Abstract
Aim
To evaluate the effect of oral semaglutide on energy intake and appetite in subjects with type 2 diabetes (T2D).
Materials and Methods
In this randomized, double‐blind, placebo‐controlled, two‐period cross‐over trial, 15 subjects with T2D received 12 weeks of treatment with once‐daily oral semaglutide (4‐week dose escalation from 3 to 7 to 14 mg) followed by placebo, or vice versa. Energy intake was measured during an ad libitum lunch, evening meal and snack box after a standard breakfast. Appetite ratings were measured using a visual analogue scale after standard and fat‐rich breakfasts. Other assessments included eating and craving control (using the Control of Eating Questionnaire), and changes in body weight and composition.
Results
Following a standard breakfast, total daily ad libitum energy intake was significantly lower (38.9%) with oral semaglutide versus placebo in 13 evaluable subjects (estimated treatment difference, −5096.0 kJ; 95% CI –7000.0, −3192.1; P = .0001). After a fat‐rich breakfast, there were significant differences in favour of oral semaglutide versus placebo for measures of satiety, hunger and for overall appetite score, with no significant differences following a standard breakfast. Fewer food cravings and better eating control were seen with oral semaglutide versus placebo. Overall, mean body weight decreased by 2.7 kg with oral semaglutide and 0.1 kg with placebo, mostly attributable to body fat mass loss.
Conclusion
After 12 weeks of treatment, ad libitum energy intake was lower with oral semaglutide versus placebo, resulting in reduced body fat mass, and was associated with increased satiety and fullness after a fat‐rich breakfast, and improved eating control.
Trial registration number
Keywords: appetite control, body composition, energy regulation, GLP‐1 analogue, incretin therapy, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 (GLP‐1) is an incretin hormone that acts via its receptor to increase insulin and decrease glucagon secretion in a glucose‐dependent manner. 1 GLP‐1 receptor agonists (GLP‐1RAs) not only improve blood glucose homeostasis, but also promote weight loss, 1 an important consideration in the treatment of type 2 diabetes (T2D). 2
When mechanisms responsible for GLP‐1–mediated weight reduction were investigated, increased satiety and reduced food intake were observed. 3 , 4 From animal studies, it appears that functional GLP‐1 receptors in specific parts of the brain are required for weight loss. 1 , 5 , 6 , 7 Furthermore, animal studies have shown that the GLP‐1RAs, liraglutide and semaglutide, can access the specific areas of the brain involved in appetite regulation. 6 , 8 In rodents, semaglutide caused weight loss without decreasing energy expenditure through an effect on both homeostatic (appetite, hunger, satiety) as well as hedonic (food choice, control) neural pathways. 8
Semaglutide has 94% structural homology with native human GLP‐1 9 and was initially approved for the treatment of T2D when given once weekly by subcutaneous (s.c.) injection. Semaglutide s.c. once weekly increased fullness and reduced hunger and energy intake in a study of 30 subjects with obesity. 10 Oral semaglutide has been developed as a co‐formulation of semaglutide with the absorption enhancer, sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate, 11 and is the first oral GLP‐1RA to be approved for the treatment of T2D. 12 , 13 Oral semaglutide has been shown to improve glycaemic control and promote weight loss in patients with T2D in several phase 3 studies. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
It was expected that the effects on appetite and energy intake, and the mechanism responsible for weight loss, would be similar with once‐daily oral formulation as with once‐weekly s.c. formulation. 10 However, given the novel formulation of the orally administered semaglutide, this required confirmation. Furthermore, the effects of s.c. semaglutide on appetite and energy intake were studied in patients with obesity 10 and it was considered important to investigate the effects of oral semaglutide in the intended population of patients with T2D. In this exploratory study, we investigated the effects of oral semaglutide on energy intake, appetite variables, food preference, control of eating and body weight in subjects with T2D.
2. MATERIALS AND METHODS
2.1. Trial design
This was a single‐centre, randomized, double‐blind, placebo‐controlled, two‐period cross‐over phase 1 trial (NCT02773381) conducted at a single study site in the UK (Covance Clinical Research Unit Ltd, Leeds, UK) from 2 June 2016 to 19 October 2018. The trial was undertaken in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki and all applicable regulatory requirements. Written informed consent was obtained from all participants before any trial‐related activities commenced.
