Abstract
Novel COVID-19 continues to intrigue medical professionals with its varied presentations. Though it affects the respiratory tract primarily, thrombogenesis has been the Achilles’ heel. A 44-year-old man diagnosed with COVID-19 presented with upper limb pain at a local hospital and was found to have thrombosis of the right axillary artery. Despite a successful embolectomy at the local hospital, there was re-occlusion of the axillary artery and the limb became ischaemic. He was referred to our institution by which time the limb became gangrenous above the elbow and had to be amputated. Extensive sloughing of the nerves was also seen in the local area. Hypercoagulability presenting with various manifestations is common in COVID-19 and needs early anticoagulation. We present this asymptomatic patient who lost a limb to this COVID-19 sequelae.
Keywords: COVID-19, vasculitis, vascular surgery, surgery, pain
Background
The first case of novel coronavirus, a pandemic that originated in China, was reported in Kerala (India) by the end of January 2020.1 Coronavirus was initially considered to be a severe viral respiratory infection leading to acute respiratory distress syndrome. Over time various other complications including cardiovascular, liver failure and renal insufficiency have been reported.2–5 Critically ill patients with COVID-19 have a hypercoagulable state with a marked elevation of D-dimer and fibrin degradation products as seen in many recent reports and may cause arterial occlusion.6 However, extensive gangrene of the upper limb as a COVID-19 disease sequela has not been reported.7 We report a case of acute upper limb ischaemia and wet gangrene above the elbow due to an arterial embolus following COVID-19 infection in a 44-year-old man with uncontrolled diabetes mellitus.
Case presentation
We are reporting the case of a 44-year-old man with uncontrolled diabetes, who presented at a peripheral hospital in mid-September with low-grade fever and fatigue for 3 days. There, he was detected to be COVID-19 reverse transcription (RT)-PCR positive and was advised oral azithromycin, nutritional supplementation and self-isolation at his residence.
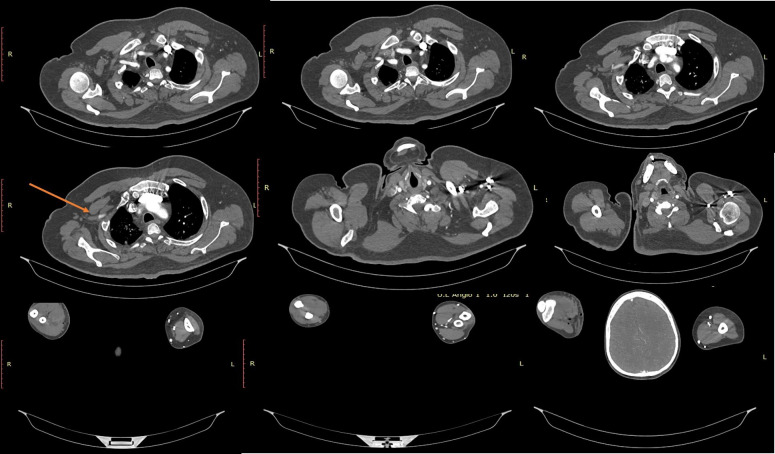
After 10 days, the patient had sudden onset pain and paraesthesia in his right upper limb. The initial diffuse pain progressively worsened and had localised to the fingers of his right upper limb. It was a severe aching type of pain and was associated with paraesthesia and numbness of his fingers. By the time of his reporting at the peripheral hospital, the condition progressed and he had developed generalised weakness of the right upper limb. He was evaluated with a CT angiogram (figure 1) which showed complete occlusion of the second and third parts of the right axillary artery. A successful embolectomy via brachial artery was done. However, after 3 hours, an ultrasonography arterial Doppler study done showed a recurrent and progressive thrombosis of the right axillary artery. With a provisional diagnosis of vasculitis, the patient was started on steroids. Over the ensuing 8 hours, his limb turned pale and became cold with a marked elevation of D-dimer later progressing to gangrene involving the right hand. He was then referred to a higher centre where he was managed conservatively with heparin infusion and prophylactic antibiotics. However, the gangrene progressed to the right forearm by this time. The patient was then referred to our centre for further evaluation and management. On arrival at our institution, the patient reported severe pain in the right upper arm. He also reported mild breathlessness and chest pain. The patient did not have a history of smoking, no family history of atherosclerosis and no history to explain thrombogenesis except this recent history of COVID-19.
