Abstract
Cardiovascular disease is a severe threat health worldwide, and circRNAs have been shown to be correlated with the development of cardiovascular disease. Expression of circ‐ITCH and miR‐17a‐5p was evaluated by RT‐qPCR. Cell viability was measured using CCK‐8. Flow cytometry was applied to measure apoptosis rate. Binding between miR‐17‐5p and circ‐ITCH was detected via luciferase reporter assays. Levels of ATP in cells were examined with ATP testing. Western blot was used to evaluate apoptosis‐related proteins and proteins in Wnt/β‐catenin signalling pathway. H2O2 induced apoptosis of H9c2 cells and lowered cell viability as well as ATP levels and circ‐ITCH expression. After overexpression, circ‐ITCH enhanced cell viability and ATP concentration. Meanwhile, apoptosis was inhibited. MiR‐17‐5p was the target of circ‐ITCH as evidenced by luciferase report assays, with higher expression in H2O2‐induced H9c2 cells. Knockdown of miR‐17‐5p could promote cell viability and level of ATP and curb apoptosis and p53 and PARP expression. Moreover, overexpressed miR‐17‐5p could reverse the function of upregulated circ‐ITCH. Wnt3a, Wnt5a and β‐catenin in Wnt/β‐catenin signalling pathway were increased after H2O2 induction. Suppression of Wnt/β‐catenin signalling pathway could initiate the process of injury in H9c2 cells. Circ‐ITCH could protect myocardial cells from injuries caused by H2O2 by suppressing apoptosis while miR‐17‐5p played a reverse role, which could upregulate apoptosis and inhibit cell viability via Wnt/β‐catenin signalling pathway.
Keywords: Adenosine Triphosphate, apoptosis, circRNA ITCH, myocardial cell
1. INTRODUCTION
Cardiovascular diseases are critical human illnesses which are increasing year by year. 1 Coronary arteriosclerosis can interrupt cardiac blood circulation, resulting necrosis of myocardium after ischaemia and hypoxia. 2 , 3 H2O2 is produced in ischaemia‐reperfusion injury (I/R), contributing to ROS in oxidative stress injury. 4 Apoptosis of myocardial cells occurs in a variety of heart diseases, such as cardiomyopathy, myocardial ischaemia/reperfusion injury, myocardial infarction and congestive heart failure. 5 , 6 , 7 Thus, it is essential to suppress excessive apoptosis in myocardial cells in cardiovascular diseases.
CircRNAs can regulate miRNAs through the molecular sponge mechanism and are involved with occurrence and development of diseases. 8 , 9 There were about 575 circRNAs according to RNA sequencing in adult mouse hearts. 10 Previously, circRNA000203 has been shown to induce its down‐stream genes Colla2 and GTGF and promote myocardial fibrosis through sponging miR‐26b. 11 CircRNA MFACR could absorb miR‐652‐5p and to inhibited myocardial cell apoptosis and to shrink the myocardial infarction area. 12 CircRNAs have also been shown to play a part in myocardial cell apoptosis in vitro, and to help cardiac repair in vivo, potentially signifying promising therapies for the diseases in the future. 13 , 14 , 15 , 16 , 17 CircRNA ITCH was widely recognized as a tumour suppressor in various cancers. 18 , 19 , 20 However, the role of circ‐ITCH in myocardial cells has not been reported previously. In this study, therefore, we evaluated the regulatory role of circ‐ITCH in H2O2‐induced myocardial cell apoptosis in vitro as a mimic of I/R and injury.
2. METHODS
2.1. Cell culture
H9c2, a rat myocardial cell line, was acquired from ATCC and cultured in DMEM medium (Gibco) containing 10% foetal bovine serum (FBS) and 100 μmol/mL penicillin and streptomycin in a humid incubator at 37°C, 5% CO2. After confluence of cells reached 80%, cells were digested by 0.25% trypsin and seeded into a 6‐well plate incubated with H2O2 (0.1 and 1 mmol/L) for 12 hours with a non‐treated cell group as a control. 16 Cells with satisfactory well growth were selected for the following experiments.
2.2. Cell transfection assays and wnt/beta‐catenin signalling inactivation
Cells with 1 mmol/L H2O2 pretreatment were selected to regulate the expression of circ‐ITCH and miR‐17‐5p. circRNA ITCH sequence was cloned into pcDNA3.1 plasmid for augmentation. miR‐17‐5p mimics, NC mimics, miR‐17‐5p inhibitor and NC inhibitor were obtained from GenePharma. Lipofectamine 3000 (Invitrogen) was used to obtain the following groups of cells, respectively: oe‐NC and oe‐circ‐ITCH by transfecting empty pcDNA3.1 and pcDNA3.1‐circ‐ITCH into the cells according to the manufacturer's instructions. Likewise, NC inhibitor, miR‐17‐5p inhibitor, NC mimics, miR‐17‐5p mimics and oe‐circ‐ITCH + miR17‐5p mimics groups were generated through transfection using Lipofectamine 3000. To study the function of wnt/beta‐catenin signalling in cells, we incubated the cells with an inhibitor of the signalling pathway, 1 μmol/L NCB‐0846, for 24 hours (Meilunbio). Cells were harvested at 48 hours after transfection or NCB‐0846 treatment for the follow‐up assays.
