Dear Editor,
We performed a metabolome‐based genome‐wide association study (metaboGWAS) in a Chinese cohort with coronary artery disease (CAD). We identified seven novel metabolite quantitative trait loci (metaboQTLs) and revealed the possible clinical significance of these metaboQTLs. Our study may add novel biological insights into the metabolic background of disease‐related variants and contribute to the development of risk prediction and treatment of CAD.
CAD is the most common type of cardiovascular disease worldwide, with high morbidity and mortality. 1 To date, genetic variants can explain approximately 10.6% of CAD heritability, 2 whereas the internal biological information is often limited. Plasma metabolites represent a functional intermediate for genetic makeup and environmental exposure to the end phenotype that can be used to uniquely identify individuals. 3 Understanding the genetic regulation of the levels of circulating metabolites in CAD can illustrate pathophysiological signals and guide the tool development for risk prediction and therapies. metaboGWAS is an effective way to discover important metaboQTLs, 4 which can provide deep insights into the biological links and molecular mechanisms between genes and metabolites. To date, hundreds of metaboQTLs have been uncovered. 5 , 6 , 7 The reported metaboQTLs exhibit greater effect size and stronger association with metabolic traits than with complex traits. However, most metaboGWASs were conducted on European populations, and a limited number were performed on Chinese populations, especially in CAD cohorts.
Here, we present the first large‐scale metaboGWAS of a Han Chinese population to identify effective metaboQTLs. Figure 1 depicts the study flow, and Figure S1 presents an overview of the patients’ enrollment. This work is a two‐stage study and involved 1551 subjects with CAD from three clinical centers (Supporting Information Methods). Table 1 provides the patient characteristics. All patients diagnosed with CAD via angiography had undergone percutaneous coronary intervention, and the exclusion criteria were the same as our previous study. 8 Plasma samples from the patients with CAD were profiled for metabolites by using widely targeted metabolomics, and DNA was collected from hemocytes for genotyping (Supporting Information Methods). Spearman correlation was used to analyze the pairwise correlation between these 161 metabolites in the discovery group (Tables S1 and S2). Table S3 summarizes the data of the two groups.
FIGURE 1.
Schematic of the study design for primary analysis. Abbreviations: CAD, coronary artery disease; MACE, major adverse cardiovascular events
TABLE 1.
Baseline characteristics of the two groups
| Value N (%) or mean ± SD | |||
|---|---|---|---|
| Characteristics | Discovery group | Validation group | p |
| Demographic data | |||
| Size | 1028 | 523 | – |
| Age | 62.98 ± 10.05 | 61.94 ± 10.14 | 0.0474 |
| Sex (male) | 818 (79.57) | 382 (73.04) | 0.0028 |
| BMI (kg/m2) | 24.27 ± 4.80 | 24.10 ± 3.07 | 0.7523 |
| Comorbidities | |||
| Arrhythmia | 92 (8.97) | 47 (8.99) | 0.9852 |
| Diabetes | 281 (27.39) | 151 (28.87) | 0.5331 |
| Heart failure | 89 (8.67) | 241 (46.08) | <0.0001 |
| Hypertension | 618 (60.18) | 315 (60.23) | 0.9574 |
| Hyperlipidemia | 119 (11.59) | 71 (13.58) | 0.2947 |
| Baseline biochemical measurements | |||
| ALT (U/L) | 27.43 ± 13.19 | 26.80 ± 23.06 | 0.5960 |
| AST (U/L) | 26.65 ± 10.65 | 26.40 ± 38.37 | 0.8622 |
| CK (U/L) | 111.47 ± 111.49 | 124.97 ± 348.94 | 0.4652 |
| eGFR (ml/min/1.73 m2) | 94.58 ± 74.06 | 94.58 ± 122.97 | 0.9993 |
| CKMB (U/L) | 7.48 ± 5.94 | 13.37 ± 17.55 | <0.0001 |
| CHOL (mmol/L) | 4.28 ± 1.13 | 4.30 ± 1.79 | 0.7706 |
