Abstract
γδ T cells can display a plethora of immune functions, but recent studies have highlighted their importance, in multiple disease models, as sources of the pro‐inflammatory cytokines, IL‐17A (IL‐17), and IFN‐γ. These are produced by distinct murine effector γδ T cell subsets that diverge during thymic γδ T cell development. Among the multiple roles these subsets play in peripheral tissues, a striking dichotomy has emerged at tumor sites: whereas IFN‐γ+ γδ T cells inhibit tumor cell growth, IL‐17+ γδ T cells promote tumor progression and metastasis formation. In this review, we discuss the main lines of evidence, mostly from preclinical studies in mouse models, for this functional dichotomy in cancer immunity. We further highlight very recent advances in our understanding how metabolic sources and pathways can impact on the balance between IFN‐γ+ and IL‐17+ γδ T cells in the tumor microenvironment, which opens a new exciting avenue to explore toward the application of γδ T cells in cancer immunotherapy.
Keywords: γδ T cells, tumor immunology, IFN‐γ, IL‐17, metabolism
γδ T cell subsets have opposite roles in cancer immunity: whereas IFN‐γ+ γδ T cells inhibit tumor cell growth, IL‐17+ γδ T cells promote tumor progression and metastasis formation. Furthermore, metabolic sources and pathways can impact on the γδ T cell subset balance in the tumor microenvironment. Manipulating γδ metabolism may pave the way toward the development of novel immunotherapies.
Introduction
γδ T cells are unconventional T cells that express a unique T‐cell receptor (TCR) composed of γ and δ chains. Although they are of low abundance in lymphoid organs, γδ T cells can play key roles in the physiology and immune surveillance of many tissues, as firmly demonstrated in mouse models [1]. In mice, γδ T cell development in the thymus involves a specific sequence of somatic rearrangement of TCR genes during ontogeny, leading to subset‐characteristic TCRγ variable region (Vγ) usage [2]. Importantly, these thymic subsets are intrinsically biased to produce either IL‐17A (IL‐17) or IFN‐γ as result of a complex process of “developmental pre‐programming” that involves TCR‐dependent and TCR‐independent mechanisms, as we [3] and others [2, 4] have reviewed elsewhere.
Distinct effector γδ T cell subsets can be defined based on the expression of the TNF‐receptor superfamily member, CD27 [5], and further resolved using additional markers such as CD44 and CD45RB [3, 6, 7]. The fate of γδ T cell progenitors is seemingly dictated by TCRγδ signaling, since strong TCRγδ signals inhibit the generation of IL‐17‐producing γδ (γδ17) T cells while promoting IFN‐γ‐producing γδ (γδIFN) T cell development [3, 6, 7]. The cellular and molecular mechanisms underpinning the differentiation of γδ17 versus γδIFN T cells have been recently reviewed elsewhere [8].
Effector γδ T cell subsets are known to play crucial roles in tissue homeostasis [1], autoimmunity [9], and cancer [10]. Here, we will concentrate on cancer immunity, where γδ T cells play crucial roles and thus represent a promising option for next‐generation immunotherapies [10, 11]. In this context, γδ T cells share functional similarities with their αβ T cell counterparts, such as cytotoxic functions and secretion of pro‐inflammatory cytokines. However, in contrast to αβ T cells, γδ T cells are mostly independent on MHC‐mediated presentation of mutated antigens, although a recent study described an exception where specific melanoma‐associated antigens were recognized by human γδ T cells in an MHC class I‐restricted manner [12]. In general, γδ T cells sense “stress‐inducible” changes, conveyed either via the TCR‐γδ or NK cell receptors, like NKG2D, to become activated and deploy rapid effector responses in transformed tissues [13, 14].
In the tumor microenvironment (TME), murine γδ T cells typically belong to the Vγ1+, Vγ4+, or Vγ6+ (using the Heilig & Tonegawa nomenclature [15]) subsets. These subpopulations have different functional biases to produce either IL‐17 or IFN‐γ: Vγ1+ T cells are IFN‐γ‐biased, whereas Vγ6+ T cells produce almost exclusively IL‐17, and Vγ4+ T cells contain sizeable IL‐17‐producing and IFN‐γ‐producing sub‐populations [2, 3]. This is particularly important because these two cytokines, and thus the corresponding γδ T cell subsets, typically play opposing roles in the TME: whereas γδIFN cells are potent antitumor type 1 cytotoxic effectors, γδ17 cells are mostly pro‐tumoral, through inducing angiogenesis, tumor cell proliferation, and recruiting immunosuppressive cells to the TME [10]. In this review, we discuss the current knowledge on tumor‐associated γδ T cell subsets, while integrating very recent discoveries on a striking metabolic dichotomy between γδIFN and γδ17 T cells [16] that define a new perspective to improve the performance of γδ T cells in cancer immunotherapy.
Pro‐tumoral functions of γδ17 cells
The TME is often conducive to IL‐17 production. In fact, high levels of IL‐17 have been detected in the different types of tumor in mice and humans [17, 18]. Interestingly, it has been recently found that increased IL‐17 in the LN microenvironment during ageing leads to accumulation of γδ17 T cells, which associates with accelerated tumor growth [19]. Two γδ17 T cell subsets can be major sources of IL‐17 at tumor sites: Vγ4+ γδ17 T cells predominate in hepatocellular carcinoma [20] and in breast tumor murine models [21], whereas Vγ6+ γδ17 T cells are enriched in lung [22], ovarian [23], and cervical [24] tumor mouse models (Fig. 1). Various cytokines have been associated with γδ17 T cell accumulation in different tumor models: IL‐1β in breast and lung metastasis [21, 25], IL‐6 in pancreatic cancer [26], IL‐1β plus IL‐23 in hepatocellular carcinoma [20], IL‐6/IL‐23/TGF‐β in fibrosarcoma and skin carcinoma [27], and IL‐1β / IL‐6 /IL‐7 in ovarian cancer [23] models. These γδ17 T cell expansions and IL‐17 secretion can have three major consequences that benefit tumor progression: promotion of angiogenesis, stimulation of tumor cell proliferation, and orchestration of an immunosuppressive TME (Fig. 2). Of note, while γδ17 T cells are rare in humans, some studies revealed that γδ T cells can constitute a major source of IL‐17 within tumor‐infiltrating lymphocytes, particularly in colorectal cancer [28, 29].
Figure 1.
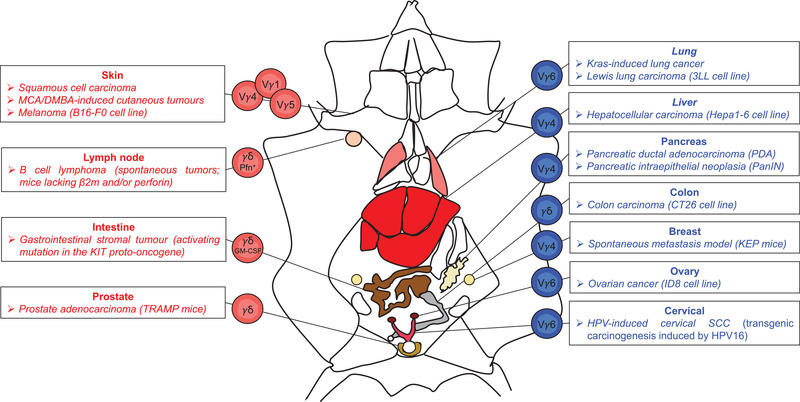
Opposite roles of murine γδ T cell subsets in cancer. Distinct γδ T cell subsets play an anti‐ (red) or pro‐ (blue) tumoural roles in various experimental mouse cancer models. Vγ1+, Vγ4+ and Vγ5+ T cell subsets producing IFN‐γ are protective in several skin cancers such as squamous cell carcinoma, 3‐methylcholanthrene (MCA)/ 7,12‐dimethylbenz]alpha]anthracene (DMBA)‐induced cutaneous tumors or B16‐F0 melanoma. γδ T cells are also overtly anti‐tumoural in: (i) spontaneous B cell lymphoma in mice lacking beta 2 microglobulin (β2m) and/ or perforin (Pfn), (ii) gastrointestinal stromal tumors due to activating mutation in the KIT proto‐oncogene, via GM‐CSF, and (iii) prostate adenocarcinoma in TRAMP mice. In contrast, Vγ6+ γδ17 T cells play pro‐tumor roles in Kras‐induced lung cancer and Lewis lung carcinoma, as well as in ID8 ovarian cancer and in Human papillomavirus (HPV)‐induced cervical squamous cell carcinoma (SCC) models. Other IL‐17‐producing γδ T cells, especially Vγ4+ T cells, are detrimental in (i) Hepa1‐6 hepatocellular carcinoma, (ii) pancreatic adenocarcinoma in PDA and pancreatic intraepithelial neoplasia (PanIN) models, and (iii) KEP breast cancer metastatic mouse model.
