Abstract
Objective
To assess the safety and efficacy of RSLV‐132, an RNase Fc fusion protein, in a phase II randomized, double‐blind, placebo‐controlled clinical trial in patients with primary Sjögren’s syndrome (SS).
Methods
Thirty patients with primary SS were randomized to receive treatment with RSLV‐132 or placebo intravenously once per week for 2 weeks, and then every 2 weeks for 12 weeks. Eight patients received placebo and 20 patients received RSLV‐132 at a dose of 10 mg/kg. Clinical efficacy measures included the European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index, EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F), Profile of Fatigue (ProF), and the Digit Symbol Substitution Test (DSST).
Results
Patients randomized to receive RSLV‐132 experienced clinically meaningful improvements in the ESSPRI score (P = 0.27), FACIT‐F score (P = 0.05), ProF score (P = 0.07), and DSST (P = 0.02) from baseline to day 99, whereas patients who received placebo showed no changes in any of these clinical efficacy measures. This improvement was significantly correlated with increased expression of selected interferon‐inducible genes (Pearson’s correlations, each P < 0.05).
Conclusion
Administration of RSLV‐132 improved severe fatigue, as determined by 4 independent patient‐reported measures of fatigue, in patients with primary SS.
INTRODUCTION
Primary Sjögren’s syndrome (SS) is a common chronic autoimmune disease that affects primarily women in the middle decades of life (1, 2). In the majority of patients with primary SS, the disease is either mild or moderate, and common symptoms include profound fatigue, joint pain, and ocular and/or oral dryness. An estimated 70% of patients with primary SS report profound, debilitating fatigue as the single symptom that has the greatest negative impact on their quality of life. The biochemical basis of profound fatigue associated with primary SS continues to be an area of intense research effort, with many investigations focusing on the role of cytokines (3).
Chronic inflammation accompanied by activation of interferon (IFN)–inducible genes, mimicking the immune response to a viral infection, are common biochemical findings in patients with primary SS (4, 5). One of the most fundamental roles of the immune system is to detect and respond to infection by retroviruses. Toll‐like receptors and other pattern‐recognition receptors are exquisitely sensitive to the presence of circulating RNA and respond with a robust activation of the innate immune system (6, 7). In the case of autoimmune disease, such as primary SS and systemic lupus erythematosus (SLE), the presence of circulating autoantibodies that present RNA autoantigens to the immune system mimics retroviral infection and drives chronic inflammation. Approximately 80% of patients with primary SS have anti‐Ro/SSA autoantibodies, which bind to autoantigens containing small noncoding RNA molecules (8, 9, 10). These immune complexes are known to trigger inflammatory cytokine production in vitro (11). Furthermore, a large observational study in SLE patients demonstrated a correlation between the presence of RNA‐containing immune complexes, chronic IFN pathway activation, and disease activity (12). In addition to RNA autoantigens, many noncoding RNAs are found in the circulation, and many of these noncoding RNAs possess inflammatory gene regulatory functions (13).
RSLV‐132 is a biologic drug composed of a full‐length, catalytically active human RNase moiety fused to the amino‐terminus of an engineered human IgG1 Fc domain. The drug is engineered to remain in the circulation and will not enter cells bearing Fc receptors. The RNase portion of RSLV‐132 maintains full enzymatic activity as compared to wild‐type human RNase, and has a serum half‐life of ~19 days (14). Given the body of evidence implicating circulating RNAs in patients with primary SS, the present study sought to evaluate the biologic and clinical impact of treating the symptoms of primary SS with RSLV‐132 nuclease therapy, which leads to significantly increased RNA digestion activity in the whole blood.
PATIENTS AND METHODS
Patients
For the study, we enrolled participants (ages 18–85 years) who were diagnosed as having primary SS according to the American–European Consensus Group 2002 classification criteria and who had elevated serum levels of anti‐Ro52/60 autoantibodies, based on the results of central laboratory testing, and a positive IFN signature at screening. A positive IFN signature was defined as expression levels of HERC5, CMPK2, and EPSTI1 that were 2 SD above the values in healthy volunteers (12). Study subjects were required to have been receiving stable concomitant medications for ≥30 days prior to the baseline visit. Patients were excluded on the basis of prior use of any of the following medications: hydroxychloroquine and glucocorticoids within 30 days of baseline; belimumab, abatacept, or tumor necrosis factor inhibitors within 90 days of baseline; or cyclophosphamide or rituximab within 180 days of baseline. Patients were also excluded if they had previously received head and neck radiation therapy or had lymphoma, graft‐versus‐host disease, or IgG4‐related disease.
Study design
Study subjects were randomized to receive the study treatment beginning on January 12, 2017, and the last subject follow‐up visit occurred on March 14, 2020. Randomization was conducted by computer algorithm and transmitted to an unblinded pharmacist at each of the 2 clinical evaluation sites. Subjects were randomized 3:1 (RSLV‐132:placebo) to receive 10 mg/kg RSLV‐132 or placebo on days 1, 8, 15, 29, 43, 53, 71, and 85 by intravenous infusion. The efficacy end points were measured on day 99, and safety follow‐ups were conducted on days 141, 176, and 211. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Guidelines for Good Clinical Practice. Ethics committee and institutional review board approval were obtained, and all patients provided written informed consent.
