Abstract
Objective Efficient, effective health care requires rapid availability of patient information. We designed, implemented, and assessed the impact of a primary care electronic medical record (EMR) in three rural Kenyan health centers.
Method Local clinicians identified data required for primary care and public health reporting. We designed paper encounter forms to capture these data in adult medicine, pediatric, and antenatal clinics. Encounter form data were hand-entered into a new primary care module in an existing EMR serving onsite clinics serving patients infected with the human immunodeficiency virus (HIV). Before subsequent visits, Summary Reports were printed containing selected patient data with reminders for needed HIV care. We assessed effects on patient flow and provider work with time-motion studies before implementation and two years later, and we surveyed providers’ satisfaction with the EMR.
Results Between September 2008 and December 2011, 72 635 primary care patients were registered and 114 480 encounter forms were completed. During 2011, 32 193 unique patients visited primary care clinics, and encounter forms were completed for all visits. Of 1031 (3.2%) who were HIV-infected, 85% received HIV care. Patient clinic time increased from 37 to 81 min/visit after EMR implementation in one health center and 56 to 106 min/visit in the other. However, outpatient visits to both health centers increased by 85%. Three-quarters of increased time was spent waiting. Despite nearly doubling visits, there was no change in clinical officers’ work patterns, but the nurses’ and the clerks’ patient care time decreased after EMR implementation. Providers were generally satisfied with the EMR but desired additional training.
Conclusions We successfully implemented a primary care EMR in three rural Kenyan health centers. Patient waiting time was dramatically lengthened while the nurses’ and the clerks’ patient care time decreased. Long-term use of EMRs in such settings will require changes in culture and workflow.
INTRODUCTION
Health care is an information business that requires effective and efficient capture, storage, management, and communication of patient data.1 Electronic medical records (EMRs) may increase the quality and efficiency of care in both developed2 and developing countries.3,4 Many of these potential improvements in care come from supporting day-to-day patient management and clinical decisions.5 However, progress towards this goal has been slow, especially in resource-constrained environments.
Capturing coded data during clinical encounters may be easier in developing countries where electronic and even long-term paper records are largely nonexistent and health care is often delivered by nurses and clinical officers, similar to US physicians’ assistants, whose work is amenable to evidence-based guidelines.6 Moreover, ministries of health (MOH) in developing countries often require their care facilities to collect and report a core set of data from patient visits to track expenditures and monitor key outcomes. Therefore, establishing EMRs in developing countries provides an opportunity to define a core dataset specific to the care being delivered to serve local health care providers, to local health systems, and to governmental authorities funding and overseeing them.
In 2001, we established an EMR in a moderate-sized rural health center in Mosoriot, Kenya.7–9 Although similar to prior clinical and research databases deployed in sub-Saharan Africa,10 to our knowledge, this was sub-Saharan Africa’s first true ambulatory EMR. Paper encounter forms mainly had check boxes and fill-in-the-blank fields (See Figure 1 in online Supplementary Data) for clinicians to record physiologic data, diagnoses, and treatments. Clinicians completed these forms while delivering care, and check-out clerks entered the data into the EMR before patients left the health center. Over time, the clinical staff added data elements to the encounter forms that assisted care delivery and clinic management. The data collected were limited to demographic information, reasons for visits, vital signs, family planning (counseling and method), ancillary services used (laboratory, radiology), antenatal care information (last menstrual period, estimated data of delivery, gestational age, etc.), child welfare (immunizations), final diagnoses, treatment, return visits and referrals, and financial information (See Figure 1 in online Supplementary Data). Space was provided for free text notes but was rarely used. After data were entered into the EMR, encounter forms were given to patients for their permanent records. No local paper charts were maintained at Kenyan MOH health centers.
In late 2001, Mosoriot’s EMR was expanded to capture data for HIV clinics established by the Academic Model Providing Access to Healthcare (AMPATH), a collaboration including Moi University School of Medicine, Moi Teaching and Referral Hospital (MTRH), and Indiana University.11,12 The minimalist primary care EMR became the AMPATH Medical Record System (AMRS) and captured extensive information on HIV-infected patients’ demographics, historical and physical findings, health risk behaviors, tests, diagnoses, and treatments.13 Four separate encounter forms were developed for initial and return visits for adult and pediatric patients. The initial form was extensive (six pages), but the follow-up two-page form (printed on both sides of one sheet of paper, see Figure 2 in online Supplementary Data) could be completed in 1–2 min.
