Abstract
Background
Atrial fibrillation (AF) during high-dose melphalan and autologous stem-cell transplantation (HDM/SCT) for light-chain (AL) amyloidosis confers significant morbidity. Traditional risk factors provide limited prediction for development of paroxysmal AF during this vulnerable period.
Objectives
We sought to assess the association of clinical and echocardiographic parameters, including left atrial (LA) mechanics and development of AF in patients undergoing HDM/SCT therapy.
Methods
Baseline echocardiograms, electrocardiograms, and electronic medical records were retrospectively assessed among patients with AL amyloidosis before HDM/SCT (n = 91). LA function analysis was performed using speckle-tracking echocardiography.
Results
In this study, 42 patients (46%) had cardiac involvement; in the peri-transplant period, 12 (13%) developed AF (7 with cardiac involvement). No significant differences in age, sex, cardiac biomarkers, or cardiac risk factors were seen between patients with and without development of AF; one-third of patients with AF peri-transplant had previous AF. Although LA reservoir strain was reduced in patients with development of AF, time to peak strain rate indexed to R-R interval (TPSRI) (p = 0.001) was prolonged in patients with development of AF compared with sinus rhythm patients in the total cohort but also in subgroups with and without cardiac involvement.
Conclusions
TPSRI, a parameter of mechanical dispersion in the early reservoir phase of LA function, is associated with development of AF among patients undergoing HDM/SCT for AL amyloidosis. These findings require validation in larger prospective cohorts.
Key Words: atrial fibrillation, left atrial strain, light-chain amyloidosis, mechanical dispersion
Abbreviations and Acronyms: AF, atrial fibrillation; AL, light-chain amyloidosis; CA, cardiac amyloid; HDM/SCT, high-dose melphalan and autologous stem cell transplantation; LA, left atrium; LV, left ventricle; TPSRI, time to peak strain rate indexed to R-R interval
Central Illustration
Development of atrial fibrillation (AF) has been an increasingly recognized complication during high-dose melphalan and autologous stem-cell transplantation (HDM/SCT) (1, 2, 3), a treatment shown to induce durable hematologic and clinical remissions in selected patients with light-chain (AL) amyloidosis (4,5). Following HDM/SCT, AF incidence has been reported at 13% and confers significant risks, including longer hospitalization days, intensive care admissions, and trend for increased mortality (6).
Risk factors for AF following SCT for a variety of hematologic malignancies have included older age, renal function, diastolic dysfunction, left atrial (LA) size, melphalan use, previous arrhythmias, and coexisting cardiac risk factors (2,7, 8, 9, 10). To our knowledge, we previously reported the only study that specifically evaluated the factors that predispose patients with AL amyloidosis to develop AF undergoing HDM/SCT. Of note, previous history of AF was the sole risk factor for development of AF in this cohort, with other typical risk factors such as age, cardiac risk factors, and standard echocardiographic parameters showing no difference between patients with AF and without AF (6). Previous history of AF was only present in 8% of patients undergoing HDM/SCT and did not account for all cases of incident AF observed; hence, improved risk-assessment tools for development of AF in these patients are warranted to help identify high-risk individuals to direct therapeutics and mitigate risk.
With the technological advances in echocardiography via deformation imaging, the complexity and importance of LA mechanical function has been increasingly recognized. LA function includes the reservoir phase (LA filling and expansion from pulmonary venous flow during LV systole when the mitral leaflets are closed), the conduit phase (left ventricular [LV] filling from the pulmonary veins in early ventricular diastole), and the booster phase (active LA contraction in late diastole) (Central Illustration). LA function and mechanics predict development of AF in individuals with and without overt cardiac disease (11, 12, 13) and are associated with worse outcomes (14, 15, 16). Patients with AL amyloidosis and cardiac involvement have a high incidence of developing cardiac arrhythmias that are often poorly tolerated (17). LA mechanics are impaired in cardiac amyloidosis (18); however, studies to date have not applied this methodology in patients with AL amyloidosis undergoing SCT. Accordingly, our objective was to evaluate echocardiographic parameters—specifically, LA mechanics via strain imaging in AL amyloidosis pre-transplant—to evaluate its association with development of AF during the peri-transplant period in patients with and without cardiac amyloidosis (CA).