2.3. RT‐qPCR
Cells pre‐exposed to 0, 0.1 and 1 mmol/L H2O2 and the sub‐groups under 1 mmol/L H2O2 after transfection or treatment with NCB‐0846 were all selected for detection of the circ‐ITCH and miR‐17‐5p expression with normalization to GAPDH and U6, respectively, by using RT‐qPCR methods. Total RNAs were isolated from cells using TRIzol reagent (Invitrogen). Afterwards, High‐Capacity RNA‐to‐cDNA™ Kit (Applied Biosystems) was used for reverse transcription with synthetic primers. Sequences were listed as follows: circ‐ITCH: forward: 5′‐AGCAATGCAGCAGTTT‐3′; reverse: 5′‐TGTAGCCCATCAAGACA‐3′ 20 ,miR‐17‐5p: forward: 5′‐GCTGAATTTGTATGGTTTATAGTTGTTA‐3′; reverse: 5′‐GCACCTTAGAACAAAAAGCACT‐3′, 8 , 9 U6: forward: 5′‐ATTGGAACGATACAGAGAAGATT‐3′; reverse: 5′‐GGAACGCTTCACGAATTTG‐3′ 21 GAPDH: forward: 5′‐ACCCAGAAGACTGTGGATGG ‐3′,reverse: 5′‐TCAGCTCAGGGATGACCTTG‐3′. PCR conditions were shown: predenaturation, 95°C, 10 minutes; denaturation, 95°C, 15 seconds; annealing, 60°C, 60 seconds; and extension, 72°C, 30 seconds, 35 cycles. Relative expressions of RNAs were quantified with 2−ΔΔCt methods.
2.4. CCK‐8
This method is applied to measure cellular viability in each group. After incubation with 0, 0.1 and 1 mmol/L H2O2, H9c2 cells were explanted into 96‐well plate at a cell density of 2 × 105 cells per well and cultured in incubators at 37°C, 5% CO2. Cells with 1 mmol/L H2O2 pretreatment were regulated through transfection assays and generated seven different sub‐groups, oe‐NC, oe‐circ‐ITCH, NC inhibitor, miR‐17‐5p inhibitor, NC mimics, miR‐17‐5p mimics and oe‐circ‐ITCH + miR17‐5p mimics groups. All the cells in the seven sub‐groups underwent the same process as normal or H2O2‐treated H9c2 cells. At specific time points (24, 48 and 72 hours), 10 μL CCK‐8 was added and the mixture was kept incubated for another hour. A microplate reader (Molecular Devices) was used for detection of optical density (OD) values of cells at 450 nm wavelength.
2.5. ATP testing
After transfection, cells were rinsed with PBS three times and lysed. Next, lysates were settled and then centrifuged at 4°C, 12 000 g for 5 minutes. Then the liquid supernatant were collected and the ATP concentrations were examined with an ATP Assay Kit (Beyotime). Luminoskan™ Microplate Luminometer (Thermo Scientific) was used to measure levels of ATP in the cells.
2.6. Luciferase report assay
3′UTR of circ‐ITCH was amplified through RT‐qPCR. In order to create a 3′UTR mutation, a Phusion Site‐Directed Mutagenesis Kit (Thermo Scientific) was applied. Furthermore, wild type and mutant type of 3’‐UTRs were inserted into psiCHECK‐2 vectors (Promega) with Lipofectamine 3000 (Invitrogen). Relative luciferase activities were checked with dual‐Luciferase Reporter Assay System (Promega).
2.7. Flow cytometry
Cell apoptosis was detected with Annexin V‐FITC Apoptosis Detection Kit (Beyotime). After cells were collected, Annexin V‐FITC and PI were applied for staining cells. In the end, CellTrace™ Blue Cell Proliferation Kit (Invitrogen) was used to analyse apoptosis of cells.
2.8. Western blot
This method was used to semi‐quantify the proteins as mentioned below, and cells were harvested and lysed by RIPA Lysis Buffer (Beyotime) to obtain total protein. After that, 40 μg of total protein was measured with BCA protein Assay Kit (Beyotime). Thereafter, proteins were isolated with 8% SDS‐PAGE and transferred into Polyvinylidene Fluoride (PVDF) membranes (Invitrogen) later. Next, 8% skimmed milk powder was selected to block membranes for 2 hours and anti‐pro‐caspase‐3 (1:1000; ab183179, Abcam), anti‐cleaved‐caspase‐3 (1:1000; ab49822), anti‐p53 (1:1000; ab26), anti‐PARP1 (1:1000; ab227224), anti‐Wnt3a (1:1000; ab219412), anti‐Wnt5a (1:1000; ab227229), anti‐β‐catenin (1:1000; ab32572) and anti‐GAPDH (1:2000; ab181602) were incubated with PVDF membranes at 4°C overnight. Afterwards, membranes were rinsed by Pierce™ Protein‐Free T20 (PBS) Blocking Buffer (Thermo Scientific) three times and incubated with secondary antibodies ‐ Goat Anti‐Rat IgG H&L (HRP) (ab97057) at room temperature for 2 hours. Finally, BeyoECL Moon (Beyotime) was used for testing protein.