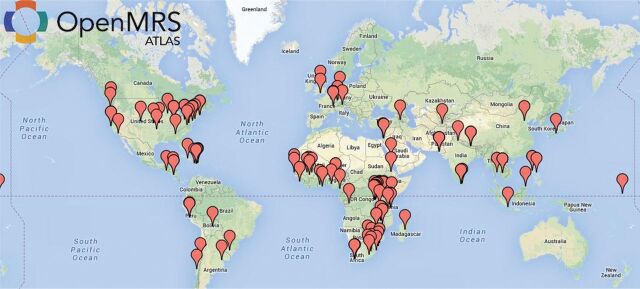
In 2004, the World Health Organization convened African and western HIV experts to develop a core dataset for outpatient HIV care.14 This guided the AMRS’ evolution, significantly expanding the variety and depth of data collected. Due to the increasing size and complexity of the AMRS, its initial MS-Access® platform became inadequate. Consequently, collaborators from the Regenstrief Institute, Partners in Health,15 and the Medical Research Council of South Africa16 created a new open-source EMR platform called OpenMRS that was based on Regenstrief’s EMR model17 and used a Java interface over a MySQL platform.18–20 We successfully installed OpenMRS in all AMPATH sites and six hospitals and health centers in Tanzania and Uganda.21 OpenMRS has attracted a global community of developers and implementers who have implemented it in more than 40 countries (Figure 1).19 OpenMRS is being implemented in all Ministry of Health venues in Kenya as well as in Rwanda, Tanzania, Uganda, Mozambique, Bangladesh, and the Philippines.
Figure 1:
OpenMRS implementation sites.
In 2007, in response to demands from patients and their communities, AMPATH expanded to include patients with chronic noncommunicable diseases including heart disease and its risk factors, pulmonary disease, diabetes, cancer, and mental health.22 Working with clinicians in these specialty areas, we developed and implemented clinic-specific OpenMRS encounter forms and data management modules for each of these specialty clinics.
In 2008, AMPATH decided to further expand its mission and provide primary care in selected health centers with AMPATH clinics. To help coordinate primary and HIV care in these clinics, we developed and implemented a primary care OpenMRS module. In this article, we answer the questions: was the primary care EMR used for all patients and visits, and what was its impact on patient flow, provider work, and user satisfaction in three rural health centers in western Kenya.
METHODS
Study Setting
This project was approved by Indiana University’s Institutional Review Board and Moi University’s Institutional Research Ethics Committee from October 2007 through December 2011 during which time AMPATH grew from 19 clinics and 61 000 enrolled patients to more than 50 clinics and 455 000 enrolled patients. Visits increased from 650 000 in 2007 to 4.9 million in 2011. Three rural health centers within 50 km of AMPATH’s headquarters in Eldoret, Kenya – Mosoriot, Turbo, and Burnt Forest – were selected for this study as were the pediatric and antenatal clinics at MTRH in Eldoret, which have been described previously.23,24 Rural health centers in Kenya provide ambulatory care to defined populations. Clinics are typically run by nurses and clinical officers, similar to US physicians’ assistants. Physicians are rare. Rural health centers provide a wide range of preventive care (e.g., childhood immunizations, family planning) and reproductive health services (e.g., antenatal clinics, labor, and delivery) and treat mostly injuries and acute illnesses (e.g., malaria flares, respiratory infections, and gastroenteritis). Chronic disease care is uncommon.
Mosoriot serves a catchment population of approximately 60 000 persons. Turbo serves a catchment population of 72 500 while Burnt forest serves a catchment population of more than 170 000. During this study, due to increasing patient visits, both Mosoriot and Burnt Forest were upgraded to sub-district hospitals. All three health centers contained AMPATH HIV clinics that used the AMRS, but only Mosoriot had previously captured any primary care data electronically.7–9
Defining the Core Dataset for Primary Care
The scope of data collected balanced the information needs of health center providers, care managers, governmental regulators, and researchers against clinician time required to record those data. Therefore, we first defined the purposes the data would serve: (1) reporting clinic activity to Kenya’s MOH; (2) supporting clinicians delivering primary care; (3) aiding health center management; and (4) facilitating quality improvement and research.
The MOH had the most direct impact on the data collected. It employed all clinic personnel and had established data reporting requirements for MOH facilities nationwide. Previously, providers entered visit data into log books located in the check-in office; the adult medicine, pediatric, antenatal, and family planning clinics; and in the laboratory, pharmacy, and financial office. Each visit had a unique number that reset to one each year; there were no unique patient identifiers. Returning patients were issued a new visit number. Consequently, patient data could not be aggregated across multiple visits. No local paper charts were maintained. Every month, clinic personnel collected the logbooks and hand-entered counts of visits to each clinic, diagnoses, vaccinations, tests performed, drugs dispensed, etc. onto standard MOH report forms.
For the primary care module, we adopted AMPATH’s unique patient identification number.20 Identifiers include patient’s name – first (Christian), middle (Kenyan), and last (tribal) – mother’s first name, gender, date of birth, and whether the birthdate was known or estimated. Once registered, each patient was given a laminated card that included his or her name and identification number (Figure 2), which used a check digit to assure typing accuracy.25
Figure 2:

AMRS identification card (front and back).