Figure 2.
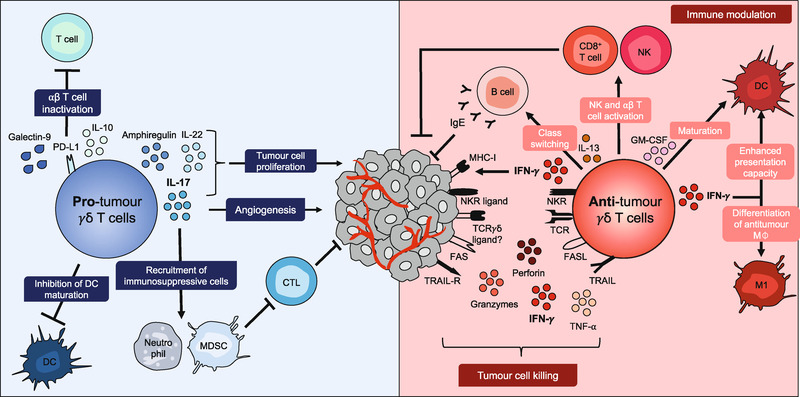
Pleiotropic functions of γδ T cells in the TME. Pro‐tumoural roles of γδ T cells (left panel in blue) include the production of IL‐17 which stimulates tumor cell proliferation, induces angiogenesis, and recruits immunosuppressive cells like myeloid‐derived suppressive cells (MDSC) that inhibit cytotoxic T lymphocytes (CTL). γδ T cells can also inhibit DC maturation and exert IL‐17‐independent pro‐tumor roles, via galectin‐9, programmed cell death protein 1 ligand 1 (PDL‐1) or IL‐10, which inhibit ⍺β T cell responses; or through production of amphiregulin or IL‐22 that promote tumour cell proliferation. Antitumoral roles of γδ T cells (right panel in red), activated through the T cell receptor (TCR) and NK cell receptors (NKRs) to kill tumor cells upon expression of perforin and granzymes, FAS ligand (FASL) and tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL), as well as the cytokines tumor necrosis factor ⍺ (TNF‐⍺) and IFN‐γ. IFN‐γ upregulates MHC class I expression on tumor cells, which enhances their recognition by cytotoxic T lymphocytes. IFN‐γ also induces the differentiation of antitumor macrophages (M) and enhances the presentation capacity of antigen‐presenting cells. Additionally, γδ T cells promote the maturation of DCs via secretion of GM‐CSF; promote activation of CD8+ T cells; increase cytotoxicity of NK cells via CD137L expression; and, through IL‐13 secretion, induce antibody class switching in B cells to autoreactive antitumor IgE.
Angiogenesis and tumor cell proliferation
Wakita and colleagues proposed for the first time, in a seminal paper, that γδ17 T cells supported tumor growth through induction of angiogenesis [27]. They showed that IL‐17 within tumors was predominantly produced by γδ T cells, and in the transplantable methylcholanthrene (MCA)‐induced CMS‐G4 tumor cell line model, mice lacking IL‐17 showed reduced tumor growth associated with decreased vascular density within the tumor tissue. Conversely, the administration of IL‐17 increased the expression of pro‐angiogenic factors such as vascular endothelial growth factor (VEGF) and angiopoietin 2 by tumor cells [27]. Our group found that γδ17 T cells can also induce angiogenesis indirectly by upregulating proangiogenic factors in small peritoneal macrophages (SPMs) that are mobilized by IL‐17 to the peritoneal cavity in the transplantable ID8 ovarian cancer model [23]. Moreover, a more recent study showed that γδ17 T cells stimulated the formation of blood vessels in the dermis underlying human papillomavirus‐induced tumor lesions [24]. Interestingly, γδ17 T cells can also promote the formation of lung metastases through tumor‐endothelial transmigration by acting on blood vessel permeability and upregulating the expression of adhesion molecules such as E‐selectin and VCAM‐1 by endothelial cells [30].
Importantly, γδ17 T cells, via IL‐17, can also directly stimulate tumor cell growth. IL‐17 (provided by γδ17 and CD4+ Th17 cells) is required for the initiation and progression of early pancreatic cancer: pancreatic cells sense IL‐17 in the TME through IL‐17 receptors and downstream signaling leads to the acceleration of pancreatic intraepithelial neoplasia [26].
Recruitment of immunosuppressive cells to the TME
γδ17 T cells can drive cancer progression by recruiting pro‐inflammatory or immunosuppressive cells to the tumor site (Fig. 2). He et al. showed that IL‐17 is necessary for both the recruitment and development of myeloid‐derived suppressor cells (MDSCs) [31]. We also found that γδ17 Vγ6+ T cells mobilize pro‐inflammatory SPMs expressing high levels of IL‐17RA, which in turn support ID8 ovarian cancer cell growth [23]. γδ17 Vγ4+ T cells also exert their pro‐tumoural functions by recruiting MDSCs in hepatocellular carcinoma, but in this case indirectly via induction of CXCL5 production by tumor cells [20]. This underpins an interesting positive feedback loop, since IL‐17‐stimulated MDSCs secrete IL‐1β and IL‐23 that support γδ17 Vγ4+ T cell expansion. Of note, human γδ17 T cells have also been associated with intra‐tumor infiltration and accumulation of MDSCs in colorectal cancer patients [28].
γδ17 T cells also recruit neutrophils to the TME (Fig. 2), and neutrophil abundance in metastatic breast cancer patients correlates with decreased survival [32]. In pre‐clinical spontaneous breast cancer murine models, Coffelt and colleagues demonstrated that γδ17 T cells drive the systemic expansion and polarization of neutrophils towards a CD8+ T cell‐suppressive phenotype, and thereby promote lung and LN metastasis [21, 33]. Consistently, the absence of γδ T cells or neutrophils substantially reduced pulmonary and LN metastases. However, neutrophils can display pleiotropic and somewhat contradictory roles in different cancer models. For example, our group found that tumor‐associated neutrophils can suppress γδ17 T cell responses through induction of oxidative stress (via ROS production), to which γδ17 T cells are particularly susceptible due to very low levels of the cellular antioxidant, glutathione [34]. Thus, the functional cross‐talks between γδ17 T cells and other immune cells, especially of the multifaceted myeloid lineage, deserves further investigation.
