Abstract
Objective
We describe our implementation of a continuous glucose monitoring (CGM) guideline to support intravenous insulin administration and reduce point of care (POC) glucose monitoring frequency in the coronavirus disease 2019 medical intensive care unit (MICU) and evaluate nurses’ experience with implementation of CGM and hybrid POC + CGM protocol using the Promoting Action on Research in Health Services framework.
Methods
A multidisciplinary team created a guideline providing criteria for establishing initial sensor-meter agreement within each individual patient followed by hybrid use of CGM and POC. POC measures were obtained hourly during initial validation, then every 6 hours. We conducted a focus group among MICU nurses to evaluate initial implementation efforts with content areas focused on initial assessment of evidence, context, and facilitation to identify barriers and facilitators. The focus group was analyzed using a qualitative descriptive approach.
Results
The protocol was integrated through a rapid cycle review process and ultimately disseminated nationally. The Diabetes Consult Service performed device set-up and nurses received just-in-time training. The majority of barriers centered on contextual factors, including limitations of the physical environment, complex device set-up, hospital firewalls, need for training, and CGM documentation. Nurses’ perceived device accuracy and utility were exceptionally high. Solutions were devised to maximize facilitation and sustainability for nurses while maintaining patient safety.
Conclusion
Outpatient CGM systems can be implemented in the MICU using a hybrid protocol implementation science approach. These efforts hold tremendous potential to reduce healthcare worker exposure while maintaining glucose control during the COVID-19 pandemic.
Key words: continuous glucose monitoring, intensive care unit, inpatient, diabetes, hyperglycemia, COVID-19
Abbreviations: BG, blood glucose; CGM, continuous glucose monitoring; CNS, clinical nurse specialist; COVID, coronavirus disease; EHR, electronic medical record; ICU, intensive care unit; IV, intravenous; MICU, medical intensive care unit; NP, nurse practitioners; PARIHS, Promoting Action on Research in Health Services; POC, point of care; PPE, personal protective equipment
Introduction
COVID-19 has proven particularly devastating for individuals with chronic illness, including diabetes.1 , 2 Individuals with diabetes have higher rates of severe COVID-19,2 account for a large percentage of hospital admissions,1, 2, 3, 4 and have higher mortality compared to the general population.1 , 5 Early data suggest that among hospitalized patients with COVID-19, poor glycemic control may be associated with higher mortality.6 , 7 For patients in intensive care units (ICU), guidelines recommend intravenous (IV) insulin with goal blood glucose (BG) 100 to 180 mg/dL.8, 9, 10
Health care workers caring for patients with COVID-19 on IV insulin assume increased risk and utilization of personal protective equipment (PPE) due to the need for hourly point of care (POC) BG testing.11 As a result, institutions have taken unprecedented measures to reduce exposure, including bundling medication administration times and positioning IV infusion pumps outside of COVID-19 patient rooms. Therefore, the ability to safely perform remote glucose monitoring would greatly support these efforts.
Continuous glucose monitoring (CGM) is a safe and effective alternative for BG testing in the outpatient setting and has potential to reduce the frequency of POC BG testing in the ICU.12 On April 1, 2020, the Food and Drug Administration notified manufacturers that it will not object to the provision of CGM systems for the treatment of patients in hospital settings to support COVID-19 health care-related efforts.13 Current evidence supports the overall safety of CGM, with similar glucose control as the standard of care in hospitals.14, 15, 16, 17, 18, 19 However, many studies are small; focused on accuracy as opposed to efficacy, safety, or implementation; and utilize older technology.14, 15, 16, 17, 18 , 20 There is concern that changes in tissue perfusion, edema, hydration, acid-base balance, and sensor compression due to patient positioning and medication interference may affect CGM performance.21 Newer generation devices demonstrate improved accuracy, provide factory calibration, and have minimized interference from acetaminophen, all key elements required for inpatient utilization.22 Moreover, the use of more frequent glucose measurement (eg, every 5 minutes), 23 setting higher threshold alerts,24 and predictive alerts25 may compensate for total error and improve safety.