2.2. Trial population
Eligible subjects were male or female, aged 18‐75 years, with T2D for at least 90 days, HbA1c 6.0%‐9.0%, body mass index (BMI) 20‐38 kg/m2, stable body weight (<3 kg change during 90 days prior to screening) and who were treated with diet and exercise and/or stable dose of metformin for more than 30 days. Key exclusion criteria included a history of chronic or acute pancreatitis; personal/family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; previous major surgical gastric procedures or the presence of clinically significant, or symptoms of, gastrointestinal disorders that may potentially affect absorption; use of any medication that could interfere with trial results; smoking or use of any nicotine products; or unusual meal habits and special diet requirements compared with the general T2D population, or an unwillingness to eat the food provided.
2.3. Interventions
Two 12‐week treatment periods were separated by a washout period of 5‐9 weeks (Figure S1). Eligible subjects were randomized 1:1 to one of two treatment sequences: oral semaglutide–placebo or placebo–oral semaglutide. The starting dose of once‐daily oral semaglutide was 3 mg (weeks 0‐4), escalating to 7 mg (weeks 4‐8) then 14 mg (weeks 8‐12) to help mitigate adverse gastrointestinal effects. Participants were instructed to take the oral semaglutide tablet in the morning, in a fasted state, with up to 120 mL of water, 30 minutes before any food, beverage or other oral medication. Water intake was allowed in the fasting period except from 2 hours prior to dosing and until 30 minutes postdosing.
At the end of each treatment period there was a 4‐day meal test period at the study site during which assessments were performed, resulting in a total treatment period for oral semaglutide 14 mg of 4 weeks and 3 days.
2.4. Assessments and endpoints
The primary endpoint of the trial was to compare the effect of oral semaglutide and placebo on postprandial glucose metabolism and these results are reported separately. 22 Secondary endpoints presented here are ad libitum energy intake, food preferences, subjective ratings of appetite and palatability variables, control of eating, and body weight and body composition. In addition, the pharmacokinetics (reported separately) 22 and safety of oral semaglutide were investigated.
During day 1 of the in‐house stay at the study site, subjects were acclimatized to standardized meals and activity. On day 2, a 5‐hour breakfast meal test was performed with a standard breakfast of ~2.2 MJ (527 kcal; macronutrient composition: ~30 energy percentage [E%] fat, 15 E% protein and 55 E% carbohydrate).
Subjective ratings of appetite variables (fullness, satiety, hunger and prospective food consumption), thirst, nausea and well‐being were assessed on a 100 mm visual analogue scale (VAS; with the ends of each line indicating the most extreme sensation that subjects have experienced) 15 minutes before and at various time points (15, 30, 60, 90, 120, 180, 240 and 300 minutes) after the standard breakfast meal. 23 The average of the four appetite ratings was used to calculate an overall appetite score: ([100‐satiety] + [100‐fullness] + hunger + prospective food consumption)/4. Palatability (taste, visual appearance and overall pleasantness) was assessed on a 100 mm VAS after the standard breakfast meal.
A weighed homogeneous ad libitum lunch was served in excess and subjects were given a self‐served ad libitum evening meal. At both ad libitum meals, subjects were instructed to eat until pleasantly satiated. Staff at the study site weighed any remaining food, consumption (g and kJ) was calculated and palatability assessments were performed. Subjects received an evening snack box (four items of 100 g each: high‐fat and sweet; low‐fat and sweet; high‐fat and non‐sweet; low‐fat and non‐sweet; individualized by preference), which they were allowed to keep until midnight. Study staff then weighed any remaining snacks and the amount of each of the food categories consumed was recorded.
On day 3, body weight and composition (whole body fat mass and lean mass, and percentage body fat) were measured in a fasted state using air displacement plethysmography (Bodpod®, Concord, CA, USA) with determination via density measurements (body density = body weight/body volume). Body weight was also measured at baseline and every 4 weeks during both treatment periods using standard weight scales. Control of eating and the degree of food cravings were also measured on day 3 in a fasted state using a validated Control of Eating Questionnaire (CoEQ), 24 which included questions related to food cravings, control of eating, hunger and fullness. Based on the previous 7 days, subjects were asked to answer 21 questions (20 rated on a 100 mm VAS and one open‐ended). On day 4, an 8‐hour meal test was performed with a fat‐rich breakfast of ~3.5 MJ (844 kcal; macronutrient composition: ~65 E% fat, 15 E% protein and 20 E% carbohydrate) assessing appetite and palatability.
