Abstract
Purpose
To ascertain the safety of soft contact lens (SCL) wear in children through a retrospective chart review including real‐world clinical practice settings.
Methods
The study reviewed clinical charts from 963 children: 782 patients in 7 US eye care clinics and 181 subjects from 2 international randomised clinical trials (RCTs). Subjects were first fitted while 8–12 years old with various SCL designs, prescriptions and replacement schedules, and observed through to age 16. Clinical records from visits with potential adverse events (AEs) were electronically scanned and reviewed to consensus by an Adjudication Panel.
Results
The study encompassed 2713 years‐of‐wear and 4611 contact lens visits. The cohort was 46% male, 60% were first fitted with daily disposable SCLs, the average age at first fitting was 10.5 years old, with a mean of 2.8 ± 1.5 years‐of‐wear of follow‐up observed. There were 122 potential ocular AEs observed from 118/963 (12.2%) subjects; the annualised rate of non‐infectious inflammatory AEs was 0.66%/year (95% CI 0.39–1.05) and 0.48%/year (0.25–0.82) for contact lens papillary conjunctivitis. After adjudication, two presumed or probable microbial keratitis (MK) cases were identified, a rate of 7.4/10 000 years‐of‐wear (95% CI 1.8–29.6). Both were in teenage boys and one resulted in a small scar without loss of visual acuity.
Conclusion
This study estimated the MK rate and the rate of other inflammatory AEs in a cohort of SCL wearers from 8 through to 16 years of age. Both rates are comparable to established rates among adults wearing SCLs.
Keywords: myopia control contact lenses, paediatric, adverse events, epidemiology
Introduction
The efficacy of myopia control soft contact lenses to slow the progression of myopia has been demonstrated in a number of clinical trials to date. 1 , 2 , 3 , 4 , 5 , 6 In 2019, the US Food & Drug Administration (FDA) approved the first soft contact lens (MiSight® 1 Day) indicated to slow the progression of myopia in children who, at the initiation of treatment, are 8–12 years of age. 7 These developments will increase the use of myopia control soft contact lenses around the world. Clinical prescribing of soft contact lenses as a myopia control strategy will continue to increase overall soft contact lens (SCL) use in children, starting at 8–12 years of age as they experience the onset of myopia. 8 , 9 Despite well‐established information on the safety of SCLs in adults, the rate of serious adverse events (AEs) with SCL use in this younger age group has not been widely studied, since there was no specific indication for their use in the paediatric population before the introduction of myopia control soft contact lenses.
Microbial keratitis (MK) related to SCL wear is the most serious AE experienced by SCL wearers, and in some instances may be sight‐threatening. 10 Fortunately, the condition is rare, but it must be differentiated from other non‐infectious corneal inflammatory events (CIEs), as MK and CIEs may require different pharmaceutical management and clinical care until the events are resolved. 11 The relative safety of SCL wear across ages 8–33 years was the focus of a large retrospective chart review conducted in 2009 by the Contact Lens Assessment in Youth (CLAY) Study Team. 12 , 13 , 14 That investigation over‐sampled for young patients aged 8–12 years old, and found that although the types of SCL‐related complications in the 8‐ to 12‐year olds was similar to that of older SCL wearers, the overall AE and CIE rates were lowest in this age group, and was less than one third of the rate for 15‐ to 25‐year‐olds. 13 , 14 This relatively low rate of complications in young SCL wearers echoed findings from an earlier study of SCL wear between 2005 and 2008, where SCL wearers from 8–12 years old again showed a much lower rate of eye care visits related to complications from SCL wear compared to older wearers. 15 Those studies were designed to estimate the rate of corneal infiltrative events (CIEs) and were not of sufficient size to determine the rate of rarer, sight‐threatening microbial keratitis (MK) in the 8‐ to 12‐year age group. The CLAY study included 243 children who were fitted between 8–12 years old, and the study of private practices had fewer than 30 wearers who were fitted at that age. 13 , 15
The purpose of this study was to ascertain the safety of SCL wear in children through a retrospective chart review that included real‐world clinical practice settings and data from randomised, controlled clinical trials.
Methods
The current study was a retrospective cohort chart review that included wearers of all types of SCLs who were fitted while they were aged 8–12 years (inclusive). It was specifically designed to be of sufficient size to estimate the rate of MK in wearers initially fitted at that age, and to characterise the AEs experienced by children and young adolescent SCL wearers. The retrospective cohort chart review utilises many of the approaches outlined by the US Food and Drug Administration’s 2017 Guidance – Use of Real‐World Evidence to Support Regulatory Decision‐Making for Medical Devices. 16 Clinical charts from young SCL wearers in seven geographically‐diverse US eye care clinics, and case report forms from two randomised clinical trials (RCTs) of MiSight® 1 Day SCLs (CooperVision, www.coopervision.co.uk) 4 , 5 conducted in Canada, Portugal, Singapore, Spain and the United Kingdom, were retrospectively reviewed to document the safety of SCL wear in children.
Eligible data were included from children aged 8–16 who were originally fitted with SCLs while they were 8–12 years old (inclusive). For each visit documented in the clinical charts and in the case report forms, the wearer’s age, visit date, chief complaint, evidence of continued SCL use, visual acuity with SCLs and results of biomicroscopy were recorded. The details of which SCLs they were wearing (refractive power, brand and replacement schedule) were only captured at the first visit. The study was expressly designed to study safety with SCL wear. No attempt to measure the progression of refractive error was made, knowing that it could not be measured precisely through a chart review.
