ABSTRACT
Vitamin D synthesis by exposure of skin to solar ultraviolet radiation (UVR) provides the majority of this hormone that is essential for bone development and maintenance but may be important for many other health outcomes. This process, which is the only well‐established benefit of solar UVR exposure, depends on many factors including genetics, age, health, and behavior. However, the most important factor is the quantity and quality of UVR reaching the skin. Vitamin D synthesis specifically requires ultraviolet B (UVB) radiation that is the minority component (<5%) of solar UVR. This waveband is also the most important for the adverse effects of solar exposure. The most obvious of which is sunburn (erythema), but UVB is also the main cause of DNA damage to the skin that is a prerequisite for most skin cancers. UVB at the Earth's surface depends on many physical and temporal factors such as latitude, altitude, season, and weather. Personal, cultural, and behavioral factors are also important. These include skin melanin, clothing, body surface area exposed, holiday habits, and sunscreen use. There is considerable disagreement in the literature about the role of some of these factors, possibly because some studies have been done by researchers with little understanding of photobiology. It can be argued that vitamin D supplementation obviates the need for solar exposure, but many studies have shown little benefit from this approach for a wide range of health outcomes. There is also increasing evidence that such exposure offers health benefits independently of vitamin D: the most important of which is blood‐pressure reduction. In any case, public health advice must optimize risk versus benefit for solar exposure. It is fortunate that the individual UVB doses necessary for maintaining optimal vitamin D status are lower than those for sunburn, irrespective of skin melanin. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.
Keywords: ACTION SPECTRUM, ENVIRONMENT, SUNSCREEN, ULTRAVIOLET RADIATION (UVR) DOSE, VITAMIN D
Introduction
Terrestrial solar ultraviolet radiation (UVR; ~295–400 nm) can be divided into ultraviolet B (UVB; 280–315 nm) and ultraviolet A (UVA; 315–400 nm), the vast majority (≥95%) of which is UVA. Exposure to sunlight has many effects on human health( 1 ) including erythema (sunburn), skin cancer, and vitamin D synthesis. Until recently, vitamin D synthesis was regarded as the only benefit from solar exposure, but there is increasing evidence for other health benefits that are independent of vitamin D,( 1 , 2 ) such as reduced blood pressure.( 3 , 4 , 5 ) All UVR effects are initiated by the absorption of UVR by chromophores.( 6 ) The absorption spectrum of a given chromophore determines the action spectrum (wavelength dependence) of the given photobiological outcome.
Solar UVB (~295–315 nm) converts 7‐dehydrocholesterol (7‐DHC), a chromophore in epidermal keratinocytes and dermal fibroblasts, into previtamin D3. ( 7 , 8 ) Figure 1 shows the action spectrum for this process,( 9 ) though the validity of this spectrum has been questioned.( 10 ) Previtamin D3 is thermally unstable and isomerizes into vitamin D3 (cholecalciferol).( 11 ) This is hydroxylated in the liver to 25‐hydroxyvitamin D [25(OH)D] and then in the kidneys to 1,25‐dihydroxyvitamin D [1,25(OH)2D], which is the active hormone. Many tissues, including skin, have the enzymes for both hydroxylations.( 12 )
Fig 1.
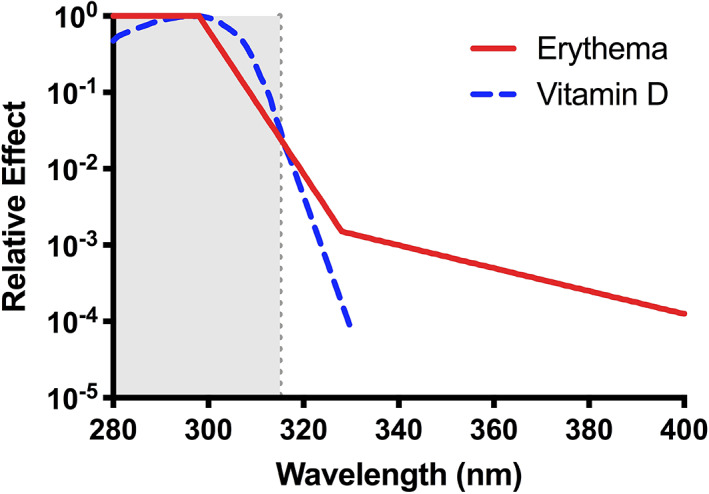
Commission Internationale de l'Eclairage (International Commission on Illumination) action spectra for erythema( 35 ) and cutaneous previtamin D3. ( 9 ) Note the considerable overlap in the solar UVB (~295–315 m) region.
Vitamin D is essential for bone health and may play a role in many diseases.( 1 ) Most 1,25(OH)2D (eg, 70%–85% in summer in the White population of the United Kingdom [UK](13 )) originates from cutaneous photosynthesis. The rest is provided by diet and supplementation: vitamin D3 from animal products and also from plants( 14 , 15 ) and vitamin D2 (ergocalciferol) from fungi.( 15 )
Solar exposure for vitamin D synthesis must be balanced against risks: The most obvious is erythema. The minimal erythemal dose (MED) is an individualized clinical measurement of personal sensitivity to UVR; it represents the lowest UVR dose (J/m2) required to cause just perceivable erythema on irradiated skin. MED shows considerable interpersonal variation.( 16 ) It is also is very dependent on the UVR source used. Thus, it is approximately 250 J/m2 with monochromatic UVB at 300 nm, whereas it is approximately 320,000 J/m2 with UVA at 360 nm.( 17 )
Many non‐UVB factors influence vitamin D status including genetics, metabolism, health, and age.( 18 , 19 , 20 , 21 ) Here we assess those factors that influence the quantity and quality of UVB reaching cutaneous 7‐DHC. These include spectral, atmospheric, geographic, and behavioral factors, as well as the skin's melanin content. In addition, we determine the minimal doses of erythemally effective UVR necessary for this purpose. Erythema, which typically peaks at approximately 24 hours after solar exposure, is the most widely used endpoint for risk assessment; its avoidance is advocated in public health campaigns for skin cancer prevention.
Vitamin D Deficiency
Serum 25(OH)D concentration gives the most accurate estimate of vitamin D status.( 22 ) There are different definitions of vitamin D insufficiency/deficiency; however, serum 25(OH)D <50 nmol/L is widely used for insufficiency.( 23 ) Suboptimal vitamin D status is widespread. A study of almost 56,000 people in Europe reported that 40.4% had insufficiency according to the above definition, especially those with darker skin.( 24 ) Globally, 37.3% had 25(OH)D concentrations <50 nmol/L, and global variability in vitamin D status showed no correlation with latitude.( 25 ) A recent report showed that Africa had much poorer vitamin D status than other parts of the world: with 34% with 25(OH)D <50 nmol/L( 26 ) but there are exceptions as discussed in the Skin Pigmentation subsection. However, there is a lack of data from Africa and South America, and for infants, children, adolescents, and pregnant women worldwide.( 27 , 28 ) The authors of a 2007 article reported that 46.6% of White UK adults (aged 45 years) had 25(OH)D <40 nmol/L in winter/spring, which improved to 15.4% of these adults in summer/autumn. The odds ratio for increased risk was 2.03 for obesity and 2.38 for living in Scotland (compared with southern England).( 29 ) More recent studies have reported 23% and 61% of UK adults (19–64 years) with serum 25(OH)D <25 nmol/L and <50 nmol/L, respectively.( 30 ) A large sample of UK South Asians (aged 40–69 years) showed 92% with serum 25(OH)D <50 nmol/L, 55% <25 nmol/L, and 20% <15 nmol/L.( 31 ) A study of 5034 Australian adults reported that 20% of participants had serum 25(OH)D <50 nmol/L.( 32 ) Newborns and the elderly living in institutions are at greatest risk of deficiency.( 25 )
UVR Spectral Factors
Irradiance and action spectra are critical considerations in photobiological research and its public health consequences. Incorrect conclusions can be reached without a good definition of these spectra and their interactions.
Irradiance spectrum
An irradiance spectrum is a plot of UVR intensity received per unit of area (measured as W/m2/nm) versus wavelength. The integral of this plot is expressed as W/m2. At the Earth's surface, the solar irradiance spectrum has a dynamic range of six orders of magnitude. Even the weaker spectral subranges may have profound biological effects (see below). Therefore, the irradiance spectrum should be measured with an instrument (spectroradiometer) that can accurately handle six orders of magnitude.
A solar UVR irradiance spectrum is shown in Fig. 2A . Many vitamin D studies have been done with broadband UVB phototherapy sources. However, as seen in Fig. 2A , these typically emit nonsolar UVB (wavelengths <295 nm) that are very effective at previtamin D3 production (Fig. 1) and can therefore give misleading results if used as a surrogate for solar UVR. Studies have also been done with a narrow band phototherapy source that is essentially monochromatic: approximately 313‐nm UVB. Tanning cabinets have also been used. Cutaneous production of vitamin D involves a complex set of photochemical reactions, of which the conversion of 7‐DHC to previtamin D3 is only one. All reactions have their own preferential wavelengths.( 33 ) Therefore, ideally, solar UVR should be used but that presents considerable logistical challenges. The best laboratory option is solar‐simulated radiation (SSR) obtained from a filtered xenon arc source. Most SSR sources are designed for sunscreen testing with very small irradiation fields. It is possible to get a good solar UVR simulation with fluorescent tubes (Fig. 2A ).
Fig 2.
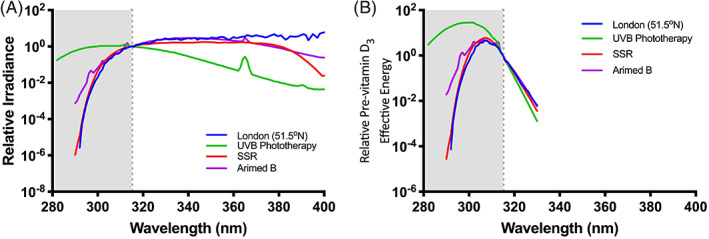
(A) Relative irradiance spectra of London (United Kingdom) noon midday midsummer solar UVR and xenon arc SSR as used to test sunscreens, fluorescent SSR (Arimed B), and a broad spectrum UVB phototherapy source. Note greater shorter nonsolar wavelength UVB content in phototherapy spectrum compared with sunlight (B). The use of the CIE action spectrum for previtamin D3 (Fig. 1) as a weighting function for the spectra is shown in (A). Note the large disproportionate effect of the nonsolar UVB with the phototherapy source. Graphs normalized at 315 nm, which is the end of shaded area. SSR = solar simulating radiation; UVB = ultraviolet B; UVR = ultraviolet radiation.
Action spectrum
Action spectrosccopy determines the wavelength dependence of a given photobiological outcome. This has two major purposes: (i) identification of a chromophore and (ii) the generation of a biological (or chemical) weighting function, which is called an action spectrum. An action spectrum for a given biological effect gives the relative efficacy of dose at any wavelength when compared with dose at a reference wavelength (which should be specified—usually the wavelength of the maximum value —and for which the absolute production should be given. The maximum value is usually normalized to 1). The effective irradiance spectrum of a given UVR source for a given biological outcome is the product of its irradiance spectrum with the action spectrum for that endpoint. The surface under this curve gives the total biological efficacy, as if the UVR source emits monochromatic radiation at the reference wavelength. The modification of an irradiance spectrum with an action spectrum is termed “spectral weighting.” The importance of spectral weighting can be found in a study that showed that the 0.8% UVB (ie, 99.2% UVA) content of a sunbed UVR source caused 75% of DNA damage in human keratinocytes in vitro.( 34 )
Action spectroscopy shows that measurements of terrestrial UVR exposure as J/m2 have little biological value per se. Thus, it is more useful to use biologically weighted UVR exposure; this is widely done for erythema as an indicator of risk. The standard erythemal dose (SED)( 35 ) is an example that is increasingly used in epidemiology. Unlike MED, this measure is independent of personal sensitivity to UVR and the irradiance spectrum. It represents a dose of 100 J/m2, of any irradiance spectrum that has been weighted by the Commission Internationale de l'Eclairage (CIE; International Commission on Illumination) erythema action spectrum (Fig. 1).( 35 ) The MED for a fair‐skinned person is approximately 3 SED.( 16 ) The CIE erythema action spectrum is also the basis for calculating the publicly available UV index (UVI): an international standardized, dimensionless scale quantifying the irradiance of erythemally effective UVR.
