Abstract
Successful tissue regeneration strategies focus on the use of novel biomaterials, structures, and a variety of cues to control cell behavior and promote regeneration. Studies discovered how biomaterial/ structure cues in the form of biomaterial chemistry, material stiffness, surface topography, pore, and degradation properties play an important role in controlling cellular events in the contest of in vitro and in vivo tissue regeneration. Advanced biomaterials structures and strategies are developed to focus on the delivery of bioactive factors, such as proteins, peptides, and even small molecules to influence cell behavior and regeneration. The present article is an effort to summarize important findings and further discuss biomaterial strategies to influence and control cell behavior directly via physical and chemical cues. This article also touches on various modern methods in biomaterials processing to include bioactive factors as signaling cues to program cell behavior for tissue engineering and regenerative medicine.
Keywords: Biomaterial Cues, Physical, Chemical, Biological, Tissue Engineering, Regenerative Medicine
Graphical Abstract.
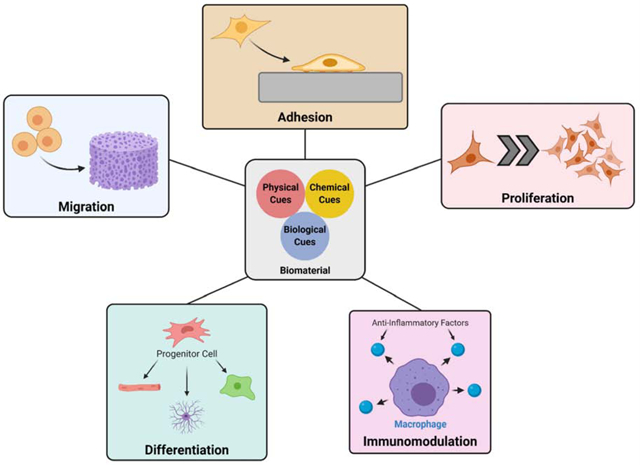
Biomaterial cues to induce cell programming. Through physical, chemical, and biological cues, a biomaterial can be tailored for regeneration of specific tissues by promoting cell migration, proliferation, and differentiation.
1. Introduction
In tissue engineering, cell behavior is often directed by signaling molecules, which include bioactive factors such as proteins, peptides and small molecules. These are expected to provide the signaling needed to enhance proliferation and differentiation of progenitor cells surrounding the defect area. Also in specific cases, these factors can serve as chemo-attractants by recruiting the needed progenitor cell population that will participate in de novo tissue formation. Growth factors, such as BMP-2 and BMP-7 have received wider attention and are being utilized as part of the commercially available products for bone defect repair and regeneration (1). Angiogenic factors, which include platelet derived growth factors (PGDF) and vascular endothelial growth factors (VEGF) are applied in tissue engineering strategies to introduce angiogenesis and vascularized tissue formation (2,3). A similar approach has been adopted in developing regenerative strategies for a number of other tissues including skin, nerve and musculoskeletal tissues (4, 5).
In tissue engineering and regenerative medicine strategies, high concentrations of growth factors are needed to achieve proper regeneration in order to maintain therapeutic levels for a sustained period of time (4). Also, the degradation of growth factors occur quickly in vivo through a variety of pathways including denaturation and proteolysis, with some growth factors such as BMP-2 and VEGF presenting a half-life of less than 30 minutes (1,5). Yet, the excessive use of growth factors lead to high costs related to production of the factors and the tissue engineering strategy as well as safety risks that are associated, with ectopic tissue regeneration and cancer being some of the side effects (6,7). The methods of growth factor delivery are also often inadequate, presenting burst delivery mechanics and failing to maintain steady therapeutic levels of the bioactive factors. This results in limited or incomplete tissue repair or regeneration (8,9).
Therefore, rather than relying so heavily on the therapeutic effects of the delivered growth factors, the modification of biomaterial scaffold’s properties are now at the forefront of tissue engineering research efforts. Although modifications of biomaterial properties have been researched in the past, the wealth of knowledge and biomaterial fabrication techniques now available make the modifications much more precise and tissue specific. The influence of a biomaterial’s physical properties on the defect site and surrounding cells are now well established, and when combined with newly developed scaffold design and fabrication methods, these properties can now be tailored to the tissue and defect types (10–13). It is widely established that biomaterial’s chemical cues, such as charge, functional group and surface chemistry also effect cell behavior and there are now established methods to achieve controlled surface chemical modification (14–16). In addition, the way in which the biomaterial interacts with the growth factors and other biologically active molecules that are being developed have also been further enhanced (17–19). Therefore, the motivation of this article stems from the need to develop biomaterial-based strategies to influence cell behavior and to achieve the required tissue regeneration. This article will summarize the recent advancements in modifying a biomaterial’s physical and chemical properties, as well as its ability to interact with bioactive factors, and the ways a biomaterial’s properties can influence and drive cell behavior.
2. Physical Cues
Studies have shown that biomaterial physical cues, such as topography, pore size and volume, scaffold architecture, and mechanical stiffness play roles in biomaterial-cell interactions and cell behavior (20). With the advent of advanced fabrication methods such as 3D printing, researchers are now able to design and develop biomaterials with desired nano/micro topography and scaffold architecture. Also, the availability of a number of natural and synthetic biomaterials makes it possible to form three-dimensional and porous structures with tunable mechanical stiffness. This section will review some of the recent developments in terms of how physical cues direct cell behavior and tissue regeneration.
2.1. Surface Texture
The physical design of a biomaterial’s surface with regards to its roughness and patterning can be tailored to aid in cell spreading/adhesion, proliferation, and/or differentiation. For osteoblasts and bone tissue engineering in general, the roughness of a biomaterial’s surface is known to promote attachment and differentiation by supporting focal adhesion complex formation and extracellular matrix (ECM) accumulation (21–26). Briefly, attachment of osteoblasts and other cells undergo indirect attachment to the biomaterial’s surface via attachment to ECM proteins adsorbed on the surface of the biomaterial, such as vitronectin, fibronectin, and collagen (23). The adsorbed proteins then bind with integrins on the surface of osteoblasts, which can further trigger downstream signaling pathways that lead to proliferation and differentiation (22). Several studies have shown that surface roughness at nanoscale increases ECM protein adsorption, which promotes osteoblast attachment (21,26). Investigations also show that nano/micro scale surface features promote MSC osteogenic differentiation when compared to a smooth surface (24). The exact mechanism of how surface roughness can modulate MSC osteogenic differentiation is not well understood, but it has been linked with downstream pathways of integrin signaling and the resulting expression of osteogenic factors such as transforming growth factor-β1 (TGF-β1) and prostaglandins (25).
Beyond just tuning the surface roughness of the biomaterial, specific topographical patterns on the surface of a biomaterial have been shown to also direct cell behavior towards tissue regeneration. Several different patterns can be made in the micro- and nanoscale, with grooves, pits, and pillars being the most commonly created and studied. The type and scale of which surface pattern benefits a certain tissue type depends strongly on the tissue and cell type being used. For instance, in cardiac tissue engineering, with its highly organized native ECM and cellular arrangement, tissue engineering materials require the proper orientation of seeded cells and produced ECM. Various studies have demonstrated the efficacies of surface patterning in developing biomaterials for muscular and cardiovascular regeneration. Specifically, a 3D-printed gelatin hydrogel containing microchannels was shown to promote human MSC alignment and differentiation into cardiomyocytes, along with spontaneous and synchronized contractions (12). In particular, microchannels caused increased sarcomere development in cardiomyocytes, and when combined with a conductive poly(pyrrole) showed evidence of cell-to-cell electrical coupling (27). In terms of vascular tissue engineering, varying responses were observed based on the type of surface pattern and the progenitor cell type used. Cellular alignment was only seen in substrates with grating patterns, and stem cell-derived endothelial cells displayed greater phenotypic maturation than either the arterial or venous endothelial cells. Further differential responses of the arterial endothelial cells and venous endothelial cells to surface patterning suggests that cell source and type must be considered for design of a biomaterial’s topography (28).
In neural tissue engineering, the viability of neural cells and development of neurites were shown to be strongly affected by nanopatterns of ridges and grooves. In a recent study, it was observed that the substrates with topographical cues assisted neural development more than a flat substrate coated with laminin as a biochemical cue (29). Yang et al. demonstrated that a combination of nanopores and microgrooves were able to induce differentiation of neural stem cells into neurons, and noted that inhibition of focal adhesion proteins eliminated the beneficial effects of topological cues (10). The height of nanofeatures was also shown to be relevant in inducing neural cell differentiation, with Song et al. reporting increased differentiation of human induced pluripotent stem cells (hiPSCs) into neurons for 560 nm tall ridges and a correlation between feature height, Yes-associated protein, and neural differentiation (30).
2.2. Architecture
Along with biomaterial surface geometry and characteristics, the biomaterial’s overall design and architecture can influence cell behavior and tissue regeneration. The pore structure of a scaffold can be a limiting factor in nutrient transport and overall cellularization. Engineered scaffolds with inadequate pore structure exhibit limited nutrient flow, waste removal, and acid build-up due to diffusion limitations. These conditions do not allow cell survival within the scaffold, which results in surface-level cellularization of the implanted scaffolds (11). Specifically, for bone tissue engineering, previous studies have shown a relationship between cell migration and differentiation with respect to overall pore size and diameter. Although cell infiltration was seen in pores as small as 40 μm in diameter, the optimal cell number and infiltration was seen in pores with diameters of 100–150 μm, both in vitro and in vivo (31). However, Murphy et al. found that the optimal pore size that promoted new bone formation was larger, 325 μm in diameter, due to even distribution of cells throughout the scaffold and less accumulation of cells on the periphery of the biomaterial (31).