Community sites were invited to participate in a chart review study of paediatric SCL wearers. To minimise any bias in chart selection, the sites were not informed that the primary focus was a review of adverse events. To ensure an adequate sample of children in the appropriate age group and to minimise missing data through treatment outside of the practice, sites were required to be practising primary eye care, be licenced to treat complications associated with SCL wear and to have a sufficient SCL patient population who were fitted while 8–12 years old. Adequate record keeping was mandatory and the Principal Site Investigators were trained in protecting the privacy of human research participants. Sites were chosen to include various practice settings, locations, sizes and geography. Table S1 lists the sites and investigators in the community clinics.
For the review of patient charts in community eye care clinics, an Institutional Review Board (IRB) Waiver of Informed Consent was obtained from Sterling IRB (SIRB#6156). Subjects were identified onsite by the research team using site‐specific search methods since each office’s search capabilities differed depending on their electronic medical record systems. In general, lists of patients who were in the age category were reviewed to find those who were fitted with SCLs before they turned 13 years old by searching backward in their medical records. All visits until they reached their 17th birthday were documented. If a child had not attended a visit in the previous nine months, the family was contacted via e‐mail or post in order to determine whether the child was still wearing SCLs. They were also asked whether they had “experienced any red or painful eye that required a visit to an eye doctor or emergency room since their last visit” to the prescribing practice. If so, records would be obtained from that treating office. Two sites did not agree to this active follow‐up of their patients.
The RCT audit included two investigations; the longitudinal study of MiSight® 1 Day SCLs 4 in Canada, Portugal, Singapore and the United Kingdom, and the MASS Study in Spain. 5 Principal investigators from other published RCTs of children in this age group were approached to determine whether their data could be included and AEs adjudicated, but none were able to share the data due to contractual or IRB‐related constraints. Both audits were covered under the original IRB approvals for the studies. The audit reviewed and documented the data listed above in all case report forms from subjects who (1) presented with any documented AEs, (2) had unscheduled visits or (3) discontinued the study before completion. In addition a random sample of 15% of all records were reviewed for possible signs of adverse response. Each visit for these subjects was documented in an Excel spreadsheet (Microsoft, www.microsoft.com) pre‐populated with response options.
The study sample size was planned around a matrix showing the MK rate and 95% confidence interval with various numbers of events and years‐of‐wear observed. The sample size was estimated for a single proportion in PASS 15.01.1 (NCSS Statistical Software, www.ncss.com). For example, for a study with 1‐3 potential MK events, the minimum years‐of‐wear was 2600 to estimate a rate with a reasonable confidence interval. Without knowing the results of the adjudication process at the time of the active chart review (and therefore the final number of MK cases), subjects were included until it was estimated that this adequate number of years of lens wear had been observed.
Definitions of adverse events
Similar to other North American post‐market surveillance studies of contact lenses, 17 , 18 , 19 the definition of MK was as follows:
“Presumed Microbial Keratitis”:
One or more corneal stromal infiltrates >1 mm with pain >mild plus 1 or more of: anterior chamber reaction >minimal or mucopurulent discharge or positive corneal culture.
The presence of a subsequent corneal scar was a requirement if follow‐up data and medical records were available. In the absence of data regarding resolution with a scar, aggressive treatment consistent with the standard of care for MK in North America was considered indicative (choice of medication, high frequency of dosing, etc.).
“Probable microbial keratitis” adjudication was allowed if not all of the criteria were met; for example, if the size of the lesion was less than 1 mm, the pain was minimal, there was no anterior chamber reaction, mucopurulent discharge or positive corneal culture.
All cases adjudicated as presumed or probable MK were included in the MK rate calculation. A classification of MK unrelated to contact lens wear was reserved for cases such as herpes simplex keratitis or staphylococcus marginal keratitis where, in the Adjudication Panel’s judgment, the aetiology was unrelated to SCL wear. These would not be counted as MK events in the rate calculation.
Table 1 shows the definitions used for adjudication of significant AEs, including inflammatory and mechanical events. Non‐significant AEs that were incorporated into this possible classification included SCL‐related dryness, corneal abrasion, superficial punctate keratitis, corneal oedema, allergic conjunctivitis, bacterial conjunctivitis, episcleritis, sub‐conjunctival haemorrhage and hordeolum. All adverse events were collected for review by the Panel regardless of whether they were specified as related to contact lens wear.
Table 1.