Action spectra for erythema, epidermal DNA photodamage,( 17 ) keratinocyte cancers( 36 ) and skin photoageing( 37 ) are broadly similar. Thus, the CIE erythema action spectrum is widely used to assess risk from solar UVR exposure.
Figure 1 shows the CIE action spectrum for the conversion of 7‐DHC to previtamin D3, ( 9 ) which is widely used in risk–benefit analyses for solar UVR exposure.( 38 , 39 , 40 ) Maximal activity was at 297 nm. However, concerns about study methodology have led to questions about its validity and its use in risk–benefit analyses.( 10 , 33 , 41 , 42 ) For example, it is based on a single ex vivo study in which human skin (unknown body site and age) was irradiated with unspecified doses of wavebands between 255 and 320 nm.( 43 ) Figure 2B shows the use of this action spectrum as a weighting function for solar UVR, a broad spectrum UVB phototherapy source, and xenon arc and fluorescent SSR (Arimed B). It can be seen that the UVB phototherapy source has a disproportionate effect, especially with shorter nonsolar UVB wavelengths.
It should be stressed that the CIE spectrum only represents the initial cutaneous photochemical step in the formation of vitamin D. It does not necessarily represent serum 25‐hydroxyvitamin D3 [25(OH)D3] because it does not consider other inevitably associated photochemical modifications, such as photo‐isomerization of excess previtamin D3 and vitamin D3 into tachysterol, lumisterol, suprasterol I and II, and 5,6‐transvitamin D3. These degradation processes may prevent vitamin D toxicity( 11 ) that may occur with supplementation( 44 ) but not with UVR exposure. If all photochemical processes are accounted for, then it can be shown that the action spectrum changes during the course of UVR exposure.( 33 ) For this reason, the use of the single CIE action spectrum to predict vitamin D production can overestimate in vivo vitamin D photosynthesis.( 33 )
Photodegradation by UVA is supported in one human study that used UVA, UVB, and mixed UVA and UVB exposures.( 45 ) The authors reported no difference in the increase of 25(OH)D between participants exposed to UVB and mixed UVA and UVB for periods of <9 minutes. Exposure for >9 minutes resulted in a significantly lower increase in 25(OH)D in the group exposed to mixed UVB and UVA compared with UVB alone. The findings of the study have, however, been called into question based on uncertainties in dosimetry.( 46 , 47 )
It should be noted that there are other less well‐established in vitro action spectra for previtamin( 48 )/vitamin D3 ( 49 ) (cholecalciferol) formation that have been used as weighting functions for human studies and compared with the CIE action spectrum.( 42 ) Different results have been obtained with different UVR spectra. A recent in vivo human study (unpublished, Young and colleagues) suggests that the CIE previtamin D action spectrum requires a 5‐nm shift to the shorter wavelengths to be applicable for serum 25(OH)D3. Furthermore, this study, done with suberythemal exposures, showed no significant spectral interaction.
An action spectrum, determined with well‐defined light‐emitting diode irradiance spectra, for vitamin D3 in pig skin showed a peak at 296 nm when tested at two UVR doses.( 50 ) It should be noted that basing an action spectrum on a value at a predetermined dose is only valid if the dose‐response curves for all wavelengths have the same slope, otherwise the action spectrum will vary with dose.
UVR Dose
UVR dose (J/m2) is the product of irradiance and exposure time in seconds. Studies in humans and pigs have reported that UVR exposure and markers of vitamin D concentration show an initial linear dose‐dependent relationship that plateaus after repeated exposures.( 50 , 51 , 52 , 53 , 54 , 55 , 56 ) Figure 3 shows linear dose responses in healthy volunteers exposed to a broadband UVB phototherapy source. A large observational study showed a very steep rise in 25(OH)D versus dose at lower doses followed by a plateau.( 57 ) Evidence suggests that the shape of the dose‐response curve depends on the individual's initial vitamin D status, with lower starting concentrations resulting in the greatest dose response.( 58 , 59 , 60 ) The plateau in the dose‐response curve only manifests in individuals with preexposure 25(OH)D concentrations of >50 nmol/L.( 59 )
Fig 3.
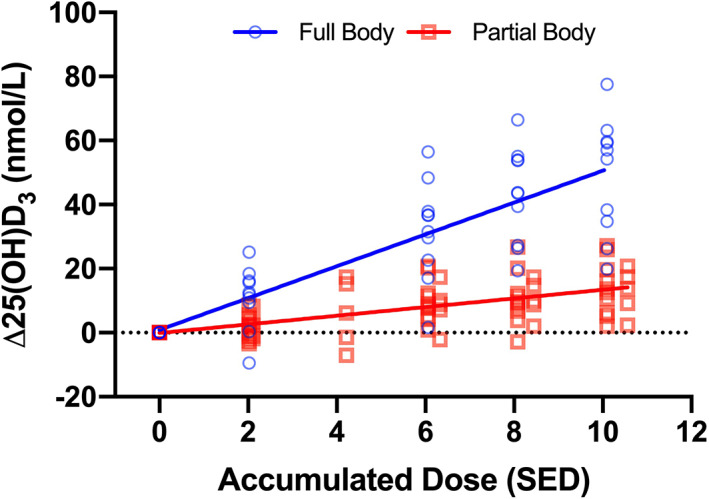
Effect of UVR dose and body surface area exposed with the same ultraviolet B phototherapy source (UV6 tubes from Waldmann GmbH & Co, Villingen‐Schwenningen, Germany). Full body (FB ~85% BSA) with n = 10 and partial body (PB ~4% BSA) with n = 15. Each site was given four to five suberythemal exposures of approximately 2 SED with intervals of 3 to 4 days, resulting in a cumulative exposure of approximately 10 SED. Increasing the exposed BSA over 20‐fold resulted in a 3.7‐fold steeper slope, which shows a nonproportional relationship between % BSA exposed and Δ25(OH)D3 (25‐hydroxyvitamin D3). Linear regression equations for FB are y = 4.97x + 0.86 (ie, 4.97 nmol/L SED), p = 1.78 × 10−13, R 2 = 0.68, and for PB are y = 1.35x − 0.09 (ie, 1.35 nmol/L SED), p = 1.67 × 10−10, R 2 = 0.41. The FB and PB slopes are significantly different: p = 2.33 × 10−32. Data from Young and colleagues (unpublished). 25(OH)D3 = 25‐hydroxyvitamin D3; SED = standard erythemal dose.
Serum 25(OH)D3 response in humans in the laboratory depends on total dose but not the dose rate (ie, irradiance).( 51 ) Such reciprocity for a given UVR dose of 300 J/m2 was also observed for vitamin D3 in pig skin using monochromatic radiation at 292, 296, and 300 nm with irradiances that varied by two orders of magnitude( 50 ) (it should be noted that these exposures would be about one MED in human skin( 17 )). Thus, equivalent increases in vitamin D3 concentration can be achieved with high irradiances of UVB over short periods and lower irradiances over long periods. However, this approach has its limits in sunshine because of changes in vitamin D effective solar‐UVB irradiance during the day.( 33 )
Dose responses are harder to determine in field conditions because personal UVR exposure must be measured or estimated in retrospect rather than irradiating participants with predetermined known doses. A study in which electronic UVB dosimeters were worn by 98 Europeans during 1‐week sun and skiing holidays showed a linear dose response when exposed body‐surface area (BSA) was taken into account.( 60 , 61 ) Another study, using personal UVR dosimeters and sun‐exposure diaries in 25 healthy White volunteers in Copenhagen (Denmark; 56oN) showed that serum 25(OH)D3 at the end of summer and winter was dependent on the UVR dose received during the previous summer.( 62 ) A study of 100 people with dark skin in South Africa showed that personal UVB exposure was highly correlated with vitamin D status.( 63 )
The difference between the CIE action spectra for erythema and previtamin D3 means that dose (SED) necessary for vitamin D synthesis varies with the irradiance spectrum of the source( 42 , 64 , 65 ) as shown in Table 1. Thus, a given level of vitamin D synthesis/SED for an artificial UVR source cannot be directly used to predict a response from solar UVR.
Table 1.
Effect of Different Spectra on Vitamin D Production
| UVR source | UVB % EEE a | Study type | BSA exposed | Dose protocol | Δ25(OH)D3/SED (nmol/L) | SED for 1 nmol/L 25(OH)D3 |
|---|---|---|---|---|---|---|
| Sunlight in March (20–27) in Tenerife (28°N) with max UVI of 9( 184 ) | 72% on March 24 at solar noon | 7‐day holiday | 85% | SPF‐15 sunscreen with high UVA‐PF transmitting more UVB than below | 7.0 | 0.14 |
| SPF‐15 sunscreen with low UVA‐PF transmitting less UVB than above | 4.8 | 0.21 | ||||
| TL01( 142 ) | 99% | Lab study | 85% |
1.5 SED × 5 Every 3–4 days |
7.9 in FST II b 5.5 in FST VI b |
0.13 0.18 |
| Fluorescent SSR( 142 ) | 78% | Lab study | 85% | 2 SED × 5 every 3–4 days |
5.0 in FST II b 4.1 in FST III b 3.9 in FST IV b 4.2 in FST V b 3.9 in FST VI b |
0.20 0.24 0.26 0.24 0.26 |
| UV6 c | 96% | Lab study | 85% | 2 SED × 5 every 3–4 days | 4.97 in FST I/II c | 0.20 |
| Fluorescent SSR c | 80% | Lab study | 85% | 2 SED × 5 every 3–4 days | 3.18 in FST I/II c | 0.31 |
| PUVA c | 45% | Lab study | 85% | 2 SED × 5 every 3–4 days | 1.99 in FST I/II c | 0.50 |
| UV6 c | 96% | Lab study | 4% | 2 SED × 5 every 3–4 days | 1.35 in FST I/II c | 0.74 |
| SSR c (Xe arc, high UVB) | 79% | Lab study | 4% | 2 SED × 5 every 3–4 days | 0.96 in FST I/II c | 1.04 |
| SSR c (Xe arc, low UVB) | 48% | Lab study | 4% | 2 SED × 5 every 3–4 days | 0.55 in FST I/II c | 1.82 |
| Fluorescent SSR( 145 ) | Unknown | Lab study | 35% | Single exposure of 0.2, 0.4, 0.6, & 0.8 MED with each exposure a month apart |
3.8 in FST I d 3.1 in FST II d 2.5 in FST III d 1.4 in FST IV d 1.1 in FST V d 0.5 in FST VI d |
0.26 0.32 0.40 0.71 0.91 2.00 |
The estimated vitamin D effectiveness per SED varies with the UVR emission spectrum and decreases with decreased UVB EEE for a given BSA exposed.
25(OH)D3 = 25‐hydroxyvitamin D3; BSA = body‐surface area; EEE = erythemally effective energy; FST = Fitzpatrick skin type; PUVA = psoralen (P) and long‐wave ultraviolet radiation (UVA); SED = standard erythemal dose; SPF = sun‐protection factor; SSR = solar‐simulated radiation; UVA = ultraviolet A; UVA‐PF = UVA protection factor; UVB = ultraviolet B; UVI = ultraviolet index; UVR = ultraviolet radiation; Xe = xenon.
EEE is the erythemally effective energy for UVB (280–315 nm)—the UVB % contribution to erythema.
Based on linear regressions [SED vs 25(OH)D3] after adjustment for baseline 25(OH)D3.
Article submitted and data based on linear regression [SED vs 25(OH)D3] without adjustment for baseline 25(OH)D3.
Personal communication from Professor Lesley Rhodes. Note: all studies are from the same group apart from the last row.