Efficacy and safety evaluations
The primary biochemical evaluations in this randomized clinical trial were the expression patterns of IFN‐inducible genes contained in 3 modules, M1.2, M3.4, and M5.12, as previously described by Chiche et al (15). Clinical efficacy measures included the European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index (ESSDAI) (16), the EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) (17), the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) (18), and the Profile of Fatigue (ProF) (19). A subset of 16 subjects completed the Digit Symbol Substitution Test (DSST), a neuropsychological evaluation of concentration and attention that measures the correct translation of numbers into symbols within 90 seconds (with results herein expressed as the mean time to complete the test) (20). Exploratory analyses included anti‐Ro52/60 autoantibody levels, erythrocyte sedimentation rate (ESR) levels, complement C3 and C4 levels, and total immunoglobulin levels. In addition, adverse events were recorded at each visit.
Analysis of gene expression
RNA sequencing of whole blood samples was performed in the laboratory at Q2 Solutions/EA Genomics in Morrisville, North Carolina. Whole blood was collected in PAXGene collection tubes on days 1 and 99 prior to study treatment. RNA was extracted and quantitated by spectrophotometry using a ThermoFisher NanoDrop 8000 instrument, and RNA integrity was assessed using an RNA 6000 Nano Assay on a Bioanalyzer 2100.
Fifty‐basepair stranded and paired‐end sequencing libraries were generated using an Illumina TruSeq Stranded Total RNA protocol with RiboZero Magnetic Gold depletion of ribosomal RNA. Libraries were sequenced on an Illumina HiSeq 2500 to a target depth of 50 million reads. Prior to gene mapping, adapter trimming, homopolymer filtering, and low‐quality read filtering were performed. Preprocessed reads were mapped to the hg38 assembly of the human genome using kallisto version 0.45.0 (21) with the use of Gencode release 31 of human reference gene annotations. For analysis of the IFN‐inducible genes, reads were mapped to Gencode release 33 (GRCh38) transcript sequences, using Salmon version 1.1.0 to obtain transcript level quantification estimates. These transcript level estimates were aggregated to gene‐level counts using Bioconductor tximport version 1.14.0. Differential gene expression analysis was carried out using Bioconductor DESeq2 version 1.26.0.
Statistical analysis
Formal hypothesis testing was not conducted in this study. To analyze the ESSDAI, ESSPRI, FACIT‐F, ProF, and DSST data, the mean values for change from baseline for each group were analyzed using separate one‐way analysis of variance models for each visit, each testing the null hypothesis that the true mean difference between treatment groups was 0, with an unadjusted significance level of α = 0.05. To analyze the rate of response to treatment in the RSLV‐132 group compared to the placebo group, a Fisher’s exact test was used for testing the null hypothesis for each clinical instrument. For analysis of gene expression data, DESeq2 estimates of the fold change in IFN‐inducible gene expression between experimental conditions using a negative binomial generalized linear regression model with a logarithmic link function were used. The fold change values and P values were derived by carrying out Wald tests on the resulting likelihood functions. In the implementation of the Wald test used by DESeq2, the maximum likelihood estimate for the fold change is divided by its standard error to obtain a test statistic, which is then compared to a standard normal distribution.
To analyze the correlation between the expression patterns of the individual genes contained in the 3 modules of IFN‐inducible genes and the scores from the patient‐reported instruments (mental fatigue, somatic fatigue, and ESSPRI), module scores for each patient were calculated as the mean normalized gene expression across all genes in each module. The normalization method used was the median of ratios method, as used by DESeq2. Pearson’s correlation coefficients were calculated to assess the correlations between changes in the mean normalized gene expression for each module and changes in the mean scores for mental fatigue, somatic fatigue, and ESSPRI.
RESULTS
Clinical efficacy outcomes
Thirty‐two subjects were screened for entry into the study, and 2 subjects not meeting the autoantibody criteria were excluded. The remaining 30 subjects were randomized into the study at 2 academic medical centers in the UK. Two subjects in the RSLV‐132 treatment group withdrew consent prior to receiving study treatment at the baseline visit. One subject in the placebo group withdrew consent after receiving at least one dose of study treatment. Twenty subjects in the RSLV‐132 group and 7 subjects in the placebo group completed the study. The modified intent‐to‐treat analysis set consisted of 28 subjects who received at least one infusion of study drug. Data from 8 subjects in the placebo group and 20 in the RSLV‐132 group were analyzed (Figure 1).
Figure 1.