Designing the Encounter Forms
We met separately with clinicians in each clinic – pediatric, adult medicine, and antenatal – to review the MOH reports and other information the clinicians desired for patient management. Initially, we intended to design separate forms for each clinic, i.e., pediatric under 5 years, pediatric over 5 years, adult medicine, antenatal, family planning, sexually transmitted infections, etc. However, except for the antenatal clinic, the information required for each clinic was similar and could be recorded on one two-sided form similar to those AMPATH clinicians had completed millions of times and found easy to navigate.20 We created a separate form for the antenatal clinics which had unique and extensive data needs. We iteratively tested and revised each form with clinicians and staff. We also vetted the forms with AMRS data technicians to maximize ease of data entry and minimize errors. The resulting primary care and antenatal encounter forms are shown in Figures 3 and 4 in the online Supplementary Data.
Clinic Workflow
When an unregistered patient presented to a health center’s check-in window, the clerk recorded registration data on the appropriate encounter form and printed and laminated an AMRS identification card. The clerk recorded the clinic(s) the patient would visit that day, who referred the patient, and the presenting problem(s). The nurse recorded vital signs and handed the encounter form to the patient to carry to the appropriate clinic(s).
Clinic personnel recorded observations and care provided in the form’s appropriate boxes. Clinicians could also write supplemental notes by hand in a comment box. Laboratory technicians entered test results. At the bottom of page two, the clinicians recorded the visit diagnoses, drugs given or prescribed, referrals, and return appointments.
The patient then carried the encounter form to the pharmacy to receive prescribed drugs, after which the pharmacist checked the form’s “Picked Up” box. For unavailable drugs, they checked the “Out of Stock” box and instructed the patient to return later. Next, the patient visited the financial officer who recorded charges and payments on the form. Finally, the patient visited the check-out window and received return appointment and referral slips, if indicated. Completed encounter forms were placed in a box where two data entry clerks typed the information into the AMRS. A Quality Management Clerk reviewed a random 5% of the forms for data entry errors. Once the data from the form had been entered into the AMRS, which typically took 1–2 days to a week, the form was placed in a box for placement in the patient’s paper chart which, to simplify storage and retrieval, was stored in a bin labeled with the last two digits of the ID number.
Previously registered patients would present their AMRS ID cards to the check-in clerk who would put the name and ID number on a blank encounter form. For patients forgetting or losing their ID card, clerks searched the AMRS computer. After check-in, the clerk printed a Summary Report of prior AMRS data (Figure 5 in the online Supplementary Data) and clipped it to the encounter form, which was handed to the patient who then proceeded to appropriate clinics and offices. We originally planned to have patients carry their paper charts to clinics so prior encounter forms would be available. However, providers were concerned about workflow and confidentiality, and because they found Summary Reports so useful, the charts remained in the chart room unless clinicians requested them.
Generating Reports
Each month, a clerk generated the MOH reports for the previous month via the AMRS’ reporting utility. These reports, which simulated the MOH’s paper reporting form previously completed by hand, were generated when all encounter forms from the previous month had been entered into the AMRS. The AMRS also contained a search program with which providers could display patient data in tabular form, which could be printed if desired.
As mentioned above, at check-in a clerk generated a Summary Report for each returning patient (Figure 5 in the online Supplementary Data) that contained identifying information, pregnancy status, summary problem list, and medications. Also included were the most recent encounters with date, primary diagnosis, and drugs/vaccines prescribed. In addition, there were listed prior vital signs and test results, and, for HIV patients, reminders for indicated tests and treatments, which have increased AMPATH clinicians’ adherence to care guidelines.26
Evaluation
We performed time-motion studies of both patients and clinicians in Burnt Forest and Turbo using the same methods we had previously used in Mosoriot9 and two sites in Uganda.27,28 We did not perform a time-motion study in Mosoriot because we had replaced their existing EMR. The pre-EMR time-motion study was performed January–March 2008. The post-EMR time-motion study was performed January–March 2010. A research assistant met the first patient entering the health center each day, explained the study, and asked if he could follow the patient. Using Android® smartphones, research assistants silently followed consenting patients, noting and time stamping each new activity from a pre-established list. No patient identifiers were stored. When the patient left the clinic, that time was noted. The research assistant then approached the next patient entering the health center. This study continued for 2 months or until a minimum of 200 patients had been included from each health center during the pre- and post-EMR implementation phases of the time-motion study.
We summed patient activities into seven categories: registration/check-in, with clinicians, in the laboratory, in the pharmacy, other care-related activities, other noncare related activities (including personal activities), and waiting. We calculated the total time in each of these categories for each visit and used Wilcoxon nonparametric tests to assess differences before and after EMR implementation, accepting a two-tailed P < 0.05 as significant. Because health care delivery and EMR implementation and use differed between venues, we performed separate analyses for Burnt Forest and Turbo.
We also performed time-motion studies of providers at Burnt Forest and Turbo. The research assistant met the provider as he or she entered the facility and followed him/her for the entire day. We studied all clinical officers, nurses, and clerks and followed each provider for at least 2 days, more for providers working shorter workdays. The number of each type of provider was small; we maintained anonymity by recording the provider’s type but not name. There were seven categories of provider activities: administration, direct patient care, indirect patient care (e.g., completing paperwork), total patient care (combination of direct and indirect), noncare related activities, personal activities, and waiting. Because the number of hours worked per day varied greatly both within each type of provider and between provider types, we calculated the time for each work category as a percent of their working day. As with patients, we assessed differences between before and after EMR implementation with nonparametric Wilcoxon tests to accommodate skewed data.