| LDLC (mmol/L) | 2.58 ± 0.93 | 2.72 ± 1.00 | 0.0103 |
| HDLC (mmol/L) | 0.97 ± 0.26 | 1.00 ± 0.25 | 0.0233 |
| TRIG (mmol/L) | 1.61 ± 1.12 | 1.83 ± 1.87 | 0.0184 |
| GLUC (mmol/L) | 6.71 ± 2.69 | 5.96 ± 2.20 | <0.0001 |
| Lpa (mg/L) | 307.16 ± 329.42 | 284.55 ± 330.01 | 0.3153 |
| APOA (g/L) | 1.04 ± 0.28 | 1.17 ± 0.24 | <0.0001 |
| BNP (pg/ml) | 777.40 ± 1604.48 | 1351.12 ± 3869.26 | 0.3297 |
| Medication | |||
| β‐blockers | 917 (89.38) | 447 (85.47) | 0.0314 |
| ACEIs | 653 (63.65) | 253 (48.37) | <0.0001 |
| CCBs | 292 (28.46) | 158 (30.21) | 0.5192 |
| PPIs | 293 (48.44) | 353 (67.50) | <0.0001 |
| SYNTAX | 16.44 ± 10.75 | 15.88 ± 13.33 | 0.8835 |
Abbreviations: ACEIs, angiotensin converting enzyme inhibitors; ALT, alanine aminotransferase; APOA, apolipoprotein a; AST, aspartate aminotransferase; BMI, body mass index; BNP, B‐type natriuretic peptide; CCBs, calcium channel blockers; CHOL, cholesterol; CK, creatine kinase; CKMB, creatine kinase MB; eGFR, estimated glomerular filtration rate; HDLC, high‐density lipoprotein cholesterol; GLUC, glucose; Lpa, lipoprotein (a); LDLC, low‐density lipoprotein cholesterol; PPIs, proton pump inhibitors; SYNTAX score, Synergy between PCI with TAXUS and Cardiac Surgery score; TRIG, triglyceride.
Primary association analysis (Supporting Information Methods) was conducted using 3,435,396 imputed single‐nucleotide polymorphisms (SNPs) with 161 metabolites and 12,280 metabolite ratios in two independent groups. The first 10 principal components, sex, age, aspartate aminotransferase (AST), antihypertensive drugs, hypertension, estimated glomerular filtration rate (eGFR), and diabetes were included as covariates in both groups. We used meta‐analysis to combine the association results of the two groups. P‐gain was used as a standard to integrate the results of association analysis between single metabolites and ratios. 9 Bonferroni p < 5 × 10−8 / 161 = 3.11 × 10−10 and p < 5 × 10−8 / 12,880 = 3.88 × 10−12 were used as thresholds for significant single metabolite and metabolite ratio associations, respectively. After heterogeneity test, P‐gain, and linkage disequilibrium (LD) analyses, the numbers of these associations collapsed to 109, including 13 metabolite and 96 ratio associations, and all of them contained 15 SNPs (Table S4). One SNP (rs73530508) was removed after LD analyses of the merged data. The association of each locus with the lowest p‐value against any metabolites and ratios was reported (Figure 2A and B and Supporting Information Figures S2–S12). We searched the GWAS Catalog 10 (last data release on August 13, 2020) for the reported or possibly novel loci. We identified 13 loci (Table 2), including seven novel loci, namely, RHPN1 with the ratio of L‐histidine and uric acid, ZBTB16 with inosine, LOC112268121 with eudesmic acid, SLC10A1 with glycocholic acid, GABRG3 with dodecanedioic acid, ACSM2B with 3‐indolepropionic acid (IPA), and PWP2 with 1,4‐dihydro‐1‐methyl‐4‐oxo‐3‐pyridinecarboxamide.
FIGURE 2.
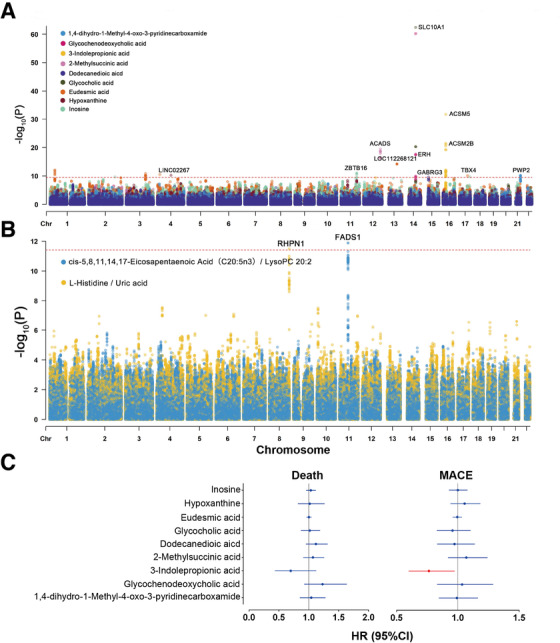
Plots for loci with metabolites/ratios and relationship between metaboQTL‐related metabolites and risk of death and MACE. (A and B) Manhattan plots for the loci in meta‐analysis. (A) Red dashed line indicates the threshold of genome‐wide significance on single metabolites (p < 3.11 × 10−10). (B) Red dashed line indicates the threshold of genome‐wide significance of metabolite ratios (p < 3.88 × 10−12). (C) Forest plots of the HR of death and MACE of metaboQTL‐related metabolites in the discovery group. The effect sizes with 95% CI were plotted by adjusting the covariant in Cox regression model, and the significant metabolites are highlighted in red
TABLE 2.