However, the implementation of these devices in the inpatient setting presents unique challenges. The purpose of this manuscript is twofold: (a) describe our experience implementing a CGM guideline to support IV insulin administration and reduce the need for fingerstick POC glucose monitoring in ICU patients with COVID-19 and (b) evaluate nurses’ experience with implementation of CGM and hybrid POC + CGM protocol using the Promoting Action on Research in Health Services (PARIHS) framework for implementation. Multisite analysis of outcomes data is underway and will be reported separately.
Description of CGM and Protocol Implementation
Assembling a Team
We assembled a multidisciplinary team of stakeholders that included representatives from endocrinology, critical care medicine, nursing, and critical care pharmacy to draft an ICU protocol. CGM was piloted within a single medical intensive care unit (MICU) at the institution, which was already positioning IV insulin pumps outside the room, enabling the greatest potential for reduced health care worker exposure and conservation of PPE. We obtained approval from the institution’s multidisciplinary COVID-19 Clinical Care Workgroup.
Training and Start-Up
Implementation team members participated in a 1-hour manufacturer training session focused on overall device implementation. Two educational documents, 1 focused on sensor insertion and initiation and the other focused on implementation of the protocol, were crafted and stored at the bedside. Content used in these handouts can be found on the manufacturer’s website (https://dexcom.com/guides). Endocrinology team members provided a single session of one-on-one training with MICU nursing leaders. This small group was tasked with device insertion, transmitter pairing, and just-in-time training to the bedside MICU nurse focused on CGM validation and use.
Dexcom CGM and Hybrid Protocol Implementation
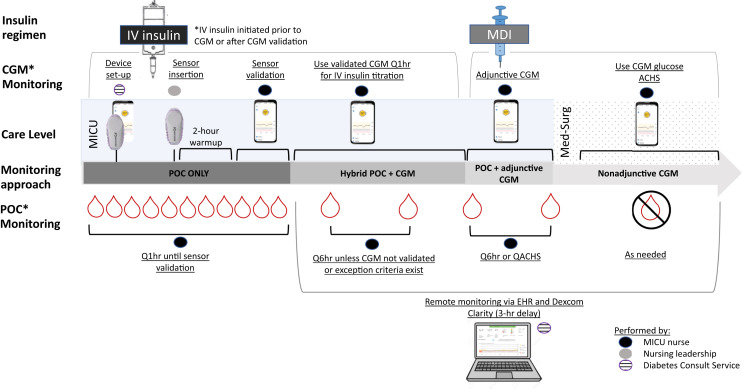
Figure 1 shows hybrid CGM plus POC glucose monitoring procedures and implementation.
Fig. 1.
Hybrid CGM + POC glucose monitoring. Hybrid CGM +POC glucose monitoring occurred in several phases. Diabetes consult service members performed device set-up tasks prior to delivering the devices to the ICU units. The nursing leadership team worked with MICU providers to identify appropriate patients with anticipated or current IV insulin orders. The nursing leadership team performed sensor insertion and paired the transmitter with the cell phone/receiver. Once paired, a 2-hour warm-up period was initiated, followed by a sensor validation period. The IV insulin infusion was titrated every hour using the POC BG value until initial validation was achieved. Then titration was performed using the CGM unless Q6 hour sensor-meter pairs were discordant or other exception criteria occurred. Additional sensors could be placed if the patient had ongoing IV insulin requirements following initial sensor expiration or removal. When patients were transitioned off IV insulin, Q6 hour POC testing was continued along with adjunctive CGM while in the MICU or until sensor expiration. Once transferred to a medical surgical floor, CGM could be used nonadjunctively (to replace POC testing) according to the institution’s global Hyperglycemia in COVID-19 Patients guideline. The Diabetes consult service utilized Dexcom Clarity, which operates on a 3-hour delay, to retrospectively visualize patient data and analyze glucose trends. BG = blood glucose; CGM = continuous glucose monitoring; COVID-19 = coronavirus disease 2019; EHR = electronic health record; ICU = intensive care unit; IV = intravenous; MDI = multiple daily injection; MICU = medical intensive care unit; POC = point of care (arterial, capillary).