Finally, using a survey instrument we developed and used for EMR implementations in Uganda,27,28 we assessed EMR users’ familiarity with computers (scored via a Likert scale from 1 = never to 4 = occasional to 7 = expert), frequency of use of the components of the primary care module (scored from 1 = never to 4 = sometimes to 7 = several times per day), and their satisfaction with the AMRS, encounter forms, and Summary Reports (from 1 = never to 4 = it varies to 7 = always). In December of 2011, we administered the questionnaire to providers at Turbo and Burnt Forest clinics. We also administered the survey to providers in the pediatric and MTRH’s antenatal clinics where the primary care module was also implemented at the same time but whose EMR use data have been reported elsewhere.23,24 Because the number of some types of providers was small, we maintained anonymity by not recording venue. Hence, we report user satisfaction data for Turbo, Burnt Forest, Mosoriot, and MTRH’s pediatric and antenatal clinics.
RESULTS
The AMRS Primary Care Module was successfully implemented at all three rural health centers in September 2008. Because MOH reporting data were captured only on the encounter forms, once implemented at a health center, the primary care encounter forms were used for all patient visits without exception. Table 1 shows the number of patients registered and visits made to each site from September 2008 through December 2011. The 72 635 primary care patients registered made 114 480 visits. The number of patients visiting the three health centers increased substantially during the study: from 404/month in the first 6 months to 749/month in the last 6 months, an 85% increase. Encounter form data were usually entered into the AMRS the same day as a visit or the next day, but almost always within a week. However, two or three times a year the data entry clerks got behind due to holidays, illness, leave, etc. When such backlogs occurred, additional clinic personnel were assigned to catch up.
Table 1:
Primary Care Patients Registered and Encounter Forms Completed from September 2008 through December 2011
| Study Health Centers | Unique Patients Registered | Visits Made from September 2008 through December 2011 |
|---|---|---|
| Mosoriot Rural Health Center | 33 795 | 52 040 |
| Burnt Forest Rural Health Center | 16 213 | 31 214 |
| Turbo Rural Health Center | 22 627 | 31 226 |
| Total, All Health Centers | 72 635 | 114 480 |
Because one goal of the primary care module was to coordinate care between the primary care clinic and the HIV clinic at each site, for the last year studied (2011), we assessed the number of patients using both primary care and HIV clinics at each site (Table 2). Only 3% of the registered primary care patients had HIV clinic visits. However, 85% of the HIV-infected patients visiting primary care also visited an HIV clinic, and almost 10% of HIV clinic patients also had primary care visits in 2011, providing opportunities for enhancing care coordination.
Table 2:
Patients Visiting Study AMPATH Primary Care and HIV/AIDS Clinics in 2011
| Primary Care Patients | HIV/AIDS Clinic Patients | ||||
|---|---|---|---|---|---|
| Study Health Centers | Total Unique Patients Seen | Those Seen Who Were HIV+, n (%) | HIV+ Patients Visiting an HIV Clinic, n (%) | Total Unique Patients Seen | Visiting a Primary Care Clinic, n (%) |
| Mosoriot Rural Health Center | 12 286 | 373 (3.0) | 299 (80) | 3050 | 299 (9.8) |
| Burnt Forest Rural Health Center | 8586 | 202 (2.3) | 170 (84) | 2054 | 170 (8.3) |
| Turbo Rural Health Center | 11 321 | 456 (4.0) | 408 (89) | 4196 | 408 (9.7) |
| Total: All Health Centers | 32 193 | 1031 (3.2) | 949 (85) | 9300 | 877 (9.4) |
Results for patient time-motion assessments are shown in Tables 3–5. At Burnt Forest, all patient times were significantly longer after EMR implementation except for time with the clinician. Overall time in clinic increased by 44 min (118%), with the majority (32 min) spent waiting. In Turbo, all times were significantly greater after EMR implementation except for time in the lab and pharmacy. Overall time in Turbo increased by 50 min (89%), again with the majority of the difference spent waiting (40 min).
Table 3:
Time-Motion Study of Burnt Forest and Turbo Patients
| Burnt Forest Patients | Turbo Patients | |||||
|---|---|---|---|---|---|---|
| Activity Category | Pre-EMR (n = 205)a | Post-EMR (n = 297)a | P-value | Pre-EMR (n = 260)a | Post-EMR (n = 191)a | P-value |
| Registration | 0 (0) | 1.7 (2.7) | <.0001 | 0.3 (0.9) | 1.7 (2.1) | <.0001 |
| Time with clinician | 6.6 (8.3) | 4.4 (6.0) | <.0001 | 5.4 (7.1) | 7.9 (5.9) | <.0001 |
| Time in lab | 0 (0) | 0 (1.4) | .0019 | 0 (0.2) | 0 (0) | .5519 |
| Time in pharmacy | 0.4 (1.8) | 2.2 (2.2) | <.0001 | 1.3 (2.7) | 0.8 (2.0) | <.0001 |
| Other care related activities | 2.7 (6.3) | 3.7 (4.3) | <.0001 | 2.6 (6.3) | 3.4 (6.9) | .0001 |
| Other non-care related activities | 0.4 (3.8) | 9.2 (30) | <.0001 | 2.2 (8.2) | 6.6 (30) | <.0001 |
| Waiting | 22 (35) | 54 (47) | <.0001 | 42 (49) | 82 (104) | <.0001 |
| Total time in clinic | 37 (44) | 81 (61) | <.0001 | 56 (63) | 106 (122) | <.0001 |
aMinutes: median (inter-quartile range). Columns do not add up to 100 due to skewness of the data.