Antitumor functions of γδ(IFN) T cells
Girardi et al. demonstrated, for the first time, that γδ T cells were host‐protective in transplantable squamous cell carcinoma and methylcholanthrene (MCA)‐induced or dimethylbenzanthracene (DMBA)‐induced cutaneous tumor models, since tumor growth and progression were markedly increased in TCRδ –/– mice [35, 36]. Since then, γδ T cells have been shown to provide tumor immunosurveillance in various other models [10, 37], also when adoptively transferred into mice bearing B16‐F0 melanomas [38] or adenocarcinomas of the prostate (TRAMP mice) [39] (Fig. 1). By generating BM chimeras and foetal liver reconstitutions varying only in their γδIFN T cell composition, Gao et al. showed that mice with IFN‐γ‐deficient γδ T cells have higher tumor incidence than those bearing γδIFN T cells [40]. Following this original study, many others followed in attributing crucial antitumor roles to γδIFN T cells [10, 37, 38], which are endowed with potent cytotoxic functions and orchestrate an overt antitumor TME (Fig. 2).
Cytotoxicity against tumor cells
The seminal studies by Girardi et al. focused on Vγ5+ dendritic epidermal γδ T cells (DETCs), which are prototypic γδIFN T cells due to thymic “developmental pre‐programming” [3, 41]. DETCs were shown to deploy potent cytotoxicity against squamous cell carcinoma, upon engagement of their signature TCR as well as the critical NK cell receptor, NKG2D [35, 36, 42]. Interestingly, recent studies have shown that rapamycin, which suppresses mTOR signaling, increases NKG2D expression on Vγ4+ γδ T cells, enhancing their cytotoxicity against multiple tumor cell lines [43]. Cytotoxicity is a common feature of all murine γδIFN T cell subsets, but some finer differences seem to exist. For example, TCRδ –/‐ mice reconstituted with Vγ4+ γδ T cells were better protected against B16 melanoma and YAC‐1 cell lymphoma cells than those reconstituted with Vγ1+ γδ T cells [38].
Human γδ T cells also combine IFN‐γ production with cytotoxicity, and have been shown to kill renal cell carcinoma [44], squamous cell carcinoma [45], colon cell carcinoma [45, 46], and acute [47] and chronic myeloid leukemia cells [48], among various other tumor types [49].
The cytotoxic mechanisms are multi‐layered, involving the perforin–granzyme axis, but also “death ligands,” namely FASL and TRAIL, which engage the “death receptors” FAS (CD95) and TRAIL [39, 48, 50, 51] (Fig. 2). Of note, human γδ T cells also express CD16 (or Fcγ receptor III) that binds the Fc region of IgG antibodies, thus enabling antibody‐dependent cellular cytotoxicity (ADCC) coupled to Granzyme production and IFN‐γ secretion [52, 53, 54].
Modulation of antitumor immunity
An important role of γδIFN T cells is to promote an antitumor TME, for which the impact of IFN‐γ on MHC class I expression makes a key contribution. For example, tumor‐infiltrating γδIFN T cells were shown to regulate (via IFN‐γ production) the expression of MHC class I on murine B16 melanoma cells, thus promoting their recognition by CD8+ T lymphocytes [55] (Fig. 2).
However, not all protective effects of γδ T cells in cancer immunity depend on IFN‐γ production. For example, Strid and colleagues demonstrated that DETCs promote antibody class switching to IgE, which is highly protective against chemically induced skin carcinogenesis mouse model [56]. Moreover, DETCs cross‐communicate with epithelial cells via the production of IL‐13 that activates a very dynamic epithelial stress response in the epidermis in mice, which is protective against cutaneous carcinogenesis [57]. This host‐defensive type 2 (atopic) response by murine DETCs is deployed upon sensing of NKG2D ligands during cutaneous epithelial stress [58]. It is still unclear if other γδ T cell subsets can similarly engage an IL‐13/ IgE pathway in antitumor immunity.
A recent study described a protective role for γδ T cells in a mouse model of gastrointestinal stromal tumor through the secretion of GM‐CSF [59]. The authors proposed a model where tumor‐associated macrophages secreting IL‐1β promoted GM‐CSF production by γδ T cells, leading to the maturation of CD103+CD11b– DCs, which were associated with effector CD8+ T cell infiltration within tumors. In humans, the main circulating γδ T cell subset, Vγ9Vδ2 T cells, have been shown to promote CD8+ T cell activation based on their DC‐like ability to cross‐present antigens on MHC class I and to express high levels of costimulatory molecules upon activation [60, 61, 62, 63, 64].
In sum, γδ T cells can display multifaceted antitumor mechanisms, with type 1 cytotoxic γδIFN T cells taking center stage (Figs. 1 and 2). The functional dichotomy between (protective) γδIFN and (pathogenic) γδ17 T cell subsets highlights the importance of their balance in tumor immunity. In this context, we have very recently unraveled how metabolic sources and pathways differentially impact on γδIFN versus γδ17 T cells and their activities in the TME [16].
Metabolic regulation of γδ T cell subsets in cancer
Until recently, the information on γδ T cell metabolism was very scarce. By contrast, many studies on αβ T cells had shown that metabolic pathways and metabolites regulate T cell signaling, survival, differentiation, and function [65]. Metabolism is highly dynamic in the life cycle of T cells: naïve T cells display a metabolic quiescent phenotype and use the available nutrients to maximize energy production through oxidative phosphorylation (OXPHOS) in mitochondria [66]; upon activation, T cells require a metabolic reprogramming in which they engage aerobic glycolysis, i.e conversion of glucose into lactate, in the cytoplasm [67]. While less efficient than OXPHOS to produce ATP, aerobic glycolysis generates crucial metabolic components, like glucose and lactate, important for cell growth and proliferation [68]. Further changes in metabolism also occur during the differentiation of memory T cells [69, 70], which primarily use OXPHOS, but undergo a glycolytic switch when deploying their rapid effector functions [71, 72, 73]. Our recent data demonstrate that γδIFN and γδ17 T cells also differentially engage in aerobic glycolysis versus mitochondrial respiration [16].
Metabolic dichotomy in γδ T cell subsets
We investigated the metabolic profile of γδ T cell subsets using a newly developed protocol, SCENITHTM (Single Cell mEtabolism by profilIng Translation inHibition), which is a simple method for deciphering the complex energy systems employed by immune cells [74]. This method uses the drug puromycin, whose incorporation into nascent proteins is a highly reliable readout of protein synthesis levels. The addition of specific chemical inhibitors allows the estimation of glucose dependence, mitochondrial dependence, glycolytic capacity, and fatty acid and amino acid oxidation capacity. Flow cytometry analysis, using a fluorescent antibody against puromycin, defines simultaneously the ex vivo phenotype and the energy metabolism of multiple cell populations in parallel [74]. Using this methodology, we found that γδ T cell subsets exhibit clearly distinct metabolic profiles that are imprinted during γδ thymic development and maintained in peripheral lymphoid organs and within tumors [16]. On one hand, γδIFN T cells are highly glycolytic, consistent with reports showing that glycolysis is required for the production of IFN‐γ by NK cells [75] and CD8+ T cells [76]. On the other hand, γδ17 T cells have higher mitochondrial mass (and membrane potential) and strongly rely on OXPHOS [16], similarly to CD4+ Th17 cells [77]. We found this dichotomy in γδ T cell subsets to have a transcriptional basis, including the segregation of two master regulators: Nrf1, which orchestrates mitochondrial DNA transcription, was enriched in γδ17 T cells; and Myc, which controls glycolysis, was highly overexpressed in γδIFN T cells [16].