Device Set-Up
Members of the Diabetes Consult Service comprised of endocrinology nurse practitioners (NP) performed the bulk of device set-up tasks (Fig. 1). Both Android phones and Dexcom receivers were paired with Dexcom G6 transmitters. To facilitate Health Insurance Portability and Accountability Act compliant remote monitoring, dummy clinical accounts were created for each device/patient. Android phone set-up required entry of individual Dexcom Clarity account information on each phone. Alert thresholds were programed into both the Android phones and receivers and set at 100 mg/dL (lower threshold) and 300 mg/dL (upper threshold). The Urgent Low Soon predictive alert was also activated, triggering an alarm when the glucose is predicted to drop below 55 mg/dL in the next 20 minutes. Device set-up ranged from 15 to 17 minutes per CGM.
Patient Identification
MICU nursing leadership identified appropriate patients during daily rounds with MICU intensivist teams. In most cases, CGM was implemented ahead of IV insulin in order to account for sensor initiation time and establish sensor-POC agreement, thereby minimizing hourly POC testing while on IV insulin.
Insertion and Pairing
The transmitter and sensor serial numbers were scanned into the Dexcom app prior to entering the room for device insertion. Android phones and receivers were both kept just outside the patient room, typically 10 to 15 feet from the transmitter and separated by a glass paneled door. The sensor was placed on the patient’s upper lateral arm (due to frequent prone positioning) using a single push inserter.26 The transmitter was paired with the Android phone Dexcom app and receiver. Pairing typically occurred in less than 1 minute. Rarely, a transmitter required several attempts to pair. After pairing, a 2-hour warm-up period began in which no CGM data were available. The sensor was worn for up to 10 days. At the time of this publication, all CGM systems initiated were successfully validated; however, time to validation analysis is underway. Each patient could receive up to 2 sensors and would be able to keep the transmitter at discharge (which may be reused for up to 90 days).
Nurse Driven Protocol
Initial sensor validation occurred using hourly paired sensor-meter readings (Fig. 1). The comparison standards (POC meter—StatStrip, Nova Biomedical, arterial blood gas) were otherwise Food and Drug Administration approved for use in each patient. The standard was compared to the CGM value obtained within 5 minutes. The threshold criterion for nonadjunctive use was defined as 2 consecutive sensor-meter pairs approximately 1-hour apart meeting either of the following criteria (Table 1 ):
-
•
CGM < 20% difference from the reference when the glucose is > 100 mg/dL
-
•
CGM < 20 mg/dL difference from the reference when the glucose is < 100 mg/dL
Table 1.
Accuracy and Glucose Testing Procedures
| Stage | POC glucose testing procedures |
|---|---|
| CGM validation | POC glucose testing Q1 hour compared to CGM glucose Proceed to Q6 hour POC testing when 2 consecutive sensor-meter pairs approximately 1-hour apart meet either of the following criteria:
|
| Sustained use | Revert from Q6 hour to Q1 hour POC testing if:
|
Abbreviations: CGM = continuous glucose monitoring; POC = point of care.
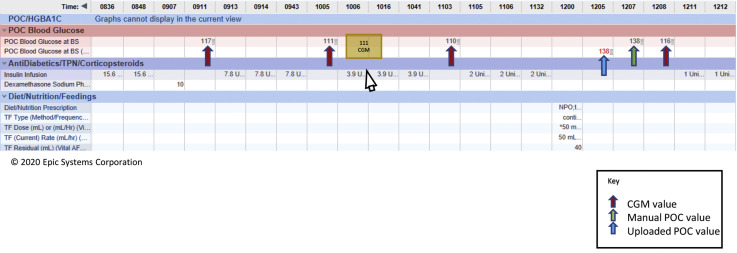
The IV insulin infusion was titrated every hour using the POC BG value until the initial validation threshold was achieved. Then titration was performed using the CGM with POC BG validation occurring every 6 hours. The organization’s standard guideline was used for IV insulin titration and no modifications were made to incorporate CGM trend data into titration guidelines. Additional POC BG values were obtained according to criteria in Table 1. POC BG monitoring reverted to hourly if any of the sensor-meter pairs were discordant. In order to distinguish CGM values from POC, nurses used a “CGM” annotation (Figure. 2 ).
Fig. 2.
Documentation of CGM values. Due to concern that CGM would be inappropriately utilized outside of patients with COVID-19, a separate row for CGM data was not added to the glucose flowsheet in the EHR. In order to distinguish CGM values from POC, nurses used a “cgm” annotation, which appears when hovering over the value with the mouse. Per usual practice, all POC BG values were manually entered into the glucose flowsheet at the time of acquisition, and POC devices were downloaded once each shift as per usual practice. Downloaded POC values also automatically populate a separate row in the glucose flowsheet. BG = blood glucose; CGM = continuous glucose monitoring; COVID-19 = coronavirus disease 2019; EHR = electronic health record; POC = point of care (arterial, capillary).