Genomic loci associated with metabolites and metabolite ratios at genome‐wide significance in meta‐analysis
| Locus | rsID | Metabolite/ratio a | Annotation b | Ref c | N | EA/OA d | Beta (SE e ) | p f |
|---|---|---|---|---|---|---|---|---|
| LINC02267 | rs117445861 | Hypoxanthine | RINT | NAS | 1551 | A/G | −0.54 (0.08) | 5.9E‐11 |
| RHPN1 | rs73365860 | L‐Histidine/uric acid | DS | NL | 1513 | T/C | −0.17 (0.02) | 3.3E‐12 |
| FADS1 | rs174548 | cis‐5,8,11,14,17‐Eicosapentaenoic acid (C20:5n3)/LysoPC 20:2 | INT | NAS | 1551 | C/G | −0.12 (0.02) | 1.3E‐12 |
| ZBTB16 | rs573195 | Inosine | INT | NL | 1499 | T/G | −0.52 (0.08) | 1.2E‐11 |
| ACADS | rs9204 | 2‐Methylsuccinic acid | UTR3 | NAS | 1551 | A/G | 0.37 (0.04) | 3.4E‐20 |
| LOC112268121 | rs9530695 | Eudesmic acid | IG | NL | 1506 | C/G | 2.50 (0.32) | 6.1E‐15 |
| ERH | rs76199614 | Glycochenodeoxycholic acid | DS | NAS | 1515 | A/G | 0.29 (0.04) | 1.2E‐10 |
| SLC10A1 | rs2296651 | Glycocholic acid | MV | NL | 1551 | A/G | −1.31 (0.08) | 4.2E‐63 |
| GABRG3 | rs58275387 | Dodecanedioic aicd | INT | NL | 1542 | T/C | −0.34 (0.05) | 2.6E‐10 |
| ACSM5 | rs9929808 | 3‐Indolepropionic acid | INT | NAS | 1551 | T/C | −0.77 (0.07) | 2.1E‐32 |
| rs6497488 | 3‐Indolepropionic acid | INT | NAS | 1500 | T/C | −0.40 (0.06) | 5.6E‐11 | |
| ACSM2B | rs11645661 | 3‐Indolepropionic acid | UTR3 | NL | 1548 | A/G | −0.69 (0.07) | 3.7E‐22 |
| TBX4 | rs8073361 | Eudesmic acid | IG | NAS | 1551 | A/C | −1.97 (0.30) | 8.8E‐11 |
| PWP2 | rs74716564 | 1,4‐dihydro‐1‐Methyl‐4‐oxo‐3‐pyridinecarboxamide | INT | NL | 1525 | A/G | −0.47 (0.07) | 6.4E‐11 |
The “/” represents a ratio relationship, the front is the numerator, the back is the denominator.
SNP annotation, UTR3 (3′ Untranslated Region), DS (downstream), IG (intergenic), INT (Intronic), MV (Missense Variant), RINT (ncRNA_intronic).
Novel Locus (NL) or Novel Association (NAS).
Effect/other allele.
Standard error.
Meta‐analysis p‐value.
We further explored the biological functions of the 13 loci. We used several databases to investigate the known biological functions and disease or pharmacological correlation of these loci (Supporting Information Methods, Table S5). We observed four loci, including three novel loci, which encode enzymes or transporters in genes. Moreover, 11 loci are related to diseases, including six novel loci. In addition, the effect on drugs of genes in two loci has been studied previously.