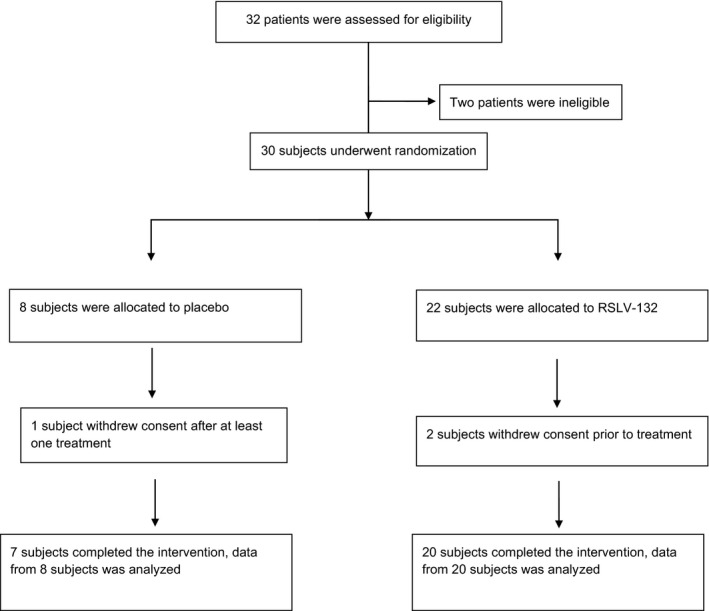
Distribution of patients with primary Sjögren’s syndrome to the randomized treatment groups, with follow‐up. Two subjects did not meet the eligibility criterion requiring serum positivity for anti‐Ro autoantibodies. Eligible patients were randomized to receive RSLV‐132 (10 mg/kg) or placebo, once weekly for 2 weeks and then every 2 weeks for 12 weeks.
Baseline demographics, disease characteristics, and biochemical data were similar between the treatment groups. The study population had mild‐to‐moderate disease activity as determined by the ESSDAI, but had high symptom burden according to the ESSPRI. Study subjects also reported experiencing profound fatigue, as indicated by scores on the FACIT‐F and ProF instruments. ESSDAI and ESSPRI scores in the placebo group were modestly higher than those in the RSLV‐132 group. Levels of C3 and C4 complement and IgG in the serum and ESR levels were similar between the 2 groups (Table 1).
Table 1.
Baseline demographic and clinical characteristics of the study patients in each randomized group*
|
Placebo (n = 8) |
RSLV‐132 (n = 20) |
|
|---|---|---|
| Age, years | 59.6 ± 8.8 | 56.5 ± 12.9 |
| Sex, % | ||
| Female | 100 | 100 |
| Male | 0 | 0 |
| Race, % | ||
| White | 87.5 | 95 |
| Asian | 12.5 | 5 |
| Ethnicity, % | ||
| Not Hispanic or Latino | 100 | 95 |
| Hispanic or Latino | 0 | 5 |
| Height, cm | 165.13 ± 4.97 | 163.22 ± 8.05 |
| Weight, kg | 81.4 ± 22.71 | 70.66 ± 13.95 |
| BMI, kg/m2 | 29.79 ± 8.20 | 26.52 ± 4.56 |
| Complement C3, mg/dl | 125.3 ± 33.1 | 134.1 ± 24.0 |
| Complement C4, mg/dl | 19.0 ± 6.2 | 19.6 ± 8.2 |
| IgG, mg/dl | 1686 ± 563 | 1683 ± 810 |
| ESR, mm/hour | 23.3 ± 12.1 | 33.2 ± 33.9 |
| ESSDAI score | 5.4 ± 4.1 | 5.0 ± 4.6 |
| Score ≤4, no. (%) | 4 (50) | 12 (60) |
| Score ≥5, no. (%) | 4 (50) | 8 (40) |
| ESSPRI score | 6.42 ± 2.48 | 5.97 ± 1.57 |
| FACIT‐F score | 23.9 ± 11.41 | 29.6 ± 12.09 |
| ProF score | 4.0 ± 1.9 | 3.5 ± 1.2 |
| Prednisone, no. (%)† | 2 (25) | 1 (5) |
Except where indicated otherwise, values are the mean ± SD. BMI = body mass index; ESR = erythrocyte sedimentation rate; ESSDAI = European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index (scale ••–••); ESSPRI = EULAR Sjögren’s Syndrome Patient Reported Index (scale ••–••); FACIT‐F = Functional Assessment of Chronic Illness Therapy–Fatigue (scale ••–••); ProF = Profile of Fatigue (scale ••–••).
Administered as a concomitant immunomodulatory medication.
The primary end point in the study was analysis of IFN‐inducible gene expression. The IFN‐inducible genes contained in the 3 modules (M1.2, M3.4, and M5.12) were analyzed for changes between study day 1 and study day 99 (Figures 2A and B; for individual genes, see Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41489/abstract). Comparison of the mean expression of the genes within these modules revealed increased expression of selected IFN‐inducible genes in the RSLV‐132–treated patients as compared to the placebo‐treated patients. For example, in module M1.2, the mean log2 fold change in gene expression between day 1 and day 99 was −0.03 in the placebo group and +0.13 in the RSLV‐132 group. For module M3.4, the mean log2 fold change in gene expression between day 1 and day 99 was −0.03 in the placebo group and +0.08 in the RSLV‐132 group. For module M5.12, the mean log2 fold change in gene expression between day 1 and day 99 was −0.06 in the placebo group and +0.03 in the RSLV‐132 group.