2.9. Statistical analysis
All data were analysed using SPSS 19.0 and displayed as mean ± SD. All experiments were repeated three times, and the differences in the mean values between or among the groups were evaluated using Student's t test and one‐way ANOVA was applied for the repetitive results inside each group. P < .05 was considered to reveal statistical significance.
3. RESULTS
3.1. H2O2 induced apoptosis of myocardial cells and downregulated level of circ‐ITCH
After cells were treated with different concentrations of H2O2, (0, 0.1, 1 mmol/L), the viability of cells decreased as H2O2 concentrations increased (Figure 1A). Increasing concentration of H2O2 induced declining ATP in H9c2 cells (Figure 1B). After that, apoptosis rates were checked, revealing that death of myocardial cells was induced by H2O2 in a concentration‐dependent fashion (Figure 1C). As H2O2 concentration rose, cleaved caspase 3, PARP and p53 proteins were activated (Figure 1D). Circ‐ITCH expression was observed to be downregulated in the H2O2 groups. The higher the concentration (1 mmol/L), the lower the expression of ITCH (Figure 1E). Based on the above results, cells with H2O2 (1 mmol/L) treatment were chosen for further experiments.
Figure 1.
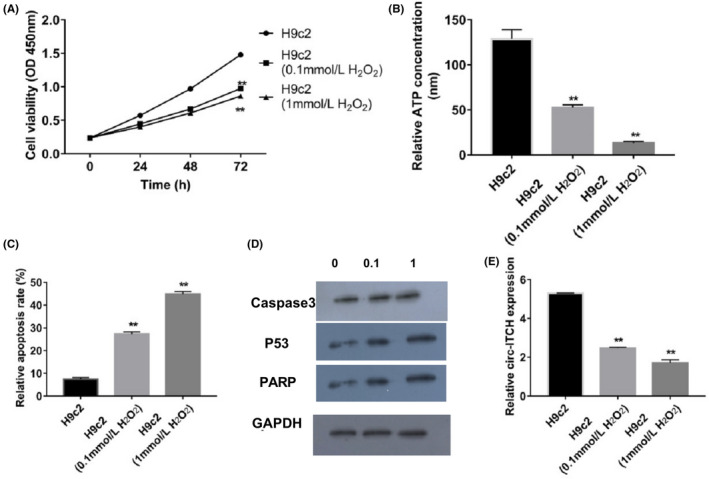
H2O2 treatment improved apoptosis of H9c2 cells and downregulated circ‐ITCH expression. A, Cell viabilities after H2O2 treatment were detected by CCK‐8, P < .05. B, ATP concentrations in H9c2 cells treated with H2O2 were analysed with ATP Assay Kit, P < .05. C, Apoptosis rate of H9c2 cells were measured by flow cytometry, P < .05. D, Caspase 3, p53 and PARP expressions in H9c2 cells treated with H2O2 were evaluated by Western blot, P < .05. E, Expressions of circ‐ITCH in H9c2 cells after H2O2 treatment were measured by RT‐qPCR, P < .05. Student's t test and one‐way ANOVA were used
3.2. Overexpression of circ‐ITCH deterred cellular apoptosis in H9c2 cells with pretreatment of H2O2
After upregulating circ‐ITCH in H9c2 cells with 1 mmol/L H2O2 pretreatment, RT‐qPCR validated the activated circ‐ITCH expression in the transfected group (Figure 2A). Besides, upregulated circ‐ITCH induced increased viability of H9c2 cells (Figure 2B). Meanwhile, concentrations of ATP were increased in the overexpressed The circ‐ITCH group (Figure 2C). Apoptosis results displayed that upregulation of circ‐ITCH could reduce apoptosis rate (Figure 2D). Cleaved caspase 3, p53 and PARP were inactivated by overexpression of circ‐ITCH (Figure 2E).
Figure 2.
