Abstract
Modular β‐borylacrylates have been validated as programmable, ambiphilic C3‐synthons in the cascade annulation of 2‐halo‐phenol derivatives to generate structurally and electronically diverse coumarins. Key to this [3+3] disconnection is the BPin unit which serves a dual purpose as both a traceless linker for C(sp2)–C(sp2) coupling, and as a chromophore extension to enable inversion of the alkene geometry via selective energy transfer catalysis. Mild isomerisation is a pre‐condition to access 3‐substituted coumarins and provides a handle for divergence. The method is showcased in the synthesis of representative natural products that contain this venerable chemotype. Facile entry into π‐expanded estrone derivatives modified at the A‐ring is disclosed to demonstrate the potential of the method in bioassay development or in drug repurposing.
Keywords: annulation, boron, catalysis, heterocycles, isomerisation
Modular β‐borylacrylates are validated as programmable, ambiphilic C3‐synthons for the annulation of 2‐halo‐phenol derivatives to generate diverse coumarins. Key to this [3+3] disconnection is the BPin unit which serves a dual purpose as both a traceless linker for C(sp2)–C(sp2) coupling, and as a chromophore extension to enable E → Z alkene isomerisation. Inverting alkene geometry is a pre‐condition to access 3‐susbtituted derivatives. This approach capitalises upon the abundance of 2‐halophenols in biology and may facilitate natural product/drug re‐purposing and the development of imaging tools.
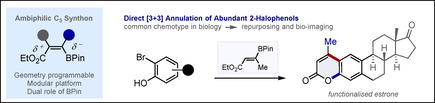
Innovative advances in annulation chemistry have been a defining feature in the evolution of organic synthesis: This reflects the importance of heterocycles in target synthesis, and in the design of functional small molecules in a broader sense. [1] As exemplified by the venerable Huisgen cycloaddition,[ 2 , 3 ] operationally simple “click” processes to construct unnatural heterocyclic scaffolds have been intensively pursued and enjoy widespread application in chemical biology. [4] Predicated on high efficiency, tolerance and atom economy, [5] these enduring processes serve as valuable guidelines for the conception of annulation routes to naturally‐occurring heterocyclic scaffolds. In particular, the revered history of the coumarin nucleus as a drug module and natural product core, together with its importance in functional materials, constitute persuasive arguments to devise more effective synthesis routes based on direct annulation. [6] The long‐standing importance of this bicyclic framework is evident from the early works of Perkin [7] and Pechmann, [8] which have since been complemented by an array of transition metal, [9] photochemical [10] and modified Knoevenagel condensation approaches. [11] To further strengthen this diverse synthesis arsenal with a direct coupling, a [3+3] approach was envisaged that would unify halogenated phenols (I) with a simple ambiphilic C3 fragment (II) via two successive bond‐forming events (Figure 1). β‐Borylacrylates (II) [12] were conceived as attractive coupling partners for a Suzuki–Miyaura/substitution cascade due to their ease of preparation and structural tenacity. [13] Moreover, the BPin unit in this modular acrylate platform serves as both a traceless linker for C(sp2)–C(sp2) coupling, and as a chromophore antenna to enable inversion of the alkene geometry [14] via selective energy transfer catalysis. [15] In the absence of base‐mediated isomerisation, [16] regulating alkene geometry is necessary to facilitate lactonisation to access 3‐substituted coumarins.
Figure 1.
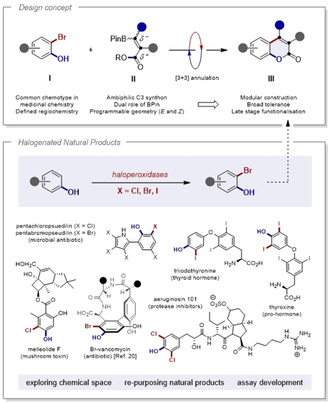
Top: Conceptual framework for a direct 2‐halo‐phenol to coumarin annulation using β‐borylacrylates. Bottom: Halogenated natural products. [17]
This disconnection capitalises upon abundance of 2‐halophenols in biology (Figure 1, bottom), [17] their regioselective halogenation chemistries[ 17 , 18 ] and the importance of the coumarin scaffold (III) in both drug discovery and molecular imaging. [19] Given the notable methodological advances in selective halogenation, [20] it was envisaged that this [3+3] annulation may facilitate natural product/drug re‐purposing. [21]
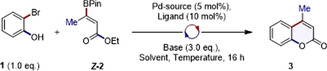
To validate the conceptual framework outlined in Figure 1, the coupling of 2‐bromophenol and β‐borylacrylate Z ‐2 (1.0 equiv.) was explored (Table 1). Gratifyingly, exposing the coupling partners to Pd(OAc)2 and SPhos in 1,4‐dioxane at 80 °C generated the desired coumarin 3 in 30 % yield after 16 h (entry 1). Switching solvent to N,N‐dimethylformamide led to a modest improvement in yield (41 %, entry 2), whereas DMSO was detrimental (32 %, entry 3). Utilising 2.0 equiv. of β‐borylacrylate Z ‐2 in DMF led to an increased yield of 53 % (entry 4) and so these parameters were fixed for the reminder of the optimisation. Variation in the Pd source (entries 5 and 6) and base (entries 7 and 8) was then explored, but these alterations did not confer a notable advantage.