Safety assessments included adverse events (AEs), hypoglycaemic events and blood pressure.
2.5. Statistical analysis
A sample size of 18 subjects was expected to give at least 90% power to detect a difference in the primary endpoint of area under the concentration–time curve from 0 to 5 hours after the start of the meal (AUC0‐5h) for glucose, except if there was a reduction in AUC0‐5h for glucose of less than 20% combined with a greater within‐subject standard deviation (>0.19), and at least 80% power to detect a difference in ad libitum energy intake of 856 kJ during a lunch meal with a within‐subject standard deviation of 850 kJ. As the trial had a long duration, a total of 22 subjects were planned to be randomized to allow for withdrawals. Further details of the sample size calculation are provided in Appendix S1.
The difference between oral semaglutide and placebo for each outcome was estimated and presented together with the corresponding two‐sided 95% confidence intervals (CI) and P‐values for the test of no difference (t‐test). Endpoints were analysed using an analysis of variance model, with the endpoint as dependent variable and treatment (oral semaglutide or placebo), treatment period (two levels) and subject as fixed factors. For the food preference endpoints of energy intake and quantity of food consumed from each of the four food categories in the evening snack box, the model additionally included interaction between treatment and fat/sweet food categories. Safety endpoints were analysed descriptively.
3. RESULTS
3.1. Subject characteristics
Following screening of 53 subjects, only 15 of the planned 22 subjects were randomized. Thirteen subjects completed the trial. One male subject was withdrawn because of an AE (acute myocardial infarction), while one subject withdrew consent before the end of the trial because of personal/other reasons. Baseline characteristics are shown in Table S1. Thirteen subjects were male (86.7%). The mean age was 58.2 years, with a mean body weight of 93.9 kg and mean BMI of 30.8 kg/m2. Mean HbA1c was 6.9% and the mean duration of T2D was 3 years.
3.2. Energy intake
Following a standard breakfast meal, total daily energy intake during ad libitum lunch, ad libitum evening meal and ad libitum snack box was significantly lower with oral semaglutide than with placebo (relative difference −38.9%; estimated treatment difference [ETD], −5096.0 kJ; 95% CI, −7000.0, −3192.1; P = .0001) (Figure 1A).
FIGURE 1.

Energy intake during A, all ad libitum meals and B, ad libitum snack box. Values are estimated means. Relative difference: estimated treatment difference (ETD)/estimated mean for placebo × 100%. Data in bold indicate significant difference (P < .05) between treatment groups. CI, confidence interval
Energy intake for all four food categories in the snack box tended to be lower with oral semaglutide than with placebo, but was significantly lower for the categories ‘high fat and sweet’, ‘high fat’ and ‘sweet’ (Figure 1B). For high‐fat sweet foods, mean energy intake was 1431.0 kJ with oral semaglutide compared with 2336.7 kJ with placebo (ETD, −905.7 kJ; 95% CI, −1538.5, −272.9; P = .0055). For high‐fat non‐sweet foods, mean energy intake was 577.6 kJ with oral semaglutide compared with 1053.7 kJ with placebo (ETD, −476.2 kJ; 95% CI, −1109.0, 156.6; P = .1384). For low‐fat sweet foods, mean energy intake was 899.8 kJ with oral semaglutide compared with 1301.9 kJ with placebo (ETD, −402.1 kJ; 95% CI, −1034.9, 230.7; P = .2100). For low‐fat non‐sweet foods, mean energy intake was 328.9 kJ with oral semaglutide compared with 518.2 kJ with placebo (ETD, −189.3 kJ; 95% CI, −822.1, 443.5; P = .5537). Overall, energy intake from high‐fat foods was 2008.6 kJ when subjects were treated with oral semaglutide, and 3390.4 kJ when subjects were receiving placebo (ETD, −1381.9 kJ; 95% CI, −2248.6, −515.1; P = .0026).