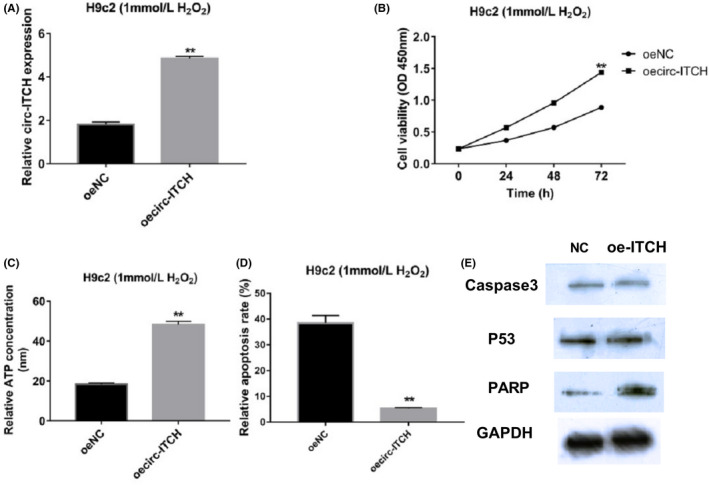
Overexpression of circ‐ITCH reduced apoptosis in H2O2‐treated H9c2 cells. A, Levels of circ‐ITCH after overexpressed transfection were validated through RT‐qPCR, P < .05. B, CCK‐8 was applied to measure cell viabilities of H9c2 cells with upregulated circ‐ITCH, P < .05. C, ATP Assay Kit was used for checking ATP concentration, P < .05. D, Flow cytometry was for examining apoptosis rate of H9c2 cells with overexpressed circ‐ITCH, P < .05. E, Western blot was applied to detect expressions of caspase 3, p53 and PARP, P < .05. Student's t test and one‐way ANOVA were used
3.3. MiR‐17‐5p was a target of circ‐ITCH and promoted apoptosis of myocardial cells
From the Bioinformatics website, Starbase (http://starbase.sysu.edu.cn/agoClipRNA.php?source=circRNA&flag=target&clade=mammal&genome=human&assembly=hg19&miRNA=hsa-miR-17-5p&clipNum=1&deNum=0&target=ITCH+), we searched the miRNA‐circRNA interactions and followed this by including the gene name of circ‐ITCH. We found that miR‐17‐5p had a putative binding site with circ‐ITCH (Figure 3A). Then, a luciferase report assay was applied for verification of the binding. We noted that luciferase activity significantly declined when miR‐17‐5p mimics and wild type of circ‐ITCH were co‐transfected into the cells, indicating the exact binding between them (Figure 3B). Therefore, miR‐17‐5p changes were evaluated which revealed that H2O2 could induce miR‐17‐5p expression in H9c2 cells in a dosage‐dependent fashion (Figure 3C). Since the 1 mmol/L group was more significantly different than the 0.1 mmol/L group, the former group was selected for the follow‐up assays (Figure 3C). Afterwards, RT‐qPCR validated the downregulation of miR‐17‐5p after interference assay in H9c2 cells (Figure 3D). Low viability of cells and ATP concentrations increased with the interference of miR‐17‐5p (Figure 3E,F). Apoptosis rate dropped as miR‐17‐5p was suppressed (Figure 3G). Furthermore, cleaved caspase 3, p53 and PARP expressions were lower with inhibition of miR‐17‐5p (Figure 3H).
Figure 3.
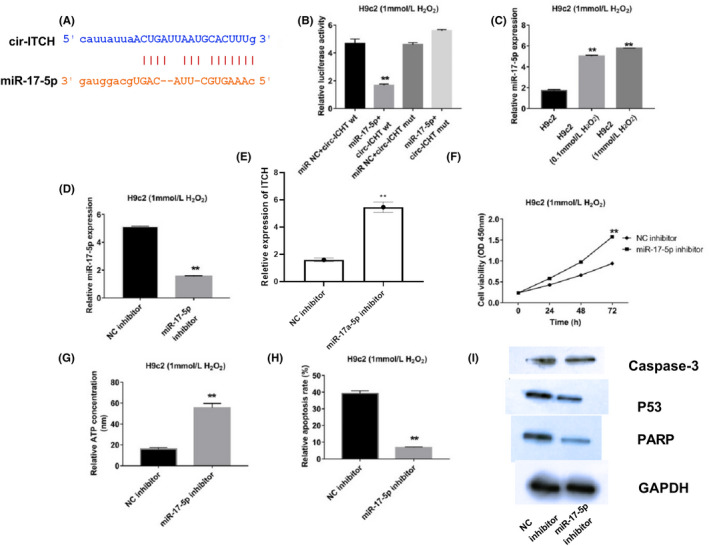
MiR‐17‐5p was the target of circ‐ITCH and promoted apoptosis of H2O2‐induced myocardial cells. A and B, Binding information was acquired from bioinformatics tools (TargetScan) and proved through luciferase report assay, P < .05. C, Expressions of miR‐17‐5p in H9c2 cells treated by H2O2 were measured with RT‐qPCR, P < .05. D, Suppressed miR‐17‐5p expression was checked through RT‐qPCR, P < .05. E, Cell viabilities were analysed by CCK‐8, P < .05. F, ATP concentrations were detected, P < .05. G, Apoptosis rate with suppressed miR‐17‐5p was measured with flow cytometry, P < .05. H, Caspase 3, p53 and PARP levels were detected by Western blot, P < .05. Student's t test and one‐way ANOVA were used
3.4. Overexpression of miR‐17‐5p counteracted protective effect of circ‐ITCH in H2O2 induced myocardial cells
After detections of miR‐17‐5p and circ‐ITCH were made individually correlation between circ‐ITCH and miR‐17‐5p was examined. First, expression of cicr‐ITCH was checked, which indicated that mimics of miR‐17‐5p could repress level of overexpressed circ‐ITCH. Meanwhile, mimics of miR‐17‐5p could reverse high cell viabilities and ATP concentration caused by high levels of circ‐ITCH. Suppressed apoptosis rate with overexpression of circ‐ITCH could be enhanced with upregulated miR‐17‐5p. Moreover, low expression of caspase 3, p53 and PARP with upregulated circ‐ITCH were seen associated with overexpression of miR‐17a‐5p (Figure 4).