Our own laboratory was able to fabricate an oxygen tension controlled (OTC) PLGA scaffold capable of maintaining optimal oxygen and pH levels throughout the graft (Figure 1) (11,32). The OTC scaffold was fabricated via “Thermal sintering and porogen leaching” technique, with overall porosity control. The developed OTC scaffolds with 100–500 μm pore size variation (Figure 1A) showed greater levels of cell survival and proliferation throughout the pore structure of the scaffold (Figure 1B). The enhanced cellularization seen in the interior of the scaffolds was attributed to the open pore structure, which enabled high levels of oxygen tension and physiological pH levels throughout the graft (Figure 1C). The in vivo evaluation of the OTC grafts also showed promising results, with increased de novo bone formation (Figure 1D, E) and evidence of increased angiogenesis (Figure 1E). These studies demonstrate the beneficial effects of tailoring a scaffold’s pore structure on cell survival to angiogenesis, and large-area bone tissue formation. In a separate study, researchers were able to take into account the degradation of the biomaterial and the amount of new tissue formed to model the optimal pore structure that will prevent mechanical failure as the structure degrades (33). Together, these studies developed a tissue engineering approach for large-area bone regeneration, and this strategy could be potentially used to treat non-union and critical-sized bone defects.
Figure 1.
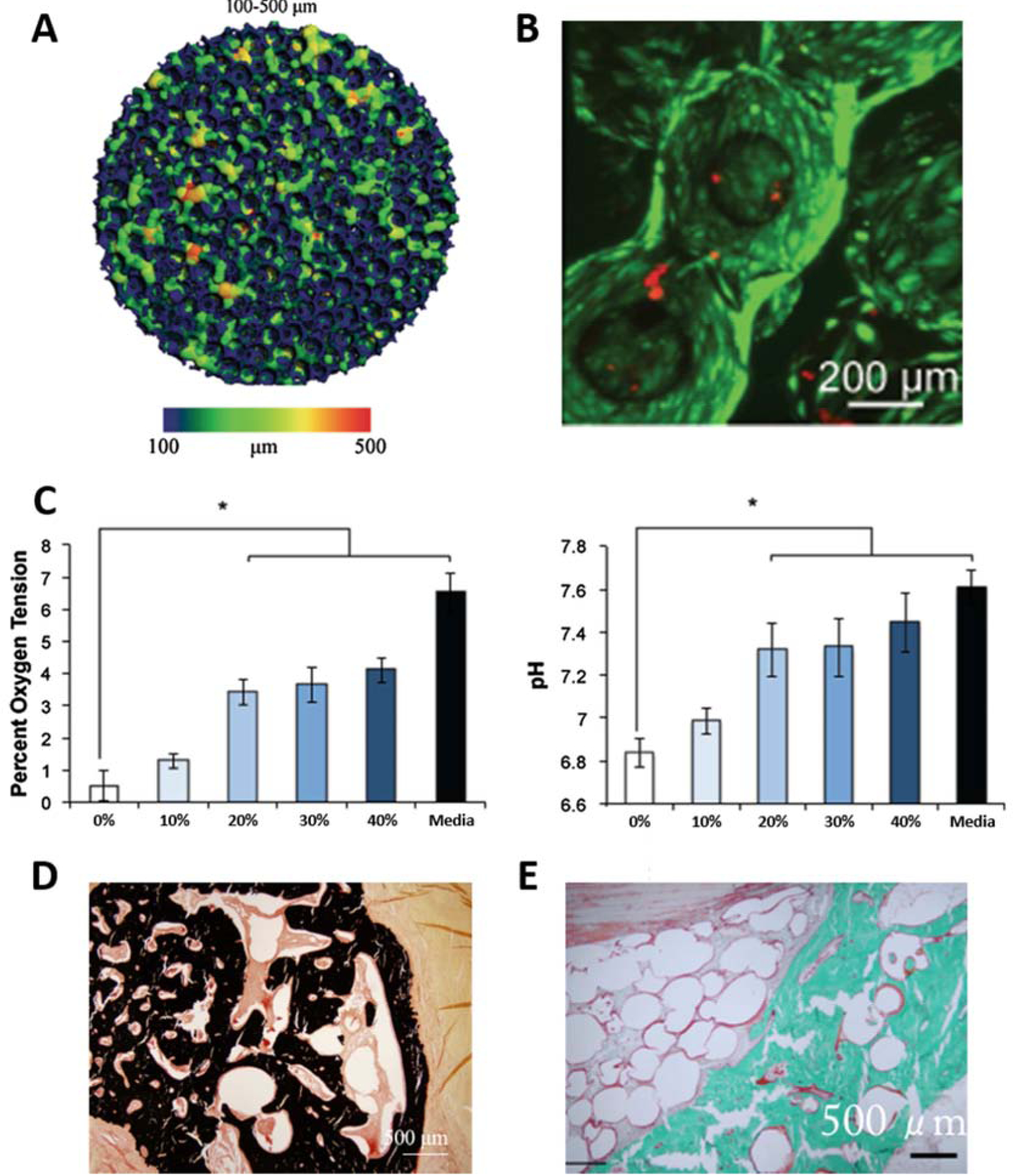
Engineering scaffold porosity to develop an oxygen tension controlled scaffold. (A) MicroCT reconstruction of available pore space in the PLGA microsphere scaffold with 20% NaCl porogen content showing variations in pore sizes ranging from 100–500 μm. (B) Live dead staining of the interior of the oxygen tension controlled scaffolds after 21 days in culture with MSCs. (C) Oxygen tension and pH levels in the interior of PLGA scaffolds made with varying concentrations of NaCl porogen after 21 days in culture with MSCs (Percentage indicates NaCl porogen content). (D–E) In vivo evaluation of oxygen tension controlled scaffold in a rabbit ulnar defect model. Von Kossa staining (D) and Goldner’s trichrome staining (E) of the defect 12 weeks after implantations shows newly formed mineral throughout the scaffold pore structure, demonstrating the ability to support large-area tissue regeneration. Parts A–C were reproduced from Ref # with permission from Springer, copyright 2014 (11). Parts D–E were reproduced from Ref # with permission from Mary Ann Liebert, copyright 2016 (32).
Besides bone tissue engineering, pore size and architecture play a critical role in the regeneration of other tissue types as well. In neural tissue engineering, nerve guide conduits (NGC), which are used to regenerate neural tissue in between two severed nerves, should present enough porosity to provide nutrient transport while preventing the invasion of external macrophages and fibroblasts. This in turn requires a graft with more than 50% porosity while maintaining flexibility and elastic modulus of 8–16 MPa, similar to human nerves. To satisfy these requirements, several studies designed highly porous systems that are mechanically compatible with pore characteristics suitable to prevent external cell infiltration and were assessed for their in vitro and in vivo performance (34,35).
Interfacial tissue engineering, such as osteochondral tissue, also requires specific pore structures to accommodate the regeneration of two different tissue types while maintaining structural integrity between the two layers. The difference in tissue types are especially apparent for osteochondral tissue, where the scaffold structures will have to facilitate the development of hard bone tissue, soft cartilage tissue, and their interface. This problem has been approached with the design of monophasic, bi-phasic, and tri-phasic scaffolds (36). But recently, osteochondral scaffolds with porosity and/or composition gradients have been developed to promote the regeneration of bone-cartilage tissue along with an interface in a single scaffold system (37–39). In one of the designs, a scaffold system with polymer and gel phases co-exist in an inverse gradient fashion, where the polymeric phase supports the osseous phase and the gel phase promotes the cartilaginous phase. The osseous phase is denser to allow differentiation of mesenchymal stem cells into osteoblasts by interacting with the stiffer template biomaterial. Meanwhile, the cartilaginous phase is designed to be more open with larger pores, which allows the use of a softer secondary phase that drives more chondrogenesis than the stiff template phase.
2.3. Mechanical Stiffness
Much like how surface topography can influence cell behavior via integrin binding, stiffness of the substrate can also drive cell behavior through a similar pathway. The cell traction force (CTF) that arises while cells migrate along a substrate serves as the initial stimulus for cell mechanotransduction (40). The generated force is then transmitted by integrins to initiate downstream pathways that lead to specific cellular behaviors (41). There is also evidence that the cell cytoskeleton, along with integrins, aids in conversion of mechanical stimuli to biochemical responses by the cell (42). After transmission of mechanical stiffness by the integrins and cytoskeleton, the downstream pathways differ greatly depending on the stiffness of the substrate, lineage of the cell, and pluripotency of the cell (43–45). However, the general consensus is that substrates that match the physiological stiffness of the target tissue will result in differentiation into said tissue type. For example, soft substrates (<1 kPa) lead to differentiation of neurocytes or brain cells, substrates with medium stiffness (1–25 kPa) cause myogenic differentiation, while stiffer substrates (25–40 kPa) can lead to osteogenic differentiation.
Mechanisms that direct osteogenic commitment by MSCs cultured on stiff substrates explored recently show that several signal transduction pathways may exist. It has been shown that adipose-derived MSCs cultured on stiff substrates (53.6–134 kPa) resulted in increased osteogenic differentiation and expression levels of RhoA and ROCK-2, proteins that respond to cytoskeletal rearrangement and tension (46). The studies also observed an increase in β-catenin expression by cells cultured on stiff substrates, and when combined with previously reported relation between the Rho/ROCK signaling system and the canonical Wnt pathway, these observations suggest that the β-catenin/Wnt pathway may be part of the mechanotransduction of MSCs towards an osteogenic response. Other signaling pathways include an increase in macrophage migration inhibitory factor (MIF) in MSCs cultured on stiff substrates, and the corresponding modulation of protein kinase B (AKT) and yes-associated protein (YAP) (47). This suggests that the MIF, in addition to various other functions controlling cell behavior, also promotes AKT/YAP signaling that in turn promotes RUNX-2 expression and osteogenesis in MSCs. Also, a recent study demonstrated that YAP promotes β-catenin levels within the nucleus, which in turn promotes osteogenic differentiation, further linking the β-catenin/Wnt pathway with osteogenic differentiation (48). However, further studies are required to fully understand the role of the Wnt pathway in mechanotransduction of substrate stiffness and osteogenesis since there are conflicting results in terms of the Wnt pathway and osteogenic differentiation (49,50).