Data Transmission and Retrieval
A Clarity Healthcare Professional account was created to visualize data, but retrieval was delayed by 3 hours and therefore only utilized by the Diabetes Consult Service.
Postinfusion Management
When patients were transitioned off IV insulin, POC testing was continued every 6 hours. Once transferred out of the MICU, CGM could be used nonadjunctively (to replace POC testing) according to the institution’s global Hyperglycemia in COVID-19 Patients guideline (Figure 1).
Diabetes Consult Service
All patients were co-managed by the inpatient Diabetes Consult Service, which, in concert with the intensivist team, determined when to transition patients off IV insulin (Figure. 1).
Nursing Protocol and CGM Implementation Focus Group Methods
We conducted a focus group qualitative evaluation among MICU nurses to evaluate initial implementation efforts using an implementation science approach with the PARIHS framework.27 The Ohio State University’s institutional review board approved the focus group protocol. A single session focus group was conducted with nurse leaders (eg, nurse managers, clinical nurse scientists [CNS], MICU staff nurses, and Diabetes Consult Service NPs. Interviews were analyzed using a qualitative descriptive approach.28 , 29
Participants
Eligibile participants included a MICU floor nurse who cared for patients with COVID-19 on CGM; a MICU nurse leader (ie, CNS, nurse manager) who assisted with patient identification, CGM insertion, or hybrid protocol staff education; and a diabetes consult NP who managed consult patients on CGM between June 1, 2020, and August 31, 2020. Nurses provided consent prior to focus group participation.
Data Collection
A single session focus group was conducted virtually. A semistructured interview guide focused on core components of the PARIHS framework: evidence, context, and facilitation. As such, questions included content regarding perceived accuracy, protocol, CGM training, workflow, facilitators, and barriers to future use. The focus group was audio recorded, professionally transcribed, and reviewed for accuracy.
Data Analysis
A code book was developed a priori based on the semistructured interview questions. Interview data, fieldnotes, and memos were imported into NVivo 12.0 (Doncaster, Australia) for data management and analyzed using a qualitative descriptive approach.28 , 29 Two authors (E.R.F. and M.M.) conducted qualitative analysis. Portions of text were coded with terms that were low inference (“data close”), then grouped into thematic categories and subthemes.28 The data were assessed using the PARIHS framework for implementation, a theoretical framework that has been widely used to implement evidence-based clinical practices in the inpatient setting.27
Results
A total of 9 nurses participated in the focus group and included 3 nurse leaders (eg, 2 nurse managers, 1 CNS), 2 diabetes consult NPs, and 4 MICU staff nurses. Participants were 89% female, ranged in age from 28 to 50 years, and had mean 11.6 years of nursing experience (range, 3-28 years). The MICU nurse leaders and staff nurses (total N = 7) had a mean of 9 years of MICU nursing experience (range, 1-26 years). Among the staff nurses, 2 had cared for patients on CGM during 4 to 6 shifts, 1 for 1 to 3 shifts, and 1 for 7 to 10 shifts. The MICU nurse leaders (N = 3) reported each assisting with CGM insertion, nursing education, or trouble shooting with 4 to 6 patients. The diabetes consult NPs (N = 2) reported caring for 7 to 10 and > 10 consult patients with CGM.
We identified 4 major themes: accuracy, nursing ownership, workflow, and barriers and suggestions, presented within the 3 core elements of the PARIHS framework (evidence, context, facilitation).27 Evidence was derived from the clinical experience of the MICU nurses, consistent with PARIHS definitions. Context included elements about organizational and unit culture, leadership, education, and initial evaluation of use. Facilitation included internal facilitation for streamlined CGM use within the unit and suggested improvements for future use.27 The major themes and interconnecting subthemes from the focus group data are presented within this PARIHS framework. Table 2 presents major themes and subthemes along with supporting nursing statements.
Table 2.
