Abstract
The insular cortex is still one of the least understood cortical regions in the human brain. This review will highlight research on taste quality representation within the human insular cortex. Much of the controversy surrounding this topic is based in the ongoing debate over different theories of peripheral taste coding. When translated to the study of gustatory cortex, this has generated a distinct set of theoretical models, namely the topographic (or ‘gustotopic’) and population coding models of taste organization. Recent investigations into this topic have employed high-resolution functional neuroimaging methods and multivariate analytic approaches to examine taste quality coding in the human brain. Collectively, these recent studies do not support the topographic model of taste quality representation, but rather one where taste quality is represented by distributed patterns of activation within gustatory regions of the insula.
Keywords: taste, Insula, fMRI, MVPA
Graphical Abstract
Introduction
Understanding how basic sensory data are represented at the neural level is one of the fundamental goals of neuroscience. For the sense of taste, chemical compounds corresponding to the five basic taste qualities – sweet, salty, sour, bitter, and umami – are detected by specialized receptors embedded in cells on the surface of the tongue, which relay this information up through the central nervous system mainly through the branches of the chorda typani (cranial nerve VII) and glossopharyngeal (CN XI) nerves (for a recent review see (1)). The first cortical destination for taste signals is an area known as the insular cortex, which is hidden underneath the sylvian fissure and surrounded by the fronto-parietal and temporal opercula. The insula’s role as primary gustatory cortex is well established in humans and non-human animal models (for reviews see (2, 3)), but unlike other primary sensory cortices, the nature of taste representation in the insula is relatively less understood. Also, unlike other primary sensory cortical areas, the insular cortex exhibits consistent involvement in a wide variety of cognitive tasks beyond gustation (4) including emotion (5) and interoception (6), the perception of visceral sensations from the body. Based on the successes within other sensory domains, somatotopy for the sense of touch, retinotopy for vision, and tonotopy for hearing, the topographic model has become a popular default hypothesis for how sensory data are represented at the cortical level. This review will focus on evaluating the topographic model of taste quality representation, focusing specifically on recent human neuroimaging studies.
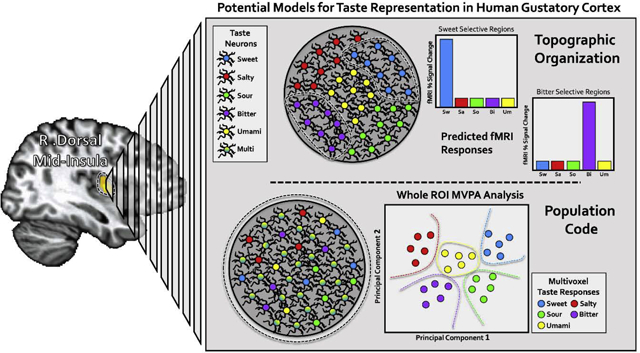
Models for taste quality representation in gustatory cortex
The approach to this problem of cortical representation has been very much shaped by competing views about peripheral taste coding. This ongoing debate has been covered in great detail (for a recent review, see (7)). Briefly, some studies have supported a labelled-line model, wherein receptor cells and peripheral neurons are tuned to specific tastes (8), and others support an across-fiber model, wherein tastes are represented by a complex combinatorial code across specifically and broadly-tuned peripheral neurons (9). At the cortical level, these competing views roughly translate to two general models for taste quality representation, one a topographic model, in which distinct tastes activate distinct cortical locations in gustatory cortex, composed of neurons specifically-tuned to a particular taste (i.e. a gustotopic organization) (10). In contrast, the population coding model posits that taste is encoded in the ensemble firing patterns of mixed populations of broadly-tuned and quality-specific neurons within gustatory cortex, with no particular spatial organization, much like olfactory representations in the piriform cortex (11).