Definitions for significant adverse events*
| Adverse Event | Signs |
|---|---|
| Inflammatory Events: | |
| Contact Lens Peripheral Ulcer (CLPU) or Sterile Infiltrative Keratitis | Single, circular focal infiltrate (up to 2 mm); peripheral or mid‐peripheral location; overlying corneal staining; surrounding diffuse infiltration; no anterior chamber reaction |
| Contact Lens Acute Red Eye (CLARE) | Diffuse infiltration in peripheral cornea; multiple small focal infiltrates; overlying corneal staining uncommon |
| Infiltrative Keratitis (IK) | Diffuse or small focal infiltration in anterior stroma, with overlying corneal staining |
| Mechanical Events: | |
| Contact Lens Papillary Conjunctivitis (CLPC) | Papillae on upper tarsus, localized to one region or evenly distributed across tarsus |
| Superior Epithelial Arcuate Lesion (SEAL) | Arc shaped, greyish white, peripheral lesion in the superior cornea. Corneal staining and surrounding diffuse infiltration |
| Corneal Erosion (CE) | Discrete area of full thickness loss of epithelium; may be single or multiple |
Adapted from Sweeney. 11
Images of patient charts were electronically scanned for all visits in which a potential AE was documented, regardless of seriousness, until the AE was resolved. These included instances where biomicroscopic signs were noted at routine visits without complaints of discomfort or redness associated with SCL wear. The chart was then redacted of all personal information, site information and lens brand. Redacted records were batched and sent to the three Adjudication Panel members for independent review for diagnoses of each AE. Panel members had been chosen for their independence from the sponsor, and depth of experience as researchers or clinicians with management of complications associated with SCL wear. The Panel was not aware of the number of wearers in the dataset or the number of years observed at any point in the adjudication process. For any cases in which a unanimous diagnosis was not achieved independently, the Panel convened via telephone to discuss the AEs until they agreed to a diagnosis through a consensus process.
Data analysis
Years‐of‐wear were computed from the fitting date up to the last visit through 16 years of age. The annualised incidence rate of MK was calculated by dividing the number of MK cases by the years‐of‐wear. A Poisson distribution was used to calculate the 95% confidence interval. The event rate for significant AEs was also calculated in a similar manner. All statistical analyses were performed using SAS software Version 9.3 (SAS Institute, www.sas.com). Non‐significant AEs were tabulated and are reported, but the rate for each category was not analysed.
Results
Charts from 963 children were reviewed: 782 patients from 7 US eye care clinics and 181 subjects from two international RCTs. All of the community clinics that were approached participated by allowing access to their clinical charts. The study included 2713 years‐of‐wear and 4611 visits. The cohort was 46% male, 60% first fitted with daily disposable SCLs on average when 10.5 years old, with a mean observation period of 2.8 ± 1.5 years of wear. Details of enrolment in each of the community clinics are shown per site in Table 2 . Due to different practice sizes, the unequal contribution of subjects was expected in this retrospective cohort review, but no site contributed more than 30% of the years of wear observed in the community clinics. After the completion of the active follow‐up query, clinical status of 728/782 (93.1%) of the cohort was current within 9 months or less. The remaining 54/782 (6.9%) either had a clinical visit more than 9 months since completion of the chart review or did not respond to the active follow‐up query. This total includes 13 (1.7%) wearers for whom we could not obtain permission from the clinical site to send an active follow‐up query.
Table 2.
Details for community clinic sites
| Location | Tucson, AZ | Atlanta, GA | Scranton, PA | Westerville, OH | Campbell, CA | Kirkland, WA | Carmel, IN |
|---|---|---|---|---|---|---|---|
| Practice type |
Multi‐OD 1 Office |
Multi‐OD 1 Office |
Multi‐OD/MD 14 Offices |
Multi‐OD 4 Offices |
Multi‐OD 1 Office |
Multi‐OD 2 Offices |
Multi‐OD 2 Offices |
| N | 59 | 92 | 230 | 158 | 77 | 40 | 126 |
| Yrs observed | 140.8 | 259.0 | 640.7 | 405.3 | 226.5 | 96.4 | 365.4 |
|
% Years* |
6.6% | 12.1% | 30.0% | 19.0% | 10.6% | 4.5% | 17.1% |
OD, Doctor of Optometry; MD, Doctor of Medicine.
% of Community Clinic Years.
The random sample of 15% of all records in the RCT reviewed for possible signs of adverse response found no new AEs that had not been identified during the conduct of the trials.
The demographics and lens wear history of the observed cohorts in the seven community clinics and two RCTs are described in Table 3 . Subjects from the community clinics had been fitted with a variety of lens types and wearing regimens. All of the RCT subjects wore lenses in a daily disposable (DD) regimen.
Table 3.
Demographics and 1st lens details for observed cohort
| Community Clinic Patients | Randomized Clinical Trial Subjects | Total Cohort | |
|---|---|---|---|
|
# of Wearers N (row%) |
782 (81.2%) | 181 (18.8%) | 963 (100%) |
|
Sex – N (%) Male (col%) |
354 (45.3%) | 90 (49.7%) | 444 (46.1%) |
| Years‐of‐Wear | |||
| Total | 2134.1 years | 578.9 years | 2713.1 years |
| Mean ± SD | 2.7 ± 1.6 years | 3.2 ± 1.4 years | 2.8 ± 1.5 years |
| Range | 0.01 to 7.6 yrs | 0.02 to 5.0 yrs | 0.01 to 7.6 yrs |
| Soft CL Design (col%) | |||
| Single Vision Sphere | 511 (65.3%) | 181 (100%)* | 682 (70.8%) |
| Toric | 213 (27.2%) | ‐ | 213 (22.1%) |
| Multi‐Focal | 47 (6.0%) | ‐ | 47 (4.9%) |
| Unknown | 11 (1.4%) | ‐ | 11 (1.1%) |
| Lens Replacement Schedule | |||
| Monthly | 196 (25.1) | ‐ | 196 (20.3%) |
| 2‐Weekly | 165 (21.1%) | ‐ | 165 (17.1%) |
| Daily | 401 (51.3%) | 181 (100%) | 582 (60.4%) |
| Unknown | 20 (2.6%) | ‐‐ | 20 (2.1%) |
Or dual focus.