Results for time-motion studies of Burnt Forest and Turbo providers are presented separately for clinical officers, nurses, and clerks. As shown in Table 4 for Burnt Forest, there were no differences in pre- vs post-EMR implementation activities for clinical officers, although there was a trend towards spending more time in direct patient care and waiting and less time in noncare related activities. Burnt Forest nurses spent 80% less time in patient care post-EMR implementation, with a concomitant 13-fold increase in personal time (P < .01 for both). Clerks trended towards spending more time post-EMR in patient care activities, with significantly less time in indirect care. However, the patient visits increased substantially and the number of each provider type more than doubled between pre- and post-EMR implementation. Such staffing changes undoubtedly effected provider work.
Table 4:
Time-Motion Study of Burnt Forest Providers
| Clinical Officers | Nurses | Clerks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity Category | Pre-EMR (n = 3)a | Post-EMR (n = 13)a | P-value | Pre-EMR (n = 9)a | Post-EMR (n = 17)a | P-value | Pre-EMR (n = 5)a | Post-EMR (n = 18)a | P-value |
| Administrative | 2 (4) | 1 (4) | 0.7915 | 2 (3) | 0 (1) | 0.0558 | 5 (2) | 6 (12) | 0.6328 |
| Total patient care | 26 (5) | 34 (9) | 0.2447 | 57 (27) | 13 (10) | 0.0015 | 30 (12) | 41 (8) | 0.0609 |
| Direct patient care | 13 (3) | 20 (6) | 0.1596 | 20 (12) | 7 (7) | 0.0700 | 11 (4) | 19 (4) | 0.0705 |
| Indirect patient care | 11 (6) | 12 (7) | 0.4322 | 15 (13) | 3 (5) | 0.0044 | 14 (3) | 21 (8) | 0.0148 |
| Noncare activities | 48 (31) | 37 (12) | 0.1596 | 34 (15) | 43 (28) | 0.0872 | 37 (19) | 26 (15) | 0.3252 |
| Personal | 26 (33) | 23 (7) | 0.7915 | 2 (6) | 26 (14) | 0.0010 | 25 (7) | 19 (9) | 0.2915 |
| Waiting | 0 (12) | 7 (4) | 0.4322 | 22 (11) | 11 (16) | 0.2149 | 5 (4) | 4 (3) | 0.8539 |
aPercent of workday: median (inter-quartile range). EMR = electronic medical record. Columns do not add up to 100 due to skewness of the data.
Time-motion study results for Turbo clinicians are shown in Table 5 below. Turbo clinical officers spent significantly more time per day waiting after EMR implementation, but otherwise there were no significant workday differences. Nurses in Turbo spent 60% less time after EMR implementation in direct and indirect patient care (P > .01 for both) and 50% more time in personal activities (P = .02). Turbo clerks spent 25% and 67% more of their workday after EMR implementation in direct patient care and personal activities, respectively (P = .02). Again, visits increased and providers more than doubled after EMR implementation.
Table 5:
Time-Motion Study of Turbo Providers
| Clinical Officers | Nurses | Clerks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity Category | Pre-EMR (n = 7)a | Post-EMR (n = 23)a | P-value | Pre-EMR (n = 7)a | Post-EMR (n = 15)a | P-value | Pre-EMR (n = 6)a | Post-EMR (n = 17)a | P-value |
| Administrative | 0 (0) | 1 (5) | .0538 | 0 (2) | 0 (1) | .8889 | 11 (15) | 7 (11) | .3210 |
| Total patient care | 32 (28) | 41 (12) | .1803 | 45 (20) | 25 (23) | .0141 | 42 (8) | 45 (5) | .3548 |
| Direct patient care | 15 (11) | 21 (8) | .3592 | 20 (27) | 8 (5) | .0054 | 16 (4) | 20 (3) | .0245 |
| Indirect patient care | 13 (7) | 12 (7) | .8841 | 11 (4) | 4 (5) | .0046 | 18 (5) | 21 (4) | .4293 |
| Non-care activities | 30 (26) | 33 (12) | .8841 | 39 (21) | 46 (33) | .1199 | 34 (14) | 30 (11) | .3909 |
| Personal | 22 (33) | 19 (9) | 0.4391 | 15 (10) | 23 (11) | .0259 | 6 (12) | 18 (7) | .0245 |
| Waiting | 2 (3) | 7 (5) | 0.0091 | 13 (17) | 12 (14) | .6766 | 6 (10) | 5 (3) | .3210 |
aPercent of workday: median (inter-quartile range). EMR = electronic medical record. Columns do not add up to 100 due to skewness of the data.