For soft tissue regeneration, hydrogels are often employed as biomaterials with tailored stiffness. For example, cartilage tissue engineering has seen success in developing hydrogels with mechanical stiffness that can promote chondrogenesis of MSCs. The optimal stiffness for chondrogenesis varies depending on the material used and other factors that are incorporated into the gel, but overall, chondrogenesis tends to occur best in relatively stiff hydrogels. However, there does seem to be a limit to hydrogel stiffness that promotes chondrogenesis, approximately 50–100 kPa (13). Beyond 100 kPa, the seeded MSCs display hypertrophic cartilage characteristics, and evidence of the osteogenic YAP/Wnt pathway, discussed previously. On the other hand, much softer hydrogels are utilized in neural tissue engineering, often in the range of 0.1–1 kPa. In fact, studies have shown that hydrogel stiffness was more important than presence of Arg-Gly-Asp (RGD) peptides in guiding neural cell attachment, morphology, and functionalization (51). Investigations have also demonstrated the relationship between hydrogel stiffness and migration of cells within them (52). In addition to movement of cells within a homogeneous hydrogel, several studies have highlighted the movement of cells along a stiffness gradient, or durotaxis. Specifically, Hadden et al. utilized hydrogels with various gradients in stiffness to determine that human adipose-derived stem cells tend to migrate towards the stiffer end of the hydrogel when cultured in steeper gradients (8.2 kPa/mm) (53). Therefore, these results suggest that stiffness gradients in hydrogels can be utilized to promote migration of cells into the innermost parts of the hydrogel both in vitro and in vivo.
Extensive investigations in the field have demonstrated that biomaterial physical cues in the form of surface texture, architecture, and mechanical stiffness play critical roles in dictating cell behavior and performance. These cues and their effects on cell behavior are illustrated and summarized in Figure 2. Studies so far established the benefit of these cues in controlling cell adhesion, growth, and differentiation, and key studies are summarized in Table 1. However, further optimization of the mentioned parameters is required to promote the desired phenotype, and biomaterial designs that utilize the above stated parameters have seldom been explored.
Figure 2.
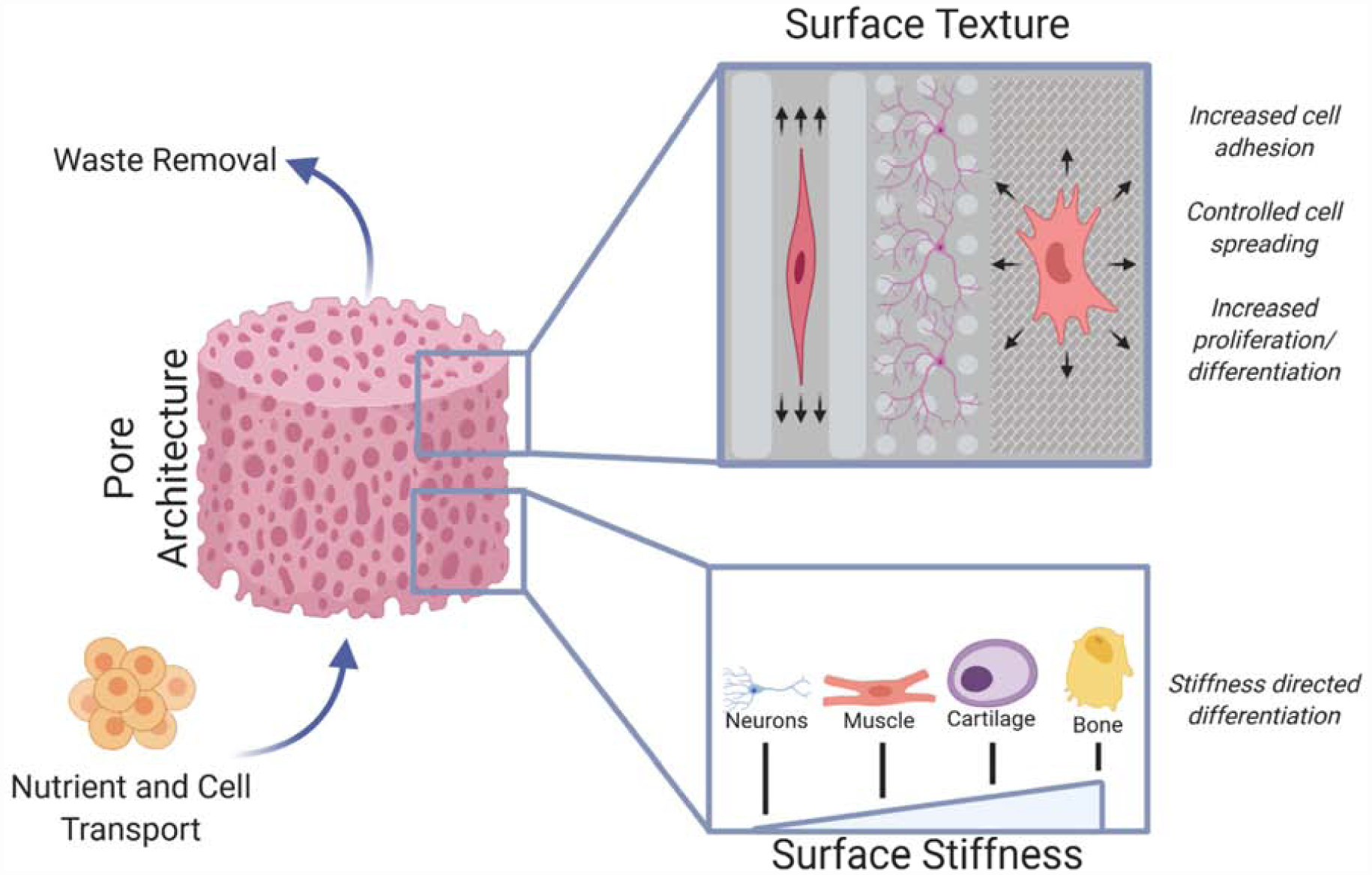
Schematic of various physical cues a biomaterial can present to the nearby cells and their effects on tissue regeneration. Overall pore architecture drives cell migration throughout the scaffold by providing ample nutrient and waste transport while providing surface area for cell attachment and growth. The attachment of cells is further enhanced by specific surface textures that aid attachment of specific cells and help guide cell growth and differentiation. The differentiation of attached cells can also be improved by tailoring the surface stiffness of the biomaterial to match that of the native tissue.
Table 1.
Summary of recent publications regarding biomaterial-induced programming of cell behavior.
| Author | Biomaterial | Results | Reference |
|---|---|---|---|
| Physical Cues | |||
| Tijore et al. | Microchanneled gelatin | Elongation and myocardial differentiation of seeded MSCs Synchronized contraction of differentiated myoblasts |
(12) |
| Lee et al. | PEG hydrogels of varying stiffness (50–100 kPa) | Chondrogenic differentiation of MSCs in softer gels Differentiation into hypertrophic chondrocyte in stiffer gels through activation of WNT pathway |
(13) |
| Stukel et al. | PEG hydrogel of varying stiffness and RGD concentration (0.1–7.9 kPa; 1–2.5 mM) | Enhanced neural differentiation of iPSCs and neurite growth in soft gels (0.1–0.8 kPa) iPSCs more responsive to gel stiffness than RGD concentration |
(51) |
| Hadden et al. | Polyacrylamide hydrogel with varying stiffness gradients (0.5–8.2 kPa/mm) | Evidence of durotactic behavior of adipose-derived MSCs Observed migration of adipose-derived MSCs to migrate towards stiffer ends of the gel when cultured in gels with steeper gradient (8.2 kPa/mm) |
(53) |
| Chemical Cues | |||
| Visalakshan et al. | Tissue culture plates coated to modify hydrophilicity | Hydrophobic surfaces increased adsorption of immunoglobulins and induced pro-inflammatory response from macrophages Hydrophilic surfaces increased albumin adsorption and promoted anti-inflammatory responses |
(55) |
| Xu et al. | PLGA/Mg composite films | Acidic degradation of PLGA neutralized by basic degradation of Mg Increased osteogenic differentiation of MC3T3-E1 cells |
(14) |
| Boffito et al. | Polyurethane scaffold conjugated with laminin-1 or gelatin | Improved cardiac progenitor cell adhesion for both protein coatings Improved proliferation and differentiation into cardiomyocytes for laminin conjugated scaffolds |
(85) |
| Flora et al. | Elastin-like recombinamer-based hydrogel | Utilized gels that degrade at different rates to direct cell infiltration | (86) |
| Biological Cues | |||
| Bai et al. | PLGA/PLA composite scaffold to induce sequential factor delivery | VEGF and FGF-2 encapsulated in PLGA experienced faster initial delivery PDGF encapsulated in PLA experienced slower delivery Sequential delivery of these factors allowed rapid formation of vasculature in vivo |
(75) |
| Vaghasiya et al. | Mesoporous silica nanoparticles (MSN) coated with collagen | Drug payload loaded onto MSN and then coated with collagen to form a “capping layer” Collagen coating digested by MMPs in vivo, releasing drug payload, allowing on-demand delivery of drug based on physiological conditions |
(82) |
| Xu et al. | Matrigel hydrogel presenting chemoattractant gradient | Chemoattractant semaphoring 3A (Sema3A) loaded onto Matrigel and gradient was established Established Sema3A gradient attracted nearby neural progenitor cells to the gel and promoted neurogenesis |
(84) |
| Zhou et al. | Collagen hydrogel with graphene oxide | TGF-β3 adhered tightly to graphene oxide, while maintaining active conformation Encapsulated cells were exposed to TGF-β3 and displayed increased chondrogenic differentiation |
(87) |
3. Chemical Cues
In addition to physical design, the chemical properties of biomaterials can have a large impact on its ability to regenerate the target tissue. Chemical properties of a biomaterial range from surface moieties and charges to degradation by-products that influence cell recruitment, proliferation, and differentiation. The goal of the research community is to design and develop biomaterials with chemical cues to impart cytocompatibility, minimal immunogenic response, and flexibility to tailor them for the desired tissue type. While there are numerous chemical cues that play a role, this section will be limited to biomaterial surface chemistry and degradation aspects and how these specific chemical cues influence cell behavior and tissue regeneration.