Atmospheric, Geographic, and Climatic Factors
Atmosphere
The stratospheric ozone (O3) layer attenuates UVB radiation. For any given solar elevation, the O3‐layer absorption is the main determinant of environmentally available surface UVB in cloud‐free and low‐aerosol conditions.( 66 , 67 ) Depletion of the O3 layer by ozone‐depleting substances (ODSs) from 1980 to 2000 resulted in greater potential exposure to UVB with possible consequent health effects, though changes were small outside the polar regions.( 68 ) By phasing out the production of ODSs, the implementation of the Montreal Protocol 1 1 will lead to the recovery of the ozone layer. The Montreal Protocol has already prevented a 20% increase in the UVI at midlatitudes between the early 1990s and today.( 69 )
The solar zenith angle (SZA) is the angle between the local vertical and the position of the sun. A small SZA provides a short path length for UVR through the atmosphere and low attenuation, whereas a larger SZA increases path length with greater attenuation. Small SZAs (approaching zero) occur only within the tropics near solar noon. At higher latitudes, where solar elevation angles are lower, the minimum SZAs are larger. For example, at latitude 45° the noon‐time SZA ranges from 21.6° in summer to 68.4° in winter. In polar regions (latitudes >66.6°), the sun remains below the horizon (SZA >90°) in the winter months; even in summer, the SZA is always >43.1° at the Arctic Circle. Terrestrial UVR is also modified by atmospheric aerosols and particulates, clouds, and surface albedo (reflectivity). Solar radiation is scattered by air and atmospheric particles in a strongly wavelength‐dependent manner. UVB is much more strongly attenuated than UVA with a greater effect when the path length is longer, such as in winter, at dawn and dusk, and at high latitudes.( 66 )
Clouds also attenuate UVR in a wavelength‐dependent way.( 41 ) Their modifying properties depend on size, depth, and composition. Although clouds influence visible light more than UVR, their attenuation of UVR contributes substantially to a reduction of UVI. In an urban location in Brazil (19.9°S), mean summer noon UVI values of a minimum of 3 and a maximum of 10 were recorded in overcast conditions,( 70 ) whereas with clear skies maximal UVI recordings in the same area were approximately 13. Reduced UVI will inevitably affect vitamin D synthesis.
Paradoxically, if the sun is not obscured, clouds can also enhance terrestrial UVB because of greater forward scattering compared with blue sky. This enhancement has been reported to be from 20%( 41 ) to 22% for erythemal UV irradiance.( 67 )
The underlying surface reflectivity also affects how much UVR arrives, though very few natural surfaces reflect significant amounts of UVB radiation. The surface albedo is the ratio of reflected‐to‐incident radiation. Snow and ice—and to a lesser extent sand—have high UVB surface albedos.( 71 , 72 ) Fresh snow in unpolluted areas can have a UVR albedo of approximately 98%.( 73 ) Such surfaces can contribute to an overall increase in UVB irradiance by reflecting radiation upwards from the Earth's surface, which is then scattered back downward by air molecules, aerosols, and cloud droplets.( 41 , 74 )
Altitude
Altitude reduces UVB atmospheric path length and increases spectral irradiance as a function of SZA and wavelength.( 75 , 76 ) For example, measurements at a valley and adjacent mountain top in Germany showed 1 km of height increased irradiance at 300 nm by 24%.( 77 ) The main contributors to lower irradiance in the valley were ozone absorption and Rayleigh scattering (ie, by atmospheric particles much smaller than the wavelength in question). The effect of altitude on vitamin D photosynthesis has not been extensively investigated. About a fourfold increase in previtamin D3 production from 7‐DHC occurs in vitro at Mount Everest in the Himalayas (China and Nepal) base camp (5300 m) as opposed to Agra, India (170 m; the 24th most‐populous city in India)( 78 ) but such measurements may be influenced by ground‐level pollution.( 75 ) Animal studies at altitudes of 2000 to 2600 m show a higher serum 25(OH)D3 in sheep( 79 , 80 ) but not goats.( 80 ) In a cohort of 73 patients with ankylosing spondylitis, a 3‐week April holiday in Bad Gastein, Austria (1000 m) significantly increased serum 25(OH)D.( 81 ) A cross‐sectional study of 372 Argentinian children living at two different altitudes, 1400 m and 3750 m, showed significant and direct association of vitamin D with altitude.( 82 ) However, vitamin D deficiency is found at high altitudes.( 83 , 84 ) Nine climbers on a 2‐week mountaineering expedition at 3200 to 4000 m showed a significant decrease in vitamin D status presumably because of their heavy clothing.( 85 )
Confounding factors must be considered. A study of 236 Bolivian children in lowlands (650 m) and highlands (4000 m), who had poor hygiene and nourishment, as well as endemic infections, found a slightly higher prevalence of vitamin D deficiency in the highlands group; the proportion in both groups was approximately 60%.( 86 ) A sample of 1222 children from two locations above 1000 m in Himachal Pradesh, India, showed a deficiency of approximately 80%.( 87 )
Latitude
Higher latitudes are associated with larger SZAs that result in lower total UVB irradiances but with an increased diffuse component caused by UVR scattering and decreased vitamin D‐effective UVB. A modeling study across Europe (35°N–69°N) showed vitamin D “winters” of 2 to 8 months from 37°N to 69°N.( 40 ) This approach has meant that latitude has been used as a surrogate for vitamin D status in population studies. For example, possible vitamin D deficiency has been linked to mortality from the SARS‐CoV‐2 virus based on the observation that death rate varies with latitude and is very low <35°N where there is no vitamin D winter.( 88 )
A study in Australian adults showed that increasing latitude south significantly decreased vitamin D status with a change of 2.28 nmol/L per degree of latitude.( 89 ) There are however caveats for the use of latitude as a surrogate for vitamin D status.( 90 ) A meta‐analysis of 394 studies investigating global vitamin D status observed no relationship over a wide latitude range after adjustment for age, gender, and ethnicity.( 91 ) However, a crude analysis showed a latitude effect for Whites but not for non‐Whites. A study of seven US locations (18°N–44°N) showed no variation in incident vitamin D‐effective UVR with increasing latitude during summer (March–October) but a significant latitude effect was seen in winter (November–February).( 90 ) A study in northern Sweden showed that 79.2% of adults (n = 1622) had serum 25(OH)D ≥50 nmol/L (January–May) while living above 63°N.( 92 ) This was probably caused by a high consumption of dietary vitamin D, supplementation, and sun holidays. Higher serum 25(OH)D concentrations were seen in children in northern Sweden (63°N) than in southern Sweden (55°N). The northern group had a greater intake of vitamin D supplementation.( 93 )
Failure to identify a correlation between vitamin D status and latitude is likely based on multiple factors. Dietary habits, sun avoidance behavior, and clothing worn vary considerably throughout the globe. Participants in nutritional studies may be more concerned with their health and vitamin D status; therefore, they are not representative. There is also a lack of standardization of 25(OH)D measurements. Studies on more homogenous populations, which would correct somewhat for these variabilities, have shown an influence of latitude.( 94 , 95 ) Other environmental features that influence vitamin D photosynthesis vary by geographical location. Pollution levels and the architecture of urban spaces both influence UVB insolation.( 96 ) Similarly, living in closer proximity to the coast has also been shown to associate with higher vitamin D‐effective irradiance and vitamin D levels.( 97 ) Therefore, the use of latitude as a surrogate for vitamin D status should be undertaken cautiously, with consideration of the potentially confounding behavioral, cultural, and environmental factors.
Temporal factors: season and time of day
SZA is dependent on season and time of day. Studies across a wide demographic, at multiple geographic locations, have reported seasonal variation in vitamin D status,( 40 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 ) particularly at higher latitudes where seasonal variations in UVB are large. This seasonality is also apparent in in tropical Brazil,( 106 ) where seasonal changes in cloud cover are probably important, and in those with dark skins in South Africa.( 63 ) At midlatitudes in both hemispheres (eg, the United States( 107 ) and New Zealand( 108 )), serum 25(OH)D is maximal in late summer and minimal at the end of winter. US data are shown in Figure 4.( 107 ) It should be noted that sun avoidance based on excessive heat may reverse this trend. A study of Saudi Arabs (24°N) with high BMI (BMI >25) showed significantly higher serum 25(OH)D in winter compared with summer.( 109 )
Fig 4.

Effect of season on vitamin D status in the United States with serum threshold set at serum 62.5 nmol/L 25(OH)D. The blue line shows data from 3,440,710 individual serum samples from all over the United States that were analyzed by the Mayo Clinic (Rochester, Minnesota, USA), which is a reference laboratory. The black line represents modeled data; the gray band is the 95% CI. Data modified from Fig. 2, Kasahara and colleagues (2013)( 107 ) with kind consent from senior author Dr Andrew Noymer. 25(OH)D/25OHD = 25‐hydroxyvitamin D.
An in vitro study in Saudi Arabia (24°N) showed that maximal conversion of 7‐DHC to previtamin D3 was at 11.00 to 12.00 in summer and winter.( 110 ) The summer % conversion at approximately 8% was twice that of winter. Peak synthesis at about this time also provides a better risk‐versus‐benefit ratio as shown by one theoretical study comparing erythema versus previtamin D3 synthesis in the absence of sunscreen.( 111 )
Personal Factors
Skin pigmentation
Skin color, which is dependent on melanin concentration, is phenotypically characterized by a Fitzpatrick skin type (FST) ranging from I (eg, Celtic) to VI (eg, African).( 112 ) Melanin, a chromophore that competes with 7‐DHC for UVB absorption, is concentrated in the basal layer of the epidermis.( 113 ) Pigmentation may be constitutive (ie, genetic) or facultative (ie, tanning acquired by sun exposure).
There is a melanin gradient with terrestrial UVB in indigenous populations. Melanin decreased as humans in prehistory dispersed away from tropical locations. One driving factor for this loss is thought to be the need to maintain adequate vitamin D synthesis.( 114 , 115 ) However, this view has recently been challenged.( 116 ) Mass migration and travel in relatively recent history has meant that people's skin color no longer necessarily matches the solar conditions under which it evolved.
A number of epidemiological studies have reported lower vitamin D status within a given latitude zone in individuals with darker skin types relative to those with lighter skins.( 117 , 118 , 119 , 120 , 121 ) Studies over long periods in the United States show that Black Americans have poorer vitamin D status than their White compatriots.( 122 ) This is also true of children living in north and south Sweden.( 93 ) Variation in given regions also exists within darker FSTs, with significantly higher increases in serum 25(OH)D in Indian children with FST IV (light brown) skin versus V (dark brown) skin( 123 ) when BSA exposed and times outdoors were similar. Limited data suggest that vitamin D status is poor in Africa.( 26 ) However, studies of traditionally living East Africans with FST VI found they had serum 25(OH)D >100 nmol/L that the authors attributed to solar UVR rather than diet.( 124 , 125 ) A study of healthy young South Africans with dark and lighter (mixed ancestry) skins showed more 25(OH)D in those with dark skin in summer (median 72.6 vs 65.5 nmol/L).( 63 ) This was possibly caused by more time outdoors and BSA exposed. One study in Nigeria compared 25(OH)D status in people with normal pigment with albinos who had similar sun‐exposure patterns. The serum values were high in all cases but were only 23% higher in the albinos (median 95.9 vs 78.25 nmol/L).( 126 ) It is difficult to interpret the protective effect of melanin without knowing when the dose‐response curves reach their plateau.
The impact of skin pigmentation, whether constitutive or facultative, on vitamin D photosynthesis is important for accurate public health messages. Facultative face pigmentation changes in FSTs I to III predicted seasonal variation in vitamin D status in a study in Scotland,( 127 ) but we lack data on the effect of tanning. Laboratory investigations of the effect of constitutive pigmentation on vitamin D production in response to controlled UVR exposure have yielded conflicting results.( 128 ) Some studies have reported that melanin inhibited vitamin D production( 56 , 129 , 130 , 131 , 132 , 133 , 134 ) whereas others did not.( 58 , 127 , 135 , 136 , 137 , 138 , 139 ) One laboratory intervention study found that variations in pigment single‐nucleotide polymorphisms showed a better relationship with vitamin D response than constitutive and facultative pigmentation.( 21 )
A systematic review concluded that studies reporting an inhibitory effect of melanin were more convincing than those that observed no influence but that insufficient evidence was available on the efficacy of vitamin D production in different skin types.( 140 ) An observational study in New Zealand showed similar dose‐response slopes for 25(OH)D versus SED for those of European, Maori, Pacific island, or Asian origin (FSTs I‐IV), suggesting no role for melanin.( 57 ) It should be noted that this study assumed linearity with BSA exposed. A recent study in France (n = 1191) reported that sun exposure and latitude were more important to vitamin D status than the FST.( 141 ) Unlike other studies, this study found better vitamin D status with a higher FST. Another recent study in FSTs II‐VI compared increases in serum 25(OH)D3 after repeated exposures to the same doses of SSR that were suberythemal in FST II.( 142 ) A melanin inhibition factor of 1.3 was obtained by comparing FSTs II and VI. This seems modest but may be enough to explain the epidemiological data. In contrast, the protection factor of melanin against basal layer DNA photodamage is about 60.( 113 ) The high concentration of melanin in the basal layer spares nuclear DNA (a major chromophore) but there is ample 7‐DHC above the basal epidermis( 143 , 144 ) which has much less melanin even in FST VI. However, another study reported that the vitamin D responses were similar in different FSTs (I‐VI) when doses of SSR were given as a function of MED.( 145 ) Thus, a comparison of vitamin D synthesis in FST I and FST VI in this study would indicate that the inhibition factor afforded by melanin was in the region of eight (see Table 1).