The strongest support for the topographic model comes from a rodent study which used 2-photon calcium imaging to identify discrete regions within gustatory cortex uniquely responsive to distinct tastes (10). However, other similar studies in rodent have identified much more widespread overlapping activations to basic tastes (12), including two more recent rodent studies, also using 2-photon calcium imaging, that have identified overlapping taste responses among populations of narrowly and broadly-tuned neurons, with no apparent spatial organization (13, 14). Though not clustered topographically, the basic tastes were clearly separable by the aggregate population response, suggesting the basis for a spatial population code (for a detailed discussion of these rodent studies, see (15)). Primate studies have echoed these latter findings, as most taste-responsive neurons in macaque gustatory cortex appear to be broadly tuned to multiple tastes, with no observable topographic organization for specific tastes (16).
Evidence from human imaging studies
Neuroscientific investigation of taste has been an area of active research since the earliest days of human functional neuroimaging (17). However, the handful of studies which examined taste quality representation in the insula relied on relativity low-resolution functional magnetic resonance imaging (fMRI) (~64mm3 voxel sizes) and small sample sizes (18, 19). One of these early studies (18), identified both substantial overlap of taste qualities within the insula, as well as substantial variability across participants in the location of clusters responding to specific tastes. This finding suggested that, while distinct tastes may be organized topographically, this representation may not be uniform across individuals. If so, this individual topography would not be observable when aggregating results at the group level but may be observed in individuals measured across multiple visits.
Recent advances in the study of cortical taste representation
The past several years has brought the increasing availability of high-field-strength MRI technology, as well as multi-band imaging and slice-acceleration techniques, which together have allowed for higher spatial and temporal resolution MR-imaging as well as generally higher signal-to-noise ratio. Bolstered by this advance in technology, over the last two years, multiple fMRI studies have re-examined the question of taste quality representation within the insular cortex.
Another recent advance has been the increasing popularity of multivoxel pattern analysis (MVPA) techniques for the study of human neuroimaging data (20)(see (21) for a recent review). The standard univariate analysis approach examines how each brain voxel independently responds to a given stimulus, whereas MVPA methods examine the combined activity of across multiple voxels to multiple stimuli. By identifying statistical regularities in the aggregate activity patterns, these methods can generate predictions about how those voxels will respond to those stimuli. Applied to the activity of a specific brain region, successful predictions by these methods indicate that region contains information which can be used to discriminate between those stimuli, which can suggest something about the underlying function of that brain region. These methods are ideal for identifying subtle differences in activity patterns that would typically be missed by traditional univariate analyses. If indeed topographic taste quality representation cannot be observed by these traditional fMRI analysis techniques, then MVPA methods could provide evidence supporting the alternative population coding model, as they did for olfaction in piriform cortex (22, 23).
One recent study employing these new techniques examined taste representation in the insula within the context of two separate experiments (24). In one experiment, performed with standard resolution 3-Tesla fMRI, the authors provided subjects with sweet, salty, bitter, and sour tastes during scanning. They first identified that these tastes (vs. baseline) activated almost entirely overlapping areas of the insula. They then used an MVPA technique to identify regions of the insula that were maximally predictive of a specific taste. Again, these regions largely overlapped within the insula, and a conjunction of these regions regions revealed areas of the anterior and middle insula in which all tastes could be reliably discriminated. Outside of the insula, they identified that only small areas of the parietal and frontal operculum also supported information which could be used to discriminate between taste types. In a second experiment, using 7-Tesla fMRI and higher spatial resolution (8mm3 voxel size), they again were able to identify regions of anterior and middle insula which could reliably discriminate sweet and bitter tastes. The authors conclude that, in contrast to rodent studies (7), their results revealed ‘a more complex gustotopic map’ as they did not observe distinct activation clusters for each taste.