3.1. Surface Chemistry
By optimizing the surface chemistry of biomaterials, events starting from initial protein and cell adhesion, immune response, and cell behavior can be controlled (Figure 3). The most basic surface chemistry that influences how the biomaterial behaves is surface wettability. The hydrophilicity/hydrophobicity of the biomaterial’s surface interacts with serum proteins by either promoting or inhibiting their adsorption and denaturation. Studies have shown that hydrophobic surfaces tend to accumulate more serum proteins, such as fibrinogen and IgG, than hydrophilic surfaces by causing conformational changes that expose hydrophobic interiors of these proteins, which may reveal their immunogenic sites (54). A recent study showed that macrophages exposed to a hydrophobic surface expressed pro-inflammatory markers while exposure to a hydrophilic surface elevated anti-inflammatory markers (55). The beneficial programming of macrophages by hydrophilic surfaces led to faster resolution of an inflammatory response and MSC recruitment (56). However, in terms of direct adhesion of cells, conflicting results have been reported, which may indicate the confounding effects of surface charge and functional groups, as well as the type of cell being used.
Figure 3.
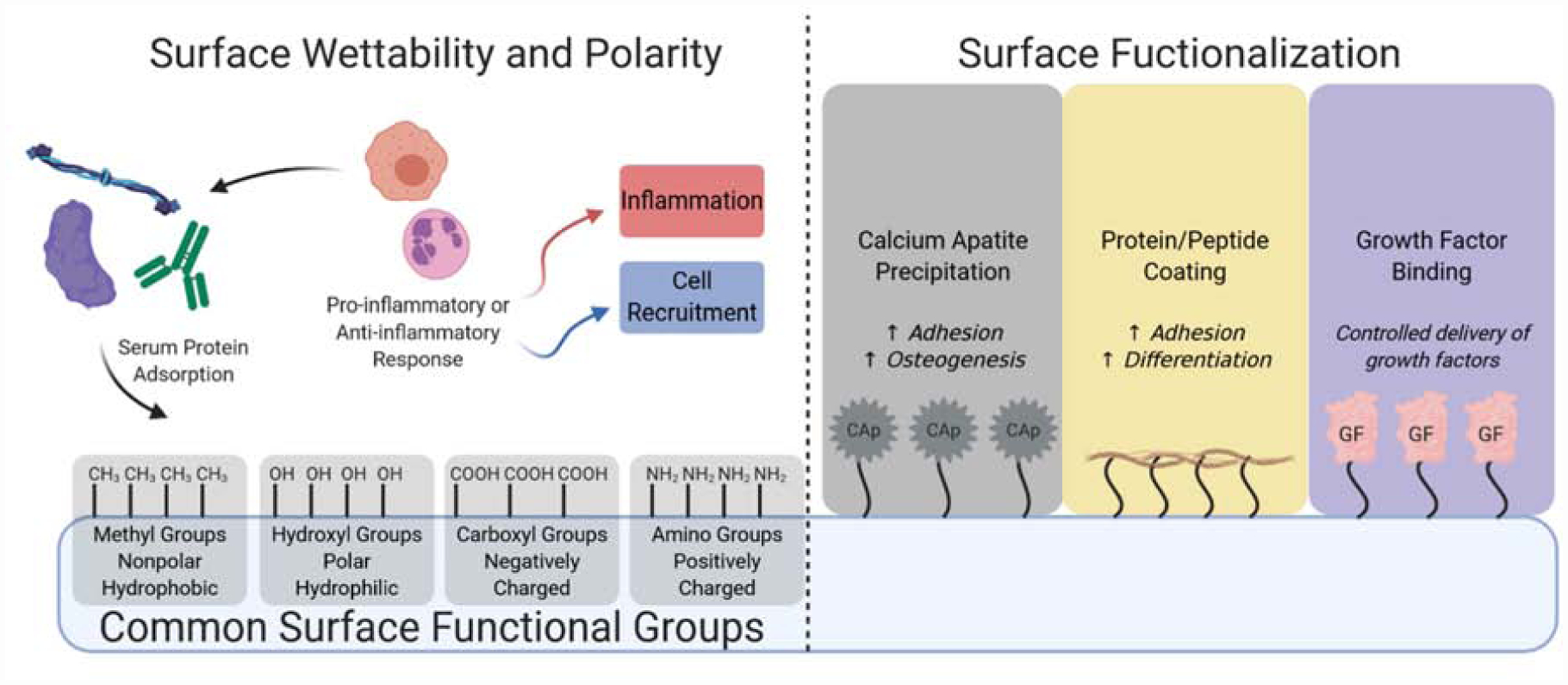
Graphical representation of biomaterial surface chemistry and associated cellular interactions. The wettability and polarity of the biomaterial surface guides attachment of serum proteins which then can aid or hinder the attachment of inflammatory cells that can help dictate whether the biomaterial will be targeted for inflammation or cell recruitment for tissue regeneration. Specific functional groups that are present on the surface can also help guide tissue regeneration by aiding/preventing inflammation. The surface of the biomaterial can be further functionalized for more specific tissue types by presenting inorganic factors like calcium apatites (Cap), ECM proteins, and/or growth factors (GF) to guide cell migration, growth, and differentiation.
In general, recent studies utilizing several cell types and materials have shown that positively charged surfaces promote greater amounts of cell adhesion and proliferation. Cernochova et al. utilized a polymerized cyclopropylamine with additional amino groups to increase its positive charge and saw a high degree of cell adhesion for fibroblasts, keratinocytes, and smooth muscle cells (57). The increase in cell adhesion on positively charged surfaces has been attributed to the amount of protein adhesion and denaturation prior to cell adhesion by others. Ishihara et al. observed an increase in fibronectin adsorption and denaturation in positively charged surfaces that correlated to adhesion of HeLa cells, indicating that the amount and degree of denaturation of fibronectin is related to cell adhesion (58). Meanwhile, it was found that positively charged surfaces attracted more α-helices to its surface, suggesting that the amount of α-helix adsorbed on the surface of biomaterials may be related to cell adhesion in addition to fibronectin, which is primarily composed of β-sheets (59). Aside from positively charged surfaces, negatively charged surfaces have been shown to be effective in adhesion of other cell types as well. Ribeiro et al. utilized poly(vinylidene fluoride) to create films with positive, negative, and neutral charges for myogenic cultures, and observed increased myogenic differentiation for the positively and negatively charged polymers; they postulated that electrostatic charges of the polymer are apt for differentiating electrically active myocytes, regardless of the charge (60). In addition, negatively charged substrates were shown to facilitate fibroblast attachment and growth while also inhibiting bacterial growth, which could aid in reducing inflammatory responses and facilitating regeneration (61).
In terms of functional groups on substrate surfaces, the simplest groups commonly used are amino (−NH2), hydroxyl (−OH), carboxyl (−COOH), and methyl groups (−CH3). These groups impart hydrophilic/hydrophobic and electrostatic properties to the biomaterial’s surface, but they have also been shown to have distinct effects on cellular behavior (Figure 3). In general, hydroxyl groups have shown high levels of cell adhesion and osteogenic response, followed by amino and carboxyl groups (62). On the other hand, amino and carboxyl groups on substrate surfaces resulted in larger reductions in inflammatory responses, with carboxylic groups inhibiting fibrous capsule formation the most in vivo (63,64). Carbon nanotubes functionalized with these groups showed in vitro and in vivo biocompatibility, when composited with biodegradable poly(lactic-co-glycolic acid) (PLGA) (65,66). These composite scaffolds also demonstrated increased compressive modulus and strength via reinforcement from the ultra-strong carbon nanotubes. In addition to chemical functional groups, surface modifications with peptide sequences have also been explored to increase biomaterial-cell interactions, with RGD sequences being the most commonly used motif for cell adhesion. However, recent developments have improved upon the commonly used chemical and biological motifs to better facilitate tissue engineering. Surface functional groups have been used to graft other polymers, peptides, and compounds to increase biomaterial-cell interactions as well as impart other bioactive properties (Figure 3). For example, Jaidev et al. modified the surface of polylactic aid (PLA) with citric acid and polyethyleneimine to increase the biomaterial’s ability to precipitate calcium phosphates from media, which in turn was used to enhance cellular proliferation and osteogenic responses (16). Similarly, biomaterial surfaces are modified with ECM proteins (collagen, gelatin, elastin, fibronectin, etc.) and synthetic moieties (calcium phosphates, ethoxysilane, polypyrrole, etc.) to improve biomaterial/construct performance in terms of biocompatibility, immune response, and in some cases even to impart electrical conductivity.