Figure 2.
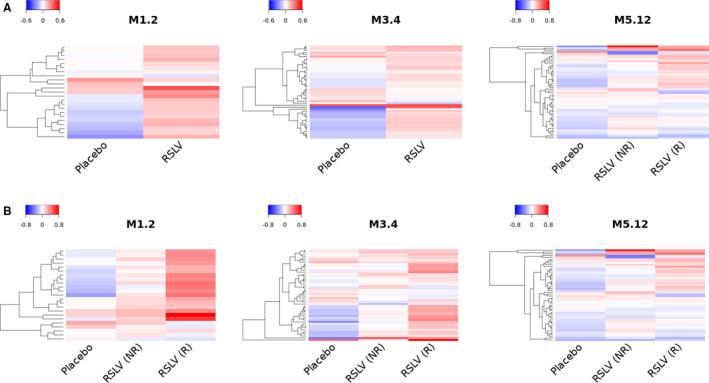
Heatmaps showing changes in expression of interferon (IFN)–inducible genes from day 1 to day 99. The log2 fold change in expression of 3 modules (M1.2, M3.4, and M5.12) of IFN‐inducible genes was assessed in whole blood samples from the placebo group (n = 7) compared to the RSLV‐132 group as a whole (n = 20) (A) or the subgroups of RSLV‐132–treated patients who either achieved a clinical response (R) or did not achieve a clinical response (NR) (B) over the follow‐up.
IFN‐inducible gene expression in the subgroup of RSLV‐132–treated patients who experienced a clinical response (designated responders; defined as subjects who achieved minimal clinically important improvement in 2 of 3 measures [ESSPRI, FACIT‐F, or ProF scores]) was compared to that in RSLV‐132–treated patients who did not experience a clinical response (designated nonresponders). The results revealed that IFN‐inducible gene up‐regulation was higher in the RSLV‐132 group compared to the placebo group. In module M1.2, the mean log2 fold change in IFN‐inducible gene expression between day 1 and day 99 was +0.07 in nonresponders and +0.25 in responders. For module M3.4, the mean log2 fold change in IFN‐inducible gene expression was +0.04 in nonresponders and +0.16 in responders. For module M5.12, the mean log2 fold change in IFN‐inducible gene expression was +0.02 in nonresponders and +0.04 in responders (Figure 2B).
The results of the Wald test analyzing the change in expression of each individual gene in each of the 3 modules failed to identify any genes that had a statistically significant difference in expression between the placebo and RSLV‐132 groups or between the responder and nonresponder subgroups over the course of the study. Further analysis of the correlation of changes in expression of these IFN‐inducible genes and performance on 3 patient‐reported outcome measures (ESSPRI, mental fatigue, and somatic fatigue scores) were conducted by analyzing the changes between day 1 and day 99 of the study. Most IFN‐inducible genes demonstrated a very weak or no correlation with the changes in the patient‐reported outcome measures, with the exception of a strong, statistically significant correlation between the mental fatigue scores and the expression of IFN‐inducible genes contained in module M1.2 among patients in the RSLV‐132–treated group (P = 0.014) (see Supplementary Table 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41489/abstract).
The secondary end point in the study was the ESSDAI, a measure of disease activity. Mean ESSDAI scores in the RSLV‐132 group remained constant, with a mean score of 5 throughout the study, whereas the mean ESSDAI score in the placebo group declined from a mean of 5 at baseline to a mean of 2.9 by day 99. This reduction was based largely on the change in ESSDAI score in 2 outlier placebo subjects who had peripheral nervous system and glandular symptoms at baseline that had resolved by day 29.
Several patient‐reported outcome instruments were used in the study to measure changes in fatigue, ocular and oral dryness, and joint pain. Subjects in the RSLV‐132 group experienced improvement in the mean ESSPRI score from baseline to day 99 (mean change from baseline −1.2 points), while subjects in the placebo group showed a mean improvement of −0.54 points (Table 2).
Table 2.
Clinical efficacy measures*
|
Placebo (n = 8) |
RSLV‐132 (n = 20) |
P between groups on day 99 |
|
|---|---|---|---|
| Mean on day 99 (mean change from baseline) | |||
| ESSDAI score | 2.9 (−2.50) | 5.0 (0.00) | 0.28† |
| ESSPRI score | 5.88 (−0.54) | 4.75 (−1.22) | 0.27† |
| ESSPRI fatigue subscale score | 6.30 (0.00) | 4.60 (−1.40) | 0.19† |
| FACIT‐F score | 25.00 (1.13) | 35.50 (5.90) | 0.05† |
| ProF score | 3.98 (−0.02) | 2.54 (−1.04) | 0.07† |
| Somatic component | 4.17 (0.00) | 2.87 (−0.80) | 0.13† |
| Mental component | 3.75 (0.06) | 2.13 (−1.53) | 0.04† |
| DSST time to complete, seconds | (+2.8) | (−16.4) | 0.02† |
| Responders on day 99, no. (%) | |||
| ≥3‐point decrease in ESSDAI | 3 (37.5) | 4 (20) | 0.75‡ |
| ≥1‐point decrease in ESSPRI | 1 (12.5) | 12 (60) | 0.06‡ |
| ≥6‐point increase in FACIT‐F | 2 (25) | 9 (45) | 0.002‡ |
| ≥1‐point decrease in ProF somatic | 2 (25) | 10 (50) | 0.37‡ |
| ≥1‐point decrease in ProF mental | 2 (25) | 11 (55) | 0.19‡ |
ESSDAI = European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index; ESSPRI = EULAR Sjögren’s Syndrome Patient Reported Index; FACIT‐F = Functional Assessment of Chronic Illness Therapy–Fatigue; ProF = Profile of Fatigue; DSST = Digit Symbol Substitution Test.