We sent surveys to 74 providers at Turbo, Burnt Forest, and MTRH’s pediatric and antenatal clinics. Of 50 anonymous responses received (68%), 35 (70%) were women. Respondents included 15 records officers, 14 nursing officers, 8 clinical officers, 5 data assistants, 3 physicians, 2 administrators, 1 laboratory technician, 1 nutritionist, 1 pharmacy technician. Survey results for all providers are shown in Figure 4. Respondents reported a wide range of familiarity with computers. They used the encounter forms frequently but varied considerably in their use of the patient Summary Reports and seldom sought additional patient information from the EMR. They generally found the AMRS both useful and reliable with a positive impact on patient care, although there were dissenters. Opinions varied concerning its effects on workflow, with no consensus on the adequacy of training. Most agreed that more training would be helpful. Overall, satisfaction with the AMRS was fairly high, with 60% of respondents scoring it 6–7 on the 7-point scale. Yet almost a quarter of respondents rated it 1–3.
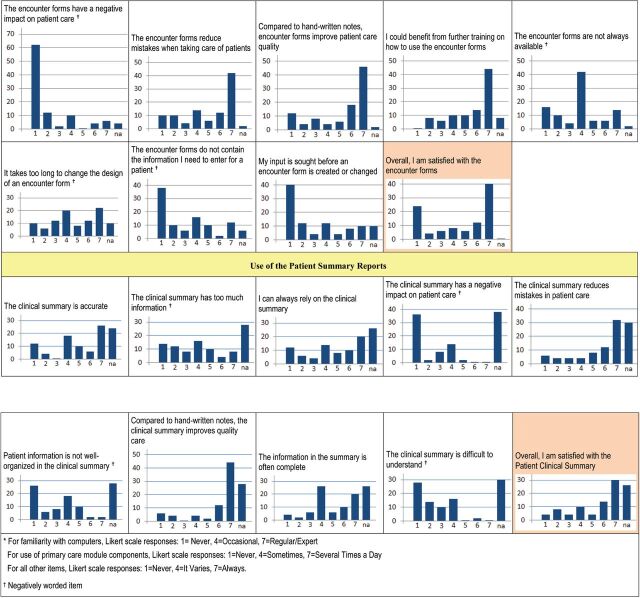
Figure 4:
Results of the primary care module user satisfaction survey.
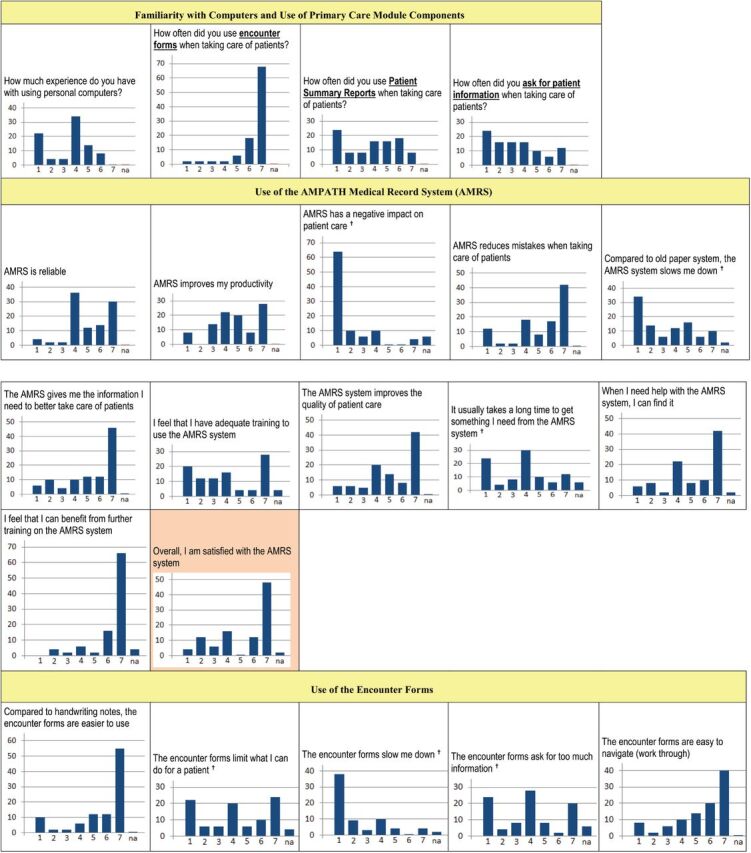
Figure 3:
AMPATH clinic locations at the beginning of this study.
