Google Scholar]
- 134. Angamuthu V, Shanmugavadivu M, Nagarajan G, Velmurugan BK. Pharmacological activities of antroquinonol – Mini review. Chem Biol Interact. 2019;297:8–15. [DOI] [PubMed] [Google Scholar]
- 135. Bailey HH, Levy D, Harris LS, et al. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern cooperative oncology group study E2E96. Gynecol Oncol. 2002;85:464–468. [DOI] [PubMed] [Google Scholar]
- 136. Musmade PB, Tumkur A, Trilok M, Bairy KL. Fusidic acid – Topical antimicrobial in the management of Staphylococcus aureus . Int J Pharm Pharmaceut Sci. 2013;5:381–390. [Google Scholar]
- 137. Salam KA, Furuta A, Noda N, et al. Inhibition of hepatitis C virus NS3 helicase by manoalide. J Nat Prod. 2012;75:650–654. [DOI] [PubMed] [Google Scholar]
- 138. Sachan AK, Das DR, Shuaib MD, Gangwar SS, Sharma R. An overview on Menthae piperitae (peppermint oil). Int J Pharmaceut Chem Biol Sci. 2013;3:834–838. [Google Scholar]
- 139. Collier NJ, Ali FR, Lear JT. Ingenol mebutate: A novel treatment for actinic keratosis. Clin Pract. 2014;11:295–306. [Google Scholar]
- 140. Burstein S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg Med Chem. 2015;23:1377–1385. [DOI] [PubMed] [Google Scholar]
- 141. Birladeanu L. The stories of santonin and santonic acid. Angew Chem Int Ed Engl. 2004;42:1202–1208. [DOI] [PubMed] [Google Scholar]
- 142. Corpas‐López V, Morillas‐Márquez F, Navarro‐Moll MC, Merino‐Espinosa G, Díaz‐Sáez V, Martín‐Sánchez J. (α)‐Bisabolol, a promising oral compound for the treatment of visceral leishmaniasis. J Nat Prod. 2015;78:1202–1207. [DOI] [PubMed] [Google Scholar]
- 143. Noori S, Hassan ZM, Mohammadi M, Habibi Z, Sohrabi N, Bayanolhagh S. Sclareol modulates the treg intra‐tumoral infiltraded cell and inhibits tumor growth in vivo . Cell Immunol. 2010;263:148–153. [DOI] [PubMed] [Google Scholar]
- 144. Valeev RF, Vostrikov NS, Miftakhov MS. Synthesis and some transformations of (−)‐carveol. Russ J Org Chem. 2009;45:810–814. [Google Scholar]
- 145. Puranam K, Rani SH. Forskolin: Its therapeutic applications. Int J Pharma Biosci. 2014;5:68–73. [Google Scholar]
- 146. Mabanglo MF, Hast MA, Lubock NB, Hellinga HW, Beese LS. Crystal structures of the fungal pathogen Aspergillus fumigatus protein farnesyltransferase complexed with substrates and inhibitors reveal features for antifungal drug design. Protein Sci. 2014;23:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sham L‐T, Butler EK, Lebar MD, Kahne D, Bernhardt TG, et al. Bacterial cell wall. MurJ is the flippase of lipid‐linked precursors for peptidoglycan biogenesis. Science. 2014;345:220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Meeske AJ, Riley EP, Robins WP, et al. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature. 2016;537:634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mohammadi T, van Dam V, Sijbrandi R, et al. Identification of FtsW as a transporter of lipid‐linked cell wall precursors across the membrane. EMBO J. 2011;30:1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Manat G, El Ghachi M, Auger R, Baouche K, Olatunji S, et al. Membrane topology and biochemical characterization of the Escherichia coli BacA undecaprenyl‐pyrophosphate phosphatase. PLoS One. 2015;10:e0142870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information