Mean values and change from baseline were compared between groups using separate one‐way analyses of variance for each visit, with each testing the null hypothesis that the true mean difference between treatment groups was 0 (unadjusted α = 0.05).
Differences in the rates of response between treatment groups were analyzed using Fisher's exact tests, with each testing the null hypothesis for each clinical instrument.
Additional clinical end points included change from baseline in the FACIT‐F score, ProF score, and DSST (measured as time to complete the test). The mean ESSPRI fatigue score was reduced in the RSLV‐132 group at day 99 (mean change from baseline −1.4 points) whereas the mean change from baseline was 0 in the placebo group (Figure 3A). Subject‐level data revealed that 25% of subjects in the placebo group and 55% of RSLV‐132–treated subjects achieved minimal clinically important improvement in the ESSPRI score by day 99 (Table 2).
Figure 3.
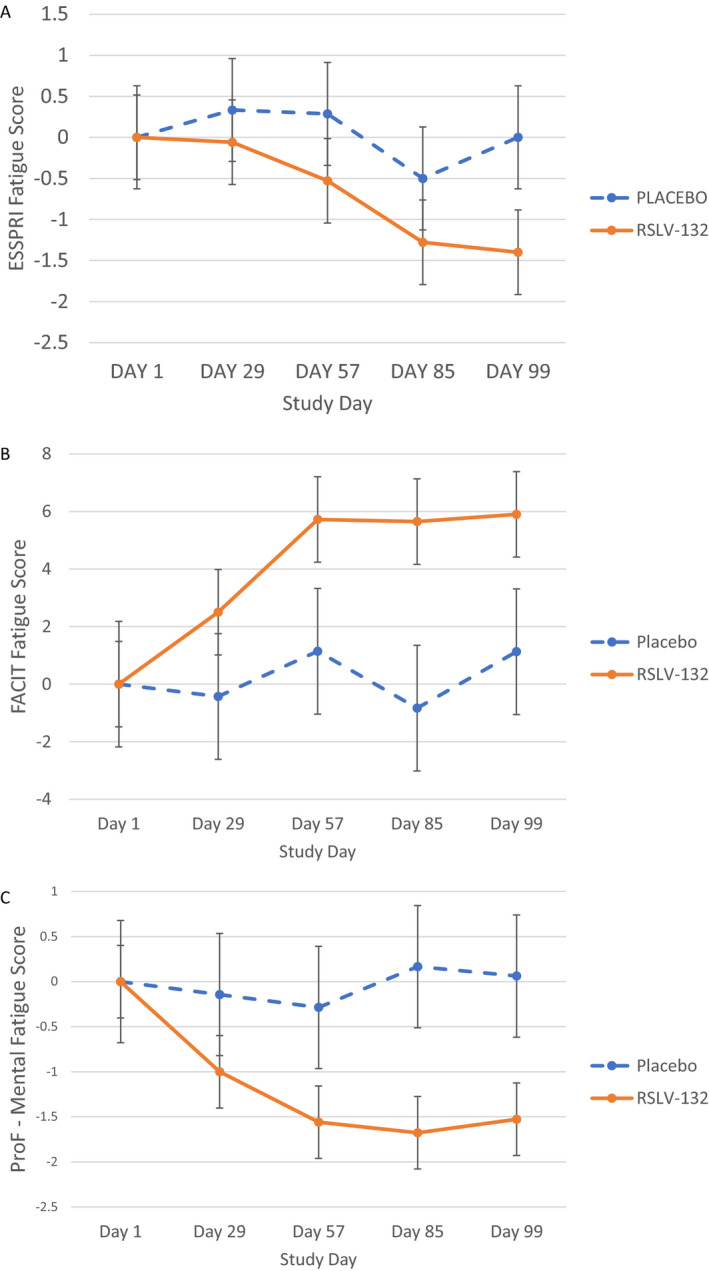
Secondary end point efficacy measures. Clinical efficacy was assessed as the mean change from baseline in the fatigue component of the European League Against Rheumatism Sjögren’s Syndrome Patient Reported Index (ESSPRI) (A), Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT‐F) (B), and mental fatigue component of the Profile of Fatigue (ProF) (C) in the RSLV‐132 and placebo treatment groups. Groups were compared using separate 1‐way analysis of variance models for each visit, each testing the null hypothesis that the true mean difference between treatment groups was 0 (unadjusted α = 0.05). Between‐group differences were as follows: P = 0.136 in A, P = 0.092 in B, and P = 0.046 in C. Results at each time point are the mean ± SEM.