Metabolic modulation of γδ T cell activities in the tumor microenvironment
It is well known that tumors adopt characteristics, including selected metabolic advantages linked to intense proliferation, that promote T cell hyporesponsiveness or dysfunction to escape immunity [78]. Tumor‐infiltrating immune cells can be shaped by nutrient availability in the TME. For example, glycolysis has been shown to regulate IFN‐γ production in T cells [79]; and antibody‐mediated blockade of the PD‐1/PD‐L1 immune checkpoint restored glycolytic and effector functions of tumor‐infiltrating T cells [80]. While performing aerobic glycolysis (“Warburg effect”), tumor masses heavily consume glucose, which deprives T cells and alters their metabolism and functionalities; thus, metabolic competition in the TME is a driver of cancer progression [80]. Consistently, resistance to adoptive T cell therapy is observed in patients with an increased tumor glycolysis [81]. Furthermore, it has been shown that high levels of lactate in the TME lead to diminished antitumor immunity by increasing the apoptosis of naïve T cells and inhibiting effector T cell functions [82, 83]. Importantly, amino acid restriction such as serine, glutamine or glycine deprivation also leads to an impaired function of CD8+ T cells and NK cells [84, 85, 86].
We have addressed the impact of the metabolic differences between effector γδ T cell subsets on their activities in the TME, particularly upon adoptive cell transfer. Given the high(er) glycolytic activity of γδIFN T cells, we explored the effect of glucose supplementation on their antitumor functions in vitro and in vivo. We found high (10‐fold higher concentration of) glucose to enhance γδIFN cell proliferation, IFN‐γ production, and antitumor cytotoxicity in vitro [16]. We did not consider injecting glucose directly into tumors given that it would likely benefit cancer proliferation progression [80]. Instead, we provided it to γδIFN T cells during a short (5 h) incubation before adoptive transfer (intra‐tumoural injection); this resulted in a substantial reduction in breast tumor growth in vivo (Fig. 3).
Figure 3.
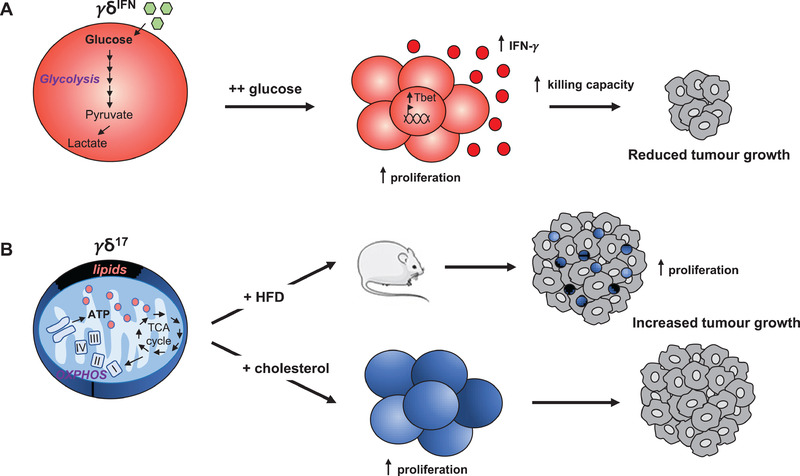
Distinct metabolic cues control γδ T cell subset expansion and functions in the TME. (A) γδIFN T cells uptake more glucose and employ aerobic glycolysis. γδIFN T cells supplemented with high dose of glucose exhibit increased (i) proliferation, (ii) Tbet expression, (iii) IFN‐γ production and (iv) killing capacity against cancer cells. Consequently, the adoptive transfer of glucose‐”boosted” γδIFN T cells substantially reduces tumor growth. (B) γδ17 T cells display high lipid uptake and intracellular lipid storage; and engage mitochondrial oxidative metabolism. γδ17 T cells are expanded in lipid‐rich environments such as in tumor‐bearing obese mice fed with high‐fat diet (HFD) leading to increase tumor growth. Likewise, cholesterol supplementation promotes γδ17 T cell proliferation and increases tumor growth after adoptive transfer.
By contrast, high glucose was detrimental to γδ17 T cell proliferation, as well as to their generation in fetal thymic organ cultures [16]. We therefore questioned which other metabolic resources could fuel γδ17 T cells and their activities in the TME. We found that lipids, including palmitate and cholesterol, are preferentially uptaken by γδ17 T cells, which become significantly expanded in tumors from high‐fat diet (HFD)‐treated obese mice (Fig. 3). Consistent with this, short‐term (5h) cholesterol treatment in vitro leads to increased proliferation of γδ17 T cells and, upon adoptive transfer, cholesterol‐treated γδ17 T cells substantially increased (compared to controls) breast tumor growth in vivo (Fig. 3). These data, suggesting that a lipid‐rich environment boosts γδ17 T cells to support tumor growth, provide a new mechanism to the body of evidence linking obesity and cancer [87], including via immunometabolism [88, 89].
In sum, we propose that the relative abundance of glucose versus lipids is a key determinant of the balance between protective γδIFN T cells and pathogenic γδ17 T cells in the TME.
Conclusions and implications for human disease
Although there are additional pro‐ versus antitumor mechanisms deployed by γδ T cells (Fig. 2; reviewed in [10]), here we focused on those contributed by γδIFN and γδ17 T cells. The concept of a functional dichotomy among γδ T cell subsets in tumor immunity may have important implications for development of γδ T cell‐based cancer immunotherapies. On that road, an outstanding question to resolve using preclinical models is how the balance between γδIFN and γδ17 T cells is regulated, particularly in the TME, toward promoting antitumor immunity. Our most recent research suggests that metabolism may provide some answers, namely by limiting lipids in the TME; and favoring aerobic glycolysis in γδ T cell‐based adoptive cell therapy (ACT) products/ strategies.
We believe that increased understanding of tumor and immune cell metabolism may be a powerful tool for the development of next‐generation immunotherapies. Targeting the metabolism of immune cells through the modulation of key metabolic pathways and/or dietary interventions in order to enhance their antitumor functions may improve cancer treatment [90]. An interesting avenue to explore within immunometabolism is the impact of different amino acids, since the TME also creates competition between tumor and T cells for essential amino acids. For example, arginine is depleted by several tumors, which leads to inhibition of T cell activation and an immunosuppressive environment [91, 92]. Thus, optimizing arginine concentrations has a direct impact on the metabolism and survival of T cells [92]; and one may consider providing additional arginine to cancer patients with reduced plasma arginine levels. Another example is glutamine, as glutaminase activity, which converts glutamine to glutamate, is required to promote Th1 and cytotoxic CD8+ T cell functions [93]. Finally, it has also been shown tumor cells express IDO, an enzyme that depletes tryptophan and thus inhibits T cell proliferation [94]. Interestingly, it has been recently shown that IDO inhibitors enhanced human γδ T cell cytotoxicity against pancreatic ductal adenocarcinoma [95]. Clinical trials with IDO inhibitors in advanced solid tumors are ongoing.
We find the context of ACT particularly interesting to modulate T‐cell metabolism without having undesired effects on tumor cells. For example, modulating activated T cells to have memory‐like metabolism, weighted toward oxidative phosphorylation (OXPHOS) and fatty acid oxidation [90], or supplementing with high doses of glucose to increase the cytotoxic activity of Th1, CD8+ or γδ T cells, may greatly improve antitumor responses in vivo (upon ACT). Thus, enhancing “metabolic fitness” of effector T cells with metabolic resources or pharmacologic agents targeting key metabolic pathways may improve the clinical efficacy of T cell‐based therapies. Like glucose supplementation, the addition of specific amino acids to expansion protocols for ACT or even as dietary supplement may also regulate γδ T cell (subset) functions in the TME. This hypothesis deserves furthern investigation.
It is of interest to combine metabolic modulation with immune checkpoint blockade to improve cancer treatment. In fact, both CTLA‐4 and PD‐1 inhibit T‐cell activation via suppression of metabolic activity, including the downregulation of AKT phosphorylation, and decreased glucose and amino acid uptake [96, 97]. On the other hand, CTLA‐4 and PD‐1 also interfere with CD28 signaling [98], which is known to sustain glycolysis and to prime mitochondria during T cell activation [99]. Interestingly, it has been recently shown that in vivo triggering of 4‐1BB costimulation, in combination with PD‐1 blockade, results in robust antitumor immunity in a B16 melanoma model [100]. Thus, metabolic modulation and immune checkpoint blockade may constitute a promising combination to increase the efficacy of cancer immunotherapy.