Table 1.
Reaction optimisation in the formation of coumarin 3.
|
Entry |
Catalyst |
Ligand |
Base |
Solvent |
H2O (equiv) |
T [°C] |
Yield [%][a] |
|---|---|---|---|---|---|---|---|
|
1[b] |
Pd(OAc)2 |
SPhos |
K3PO4 |
1,4‐dioxane |
5.0 |
80 |
30 |
|
2[b] |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMF |
5.0 |
80 |
41 |
|
3[b] |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMSO |
5.0 |
80 |
32 |
|
4 |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMF |
5.0 |
80 |
53 |
|
5 |
Pd(PPh3)4 |
– |
K3PO4 |
DMF |
5.0 |
80 |
37 |
|
6 |
Pd(dppf)Cl2 [c] |
– |
K3PO4 |
DMF |
5.0 |
80 |
45 |
|
7 |
Pd(OAc)2 |
SPhos |
K2CO3 |
DMF |
5.0 |
80 |
50 |
|
8 |
Pd(OAc)2 |
SPhos |
CsCO3 |
DMF |
5.0 |
80 |
31 |
|
9 |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMF |
5.0 |
50 |
41 |
|
10 |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMF |
5.0 |
100 |
39 |
|
11 |
Pd(OAc)2 |
SPhos |
K3PO4 |
DMF |
/ |
80 |
82(76)[d] |
[a] Yields determined by NMR using 1,3,5‐trimethoxybenzene as internal standard. [b] 1.0 equiv. of Z ‐2 was used. [c] DCM complex. [d] Numbers in parenthesis are isolated yields.
Altering the temperature had a negative impact on yield (entries 9 and 10), but exclusion of water proved to be beneficial (82 %, entry 11). This observation, together with the yield enhancement when using 2.0 equiv., suggests that Z ‐2 displays limited stability under basic aqueous conditions. Given the involvement of an oxopalladium complex in the Suzuki–Miyaura mechanism, [22] it is pertinent to note that the DMF used is not fully anhydrous and thus water is likely present in the reaction mixture.
Having identified optimised reaction conditions for the catalytic annulation, the scope and limitations were investigated using a range of electronically and structurally modified 2‐bromophenols (Figure 2). Systematically increasing the steric demand of the β‐substituent of the β‐borylacrylate from n‐propyl (4) to cyclopropyl (5) and methylcyclohexyl (6) was well tolerated (69 %, 67 % and 75 % yield, respectively). Incorporation of an aryl group furnished 7 in 73 % yield. The addition of an α‐substituent was also well tolerated enabling the formation of 3,4‐dimethylcoumarin (8) in 83 %. Given the importance of fluorinated aryl systems in drug discovery, [23] the influence of bioisosteric H to F substitution on the phenol coupling partner was examined. This led to smooth formation of 9 in 76 % yield. Free amines were also compatible with the general conditions (10, 71 %), as were anisole derivatives enabling formation of 11 (63 %). Expected chemoselective C(sp2)–C(sp2) coupling was confirmed by formation of the chlorinated derivative 12 (63 %). To demonstrate the utility of the method in accessing more sterically congested, 2‐bromo‐6‐methylphenol and 1‐bromo‐2‐naphtol were exposed to the standard annulation conditions enabling formation of 13 (85 %) and 14 (80 %), respectively. The latter system demonstrates the suitability of this approach in accessing the naphthopyran‐2‐one class of photochromic dyes. [24] Furthermore, the transformation enables formation of π‐expanded systems such as the biaryl derivative 15 in 68 %. [6b]
Figure 2.