Around the same time, a recent study from my lab set out to directly evaluate the topographic and population coding models, with an approach which combined both univariate conjunction analyses and multivariate pattern analyses (25). As with the second experiment in (24), our study used 7T fMRI, combined with extremely high voxel resolution (1.73mm3 voxel size) and gustatory stimulus delivery using a block design intended to maximize statistical power at the participant level. We demonstrated that sweet, sour, and salty tastants (vs. a neutral solution) reliably activated gustatory regions of the bilateral dorsal mid-insula (Figure 1A,B). The activation for each taste vs. the neutral taste largely overlapped within these regions (Figure 1C). However, our group conjunction analyses, designed to identify voxels selective for a particular taste, identified only a sparse collection of voxels scattered across the brain without any apparent organization, that only appeared at very low statistical thresholds (Figure 1D). We failed to identify any areas within the brain exhibiting a reliable preference for any taste. We performed these same conjunction analyses at the subject-level, and additionally scanned several of our participants during two scanning sessions, to examine the possibility of stable individual-level topography, suggested by a previous study (18). As with that previous study, we observed highly variable patterns of taste-specific responses across subjects, but we also observed equally variable patterns when subjects were examined across multiple sessions. However, our multivariate pattern analyses, performed at both the region-of-interest and searchlight level, were able to reliably classify taste responses within the bilateral dorsal mid-insula (Figure 1C), as well as other sensory regions such as the somatosensory and piriform cortex, and regions downstream of the taste pathway such as the amygdala and orbitofrontal cortex (OFC). These results indicated that, in contrast to the results of the earlier study (24), taste-predictive activity patterns could be found throughout the brain in regions involved in various aspects of food sensation and reward. As mentioned above, the nature of the hemodynamic response sets inherent limits on the spatial resolution of even very high-resolution fMRI studies. However, we can still expect that, if tastes selectively activated specific ‘hot spots’ within gustatory cortex on a scale comparable to that observed in rodents (7), we would have observed them with the functional resolution employed in our study. What we observed instead were overlapping patterns of activation for all tastes, which could be reliably discriminated using MVPA techniques, in keeping with more recent results in rodent gustatory cortex (13) and strongly supporting the population coding model of taste quality representation.