Although not detailed in this review, another major factor to consider is the microbiome, based on a very interesting link recently uncovered between commensal microbiota and pro‐tumoural γδ17 T cells in mouse models of lung cancer. The bacteria were shown to induce the production of IL‐1β and IL‐23 by myeloid cells, which triggered high proliferation and activation of Vγ6+Vδ1+ tissue‐resident γδ17 T cells that supported tumor growth [22].
Pre‐clinical models are also extremely valuable to dissect the cellular crosstalks that may control the balance between tumor‐infiltrating γδIFN and γδ17 T cells. In this regard, we have demonstrated that neutrophils can suppress γδ17 T cell responses, while not affecting γδIFN T cells, through ROS‐mediated oxidative stress [34]. Other positive or negative regulators of γδ T cell subsets in the TME, including regulatory T cells [101], should be better defined in future research.
Ultimately, one should consider the relevance of such findings on mouse γδ T cells to human cancer, particularly as the correspondence between murine and human γδ T cell subsets is not straightforward. Namely, the Vγ‐based definition of murine γδ T cell subsets does not apply to humans, where the main sub‐populations are defined by the Vδ (segment) usage of their TCR [102]. Thus, in humans, Vδ1+ T cells are enriched within healthy (as well as malignant) tissues, whereas Vδ2+ T cells (mostly Vγ9+) predominate in the peripheral blood [14]. Both subsets have been clearly implicated in cancer immunity [10, 11, 103], especially given their very strong cytotoxic type 1 (γδIFN) biases [10, 104]. In fact, γδ17 T cells are much less frequent in humans than in mice, and the presence of human γδ17 T cells in cancer is somewhat controversial. While Wu and colleagues reported that human γδ T cells are the major cellular source of IL‐17 in colorectal cancer [28], a more recent study demonstrated by using three different flow cytometry gating strategies that the majority of IL‐17‐producing leukocytes in colorectal cancer were CD3+ but not Vδ1+ or Vδ2+ T cells, suggesting they are mostly Th17 cells [29]. Nonetheless, γδ17 T cell infiltration was shown to correlate with poor survival in gallbladder cancer in humans, in sharp contrast with γδIFN T cells [105]. Thus, the balance between γδIFN and γδ17 T cells may be informative also in human cancer, although its value as a potential biomarker remains to be established.
Author contributions
N.L. and B.S.‐S. conceived and wrote the manuscript.
Conflicts of interest
B.S.‐S. is co‐founder and shareholder of Lymphact S.A., a start‐up company acquired by GammaDelta Therapeutics (London, UK). N.L. declared no commercial or financial conflicts of interest.
Abbreviations
- γδ
gamma delta
- ACT
adoptive cell therapy
- DETC
dendritic epidermal γδ T cell
- HFD
high‐fat diet
- IFN‐γ
interferon‐gamma
- IL‐17
interleukin‐17
- LN
lymph node
- MCA
methylcholanthrene
- MDSC
myeloid‐derived suppressor cell
- OXPHOS
oxidative phosphorylation
- SCENITH
single cell metabolism by profiling translation inhibition
- SPM
small peritoneal macrophage
- TCR
T‐cell receptor
- TME
tumor microenvironment
- VEGF
vascular endothelial growth factor
Acknowledgments
We thank Sofia Mensurado and Karine Serre for helpful discussions on this topic. Our work is supported by “la Caixa” Foundation's Health Research Program, project HR18‐00069; PAC‐PRECISE LISBOA‐01‐0145‐FEDER‐016394, co‐funded by FEDER (POR Lisboa 2020) and Fundação para a Ciência e a Tecnologia (Portugal); and N.L. received an EMBO longterm fellowship (ALTF 752–2018).
References
- 1. Ribot, J. C. , Lopes, N. and Silva‐Santos, B. , γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2020. (in press). [DOI] [PubMed] [Google Scholar]
- 2. Prinz, I. , Silva‐Santos, B. and Pennington, D. J. , Functional development of gammadelta T cells. Eur. J. Immunol. 2013. 43: 1988–1994. [DOI] [PubMed] [Google Scholar]
- 3. Munoz‐Ruiz, M. , Sumaria, N. , Pennington, D. J. and Silva‐Santos, B. , Thymic determinants of gammadelta T cell differentiation. Trends Immunol. 2017. 38: 336–344. [DOI] [PubMed] [Google Scholar]
- 4. Sumaria, N. , Martin, S. and Pennington, D. J. , Developmental origins of murine gammadelta T‐cell subsets. Immunology 2019. 156: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribot, J. C. , deBarros, A. , Pang, D. J. , Neves, J. F. , Peperzak, V. , Roberts, S. J. , Girardi, M. et al., CD27 is a thymic determinant of the balance between interferon‐gamma‐ and interleukin 17‐producing gammadelta T cell subsets. Nat. Immunol. 2009. 10: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sumaria, N. , Grandjean, C. L. , Silva‐Santos, B. and Pennington, D. J. , Strong TCRgammadelta signaling prohibits thymic development of IL‐17A‐secreting gammadelta T cells. Cell Rep. 2017. 19: 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munoz‐Ruiz, M. , Ribot, J. C. , Grosso, A. R. , Goncalves‐Sousa, N. , Pamplona, A. , Pennington, D. J. , Regueiro, J. R. et al., TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat. Immunol. 2016. 17: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiala, G. J. , Gomes A.Q. and Silva‐Santos, B. , From thymus to periphery: molecular basis of effector γδ T cell differentiation. Immunol. Rev. 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papotto, P. H. , Ribot, J. C. and Silva‐Santos, B. , IL‐17(+) gammadelta T cells as kick‐starters of inflammation. Nat. Immunol. 2017. 18: 604–611. [DOI] [PubMed] [Google Scholar]
- 10. Silva‐Santos, B. , Mensurado, S. and Coffelt, S. B. , gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer 2019. 19: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sebestyen, Z. , Prinz, I. , Dechanet‐Merville, J. , Silva‐Santos, B. and Kuball, J. , Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2020. 19: 169–184. [DOI] [PubMed] [Google Scholar]
- 12. Benveniste, P. M. , Roy, S. , Nakatsugawa, M. , Chen, E. L. Y. , Nguyen, L. , Millar, D. G. , Ohashi, P. S. et al., Generation and molecular recognition of melanoma‐associated antigen‐specific human gammadelta T cells. Sci. Immunol. 2018. 3: 333–336. [DOI] [PubMed] [Google Scholar]
- 13. Hayday, A. C. , Gammadelta T cells and the lymphoid stress‐surveillance response. Immunity 2009. 31: 184–196. [DOI] [PubMed] [Google Scholar]
- 14. Silva‐Santos, B. , Serre, K. and Norell, H. , gammadelta T cells in cancer. Nat. Rev. Immunol. 2015. 15: 683–691. [DOI] [PubMed] [Google Scholar]
- 15. Heilig, J. S. and Tonegawa, S. , Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 1986. 322: 836–840. [DOI] [PubMed] [Google Scholar]
- 16. Lopes, N. , McIntyre, C. , Martin, S. , Raverdeau, M. , Sumaria, N. , Kohlgruber, AC. , Fiala, G. et al., Distinct metabolic programmes established in the thymus control the effector functions of γδ T cell subsets in tumour microenvironments. Nat. Immunol. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kato, T. , Furumoto, H. , Ogura, T. , Onishi, Y. , Irahara, M. , Yamano, S. , Kamada, M. et al., Expression of IL‐17 mRNA in ovarian cancer. Biochem. Biophys. Res. Commun. 2001. 282: 735–738. [DOI] [PubMed] [Google Scholar]