3.2. Degradation
The in vivo degradation of a biomaterial is pivotal in the design of a potential tissue engineering strategy, and involves the replacement of the implant with the host tissue. Advances in biodegradable biomaterials have explored the timing of the biomaterial’s degradation and how its degradation byproducts can react with its surroundings to aid in tissue regeneration. One of the most common biodegradable materials used for tissue engineering is PLGA, which hydrolytically degrades into lactic and glycolic acid when exposed to an aqueous environment. However, its degradation byproducts often lower the surrounding pH, and may cause cell death and tissue necrosis, so several recent studies have looked into a modified PLGA scaffold to self-neutralize its degradation byproducts (14,67). One of the developed strategies utilized basic degradation byproducts of magnesium-based biomaterials by compositing it with the PLGA to counteract its acidic byproducts (Figure 4) (14). The Mg:PLGA composite was developed by incorporating pure magnesium microparticles into PLGA during the solvent-casting method. The developed film was capable of maintaining near physiological pH levels while degrading, while the pure PLGA films resulted in sudden decreases in pH, as expected by its acidic degradation byproducts (Figure 1A). The inclusion of Mg also enhanced the bioactivity of the composite, as evidenced by high amounts of calcium apatite deposition when immersed in simulated body fluid (SBF) for 14 days (Figure 4B). These calcium apatite deposits were co-localized with the Mg atoms present in the scaffold, demonstrating the involvement of Mg in the precipitation of calcium apatite (Figure 4C). The in vitro assessment of the Mg:PLGA composites also confirmed its bioactivity as it resulted in significantly higher levels of cell induced mineralization, assessed through alizarin red quantification (Figure 4D). Therefore, novel biodegradable polymer - biodegradable metal (PLGA-MG) composite biomaterials, which demonstrated a neutral degradation profile while also taking advantage of Mg’s bone compatible mechanical and osteogenic properties. In addition to Mg, inorganic molecules such as calcium phosphates are also commonly used alone or in combination with other biomaterials and they degrade in vivo via a variety of mechanisms including dissolution of constituent ions and resorption by osteoclasts. The release of ions such as Ca2+ and PO42− into the surrounding environment during its degradation has shown to promote osteogenic activity, leading to frequent use as a bone tissue engineering material (68,69).
Figure 4.
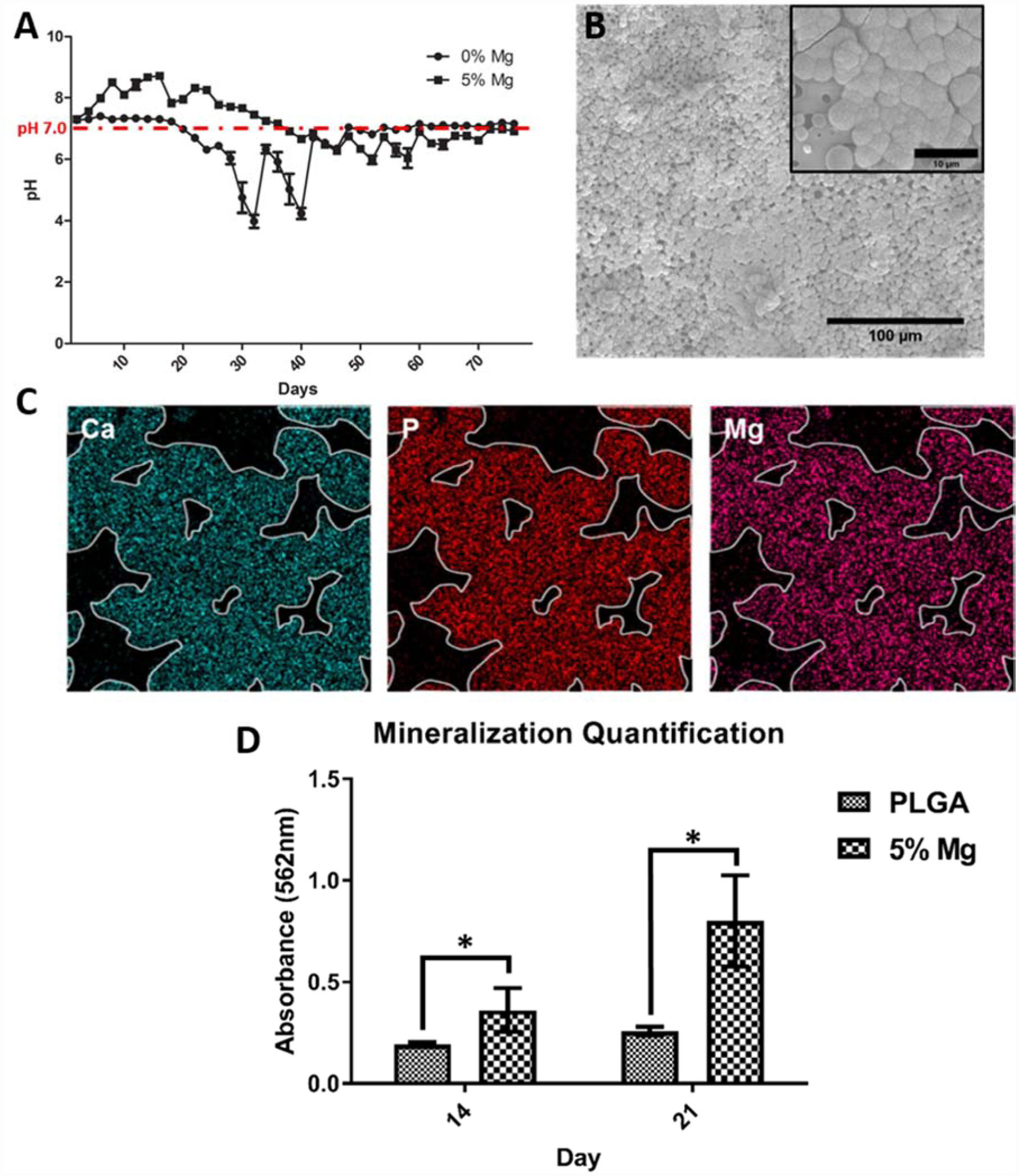
PLGA-Mg composite biomaterial neutral degradation. (A) Degradation profile of PLGA and PLGA containing 5wt% of Mg. (B) SEM image of 5% Mg:PLGA after 14 days immersed in Simulated Body Fluid (SBF), insert clearly show apatite crystal growth. (C) EDAX spectra of calcium apatite deposits on 5% Mg:PLGA after immersion in SBF (D) Alizarin red quantification of PLGA and Mg:PLGA samples after culture with MC3T3-E1 cells. Reproduced from Ref # with permission of IOP Publishing, copyright 2018 (14).
Another mechanism of degradation for biomaterials is enzymatic degradation, where the biomaterial is degraded by enzymes that are already present in the body. With recent advancements in polymer and hydrogel engineering, new enzymatically degradable biomaterials have been developed that can utilize enzymatic degradation for a variety of purposes. Many enzymatically degradable biomaterials allow greater control over their degradation rate by controlling the amount of cleavage sites. For example, Pereira et al. tuned the degradation rate by varying the amount of matrix metalloproteinase (MMP) cleavage sites that were used to crosslink the gel (15). Enzymatic degradation could also be used to impart degradability to typically non-degradable materials. For example, an alginate-based hydrogel, which is typically nondegradable in a mammalian system due to lack of enzymes capable of digesting alginate, was developed with peptide crosslinkers that could be cleaved by MMPs and allowed tunable degradation rates (70). The in vivo testing of the alginate-peptide hydrogel also showed an increase in host tissue infiltration versus the non-degradable alginate gel, which suggests that the hydrogel would degrade with cellular activity and allow more tissue infill with time. Madl et al. have developed an enzymatically degradable structure that would also aid in cell infiltration, spreading, and growth by designing cleavable peptide crosslinks that would result in the release of degraded fragments (71). This allowed more room in the hydrogel as it degraded, thereby allowing greater cell growth and infiltration.
Biomaterial/scaffold chemical cues, such as surface chemistry and degradation play an important role in the tissue regeneration process. Functional groups present on a biomaterial/scaffold surface control hydrophilicity/hydrophobicity and protein adsorption, which dictate cell adhesion and growth characteristics. Biomaterial degradation offers the space required for new tissue formation in a progressive manner while providing cell recruitment and induction cues through degradation products. Advances in biomaterial chemical cues have resulted in surfaces with varied types and levels of functional groups and degradability (Table 1). However, further characterization of tissue-specific responses to surface chemistry and degradation are needed to design biomaterial implants that can be precisely tailored for specific tissue engineering applications.
4. Biological Cues
Tissue engineering strategies often include biological factors as cues to promote and direct the desired tissue formation. These factors include protein growth factors as well as smaller signaling molecules that can either recruit cells or stimulate cell proliferation and differentiation. Therefore, there is a significant effort in tissue engineering to effectively utilize growth factors/signaling molecules in conjunction with biomaterials.
4.1. Growth Factors and Small Molecule Delivery
Growth factors are signaling proteins typically produced by cells that serve a critical role in tissue development, repair, and regeneration. Several growth factors have been well characterized and commonly used in tissue engineering depending on the type of tissue being regenerated. Most growth factors are able to influence cell behavior in multiple stages of regeneration, such as recruitment, proliferation, and differentiation. On the other hand, small molecules are organic molecules that may or may not be naturally occurring and serve as ligands, cofactors, and/or inhibitors in cellular signaling to influence cell behavior. Unlike growth factors, small molecules are inexpensive and can withstand harsh biomaterial processing methods without losing biological activity. Biomaterials are specifically engineered to present biological cues in the mode required for specific tissue repair and regeneration types, such as burst release, sustained release, or their combination.