Nursing CGM Implementation Themes, Subthemes, and Supporting Nursing Statements Within the PARIHS Framework for Evidence
| Theme | Subtheme | Exemplar statement |
|---|---|---|
| Accuracy | Initial validation |
|
| Patient condition |
|
|
| Watch it |
|
Abbreviations: CGM = continuous glucose monitoring; MICU = medical intensive care unit; NP = nurse practitioners; PARIHS = Promoting Action on Research in Health Services.
Evidence
Accuracy
All participants had positive perceptions regarding the accuracy of the CGM systems. Six of the 9 participants commented that they never experienced any inaccuracies in which the POC and CGM values fell outside the 20% or 20 mg/dL threshold for values under 100 mg/dL. Table 2 presents accuracy’s subthemes along with supporting nursing statements.
Initial Validation
While 5 nursing participants stated they were aware that the CGM values might not be as accurate during the first 24 hours, most reported the CGM systems were validated quickly, typically 2 hours after the first CGM reading or 4 hours after insertion. While device calibration was not mentioned in the protocol or educational materials, several MICU leaders did mention occasionally calibrating CGM systems if accuracy was not initially obtained.
Patient Condition
Two participants, a MICU staff nurse and diabetes NP, discussed 2 examples of inaccuracy. In 1 case, the CGM was validated within the first 2 POC readings; however, values for the subsequent 12 hours were slightly outside the 20% required range before finally regaining and sustaining accuracy. Inaccuracies in this case were attributed to changes in the patient’s condition prompting adjustments in the tube feed regimen and initiation of steroids. In the second instance, the NP reported inaccuracies attributed to the close proximity of a cooling blanket. The sensor was repositioned on the patient’s abdomen, which resolved the issue.
Watch It
Nurses stated they were likely to accept the CGM glucose values and use those values to titrate the IV insulin, knowing they could continue to monitor the readings. Nurses felt that the ability to continuously monitor glucose values presented a safer alternative to traditional POC glucose testing.
Context
The major themes of nursing ownership and workflow, their subthemes, and supporting nursing statements are presented in Table 3 .
Table 3.
Nursing CGM Implementation Themes, Subthemes, and Supporting Nursing Statements Within the PARIHS Framework for Context
| Theme | Subtheme | Exemplar statement |
|---|---|---|
| Nursing ownership | Acceptance |
|
| Training |
|
|
| Patient selection |
|
|
| Workflow | Decreased exposure |
|
| Frequent monitoring |
|
|
| Eyeball |
|
|
| CGM insertion |
|
Abbreviations: CGM = continuous glucose monitoring; CNS = clinical nurse specialist; MICU = medical intensive care unit; NP = nurse practitioners; PARIHS = Promoting Action on Research in Health Services.
Nursing Ownership
Despite its novelty, there was surprising acceptance of CGM technology among MICU nurses. Interestingly, that acceptance did not as readily extend to other members of the MICU care team. Nurses readily and organically took on the bulk of implementation activities including training and patient selection.
Acceptance
Nurses seemed to readily incorporate the technology into the MICU and expressed a desire for continued and expanded use beyond the COVID pandemic and population. While there was general sentiment and description of the technology within the nursing domain, 1 nurse expressed a desire for “nursing ownership.”
Training
Training on both the CGM and the protocol appeared to be very organic and nurse driven. Several nurses described receiving initial training from a CNS or from a nurse manager; however, others described receiving training from other nurses who had developed CGM experience. The diabetes consult NP team played an essential role in ongoing support, especially when managing more difficult patients or trouble shooting a device issue.
Patient Selection
Patient selection and CGM initiation were described as nursing-driven processes. Initially, patient selection was driven by the MICU CNS team; however, once nurses became more comfortable and gained experience using the CGM systems, they began promoting use. The MICU intensivist teams had little involvement in initiating CGM, and nurses described encountering confusion and occasionally pushback from MICU providers regarding initiation of therapy.
Workflow
Nurses discussed use of the devices and protocol and how CGM changed aspects of their workflow.
Decreased Exposure
Nurses described significant changes to workflow and glucose management associated with the addition of CGM. The ability to monitor glucose from outside the COVID patient rooms allowed nurses to spend significantly less time in the room.