Figure 1.
CT angiogram showing occlusion of second and third part of axillary artery.
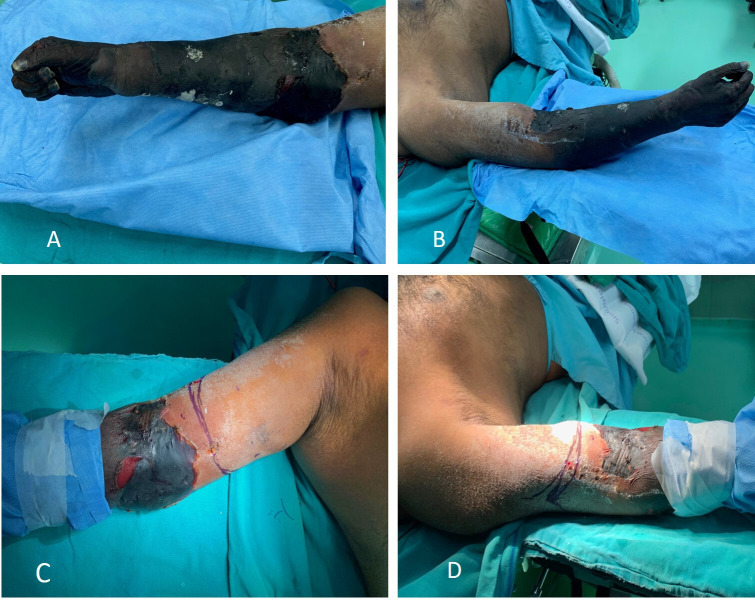
On clinical examination, he had tachycardia and tachypnoea with an oxygen saturation of 88% at room air. On local examination, wet gangrene of the distal part of the arm, forearm and hand was noted with redness, oedema and rise of temperature in the proximal arm (figure 2A, B). The distal limb was cold to touch. There was a sensory deficit with absent motor activity in all the muscles below the elbow. There were no pulses palpable in the right upper limb except a feeble pulsation of the right axillary artery. Hyperaesthesia was noted in the arm over the deltoid region. The left upper limb was normal. All other peripheral pulsations were palpable. There was a reduced air entry with absent breath sounds in the left lower lung fields.
Figure 2.
(A) Anterior and medial aspect of the upper limb showing gangrene extending above the elbow. (B) Posterior and lateral aspect of the upper limb showing gangrene extending above the elbow. (C, D) Level of incision marked.
Investigations
COVID-19 RT-PCR positive (07 October 2020).
Total count (TC) at the time of admission was 20.49 ×109/L (4.0–10.0 ×109/L) with elevated C reactive protein (CRP) of 167.81 mg/L (0.0–1.0 mg/L). By the time of discharge it had come down to near normal levels with TC of 8.83 ×109/L (4.0–10.0 ×109/L) and CRP at 39.59 mg/L (0.0–1.0 mg/L).
At admission, D-dimer was 932 µg/mL (normal 500 ng/mL), activated partial thromboplastin—42.1 s (25.35–33.73 s) and prothrombin time—15.00 s (11.24–14.87 s).
High-sensitivity troponin T (hs-Trop T—0.0260 ng/mL (0.0127–0.0250 ng/mL), repeat hs-Trop T after 6 hours–0.0180 ng/mL.
Creatine kinase-MB (CK-MB)—1.74 ng/mL (0.0–4.94 ng/mL), repeat CK-MB after 6 hours–1.67 ng/mL.
CT angiogram of the right upper limb showed complete occlusion of the second and third part of the right axillary artery with absent contrast opacification in distal brachial, radial and ulnar arteries.