- 18. Numasaki, M. , Watanabe, M. , Suzuki, T. , Takahashi, H. , Nakamura, A. , McAllister, F. , Hishinuma, T. et al., IL‐17 enhances the net angiogenic activity and in vivo growth of human non‐small cell lung cancer in SCID mice through promoting CXCR‐2‐dependent angiogenesis. J. Immunol. 2005. 175: 6177–6189. [DOI] [PubMed] [Google Scholar]
- 19. Chen, H. C. , Eling, N. , Martinez‐Jimenez, C. P. , O'Brien, L. M. , Carbonaro, V. , Marioni, J. C. , Odom, D. T. et al., IL‐7‐dependent compositional changes within the gammadelta T cell pool in lymph nodes during ageing lead to an unbalanced anti‐tumour response. EMBO Rep. 2019. 20: e47379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma, S. , Cheng, Q. , Cai, Y. , Gong, H. , Wu, Y. , Yu, X. , Shi, L. et al., IL‐17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014. 74: 1969–1982. [DOI] [PubMed] [Google Scholar]
- 21. Coffelt, S. B. , Kersten, K. , Doornebal, C. W. , Weiden, J. , Vrijland, K. , Hau, C. S. , Verstegen, N. J. M. et al., IL‐17‐producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015. 522: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin, C. , Lagoudas, G. K. , Zhao, C. , Bullman, S. , Bhutkar, A. , Hu, B. , Ameh, S. et al., Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 2019. 176: 998–1013 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rei, M. , Goncalves‐Sousa, N. , Lanca, T. , Thompson, R. G. , Mensurado, S. , Balkwill, F. R. , Kulbe, H. et al., Murine CD27(−) Vgamma6(+) gammadelta T cells producing IL‐17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl. Acad. Sci. U. S. A. 2014. 111: E3562–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Hede, D. , Polese, B. , Humblet, C. , Wilharm, A. , Renoux, V. , Dortu, E. , de Leval, L. et al., Human papillomavirus oncoproteins induce a reorganization of epithelial‐associated gammadelta T cells promoting tumor formation. Proc. Natl. Acad. Sci. U. S. A. 2017. 114: E9056–E9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carmi, Y. , Rinott, G. , Dotan, S. , Elkabets, M. , Rider, P. , Voronov, E. and Apte, R. N. , Microenvironment‐derived IL‐1 and IL‐17 interact in the control of lung metastasis. J. Immunol. 2011. 186: 3462–3471. [DOI] [PubMed] [Google Scholar]
- 26. McAllister, F. , Bailey, J. M. , Alsina, J. , Nirschl, C. J. , Sharma, R. , Fan, H. , Rattigan, Y. et al., Oncogenic Kras activates a hematopoietic‐to‐epithelial IL‐17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 2014. 25: 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakita, D. , Sumida, K. , Iwakura, Y. , Nishikawa, H. , Ohkuri, T. , Chamoto, K. , Kitamura, H. et al., Tumor‐infiltrating IL‐17‐producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010. 40: 1927–1937. [DOI] [PubMed] [Google Scholar]
- 28. Wu, P. , Wu, D. , Ni, C. , Ye, J. , Chen, W. , Hu, G. , Wang, Z. et al., gammadeltaT17 cells promote the accumulation and expansion of myeloid‐derived suppressor cells in human colorectal cancer. Immunity 2014. 40: 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meraviglia, S. , Lo Presti, E. , Tosolini, M. , La Mendola, C. , Orlando, V. , Todaro, M. , Catalano, V. et al., eDistinctive features of tumor‐infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology 2017. 6: e1347742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulig, P. , Burkhard, S. , Mikita‐Geoffroy, J. , Croxford, A. L. , Hovelmeyer, N. , Gyulveszi, G. , Gorzelanny, C. et al., IL17A‐mediated endothelial breach promotes metastasis formation. Cancer Immunol. Res. 2016. 4: 26–32. [DOI] [PubMed] [Google Scholar]
- 31. He, D. , Li, H. , Yusuf, N. , Elmets, C. A. , Li, J. , Mountz, J. D. and Xu, H. , IL‐17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid‐derived suppressor cells. J. Immunol. 2010. 184: 2281–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azab, B. , Bhatt, V. R. , Phookan, J. , Murukutla, S. , Kohn, N. , Terjanian, T. and Widmann, W. D. , Usefulness of the neutrophil‐to‐lymphocyte ratio in predicting short‐ and long‐term mortality in breast cancer patients. Ann. Surg. Oncol. 2012. 19: 217–224. [DOI] [PubMed] [Google Scholar]
- 33. Benevides, L. , da Fonseca, D. M. , Donate, P. B. , Tiezzi, D. G. , De Carvalho, D. D. , de Andrade, J. M. , Martins, G. A. et al., IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015. 75: 3788–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mensurado, S. , Rei, M. , Lanca, T. , Ioannou, M. , Goncalves‐Sousa, N. , Kubo, H. , Malissen, M. et al., Tumor‐associated neutrophils suppress pro‐tumoral IL‐17+ gammadelta T cells through induction of oxidative stress. PLoS Biol. 2018. 16: e2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Girardi, M. , Glusac, E. , Filler, R. B. , Roberts, S. J. , Propperova, I. , Lewis, J. , Tigelaar, R. E. et al., The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. J. Exp. Med. 2003. 198: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Girardi, M. , Oppenheim, D. E. , Steele, C. R. , Lewis, J. M. , Glusac, E. , Filler, R. , Hobby, P. et al., Regulation of cutaneous malignancy by gammadelta T cells. Science 2001. 294: 605–609. [DOI] [PubMed] [Google Scholar]
- 37. Street, S. E. , Hayakawa, Y. , Zhan, Y. , Lew, A. M. , MacGregor, D. , Jamieson, A. M. , Diefenbach, A. et al., Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004. 199: 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He, W. , Hao, J. , Dong, S. , Gao, Y. , Tao, J. , Chi, H. , Flavell, R. et al., Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J. Immunol. 2010. 185: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu, Z. , Eltoum, I. E. , Guo, B. , Beck, B. H. , Cloud, G. A. and Lopez, R. D. , Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J. Immunol. 2008. 180: 6044–6053. [DOI] [PubMed] [Google Scholar]
- 40. Gao, Y. , Yang, W. , Pan, M. , Scully, E. , Girardi, M. , Augenlicht, L. H. , Craft, J. et al., Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003. 198: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turchinovich, G. and Hayday, A. C. , Skint‐1 identifies a common molecular mechanism for the development of interferon‐gamma‐secreting versus interleukin‐17‐secreting gammadelta T cells. Immunity 2011. 35: 59–68. [DOI] [PubMed] [Google Scholar]
- 42. Strid, J. , Roberts, S. J. , Filler, R. B. , Lewis, J. M. , Kwong, B. Y. , Schpero, W. , Kaplan, D. H. et al., Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008. 9: 146–154. [DOI] [PubMed] [Google Scholar]
- 43. Cao, G. , Wang, Q. , Li, G. , Meng, Z. , Liu, H. , Tong, J. , Huang, W. et al., mTOR inhibition potentiates cytotoxicity of Vgamma4 gammadelta T cells via up‐regulating NKG2D and TNF‐alpha. J. Leukoc. Biol. 2016. 100: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 44. Viey, E. , Fromont, G. , Escudier, B. , Morel, Y. , Da Rocha, S. , Chouaib, S. and Caignard, A. , Phosphostim‐activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J. Immunol. 2005. 174: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 45. Alexander, A. A. , Maniar, A. , Cummings, J. S. , Hebbeler, A. M. , Schulze, D. H. , Gastman, B. R. , Pauza, C. D. et al., Isopentenyl pyrophosphate‐activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 2008. 14: 4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Todaro, M. , D'Asaro, M. , Caccamo, N. , Iovino, F. , Francipane, M. G. , Meraviglia, S. , Orlando, V. et al., Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J. Immunol. 2009. 182: 7287–7296. [DOI] [PubMed] [Google Scholar]