Sun behavior
Season and latitude may be poor markers of vitamin D because of confounding behavioral factors.( 146 ) Low supplement use, poor dietary intake of vitamin D, and sun‐avoidance behaviors are associated with low vitamin D status.( 147 , 148 , 149 ) Despite any effects of latitude, certain populations in northern Europe have better vitamin D status than populations in southern Europe( 150 ) because of vitamin D‐rich diets.( 104 )
People from mid to high latitudes often take sun holidays that can result in a greater summer vitamin D peak.( 105 , 127 ) A sun holiday in 2019 increased winter 25(OH)D by 20 to 30 nmol/L in native and immigrant Swedes from Uppsala (60°N).( 151 ) Similarly, summer sun holidays were shown to improve vitamin D status in postmenopausal women in Aberdeen, Scotland (57°N).( 99 ) Summer holidays abroad by people living in Orkney (57°N –59°N), an archipelago in northern Scotland, may account for better vitamin D status than mainland Scotland.( 152 ) Summer and winter serum 25(OH)D were greater in UK adolescents who had taken a holiday; it accounted for a 17% variation in peak vitamin D status.( 105 ) A beach holiday in the previous year predicted 6.4% of the variance in vitamin D levels in an Italian cohort of 620 participants.( 153 )
However, sun holidays result in short periods of intense UVR exposure, which has been shown to associate with skin cancer at all latitudes.( 60 ) Petersen and colleagues found a strong correlation between holiday UVB exposure, positive vitamin D response, and an increase in DNA damage over a 6‐day March holiday.( 60 ) It should be noted that the Danes who participated in the holiday study (in Tenerife in the Canary Islands, Spain) had 43% of the annual UVR burden of a Danish indoor worker.( 154 ) A 12‐day summer holiday taken by Polish children on the Baltic Sea with a modest daily‐exposure dose of 2.4 SED (without taking any effect of sunscreen into account) showed a change from 64.7 to 79.3 in nmol/L serum 25(OH)D3 (mean increase of 14.7 ± 12.4),( 155 ) which had fallen to 68.2 nmol/L in October. The modest summer increase, perhaps because of a high baseline, was accompanied by a 12.6‐fold increase in DNA damage. Further investigation of the risks and benefits of holiday UVR exposure is required.
Clothing plays a determinant role in vitamin D status. This is affected by many complex interactions: For example, among Austrian women increases in temperature led to less body coverage but also to less time outdoors.( 156 ) Cultural factors are also important. Vitamin D deficiency was found to be prevalent in Kuwaiti women wearing Western and traditional clothing but was more marked in the latter group.( 157 ) Similarly, hijab‐wearing women in Nova‐Scotia (Canada) had less good vitamin D status than Western‐dressing counterparts.( 158 ) Another factor to consider is physical activity, which may contribute to better vitamin D status independently of sunlight and dietary intake.( 159 ) In addition, poor public knowledge of vitamin D may contribute to low prevalence of vitamin D supplementation and therefore lower vitamin D status.( 160 )
Body site and surface area exposed
Knowledge of the relationship between UVR dose, body site, and BSA irradiated, and vitamin D response is important for accurate public health messages.( 68 ) This relationship has been investigated in vivo under laboratory conditions by exposing variable proportions of BSA to UVR.( 161 , 162 , 163 , 164 , 165 , 166 ) In general, a positive correlation has been observed between BSA exposed and vitamin D response with some variation depending on anatomical location.
A winter study in Finland exposed different groups of FST II and FST III women to seven consecutive daily exposures (1 SED 1st day and 2 SEDs thereafter) of narrow band UVB to different body areas. The increase in the group with abdominal exposure was 4 nmol/L. Increases of approximately 11 nmol/L 25(OH)D were observed in groups that either had full‐body or head plus arms exposures. The same protocol to face plus arms group with SSR showed a smaller increase of 3.8 nmol/L.( 162 ) Increasing BSA exposed resulted in better end‐of‐summer vitamin D status in a study of office workers in Australia with values of approximately 47 and approximately 89 nmol/L of serum 25(OH)D for face plus hands and whole‐body exposure, respectively,( 167 ) suggesting a nonlinear relationship.
Increasing BSA (6%, 12%, and 24%) showed a linear relationship with increased serum 25(OH)D after four exposures of 0.75 SED broadband UVB but not with 1.5 and 3 SED.( 164 ) Dose‐response analyses in the same study showed significant linear responses at 6% and 12% BSA but no effect at 24% BSA. These results suggest a complex relationship between UVB dose and BSA exposed. Figure 5 suggests a linear relationship between increase in 25(OH)D3 and the log10 product of SED and BSA exposed when BSA is between 6% to 25%. Small but nonsignificant increases in vitamin D response have been seen with increased BSA (15 vs 30%) exposure to sunlight in children in India with FST IV and FST V skin.( 123 ) Figure 3 shows the UVR dose–response curves for vitamin D with 3.7% (defined area on trunk) and 85% BSA exposure (underwear only). It can be seen that a greater than 20‐fold increase in BSA results in a 3.7‐fold increase in the rate of 25(OH)D3 production, suggesting a positive but nonproportional relationship between BSA and vitamin D photosynthesis. Therefore, a comparison of Figs. 3 and 5 suggests a failure of reciprocity for UVR dose × BSA exposed when the BSA are extremes. This also suggests a homeostatic process that limits systemic vitamin D toxicity. CYP2R1 is the gene that codes for the enzyme that converts vitamin D to 25(OH)D. Exposure of FST I/II skin to 6 SED SSR reduced cutaneous CYP2R1 mRNA expression (at 6 and 24 hours postexposure), which also suggests a local feedback mechanism.( 168 )
Fig 5.
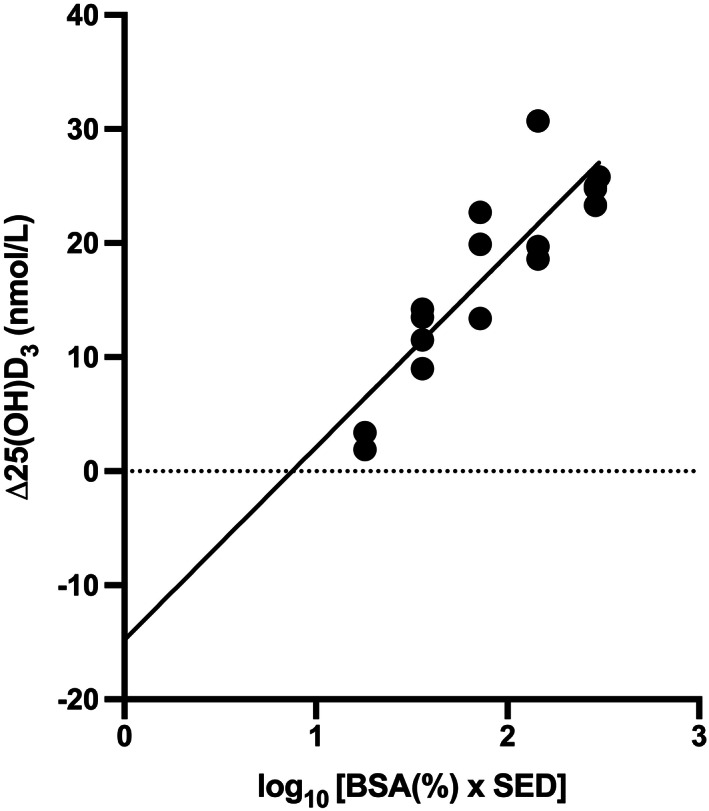
Linear relationship between increase of 25(OH)D3 with log10 product of total exposure dose (SED) and BSA exposed (6, 12, and 24%–25%). Regression equation; y = 16.89x – 14.80, R 2 = 0.7739, p < 0.0001. Each volunteer was exposed to four equal doses (0.375–3.0 SED) of a broadband ultraviolet B phototherapy source. Data taken from several studies from the same laboratory.( 51 , 58 , 64 , 164 , 198 ) 25(OH)D3 = 25‐hydroxyvitamin D3; BSA = body‐surface area; SED = standard erythemal dose.
Factors other than BSA are likely to be important. 7‐DHC concentration may vary with body site. For example, a study in chickens reported that its concentration in leg and feet skin was 30 times greater than the back.( 169 ) Consequently, whole‐body exposure with a given UVB dose to defeathered birds resulted in previtamin D photosynthesis in the legs and feet but not in the skin on the back.
In humans, UVR transmission varies with body site, and this will impact on vitamin D synthesis.( 33 ) A significantly steeper vitamin D3 (cholecalciferol) response was produced in the upper body and full body compared to the hands plus face( 166 ) (data from acute erythemal and repeated sun‐erythemal broadband UVB exposures combined). In another study, in which different body sites were differentiated by sunscreen protection, significant increases in serum vitamin D3 (cholecalciferol) were seen in trunk, legs, and whole body of participants after a single suberythemal UVR dose (260–360 nm), but not for the arms or head plus neck.( 161 ) Another study with three consecutive suberythemal broadband UVB exposures showed different results with serum 25(OH)D3 and vitamin D3. Whole‐body exposure gave the same results as the face plus hands with 25(OH)D3 but was considerably more effective than face and hands for vitamin D3.( 163 )
Sunscreen use
Sunscreen efficacy is measured by sun‐protection factor (SPF). This is the ratio of MED with and without sunscreen applied under laboratory conditions using SSR.( 170 ) Erythema is primarily induced by UVB (Fig. 1), making SPF mainly, but not exclusively, a measure of UVB protection.
The SPF is calculated at 2 mg/cm2 but users typically apply much less( 171 ) (eg, 0.79 mg/cm2 by Danes on holiday in Egypt( 172 )), and so receive suboptimal protection.( 172 , 173 , 174 ) Application of an SPF 50 sunscreen at 0.75 mg/cm2 will provide a real SPF of approximately 20.( 175 )
The overlap of the erythema and previtamin D3 action spectra in the UVB region, suggests that sunscreens block vitamin D photosynthesis (Fig. 1). Indeed, sunscreens and other types of photoprotection have been shown to associate with low vitamin D status.( 161 , 176 , 177 , 178 , 179 ) Some laboratory studies have used inappropriate nonsolar UVB sources and their conclusions can be considered invalid.( 23 ) In a study of 5920 adults, seeking shade and wearing long‐sleeved clothing was associated with lower 25(OH)D levels but frequent low, moderate, or high sunscreen use had no effect.( 180 ) Reviews on sunscreens and vitamin D status have concluded that typical use has little or no impact.( 23 , 128 , 181 , 182 ) However, sunscreen use in Australia may be associated with higher vitamin D status because people spend more time outdoors.( 167 ) Sunscreen use in a study in Scotland also resulted in better vitamin D status.( 127 ) A field study in Tenerife compared discretionary sunscreen use with interventional guided optimal use.( 183 , 184 ) Sunburn was observed in the former but not in the latter group. Vitamin D status improved significantly in both groups, though the increase was greater in the group with sunburn. The reason for the modest effect of sunscreens is that the dose threshold for vitamin D synthesis is much lower than that for erythema.