Figure 4.
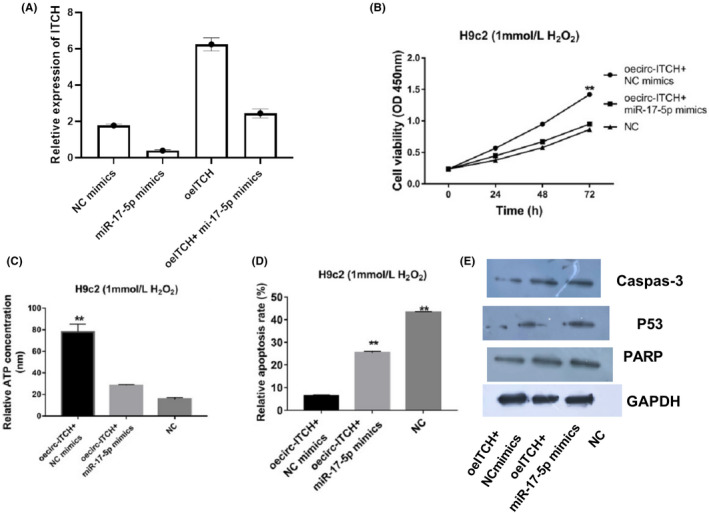
Overexpression of miR‐17‐5p downregulated protection of circ‐ITCH to H2O2‐induced myocardial cells. A, Circ‐ITCH expressions with mimics of miR‐17‐5p were examined with RT‐qPCR, P < .05. B, Cell viabilities were detected by CCK‐8, P < .05. C, ATP Assay Kit was applied to check ATP concentrations in H9c2 cells with transfected circ‐ITCH and miR‐17‐5p, P < .05. D, Flow cytometry was used to check apoptosis rate in H9c2 cells with transfected circ‐ITCH and miR‐17‐5p, P < .05. E, Western blot was used for measuring levels of caspase 3, p53 and PARP, P < .05. Student's t test and one‐way ANOVA were used
3.5. Wnt/β‐catenin signalling pathway participated in apoptosis of myocardial cells
Western blot results showed that H2O2 led to higher Wnt3a, Wnt5a and β‐catenin protein levels and when H2O2 concentration was raised from 0.1 to 1 mmol/L, the changes in Wnt3a, Wnt5a and β‐catenin protein expression were more significant (Figure 5A). Therefore, interactions between circ‐ITCH, miR‐17a‐5p and the Wnt/β‐catenin signalling pathway were investigated in cells with 1mmol/L H2O2 treatment. Comparing the NC and oe‐ITCH groups, the changes showed that overexpression of circ‐ITCH could downregulate Wnt3a, Wnt5a and β‐catenin. When comparing the co‐transfected group where circ‐ITCH and miR‐17‐5p were both upregulated with the oe‐ITCH group, we showed that miR‐17‐5p mimics could reverse the fall in signalling proteins induced by ITCH overexpression (Figure 5B). In order to confirm that the Wnt/β‐catenin signalling pathway was involved in injury to myocardial cells, NCB‐0846, a Wnt/β‐catenin signalling inhibitor was applied to silence the signalling. Wnt3a, Wnt5a and β‐catenin all decreased in NCB‐0846 group in comparison with the NC group (Figure 5B). Functional assays were adopted to study the regulatory role of wnt/β‐catenin signalling in the myocardial cells, which revealed that cell viability and ATP concentrations were enhanced while apoptosis rate dropped, accompanied by lower caspase 3, p53 and PARP proteins when signalling pathway was inactivated (Figure 5C‐F).
Figure 5.