Age at first SCL fitting is shown in Figure 1 . The US Census data on the racial and socio‐economic demographics of the community clinic cities are shown in Table S2. This data is included to demonstrate our attempt to observe a diverse racial, economic and geographic sample of SCL wearers. Race and socioeconomic status were not captured in eye care clinic charts.
Figure 1.
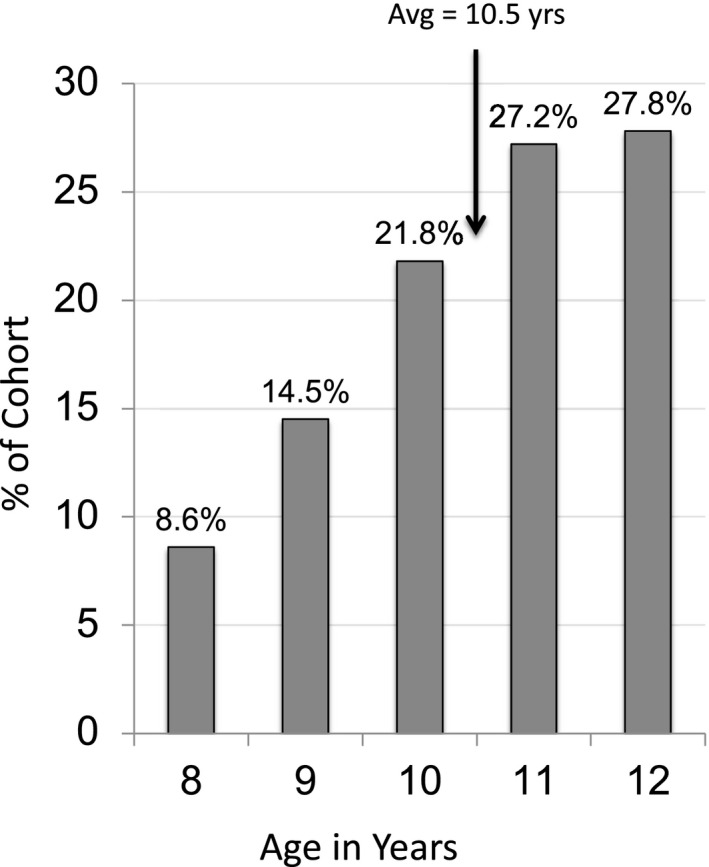
Age at 1st SCL Fitting N = 963.
A unanimous agreement from the independent adjudication was reached on less than half of the diagnoses, requiring telephone discussion to reach a consensus. Conditions that were more likely to have agreement during the independent adjudication were those that presented with a clear history and succinct signs such as sub‐conjunctival haemorrhage, hordeolum and corneal foreign body. Differences were often minor, e.g., conjunctivitis vs allergic conjunctivitis. Differentiating inflammatory conditions into the categories listed in Table 1 usually required discussion.
After adjudication, two microbial keratitis (MK) cases (one presumed and one probable) were identified from the community sites; a rate of 7.4/10 000 years‐of‐wear (95% CI 1.8–29.6). The details of each case follow. Neither of the MK events was unanimously adjudicated as MK on the initial review by the expert Panel, but these diagnoses were reached after the Panel’s independent consensus discussion. Figure 2 shows the annualised rate of MK in children wearing soft contact lenses and the other post‐market safety CL study in children utilising overnight orthokeratology lenses. 19
Figure 2.
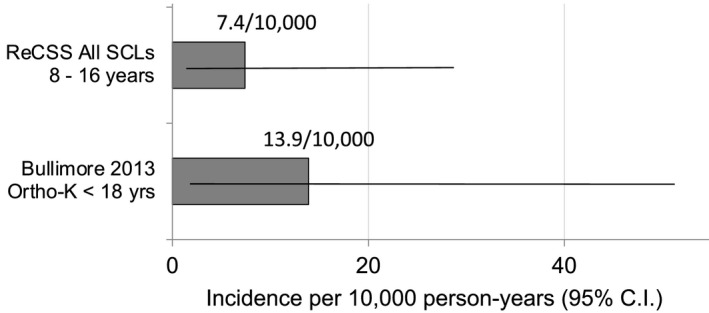
Comparison of annualised MK rate in ReCSS and other contact lens studies. Horizontal lines show the 95% confidence interval. 95% CI for ReCSS Study (N = 963): 1.8 to 29.6/10 000; Bullimore 19 2013 (N = 677): 1.7–50.4/10 000.
The first case was considered a presumed MK in a 14‐year old male who reportedly had been sleeping in his daily disposable lenses. He presented with light sensitivity and pain, a marginal ulcer in the inferior central region and no anterior chamber reaction. During an emergency room visit he was treated with ofloxacin every 30 min, then with besifloxacin every 2 h and oral ibuprofen the following day at his eye care practitioner. At resolution he had 6/6‐ acuity and an off‐axis mild scar. During the initial adjudication round, this case was judged to be a probable MK, CLPU and MK by the three adjudicators.
The second case was a probable MK in a 13‐year‐old male who wore daily disposable lenses. He presented with an irritated eye that started hurting 1 day previously, and a mid‐peripheral small infiltrate, trace cells and flare. He was initially treated with besifloxacin every 30 min for 2 h, and then every 2 h for the rest of that day. The following day he received one drop of homatropine, loteprednol every 8 h as well as combined loteprednol and tobramycin (Tobradex) every 2 h for 2 days, which was then tapered to every 6 h. At resolution he had 6/6 acuity and no scaring was noted. During the initial adjudication round, this case was judged to be IK, corneal infiltrate and probable MK.