The absorption spectra of sunscreens depend on their mix of active ingredients. It has been suggested that based on the CIE action spectrum for previtamin D3, their absorption spectra can be tailored to maximize vitamin D synthesis.( 185 ) There were two intervention SPF = 15 sunscreens in the Tenerife study referred to above.( 184 ) However, one transmitted more UVB and enabled significantly more vitamin D synthesis (Table 1).
How Much Sunlight Is Necessary to Maintain Optimal Vitamin D Status?
There are different methods to determine the minimal amount of solar UVB necessary for maintaining vitamin D status. One approach is climatic modeling using the previtamin D3 action spectrum.( 186 ) This depends on the accuracy of this spectrum for serum 25(OH)D3 and assumes no spectral interactions ( eg, significant photodegradation by UVA). Many studies have used UVB phototherapy sources but these are likely to overestimate vitamin D production for a given erythemal exposure protocol (see Table 1). The use of SSR sources is likely to give a better estimation. The ideal approach is sunlight in which change of vitamin D status is compared with personal exposure and BSA exposed.
The number of physical variables that affect vitamin D status makes it hard to give precise UVR exposure recommendations for optimal vitamin D status. Studies use different designs, assumptions, and target endpoints. This is further complicated by biological (eg, genetics( 187 )) and clinical factors (eg, BMI( 188 ) and disorders( 23 )). Given seasonal variation, it is also important to generate sufficient vitamin D in summer to provide winter reserves.
Vitamin D status can be maintained above 50 nmol/L throughout the UK winter if sufficient stores are generated during the warm months but the majority of the population fails to do so.( 100 ) Increasing daily summer sun exposure has been reported to have minimal effect on winter vitamin D levels and may increase the risk of the negative consequences of UVR exposure.( 189 , 190 ) A large New Zealand observational study suggests regular exposures of <2 SED solar UVR/week (adjusted for BSA exposed) is sufficient for good vitamin synthesis( 57 ) but increasing the weekly exposure dose brought diminishing returns.
Using a UK climatic model, it has been estimated that 1 SED daily at lunchtime for those with light skins (9–13 minutes of exposure depending on latitude) is sufficient from March to September in season‐appropriate clothing to achieve an end‐of‐summer target of 80.5 nmol/L of 25(OH)D to maintain a winter vitamin D status of 25 nmol/L.( 186 ) A similar analysis for the same winter target for FST V showed a daily dose of 2.75 SED (25–40 minutes) is needed to achieve an end‐of ‐summer target of 85.8 nmol/L 25(OH)D.( 191 ) In all cases, approximately 35% BSA (eg, forearms and lower legs) would need to be exposed between June and August.
One study in central England exposed 35% BSA of FST V with low baseline 25(OH)D to different SSR doses (0.65–3.9 SED) 3 times per week for 6 weeks. In general, there was a dose‐dependent effect, though the best response was with 3.25 rather than 3.9 SED. However, in no case did mean serum 25(OH)D reach 50 nmol/L.( 56 )
A study of Danish girls and older women suggested a summer peak of 100 nmol/L was necessary to obtain a winter level of 50 nmol/L.( 98 ) This is similar to results from data and modeling for central England.( 192 ) These data showed serum 25(OH)D <50 nmol/L from November to May. Modeling estimated that daily oral intake of 3 μg plus 2 hours local sun exposure on weekdays and 3 hours on weekend days on unprotected skin with a maximum of 20% BSA exposed would increase the summer peak to >100 nmol/L and February nadir from approximately 38 to 58 nmol/L. In contrast, the same dietary intake with weekday and weekend times of 30 and 45 minutes, respectively, would not result in 50 nmol/L at any time. A study of indoor workers in Sydney, Australia (33.9°S) showed a significant linear relationship between end‐of‐summer and end‐of‐winter serum 25(OH)D.( 167 ) An end of summer value of approximately 60 nmol/L was associated with an end‐of‐winter status of 50 nmol/L, which suggests that some vitamin D synthesis occurred in autumn/winter.
Sun holiday studies,( 60 , 184 ) in which typically a very high BSA is exposed, have shown a significant increase in vitamin D status that is accompanied by sunburn: i.e., excess UVB. In one study, in Tenerife in March, some participants were given sunscreen with SPF‐15 and instructions on optimal use.( 184 ) Participants did not sunburn, and they showed a highly significant improvement in vitamin D status over 1 week. Personal exposure was measured as SED. It is therefore possible to estimate erythemal dose to the skin through the sunscreen and equate this with change in vitamin D status. This was approximately 0.4 SED/day through the sunscreen in the study described above,( 184 ) which equates to about 0.13 MED in a FST II person.( 16 ) This resulted in an increase of 4.8 to 7.0 nmol/L 25(OH)D3 per SED, depending on the optical properties of the sunscreen (Table 1). An important advantage of repeated low‐dose exposure (eg, 1.3 SED SSR thrice weekly) to maintain vitamin D status is a reduction in DNA damage.( 193 )
Given the uncertainties of the vitamin D action spectrum, the effect of BSA exposed, and the role of melanin, it is difficult to give precise exposure recommendations. However, it is important to note that considerable vitamin D synthesis occurs with suberythemal exposures.
The most prudent advice may be to encourage suberythemal sun exposure during the summer and autumn and increase dietary intake and supplementation during the winter.( 108 , 190 )
Conclusions
Multiple factors influence the quantity and quality of solar UVB reaching the skin for vitamin D photosynthesis. The effect of quality of UVR in terms vitamin D efficacy per SED is shown in Table 1. UVR dose‐response studies show an initial linear response followed by a plateau, possibly because of UVA‐mediated regulatory mechanisms.( 11 , 45 ) Vitamin D production is dependent on dose but not dose rate, which in theory means that longer periods of lower irradiance sun exposure are as effective as and safer than short periods of high‐intensity exposure. However, solar irradiance and emission spectrum varies with SZA, which is constantly changing at a given latitude, so the balance between the effects of exposure time on benefit and risk fluctuates. Public health advice must take these factors into account. BSA is positively correlated with vitamin D response but the relationship is nonlinear. It is likely that production of vitamin D differs depending on anatomical location, owing to varying optical properties of skin and different levels of cutaneous 7‐DHC in different skin layers.( 144 ) Furthermore, UVR exposure may alter the skin's UVR transmission properties.( 194 ) This may in part explain the nonlinear relationship between BSA and vitamin D response. However, real‐life world data on dose responses are lacking, and further investigation is needed to assess the variability in response between exposure sites.
Direct investigation of the relationship between skin pigmentation and vitamin D response has produced conflicting results.( 128 ) The degree of inhibition by melanin on vitamin D photosynthesis requires further investigation in high‐quality studies with large sample sizes in conditions representative of real life.( 140 ) The consensus on sunscreen use is that it has limited effects but intervention studies are required at temperate latitudes( 195 ) and with high SPF sunscreens. Photoprotection by behavior and clothing has inhibitory effects even in countries with high insolation.
Laboratory studies can give valuable results but the irradiance spectrum in such studies needs careful consideration to avoid misleading conclusions. The action spectrum for vitamin D3 production is under debate. An incorrect action spectrum will lead to misleading risk/benefit calculations from solar exposure.
Laboratory and outdoor studies show that repeated suberythemal exposures are sufficient to improve and maintain optimal vitamin D status. This is in line with public health advice to avoid sunburn to minimize skin cancer risk in those with light skins. Supplementation may provide a safer means of obtaining an adequate vitamin D status in those who are suboptimal. However, this approach may avoid benefits of solar exposure that are independent of vitamin D. There are reports of increasing toxicity( 44 ) although this is rare and may be caused by manufacturing and labeling errors.( 196 , 197 )
Improved knowledge of the factors that influence the interactions of UVB with cutaneous 7‐DHC will result in better public health messages to maintain safer levels of solar exposure for given populations.
There is a need for a better understanding of vitamin D synthesis in different body sites. Obviously, further investigation is required to clarify whether, and in what contexts, certain anatomical locations are more effective at photosynthesizing vitamin D. That a proportionately greater increase in vitamin D status is achieved with larger BSA exposure—especially at low doses of UVB—remains unquestionable.
Disclosures
None of the authors has any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Jonathan Neville: Conceptualization; data curation; investigation; methodology; resources; writing‐original draft. Tommaso Palmieri: Data curation; formal analysis; investigation; methodology; writing‐review and editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbm4.10460.
Acknowledgments
We thank Drs Arjan van Dijk, Germar Bernhard, and Richard McKenzie for their valuable comments during the preparation of this review. We also thank Dr Andrew Noymer for allowing us to use Fig. 4, Dr Peter Philipsen for generating the data for Fig. 3, and Dr Karl Lawrence for preparing the figures.
Authors' Roles: ARY conceived the idea for the paper and oversaw its writing and revision. JJN sourced the literature and wrote the first draft. TP assisted with the editing and specifically researched the literature on the effects of UVR dose and body surface area on vitamin D responses.
Footnotes
The Montreal Protocol on Substances that Deplete the Ozone Layer is the landmark multilateral environmental agreement that regulates the production and consumption of nearly 100 man‐made chemicals referred to as ozone depleting substances (ODS). When released to the atmosphere, those chemicals damage the stratospheric ozone layer, Earth's protective shield that protects humans and the environment from harmful levels of UVR from the sun. Adopted on 15 September 1987, the Protocol is to date the only UN treaty ever that has been ratified every country on Earth ‐ all 198 UN Member States.