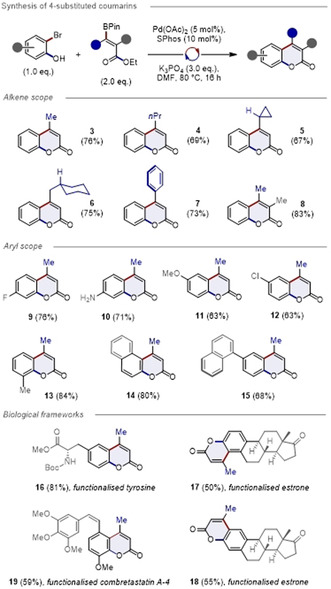
Scope of the annulation and application in natural product synthesis and derivatisation.
To demonstrate the synthetic utility of β‐borylacrylates in derivatising biological frameworks, commercially available 3‐bromotyrosine was processed to 16 in 81 %. Moreover, the terpene derivatives 2‐ and 4‐bromoestrone could be smoothly transformed into 17 and 18 (50 % and 55 %, respectively), enabling entry into novel π‐extended analogs of the parent hormone. A brominated derivative of the anti‐tumour agent combretastatin A‐4 underwent smooth annulation, enabling formation of the coumarin derivative 19 in 59 % yield. It is pertinent to note that combretastatin A‐4‐phosphate is currently in clinical development for ovarian cancer. [25]
The structure of compound 18 was unequivocally established by single‐crystal X‐ray analysis (CCDC number 2022960, Figure 3, top). This compound crystallised in the chiral space group P212121 (Flack parameter was refined to 0.00(6)). In the packing diagram the formation of linear chains along the a‐axis involving π⋅⋅⋅π interactions (Cg1⋅⋅⋅ Cg1 3.286 Å) supported by additional C−H⋅⋅⋅O interactions (C8–H8B⋅⋅⋅O2 3.418(2) Å; 157.9° and C22–H22B⋅⋅⋅O2 3.434(2) Å; 159.6°) were observed (Figure 3, bottom). The prominence of π⋅⋅⋅π interactions between the aromatic units of estrone derivative 18 is presented in Figure 2 d in the Supporting Information. The 3D network of estrone derivative 18 is compact, Moreover, the influence of the coumarin in generating linear chains due to additional C−H⋅⋅⋅O interactions is evident from the extended packing analysis (Figure 3, bottom).
Figure 3.
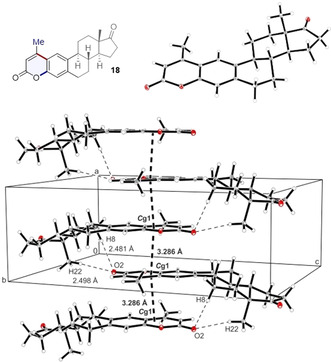
Top: The X‐ray crystal structure of estrone derivative 18 (Deposition Number 2022960 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.). Thermal ellipsoids are shown at 30 % probability. Bottom: Excerpt of the packing diagram of compound 18 presenting the formation of a linear chain along a‐axis involving π⋅⋅⋅π and C−H⋅⋅⋅O interactions.
In the course of this scope investigation, it was noted that β‐borylacrylates that were unsubstituted in the β‐position were problematic (Figure 4). Indeed, exposing compound E ‐20 (R=H) to the general reaction conditions yielded the cross‐coupled product 22 exclusively, indicating that in situ isomerisation does not occur. This selectivity was also noted for E ‐21 (R=Me), generating product 23 in 58 %. Although attractive from the perspective of divergence, a solution was required to expand the substrate scope. To that end, a photocatalytic isomerisation of the β‐borylacrylate was performed under the auspices of selective energy transfer catalysis to generate substrates Z ‐20 and Z ‐21. [15] Exposure to the general condition reported above enabled formation of the desired coumarins 24 and 25 (55 % and 90 %, respectively). The direct annulation was employed in a short natural product synthesis as indicated in Figure 4 (bottom). Since the 3‐ and 4‐positions of angelicin (26) are unsubstituted, the Z‐configured C3‐synthon Z ‐20 was employed. Under the general conditions developed, this direct [3+3] annulation proceeded smoothly to deliver the target natural products Angelicin 26. By employing the geometric isomer E ‐20, it was possible to access compound 27 which is the core of Pongamol and Lanceolatin B. [26]
Figure 4.