3.3. Appetite, palatability and control of eating
VAS scores for subjective fasting ratings of appetite were not significantly different between oral semaglutide and placebo before the standard breakfast and 0‐5 hours after the standard breakfast (Figure 2A; Figure S2). After the fat‐rich breakfast, there were significant differences in favour of oral semaglutide versus placebo for the mean postprandial overall appetite score, as well as all four individual mean postprandial ratings of satiety, fullness, hunger and prospective food consumption (Figure 2B; Figure S3). Overall appetite scores remained lower at all time points with oral semaglutide compared with placebo, particularly after the fat‐rich breakfast (Figure S3).
FIGURE 2.
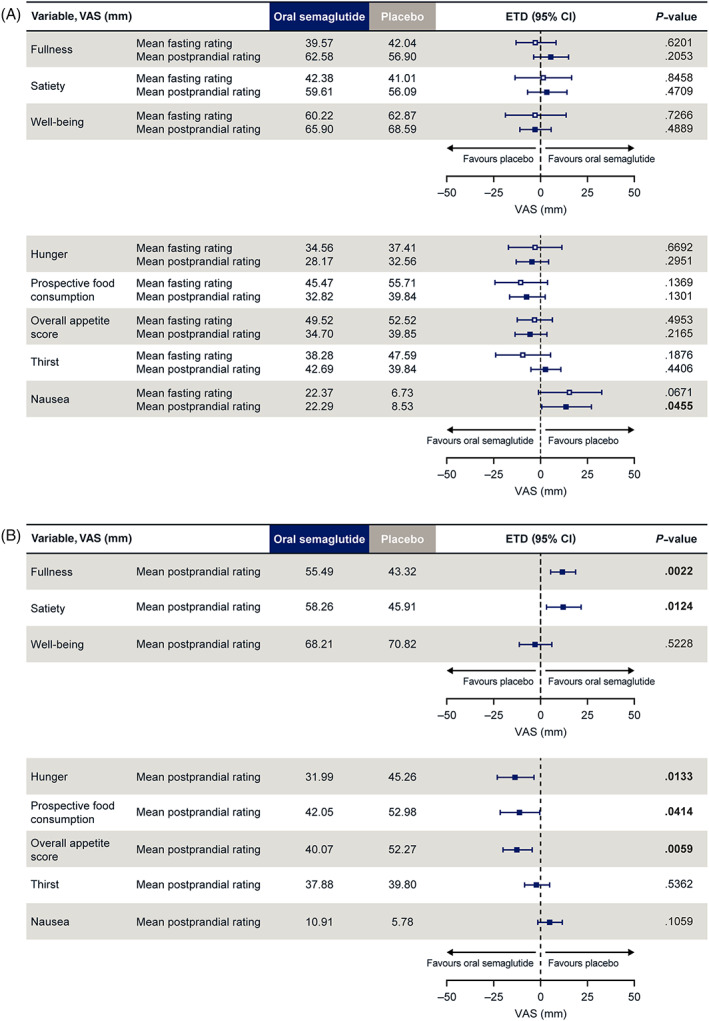
Mean fasting and postprandial appetite ratings after A, standard breakfast and B, fat‐rich breakfast. A, after standard breakfast:mean postprandial rating = AUC15–300 min/285 min (postprandial time span). Overall appetite score = ([100‐satiety] + [100‐fullness] + hunger + prospective food consumption)/4; B, after fat‐rich breakfast:mean postprandial rating = AUC15–480 min/465 min (postprandial time span). Overall appetite score = ([100‐satiety] + [100‐fullness] + hunger + prospective food consumption)/4. Values are estimated means. Data in bold indicate significant difference (P < .05) between treatment groups.AUC, area under the concentration–time curve; CI, confidence interval; ETD, estimated treatment difference; VAS, visual analogue scale
Palatability (taste, visual appearance and overall pleasantness) of the breakfasts, ad libitum lunch and evening meal, and evening snack box appeared similar for oral semaglutide and placebo (Table S2). No mean VAS scores of less than 50 mm were reported for palatability with either treatment, indicating no general food aversion.
When control of eating was evaluated with the CoEQ after a standard breakfast, there were significant differences in four out of the 20 closed questions indicating fewer and less strong food cravings, better control of eating and less difficulty resisting food when receiving oral semaglutide versus placebo (Figure S4).