Figure 1: Taste quality decoding within gustatory regions of the human insula A.
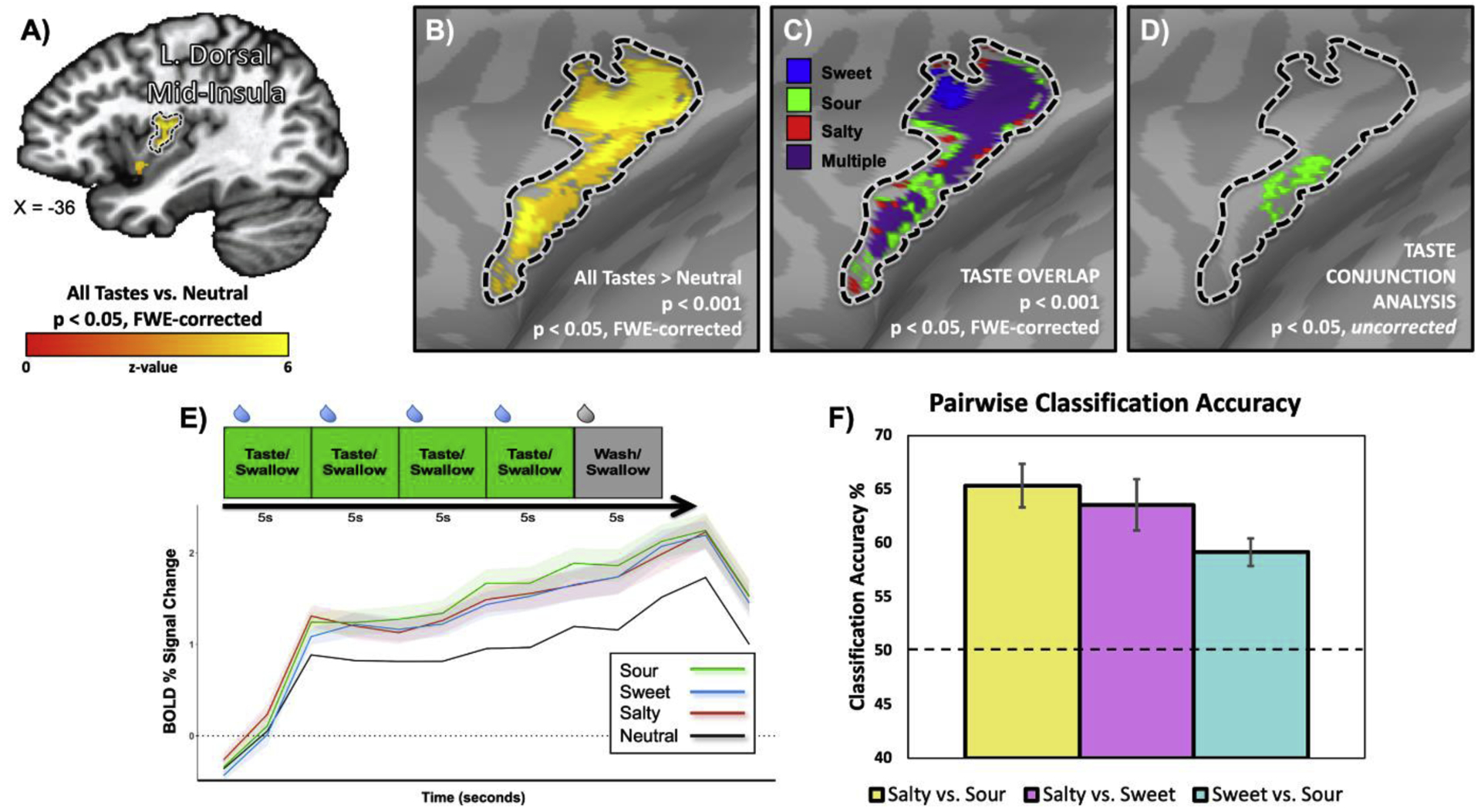
The average activation of sweet, salty, and sour tastes (vs. a neutral solution) identified gustatory regions of the dorsal mid-insular cortex. B. Same results as A, projected on the cortical surface. C. Activations for each taste vs. the neutral solution largely overlap within the left mid-insula. D. Taste conjunctions, designed to detect voxels specifically activated by sweet, sour, or salty tastes, identified only sparse collections of voxels at low statistical thresholds. E. The average time course of the taste response within the left mid-insula did not differ between sweet, sour, and salty tastes. E. However, multivoxel pattern analyses (MVPA) can reliably discriminate between tastes based on the spatial patterns of activation within this region. Adapted from Avery et al., 2020.
Following this, a third 7-Telsa fMRI study also examined the multivariate representation of taste in the human insula at high resolution (2.81mm3 voxel size), using MVPA methods as a basis for mapping the taste selectivity of various areas of the insula (26). The investigators in this study tested participants with both high and low concentrations of sweet, sour, salty, and bitter tastes on two separate days, using nearly identical in-scanner paradigms. Using an MVPA technique designed to determine the selectivity of individual voxels for specific tastes, they identified patterns of patchy regions intermingled along the surface of the insula which appeared preferentially tuned to specific tastes. Importantly, they were able to identify these patterns by training and testing across multiple days, which suggests that these patterns are somewhat stable within participants. These patterns did not exhibit any clear spatial layout or gradient at the group level however, and the layout reportedly was not consistent between participants. Surprisingly, they also observed little to no overlap of the taste selective spheres for high and low taste concentrations, and some spheres flipped their preferred taste with a change in concentration. Interestingly, this result is similar to that observed by a recent 3-Tesla taste fMRI study (27) that identified a spatial gradient with increasing concentrations of sweet and bitter tastes, with greater concentrations shifting activation to more superior and anterior areas in the insula. While Porcu and colleagues did attempt to identify a rough topographic organization for taste using their selectivity approach (26), they noted that the lack of overlap in selectivity across concentrations did not support a stable topographic model, but rather a model where taste quality is coded by distributed patterns of activity within the insular cortex.