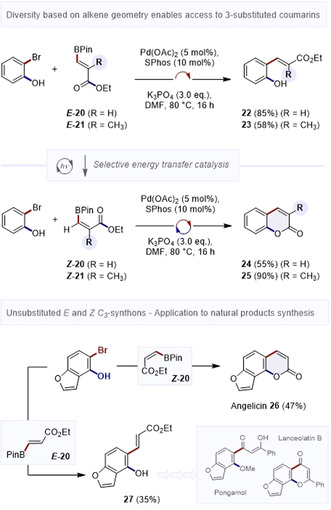
Merging energy transfer catalysis with the Suzuki–Miyaura/substitution cascade to enable divergence to 3‐substuted coumarins.
Finally, preliminary validation of the strategy in modulating the photophysical properties of selected systems was conducted (Figure 5). As illustrated, clear peaks that correspond to the coumarin scaffold are clearly visible in 14, 16, 17 and 18 which supports the notion that this simple annulation strategy based on β‐borylacrylates may find application in the development of tools for molecular imaging.
Figure 5.
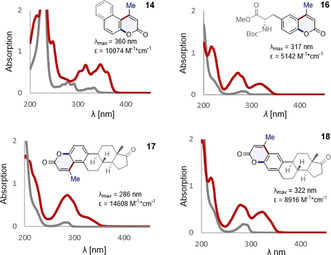
Comparative analysis of the UV/Vis spectra of selected 2‐halophenols (shown in grey) and the corresponding coumarin adducts 14, 16, 17 and 18 (shown in red).
In conclusion, a direct annulation of 2‐halophenols has been devised utilising simple, modular β‐borylacrylates as ambiphilic C3‐synthons. This study contributes to the venerable role of organoborons in creating well‐defined synthons for organic synthesis.[ 27 , 28 ] In this modular platform, the boron substituent serves a dual purpose as both a traceless linker for C(sp2)–C(sp2) coupling, and as a chromophore extension to enable inversion of the alkene geometry via selective energy transfer catalysis. This latter attribute enables access to C‐3 and C‐4 unsubstituted coumarins and provides a basis for divergence. The transformation is operationally simple, displays high functional group tolerance and can be employed for late‐stage functionalisation. Application in short natural product syntheses is showcased and also in the facile conversion of the hormone estrone to a π‐extended analogue. It is envisaged that such transformations may be valuable in re‐purposing existing bioactive small molecules or in modifying them for analytical purposes.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We acknowledge generous financial support from the Westfälische Wilhelms‐Universität Münster, the DFG (SFB 858 and Excellence Cluster EXC 1003 “Cells in Motion”) and the Alexander von Humboldt Foundation (post‐doctoral research fellowship to J.J.M.). Open access funding enabled and organized by Projekt DEAL.
M. Wienhold, J. J. Molloy, C. G. Daniliuc, R. Gilmour, Angew. Chem. Int. Ed. 2021, 60, 685.
Dedicated to Prof. Dr. Albert Eschenmoser on the occasion of his 95th birthday
Contributor Information
M. Sc. Max Wienhold, https://www.uni‐muenster.de/Chemie.oc/gilmour/
Prof. Dr. Ryan Gilmour, Email: ryan.gilmour@uni-muenster.de.
References
- 1. Eschenmoser A. in Chemical Synthesis Gnosis to Prognosis (Ed.: Chatgilialoglu C., Snieckus V.), NATO ASI, Kluwer Academic Publications, Dordrecht, 1994, pp. 231–232. [Google Scholar]
- 2. Huisgen R., Proc. Chem. Soc. 1961, 357–396. [Google Scholar]
- 3. Breugst M., Reissig H.-U., Angew. Chem. Int. Ed. 2020, 59, 12293–12307; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 12389–12404. [Google Scholar]
- 4. Jewett J. C., Bertozzi C. R., Chem. Soc. Rev. 2010, 39, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolb H. C., Finn M. G., Sharpless K. B., Angew. Chem. Int. Ed. 2001, 40, 2004–2021; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2056–2075. [Google Scholar]
- 6.