References
- 1. Lucas RM, Yazar S, Young AR, et al. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem Photobiol Sci. 2019;18(3):641–80. [DOI] [PubMed] [Google Scholar]
- 2. Alfredsson L, Armstrong BK, Butterfield DA, et al. Insufficient sun exposure has become a real public health problem. Int J Environ Res Public Health. 2020;17(14):5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weller RB, Wang Y, He J, et al. Does incident solar ultraviolet radiation lower blood pressure? J Am Heart Assoc. 2020;9(5):e013837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weller RB. The health benefits of UV radiation exposure through vitamin D production or non‐vitamin D pathways. Blood pressure and cardiovascular disease. Photochem Photobiol Sci. 2017;16(3):374–80. [DOI] [PubMed] [Google Scholar]
- 5. Kapil V, Gupta AK. Solar UV radiation: a potential modifiable risk factor for hypertension. J Am Heart Assoc. 2020;9(5):e015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young AR. Chromophores in human skin. Phys Med Biol. 1997;42(5):789–802. [DOI] [PubMed] [Google Scholar]
- 7. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. [DOI] [PubMed] [Google Scholar]
- 8. Glossmann HH. Origin of 7‐dehydrocholesterol (provitamin D) in the skin. J Invest Dermatol. 2010;130(8):2139–41. [DOI] [PubMed] [Google Scholar]
- 9. International Commission on Illumination (CIE) . Action spectrum for the production of previtamin D3 in human skin. Vienna, Austria: CIE; 2006. [Google Scholar]
- 10. Norval M, Bjorn LO, de Gruijl FR. Is the action spectrum for the UV‐induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9(1):11–7. [DOI] [PubMed] [Google Scholar]
- 11. Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68(5):882–7. [DOI] [PubMed] [Google Scholar]
- 12. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1–2):80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int. 2013;92(2):163–76. [DOI] [PubMed] [Google Scholar]
- 14. Ljubic A, Jacobsen C, Holdt SL, Jakobsen J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020;320:126627. [DOI] [PubMed] [Google Scholar]
- 15. Japelt RB, Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013;4:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrison GI, Young AR. Ultraviolet radiation‐induced erythema in human skin. Methods. 2002;28(1):14–9. [DOI] [PubMed] [Google Scholar]
- 17. Young AR, Chadwick CA, Harrison GI, Nikaido O, Ramsden J, Potten CS. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J Invest Dermatol. 1998;111(6):982–8. [DOI] [PubMed] [Google Scholar]
- 18. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abboud M, Rybchyn MS, Rizk R, Fraser DR, Mason RS. Sunlight exposure is just one of the factors which influence vitamin D status. Photochem Photobiol Sci. 2017;16(3):302–13. [DOI] [PubMed] [Google Scholar]
- 20. Jiang X, Kiel DP, Kraft P. The genetics of vitamin D. Bone. 2019;126:59–77. [DOI] [PubMed] [Google Scholar]
- 21. Datta P, Philipsen PA, Olsen P, et al. Pigment genes not skin pigmentation affect UVB‐induced vitamin D. Photochem Photobiol Sci. 2019;18(2):448–58. [DOI] [PubMed] [Google Scholar]
- 22. Fraser WD, Milan AM. Vitamin D assays: past and present debates, difficulties, and developments. Calcif Tissue Int. 2013;92(2):118–27. [DOI] [PubMed] [Google Scholar]
- 23. Passeron T, Bouillon R, Callender V, et al. Sunscreen photoprotection and vitamin D status. Br J Dermatol. 2019;181(5):916–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23–45. [DOI] [PubMed] [Google Scholar]
- 26. Bouillon R. Vitamin D status in Africa is worse than in other continents. Lancet Glob Health. 2020;8(1):e20–e1. [DOI] [PubMed] [Google Scholar]
- 27. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health. J Steroid Biochem Mol Biol. 2014;144:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wahl DA, Cooper C, Ebeling PR, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos. 2012;7:155–72. [DOI] [PubMed] [Google Scholar]
- 29. Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85(3):860–8. [DOI] [PubMed] [Google Scholar]
- 30. Calame W, Street L, Hulshof T, Vitamin D. Serum levels in the UK population, including a mathematical approach to evaluate the impact of vitamin D fortified ready‐to‐eat breakfast cereals: application of the NDNS database. Nutrients. 2020;12(6):1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darling AL, Blackbourn DJ, Ahmadi KR, Lanham‐New SA. Very high prevalence of 25‐hydroxyvitamin D deficiency in n 6433 UK South Asian adults: analysis of the UK Biobank Cohort. Br J Nutr. 2020;22:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malacova E, Cheang PR, Dunlop E, et al. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of adults participating in the 2011‐2013 Australian Health Survey. Br J Nutr. 2019;121(8):894–904. [DOI] [PubMed] [Google Scholar]
- 33. van Dijk A, den Outer P, van Kranen H, Slaper H. The action spectrum for vitamin D3: initial skin reaction and prolonged exposure. Photochem Photobiol Sci. 2016;15(7):896–909. [DOI] [PubMed] [Google Scholar]
- 34. Woollons A, Kipp C, Young AR, et al. The 0.8% ultraviolet B content of an ultraviolet A sunlamp induces 75% of cyclobutane pyrimidine dimers in human keratinocytes in vitro. Br J Dermatol. 1999;140(6):1023–30. [DOI] [PubMed] [Google Scholar]
- 35. Commission Internationale de l'Eclairage (CIE) . erythema reference action spectrum and standard erythema dose. Vienna, Austria: CIE; 1998. [Google Scholar]
- 36. de Gruijl FR, Sterenborg HJ, Forbes PD, et al. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53(1):53–60. [PubMed] [Google Scholar]
- 37. Tewari A, Lahmann C, Sarkany R, Bergemann J, Young AR. Human erythema and matrix metalloproteinase‐1 mRNA induction, in vivo, share an action spectrum which suggests common chromophores. Photochem Photobiol Sci. 2012;11(1):216–23. [DOI] [PubMed] [Google Scholar]
- 38. McKenzie RL, Liley JB, Bjorn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85(1):88–98. [DOI] [PubMed] [Google Scholar]
- 39. Kazantzidis A, Smedley A, Kift R, et al. A modeling approach to determine how much UV radiation is available across the UK and Ireland for health risk and benefit studies. Photochem Photobiol. 2015;14(6):1073–81. [DOI] [PubMed] [Google Scholar]
- 40. O'Neill CM, Kazantzidis A, Ryan MJ, et al. Seasonal changes in vitamin D‐effective UVB availability in Europe and associations with population serum 25‐hydroxyvitamin D. Nutrients. 2016;8(9):533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bais AF, Bernhard G, McKenzie RL, et al. Ozone‐climate interactions and effects on solar ultraviolet radiation. Photochem Photobiol Sci. 2019;18(3):602–40. [DOI] [PubMed] [Google Scholar]
- 42. McKenzie R, Liley B, Johnston P, et al. Small doses from artificial UV sources elucidate the photo‐production of vitamin D. Photochem Photobiol Sci. 2013;12(9):1726–37. [DOI] [PubMed] [Google Scholar]
- 43. MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216(4549):1001–3. [DOI] [PubMed] [Google Scholar]
- 44. Taylor PN, Davies JS. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br J Clin Pharmacol. 2018;84(6):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sallander E, Wester U, Bengtsson E, Wiegleb Edstrom D. Vitamin D levels after UVB radiation: effects by UVA additions in a randomized controlled trial. Photodermatol Photoimmunol Photomed. 2013;29(6):323–9. [DOI] [PubMed] [Google Scholar]
- 46. Norval M, de Gruijl FR. Comment on Sallander et al. 'Vitamin D levels after UVB radiation: effects by UVA additions in a randomized controlled trial.'. Photodermatol Photoimmunol Photomed. 2014;30(4):176–7. [DOI] [PubMed] [Google Scholar]
- 47. Wester U, Wiegleb Edstrom D, Bengtsson E, Sallander E. Response to Norval and de Gruijl's ‘Comment on Sallander et al.’. Photodermatol Photoimmunol Photomed. 2014;30(4):178–9. [DOI] [PubMed] [Google Scholar]
- 48. Bolsee D, Webb AR, Gillotay D, et al. Laboratory facilities and recommendations for the characterization of biological ultraviolet dosimeters. Appl Optics. 2000;39(16):2813–22. [DOI] [PubMed] [Google Scholar]
- 49. Olds WJ. Elucidating the links between UV radiation and vitamin D synthesis: using an in vitro model. Brisbane, Australia: Queensland University of Technology; 2010. [Google Scholar]
- 50. Barnkob LL, Argyraki A, Petersen PM, Jakobsen J. Investigation of the effect of UV‐LED exposure conditions on the production of vitamin D in pig skin. Food Chem. 2016;212:386–91. [DOI] [PubMed] [Google Scholar]
- 51. Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production depends on ultraviolet‐B dose but not on dose rate: a randomized controlled trial. Exp Dermatol. 2011;20(1):14–8. [DOI] [PubMed] [Google Scholar]
- 52. McKenzie R, Scragg R, Liley B, et al. Serum 25‐hydroxyvitamin‐D responses to multiple UV exposures from solaria: inferences for exposure to sunlight. Photochem Photobiol Sci. 2012;11(7):1174–85. [DOI] [PubMed] [Google Scholar]
- 53. Rhodes LE, Webb AR, Fraser HI, et al. Recommended summer sunlight exposure levels can produce sufficient (> or =20 ng ml(−1)) but not the proposed optimal (> or =32 ng ml(−1)) 25(OH)D levels at UK latitudes. J Invest Dermatol. 2010;130(5):1411–8. [DOI] [PubMed] [Google Scholar]
- 54. Porojnicu AC, Bruland OS, Aksnes L, Grant WB, Moan J. Sun beds and cod liver oil as vitamin D sources. J Photochem Photobiol B Biol. 2008;91(2–3):125–31. [DOI] [PubMed] [Google Scholar]
- 55. Orlova T, Moan J, Lagunova Z, Aksnes L, Terenetskaya I, Juzeniene A. Increase in serum 25‐hydroxyvitamin‐D3 in humans after sunbed exposures compared to previtamin D3 synthesis in vitro. J Photochem Photobiol B Biol. 2013;122:32–6. [DOI] [PubMed] [Google Scholar]
- 56. Farrar MD, Webb AR, Kift R, et al. Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure. Am J Clin Nutr. 2013;97(6):1210–6. [DOI] [PubMed] [Google Scholar]
- 57. Scragg RKR, Stewart AW, McKenzie RL, Reeder AI, Liley JB, Allen MW. Sun exposure and 25‐hydroxyvitamin D3 levels in a community sample: quantifying the association with electronic dosimeters. J Expo Sci Environ Epidemiol. 2017;27(5):471–7. [DOI] [PubMed] [Google Scholar]
- 58. Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol. 2010;130(2):546–53. [DOI] [PubMed] [Google Scholar]
- 59. Moan J, Lagunova Z, Cicarma E, et al. Sunbeds as vitamin D sources. Photochem Photobiol. 2009;85(6):1474–9. [DOI] [PubMed] [Google Scholar]
- 60. Petersen B, Wulf HC, Triguero‐Mas M, et al. Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage. J Invest Dermatol. 2014;134(11):2806–13. [DOI] [PubMed] [Google Scholar]
- 61. Petersen B, Triguero‐Mas M, Maier B, et al. Sun behaviour and personal UVR exposure among Europeans on short term holidays. J Photochem Photobiol B Biol. 2015;151:264–9. [DOI] [PubMed] [Google Scholar]
- 62. Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85(6):1480–4. [DOI] [PubMed] [Google Scholar]
- 63. Coussens AK, Naude CE, Goliath R, Chaplin G, Wilkinson RJ, Jablonski NG. High‐dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV‐1 replication in urban Southern Africans. Proc Natl Acad Sci U S A. 2015;112(26):8052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Datta P, Bogh MK, Olsen P, et al. Increase in serum 25‐hydroxyvitamin‐D3 in humans after solar exposure under natural conditions compared to artificial UVB exposure of hands and face. Photochem Photobiol Sci. 2012;11(12):1817–24. [DOI] [PubMed] [Google Scholar]
- 65. Grigalavicius M, Moan J, Dahlback A, Juzeniene A. Vitamin D and ultraviolet phototherapy in Caucasians. J Photochem Photobiol B. 2015;147:69–74. [DOI] [PubMed] [Google Scholar]
- 66. Bais AF, McKenzie RL, Bernhard G, et al. Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci. 2015;14(1):19–52. [DOI] [PubMed] [Google Scholar]
- 67. de Miguel A, Román R, Bilbao J, Mateos D. Evolution of erythemal and total shortwave solar radiation in Valladolid, Spain: effects of atmospheric factors. J Atmos Sol Terr Phys. 2011;73(5–6):578–86. [Google Scholar]
- 68. Lucas RM, Norval M, Neale RE, et al. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem Photobiol Sci. 2015;14(1):53–87. [DOI] [PubMed] [Google Scholar]
- 69. McKenzie R, Bernhard G, Liley B, et al. Success of Montreal protocol demonstrated by comparing high‐quality UV measurements with "world avoided" calculations from two chemistry‐climate models. Sci Rep. 2019;9(1):12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Silva AA. Erythemal dose rate under noon overcast skies. Photochem Photobiol Sci. 2013;12(5):777–86. [DOI] [PubMed] [Google Scholar]
- 71. McKenzie RL, Kotkamp M, Ireland W. Upwelling UV spectral irradiances and surface albedo measurements at Lauder, New Zealand. Geophys Res Lett. 1996;23(14):1757–60. [Google Scholar]