One of the current facets of biological factor delivery research is the sustained delivery of factors and extended maintenance of biological activity of the delivered factors. Since biological factors, specifically growth factors, rely heavily on their conformation for their bioactivity, the structure of these factors must be maintained; this can be a challenge considering the relatively short half-lives of many growth factors (1,5). As for the sustained release of the incorporated factors, a variety of strategies have been used, including enhancing electrostatic interactions with the loaded factor, covalent conjugation of the factor, and advanced fabrication techniques. Electrostatic interactions were used by Lin et al. to encapsulate vascular endothelial growth factor (VEGF) in functionally modified alginate with a variable carboxyl and amino group ratio, creating a tunable surface charge which allowed sustained delivery of VEGF (19). Mesoporous silica nanoparticles have also been used as a drug delivery vehicle since their nanoporous structure features high surface area, efficient drug loading, and physical diffusion barriers for controlled release (72). Several studies have also confirmed the sustained delivery and beneficial effects of covalent conjugation of biologic factors onto the biomaterial (73). However, this method of growth factor incorporation often results in loss of bioactivity, which needs to be accounted for while designing biomaterials/scaffolds with conjugated growth factors.
Multiple factor delivery has garnered attention as a way to present specific biological cues for certain events during the regeneration process or for regeneration of multiple tissue types/complex tissues. Multiple factor delivery strategies can be divided into three main categories. The first one is to deliver multiple factors at the same time to enhance the cellular response. Simultaneous delivery of BMP-2 and VEGF promoted osteogenesis and angiogenesis, which presents possible solutions for regeneration of vascularized bone tissue for larger sized defects (74). The second strategy is to deliver multiple factors sequentially to enhance the regenerative process. A sequential release of VEGF and FGF-2, followed by platelet derived growth factor (PDGF), stimulated the migration and proliferation of endothelial cells followed by maturation and vasculature development, leading to a more robust angiogenic response (75). Other temporally controlled factor delivery systems focus on sequential regeneration of multiple tissues, as shown by Yao et al., who utilized initial delivery of an angiogenic drug, deferoxamine (DFO), to prime vasculature formation before release of bone morphogenic protein-2 (BMP-2) for increased osteogenesis (18). The third form of multiple factor delivery is spatially directed delivery, where factors are limited to different portions of the biomaterial so more than one tissue type can be formed in the same graft. These delivery systems have been mainly used in interfacial tissue engineering where the regeneration of various tissue types and their transitional areas are crucial for proper function. For instance, growth factor combination of TGF-β3/BMP-2 and TGF-β1/osteogenic peptide were delivered in isolated locations along the scaffold (76,77) for osteochondral tissue, while BMP-4 and TGF-β3 were delivered in an isolated manner (78) for tendon-bone tissue-tissue constructs.
Release of factors based on external stimuli has also gained attention for possible uses in tissue engineering, drug delivery, and in vivo imaging by precisely targeting certain areas, conditions, and cells. A variety of strategies have been developed in accordance with multiple specific triggering stimuli, such as pH and enzyme activity. pH-specific release of drugs has mainly been developed for chemotherapy solutions to target acidic environments near tumors (79), but some pH-responsive delivery vehicles were also developed for oral medications that target the almost neutral intestinal mucosa pH while avoiding acidic pH of the stomach (80). These pH-responsive materials may be used in tissue engineering applications to deliver biological cues in response to changes in the pH brought on by tissue degeneration or abnormal events. Enzyme-activated delivery of factors utilizes the presence of certain enzymes to trigger the release of the incorporated factors. The exact design differs based on the enzymes that are present in the tissue, but enzyme-activated delivery systems have been designed to target hyaluronidase and MMP activities for cancer therapy (81,82). These designs may be useful for delivering matrix anabolic factors in response to an increase in matrix catabolic events and presence of the associated enzymes/proteins in the defect site.
4.2. Biologics Delivery for Cell Recruitment
In addition to aiding the proliferation and differentiation of progenitor cells, various factors have also been studied for their use in recruiting host cells to the implanted biomaterial. By utilizing endogenous cells, the implanted biomaterial can be cellularized without being cultured in vitro, thereby avoiding exogenous cell culture before implantation. Various factors have been identified to be chemotactic, with some attracting a wide range of progenitor cells while others attract tissue-specific cells. However, the use of chemotactic factors requires the establishment of a chemotactic gradient that the surrounding cells can sense and move towards. To that end, coordination between the loaded chemotactic factor and the biomaterial is required to provide sustained delivery of the factor for the requisite period to establish a gradient that recruits surrounding cells.
One method for establishing the chemotactic gradient is via steady release of the loaded chemotactic factor and relying on diffusion to form the gradient. A recent study adopted this approach to attract MSCs for articular cartilage repair using erythropoietin (EPO) incorporated into a hyaluronic acid hydrogel (83). This study demonstrated an initial burst followed by subsequent release of EPO, which was able to attract MSCs to the injury site. Similarly, Cipitria et al. utilized sustained release of stromal cell-derived factor-1α (SDF-1α), a commonly used chemoattractant for a variety of progenitor cells, to recruit MSCs for bone regeneration purposes (17). In this case, an alginate hydrogel with stiffness tailored for bone tissue engineering, was loaded with SDF-1α and was able to continually release the factor after a period of burst release, which resulted in increased recruitment of endogenous MSCs and bone regeneration. Both studies report that the initial burst release of the factor quickly established the chemotactic gradient, while the following sustained release maintained the gradient throughout the cell recruitment phase. In both of these studies, the implanted biomaterial is able to recruit host cells by establishing a biological cue gradient in the environment. On the other hand, Xu et al. utilized a different approach by establishing a gradient of chemoattractant within the biomaterial itself (84). In this study, semaphorin 3A (Sema3A) was loaded onto polymerized Matrigel in a way that resulted in a concentration gradient. The established gradient of Sema3A, a neural progenitor cell attractant, was maintained for an extended period of time and was able to promote migration of neural progenitor cells deeper into the matrix. Therefore, by utilizing a variety of methods to establish a chemoattractant gradient and by utilizing the proper attractant, specific progenitor cells can be directed towards an acellular implant and allow for more complete regeneration.
Biological cues in the form of growth factors and small drug molecules control and direct cell behavior for the purpose of tissue repair and regeneration. However, the delivery of biological factors rely heavily on the relationship between the biomaterial delivery vehicle and the loaded biological factor, and much thought has gone into the optimization of this relationship. Some of the developed methods include direct loading, covalent bonding, and surface functionalization (Table 1). By these methods, the recruitment and induction of cells have been demonstrated, but further research is required to understand the ideal dosage, rate of release, synergies, release conditions, and other variables.
5. Conclusions and Future Directions
Biomaterials have been established as principal components of tissue engineering along with cells and signaling molecules. Over the last decade, biomaterial research efforts have significantly expanded the ways to introduce physical, chemical, and biological cues. The physical cues – in the form of surface topography, porosity, and material stiffness – are proven to influence cell behavior in terms of adhesion and growth. Chemical cues also affect various aspects of cell behavior, while overall chemical composition of materials can impart degradability and bioactivity to help coordinate regeneration and replacement of the defect with de novo tissue. On the other hand, biomaterials/scaffolds are combined with signaling molecules in a number of ways to influence or control cell behaviors via growth factors or small drug molecules.
There has been significant progress in terms of developing methods/techniques to fabricate biomaterials with various physical, chemical and biological cues; however, efforts are mostly limited to introducing them and studying cell responses in isolation. It is critical that combinations of physical, chemical, and biological cues are studied to establish synergistic effects for tissue regeneration. Therefore, future research efforts may focus on the interplay between the various cues and their synergistic effects on various tissues. Further research may also utilize the cell/tissue-specific responses to these cues to develop more specialized biomaterials and biomaterial structures that are optimized for specific tissue/defect repair and regeneration. Also, in addition to cues delivered by the implanted biomaterials, recent research has also highlighted the efficacy of external cues in the form of electrical or magnetic stimulation on the regeneration of tissues, which opens up another avenue for research and exploration.
Acknowledgements
The authors acknowledge support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (#R01EB030060 & #R01EB020640). Dr. Nukavarapu also acknowledges funding from NSF EFMA (#1640008 & 1908454). The authors thank Drs. Bonin and Hargis for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- 1.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of The Royal Society Interface. 2011. February 6;8(55):153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Cheng N, Miron R, Shi B, Cheng X. Delivery of PDGF-B and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials. 2012. October 1;33(28):6698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García JR, Clark AY, García AJ. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. Journal of Biomedical Materials Research Part A. 2016;104(4):889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeherman H, Wozney J, Li R. Bone Morphogenetic Protein Delivery Systems. Spine. 2002. August 15;27(16S):S16. [DOI] [PubMed] [Google Scholar]
- 5.El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm Res. 2017;34(6):1152–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu Y, Li Q, Ding Y, Dong L, Wang C. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Advanced Drug Delivery Reviews. 2019. June 1;146:190–208. [DOI] [PubMed] [Google Scholar]
- 7.Ren X, Zhao M, Lash B, Martino MM, Julier Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front Bioeng Biotechnol [Internet]. 2020. [cited 2020 Nov 5];7 Available from: https://www.frontiersin.org/articles/10.3389/fbioe.2019.00469/full?utm_source=S-TWT&utm_medium=SNET&utm_campaign=ECO_FBIOE_xrefXX_auto-dlvrit [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon O, Song SJ, Yang HS, Bhang S-H, Kang S-W, Sung MA, et al. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochemical and Biophysical Research Communications. 2008. May 2;369(2):774–80. [DOI] [PubMed] [Google Scholar]
- 9.Kowalczewski CJ, Saul JM. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front Pharmacol [Internet]. 2018. [cited 2020 Nov 5];9 Available from: https://www.frontiersin.org/articles/10.3389/fphar.2018.00513/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang K, Jung H, Lee H-R, Lee JS, Kim SR, Song KY, et al. Multiscale, Hierarchically Patterned Topography for Directing Human Neural Stem Cells into Functional Neurons. ACS Nano. 2014. August 26;8(8):7809–22. [DOI] [PubMed] [Google Scholar]
- 11.Amini AR, Nukavarapu SP. Oxygen-tension controlled matrices for enhanced osteogenic cell survival and performance. Ann Biomed Eng. 2014. June;42(6):1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. 2018. January;10(2):025003. [DOI] [PubMed] [Google Scholar]
- 13.**.Lee J, Jeon O, Kong M, Abdeen AA, Shin J-Y, Lee HN, et al. Combinatorial screening of biochemical and physical signals for phenotypic regulation of stem cell–based cartilage tissue engineering. Science Advances. 2020. May 1;6(21):eaaz5913. [DOI] [PMC free article] [PubMed] [Google Scholar]; Utilized PEG:alginate hydrogel to tune degradation rate and stiffness to study their effects on MSC chondrogenesis. Found that fast degrading/softer hydrogels induced chondrogenesis while slow degrading/stiffer hydrogels induced hypertrophic chondrocytes.