3.4. Body weight and composition
Overall, for both treatment periods, mean body weight (as measured by Bodpod®) decreased by 2.7 kg with oral semaglutide and 0.1 kg with placebo (Table 1). Weight loss with oral semaglutide was attributable to a 2.6 kg reduction in whole body fat mass, whereas whole body lean mass was not reduced. Mean fat percentage was reduced by 2.0% with oral semaglutide and by 0.8% with placebo (Table 1). Changes in body weight from baseline over time in subjects completing the trial are shown in Figure S5. For subjects receiving oral semaglutide, a similar decrease in body weight was seen in both treatment periods. For subjects receiving placebo in the first treatment period, body weight remained near baseline during the entire 12‐week treatment period. For subjects receiving placebo during the second 12‐week treatment period, there was a rebound increase in body weight after the end of the first oral semaglutide treatment period. The washout period between treatments was of insufficient duration for body weight to return to baseline level and weight gain continued on placebo during the second treatment period, returning to near baseline. A similar rebound effect was seen with waist circumference measurements. Across both treatment periods, mean observed waist circumference decreased with both oral semaglutide (2.4 cm) and placebo (2.0 cm). In subjects receiving placebo during the first treatment period, waist circumference decreased, then increased during the washout period, before decreasing with oral semaglutide during the second 12‐week treatment period. For subjects receiving oral semaglutide during the first 12‐week treatment period, there was a rebound increase in waist circumference in the washout period that continued on placebo during the second 12‐week treatment period.
TABLE 1.
Changes from baseline in body weight and body composition as measured by Bodpod® and waist circumference at week 12 (day 3)
| Mean ± standard deviation | ||
|---|---|---|
| Oral semaglutide (N = 13) | Placebo (N = 14) | |
| Body weight, kg | −2.7 ± 3.6 | −0.1 ± 2.7 |
| Whole body fat mass, kg | −2.6 ± 2.5 | −0.6 ± 2.6 |
| Whole body lean mass, kg | −0.1 ± 1.7 | 0.5 ± 1.2 |
| Fat percentage | −2.0 ± 1.8 | −0.8 ± 2.2 |
| Waist circumference, cm | −2.4 ± 2.1 | −2.0 ± 4.7 |
Abbreviation: N, number of subjects contributing to the summary statistic.
Note: Values are observed means.
3.5. Safety
More AEs were reported in subjects when receiving oral semaglutide versus placebo (93 events in 14 [93.3%] subjects vs. 51 events in 13 [92.9%] subjects) (Table S3). Typical of the GLP‐1RA class, gastrointestinal AEs were more frequently reported with oral semaglutide (47 events in 10 [66.7%] subjects) than with placebo (17 events in 7 [50.0%] subjects) (Table S3). The apparent difference in the number of events between oral semaglutide versus placebo treatments was primarily driven by subjects reporting nausea (11 vs. three events), vomiting (six vs. one event), abdominal pain (six vs. one event), eructation (three vs. no events) and flatulence (four vs. no events).
There was one serious AE (acute myocardial infarction), which occurred during treatment with oral semaglutide; this was considered a severe AE and led to trial withdrawal. All other AEs reported were of mild or moderate severity. No deaths were reported. There were no clinically relevant changes in vital signs including blood pressure (systolic and diastolic), pulse rate and body temperature.
4. DISCUSSION
The main finding reported here was that ad libitum energy intake throughout the day was lower during treatment with oral semaglutide than with placebo in subjects with T2D. These findings are consistent with our previous study that found a similarly lower ad libitum energy intake following 12 weeks of treatment with s.c. once‐weekly semaglutide versus placebo in 30 subjects with obesity. 10 Thus, it appears that the effects of semaglutide are similar whether given by once‐weekly s.c. injection or via the novel once‐daily oral formulation and in patients with obesity 10 or T2D.
Of note, the lower energy intake seen with semaglutide was associated with changes in food preferences and feelings of appetite control. In the evening snack box assessment, the lower preference for high‐fat snacks with oral semaglutide versus placebo was significant. In addition, there was also less preference for sweet foods with oral semaglutide than placebo. A lower preference for high‐fat food was also seen in the trial with s.c. semaglutide, where the Leeds Food Preference Questionnaire indicated a significantly lower explicit liking for high‐fat and non‐sweet foods with s.c. semaglutide versus placebo. 10 Furthermore, ratings of implicit wanting were significantly lower for high‐fat and non‐sweet foods and higher for low‐fat and sweet foods with s.c. semaglutide versus placebo.