Do we really need a gustotopic map?
The recent human fMRI studies reviewed above, along with numerous rodent and monkey studies, provide little evidence for the topographic model of cortical taste representation. This prompts an obvious question, why are some sensory modalities topographically organized and others not? Retinopy for vision and somatotopy for touch are both based upon the mapping of an area of two-dimensional space onto the cortical surface. This provides a distinct advantage for an organism, as it allows for the direction of an appropriate behavioral response to a stimulus appearing in that spatial location. But, for a sensory system concerned with detecting of helpful and harmful chemicals, the more important question is what, rather than where. Much like olfaction (11), there is no spatial correlate or meaningful representation of taste in two-dimensions, as receptors for all tastes are represented across the surface of the tongue (8). The basic tastes are also discrete qualities, rather than a continuous measure such as auditory frequency, the basis for tonotopic representation. Thus, there would seem to be little advantage to organizing taste topographically.
The cortical representation of taste also does not appear to be static, as in other sensory modalities. According to one recent study (27), these patterns not only vary across individuals, they seem to vary significantly according to taste concentration. This finding of concentration-dependence is somewhat supported by rodent studies in which peripheral neurons appear as specialists at one concentration and generalists at higher concentrations (28, 29). The response profile to a taste in rodent cortex can also vary greatly during acquisition and extinction of a conditioned taste aversion (30), which also underscores a more fundamental role the insular cortex plays in representing the current and predicted homeostatic state of the body (31).
Taste Quality and Valence
Recent studies in rodents suggest that cortical taste responses might be organized according to hedonic valence, rather than taste quality, with pleasant and aversive tastes activating different regions along the rostro-caudal axis of the insula (32). These distinct regions exhibit corresponding connectivity to specific sub-regions of the amygdala involved in appetitive and aversive behavioral responses (33). This accords well with the insula’s role in homeostatic maintenance and suggests that hedonic valence could provide the fundamental organizing principle behind cortical taste representation. However, none of the recent human fMRI studies reported any spatial gradient associated with taste pleasantness. This result may partly be due to the difficulty dissociating taste quality with pleasantness, within the context of these human imaging studies. Part of the difficulty may stem from the very artificiality of the pure taste solutions used in these studies. Food is by nature a multisensory stimulus, with strong olfactory and tactile components that combine with taste to form a unified flavor percept (34). There is some evidence that our taste systems may be optimized for responding to these more naturalistic compound stimuli (35). One recent model suggests that the convergence of both olfactory and gustatory inputs in the ventral anterior insula and OFC is required for normal sensory and hedonic processing of flavors (34). Accordingly, there is some evidence that the hedonic valence of tastes may be represented as spatially distributed patterns within the ventral anterior insula and OFC (36). Further exploring the relationship between hedonic valence and taste representation, perhaps by using more naturalistic flavor stimuli, will be an important avenue of research for future human fMRI studies.
In addition to spatial population coding, temporal coding of tastes, discussed elsewhere in this issue (Ohla, 2020), may also play a large factor in coding both taste quality and valence. In the human brain, taste evoked electrical responses can be decoded on a timescale from 150ms to 200ms (37), well below the typical sampling frequencies of fMRI methods. Furthermore, the ensemble firing rates of cortical taste neurons may encode information about taste quality and palatability within different temporal epochs of individual taste responses (38), much of which can be lost when averaging over multiple trials.