Metabolites may affect the prognosis of CAD, but changes in the concentration of certain metabolites may be a concomitant state. To explore whether or not these metaboQTL‐related metabolites affect the occurrence of end‐point events, we used Mendelian randomization (MR) approaches to explain the causal relationship between them. First, we explored the association between metaboQTL‐related metabolites and death and major adverse cardiovascular events (MACE) in the discovery group through Cox regression model (Table S6), adjusting for sex, age, eGFR, AST, hypertension, diabetes, cardiovascular family history, and smoking history. We observed that IPA may reduce the risk of MACE (HR [95%CI], 0.76 [0.60–0.97]) (Figure 2C) and then used two MR approaches (Supporting Information Methods) to explore their causal relationship. IPA was found related to the risk of MACE in the one‐sample MR analysis (HR [95%CI], 0.70 [0.55–0.89]). However, no strong evidence was found in the two‐sample MR analysis. The effect value of the inverse variance weighted method in the two‐sample MR analysis opposed the expectation, and no similar effect was observed in the four other models (Figure S13 and Tables S7 and S8). A large sample size in the future may provide deep insights into the relationship between IPA and MACE, and further work is needed to uncover the role of IPA in the pathogenesis of the disorder.
In sum, this study represents the first large‐scale metaboGWAS study of Chinese populations. We identified seven novel loci that may be unique to Chinese populations with CAD. Using the one‐sample MR approach, we found that IPA was related to the risk of MACE in patients with CAD. The results strengthen our knowledge of the relationships between genetic variations and individualized metabolism in patients with CAD and potentially facilitate the establishment of personalized explanation or markers for biological differences in disease status.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Medical Ethical Review Committee of Guangdong Provincial People's Hospital (Nos. GDREC2010137 and GDREC2017071H) and conducted according to the Declaration of Helsinki. Informed consent (Nos. 20100910 and 20170211) was obtained from all individual participants included in the study.
AUTHOR CONTRIBUTIONS
Zixian Wang participated in patient recruitment, performed experiment, analyzed the data, and wrote the original draft of the manuscript. Qian Zhu participated in patient recruitment, performed experiment, supervised the data, and revised manuscript. Yibin Liu performed experiment and analyzed the data. Shiyu Chen analyzed the data. Ying Zhang curated the data. Qilin Ma provided resources. Xiaoping Chen curated the data. Chen Liu curated the data. Heping Lei curated the data. Hui Chen established methodology. Jing Wang performed experiment. Shufen Zheng curated the data. Zehua Li made investigation. Lingjuan Xiong curated the data. Weihua Lai provided resources. Shilong Zhong contributed to the conception of the project, design of the study, provision of resources, analyses of the data, and writing of the manuscript.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study was funded by the National Nature Science Foundation of China (No. 81872934 [Principle investigator: Shilong Zhong], 81673514 [Principle investigator: Shilong Zhong]; http://www.nsfc.gov.cn/), the National key research and development program (No. 2017YFC0909301 [Principle investigator: Shilong Zhong]; https://service.most.gov. cn/index/), and the Key research and development program of Guangdong Province, China (2019B020229003 [Principle investigator: Shilong Zhong]; http://pro.gdstc.gd.gov.cn/egrantweb/). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139‐e596. [DOI] [PubMed] [Google Scholar]
- 2. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122:433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tremblay BL, Guénard F, Lamarche B, et al. Familial resemblances in human plasma metabolites are attributable to both genetic and common environmental effects. Nutr Res. 2019;61:22‐30. [DOI] [PubMed] [Google Scholar]
- 4. Adamski J. Genome‐wide association studies with metabolomics. Genome Med. 2012;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long T, Hicks M, Yu HC, et al. Whole‐genome sequencing identifies common‐to‐rare variants associated with human blood metabolites. Nat Genet. 2017;49:568‐578. [DOI] [PubMed] [Google Scholar]
- 7. Yousri NA, Fakhro KA, Robay A, et al. Whole‐exome sequencing identifies common and rare variant metabolic QTLs in a Middle Eastern population. Nat Commun. 2018;9:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin M, Zhu Q, Lai W, et al. Insights into the prognosis of lipidomic dysregulation for death risk in patients with coronary artery disease. Clin Transl Med. 2020;10:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen AK, Krumsiek J, Wägele B, et al. On the hypothesis‐free testing of metabolite ratios in genome‐wide and metabolome‐wide association studies. BMC Bioinform. 2012;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Welter D, MacArthur J, Morales J, et al. The NHGRI GWAS Catalog, a curated resource of SNP‐trait associations. Nucl Acids Res. 2014;42:D1001‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