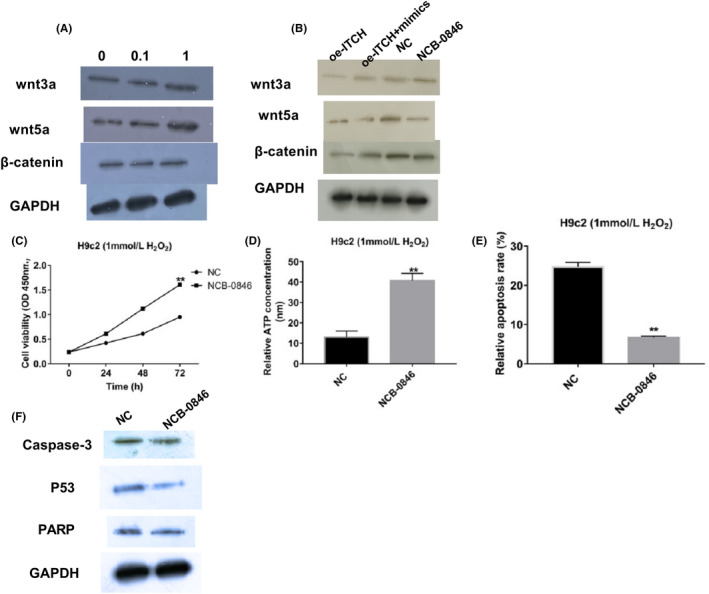
Wnt/β‐catenin signalling pathway took part in apoptosis of H2O2‐induced myocardial cells. A, Expressions of Wnt3a, Wnt5a and β‐catenin in H9c2 cells treated after H2O2 were measured with Western blot, P < .05. B, Levels of Wnt3a, Wnt5a and β‐catenin with transfected circ‐ITCH and miR‐17‐5p and after NCB‐0846 treatment were measured using Western blot, P < .05. C, Cell viabilities after NCB‐0846 treatment were analysed with CCK‐8, P < .05. D, ATP concentrations with NCB‐0846 added were analysed through ATP Assay Kit, P < .05. E, Apoptosis rate were evaluated using flow cytometry, P < .05. F, Western blot was performed for measuring expressions of caspase 3, p53 and PARP, P < .05. Student's t test and one‐way ANOVA were used
4. DISCUSSION
Myocardial ischaemia‐reperfusion injury (I/R) refers to aggravated ischaemic myocardial injury caused by restored blood perfusion after ischaemia, and is associated with clinical features such as abnormal heart rate, myocardial systolic dysfunction. This becomes irreversible reperfusion injury and so on. 22 With increased applications of thrombolytic therapy, cardiovascular surgery and cardiopulmonary bypass (CPB), reperfusion injuries have become more common than before. 23 Until now, mechanisms of reperfusion injury have included overproduction of reactive oxygen species, Ca2+ overload, excessive apoptosis of myocardial cells and inflammatory reactions, in 24 , 25 recent years, researchers have revealed that oxidative stress played an important role in development of ischaemic heart disease, and was the main element inducing apoptosis in myocardial cells. 26 In this study, H2O2 was applied to cause oxidative stress in H9c2 cells. H2O2 treatment could decrease cell viability as well as ATP concentrations, but increased apoptosis rate, as evidenced by higher levels of caspase 3, p53 and PARP. Results from those experiments revealed that H2O2 treatment lead to increased apoptosis in myocardial cells with suppression of ATP as well as cell viability.
Circular RNA is a type of single‐stranded, closed RNA formed by reverse cleavage of the exon at the maturation of the precursor mRNA, whose formation contains intron pairing driving ring formation, RNA‐binding protein pairs driving ring formation and lasso‐driving ring formation. 27 , 28 , 29 In cardiac muscle tissues of the heart‐failing mouse, lots of misaligned circRNAs were discovered. Among 1163 kinds of circRNAs tested, expressions of 29 kinds of circRNAs were upregulated and 34 kinds of circRNAs were decreased. 30 CircRNA‐010567 could enhance ischaemia‐reperfusion injury of cells through sponging miR‐141, indicating that it might be an important regulator in heart failure after myocardial infarction. 31 Moreover, circRNA HRCR was shown to retard heart failure as well as myocardial hypertrophy by absorbing miR‐223 and increasing ARC. 32 Circ‐ITCH was also detected as a suppressor in many kinds of cancers, such as bladder cancer, ovarian cancer and osteosarcoma. 33 , 34 However, research about circ‐ITCH is rare in cardiovascular diseases. In this study, circ‐ITCH was found to be downregulated after treatment with H2O2, which indicated that circ‐ITCH might have a role in myocardial cells. Therefore, the functions of circ‐ITCH were evaluated. Upregulated circ‐ITCH could increase cell viability as well as ATP concentrations in H2O2 treated H9c2 cells. Meanwhile, decreased apoptosis rate and low expression of caspase 3, p53 and PARP reminded that circ‐ITCH could repress oxidative stress injury of myocardial cells, suggesting that circ‐ITCH might be a protector of myocardial cells.