The impact of RSLV‐132 on fatigue in patients with primary SS was further evaluated using 2 additional patient‐reported outcome measures, the FACIT‐F and the ProF mental fatigue scores. Mean FACIT‐F scores increased from baseline by a mean 1.13 points in the placebo group compared to a mean increase of 5.90 points in the RSLV‐132 group (Figure 3B). Subject‐level data revealed that 25% of subjects in the placebo group and 45% of the RSLV‐132–treated subjects achieved minimal clinically important improvement in the FACIT‐F score by day 99.
With respect to the mean change from baseline in the ProF mental fatigue score, the placebo group experienced a mean reduction of 0.02 points, while the RSLV‐132–treated group experienced a mean reduction of 1.04 points. The ProF can be subdivided into somatic and mental components. The placebo group did not experience an improvement in the somatic fatigue score, whereas the RSLV‐132 group had a mean decrease in the somatic fatigue score of 0.8 points. The largest change was observed in the mental fatigue component, in which the placebo group experienced a mean decrease in the mental fatigue score of 0.06 points, while the RSLV‐132 group experienced a mean decrease in the mental fatigue score of 1.53 points (Figure 3C).
Among the subset of 16 patients who were administered the DSST, patients in the placebo group were observed to have a slight worsening in the mean time to complete the DSST (mean change from baseline +2.8 seconds), whereas patients in the RSLV‐132 group showed improvement in the mean time to complete the DSST (mean change from baseline −16.4 seconds) (Table 2).
Exploratory end points included anti‐Ro/SSA levels, immunoglobulin levels, and ESR. There were no significant changes in autoantibody, immunoglobulin, or ESR levels in either treatment group during the study (data not shown). Ocular and oral dryness were measured using the Schirmer’s test (for eye dryness) and stimulated and unstimulated salivary flow tests (for mouth dryness). No meaningful differences between the RSLV‐132 and placebo groups were observed for any of these measures (data not shown).
Safety and tolerability
The incidence of treatment‐emergent adverse events, serious adverse events, and drug‐related adverse events were comparable between the RSLV‐132 and placebo treatment groups (Table 3). No deaths occurred during the study. There were no serious infections or infusion reactions observed in either treatment group during the study. No patients discontinued the study drug due to an adverse event. One patient in the RSLV‐132 group experienced a serious adverse event and was hospitalized for parotitis 88 days after receiving the last dose of study drug.
Table 3.
Treatment‐emergent adverse events (TEAEs) in the safety analysis set*
|
Placebo (n = 8) |
RSLV‐132 (n = 20) |
|
|---|---|---|
| At least one TEAE | 8 (100) | 20 (100) |
| At least one drug‐related TEAE | 5 (62.5) | 13 (65) |
| At least one serious AE | 0 | 1 (5)† |
| At least one drug‐related serious TEAE | 0 | 0 |
| Infections | 6 (75) | 16 (80) |
| Deaths | 0 | 0 |
| Most common AEs | ||
| Fatigue | 1 (12.5) | 6 (30) |
| URTI | 2 (25) | 5 (25) |
| Arthralgia | 0 | 5 (25) |
| Viral URI | 1 (13) | 4 (20) |
| Conjunctivitis | 1 (13) | 3 (15) |
| Headache | 1 (13) | 3 (15) |
| LRTI | 3 (38) | 1 (5) |
| Worsening of Sjögren’s syndrome | 2 (25) | 0 |
Values are the number (%) of patients. URTI = upper respiratory tract infection; URI = upper respiratory infection; LRTI = lower respiratory tract infection.
Hospitalized for parotitis 88 days after receiving the last dose of study drug; the event was unrelated to the study drug.
Pharmacokinetics and immunogenicity
RSLV‐132 protein concentrations in the serum were measured using a validated electrochemiluminescence‐based assay. In addition, RSLV‐132 catalytic RNase activity was measured using an RNase enzyme assay. We found that the 2 measurements were highly correlated. The median RSLV‐132 serum concentration, as determined by enzyme‐linked immunosorbent assay in the serum at steady state, was 4 μg/ml (1.3–11.0 μg/ml). The RNase catalytic enzyme activity measurement of serum drug levels was 4.6 μg/ml (2.8–12 μg/ml) at steady state on day 57 (data not shown). A clear correlation between serum RSLV‐132 levels and clinical responses was not observed in this study. Serum was assayed at multiple time points for anti–RSLV‐132 antibodies using an assay validated in accordance with US Food and Drug Administration guidance. None of the subjects in the study were positive for anti–RSLV‐132 antibodies (data not shown).