- 14.*.Xu TO, Kim HS, Stahl T, Nukavarapu SP. Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering. Biomed Mater. 2018. March 16;13(3):035013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Developed PLGA:Mg composite scaffolds that was able to neutralize the acidic degradation byproducts of PLGA. Also showed evidence of increased osteogenic differentiation of seeded MC3T3-E1 preosteoblasts. The PLGA:Mg composite also showed increased ability to precipitate calcium apatites when exposed to physiological conditions.
- 15.Pereira RF, Barrias CC, Bártolo PJ, Granja PL. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomaterialia. 2018. January 15;66:282–93. [DOI] [PubMed] [Google Scholar]
- 16.Jaidev LR, Chatterjee K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Materials & Design. 2019. January 5;161:44–54. [Google Scholar]
- 17.Cipitria A, Boettcher K, Schoenhals S, Garske DS, Schmidt-Bleek K, Ellinghaus A, et al. In-situ tissue regeneration through SDF-1α driven cell recruitment and stiffness-mediated bone regeneration in a critical-sized segmental femoral defect. Acta Biomaterialia. 2017. September 15;60:50–63. [DOI] [PubMed] [Google Scholar]
- 18.*.Yao Q, Liu Y, Selvaratnam B, Koodali RT, Sun H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. Journal of Controlled Release. 2018. June 10;279:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]; Loaded BMP-2 into MSNs for sustained release and DFO was incorporated into chitosan for rapid burst release. Initial release of DFO provided angiogenic cues while longer sustained release of BMP-2 encouraged bone regeneration. Synergy between the two growth factors were also seen.
- 19.Lin Z, Wu M, He H, Liang Q, Hu C, Zeng Z, et al. 3D Printing of Mechanically Stable Calcium-Free Alginate-Based Scaffolds with Tunable Surface Charge to Enable Cell Adhesion and Facile Biofunctionalization. Advanced Functional Materials. 2019;29(9):1808439. [Google Scholar]
- 20.Igwe J, Amini A, Mikael P, Laurencin C, Nukavarapu S. Nanostructured Scaffolds for Bone Tissue Engineering In: Zilberman M, editor. Active Implants and Scaffolds for Tissue Regeneration [Internet]. Berlin, Heidelberg: Springer; 2011. [cited 2020 Sep 14]. p. 169–92. (Studies in Mechanobiology, Tissue Engineering and Biomaterials). Available from: 10.1007/8415_2010_60 [DOI] [Google Scholar]
- 21.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000. September 5;51(3):475–83. [DOI] [PubMed] [Google Scholar]
- 22.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nature Reviews Molecular Cell Biology. 2001;2(11):793–805. [DOI] [PubMed] [Google Scholar]
- 23.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Engineering. 2005. January 1;11(1–2):1–18. [DOI] [PubMed] [Google Scholar]
- 24.Faia-Torres AB, Guimond-Lischer S, Rottmar M, Charnley M, Goren T, Maniura-Weber K, et al. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014. November 1;35(33):9023–32. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Guo Y, Liu R, Wu S, Fang J, Huang B, et al. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf B Biointerfaces. 2018. April 1;164:58–69. [DOI] [PubMed] [Google Scholar]
- 26.Tian Y, Liu H, Sheldon BW, Webster TJ, Yang S, Yang H, et al. Surface energy-mediated fibronectin adsorption and osteoblast responses on nanostructured diamond. Journal of Materials Science & Technology. 2019. May 1;35(5):817–23. [Google Scholar]
- 27.Tsui JH, Ostrovsky-Snider NA, Yama DM P, Donohue JD, Seob Choi J, Chavanachat R, et al. Conductive silk-polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. Journal of Materials Chemistry B. 2018;6(44):7185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora S, Lin S, Cheung C, Yim EKF, Toh Y-C. Topography elicits distinct phenotypes and functions in human primary and stem cell derived endothelial cells. Biomaterials. 2020. March 1;234:119747. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Choi KS, Kim D, Kim W, Lee D, Kim H-N, et al. Controlled extracellular topographical and chemical cues for acceleration of neuronal development. Journal of Industrial and Engineering Chemistry. 2018. May 25;61:65–70. [Google Scholar]
- 30.Song L, Wang K, Li Y, Yang Y. Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids and Surfaces B: Biointerfaces. 2016. December 1;148:49–58. [DOI] [PubMed] [Google Scholar]
- 31.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010. January 1;31(3):461–6. [DOI] [PubMed] [Google Scholar]
- 32.Amini AR, Xu TO, Chidambaram RM, Nukavarapu SP. Oxygen Tension-Controlled Matrices with Osteogenic and Vasculogenic Cells for Vascularized Bone Regeneration In Vivo. Tissue Eng Part A. 2016. April;22(7–8):610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz C, Duda GN, Checa S. Towards multi-dynamic mechano-biological optimization of 3D-printed scaffolds to foster bone regeneration. Acta Biomaterialia. 2020. January 1;101:117–27. [DOI] [PubMed] [Google Scholar]
- 34.Wang G-W, Yang H, Wu W-F, Zhang P, Wang J-Y. Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials. 2017. July 1;131:145–59. [DOI] [PubMed] [Google Scholar]
- 35.Oh SH, Kang JG, Kim TH, Namgung U, Song KS, Jeon BH, et al. Enhanced peripheral nerve regeneration through asymmetrically porous nerve guide conduit with nerve growth factor gradient. Journal of Biomedical Materials Research Part A. 2018. January 1;106(1):52–64. [DOI] [PubMed] [Google Scholar]
- 36.Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013. October;31(5):706–21. [DOI] [PubMed] [Google Scholar]
- 37.Nukavarapu SP, Laurencin CT, Amini AR, Dorcemus DL. Gradient Porous Scaffolds [Internet]. US20170239395A1, 2017. [cited 2019 Jan 7]. Available from: https://patents.google.com/patent/US20170239395A1/en [Google Scholar]
- 38.Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, et al. Selective Laser Sintering Scaffold with Hierarchical Architecture and Gradient Composition for Osteochondral Repair in Rabbits. Biomaterials. 2017. August;137:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Huang J, Narayan RJ. Gradient scaffolds for osteochondral tissue engineering and regeneration. Journal of Materials Chemistry B [Internet]. 2020. [cited 2020 Sep 14]; Available from: https://pubs.rsc.org/en/content/articlelanding/2020/tb/d0tb00688b [DOI] [PubMed] [Google Scholar]
- 40.Wang JH-C. Cell Traction Forces (CTFs) and CTF Microscopy Applications in Musculoskeletal Research. Oper Tech Orthop. 2010. June 1;20(2):106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Research & Therapy. 2015. May 27;6(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathieu PS, Loboa EG. Cytoskeletal and Focal Adhesion Influences on Mesenchymal Stem Cell Shape, Mechanical Properties, and Differentiation Down Osteogenic, Adipogenic, and Chondrogenic Pathways. Tissue Engineering Part B: Reviews. 2012. June 28;18(6):436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009. December 1;30(36):6867–78. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Wang G, Luo X, Qiu J, Tang C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns. 2012. May 1;38(3):414–20. [DOI] [PubMed] [Google Scholar]
- 45.Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. MBoC. 2012. July 25;23(18):3731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T, Lin S, Shao X, Shi S, Zhang Q, Xue C, et al. Regulating osteogenesis and adipogenesis in adipose-derived stem cells by controlling underlying substrate stiffness. Journal of Cellular Physiology. 2018;233(4):3418–28. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H, Zhou Y, Lee M-S, Zhang Y, Li W-J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomaterialia. 2016. September 15;42:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan J-X, Xiong L, Zhao K, Zeng P, Wang B, Tang F-L, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Research. 2018. June 1;6(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. Journal of Cellular Biochemistry. 2004;93(6):1210–30. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Liu W, Zhao J, Ma X, Shen L, Zhang Y, et al. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/β-catenin pathway. Biochimica et Biophysica Acta (BBA) - General Subjects. 2016. October 1;1860(10):2211–9. [DOI] [PubMed] [Google Scholar]
- 51.*.Stukel JM, Willits RK. The interplay of peptide affinity and scaffold stiffness on neuronal differentiation of neural stem cells. Biomed Mater. 2018. February;13(2):024102. [DOI] [PubMed] [Google Scholar]; In vitro analysis of neural stem cell differentiation while varying RGD concentration and stiffness of PEGDA hydrogel. Showed that neural differentiation and development was primarily affected by hydrogel stiffness.
- 52.Zaman MH, Matsudaira P, Lauffenburger DA. Understanding effects of matrix protease and matrix organization on directional persistence and translational speed in three-dimensional cell migration. Ann Biomed Eng. 2007. January;35(1):91–100. [DOI] [PubMed] [Google Scholar]
- 53.**.Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. PNAS. 2017. May 30;114(22):5647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; Found evidence of durotaxis on hydrogels fabricated with steeper gradients in gel stiffness while shallower gradients did not show durotaxis. Also analyzed MSC morphology in response to hydrogel stiffness and mechanotransduction pathways of MSCs.