- 6a. Thakur A., Singla R., Jaitak V., Eur. J. Med. Chem. 2015, 101, 475–495; [DOI] [PubMed] [Google Scholar]
- 6b. Tasior M., Kim D., Singha S., Krzeszewski M., Ahn K. H., Gryko D. T., J. Mater. Chem. C 2015, 3, 1421–1446; [Google Scholar]
- 6c. Sadler J. C., Chung C.-w. H., Mosley J. E., Burley G. A., Humphreys L. D., ACS Chem. Biol. 2017, 12, 374–379; [DOI] [PubMed] [Google Scholar]
- 6d. Pereira T. M., Franco D. P., Vitorio F. E., Kummerle A., Curr. Top. Med. Chem. 2018, 18, 124–148; [DOI] [PubMed] [Google Scholar]
- 6e. Cao D., Liu Z., Verwilst P., Koo S., Jangjili P., Kim J. S., Lin W., Chem. Rev. 2019, 119, 10403–10519. [DOI] [PubMed] [Google Scholar]
- 7. Perkin W. H., J. Chem. Soc. 1868, 21, 53–63. [Google Scholar]
- 8. v. Pechmann H., Ber. Dtsch. Chem. Ges. 1884, 17, 929–936. [Google Scholar]
- 9.For selected examples see:
- 9a. Trost B. M., Toste F. D., Greenman K., J. Am. Chem. Soc. 2003, 125, 4518–4528; [DOI] [PubMed] [Google Scholar]
- 9b. Battistuzzi G., Cacchi S., De Salve I., Fabrizi G., Parisi L. M., Adv. Synth. Catal. 2005, 347, 308–312; [Google Scholar]
- 9c. Ferguson J., Zeng F., Alper H., Org. Lett. 2012, 14, 5602–5605; [DOI] [PubMed] [Google Scholar]
- 9d. Sasano K., Takaya J., Iwasawa N., J. Am. Chem. Soc. 2013, 135, 10954–10957; [DOI] [PubMed] [Google Scholar]
- 9e. Gadakh S. K., Dey S., Sudalai A., J. Org. Chem. 2015, 80, 11544–11550. [DOI] [PubMed] [Google Scholar]
- 10.For selected examples see:
- 10a. Mi X., Wang C., Huang M., Wu Y., Wu Y., J. Org. Chem. 2015, 80, 148–155; [DOI] [PubMed] [Google Scholar]
- 10b. Metternich J. B., Gilmour R., J. Am. Chem. Soc. 2016, 138, 1040–1045; [DOI] [PubMed] [Google Scholar]
- 10c. Hou J., Ee A., Feng W., Xu J., Zhao Y., Wu J., J. Am. Chem. Soc. 2018, 140, 5257–5263; [DOI] [PubMed] [Google Scholar]
- 10d. Kawaai K., Yamaguchi T., Yamaguchi E., Endo S., Tada N., Ikari A., Itoh A., J. Org. Chem. 2018, 83, 1988–1996; [DOI] [PubMed] [Google Scholar]
- 10e. Chen L., Wu L., Duan W., Wang T., Li L., Zhang K., Zhu J., Peng Z., Xiong F., J. Org. Chem. 2018, 83, 8607–8614; [DOI] [PubMed] [Google Scholar]
- 10f. Neveselý T., Daniliuc C. G., Gilmour R., Org. Lett. 2019, 21, 9724–9728. [DOI] [PubMed] [Google Scholar]
- 11. Ranu B. C., Jana R., Eur. J. Org. Chem. 2006, 3767–3770. [Google Scholar]
- 12.For selected strategies, see:
- 12a. Nagao K., Yamazaki A., Ohmiya H., Sawamura M., Org. Lett. 2018, 20, 1861–1865; [DOI] [PubMed] [Google Scholar]
- 12b. Grams R. J., Fritzemeier R. G., Slebodnick C., Santos W. L., Org. Lett. 2019, 21, 6795–6799. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Lennox A. J. J., Lloyd-Jones G. C., Angew. Chem. Int. Ed. 2013, 52, 7362–7370; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 7506–7515; [Google Scholar]
- 13b. Fyfe J. W. B., Watson A. J. B., Chem 2017, 3, 31–55. [Google Scholar]
- 14.