There were divergent opinions regarding the encounter forms, with half giving them an overall rating of 6 or 7; yet a quarter gave an overall rating of 1. Most respondents found the encounter forms easy to use and did not slow them down. Opinions varied on whether they required too much information. Almost two-thirds of respondents felt that the encounter forms increased the quality of care (scores 6–7). There was a wide range of opinions on the encounter forms’ availability, and most respondents agreed that more training would be helpful.
There was less satisfaction with the Summary Reports, only 44% gave them an overall rating of 6–7. Respondents generally felt the summaries improved care but varied on whether the summary information was complete and easy to understand. Respondents also differed widely on their reliance on the summaries for patient care.
DISCUSSION
We successfully designed and implemented a comprehensive primary care EMR in three rural Kenyan health centers that captured information for clinical care, health center management, and MOH reporting. We collaborated with the Kenyan clinicians to design their EMR system with encounter forms that were designed by them rather than imposing a foreign system on them. This design process took many months and required many rounds of developing and field-testing the encounter forms. It eventually yielded forms that the clinicians felt were easily navigated and enhanced data entry through the use of tick boxes and single word/number entries. Very little writing was required.
However, the time-motion studies showed that patient time in the health centers increased dramatically after EMR implementation. This could have been due to the failure of the health centers to adapt their workflow to the new information system, especially in light of the increasing demand for clinical services. The number of patient visits per month increased dramatically between the pre- and post-EMR periods, as did the number of health center staff. Yet the facilities did not expand their physical footprint, which could have led to crowding and disruption of patient flow as evidenced by the increase in patient waiting time at both health centers. It is unclear from our before-and-after time-motion study whether increased traffic or the EMR contributed to the increase in patient time. The EMR had limited effects on physician work but may have reduced the time nurses and clerks spent in patient care activities which both declined (and personal time increased) despite increasing patient visits. However, these differences could have been due to increased numbers of providers post-EMR. Regardless, because health care is an information business,1 implementing EMRs can be expected to change health care delivery, and implementers must anticipate and ameliorate adverse effects on workflow.
The greatest barrier to creating and implementing the primary care module and was the rural health centers’ existing patterns of care. There were no prior patient charts due to the dearth of chronic disease management that would have required longitudinal data. Also, most clinicians found the patient summaries adequate for providing past visit information. Clinicians neither wanted nor needed paper charts, and because staffing issues resulted in piles of data-entered but unfiled encounter forms, we eliminated the charts and stored all completed encounter forms chronologically in a single box. It remains to be seen whether avoiding charts and relying on patient summaries will suffice if, as expected, the prevalence of non-communicable, chronic conditions needing care increases in sub-Saharan Africa.29,30
This study had important limitations. We studied a single health system (AMPATH) in one East African country. Results may not generalize to other venues. There were dramatic increases in patient visits and providers in the study clinics during the study, which confounded the time-motion study results. We also made no attempt to assess the impact of the EMR on the quality of care. Nevertheless, the primary care module was successfully implemented in heavily used clinics in a resource-constrained environment and is an available tool for capturing clinical data as the size and scope of their health care delivery evolves.
Effective and efficient patient care management requires information. Improving information capture and flow should allow low-resource countries to deliver the most care and realize the best outcomes possible for the restricted funds available for health care. EMRs can enhance the timely capture and use of key medical data by providers and health system managers. Much additional research and development is needed before EMRs can be most useful, fully implemented into developing countries’ health care settings, and used to manage and improve patient care.
This study was funded by the US Centers for Disease Control and Prevention grant number R18 HK000058.
COMPETING INTERESTS
None.
CONTRIBUTORS
All of the authors provided substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; approved of the final version, and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplementary Material
ACKNOWLEDGEMENTS
The opinions expressed herein are those of the authors and do not necessarily reflect those of the authors’ institutions or funding agency. We wish to thank the clinicians and staff at the Kenyan rural health centers who were so supportive of this work. We also thank the staff of the Academic Model Providing Access to Healthcare Research Program for their unflagging support.
SUPPLEMENTARY MATERIAL
Supplementary Data is available online at http://jamia.oxfordjournals.org/.