- 72. McKenzie RL, Paulin KJ, Madronich S. Effects of snow cover on UV radiation and surface albedo: a case study. J Geophys Res. 1998;103(D22):28785–92. [Google Scholar]
- 73. Grenfell TC, Warren SG, Mullen PC. Reflection of solar radiation by the Antarctic snow surface at ultraviolet, visible, and near‐infrared wavelengths. J Geophys Res. 1994;99:18669–84. [Google Scholar]
- 74. Webb AR. Who, what, where and when—influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. [DOI] [PubMed] [Google Scholar]
- 75. McKenzie RL, Johnston PV, Smale D, Bodhaine BA, Madronich S. Altitude effects on UV spectral irradiance deduced from measurements at Lauder, New Zealand, and at Mauna Loa Observatory, Hawaii. J Geophys Res Atmos. 2001;106(D19):22845–60. [Google Scholar]
- 76. Wagner JE, Angelini F, Arola A, et al. Comparison of surface UV irradiance in mountainous regions derived from satellite observations and model calculations with ground‐based measurements. Meteorol Z. 2010;19:481–90. [Google Scholar]
- 77. Blumthaler M, Webb AR, Seckmeyer G, Bais AF, Huber M, Mayer B. Simultaneous spectroradiometry: a study of solar UV irradiance at two altitudes. Geophys Res Letts. 1994;21:2805–8. [Google Scholar]
- 78. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D‐lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. [DOI] [PubMed] [Google Scholar]
- 79. Liesegang A, Huttenmoser D, Risteli J, Leiber F, Kreuzer M, Wanner M. Influence of high‐altitude grazing on bone metabolism of growing sheep. J Anim Physiol Anim Nutr. 2013;97(1):58–66. [DOI] [PubMed] [Google Scholar]
- 80. Kohler M, Leiber F, Willems H, Merbold L, Liesegang A. Influence of altitude on vitamin D and bone metabolism of lactating sheep and goats. J Anim Sci. 2013;91(11):5259–68. [DOI] [PubMed] [Google Scholar]
- 81. Falkenbach A, Tripathi R, Sedlmeyer A, Staudinger M, Herold M. Serum 25‐hydroxyvitamin D and parathyroid hormone in patients with ankylosing spondylitis before and after a three‐week rehabilitation treatment at high altitude during winter and spring. Wien Klin Wochenschr. 2001;113(9):328–32. [PubMed] [Google Scholar]
- 82. Hirschler V, Molinari C, Maccallini G, Intersimone P, Gonzalez CD. Vitamin D levels and cardiometabolic markers in indigenous Argentinean children living at different altitudes. Glob Pediatr Health. 2019;6:2333794X18821942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kapil U, Pandey RM, Goswami R, et al. Prevalence of Vitamin D deficiency and associated risk factors among children residing at high altitude in Shimla district, Himachal Pradesh, India. Indian J Endocrinol Metab. 2017;21(1):178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alp H, Tekgunduz KS, Akkar MK. Maternal and cord blood vitamin D status in high‐altitude pregnancy. J Matern Fetal Neonatal Med. 2016;29(4):571–5. [DOI] [PubMed] [Google Scholar]
- 85. Kasprzak Z, Sliwicka E, Hennig K, Pilaczynska‐Szczesniak L, Huta‐Osiecka A, Nowak A. Vitamin D, iron metabolism, and diet in alpinists during a 2‐week high‐altitude climb. High Alt Med Biol. 2015;16(3):230–5. [DOI] [PubMed] [Google Scholar]
- 86. Teran G, Cuna W, Branez F, et al. Differences in nutritional and health status in school children from the highlands and lowlands of Bolivia. Am J Trop Med Hyg. 2018;98(1):326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kapil U, Pandey RM, Sharma B, et al. Prevalence of vitamin D deficiency in children (6‐18 years) residing in Kullu and Kangra Districts of Himachal Pradesh, India. Indian J Pediatr. 2018;85(5):344–50. [DOI] [PubMed] [Google Scholar]
- 88. Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population mortality from COVID‐19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lucas RM, Ponsonby AL, Dear K, et al. Vitamin D status: multifactorial contribution of environment, genes and other factors in healthy Australian adults across a latitude gradient. J Steroid Biochem Mol Biol. 2013;136:300–8. [DOI] [PubMed] [Google Scholar]
- 90. Kimlin MG, Olds WJ, Moore MR. Location and vitamin D synthesis: is the hypothesis validated by geophysical data? J Photochem Photobiol B. 2007;86(3):234–9. [DOI] [PubMed] [Google Scholar]
- 91. Hagenau T, Vest R, Gissel TN, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta‐regression analysis. Osteoporos Int. 2009;20(1):133–40. [DOI] [PubMed] [Google Scholar]
- 92. Ramnemark A, Norberg M, Pettersson‐Kymmer U, Eliasson M. Adequate vitamin D levels in a Swedish population living above latitude 63 degrees N: the 2009 Northern Sweden MONICA study. Int J Circumpolar Health. 2015;74:27963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Akeson PK, Lind T, Hernell O, Silfverdal SA, Ohlund I. Serum vitamin D depends less on latitude than on skin color and dietary intake during early winter in Northern Europe. J Pediatr Gastroenterol Nutr. 2016;62(4):643–9. [DOI] [PubMed] [Google Scholar]
- 94. Leary PF, Zamfirova I, Au J, McCracken WH. Effect of latitude on vitamin D levels. J Am Osteopath Assoc. 2017;117(7):433–9. [DOI] [PubMed] [Google Scholar]
- 95. Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–43. [DOI] [PubMed] [Google Scholar]
- 96. Mendes MM, Darling AL, Hart KH, Morse S, Murphy RJ, Lanham‐New SA. Impact of high latitude, urban living and ethnicity on 25‐hydroxyvitamin D status: a need for multidisciplinary action? J Steroid Biochem Mol Biol. 2019;188:95–102. [DOI] [PubMed] [Google Scholar]
- 97. Cherrie MP, Wheeler BW, White MP, Sarran CE, Osborne NJ. Coastal climate is associated with elevated solar irradiance and higher 25(OH)D level. Environ Int. 2015;77:76–84. [DOI] [PubMed] [Google Scholar]
- 98. Andersen R, Brot C, Jakobsen J, et al. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: the influence of sun exposure and vitamin D intake. Eur J Clin Nutr. 2013;67(3):270–4. [DOI] [PubMed] [Google Scholar]
- 99. Mavroeidi A, Aucott L, Black AJ, Fraser WD, Reid DM, Macdonald HM. Seasonal variation in 25(OH)D at Aberdeen (57 degrees N) and bone health indicators—could holidays in the sun and cod liver oil supplements alleviate deficiency? PLoS One. 2013;8(1):e53381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Webb AR, Kift R, Durkin MT, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163(5):1050–5. [DOI] [PubMed] [Google Scholar]
- 101. Lucas RM, Valery P, van der Mei I, et al. Sun exposure over a lifetime in Australian adults from latitudinally diverse regions. Photochem Photobiol. 2013;89(3):737–44. [DOI] [PubMed] [Google Scholar]
- 102. Rabenberg M, Scheidt‐Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GB. Vitamin D status among adults in Germany—results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health. 2015;15:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Thiering E, Bruske I, Kratzsch J, et al. Associations between serum 25‐hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci Rep. 2015;5:18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Andersen S, Jakobsen A, Laurberg P. Vitamin D status in North Greenland is influenced by diet and season: indicators of dermal 25‐hydroxy vitamin D production north of the Arctic Circle. Br J Nutr. 2013;110(1):50–7. [DOI] [PubMed] [Google Scholar]
- 105. Farrar MD, Mughal MZ, Adams JE, et al. Sun exposure behavior, seasonal vitamin D deficiency, and relationship to bone health in adolescents. J Clin Endocrinol Metab. 2016;101(8):3105–13. [DOI] [PubMed] [Google Scholar]
- 106. Eloi M, Horvath DV, Szejnfeld VL, et al. Vitamin D deficiency and seasonal variation over the years in Sao Paulo, Brazil. Osteoporos Int. 2016;27(12):3449–56. [DOI] [PubMed] [Google Scholar]
- 107. Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) serum seasonality in the United States. PLoS One. 2013;8(6):e65785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Livesey J, Elder P, Ellis MJ, McKenzie R, Liley B, Florkowski C. Seasonal variation in vitamin D levels in the Canterbury, New Zealand population in relation to available UV radiation. N Z Med J. 2007;120(1262):U2733. [PubMed] [Google Scholar]
- 109. Al‐Daghri NM, Al‐Attas OS, Alokail MS, et al. Increased vitamin D supplementation recommended during summer season in the gulf region: a counterintuitive seasonal effect in vitamin D levels in adult, overweight and obese Middle Eastern residents. Clin Endocrinol (Oxf). 2012;76(3):346–50. [DOI] [PubMed] [Google Scholar]
- 110. Alshahrani FM, Almalki MH, Aljohani N, et al. Light side and best time of sunshine in Riyadh, Saudi Arabia. Dermatoendocrinol. 2013;5(1):177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sayre RM, Dowdy JC. Darkness at noon: sunscreens and vitamin D3. Photochem Photobiol. 2007;83(2):459–63. [DOI] [PubMed] [Google Scholar]
- 112. Fajuyigbe D, Young AR. The impact of skin colour on human photobiological responses. Pigment Cell Melanoma Res. 2016;29(6):607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fajuyigbe D, Lwin SM, Diffey BL, et al. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018;32(7):3700–6. [DOI] [PubMed] [Google Scholar]
- 114. Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39(1):57–106. [DOI] [PubMed] [Google Scholar]
- 116. Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29(9):864–75. [DOI] [PubMed] [Google Scholar]
- 117. Harris SS, Dawson‐Hughes B. Seasonal changes in plasma 25‐hydroxyvitamin D concentrations of young American Black and White women. Am J Clin Nutr. 1998;67(6):1232–6. [DOI] [PubMed] [Google Scholar]
- 118. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25‐hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Renzaho AM, Halliday JA, Nowson C. Vitamin D, obesity, and obesity‐related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27(9):868–79. [DOI] [PubMed] [Google Scholar]
- 120. Brown LL, Cohen B, Tabor D, Zappala G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems‐based approach to investigating clinical practice, research, and public health–expert panel meeting report. BMC Proc. 2018;12(Suppl 6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25‐hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988‐2004. Arch Intern Med. 2009;169(6):626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Marwaha RK, Sreenivas V, Talwar D, et al. Impact of solar ultraviolet B radiation (290‐320 nm) on vitamin D synthesis in children with type IV and V skin. Br J Dermatol. 2015;173(2):604–6. [DOI] [PubMed] [Google Scholar]
- 124. Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck‐Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr. 2013;52(3):1115–25. [DOI] [PubMed] [Google Scholar]
- 125. Luxwolda MF, Kuipers RS, Kema IP, Dijck‐Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25‐hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012;108(9):1557–61. [DOI] [PubMed] [Google Scholar]
- 126. Enechukwu N, Cockburn M, Ogun G, Ezejiofor OI, George A, Ogunbiyi A. Higher vitamin D levels in Nigerian albinos compared with pigmented controls. Int J Dermatol. 2019;58(10):1148–52. [DOI] [PubMed] [Google Scholar]
- 127. Macdonald HM, Mavroeidi A, Aucott LA, et al. Skin color change in Caucasian postmenopausal women predicts summer‐winter change in 25‐hydroxyvitamin D: findings from the ANSAViD cohort study. J Clin Endocrinol Metab. 2011;96(6):1677–86. [DOI] [PubMed] [Google Scholar]
- 128. Springbett P, Buglass S, Young AR. Photoprotection and vitamin D status. J Photochem Photobiol B Biol. 2010;101(2):160–8. [DOI] [PubMed] [Google Scholar]
- 129. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–6. [DOI] [PubMed] [Google Scholar]
- 130. Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127(4):536–8. [PubMed] [Google Scholar]
- 131. Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Armas LA, Dowell S, Akhter M, et al. Ultraviolet‐B radiation increases serum 25‐hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57(4):588–93. [DOI] [PubMed] [Google Scholar]
- 133. Farrar MD, Kift R, Felton SJ, et al. Recommended summer sunlight exposure amounts fail to produce sufficient vitamin D status in UK adults of South Asian origin. Am J Clin Nutr. 2011;94(5):1219–24. [DOI] [PubMed] [Google Scholar]
- 134. Libon F, Cavalier E, Nikkels AF. Skin color is relevant to vitamin D synthesis. Dermatology. 2013;227(3):250–4. [DOI] [PubMed] [Google Scholar]
- 135. Stamp TC. Factors in human vitamin D nutrition and in the production and cure of classical rickets. Proc Nutr Soc. 1975;34(2):119–30. [DOI] [PubMed] [Google Scholar]
- 136. Lo CW, Paris PW, Holick MF. Indian and Pakistani immigrants have the same capacity as Caucasians to produce vitamin D in response to ultraviolet irradiation. Am J Clin Nutr. 1986;44(5):683–5. [DOI] [PubMed] [Google Scholar]