- 47. Gertner‐Dardenne, J. , Castellano, R. , Mamessier, E. , Garbit, S. , Kochbati, E. , Etienne, A. , Charbonnier, A. et al., Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J. Immunol. 2012. 188: 4701–4708. [DOI] [PubMed] [Google Scholar]
- 48. D'Asaro, M. , La Mendola, C. , Di Liberto, D. , Orlando, V. , Todaro, M. , Spina, M. , Guggino, G. et al., V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate‐sensitized, imatinib‐sensitive, and imatinib‐resistant chronic myelogenous leukemia cells. J. Immunol. 2010. 184: 3260–3268. [DOI] [PubMed] [Google Scholar]
- 49. Lo Presti, E. , Dieli, F. and Meraviglia, S. , Tumor‐infiltrating gammadelta T lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front. Immunol. 2014. 5: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pennington, D. J. , Vermijlen, D. , Wise, E. L. , Clarke, S. L. , Tigelaar, R. E. and Hayday, A. C. , The integration of conventional and unconventional T cells that characterizes cell‐mediated responses. Adv. Immunol. 2005. 87: 27–59. [DOI] [PubMed] [Google Scholar]
- 51. Li, Z. , Xu, Q. , Peng, H. , Cheng, R. , Sun, Z. and Ye, Z. , IFN‐gamma enhances HOS and U2OS cell lines susceptibility to gammadelta T cell‐mediated killing through the Fas/Fas ligand pathway. Int. Immunopharmacol. 2011. 11: 496–503. [DOI] [PubMed] [Google Scholar]
- 52. Couzi, L. , Pitard, V. , Sicard, X. , Garrigue, I. , Hawchar, O. , Merville, P. , Moreau, J. F. et al., Antibody‐dependent anti‐cytomegalovirus activity of human gammadelta T cells expressing CD16 (FcgammaRIIIa). Blood 2012. 119: 1418–1427. [DOI] [PubMed] [Google Scholar]
- 53. Gertner‐Dardenne, J. , Bonnafous, C. , Bezombes, C. , Capietto, A. H. , Scaglione, V. , Ingoure, S. , Cendron, D. et al., Bromohydrin pyrophosphate enhances antibody‐dependent cell‐mediated cytotoxicity induced by therapeutic antibodies. Blood 2009. 113: 4875–4884. [DOI] [PubMed] [Google Scholar]
- 54. Schiller, C. B. , Braciak, T. A. , Fenn, N. C. , Seidel, U. J. , Roskopf, C. C. , Wildenhain, S. , Honegger, A. et al., CD19‐specific triplebody SPM‐1 engages NK and gammadelta T cells for rapid and efficient lysis of malignant B‐lymphoid cells. Oncotarget 2016. 7: 83392–83408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riond, J. , Rodriguez, S. , Nicolau, M. L. , al Saati, T. and Gairin, J. E. , In vivo major histocompatibility complex class I (MHCI) expression on MHCIlow tumor cells is regulated by gammadelta T and NK cells during the early steps of tumor growth. Cancer Immun. 2009. 9: 10. [PMC free article] [PubMed] [Google Scholar]
- 56. Crawford, G. , Hayes, M. D. , Seoane, R. C. , Ward, S. , Dalessandri, T. , Lai, C. , Healy, E. et al., Epithelial damage and tissue gammadelta T cells promote a unique tumor‐protective IgE response. Nat. Immunol. 2018. 19: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dalessandri, T. , Crawford, G. , Hayes, M. , Castro Seoane, R. and Strid, J. , IL‐13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat. Commun. 2016. 7: 12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strid, J. , Sobolev, O. , Zafirova, B. , Polic, B. and Hayday, A. , The intraepithelial T cell response to NKG2D‐ligands links lymphoid stress surveillance to atopy. Science 2011. 334: 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Medina, B. D. , Liu, M. , Vitiello, G. A. , Seifert, A. M. , Zeng, S. , Bowler, T. , Zhang, J. Q. et al., Oncogenic kinase inhibition limits Batf3‐dependent dendritic cell development and antitumor immunity. J. Exp. Med. 2019. 216: 1359–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brandes, M. , Willimann, K. and Moser, B. , Professional antigen‐presentation function by human gammadelta T Cells. Science 2005. 309: 264–268. [DOI] [PubMed] [Google Scholar]
- 61. Altvater, B. , Pscherer, S. , Landmeier, S. , Kailayangiri, S. , Savoldo, B. , Juergens, H. and Rossig, C. , Activated human gammadelta T cells induce peptide‐specific CD8+ T‐cell responses to tumor‐associated self‐antigens. Cancer Immunol. Immunother. 2012. 61: 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mao, C. , Mou, X. , Zhou, Y. , Yuan, G. , Xu, C. , Liu, H. , Zheng, T. et al., Tumor‐activated TCRgammadelta(+) T cells from gastric cancer patients induce the antitumor immune response of TCRalphabeta(+) T cells via their antigen‐presenting cell‐like effects. J. Immunol. Res. 2014. 2014: 593562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Himoudi, N. , Morgenstern, D. A. , Yan, M. , Vernay, B. , Saraiva, L. , Wu, Y. , Cohen, C. J. et al., Human gammadelta T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J. Immunol. 2012. 188: 1708–1716. [DOI] [PubMed] [Google Scholar]
- 64. Muto, M. , Baghdadi, M. , Maekawa, R. , Wada, H. and Seino, K. , Myeloid molecular characteristics of human gammadelta T cells support their acquisition of tumor antigen‐presenting capacity. Cancer Immunol. Immunother. 2015. 64: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Almeida, L. , Lochner, M. , Berod, L. and Sparwasser, T. , Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016. 28: 514–524. [DOI] [PubMed] [Google Scholar]
- 66. van der Windt, G. J. and Pearce, E. L. , Metabolic switching and fuel choice during T‐cell differentiation and memory development. Immunol. Rev. 2012. 249: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Geltink, R. I. K. , Kyle, R. L. and Pearce, E. L. , Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 2018. 36: 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vander Heiden, M. G. , Cantley, L. C. and Thompson, C. B. , Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009. 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Araki, K. , Turner, A. P. , Shaffer, V. O. , Gangappa, S. , Keller, S. A. , Bachmann, M. F. , Larsen, C. P. et al., mTOR regulates memory CD8 T‐cell differentiation. Nature 2009. 460: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pearce, E. L. , Walsh, M. C. , Cejas, P. J. , Harms, G. M. , Shen, H. , Wang, L. S. , Jones, R. G. et al., Enhancing CD8 T‐cell memory by modulating fatty acid metabolism. Nature 2009. 460: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van der Windt, G. J. , Everts, B. , Chang, C. H. , Curtis, J. D. , Freitas, T. C. , Amiel, E. , Pearce, E. J. et al., Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012. 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Windt, G. J. , O'Sullivan, D. , Everts, B. , Huang, S. C. , Buck, M. D. , Curtis, J. D. , Chang, C. H. et al., CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. U. S. A. 2013. 110: 14336–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gubser, P. M. , Bantug, G. R. , Razik, L. , Fischer, M. , Dimeloe, S. , Hoenger, G. , Durovic, B. et al., Rapid effector function of memory CD8+ T cells requires an immediate‐early glycolytic switch. Nat. Immunol. 2013. 14: 1064–1072. [DOI] [PubMed] [Google Scholar]