Conclusion - The Cartesian Restaurant
The notion of the ‘Cartesian Theater’ comes from Daniel Dennett, as a critique of Cartesian Materialism, an idea that there exists a place in the brain within which all the contents of consciousness come together as a clear and unified whole, fit for display, as in a theater (39). Based on the emerging evidence from rodent, primate, and human studies, gustatory cortex appears to consist of dispersed populations of generalist and specialist neurons which represent taste quality and valence through a complex spatiotemporal code. With no clear need for a spatial organization of taste, it seems ultimately unlikely that we will find any location in the brain where the various qualities represented by the sense of taste come together in a clear topographic display.
Acknowledgements:
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The author has no financial or commercial interests relevant to the present manuscript.
References
Papers of particular interest, published within the period of review, have been highlighted as: [*] of special interest.
- 1.Roper SD, & Chaudhari N (2017). Taste buds: cells, signals and synapses. Nature Reviews Neuroscience, 18(8), 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Small DM (2010) Taste representation in the human insula. Brain Struct Funct 214(5–6):551–561. [DOI] [PubMed] [Google Scholar]
- 3.De Araujo IE, Geha P, Small DM (2012) Orosensory and Homeostatic Functions of the Insular Taste Cortex. Chem Percept 5(1):64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214(5–6):519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer T, Critchley HD, Preuschoff K (2009) A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences 13(8):334–340. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD (2003) Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology 13(4):500–505. [DOI] [PubMed] [Google Scholar]
- 7.Ohla K, Yoshida R, Roper SD, Di Lorenzo PM, Victor JD, Boughter JD, … & Chaudhari N (2019). Recognizing taste: coding patterns along the neural axis in mammals. Chemical senses, 44(4), 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrashekar J, Hoon MA, Ryba NJ, & Zuker CS (2006). The receptors and cells for mammalian taste. Nature, 444(7117), 288–294. [DOI] [PubMed] [Google Scholar]
- 9.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD (2007) Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27(40):10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Gabitto M, Peng Y, Ryba NJ, & Zuker CS (2011). A gustotopic map of taste qualities in the mammalian brain. Science, 333(6047), 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stettler DD, & Axel R (2009). Representations of odor in the piriform cortex. Neuron, 63(6), 854–864. [DOI] [PubMed] [Google Scholar]
- 12.Accolla R, Bathellier B, Petersen CCH, Carleton A (2007) Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 27(6):1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher ML, Ogg MC, Lu L, Ogg RJ, & Boughter JD (2017). Overlapping representation of primary tastes in a defined region of the gustatory cortex. J Neurosci, 37(32), 7595–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen K, Kogan JF, & Fontanini A (2020). Spatially Distributed Representation of Taste Quality in the Gustatory Insular Cortex of Behaving Mice. Current Biology (online only). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staszko SM, Boughter JD, Fletcher ML (2020) Taste coding strategies in insular cortex. Exp Biol Med (Maywood) 245(5):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott TR, Plata-Salamán CR (1999) Taste in the monkey cortex. Physiology & Behavior 67(4):489–511. [DOI] [PubMed] [Google Scholar]
- 17.Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, & Petrides M (1999). Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport, 10(1), 7–13. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld MA, Neuer G, Tempelmann C, Schüssler K, Noesselt T, Hopf JM, & Heinze HJ (2004). Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience, 127(2), 347–353. [DOI] [PubMed] [Google Scholar]
- 19.Prinster A, Cantone E, Verlezza V, Magliulo M, Sarnelli G, Iengo M, … & Esposito F (2017). Cortical representation of different taste modalities on the gustatory cortex: A pilot study. PLoS One, 12(12), e0190164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriegeskorte N, Goebel R, Bandettini P (2006) Information-based functional brain mapping. Proc Natl Acad Sci USA 103(10):3863–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebart MN, Baker CI (2018) Deconstructing multivariate decoding for the study of brain function. NeuroImage 180(Part A):4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review of multivariate decoding methods emphasizes the specific ways MVPA methods have been used and misused for the study of brain function. They identify common pitfalls and confusion about of the results of MVPA methods, and how they can impact the interpretation of neuroimaging data.