Figure 3 shows the annualised rates of significant AEs observed in this study. The combined CIE events (IK, CLARE, CLPU) occurred at a combined annual rate of 0.66%/yr. Figure 4 illustrates the age at onset for all of the MK + CIE events.
Figure 3.
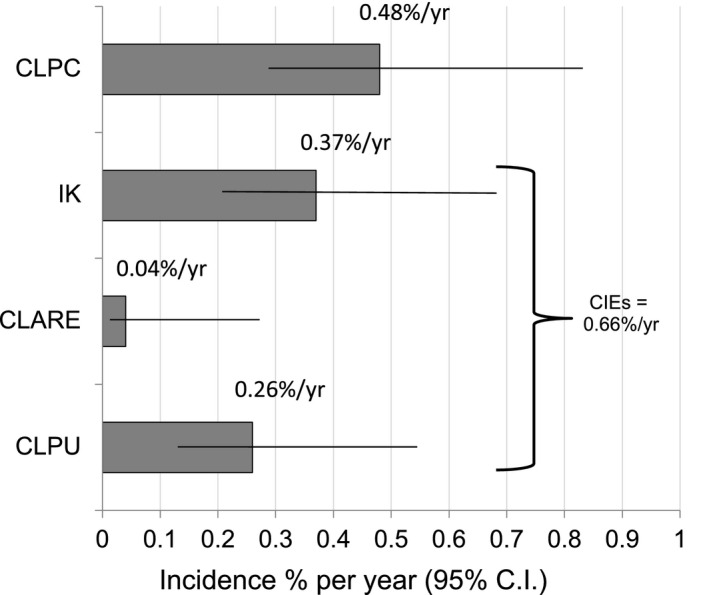
Annualised rate of Significant Adverse Events in the ReCSS Study. Horizontal lines show the 95% confidence interval. CLPC = contact lens papillary conjunctivitis, IK = infiltrative keratitis, CLARE = contact lens acute red eye, CLPU = contact lens peripheral ulcer.
Figure 4.
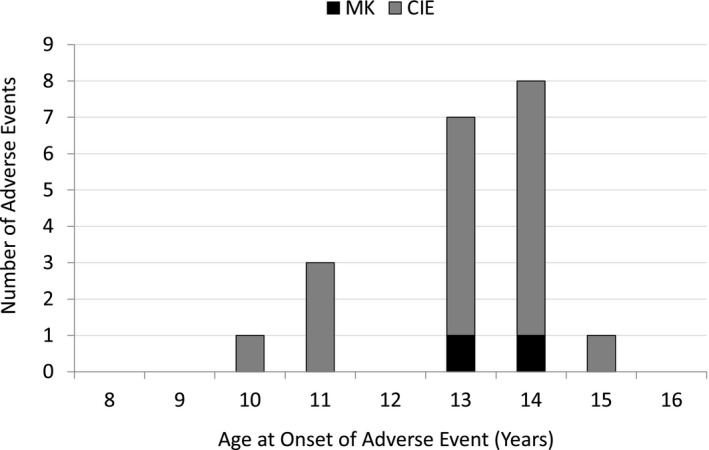
Corneal infiltrative and microbial keratitis events observed by age of onset in ReCSS Study. N = 963.
Table S3 lists the frequency of non‐significant AEs and AEs that were not related to SCL wear. A wide variety of AEs was observed, most of them easily managed by temporary discontinuation of lens wear (e.g., superficial punctate keratitis, abrasion) or therapeutic management (e.g., conjunctivitis, blepharitis). The bottom of Table S3 lists the AEs that were not related to SCL wear; three anterior chamber AEs (Herpes Zoster, post‐traumatic hyphema and a non‐specific iritis with no corneal involvement) and other cases of headache and general discomfort.
Discussion
Leading researchers in this field have concluded that, “one of the major challenges for improved uptake and acceptance of contact lenses centres on the perceived risk of complications with lens wear” 20 and that “safety of all refractive correction and management options must also remain at the forefront of practitioners’ recommendations”. 21 The current study fills a significant gap in understanding of the safety of SCL wear specifically in children and young teens who began to use lenses before they turned 13 years of age.
This retrospective cohort study design allowed the accumulation of sufficient real‐world patient experience to estimate MK with a reasonable margin of error. The age range studied aligns with the Indications for Use of the only soft contact lens currently approved for myopia control by the United States Food and Drug Administration. 7 This study of existing SCLs can be considered applicable to new myopia control soft contact lenses that use currently marketed materials with only slightly modified optics. Results from this chart review of typical SCLs should be applicable for myopia control lenses whose myopia control function is accomplished through optical designs, but are otherwise similar in all other aspects to marketed SCLs. 1 , 2 , 3 , 4 , 5 , 6
This study observed the safety outcomes in the medical charts of 782 young SCL wearers in US clinical practices and 181 subjects in two RCTs. Every effort was made to find these young wearers in a variety of clinical practice settings (size and location of practices, number of practitioners, number of locations within the practice, age of practitioners, etc.). The children were observed for an average of 2.8 years and up to 7.6 years for a total of 2713 wearer‐years. In comparison, the largest previous effort to study AEs in young SCL wearers, the CLAY 2011 study, had only 243 wearers and approximately 200 years‐of‐wear in the 8–12 year old age group; that was appropriate to find overall AE rates but was too small to estimate an annualized rate of MK. 14 The 2013 post‐market surveillance study by Bullimore et al reported on 1145 years of wear in minors up to age 18, but the proportion fitted before age 13 was not noted. 19 Figure 2 shows the rate of MK in that clinical trial relative to the current study. Additionally, in a 2017 review of serious AEs in children wearing SCLs, Bullimore found that there were no MK events reported among minors in any of the prospective clinical trials reviewed (over 1800 years of wear). 20
Figure 5 shows the annualised rates of all CIEs + MK combined in this study with the CLAY study cohort by age group. 10
Figure 5.