- 14a. Pearson C. M., Snaddon T. N., ACS Cent. Sci. 2017, 3, 922; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14b. Molloy J. J., Morack T., Gilmour R., Angew. Chem. Int. Ed. 2019, 58, 13654–13664; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 13789–13800. [Google Scholar]
- 15. Molloy J. J., Schäfer M., Wienhold M., Morack T., Daniliuc C. G., Gilmour R., Science 2020, 369, 302–306. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Stoker J. R., Bellis D. M., J. Biol. Chem. 1962, 237, 2303; [PubMed] [Google Scholar]
- 16b. Horaguchi T., Hosokawa N., Tanemura K., Suzuki T., J. Heterocycl. Chem. 2002, 39, 61–67; [Google Scholar]
- 16c. Boeck F., Blazejak M., Anneser M. R., Hintermann L., Beilstein J. Org. Chem. 2012, 8, 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Vaillancourt F. H., Yeh E., Vosburg D. A., Garneau-Tsodikova S., Walsh C. T., Chem. Rev. 2006, 106, 3364–3378; [DOI] [PubMed] [Google Scholar]
- 17b. Agarwal V., El Gamal A. A., Yamanaka K., Poth D., Kesten R. D., Schorn M., Allen E. E., Moore B. S., Nat. Chem. Biol. 2014, 10, 640–647; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17c. Agarwal V., Miles Z. D., Winter J. M., Eustáquio A. S., El Gamal A. A., Moore B. S., Chem. Rev. 2017, 117, 5619–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chung W.-j., Vanderwal C. D., Angew. Chem. Int. Ed. 2016, 55, 4396–4434; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 4470–4510. [Google Scholar]
- 19.For selected examples, see:
- 19a. Gee K. R., Sun W., Bhalgat M. K., Upson R. H., Klaubert D. H., Latham K. A., Haugland R. P., Anal. Biochem. 1999, 273, 41–48; [DOI] [PubMed] [Google Scholar]
- 19b. Volpe M. R., Wilson M. R., Brotherton C. A., Winter E. S., Johnson S. E., Balskus E. P., ACS Chem. Biol. 2019, 14, 1097–1101; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19c. Laguerre A., Hauke S., Qui J., Kelly M. J., Schultz C., J. Am. Chem. Soc. 2019, 141, 16544–16547; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19d. Sun X.-y., Liu T., Sun J., Wang X.-j., RSC Adv. 2020, 10, 10826–10847; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19e. Tang X.-J., Wu Y., Kou X., Dong Z., Zhou W., Zhang Z., Tan W., Fang X., Angew. Chem. Int. Ed. 2020, 59, 18386–18389; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 18544–18547. [Google Scholar]
- 20.For a striking example of the selective bromination of vancomycin, see: Pathak T. P., Miler S. J., J. Am. Chem. Soc. 2012, 134, 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pushpakom S., Iorio F., Eyers P. A., Escott K. J., Hopper S., Wells A., Doig A., Guiliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M., Nat. Rev. Drug Discovery 2019, 18, 41–58. [DOI] [PubMed] [Google Scholar]
- 22. Thomas A. A., Denmark S. E., Science 2016, 352, 329–332. [DOI] [PubMed] [Google Scholar]
- 23. Purser S., Moore P. R., Swallow S., Gouveneur V., Chem. Soc. Rev. 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]
- 24. Saleh R. M., Soliman A. Y., El Nagdy S., Bakeer H. M., Mostafa M. M., Phosphorus Sulfur Silicon Relat. Elem. 1990, 48, 285–288. [Google Scholar]
- 25. Grisham R., Ky B., Tewari K. S., Chaplin D. J., Walker J., Gynecol. Oncol. Res. Pract. 2018, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heravi M. M., Zadsirjan V., Hamidi H., Amiri P. H. T., RSC Adv. 2017, 7, 24470–24521. [Google Scholar]
- 27.For examples of a C1 organoboron synthons see:
- 27a. St. Denis J. D., He Z., Yudin A. K., ACS Catal. 2015, 5, 5373–5379; [Google Scholar]
- 27b. Holownia A., Tien C.-H., Diaz D. B., Larson R. T., Yudin A. K., Angew. Chem. Int. Ed. 2019, 58, 15148–15153; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 15292–15297; [Google Scholar]
- 27c. Ivon Y. M., Mazurenko I. V., Kuchkovska Y. O., Voitenko Z. V., Grygorenko O. O., Angew. Chem. Int. Ed. 2020, 59, 18016–18022; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 18172–18178. [DOI] [PubMed] [Google Scholar]
- 28.
- 28a. He Z., Zajdlik A., Yudin A. K., Acc. Chem. Res. 2014, 47, 1029–1040; [DOI] [PubMed] [Google Scholar]
- 28b. Trinchera P., Corless V. B., Yudin A. K., Angew. Chem. Int. Ed. 2015, 54, 9038–9041; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 9166–9169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