The right subclavian and the first part of the axillary artery showed normal calibre and luminal contrast filling. First part of the axillary artery proximal to pectoralis minor showed normal contrast opacification (figure 1) and length of the patent segment measured approximately 33 mm.
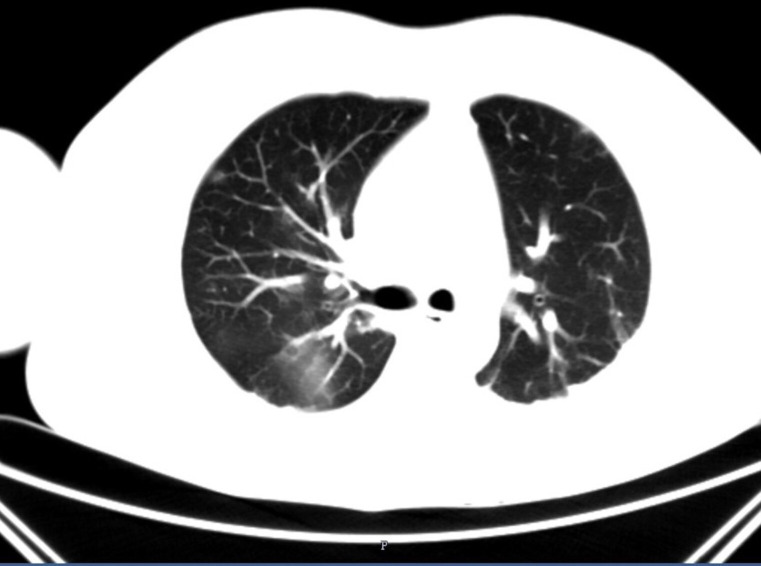
A multidetector CT pulmonary angiogram ruled out pulmonary thromboembolism. However, subtle areas of ground-glass opacities in the right upper lobe (figure 3) and subsegmental atelectasis in bilateral basal lungs with a thin rim of pleural effusion in the left lobe were noted.
Figure 3.
Multidetector CT pulmonary angiogram showing ground-glass opacities.
Since viral myocarditis was a distinct possibility as a post-COVID sequela, a screening Echocardiogram (ECHO) was done. Regional wall motion abnormalities, mid and basal inferior wall hypokinesia with fair left ventricular systolic function were noted. No clots were seen.
Thrombophilia work-up:
Lupus anticoagulant—positive.
Factor V Leiden mutation—not detected.
Prothrombin mutation—not detected.
Anti-cardiolipin IgG and IgM—negative.
Antineutrophil cytoplasmic antibody (C-ANCA), perinuclear ANCA (P-ANCA)—negative.
Antinuclear antibody screen (indirect fluorescent antibody)—negative.
Beta 2 glycoprotein IgM—0.7 (<20 SG units).
Beta 2 glycoprotein IgG—4.7 (<20 SG units).
Serum homocysteine—8.77 µmol/L (5.08–15.39 µmol/L).
Protein C—90% (70%–130%).
Protein S—105% (60%–140%).
Antithrombin III—84% (80%–120%).
Peripheral smear done showed normocytic normochromic anaemia with neutrophilic leucocytosis and thrombocytosis.
Red blood cell—polychromatophils present, rouleaux formation present, no agglutination.
White blood cell—total count 18 ×109/L, no blasts seen.
Platelets—650×109 /L seen singly.
Differential diagnosis
Vasculitis as a cause was considered since lupus anticoagulant was positive for this patient. However, since his symptoms started 10 days after the detection of COVID-19, we attributed the arterial occlusion to increased thrombogenicity of COVID-19. Literature also proves that lupus anticoagulant positivity is high in patients with COVID-19.8 Viral myocarditis was considered as one of the differential diagnoses, given a history of mild chest pain. This was however ruled out by a 2D ECHO. Pre-existing arterial disease was ruled out as the patient was a non-smoker and his angiogram showed no features of thromboangitis obliterans (Buerger’s disease) or atherosclerosis, common in this age. CT done did not reveal any other extrinsic cause of arterial obstruction. Thrombophilia work-up did not reveal any thrombogenic state.