DISCUSSION
In the present study, RSLV‐132–induced up‐regulation of selected IFN‐inducible genes was observed, a finding that was unexpected based on the mechanism of action of RSLV‐132. Although increased expression of IFN‐inducible genes has historically been thought to be associated with higher disease activity, recent studies have highlighted a counterintuitive relationship between cytokines and fatigue in patients with primary SS. For example, in an observational study of 159 patients with primary SS, the serum levels of several cytokines were observed to increase as fatigue decreased (22, 23). In another observational study of 2 European cohorts of patients with primary SS, fatigue was observed to decrease as systemic IFN activity increased (24). In a third large observational study from the UK, conducted in 608 patients with primary SS, patients with the lowest symptom burden had the highest expression of IFN‐inducible genes (25). In a recent clinical study of hydroxychloroquine for the treatment of primary SS, it was observed that hydroxychloroquine treatment resulted in a significant decrease in IFN‐inducible gene expression, but no clinical improvement (26).
Furthermore, in a phase III clinical trial involving treatment of patients with SLE with anifrolumab, an anti–IFN receptor antibody, improvement in the British Isles Lupus Assessment Group–based Composite Lupus Assessment score at week 52 was greater in the active treatment group as compared to the placebo group (47.8% versus 31.5%) (27). It is unclear what impact anifrolumab might have on fatigue and other patient‐reported outcome measures, as these data await further study. Interestingly, at a biochemical level, the majority of anifrolumab‐treated patients in that previous study had a significant reduction in IFN‐inducible gene expression, although only a small subset of those subjects experienced clinical benefit incremental to placebo treatment (27). The cumulative data on the role of the IFN signature in patients with primary SS suggest that increased activation of this pathway may be a homeostatic compensatory mechanism to overcome the disease, since increased activation of the pathway is correlated with improved symptoms in primary SS.
RSLV‐132 contains a catalytically active RNase enzyme moiety, which in the context of primary SS was hypothesized to digest RNA associated with immune complexes that are inducing IFN expression from immune system cells. This would be expected to decrease IFN‐inducible gene activation. However, in the present study, increased RNase activity in the circulation resulted in an increase, not decrease, in the expression of IFN‐inducible genes in module M1.2. Since our analysis did not measure IFNα directly, but rather analyzed the expression of genes under the transcriptional regulation of IFNα as a proxy for its increase, we cannot rule out the possibility that RNA molecules in the circulation that have a negative regulatory effect on these IFN‐inducible genes were removed or decreased by RSLV‐132 treatment, thereby resulting in the increased expression of selected IFN‐inducible genes.
The presence of circulating microRNAs and their sensitivity to RNase digestion is well established, as is the regulation of various Toll‐like receptor pathways by these RNAs (6, 28). The RSLV‐132–induced increase in selected IFN‐inducible genes correlated with decreased fatigue based on several different patient‐reported outcome measures. Broad‐based improvements in the RSLV‐132 treatment group were noted in the ESSPRI scores, FACIT‐F scores, ProF scores, and time to complete the DSST test. Of note, one limitation of this study was the relatively small number of patients included in the analyses.
Notwithstanding the derivative nature and complexity of the biomarker data, the present study presents the first compelling evidence from an interventional randomized clinical trial to show that the profound fatigue experienced by patients with primary SS can be improved by pharmacologic intervention with nuclease therapy such as RSLV‐132. The data support continued development of RSLV‐132 as a treatment strategy in patients with primary SS, with future testing in additional, larger randomized clinical trials.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Posada had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Posada, Burge, Fisher, Ng.
Acquisition of data
Posada, Valadkhan, Burge, Davies, Tarn, Casement, Jobling, Gallagher, Wilson, Barone, Fisher, Ng.
Analysis and interpretation of data
Posada, Burge, Fisher, Ng.
ROLE OF THE STUDY SPONSOR
Resolve Therapeutics, LLC funded the study but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Resolve Therapeutics, LLC.
ADDITIONAL DISCLOSURE
Author Wilson is an employee of Q2 Solutions.
Supporting information
Fig S1
Table S1
Acknowledgment
We thank the patients who participated in the study and who agreed to make the data available to the scientific community.
Clinicaltrials.gov identifier: NCT03247686.
Supported by Resolve Therapeutics, LLC.
Dr. Posada owns stock or stock options in Resolve Therapeutics. Dr. Fisher has received consulting fees from Novartis, Roche, Bristol Myers Squibb, and Servier (less than $10,000 each). No other disclosures relevant to this article were reported.