Frequent Monitoring
The ability to continuously view glucose values changed the frequency of glucose monitoring. Although the management of IV insulin requires hourly monitoring, nurses reported viewing CGM glucose much more frequently. Changes in patient clinical condition often prompted more frequent glucose monitoring. Alarms played a minor role in glucose monitoring, which was attributed to the fact that alarms were triggered only when readings were “really high.”
Eyeball
In addition to viewing the glucose value, nurses evaluated glucose trends. They all referred to the trend arrow as the “eyeball,” due to the shape of the icon on the display. Most nurses reported that while they were aware of the trend arrow, it did not really impact their treatment or IV insulin titration. However, 1 nurse indicated that the trend arrows did factor into an adjustment she might make within the IV guideline parameters.
CGM Insertion
Sensor insertion was thought to be very easy and quick. There was a sense that the time savings experienced once the device was validated was well worth the small amount of time required for insertion. Training surrounding device insertion was transmitted between nurse managers and CNSs through demonstration and the sharing of valuable lessons learned. One diabetes NP shared 2 instances in which she experienced difficulty during device insertion despite having worn a Dexcom CGM for many years herself. One involved the novelty of wearing gloves while managing adhesive backing and the other involved not fully pushing the transmitter in place.
Facilitation
Barriers & Recommendations
Nursing participants were asked what changes they would recommend for current and longstanding CGM use in the MICU. Table 4 presents subthemes along with supporting nursing statements. Perceived barriers and nursing recommendations for future use were as follows.
Table 4.
Nursing CGM Implementation Themes, Subthemes, and Supporting Nursing Statements Within the PARIHS Framework for Facilitation
| Theme | Subtheme | Exemplar statement |
|---|---|---|
| Barriers & recommendations | EHR & technology integration |
|
| Protocol simplification |
|
|
| CGM insertion |
|
Abbreviations: CGM = continuous glucose monitoring; CNS = clinical nurse specialist; COVID = coronavirus disease; EHR = electronic medical record; MICU = medical intensive care unit; NP = nurse practitioners; PARIHS = Promoting Action on Research in Health Services.
Electronic Health Record and Technology Integration
Suggestions included the need for an improved documentation system and EHR integration. The need for nurses to annotate “cgm” within the POC glucose EHR field was seen as cumbersome and was thus inconsistently performed. Nurses commented that while they appreciated the technology, the receiver, phone, cords, and associated protocols and training materials crowded their workstations. Nurses exclusively used the receivers to view CGM data and remarked that the phones “never worked” and were rarely successfully paired.
Protocol Simplification
There was unanimous consensus that the hybrid CGM + POC protocol was excessively lengthy and nurses expressed a desire for simplification. Nurses reported that confusion surrounding CGM use and the protocol was most likely to occur after insertion during the validation period. This was especially true if the validation period coincided with nursing shift change. Additionally, it was acknowledged that the need to perform mathematical calculations could be a barrier for some nurses. One nurse suggested automatic EHR mathematical calculations in the future.
CGM Insertion
There was general consensus that the CGM sensor insertion process was uncomplicated and quick. CGM sensor insertion was being done by MICU CNSs, nurse managers, and diabetes consult NPs. Participants felt MICU staff nurses were absolutely capable of performing CGM sensor insertions; however, it was recommended that for future and potentially more widespread use, training be extended to only charge nurses initially.
Discussion
This report and evaluation highlight successful implementation of CGM in a MICU among patients with COVID-19 using a hybrid POC plus CGM approach. Expanded inpatient CGM use holds promise to reduce health care worker exposure, conserve PPE, improve hospital efficiencies, and reduce costs, while improving glycemic control.
The cohort of nurses who participated in the MICU focus group represented 3 distinct subsets of nursing care in the MICU (eg, nursing leaders, staff nurses, diabetes consult NPs). Focus group data demonstrated the distinct but integrated roles of these 3 nursing groups in CGM implementation. Nurses appeared to readily accept and integrate CGM technology into the MICU environment. They felt the devices were accurate, with the majority reporting all CGM/POC paired values within target. Interestingly, any reported inaccuracies were attributed to the critical nature of the patients rather than the technology itself.