REFERENCES
- 1. Berwick DM. Escape Fire: Lessons for the Future of Health. New York: The Commonwealth Fund; 2002. [Google Scholar]
- 2. Buntin MB Jain SH Blumenthal D. Health information technology: laying the infrastructure for national health reform. Health Aff. 2010;29:1214–1219. [DOI] [PubMed] [Google Scholar]
- 3. Tierney WM Kanter AS Fraser HSF et al. A toolkit for e-health partnerships in low-income nations. Health Aff. 2010;29:272–277. [DOI] [PubMed] [Google Scholar]
- 4. Oluoch T Katana A Ssempijja V et al. Electronic medical record systems are associated with appropriate placement of HIV patients on antiretroviral therapy in rural health facilities in Kenya: a retrospective pre-post study. JAMIA. 2014;21:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhry B Wang J Wu S et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742–752. [DOI] [PubMed] [Google Scholar]
- 6. Wools-Kaloustian K Kimaiyo S Diero L et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20:41–48. [DOI] [PubMed] [Google Scholar]
- 7. Hannan TJ Rotich JK Odero WW et al. The Mosoriot medical record system: design and initial implementation of an outpatient electronic record system in rural Kenya. Int J Med Informat. 2000;60:21–28. [DOI] [PubMed] [Google Scholar]
- 8. Tierney WM Rotich JK Smith FE Bii J et al. Crossing the “digital divide:” Implementing an electronic medical record system in a rural Kenyan health center to support clinical care and research. AMIA Annu Symp Proc. 2002:792–795. [PMC free article] [PubMed] [Google Scholar]
- 9. Rotich JK Hannan TJ Smith FE et al. Installing and implementing a computer-based patient record system in sub-Saharan Africa: the Mosoriot Medical Record System. JAMIA. 2003;10:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanter AS Spencer DC Steinberg MH. Development of an HIV clinical and research database for South Africa. Methods Inf Med. 1997;36:144–148. [PubMed] [Google Scholar]
- 11. Einterz RM Kimaiyo S Mengech HNK et al. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82:812–818. [DOI] [PubMed] [Google Scholar]
- 12. Quigley F. Walking Together, Walking Far: How a U.S. and African Medical School Partnership is Winning the Fight Against HIV/AIDS. Bloomington, IN: Indiana University Press; 2009. [Google Scholar]
- 13. Siika AM Rotich JK Simiyu CJ et al. An electronic medical record system for ambulatory care of HIV-infected patients in Kenya. Int J Med Informat. 2005;74:345–355. [DOI] [PubMed] [Google Scholar]
- 14. Tierney WM Beck EJ Gardner RM et al. Viewpoint: a pragmatic approach to constructing a minimum data set for care of patients with HIV in developing countries. JAMIA. 2006;13(3):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen C Jazayeri D Miranda J et al. Experience in implementing the OpenMRS medical record system to support HIV treatment in Rwanda. Stud Health Technol Inform. 2007;129:382–386. [PubMed] [Google Scholar]
- 16. Seebregts CJ Mamlin BW Biondich PG et al. The OpenMRS Implementers Network. Int J Med Inform. 2009;78:711–720. [DOI] [PubMed] [Google Scholar]
- 17. McDonald CJ Overhage JM Tierney WM et al. The Regenstrief Medical Record System: A quarter century experience. Int J Med Informat. 1999;54:225–253. [DOI] [PubMed] [Google Scholar]
- 18. Mamlin BW Biondich PG Wolfe BA et al. Cooking up an open-source EMR for developing countries: a recipe for successful collaboration. Proc AMIA Symp. 2006;529–533. [PMC free article] [PubMed] [Google Scholar]
- 19. OpenMRS. http://openmrs.org. Accessed October 30, 2014.
- 20. Tierney WM Rotich JK Hannan TJ et al. The AMPATH Medical Record System: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform. 2007;129(Pt 1):372–376. [PubMed] [Google Scholar]
- 21. Tierney WM Achieng M Baker E et al. for the Tanzania-Uganda OpenMRS Consortium. Experience implementing electronic health records in three East African countries. Stud Health Technol Inform. 2010;160:371–375. [PubMed] [Google Scholar]
- 22. Inui TS Sidle JE Nyandiko WM et al. “Triangulating” AMPATH: demonstration of a multi-perspective strategic program evaluation method. SAHARA J. 2009;6:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chemwolo B Caloia D Wools-Kaloustian K et al. Experience implementing electronic health records in the antenatal clinic of a teaching and referral hospital in Kenya. Stud Health Technol Inform. 2013;192:1126. [PubMed] [Google Scholar]
- 24. Gray A Henshaw C Wright J et al. Effect of EMR implementation on clinic time, patient and staff satisfaction, and chart completeness in a resource-limited antenatal clinic in Kenya. Stud Health Technol Inform. 2013;192:1223. [PubMed] [Google Scholar]
- 25. Board of Directors of the American Medical Informatics Association. Standards for Medical Identifiers, Codes, and Messages Needed to Create an Efficient Computer-Stored Medical Record. JAMIA. 1994;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Were MC Nyandiko WM Huang KTL et al. Computer-generated reminders improve quality of HIV care for pediatric patients in a resource-limited setting. Pediatrics. 2013;131:e789–e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Were MC Sutherland JM Bwana M et al. Patterns of care in two HIV continuity clinics in Uganda, Africa: a time-motion study. AIDS Care. 2008;20:677–682. [DOI] [PubMed] [Google Scholar]
- 28. Were MC Shen C Bwana M et al. Creation and evaluation of EMR-based summaries to support HIV care in Uganda, Africa. Int J Med Inform. 2010;79:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization. A comprehensive global monitoring framework, including indicators, and a set of voluntary targets for the prevention and control of non-communicable diseases. http://www.who.int/nmh/events/2012/discussion_paper3.pdf. Accessed November 20, 2014. [Google Scholar]
- 30. Murray CJL Lopez AD, eds. The Global Burden of Disease: a Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge: Harvard University Press on behalf of the World Health Organization and the World Bank; 1996: 23–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.