- 54.Ouberai MM, Xu K, Welland ME. Effect of the interplay between protein and surface on the properties of adsorbed protein layers. Biomaterials. 2014. August;35(24):6157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visalakshan RM, MacGregor MN, Sasidharan S, Ghazaryan A, Mierczynska-Vasilev AM, Morsbach S, et al. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses. ACS Appl Mater Interfaces. 2019. August 7;11(31):27615–23. [DOI] [PubMed] [Google Scholar]
- 56.Hotchkiss KM, Clark NM, Olivares-Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials. 2018. November 1;182:202–15. [DOI] [PubMed] [Google Scholar]
- 57.Černochová P, Blahová L, Medalová J, Nečas D, Michlíček M, Kaushik P, et al. Cell type specific adhesion to surfaces functionalised by amine plasma polymers. Scientific Reports. 2020. June 9;10(1):9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishihara K, Mitera K, Inoue Y, Fukazawa K. Effects of molecular interactions at various polymer brush surfaces on fibronectin adsorption induced cell adhesion. Colloids and Surfaces B: Biointerfaces. 2020. October 1;194:111205. [DOI] [PubMed] [Google Scholar]
- 59.Hasan A, Pattanayek SK, Pandey LM. Effect of Functional Groups of Self-Assembled Monolayers on Protein Adsorption and Initial Cell Adhesion. ACS Biomater Sci Eng. 2018. September 10;4(9):3224–33. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro S, Gomes AC, Etxebarria I, Lanceros-Méndez S, Ribeiro C. Electroactive biomaterial surface engineering effects on muscle cells differentiation. Materials Science and Engineering: C. 2018. November 1;92:868–74. [DOI] [PubMed] [Google Scholar]
- 61.Guo S, Kwek MY, Toh ZQ, Pranantyo D, Kang E-T, Loh XJ, et al. Tailoring Polyelectrolyte Architecture To Promote Cell Growth and Inhibit Bacterial Adhesion. ACS Appl Mater Interfaces. 2018. March 7;10(9):7882–91. [DOI] [PubMed] [Google Scholar]
- 62.Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int J Mol Sci [Internet]. 2019. February 1 [cited 2020 Apr 20];20(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6386828/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbosa JN, Madureira P, Barbosa MA, Aguas AP. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J Biomed Mater Res A. 2006. March 15;76(4):737–43. [DOI] [PubMed] [Google Scholar]
- 64.Kamath S, Bhattacharyya D, Padukudru C, Timmons RB, Tang L. Surface chemistry influences implant-mediated host tissue responses. J Biomed Mater Res A. 2008. September;86(3):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikael P, Nukavarapu S. Functionalized Carbon Nanotube Composite Scaffolds for Bone Tissue Engineering: Prospects and Progress. JBT. 2011. June 1;1:76–85. [Google Scholar]
- 66.Laurencin CT, Nukavarapu SP, Kumbar SG. Carbon nanotube composite scaffolds for bone tissue engineering [Internet]. US8614189B2, 2013. [cited 2020 Sep 14]. Available from: https://patents.google.com/patent/US8614189B2/en [Google Scholar]
- 67.Lee SK, Han C-M, Park W, Kim IH, Joung YK, Han DK. Synergistically enhanced osteoconductivity and anti-inflammation of PLGA/β-TCP/Mg(OH)2 composite for orthopedic applications. Materials Science and Engineering: C. 2019. January 1;94:65–75. [DOI] [PubMed] [Google Scholar]
- 68.Gonda Y, Ioku K, Shibata Y, Okuda T, Kawachi G, Kamitakahara M, et al. Stimulatory effect of hydrothermally synthesized biodegradable hydroxyapatite granules on osteogenesis and direct association with osteoclasts. Biomaterials. 2009. September 1;30(26):4390–400. [DOI] [PubMed] [Google Scholar]
- 69.Fukuda C, Akiyama N, Takemoto M, Fujibayashi S, Neo M, Nakamura T. Local Application of Alendronate on β-Tricalcium Phosphate Accelerated Induction of Osteogenesis with Formation of Giant Osteoclast-Like Cell. 2012. April 28 [cited 2020 Jun 30];2012 Available from: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=18936 [Google Scholar]
- 70.Lueckgen A, Garske DS, Ellinghaus A, Mooney DJ, Duda GN, Cipitria A. Enzymatically-degradable alginate hydrogels promote cell spreading and in vivo tissue infiltration. Biomaterials. 2019. October 1;217:119294. [DOI] [PubMed] [Google Scholar]
- 71.Madl CM, Katz LM, Heilshorn SC. Tuning Bulk Hydrogel Degradation by Simultaneous Control of Proteolytic Cleavage Kinetics and Hydrogel Network Architecture. ACS Macro Lett. 2018. November 20;7(11):1302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui W, Liu Q, Yang L, Wang K, Sun T, Ji Y, et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater Sci Eng. 2018. January 8;4(1):211–21. [DOI] [PubMed] [Google Scholar]
- 73.Igwe JC, Mikael PE, Nukavarapu SP. Design, fabrication and in vitro evaluation of a novel polymer-hydrogel hybrid scaffold for bone tissue engineering. J Tissue Eng Regen Med. 2014. February;8(2):131–42. [DOI] [PubMed] [Google Scholar]
- 74.Lv J, Xiu P, Tan J, Jia Z, Cai H, Liu Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: implantation of electron beam melting-fabricated porous Ti 6 Al 4 V scaffolds incorporating growth factor-doped fibrin glue. Biomed Mater. 2015. June;10(3):035013. [DOI] [PubMed] [Google Scholar]
- 75.*.Bai Y, Bai L, Zhou J, Chen H, Zhang L. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cellular Immunology. 2018. January 1;323:19–32. [DOI] [PubMed] [Google Scholar]; Utilized two different drug delivery kinetics to provide VEGF and FGF-2 in the initial stages of tissue engineering to attract and prime surrounding cells for angiogenesis. Then followed up with delayed PDGF delivery to induce angiogenesis and maturation.
- 76.Stüdle C, Vallmajó-Martín Q, Haumer A, Guerrero J, Centola M, Mehrkens A, et al. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomaterials. 2018. July 1;171:219–29. [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Yue H, Huang W, Lin X, Xie X, He Z, et al. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-$\upbeta$1 for osteochondral tissue regeneration. Biofabrication. 2020. March;12(2):025030. [DOI] [PubMed] [Google Scholar]
- 78.Hurley-Novatny AC, Arumugasaamy N, Kimicata M, Baker H, Mikos AG, Fisher JP. Concurrent multi-lineage differentiation of mesenchymal stem cells through spatial presentation of growth factors. Biomed Mater [Internet]. 2020. [cited 2020 Jul 1]; Available from: http://iopscience.iop.org/10.1088/1748-605X/ab9bb0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Seminars in Cancer Biology. 2017. April 1;43:74–89. [DOI] [PubMed] [Google Scholar]
- 80.Ajji Z, Maarouf M, Khattab A, Ghazal H. Synthesis of pH-responsive hydrogel based on PVP grafted with crotonic acid for controlled drug delivery. Radiation Physics and Chemistry. 2020. May 1;170:108612. [Google Scholar]
- 81.Hu C, Cun X, Ruan S, Liu R, Xiao W, Yang X, et al. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018. June 1;168:64–75. [DOI] [PubMed] [Google Scholar]
- 82.Vaghasiya K, Ray E, Sharma A, Katare OP, Verma RK. Matrix metalloproteinase responsive mesoporous silica nanoparticles cloaked with cleavable-protein for “Self-actuating” on-demand controlled drug delivery for cancer therapy. ACS Appl Bio Mater [Internet]. 2020. June 26 [cited 2020 Jul 1]; Available from: 10.1021/acsabm.0c00497 [DOI] [PubMed] [Google Scholar]
- 83.Nair AM, Tsai Y-T, Shah KM, Shen J, Weng H, Zhou J, et al. The effect of erythropoietin on autologous stem cell-mediated bone regeneration. Biomaterials. 2013. October 1;34(30):7364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.*.Xu Z, Wang W, Ren Y, Zhang W, Fang P, Huang L, et al. Regeneration of cortical tissue from brain injury by implantation of defined molecular gradient of semaphorin 3A. Biomaterials. 2018. March 1;157:125–35. [DOI] [PubMed] [Google Scholar]; Encapsulated Sema3A in Matrigel to present a gradient of the neural chemoattractant that was able to attract surrounding neural progenitor cells to the defect site in vivo. The loaded Sema3A also assisted in neurogenesis of the attracted progenitor cells.
- 85.Boffito M, Meglio FD, Mozetic P, Giannitelli SM, Carmagnola I, Castaldo C, et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLOS ONE. 2018. July 6;13(7):e0199896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.**.Flora T, Torre IG de, Alonso M, Rodríguez-Cabello JC. Use of proteolytic sequences with different cleavage kinetics as a way to generate hydrogels with preprogrammed cell-infiltration patterns imparted over their given 3D spatial structure. Biofabrication. 2019. April;11(3):035008. [DOI] [PubMed] [Google Scholar]; Composited two hydrogels with different degradation kinetics to develop a composite that could control cell migration into the scaffold and regeneration. Provides biomaterial model for more precise temporal control over regeneration of the defect.
- 87.**.Zhou M, Lozano N, Wychowaniec JK, Hodgkinson T, Richardson SM, Kostarelos K, et al. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomaterialia. 2019. September 15;96:271–80. [DOI] [PubMed] [Google Scholar]; Adsorbed TGF-β3 onto graphene oxide flakes and incorporated into a collagen 1 hydrogel. The adsorbed growth factor showed minimal release while maintaining conformation and chondrogenic activity for extended periods of time.