Treatment
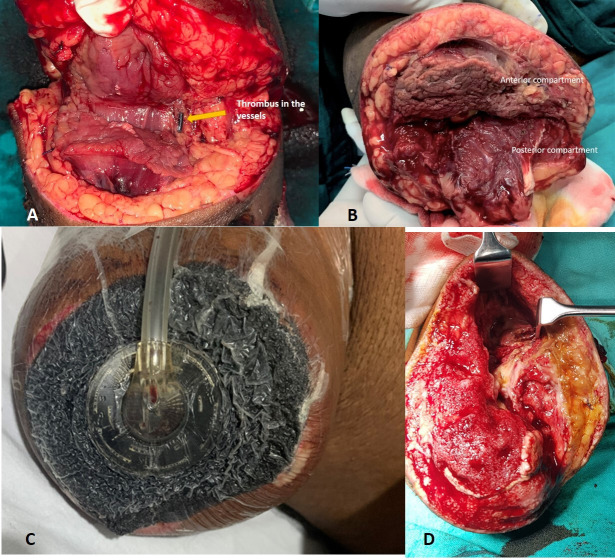
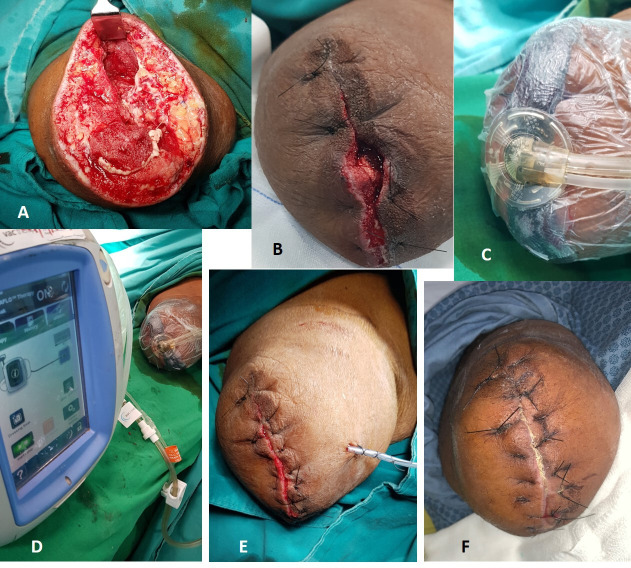
Since the limb was non-salvageable and no further vascular intervention was possible at this stage, a right above elbow amputation was done under general anaesthesia. Intraoperatively, brachial artery, paired brachial veins and the cephalic vein at the level of amputation were found to be filled with thrombus (figure 4A), and the muscles of the anterior compartment (biceps) were found to be necrosed with no response to stimulation and a putrid smell (figure 4B). Nerve sheaths of median and ulnar nerve were found to be sloughed off and seemed like butter when touched. However, the muscles of the posterior compartment seemed viable with mild to moderate response to stimulation (figure 4B). All anterior compartment muscles were removed. The stump was left open in view of poor vascularity, and negative pressure wound therapy was initiated (figure 4C, D). Injectable cefoperazone+sulbactam (1 g+0.5 g) was given prophylactically in the postoperative period. The patient was continued on injectable low-molecular-weight heparin. Since oxygen saturation was around 88% and ground-glass opacities were seen in CT of the chest, suspicious of lung fibrosis post-COVID-19 (figure 3), he was started on pirfenidone at 400 mg three times per day. Culture sensitivity revealed growth of Klebsiella oxytoca sensitive only to colistin and tigecycline.
Figure 4.
(A) Thrombus in the vessels. (B) Ischaemic anterior compartment muscle (biceps). (C) Negative pressure wound therapy applied in the first sitting. (D) Healthy tissues after removal of first sitting of negative pressure wound therapy after excision of necrotic anterior compartment muscles.
The wound was reassessed after 5 days. Negative pressure wound therapy with colistin instillation (NPWTi—KCI VAC veroflo therapy) was initiated in view of infected muscle compartments.