In the current study, the reduced food intake and the associated weight loss did not appear to induce a compensatory increase in the drive to eat. In fact, mean postprandial overall appetite score (including all four individual ratings of satiety, fullness, hunger and prospective food consumption) was more favourable following treatment with oral semaglutide versus placebo, indicating reduced appetite and improved satiety with oral semaglutide, but these differences were more prominent after the fat‐rich, high‐calorie breakfast and not the standard lower‐calorie breakfast.
Overall appetite scores remained lower at all time points with oral semaglutide compared with placebo after both the standard and fat‐rich breakfasts, although these only reached statistical significance after the fat‐rich breakfast. These findings are largely consistent with those previously observed for s.c. semaglutide in obese subjects, in which the fasting overall appetite suppression score was significantly higher with semaglutide versus placebo after a standard breakfast (not assessed after fat‐rich breakfast). 10 It should be noted that in the current trial, an overall appetite score was prespecified, whereas the trial with s.c. semaglutide reported the inverse (i.e. overall appetite suppression score). The reason for selecting the appetite score in this trial was that it was considered more intuitive to investigate the change in appetite as opposed to appetite suppression.
Control of eating was improved during treatment with oral semaglutide compared with placebo, which is in line with findings with s.c. semaglutide. 10 With both oral and s.c. administered semaglutide versus placebo, palatability ratings were similar and all mean ratings were above 50 mm for both treatments, indicating that meals were well liked. Thus, it appears that nausea or food aversion were not the cause of the lower energy intake.
Reduced appetite and energy intake, with less preference for energy‐rich foods, provides a possible mechanism to explain the weight loss observed with oral semaglutide. Treatment with oral semaglutide over 12 weeks led to weight reductions of 2.7 kg in this trial, which is consistent with a weight loss of 3.3‐4.7 kg observed over 26 weeks with oral semaglutide 14 mg in phase 3 trials. 14 , 15 , 16 , 17 , 18 , 20 In the current study, body weight reductions were mainly driven by reductions in body fat mass, which was also seen in the study with s.c. semaglutide. Long‐term effects on body weight were seen in phase 3 trials with oral semaglutide, suggesting that the proposed mechanism is clinically relevant, helping to maintain sustainable weight loss, an important attribute in the overall management of T2D. Greater body weight reductions were seen in the s.c. semaglutide trial (5.0 kg) than with oral semaglutide, but this may reflect the different populations studied (obese vs. T2D). Although acting as subjects' own controls is a strength of the cross‐over design for most variables, in the current study the cross‐over limited weight loss and waist circumference assessments. The changes for subjects when treated with placebo should be interpreted with caution because of the rebound effect of subjects treated with oral semaglutide in the first treatment period. A longer washout period may have avoided this.
Regarding safety, as expected there were more AEs with oral semaglutide than placebo, which were mostly mild or moderate gastrointestinal AEs. One serious AE of acute myocardial infarction was reported in a subject with underlying risk factors of longstanding T2D, hypertension, prior hypercholesterolaemia and obesity. Overall, the safety profile of oral semaglutide from the phase 3 PIONEER programme is consistent with the GLP‐1RA class as a whole. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 This includes cardiovascular safety, with several GLP‐1RAs including s.c. semaglutide having a proven CV benefit.25 Exposure of semaglutide, which is reported elsewhere, 22 was consistent with previously published data.26
A limitation of the trial is that the power to detect differences was reduced as only 15 of the planned 22 subjects were randomized. However, the results are generally consistent with the findings of the previous trial for s.c. semaglutide in 30 subjects with obesity. 10 In addition, the results were comparable for the two different populations in each trial. A further limitation was the short duration of the treatment period, which may not have been sufficient to determine if there was a plateau effect on body weight with oral semaglutide. In addition, the trial population was mostly male and entirely white, and may not be representative of the general population. No adjustments of hypothesis tests for multiplicity were performed; rather, this exploratory trial was intended to generate evidence on possible mechanisms. As a broad scope of food‐related endpoints was studied, there is a risk of false positive findings.