- 137. Brazerol WF, McPhee AJ, Mimouni F, Specker BL, Tsang RC. Serial ultraviolet B exposure and serum 25 hydroxyvitamin D response in young adult American Blacks and Whites: no racial differences. J Am Coll Nutr. 1988;7(2):111–8. [DOI] [PubMed] [Google Scholar]
- 138. Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. Skin types and epidermal photosynthesis of vitamin D3. J Am Acad Dermatol. 1990;23(3 Pt 1):525–6. [DOI] [PubMed] [Google Scholar]
- 139. Hakim OA, Hart K, McCabe P, et al. Vitamin D production in UK Caucasian and South Asian women following UVR exposure. J Steroid Biochem Mol Biol. 2016;164:223–9. [DOI] [PubMed] [Google Scholar]
- 140. Xiang F, Lucas R, de Gruijl F, Norval M. A systematic review of the influence of skin pigmentation on changes in the concentrations of vitamin D and 25‐hydroxyvitamin D in plasma/serum following experimental UV irradiation. Photochem Photobiol Sci. 2015;14(12):2138–46. [DOI] [PubMed] [Google Scholar]
- 141. Malvy DJ, Guinot C, Preziosi P, et al. Relationship between vitamin D status and skin phototype in general adult population. Photochem Photobiol. 2000;71(4):466–9. [DOI] [PubMed] [Google Scholar]
- 142. Young AR, Morgan KA, Ho TW, et al. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: a comparison of extreme phenotypes. J Invest Dermatol. 2020;140(7):1418–26.e1. [DOI] [PubMed] [Google Scholar]
- 143. Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–5. [DOI] [PubMed] [Google Scholar]
- 144. Meinhardt‐Wollweber M, Krebs R. A computational model for previtamin D(3) production in skin. Photochem Photobiol Sci. 2012;11(4):731–7. [DOI] [PubMed] [Google Scholar]
- 145. Shih BB, Farrar MD, Cooke MS, et al. Fractional sunburn threshold UVR doses generate equivalent vitamin D and DNA damage in skin types I‐VI but with epidermal DNA damage gradient correlated to skin darkness. J Invest Dermatol. 2018;138(10):2244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. van der Mei IA, Ponsonby AL, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115(8):1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kift R, Berry JL, Vail A, Durkin MT, Rhodes LE, Webb AR. Lifestyle factors including less cutaneous sun exposure contribute to starkly lower vitamin D levels in U.K. South Asians compared with the white population. Br J Dermatol. 2013;169(6):1272–8. [DOI] [PubMed] [Google Scholar]
- 148. Norsang G, Ma L, Dahlback A, et al. The vitamin D status among Tibetans. Photochem Photobiol. 2009;85(4):1028–31. [DOI] [PubMed] [Google Scholar]
- 149. Bjork A, Andersson A, Johansson G, Bjorkegren K, Bardel A, Kristiansson P. Evaluation of sun holiday, diet habits, origin and other factors as determinants of vitamin D status in Swedish primary health care patients: a cross‐sectional study with regression analysis of ethnic Swedish and immigrant women. BMC Fam Pract. 2013;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Toss G, Magnusson P. Vitamin D status: sunshine is nice but other factors prevail. Eur J Nutr. 2012;51:255–6. [DOI] [PubMed] [Google Scholar]
- 151. Björk A, Andersson Å, Johansson G, Björkegren K, Bardel A, Kristiansson P. Evaluation of sun holiday, diet habits, origin and other factors as determinants of vitamin D status in Swedish primary health care patients: a cross‐sectional study with regression analysis of ethnic Swedish and immigrant women. BMC Fam Pract. 2013;14(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Weiss E, Zgaga L, Read S, et al. Farming, foreign holidays, and vitamin D in Orkney. PLoS One. 2016;11(5):e0155633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vignali E, Macchia E, Cetani F, et al. Development of an algorithm to predict serum vitamin D levels using a simple questionnaire based on sunlight exposure. Endocrine. 2017;55(1):85–92. [DOI] [PubMed] [Google Scholar]
- 154. Petersen B, Thieden E, Philipsen PA, Heydenreich J, Wulf HC, Young AR. Determinants of personal ultraviolet‐radiation exposure doses on a sun holiday. Br J Dermatol. 2013;168(5):1073–9. [DOI] [PubMed] [Google Scholar]
- 155. Narbutt J, Philipsen PA, Lesiak A, et al. Children sustain high levels of skin DNA photodamage, with a modest increase of serum 25‐hydroxyvitamin D3, after a summer holiday in Northern Europe. Br J Dermatol. 2018;179(4):940–50. [DOI] [PubMed] [Google Scholar]
- 156. Schmalwieser AW, Schmalwieser VT, Schmalwieser SS. Influence of air temperature on the UV exposure of different body sites due to clothing of young women during daily errands. Photochem Photobiol. 2019;95(4):1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Al‐Yatama FI, AlOtaibi F, Al‐Bader MD, Al‐Shoumer KA. The effect of clothing on vitamin D status, bone turnover markers, and bone mineral density in young Kuwaiti females. Int J Endocrinol. 2019;2019:6794837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ojah RC, Welch JM. Vitamin D and musculoskeletal status in Nova Scotian women who wear concealing clothing. Nutrients. 2012;4(5):399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hibler EA, Sardo Molmenti CL, Dai Q, et al. Physical activity, sedentary behavior, and vitamin D metabolites. Bone. 2016;83:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Aljefree NM, Lee P, Ahmed F. Knowledge and attitudes about vitamin D, and behaviors related to vitamin D in adults with and without coronary heart disease in Saudi Arabia. BMC Public Health. 2017;17(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Matsuoka LY, Wortsman J, Hollis BW. Use of topical sunscreen for the evaluation of regional synthesis of vitamin D3. J Am Acad Dermatol. 1990;22(5 Pt 1):772–5. [DOI] [PubMed] [Google Scholar]
- 162. Vahavihu K, Ylianttila L, Kautiainen H, et al. Narrowband ultraviolet B course improves vitamin D balance in women in winter. Br J Dermatol. 2010;162(4):848–53. [DOI] [PubMed] [Google Scholar]
- 163. Osmancevic A, Sandstrom K, Gillstedt M, et al. Vitamin D production after UVB exposure ‐ a comparison of exposed skin regions. J Photochem Photobiol B Biol. 2015;143:38–43. [DOI] [PubMed] [Google Scholar]
- 164. Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial. Br J Dermatol. 2011;164(1):163–9. [DOI] [PubMed] [Google Scholar]
- 165. Libon F, Courtois J, Le Goff C, et al. Effect of body site and surface on vitamin D and 25‐hydroxyvitamin D production after a single narrowband UVB‐exposure. J Invest Dermatol. 2017;137(6):1391–3. [DOI] [PubMed] [Google Scholar]
- 166. Osmancevic A, Gillstedt M, Landin‐Wilhelmsen K, et al. Size of the exposed body surface area, skin erythema and body mass index predict skin production of vitamin D. J Photochem Photobiol B Biol. 2015;149:224–9. [DOI] [PubMed] [Google Scholar]
- 167. Fayet‐Moore F, Brock KE, Wright J, et al. Determinants of vitamin D status of healthy office workers in Sydney, Australia. J Steroid Biochem Mol Biol. 2019;189:127–34. [DOI] [PubMed] [Google Scholar]
- 168. Bustamante M, Hernandez‐Ferrer C, Tewari A, et al. Dose and time effects of solar‐simulated ultraviolet radiation on the in vivo human skin transcriptome. Br J Dermatol. 2020;182(6):1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tian XQ, Chen TC, Lu Z, Shao Q, Holick MF. Characterization of the translocation process of vitamin D3 from the skin into the circulation. Endocrinology. 1994;135(2):655–61. [DOI] [PubMed] [Google Scholar]
- 170. Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100–S9. [DOI] [PubMed] [Google Scholar]
- 171. Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30(2–3):96–101. [DOI] [PubMed] [Google Scholar]
- 172. Petersen B, Datta P, Philipsen PA, Wulf HC. Sunscreen use and failures—on site observations on a sun‐holiday. Photochem Photobiol Sci. 2013;12(1):190–6. [DOI] [PubMed] [Google Scholar]
- 173. Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol. 2002;138(10):1319–25. [DOI] [PubMed] [Google Scholar]
- 174. Lademann J, Schanzer S, Richter H, et al. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3(2):62–8. [DOI] [PubMed] [Google Scholar]
- 175. Ou‐Yang H, Stanfield J, Cole C, Appa Y, Rigel D. High‐SPF sunscreens (SPF >/= 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67(6):1220–7. [DOI] [PubMed] [Google Scholar]
- 176. Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25‐hydroxyvitamin D. A preliminary study. Arch Dermatol. 1988;124(12):1802–4. [PubMed] [Google Scholar]
- 177. Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165–8. [DOI] [PubMed] [Google Scholar]
- 178. Cusack C, Danby C, Fallon JC, et al. Photoprotective behaviour and sunscreen use: impact on vitamin D levels in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed. 2008;24(5):260–7. [DOI] [PubMed] [Google Scholar]
- 179. Holme SA, Anstey AV, Badminton MN, Elder GH. Serum 25‐hydroxyvitamin D in erythropoietic protoporphyria. Br J Dermatol. 2008;159(1):211–3. [DOI] [PubMed] [Google Scholar]
- 180. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003‐2006. Cancer Causes Control. 2012;23(1):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732–6. [DOI] [PubMed] [Google Scholar]
- 182. Neale RE, Khan SR, Lucas RM, Waterhouse M, Whiteman DC, Olsen CM. The effect of sunscreen on vitamin D: a review. Br J Dermatol. 2019;181(5):907–15. [DOI] [PubMed] [Google Scholar]
- 183. Narbutt J, Philipsen PA, Harrison GI, et al. Sunscreen applied at >/= 2 mg cm(−2) during a sunny holiday prevents erythema, a biomarker of ultraviolet radiation‐induced DNA damage and suppression of acquired immunity. Br J Dermatol. 2019;180(3):604–14. [DOI] [PubMed] [Google Scholar]
- 184. Young AR, Narbutt J, Harrison GI, et al. Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br J Dermatol. 2019;181(5):1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kockott D, Herzog B, Reichrath J, Keane K, Holick MF. New approach to develop optimized sunscreens that enable cutaneous Vitamin D formation with minimal erythema risk. PLoS One. 2016;11(1):e0145509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Meeting vitamin D requirements in White Caucasians at UK latitudes: providing a choice. Nutrients. 2018;10(4):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Manousaki D, Mitchell R, Dudding T, et al. Genome‐wide association study for vitamin D levels reveals 69 independent loci. Am J Hum Genet. 2020;106(3):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. de Oliveira LF, de Azevedo LG, da Mota Santana J, de Sales LPC, Pereira‐Santos M. Obesity and overweight decreases the effect of vitamin D supplementation in adults: systematic review and meta‐analysis of randomized controlled trials. Rev Endocr Metab Disord. 2020;21(1):67–76. [DOI] [PubMed] [Google Scholar]
- 189. Krzyscin JW, Jaroslawski J, Sobolewski PS. A mathematical model for seasonal variability of vitamin D due to solar radiation. J Photochem Photobiol B Biol. 2011;105(1):106–12. [DOI] [PubMed] [Google Scholar]
- 190. Diffey BL. Modelling the seasonal variation of vitamin D due to sun exposure. Br J Dermatol. 2010;162(6):1342–8. [DOI] [PubMed] [Google Scholar]
- 191. Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Colour counts: sunlight and skin type as drivers of vitamin D deficiency at UK latitudes. Nutrients. 2018;10(4):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Diffey BL. Modelling vitamin D status due to oral intake and sun exposure in an adult British population. Br J Nutr. 2013;110(3):569–77. [DOI] [PubMed] [Google Scholar]
- 193. Felton SJ, Cooke MS, Kift R, et al. Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low‐level summer sunlight exposures. Br J Dermatol. 2016;175(6):1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Garmyn M, Young AR, Miller SA. Mechanisms of and variables affecting UVR photoadaptation in human skin. Photochem Photobiol Sci. 2018;17(12):1932–40. [DOI] [PubMed] [Google Scholar]
- 195. Young AR, Passeron T. Everyday sunscreen use may compromise vitamin D in temperate climes: reply from authors. Br J Dermatol. 2019. 10.1111/BJD.18815. [DOI] [PubMed] [Google Scholar]
- 196. Araki T, Holick MF, Alfonso BD, et al. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metab. 2011;96(12):3603–8. [DOI] [PubMed] [Google Scholar]
- 197. Galior K, Grebe S, Singh R. Development of vitamin D toxicity from overcorrection of vitamin D deficiency: a review of case reports. Nutrients. 2018;10(8):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Faurschou A, Beyer DM, Schmedes A, Bogh MK, Philipsen PA, Wulf HC. The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: a randomized clinical trial. Br J Dermatol. 2012;167(2):391–5. [DOI] [PubMed] [Google Scholar]