MicroRNAs have been shown to participate in pathological and physiological processes of cardiovascular diseases, such as cardiac hypertrophy, arrhythmia, myocardial ischaemia and myocardial fibrosis. 35 MiRNA‐195 overexpression could activate myocardial hypertrophy, accelerating the progression of heart failure. 36 High MiR‐210 expression in myocardial cells from living mice is associated with upregulating of many kinds of angiogenic factor and suppression of activation of caspase 3/7, indicating that miR‐210 could be a potential therapy in cardiovascular diseases. 37 MiR‐17‐5p was a promoter in hypoxia‐induced myocardial cells, which was significantly upregulated in AMI patients. 38 Moreover, the luciferase report assay proved that miR‐17‐5p was the target of circ‐ITCH and expressions of miR‐17‐5p were upregulated in H2O2 treated H9c2 cells. Furthermore, suppression of mIR‐16‐5p could increase cell viability and ATP levels with reduced apoptosis and decreased expression of caspase 3, p53 and PARP. In addition correlation between miR‐17‐5p and circ‐ITCH revealed that miR‐17‐5p could restore the protective functions of circ‐ITCH in myocardial cells. According to previous studies, Wnt/β‐catenin signalling pathway could accelerate process of cardiovascular diseases. 39 , 40 In this study, we also measured proteins in the Wnt/β‐catenin signalling pathway, which revealed that Wnt3a, Wnt5a and β‐catenin levels were all upregulated. After Wnt inhibitor, NCB‐0846 was applied, levels of Wnt3a, Wnt5a and β‐catenin were decreased, which upregulated viability of H9c2 cells as well as ATP concentrations and suppressed apoptosis rate and related protein expression. The results indicated that miR‐17‐5p took part in the process of oxidative stress injury of myocardial cells through the Wnt/β‐catenin signalling pathway and negative regulation by circ‐ITCH.
5. CONCLUSION
Circ‐ITCH could retard the injuries seen in H2O2‐treated myocardial cells in suppressing apoptosis through absorbing miR‐17‐5p via negative regulating factors in the Wnt/β‐catenin signalling pathway. This suggests that it could be a potential factors in release from oxidative stress injury to myocardial cells.
CONFLICT OF INTEREST
None
ETHICAL APPROVAL
Ethical approval was not required for this study.
ACKNOWLEDGMENTS
This research did not involved outside funding.
Zhang N, Wang X. Circular RNA ITCH mediates H2O2‐induced myocardial cell apoptosis by targeting miR‐17‐5p via wnt/β‐catenin signalling pathway. Int J Exp Path.2021;102:22–31. 10.1111/iep.12367
REFERENCES
- 1. Tofield A. Cancer overtakes cardiovascular disease as the main cause of death in 12 European Union countries. Eur Heart J. 2016;37:3183‐3184. [DOI] [PubMed] [Google Scholar]
- 2. Plowright AT, Engkvist O, Gill A, Knerr L, Wang QD. Heart regeneration: opportunities and challenges for drug discovery with novel chemical and therapeutic methods or agents. Angew Chem. 2014;53:4056‐4075. [DOI] [PubMed] [Google Scholar]
- 3. Shen E, Diao X, Wei C, Wu Z, Zhang L, Hu B. MicroRNAs target gene and signaling pathway by bioinformatics analysis in the cardiac hypertrophy. Biochem Biophys Res Comm. 2010;397:380‐385. [DOI] [PubMed] [Google Scholar]
- 4. Wu Z, Wang H, Fang S, Xu C. Roles of endoplasmic reticulum stress and autophagy on H2O2‐induced oxidative stress injury in HepG2 cells. Mol Med Rep. 2018;18:4163‐4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner JL, Lang CH. Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int J Biochem Cell Biol. 2017;89:125‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Hu Y, Zeng Z, et al. Influence of androgen on myocardial apoptosis and expression of myocardial IR and IRS‐1 in chronic heart failure rat models. Mol Med Rep. 2018;17:1057‐1064. [DOI] [PubMed] [Google Scholar]
- 7. Wu T, Wu D, Wu Q, et al. Knockdown of long non‐coding RNA‐ZFAS1 protects cardiomyocytes against acute myocardial infarction via anti‐apoptosis by regulating miR‐150/CRP. J Cell Biochem. 2017;118:3281‐3289. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Lai Y, Ma J, et al. miR‐17‐5p suppresses cell proliferation and invasion by targeting ETV1 in triple‐negative breast cancer. BMC Cancer. 2017;17:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li LJ, Leng RX, Fan YG, Pan HF, Ye DQ. Translation of noncoding RNAs: focus on lncRNAs, pri‐miRNAs, and circRNAs. Exp Cell Res. 2017;361:1‐8. [DOI] [PubMed] [Google Scholar]