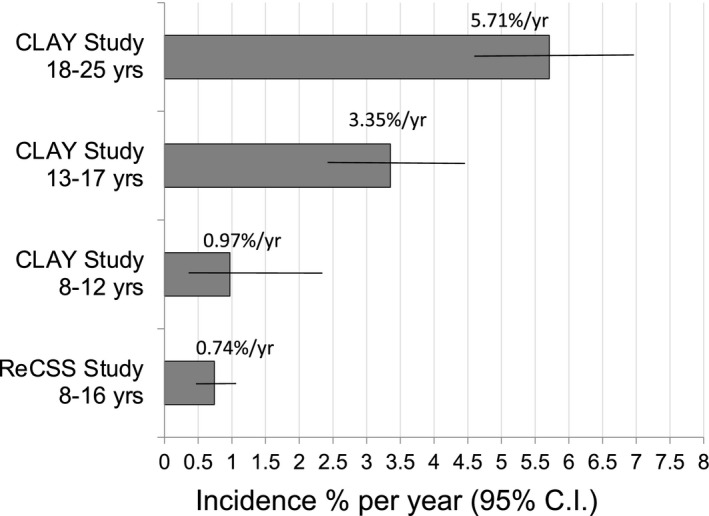
Comparison of annualised rate of Significant Inflammatory Events (All CIEs + MK) in ReCSS and the CLAY study 14 by age group. Horizontal lines show the 95% confidence interval. Sample sizes: CLAY Study 14 18–25 years N = 1129; 13–17 years N = 811; 8–12 N = 243, ReCSS Study 8–16 years N = 963.
Clinical trials of the efficacy of myopia control soft contact lenses have been designed to test the effect of lens wear on refractive error, and are typically in the range of 200 subjects followed longitudinally for at least 2 years. 21 , 22 This retrospective cohort study design allowed us to follow this large diverse cohort of young wearers fitted by many practitioners in practice. The observed follow‐up times ranged from a few days (for a small number of subjects that discontinued early in the RCT) to over seven years.
Weaknesses in the retrospective cohort approach include the inability to balance the age at first fitting across the age group from 8–12 year olds. An observational study can only examine those patients who were actually prescribed and wearing the SCLs under investigation. Also, due to the unknown number of young SCL patients that would be identified in each practice, the sequential review yielded unequal numbers of wearers at each site, because there were different numbers of SCL‐wearing children in those practices. Unequal sample sizes between sites is typical in observational studies. There were no more than 30% of the years of wear observed in any one practice. Additionally, in this type of study, without a concurrent questionnaire at the time of the events regarding how SCLs were being used (overnight wear, swimming, etc.) it is not possible to study risk factors for the AEs that were observed.
Most professional organisations recommend at least annual visits for children and SCL wearers. 23 One strength of the study was the addition of an active follow‐up step for any children who were still wearing lenses but had not been seen by the practice in the past nine months, which we were able to do in five of the seven community clinic sites. This outreach step resulted in only a small percentage (6.9%) of subjects who either did not respond with their current disposition or had not been examined in nine months, an acceptable rate for a retrospective cohort study. 24
The annualized rate of MK in this study, 7.4/10 000 years‐of‐wear, was computed from two cases; one “presumed” and one “probable” case. This rate is slightly lower, but not significantly different, from the MK rate from the other post‐market study with overnight orthokeratology CLs in children shown in Figure 2 . 19 The current study provides a lower rate and tighter confidence interval than other paediatric post‐market studies, which may offer reassurance to clinicians who are counselling parents of young children regarding the safety of myopia control soft contact lenses. The rate is also comparable to established rates of MK in adults, from 1–4/10 000 for adults with daily wear hydrogel lenses 18 and 18‐20/10 000 for overnight wear of silicone hydrogel lenses. 10 , 17 , 18
It is notable that the two MK events occurred in young teens aged 13 and 14 years old from community practices, one of whom reported overnight wear of his DD SCLs. During the adjudication process, these AEs were not unanimously judged as MK cases by all three experts but were discussed by the panel to reach that consensus diagnosis. This lack of early agreement on the diagnosis amongst the panel reflects the relatively mild severity and positive outcomes that did not affect visual acuity in either case. Most of the CIEs occurred in this age range as well.