- 22.Howard JD, Plailly J, Grueschow M, Haynes JD, & Gottfried JA (2009). Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci, 12(7):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahnt T, Gottfried JA, Howard JD (2019) Converging prefrontal pathways support associative and perceptual features of conditioned stimuli. Nature Communications, 7(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chikazoe J, Lee DH, Kriegeskorte N, & Anderson AK (2019). Distinct representations of basic taste qualities in human gustatory cortex. Nature Communications 10(1):1048. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses multivariate pattern analysis to identify insula regions differentially predictive of different taste qualities. They identified regions in the anterior and middle insula which discriminated all tastes, a finding which they were able to replicate within a follow-up study performed with 7T fMRI.
- 25.Avery JA, Liu AG, Ingeholm JE, Riddell CD, Gotts SJ, & Martin A (2020). Taste quality representation in the human brain. J Neurosci, 40(5), 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porcu E, Benz KM, Ball F, Tempelmann C, Hanke M, & Noesselt T (2020). Macroscopic information-based taste representations in insular cortex are shaped by stimulus concentration. Proceedings of the National Academy of Sciences, 117(13), 7409–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used a multivariate information mapping technique to find taste selective regions of the insula. They identified a patchy organization of regions selective for tastes at low concentrations, and a remarkably different organization of regions selective for taste at high concentrations.
- 27.Canna A, Prinster A, Cantone E, Ponticorvo S, Russo AG, Di Salle F, & Esposito F (2019). Intensity-related distribution of sweet and bitter taste fMRI responses in the insular cortex. Human brain mapping, 40(12), 3631–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors of this study tested the insular response to sweet and bitter tastes at increasing levels of concentration. They identified a spatial gradient with increasing stimulus concentration, with greater concentrations generating activations in more superior areas in the right insula and more anterior areas in the left insula.
- 28.Wu A, Dvoryanchikov G, Pereira E, Chaudhari N, & Roper SD (2015). Breadth of tuning in taste afferent neurons varies with stimulus strength. Nature communications, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonseca E, de Lafuente V, Simon SA, & Gutierrez R (2018). Sucrose Intensity coding and decision-making in taste cortices. bioRxiv, doi: 10.1101/391342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accolla R, & Carleton A (2008). Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proceedings of the National Academy of Sciences, 105(10), 4010–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, … & Andermann ML (2020). Estimation of current and future physiological states in insular cortex. Neuron, 105(6), 1094–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this rodent study, the authors identify distinct population codes within the insular cortex representing the physiological states of hunger and thirst. These population codes are shown to track the deviation from and gradual return to homeostasis. During hunger and thirst, external cues predictive of food or water transiently shift the population activity of these insular neurons towards a state of satiety. These results demonstrate how the insula represents the current state of the body and estimates the potential impact of external stimuli upon homeostasis.
- 32.Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJ, & Zuker CS (2015). Sweet and bitter taste in the brain of awake behaving animals. Nature, 527(7579), 512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, … & Zuker CS (2018). The coding of valence and identity in the mammalian taste system. Nature, 558(7708), 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small DM, & Green BG (2012). A proposed model of a flavor modality In The neural bases of multisensory processes. CRC Press/Taylor & Francis. [Google Scholar]
- 35.Escanilla OD, Victor JD, & Di Lorenzo PM (2015). Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. Journal of Neuroscience, 35(16), 6284–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chikazoe J, Lee DH, Kriegeskorte N, & Anderson AK (2014). Population coding of affect across stimuli, modalities and individuals. Nature Neuroscience, 17(8), 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallroth R, & Ohla K (2018). As soon as you taste it: Evidence for sequential and parallel processing of gustatory information. eNeuro 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, & Miller P (2016). The behavioral relevance of cortical neural ensemble responses emerges suddenly. Journal of Neuroscience, 36(3), 655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennett DC (1993). Consciousness Explained. Penguin; uk. [Google Scholar]