There was a strong sense that this was a nursing intervention and a desire to keep the technology as a readily available tool. Patient selection was primarily driven by nurses, initially by the CNS and then the MICU staff nurse. Barriers encountered in patient selection included insufficient knowledge among the ICU care teams regarding CGM availability and use. Rather than using CGM to facilitate IV insulin, teams tended to use subcutaneous insulin in patients without severe refractory hyperglycemia. This was compounded by a lack of strong evidence from randomized controlled trials for a specific glucose target in MICU patients.30, 31, 32 In addition, MICU teams pulled IV insulin pumps outside of the room only for patients with central IV access, thus making the perception of CGM less valuable when nurses needed to enter the room to titrate IV insulin. Thus, it is important to have an engaged leadership team and open lines of communication.
There was a sense that CGM devices provided an overall time savings for nurses and decreased the time spent in COVID-19 rooms. At the same time, nurses reported that the ability to continuously view CGM glucose data from outside the rooms prompted more frequent glucose monitoring. Additional analysis quantifying these aspects of implementation and time savings is greatly needed. This would include a formal analysis of time spent on each component of CGM implementation (eg, device set-up, insertion, pairing, CGM validation, monitoring, documentation).
Recommendations for future use included improvements to EHR documentation. While nurses did appear to be documenting CGM values as a substitute for hourly POC, the annotation of “cgm” did not consistently occur despite frequent reinforcement. This was certainly substantiated by focus group data. A distinct field in the EHR dedicated to CGM glucose would help alleviate many documentation issues for nursing, but it was not possible to customize this for patients with COVID-19 only. The failure of the Android phones to pair in many cases (13 or 19 phones did not pair), despite their connection to secure Wifi, was likely due to firewall protections that will need to be considered in the future. Glucose telemetry systems using the Dexcom Share feature have also been described.33 However, no commercial system currently integrates with the EHR or with IV insulin software programs.
Additional considerations not addressed in the focus group data include streamlining the device set-up procedures. While nurses reported successful implementation of device insertion and receiver pairing, retooling Dexcom G6 personal CGM for inpatient use required considerable time and effort, and this process should be streamlined to facilitate widespread implementation.
Limitations
The study was limited by several factors, including the small sample size that is inherent to focus groups. Additionally, the focus group was at a single large academic medical center, which could limit generalizability of experiences and implementation strategies.
Conclusion
This report demonstrates the feasibility of CGM integration in the ICU in response to the COVID-19 pandemic using an implementation science approach. The emerging use of CGM in the critical care environment holds tremendous promise to improve health care efficiency, reduce cost, and improve glycemic control; however, there is need for further research to quantify changes to nursing workflow, implementation burden, and associated economic implications. Finally, there is a need for prospective multicenter controlled trials to demonstrate feasibility, safety, and efficacy in the broader ICU population.
Acknowledgment
We would like to thank the MICU nursing staff at The Ohio State University Medical Center. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Team C.C.-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARSCoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epidemiology Working Group for Ncip Epidemic Response CCfDC, Prevention [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Bode B., Garrett V., Messler J. Glycemic characteristics and clinical outcomes of covid-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L., She Z.G., Cheng X. Association of blood glucose control and outcomes in patients with covid-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077 e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeland B., Penprase B.B., Anthony M. Nursing practice patterns: timing of insulin administration and glucose monitoring in the hospital. Diabetes Educ. 2011;37:357–362. doi: 10.1177/0145721711401669. [DOI] [PubMed] [Google Scholar]
- 9.Cobaugh D.J., Maynard G., Cooper L. Enhancing insulin-use safety in hospitals: practical recommendations from an ASHP Foundation expert consensus panel. Am J Health Syst Pharm. 2013;70:1404–1413. doi: 10.2146/ajhp130169. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes A., Evans L.E., Alhazzani W. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 11.Coronavirus disease 2019 (COVID-19): cases in the U.S. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html0 Accessed June 28, 2020.
- 12.Galindo R.J., Migdal A.L., Davis G.M. Comparison of the freestyle libre pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43:2730–2735. doi: 10.2337/dc19-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dexcom. Fact Sheet for Healthcare Providers: Use of Dexcom Continuous Glucose Monitoring Systems During the COVID-19 Pandemic. Accessed April 23, 2020, https://www.dexcom.com/hospitalfacts.