- 74. Argüello, R. J. , Combes, A.J. , Char, R. , Gigan, JP. , Baaziz, A.I. , Bousiquot, E. , Camosseto, V. et al., SCENITH: A flow cytometry based method for functional profiling energy metabolism with single cell resolution. Cell Metabolism 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Donnelly, R. P. , Loftus, R. M. , Keating, S. E. , Liou, K. T. , Biron, C. A. , Gardiner, C. M. and Finlay, D. K. , mTORC1‐dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 2014. 193: 4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cham, C. M. and Gajewski, T. F. , Glucose availability regulates IFN‐gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005. 174: 4670–4677. [DOI] [PubMed] [Google Scholar]
- 77. Shin, B. , Benavides, G. A. , Geng, J. , Koralov, S. B. , Hu, H. , Darley‐Usmar, V. M. and Harrington, L. E. , Mitochondrial oxidative phosphorylation regulates the fate decision between pathogenic Th17 and regulatory T cells. Cell Rep. 2020. 30: 1898–1909 e1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O'Sullivan, D. , Sanin, D. E. , Pearce, E. J. and Pearce, E. L. , Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 2019. 19: 324–335. [DOI] [PubMed] [Google Scholar]
- 79. Chang, C. H. , Curtis, J. D. , Maggi, L. B., Jr. , Faubert, B. , Villarino, A. V. , O'Sullivan, D. , Huang, S. C. et al., Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013. 153: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chang, C. H. , Qiu, J. , O'Sullivan, D. , Buck, M. D. , Noguchi, T. , Curtis, J. D. , Chen, Q. et al., Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015. 162: 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cascone, T. , McKenzie, J. A. , Mbofung, R. M. , Punt, S. , Wang, Z. , Xu, C. , Williams, L. J. et al., Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018. 27: 977–987 e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xia, H. , Wang, W. , Crespo, J. , Kryczek, I. , Li, W. , Wei, S. , Bian, Z. et al., Suppression of FIP200 and autophagy by tumor‐derived lactate promotes naive T cell apoptosis and affects tumor immunity. Sci. Immunol. 2017. 2: eaan4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haas, R. , Smith, J. , Rocher‐Ros, V. , Nadkarni, S. , Montero‐Melendez, T. , D'Acquisto, F. , Bland, E. J. et al., Lactate regulates metabolic and pro‐inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015. 13: e1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma, E. H. , Bantug, G. , Griss, T. , Condotta, S. , Johnson, R. M. , Samborska, B. , Mainolfi, N. et al., Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017. 25: 345–357. [DOI] [PubMed] [Google Scholar]
- 85. Swamy, M. , Pathak, S. , Grzes, K. M. , Damerow, S. , Sinclair, L. V. , van Aalten, D. M. and Cantrell, D. A. , Glucose and glutamine fuel protein O‐GlcNAcylation to control T cell self‐renewal and malignancy. Nat. Immunol. 2016. 17: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Loftus, R. M. , Assmann, N. , Kedia‐Mehta, N. , O'Brien, K. L. , Garcia, A. , Gillespie, C. , Hukelmann, J. L. et al., Amino acid‐dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 2018. 9: 2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Avgerinos, K. I. , Spyrou, N. , Mantzoros, C. S. and Dalamaga, M. , Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019. 92: 121–135. [DOI] [PubMed] [Google Scholar]
- 88. Dyck, L. and Lynch, L. , Cancer, obesity and immunometabolism ‐ Connecting the dots. Cancer Lett. 2018. 417: 11–20. [DOI] [PubMed] [Google Scholar]
- 89. Michelet, X. , Dyck, L. , Hogan, A. , Loftus, R. M. , Duquette, D. , Wei, K. , Beyaz, S. et al., Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018. 19: 1330–1340. [DOI] [PubMed] [Google Scholar]
- 90. Sukumar, M. , Kishton, R. J. and Restifo, N. P. , Metabolic reprograming of anti‐tumor immunity. Curr. Opin. Immunol. 2017. 46: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fletcher, M. , Ramirez, M. E. , Sierra, R. A. , Raber, P. , Thevenot, P. , Al‐Khami, A. A. , Sanchez‐Pino, D. et al., l‐Arginine depletion blunts antitumor T‐cell responses by inducing myeloid‐derived suppressor cells. Cancer Res. 2015. 75: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Geiger, R. , Rieckmann, J. C. , Wolf, T. , Basso, C. , Feng, Y. , Fuhrer, T. , Kogadeeva, M. et al., L‐arginine modulates T cell metabolism and enhances survival and anti‐tumor activity. Cell 2016. 167: 829–842 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Johnson, M. O. , Wolf, M. M. , Madden, M. Z. , Andrejeva, G. , Sugiura, A. , Contreras, D. C. , Maseda, D. et al., Distinct regulation of Th17 and Th1 cell differentiation by glutaminase‐dependent metabolism. Cell 2018. 175: 1780–1795 e1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Munn, D. H. and Mellor, A. L. , Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013. 34: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jonescheit, H. , Oberg, H. H. , Gonnermann, D. , Hermes, M. , Sulaj, V. , Peters, C. , Kabelitz, D. et al., Influence of indoleamine‐2,3‐dioxygenase and its metabolite kynurenine on gammadelta T cell cytotoxicity against ductal pancreatic adenocarcinoma cells. Cells 2020. 9: 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patsoukis, N. , Bardhan, K. , Chatterjee, P. , Sari, D. , Liu, B. , Bell, L. N. , Karoly, E. D. et al., PD‐1 alters T‐cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015. 6: 6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Parry, R. V. , Chemnitz, J. M. , Frauwirth, K. A. , Lanfranco, A. R. , Braunstein, I. , Kobayashi, S. V. , Linsley, P. S. et al., CTLA‐4 and PD‐1 receptors inhibit T‐cell activation by distinct mechanisms. Mol. Cell. Biol. 2005. 25: 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hui, E. , Cheung, J. , Zhu, J. , Su, X. , Taylor, M. J. , Wallweber, H. A. , Sasmal, D. K. et al., T cell costimulatory receptor CD28 is a primary target for PD‐1‐mediated inhibition. Science 2017. 355: 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klein Geltink, R. I. , O'Sullivan, D. , Corrado, M. , Bremser, A. , Buck, M. D. , Buescher, J. M. , Firat, E. et al., Mitochondrial Priming by CD28. Cell 2017. 171: 385–397 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Menk, A. V. , Scharping, N. E. , Rivadeneira, D. B. , Calderon, M. J. , Watson, M. J. , Dunstane, D. , Watkins, S. C. et al., 4‐1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018. 215: 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yi, Y. , He, H. W. , Wang, J. X. , Cai, X. Y. , Li, Y. W. , Zhou, J. , Cheng, Y. F. et al., The functional impairment of HCC‐infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta‐ and IL‐10‐dependent manner. J. Hepatol. 2013. 58: 977–983. [DOI] [PubMed] [Google Scholar]
- 102. Adams, E. J. , Gu, S. and Luoma, A. M. , Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015. 296: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bonneville, M. and Scotet, E. , Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006. 18: 539–546. [DOI] [PubMed] [Google Scholar]
- 104. Wu, Y. , Kyle‐Cezar, F. , Woolf, R. T. , Naceur‐Lombardelli, C. , Owen, J. , Biswas, D. , Lorenc, A. et al., An innate‐like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple‐negative breast cancer. Sci. Transl. Med. 2019. 11: eaax9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Patil, R. S. , Shah, S. U. , Shrikhande, S. V. , Goel, M. , Dikshit, R. P. and Chiplunkar, S. V. , IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 2016. 139: 869–881. [DOI] [PubMed] [Google Scholar]