On reassessing the wound after 5 days (figure 5A), good granulation was seen and the wound was partially closed (figure 5B) reinitiating NPWTi with colistin instillation (figure 5C, D). The wound appeared healthy with good granulation tissue after this second sitting. After a thorough wash, the wound was closed with non-absorbable sutures (figure 5E). The patient was started on newer oral anticoagulant—apixaban (Eliquis) and was sent home.
Figure 5.
(A) Wound at reinspection after negative pressure wound therapy, (B) partially closed wound, (C, D) negative pressure wound therapy with colistin instillation on the partially closed wound, (E) wound closure with a suction drain in situ, (F) wound at suture removal.
Outcome and follow-up
The patient was reviewed 10 days after discharge (figure 5F). His sutures were removed and he was referred to physical medicine and rehabilitation department for muscle strengthening and stump prosthesis fitment. His oxygen saturation has improved to 99% in room air, but he has been continued on pirfenidone by the pulmonologists.
Discussion
COVID-19, declared as a pandemic in March 2020, exhibits symptoms ranging from mild to moderate (fever, dry cough, headache, anosmia, myalgia) to very severe. Systemic involvement including pneumonia, viral myocarditis and various other coagulation abnormalities leading to stroke and myocardial infarction have also become common.9 The SARS-CoV-2 virus is thought to infect the human cells via the ACE-2 receptors, which are expressed in cells of the kidney, heart, vascular endothelium, testis and small intestine.10
Various studies have shown a direct link of the inflammatory process induced by the virus to prothrombotic states. Inflammation leads to microvascular and macrovascular endothelial damage which then leads to disturbances in the coagulation cascade.11 There is also inhibition of fibrinolysis in this condition often resulting in extensive thrombosis. The interleukin 6-related inflammatory storm stimulates the liver to produce fibrinogen and thrombopoietin, and also disrupts the endothelium of the vessels leading to activation of the extrinsic pathway of coagulation, causing catastrophic thrombotic events.12
Though literature reports ischaemia related to COVID-19, more commonly in the lower limb, there are very few reports of upper limb involvement.13 Even with prophylactic or therapeutic anticoagulation, thrombosis rates are found to be high.14 Though most studies show this condition as being amenable to limb salvage, there is always a high probability of loss of limb.12 13
As our patient was asymptomatic, he was placed in home isolation without any major medications though he was detected to be positive for COVID-19. The symptoms and signs related to limb ischaemia started on the 10th day after detection of COVID-19. Despite anticoagulation and embolectomy, the ischaemia progressed to gangrene requiring amputation of the upper limb. If anticoagulation had been started on the day of detecting COVID-19, this catastrophic event could have been prevented.
It is highly recommended to start anticoagulation even for asymptomatic to mild patients with COVID-19 to prevent this untoward complication. While anticoagulation may suffice, the occasional patient progresses to gangrene needing limb amputation. Since COVID-19 infection is a highly aggressive thrombogenic and inflammatory state with a propensity for recurrence as can be seen in our patient, the prognosis for the condition is guarded despite attempting an embolectomy or a thrombectomy.10–14 Lupus anticoagulant positivity has been proven in a significant number of cases with COVID-19-associated thrombosis.8
Perioperatively, the patient had severe hyperaesthesia of skin around the stump, unresponsive to regional nerve blocks, necessitating general anaesthesia even for a dressing change. However, this hyperaesthesia was limited to the skin around the stump and was not seen elsewhere in the body. Another interesting finding that we noted in the operating room was the butter-like feel of the median and ulnar nerve sheaths. Zanin et al reported that SARS-CoV-2 can attack the nervous system and cause extensive demyelination. This may explain our intraoperative findings.15
Secondary infection and extensive inflammatory reaction in the tissues near the stump prevented primary closure of the wound. The culture sent from the stump region showed multidrug-resistant (MDR) K. oxytoca sensitive only to colistin. Since the lung function was compromised post-COVID-19, there was fear of respiratory failure associated with the administration of intravenous colistin.16 Also, there are reports of local application of colistin giving good results in MDR Klebsiella.17 Hence, NPWTi with colistin instillation was considered for this patient. With three sittings of NPWTi, the wound granulated very well and the inflammation subsided. Secondary suturing of the wound was then done.