The current results were observed in patients using purchased lenses in the “real world” and not exclusively in RCTs where lenses are often supplied for free. The authors assume that there were examples of non‐compliance of SCL‐related behaviours in this age group as in adults; some patients sleeping while wearing SCLs or wearing them while swimming, etc. 25 In fact, these young wearers may be more compliant, as the CLAY Study team, using a validated contact lens risk questionnaire, found that CL compliance was related to age, with the youngest group showing the highest level of compliance. 26 The patients’ young age does give the eye care practitioner the opportunity to engage parents as additional support for supervision of compliant lens wear. A rigorous programme of training and retraining on the best practices for safe SCL use that is engaging to young wearers will be important to the long‐term safety and success of myopia control soft contact lenses as a management option for myopia.
When combining MK plus other inflammatory AEs in this age group, the annualised rate was 0.74%/yr, which compares very well with the 0.97%/yr and 3.35%/yr reported in the CLAY study groups that were between 8–12 and 13–17 years old respectively (Figure 5 ). 14 The CLAY study was selected for comparison because it included a similar retrospective chart review study design, and had a substantial number of wearers who were fitted while they were 8–12 years old and reported events by age groups when they experienced an AE. The current findings are very similar to but slightly lower than rates from the two youngest age groups reported in the CLAY study, as shown in Figure 5 . This could be attributed to the fact that the current study had a much higher proportion of DD lens use compared with the CLAY study (8–12 year olds; 60.8% vs 15.8%). 12 This may explain the lower rate in the current study as the use of DD lenses is associated with a reduced rate of inflammatory events. 14 , 25 , 26 , 27 Inflammatory AEs are of special interest as they typically require pharmaceutical management and differential diagnosis with MK to assure there is no risk of vision loss.
The summary of non‐significant AEs experienced by these young wearers shows a relatively high rate of conjunctivitis (19 cases) and abrasion/foreign body events (14 cases), which seem reasonable in a sample of school aged children. These AE results show the safety outcomes primarily derived from routine eye care practice visits and not exclusively from clinical trials with frequent defined visits; most of these youngsters received eye examinations approximately annually, and only presented for interim visits when they experienced problems. Compared to safety results derived exclusively from clinical trials, these results are likely to be more generalizable to the post‐market experience after myopia control soft contact lenses are prescribed more widely to young patients in practice.
These results give assurance of an acceptable range of safety during SCL wear in children which will be reinforced with teaching protocols that emphasise best practices for safe SCL wear. Much of the safety discussion amongst myopia control researchers promotes the potential long‐term safety implications of reduced retinal disease and other sight‐threatening ocular abnormalities if higher levels of myopia can be avoided; 20 , 22 although it may be difficult for families and eye care practitioners to imagine that far into the future. The results of the current study help to answer parents’ and practitioners' concerns about the risk/benefit of real‐world SCL use in children and young teens and assure the relative safety of SCL use in this age group.
Author contribution
Robin L Chalmers: Conceptualization (equal); Data curation (lead); Investigation (lead); Methodology (equal); Project administration (lead); Writing‐original draft (lead); Writing‐review & editing (equal). John J McNally: Conceptualization (equal); Funding acquisition (lead); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). Paul Chamberlain: Conceptualization (equal); Funding acquisition (lead); Investigation (supporting); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). Lisa Keay: Formal analysis (equal); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting).
Disclosures
Robin Chalmers is a consultant for AcuFocus Inc., Alcon Research, Ltd., CooperVision Inc., Johnson & Johnson Vision, Inc., and Vision Service Plan, Inc. John McNally and Paul Chamberlain are employees of CooperVision, Inc. Lisa Keay is a consultant for CooperVision, Inc.
Supporting information
Table S1 Clinical Trial Sites & Investigators
Table S2 US Census data for community clinic cities
Table S3 Non‐significant adverse events (AEs) and events not related to soft contact lens wear
Acknowledgements
This study was funded by CooperVision, Inc. We thank all the eye care practitioners listed in Table S1 for their cooperation with the study. Members of the Adjudication Panel were Thomas G. Quinn, OD, MS, FAAO, Dip(CCLRT) in private practice in Athens, OH; Oliver D. Schein, MD, MPH of Johns Hopkins University Bloomberg School of Public Health in Baltimore, MD; and Loretta Szczotka‐Flynn, OD, PhD, FAAO, Dip(CCLRT) of Case Western Reserve University in Cleveland, OH. Regulatory assistance was provided by Foresight Regulatory Strategies, Inc. Access to the RCTs was facilitated by the staff of VisionCare Research, Inc of Farnham, Surrey, UK and the European University of Madrid School of Optometry. Data management assistance was provided by Julia Fulmer, Corrin Lesjak, Rebecca Purser, Nathan Sinyangwe, Cheryl Taylor and Ross Wofford.
Chalmers RL, McNally JJ, Chamberlain P, & Keay L. Adverse event rates in the retrospective cohort study of safety of paediatric soft contact lens wear: the ReCSS study. Ophthalmic Physiol Opt. 2020; 41: 84–92. 10.1111/opo.12753
References
- 1. Anstice N & Phillips J. Effect of dual‐focus soft contact lenses on axial myopia progression in children. Ophthalmology 2011; 118: 1152–1161. [DOI] [PubMed] [Google Scholar]
- 2. Walline JJ, Greiner KL, McVey KL & Jones‐Jordan LA. Multifocal contact lens myopia control. Optom Vis Sci 2013; 90: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 3. Aller TA & Wildsoet C. Myopia control with bifocal soft contact lenses: a randomized clinical trial. Optom Vis Sci 2016; 93: 344–352. [DOI] [PubMed] [Google Scholar]
- 4. Chamberlain P, Back A, Lazon P et al A 3‐year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci 2019; 96: 556–567. [DOI] [PubMed] [Google Scholar]
- 5. Ruiz‐Pomeda A, Perez‐Sanchez B, Valls I, Prieto‐Garrido FL, Gutiérrez‐Ortega R & Villa‐Collar C. MiSight Assessment Study Spain (MASS). A 2‐year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 2018; 256: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 6. Sankaridurg P, Bakaraju RC, Naduvilath T et al Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt 2019; 39: 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180035A.pdf.