- 10. Jakobi T, Czaja‐Hasse LF, Reinhardt R, Dieterich C. Profiling and validation of the circular RNA repertoire in adult murine hearts. Genom Proteom Bioinform. 2016;14:216‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang CM, Zhang M, Huang L, et al. CircRNA_000203 enhances the expression of fibrosis‐associated genes by derepressing targets of miR‐26b‐5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci Rep. 2017;7:40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Gan TY, Li N, et al. Circular RNA mediates cardiomyocyte death via miRNA‐dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24:1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayoumi AS, Aonuma T, Teoh JP, Tang YL, Kim IM. Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol Sin. 2018;39:1100‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bei Y, Yang T, Wang L, et al. Circular RNAs as potential theranostics in the cardiovascular system. Mol Ther Nucl Acids. 2018;13:407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garikipati VNS, Verma SK, Cheng Z, et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF‐A axis. Nat Commun. 2019;10:4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M, Ding W, Tariq MA, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR‐133a‐3p. Theranostics. 2018;8:5855‐5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Q, Zhang Z, Bei Y, Li G, Wang T. Circular RNAs as novel biomarkers for cardiovascular diseases. Adv Exp Med Biol. 2018;1087:159‐170. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Ge YZ, Xu L, Jia R. Circular RNA ITCH: a novel tumor suppressor in multiple cancers. Life Sci. 2020;254:117176. [DOI] [PubMed] [Google Scholar]
- 19. Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Circular RNA‐ITCH suppresses lung cancer proliferation via inhibiting the Wnt/β‐catenin pathway. Biomed Res Int. 2016;2016:1579490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang C, Yuan W, Yang X, et al. Circular RNA circ‐ITCH inhibits bladder cancer progression by sponging miR‐17/miR‐224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang QH, Yang M, Zhang LL, Xiao MC, Zhao Y, Yan DX. The mechanism of miR‐23a in regulating myocardial cell apoptosis through targeting FoxO3. Eur Rev Med Pharmacol Sci. 2017;21:5789‐5797. [DOI] [PubMed] [Google Scholar]
- 22. Cao Y, Bojjireddy N, Kim M, et al. Activation of γ2‐AMPK suppresses ribosome biogenesis and protects against myocardial ischemia/reperfusion injury. Circ Res. 2017;121:1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gewirtz H, Dilsizian V. Myocardial viability: survival mechanisms and molecular imaging targets in acute and chronic ischemia. Circ Res. 2017;120:1197‐1212. [DOI] [PubMed] [Google Scholar]
- 24. Consolini AE, Ragone MI, Bonazzola P, Colareda GA. Mitochondrial bioenergetics during ischemia and reperfusion. Adv Exp Med Biol. 2017;982:141‐167. [DOI] [PubMed] [Google Scholar]
- 25. Morciano G, Bonora M, Campo G, et al. Mechanistic role of mPTP in ischemia‐reperfusion injury. Adv Exp Med Biol. 2017;982:169‐189. [DOI] [PubMed] [Google Scholar]
- 26. Wattanapitayakul SK, Bauer JA. Oxidative pathways in cardiovascular disease: roles, mechanisms, and therapeutic implications. Pharmacol Ther. 2001;89:187‐206. [DOI] [PubMed] [Google Scholar]
- 27. Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan MA, Reckman YJ, Aufiero S, et al. RBM20 regulates circular RNA production from the titin gene. Circ Res. 2016;119:996‐1003. [DOI] [PubMed] [Google Scholar]
- 29. Liang D, Tatomer DC, Luo Z, et al. The output of protein‐coding genes shifts to circular RNAs when the Pre‐mRNA processing machinery is limiting. Mol Cell. 2017;68:940‐954. e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu HJ, Zhang CY, Zhang S, Chang M, Wang HY. Microarray expression profile of circular RNAs in heart tissue of mice with myocardial infarction‐induced heart failure. Cell Physiol Biochem. 2016;39:205‐216. [DOI] [PubMed] [Google Scholar]
- 31. Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR‐141 by targeting TGF‐β1. Biochem Biophys Res Comm. 2017;487:769‐775. [DOI] [PubMed] [Google Scholar]
- 32. Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR‐223. Eur Heart J. 2016;37:2602‐2611. [DOI] [PubMed] [Google Scholar]
- 33. Hu J, Wang L, Chen J, et al. The circular RNA circ‐ITCH suppresses ovarian carcinoma progression through targeting miR‐145/RASA1 signaling. Biochem Biophys Res Comm. 2018;505:222‐228. [DOI] [PubMed] [Google Scholar]
- 34. Ren C, Liu J, Zheng B, Yan P, Sun Y, Yue B. The circular RNA circ‐ITCH acts as a tumour suppressor in osteosarcoma via regulating miR‐22. Art Cells Nanomed Biotechnol. 2019;47:3359‐3367. [DOI] [PubMed] [Google Scholar]
- 35. Barringhaus KG, Zamore PD. MicroRNAs: regulating a change of heart. Circulation. 2009;119:2217‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress‐responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255‐18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu S, Huang M, Li Z, et al. MicroRNA‐210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124‐S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xue S, Liu D, Zhu W, et al. Circulating MiR‐17‐5p, MiR‐126‐5p and MiR‐145‐3p are novel biomarkers for diagnosis of acute myocardial infarction. Front Physiol. 2019;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan J, Qiu L, Shu H, et al. Recombinant frizzled1 protein attenuated cardiac hypertrophy after myocardial infarction via the canonical Wnt signaling pathway. Oncotarget. 2018;9(3)::3069‐3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhuang YS, Liao YY, Liu BY, et al. MicroRNA‐27a mediates the Wnt/β‐catenin pathway to affect the myocardial fibrosis in rats with chronic heart failure. Cardiovasc Ther. 2018;e12468. [DOI] [PubMed] [Google Scholar]