REFERENCES
- 1. Haldorsen K, Bjelland I, Bolstad AI, Jonsson R, Brun JG. A five‐year prospective study of fatigue in primary Sjögren’s syndrome. Arthritis Res Ther 2011;13:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Overman CL, Kool MB, da Silva JA, Geenen R. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol 2016;35:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodewes IL, van der Spek PJ, Leon LG, Wijkhuijs AJ, van Helden‐Meeuwsen CG, Tas L, et al. Fatigue in Sjögren’s syndrome: a search for biomarkers and treatment targets. Front Immunol 2019;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nezos A, Gravani F, Tassidou A, Kapsogeorgou EK, Voulgarelis M, Koutsilieris M, et al. Type I and II interferon signatures in Sjögren’s syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjögren’s related lymphomagenesis. J Autoimmun 2015;63:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JC, Baer AN, Shah AA, Criswell LA, Shiboski CH, Rosen A, et al. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis Rheumatol 2015;67:2437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Driedonks TA, Hoen EN. Circulating Y‐RNAs in extracellular vesicles and ribonucleoprotein complexes: implications for the immune system [review]. Front Immunol 2019;9:3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katze MG, Fornek JL, Palermo RE, Walters KA, Korth MJ. Innate immune modulation by RNA viruses: emerging insights from functional genomics [review]. Nat Rev Immunol 2008;8:644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moutsopoulos HM, Zerva LV. Anti‐Ro (SSA)/La (SSB) antibodies and Sjögren’s syndrome. Clin Rheumatol 1990;9:123–30. [DOI] [PubMed] [Google Scholar]
- 9. Kattah NH, Kattah MG, Utz PJ. The U1‐snRNP complex: structural properties relating to autoimmune pathogenesis in rheumatic diseases [review]. Immunol Rev 2010;233:126–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noll F, Behnke J, Leiting S, Troidl K, Alves GT, Müller‐Redetzky H, et al. Self‐extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PLoS One 2017;12:e0190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eloranta ML, Lövgren T, Finke D, Mathsson L, Rönnelid J, Kastner B, et al. Regulation of the interferon‐α production induced by RNA‐containing immune complexes in plasmacytoid dendritic cells. Arthritis Rheum 2009;60:2418–27. [DOI] [PubMed] [Google Scholar]
- 12. Doedens JR, Jones WD, Hill K, Mason MJ, Gersuk VH, Mease PJ, et al. Blood‐borne RNA correlates with disease activity and IFN‐stimulated gene expression in systemic lupus erythematosus. J Immunol 2016;197:2854–63. [DOI] [PubMed] [Google Scholar]
- 13. Hur K, Kim SH, Kim JM. Potential implications of long noncoding RNAs in autoimmune diseases [review]. Immune Netw 2019;19:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burge DJ, Eisenman J, Byrnes‐Blake K, Smolak P, Lau K, Cohen SB, et al. Safety, pharmacokinetics, and pharmacodynamics of RSLV‐132, an RNase‐Fc fusion protein in systemic lupus erythematosus: a randomized, double‐blind, placebo‐controlled study. Lupus 2017;26:825–34. [DOI] [PubMed] [Google Scholar]
- 15. Chiche L, Jourde‐Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol 2014;66:1583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al, on behalf of the EULAR Sjögren’s Task Force . EULAR Sjögren’s Syndrome Disease Activity Index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann Rheum Dis 2010;69:1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seror R, Ravaud P, Mariette X, Bootsma H, Theander E, Hansen A, et al, on behalf of the EULAR Sjögren’s Task Force . EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjögren’s syndrome. Ann Rheum Dis 2011;70:968–72. [DOI] [PubMed] [Google Scholar]
- 18. Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811–9. [PubMed] [Google Scholar]
- 19. Bowman SJ, Booth DA, Platts RG, UK Sjögren’s Interest Group . Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology (Oxford) 2004;43:758–64. [DOI] [PubMed] [Google Scholar]
- 20. Wechsler D. Wechsler Adult Intelligence Scale‐Revised. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 21. Bray NL, Pimentel H, Melsted P, Pachter L. Near‐optimal probabilistic RNA‐seq quantification. Nat Biotechnol 2016;34:525–7. [DOI] [PubMed] [Google Scholar]
- 22. Tripp HN, Tarn J, Natasari A, Gillespie C, Mitchell S, Hackett KL, et al. Fatigue in primary Sjögren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open 2016;2:e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies K, Mirza K, Tarn J, Howard‐Tripp N, Bowman SJ, Lendrem D, et al. Fatigue in primary Sjögren’s syndrome (pSS) is associated with lower levels of proinflammatory cytokines: a validation study. Rheumatol Int 2019;39:1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bodewes IL, Al‐Ali S, van Helden‐Meeuwsen CG, Maria NI, Tarn J, Lendrem DW, et al. Systemic interferon type I and type II signatures in primary Sjögren’s syndrome reveal differences in biological disease activity. Rheumatology (Oxford) 2018;57:921–30. [DOI] [PubMed] [Google Scholar]
- 25. Tarn JR, Howard‐Tripp N, Lendrem DW, Mariette X, Saraux A, Devauchelle‐Pensec V, et al. Symptom‐based stratification of patients with primary Sjögren’s syndrome: multi‐dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol 2019;1:e85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bodewes IL, Gottenberg JE, van Helden‐Meeuwsen CG, Mariette X, Versnel MA. Hydroxychloroquine treatment downregulates systemic interferon activation in primary Sjögren’s syndrome in the JOQUER randomized trial. Rheumatology (Oxford) 2020;59:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 28. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Givson DF, et al. Argonaut‐2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011;108:5003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