- 8. Wolffsohn JS, Calossi A, Cho P et al Global trends in myopia management attitudes and strategies in clinical practice – 2019 update. Cont Lens Anterior Eye 2020; 43: 9–17. Epub 2019 Nov 21. https://pubmed.ncbi.nlm.nih.gov/31761738/?from_term=Wolffsohn%2C+contact+lens&from_page=5&from_pos=8#affiliation‐5 [DOI] [PubMed] [Google Scholar]
- 9. Sindt CW & Riley CM. Practitioner attitudes on children and contact lenses. Optometry 2011; 82: 44–51. [DOI] [PubMed] [Google Scholar]
- 10. Stapleton F, Keay L, Edwards K et al The incidence of contact lens related microbial keratitis. Opthalmology 2008; 115: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 11. Sweeney DF, Jalbert I, Covey M et al Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea 2003; 22: 435–442. [DOI] [PubMed] [Google Scholar]
- 12. Lam DY, Kinoshita BT, Jansen ME et al Contact lens assessment in youth: methods and baseline findings. Optom Vis Sci 2011; 88: 708–715. [DOI] [PubMed] [Google Scholar]
- 13. Wagner H, Chalmers R, Mitchell L et al Risk factors for interruption to soft contact lens wear in children and young adults. Optom Vis Sci 2011; 88: 973–980. [DOI] [PubMed] [Google Scholar]
- 14. Chalmers RL, Wagner H, Mitchell GL et al Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the contact lens assessment in youth (CLAY) study. Invest Ophthalmol Vis Sci 2011; 52: 6690–6696. [DOI] [PubMed] [Google Scholar]
- 15. Chalmers RL, Keay L, Long B, Bergenske P, Giles T & Bullimore MA. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci 2010; 87: 725–735. [DOI] [PubMed] [Google Scholar]
- 16. Use of Real‐World Evidence to Support Decision‐Making for Medical Devices . Guidance for Industry and Food and Drug Administration Staff. Issued August 31, 2017. (https://www.fda.gov/news‐events/fda‐brief/fda‐brief‐fda‐issues‐new‐guidance‐facilitate‐expanded‐use‐real‐world‐evidence).
- 17. Schein OD, McNally JJ, Katz J et al The Incidence Of Microbial Keratitis among wearers of a 30‐day silicone hydrogel extended‐wear contact lens. Ophthalmology 2005; 112: 2172–2179. [DOI] [PubMed] [Google Scholar]
- 18. Poggio EC, Glynn RJ, Schein OD et al The incidence of ulcerative keratitis among users of daily‐wear and extended‐wear soft contact lenses. N Engl J Med 1989; 321: 779–783. [DOI] [PubMed] [Google Scholar]
- 19. Bullimore MA, Sinnott LT & Jones‐Jordan LA. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom Vis Sci 2013; 90: 937–944. [DOI] [PubMed] [Google Scholar]
- 20. Bullimore MA. Safety of soft contact lenses in children. Optom Vis Sci 2017; 94: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sankaridurg P. Contact lenses to slow progression of myopia. Clin Exp Optom 2017; 100: 432–437. [DOI] [PubMed] [Google Scholar]
- 22. Bullimore MA & Richdale K. Myopia control 2020: where are we and where are we heading? Ophthal Physio Opt 2020; 40: 254–270. [DOI] [PubMed] [Google Scholar]
- 23. https://www.aoa.org/patients‐and‐public/caring‐for‐your‐vision/comprehensive‐eye‐and‐vision‐examination/recommended‐examination‐frequency‐for‐pediatric‐patients‐and‐adults. Accessed on June 15, 2020.
- 24. Kristman V, Manno M & Côté P. Loss to follow‐up in cohort studies: how much is too much? Eur J Epidemiol 2004; 19: 751–760. [DOI] [PubMed] [Google Scholar]
- 25. Chalmers RL, Hickson‐Curran SB, Keay L, Gleason WJ & Albright R. Rates of adverse events with hydrogel and silicone hydrogel daily disposable lenses in a large postmarket surveillance registry: the TEMPO Registry. Invest Ophthalmol Vis Sci 2015; 56: 654–663. [DOI] [PubMed] [Google Scholar]
- 26. Wagner H, Richdale K, Mitchell GL et al Age, behavior, environment, and health factors in the soft contact lens risk survey. Optom Vis Sci 2014; 91: 252–261. [DOI] [PubMed] [Google Scholar]
- 27. Chalmers RL, Keay L, McNally J & Kern J. Multicenter case‐control study of the role of lens materials and care products on the development of corneal infiltrates. Optom Vis Sci 2012; 89: 316–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinical Trial Sites & Investigators
Table S2 US Census data for community clinic cities
Table S3 Non‐significant adverse events (AEs) and events not related to soft contact lens wear