In conclusion, ad libitum energy intake throughout the day was lower during treatment with oral semaglutide versus placebo. The reduced food intake observed with oral semaglutide did not result in an increased desire to eat, but instead was associated with improved eating control, satiety and fullness, and reduced hunger and preference for energy‐dense foods, resulting in a greater reduction in body weight after 12 weeks of treatment, mainly accounted for by a reduction in whole body fat mass. These findings provide a possible mechanism for the sustained weight loss seen with oral semaglutide in phase 3 trials.
CONFLICT OF INTEREST
JB and CG received research grants from Novo Nordisk for the current study. JB served on a Novo Nordisk Obesity Advisory Board and received personal fees from Novo Nordisk, outside the submitted work. STH, KD, RB and TB are employees, and KD, STH and TB shareholders, of Novo Nordisk A/S, the sponsor of this trial.
AUTHOR CONTRIBUTIONS
CG, JB, STH and TB designed the trial. CG, JB, KD and TB were responsible for the conduct of the trial. CG, JB, STH, KD, RB and TB were responsible for the collection, analysis and interpretation of data. All the authors were involved in the writing, revision and final approval of the manuscript, and agree to be accountable for all aspects of the work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14255.
Supporting information
Appendix S1. Supporting information.
ACKNOWLEDGEMENTS
Emisphere is acknowledged for providing a licence to the Eligen technology, the sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate component of oral semaglutide. The authors would like to thank the subjects participating in this trial, the investigators Firas Almazedi MBChB MSc DPM and Ashley Brooks MBChB, all trial site staff and all Novo Nordisk employees involved in the trial. The authors would also like to thank Christin Løth Hertz MD PhD of Novo Nordisk for reviewing the manuscript, and Andy Bond MSc of Axis, a division of Spirit Medical Communications Group Limited, for assistance with medical writing and editorial support (funded by Novo Nordisk A/S).
Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Bækdal T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23:581–588. 10.1111/dom.14255
Funding information Novo Nordisk A/S
DATA AVAILABILITY STATEMENT
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymized format.
REFERENCES
- 1. Baggio LL, Drucker DJ. Glucagon‐like peptide‐1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124:4223‐4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515‐520.9449682 [Google Scholar]
- 4. Gutzwiller JP, Drewe J, Göke B, et al. Glucagon‐like peptide‐1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541‐R1544. [DOI] [PubMed] [Google Scholar]
- 5. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon‐like peptide 1 (GLP‐1) analogue, exendin‐ 4, decreases the rewarding value of food: a new role for mesolimbic GLP‐1 receptors. J Neurosci. 2012;32:4812‐4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sisley S, Gutierrez‐Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. J Clin Invest. 2014;124:2456‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest. 2014;124:4473‐4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 10. Blundell J, Finlayson G, Axelsen M, et al. Effects of once‐weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19:1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon‐like peptide‐1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. [DOI] [PubMed] [Google Scholar]
- 12. Rybelsus® prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf. Accessed June 15, 2020.
- 13. Rybelsus summary of product characteristics. https://ec.europa.eu/health/documents/community-register/2020/20200403147411/anx_147411_en.pdf. Accessed June 15, 2020.
- 14. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724‐1732. [DOI] [PubMed] [Google Scholar]
- 15. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272‐2281. [DOI] [PubMed] [Google Scholar]
- 16. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394:39‐50. [DOI] [PubMed] [Google Scholar]
- 18. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515‐527. [DOI] [PubMed] [Google Scholar]
- 19. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528‐539. [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 22. Dahl K, Brooks A, Almazedi F, Tetens Hoff ST, Boschini C, Bækdal T. 4248 metabolism placeholder. TBC 2020;TBC:TBC. (In Press).
- 23. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38‐48. [DOI] [PubMed] [Google Scholar]
- 24. Dalton M, Finlayson G, Hill A, Blundell J. Preliminary validation and principal components analysis of the control of eating questionnaire (CoEQ) for the experience of food craving. Eur J Clin Nutr. 2015;69:1313‐1317. [DOI] [PubMed] [Google Scholar]
- 25. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 26. Granhall C, Donsmark M, Blicher TM, et al. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP‐1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58:781‐791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Data Availability Statement
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymized format.