Patient’s perspective.
I would like to express my experience regarding my condition. I had an episode of fever in mid of September and had gone to a nearby government hospital. They started me on some paracetamol tablets. However they asked me to get a COVID test done and it was found to be positive. Since my symptoms had come down, the government health officials asked me to take rest and get quarantined at home. So I was staying in the first floor of my own house. Around 10 days after I was found to be positive, I had some tingling sensation, pain and weakness of my right hands and fingers. As my pain had increased, I went to a nearby hospital. They asked me to get admitted in the hospital. In the next 2–3 days, I saw that my fingers and wrist skin colour was changing. It changed to a black colour. They did a scan there and told me that there was a block in my right upper hand artery. They did a procedure named embolectomy to remove the block in my hand. After the procedure I felt some relief of symptoms, but around 3 hours later, my pain had again increased. Next day morning I noticed that my skin colour had again changed. They sent me to another hospital where they gave some injections and some medicines. My hand was still black and so they referred me to Amrita hospital. My condition had worsened and the black colour went above the elbow region. I also had some fever and breathlessness. My doctors told that I will have to undergo an amputation for this and there is no other way to prevent the infection. My family personnel were consulted and I underwent an amputation for this. After the amputation was done, doctors told me that my muscles were infected and sponge with suction (vacuum therapy) was started. Over the due course of time, my wound improved with all the new modalities used here. Finally my doctors had closed my wound and I was so happy with all my doctors who had treated me very well. I am now looking forward to prosthetic fitment to my limb.
Learning points.
COVID-19 need not always present as respiratory disease and can present as limb-threatening arterial thrombosis.
Early anticoagulation in COVID-19 can save a life and limb.
Local application of colistin is effective in multidrug-resistant Klebsiella infection.
Demyelination of nerves was seen in the affected area. The patient did not get adequate analgesia with regional block and needed general anaesthesia. Whether this too is a sequela or not is unclear.
Footnotes
Contributors: RR—conception or design of the work; acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work. AVP—conception or design of the work; acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work. SR—conception or design of the work; acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work. SS—conception or design of the work; acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.India Today Kerala confirmed first novel coronavirus case in India. Available: https://www.indiatoday.in/india/story/kerala-reports-first-confirmed-novel-coronavirus-case-in-india-1641593-2020-01-30 [Accessed 30 Jan 2020].
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun P, Qie S, Liu Z. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. J Med Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831. 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ 2020;192:E583. 10.1503/cmaj.200685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanis N, Stavraka C, Agathangelidis F, et al. Coagulopathy in COVID-19 infection: a case of acute upper limb ischemia. J Surg Case Rep 2020;2020:rjaa204. 10.1093/jscr/rjaa204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 2020;383:288–90. 10.1056/NEJMc2013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020;7:e438–40. 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sypniewska G. Pro-Inflammatory and prothrombotic factors and metabolic syndrome. EJIFCC 2007;18:39–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Anwar S, Acharya S, Shabih S, et al. Acute limb ischemia in COVID-19 disease: a mysterious coagulopathy. Cureus 2020;12:e9167. 10.7759/cureus.9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg 2020;72:1864–72. 10.1016/j.jvs.2020.04.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–98. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir 2020;162:1491–4. 10.1007/s00701-020-04374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha A, Soriano SM, Song M, et al. Intravenous colistin-induced acute respiratory failure: a case report and a review of literature. Int J Crit Illn Inj Sci 2014;4:266–70. 10.4103/2229-5151.141487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali S, Pillai AV, Ramachandran R. Efficacy of colistimethate sodium as local application in necrotising fasciitis. BMJ Case Rep 2019;12:e232354. 10.1136/bcr-2019-232354 [DOI] [PMC free article] [PubMed] [Google Scholar]