- 14.Kopecky P., Mraz M., Blaha J. The use of continuous glucose monitoring combined with computer-based eMPC algorithm for tight glucose control in cardiosurgical ICU. Biomed Res Int. 2013;2013:186439. doi: 10.1155/2013/186439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabiee A., Andreasik V., Abu-Hamdah R. Numerical and clinical accuracy of a continuous glucose monitoring system during intravenous insulin therapy in the surgical and burn intensive care units. J Diabetes Sci Technol. 2009;3:951–959. doi: 10.1177/193229680900300443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Quintanilla K.A., Lavalle-Gonzalez F.J., Mancillas-Adame L.G., Zapata-Garrido A.J., Villarreal-Perez J.Z., Tamez-Perez H.E. Continuous glucose monitoring in acute coronary syndrome. Arch Cardiol Mex. 2013;83:237–243. doi: 10.1016/j.acmx.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.De Block C., Manuel Y.K.B., Van Gaal L., Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29:1750–1756. doi: 10.2337/dc05-2353. [DOI] [PubMed] [Google Scholar]
- 18.Holzinger U., Warszawska J., Kitzberger R., Herkner H., Metnitz P.G., Madl C. Impact of shock requiring norepinephrine on the accuracy and reliability of subcutaneous continuous glucose monitoring. Intensive Care Med. 2009;35:1383–1389. doi: 10.1007/s00134-009-1471-y. [DOI] [PubMed] [Google Scholar]
- 19.Holzinger U., Warszawska J., Kitzberger R. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33:467–472. doi: 10.2337/dc09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis G.M., Galindo R.J., Migdal A.L., Umpierrez G.E. Diabetes technology in the inpatient setting for management of hyperglycemia. Endocrinol Metab Clin North Am. 2020;49:79–93. doi: 10.1016/j.ecl.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallia A., Umpierrez G.E., Nasraway S.A., Klonoff D.C., Investigators P. Round table discussion on inpatient use of continuous glucose monitoring at the international hospital diabetes meeting. J Diabetes Sci Technol. 2016;10:1174–1181. doi: 10.1177/1932296816656380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh J.B., Gao P., Derdzinski M. Accuracy, utilization, and effectiveness comparisons of different continuous glucose monitoring systems. Diabetes Technol Ther. 2019;21:128–132. doi: 10.1089/dia.2018.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krinsley J.S., Bruns D.E., Boyd J.C. The impact of measurement frequency on the domains of glycemic control in the critically ill--a Monte Carlo simulation. J Diabetes Sci Technol. 2015;9:237–245. doi: 10.1177/1932296814566507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyser T.A., Nakamura K., Price D., Bohnett L.C., Hirsch I.B., Balo A. Hypoglycemic accuracy and improved low glucose alerts of the latest Dexcom G4 Platinum Continuous Glucose Monitoring System. Diabetes Technol Ther. 2015;17:548–554. doi: 10.1089/dia.2014.0415. [DOI] [PubMed] [Google Scholar]
- 25.Puhr S., Derdzinski M., Welsh J.B., Parker A.S., Walker T., Price D.A. Real-world hypoglycemia avoidance with a continuous glucose monitoring system’s predictive low glucose alert. Diabetes Technol Ther. 2019;21:155–158. doi: 10.1089/dia.2018.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steineck I.I.K., Mahmoudi Z., Ranjan A., Schmidt S., Jorgensen J.B., Norgaard K. Comparison of continuous glucose monitoring accuracy between abdominal and upper arm insertion sites. Diabetes Technol Ther. 2019;21:295–302. doi: 10.1089/dia.2019.0014. [DOI] [PubMed] [Google Scholar]
- 27.Helfrich C.D., Li Y.F., Sharp N.D., Sales A.E. Organizational readiness to change assessment (ORCA): development of an instrument based on the Promoting Action on Research in Health Services (PARIHS) framework. Implement Sci. 2009;4:38. doi: 10.1186/1748-5908-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Colorafi K.J., Evans B. Qualitative descriptive methods in health science research. HERD. 2016;9:16–25. doi: 10.1177/1937586715614171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Investigators N.-S.S., Finfer S., Chittock D.R. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 31.Investigators C.S., Annane D., Cariou A. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303:341–348. doi: 10.1001/jama.2010.2. [DOI] [PubMed] [Google Scholar]
- 32.Brunkhorst F.M., Engel C., Bloos F. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 33.Singh L.G., Satyarengga M., Marcano I. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43:2736–2743. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]