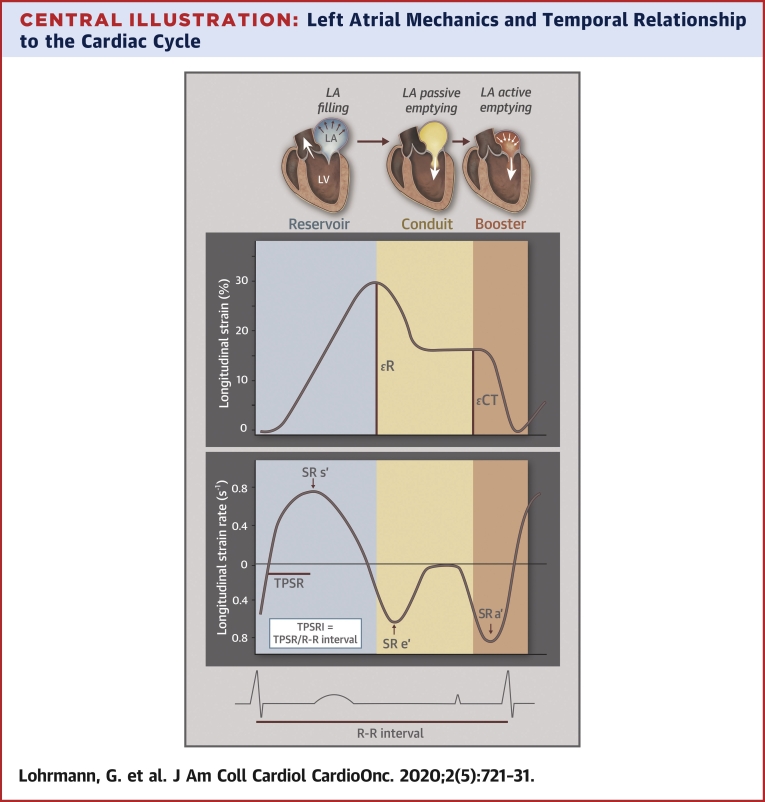
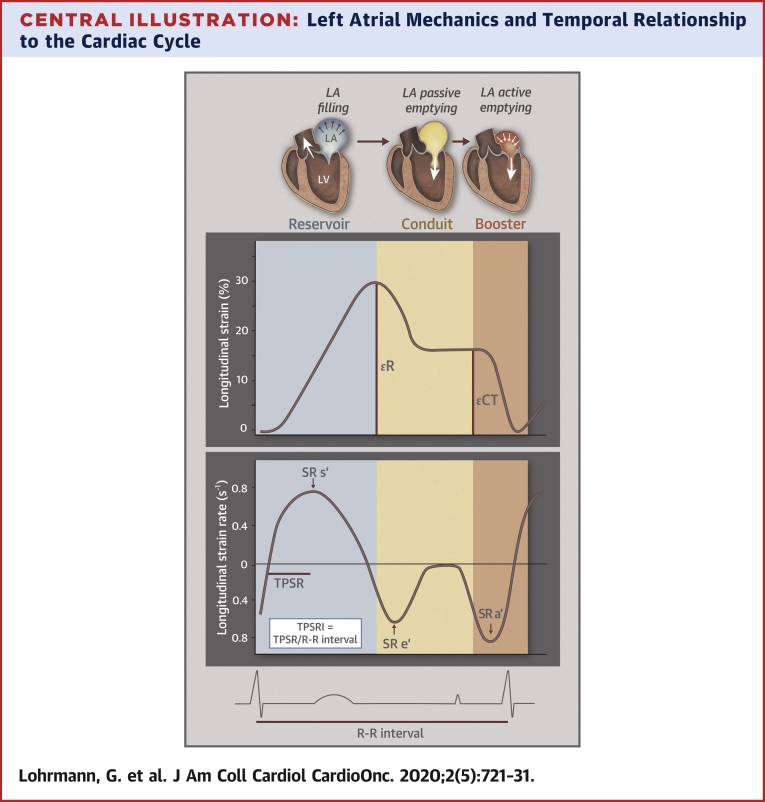
Central Illustration.
Left Atrial Mechanics and Temporal Relationship to the Cardiac Cycle
Left atrial mechanics in relation to the left ventricle throughout the cardiac cycle. R represents left atrial reservoir strain (peak left atrial longitudinal strain during ventricular systole); CT represents left atrial contractile strain (peak left atrial longitudinal strain during atrial contraction). Left atrial strain rate, time-to-peak strain rate, and time-to-peak strain rate index description in relation to the cardiac cycle. Vertical colors designate reservoir (blue), conduit (yellow), and booster (orange) pump functions, respectively. TPSRI = time-to-peak strain rate indexed to R-R interval.
Methods
Study population
Electronic medical records, electrocardiograms, and baseline transthoracic echocardiograms were analyzed for LV and LA strain from a retrospective cohort of 91 consecutive patients in sinus rhythm with systemic AL amyloidosis undergoing HDM/SCT between January 2011 and June 2016 at Boston Medical Center. Eligibility criteria, protocols for HDM/SCT, and outcomes of these patients have been reported previously (4,6). Of the total cohort, 42 patients had cardiac involvement. Paroxysmal AF (defined as noted AF that terminated spontaneously and was present for 7 days or fewer) was noted in the peri-transplant period in 12 patients (13%), 58% (7 of 12 had cardiac involvement) who had cardiac involvement. Peri-transplant period was defined as the first day of stem-cell mobilization until time of neutrophil engraftment (absolute neutrophil count >500 for 2 consecutive days) after HDM/SCT. No patients undergoing SCT had persistent AF. The Boston University Medical Center Institutional Review Board approved this study, and all participants provided informed consent at the time of first visit at the Boston University Amyloidosis Center.
Assessment of cardiac structure and function
Conventional transthoracic echocardiography
Patients underwent comprehensive transthoracic echocardiography before initiation of HDM/SCT therapy with 2-dimensional (2D), color, and tissue Doppler measurements, acquired according to American Society of Echocardiography (ASE) guideline recommendations for chamber quantification, diastolic function, and systolic function (19,20). Echocardiograms were obtained on all patients using a 1- to 5-MHz transducer and commercially available ultrasound machine (iE33, Phillips Medical System, Andover, Massachusetts).
2D speckle-tracking echocardiography
LV and LA strain analyses of patients were performed offline and blinded to clinical characteristics, using the TomTec software package (Cardiac Performance Analysis, Version 4.2, Unterschleissheim, Germany) by a single analyst (G.L.). LV and LA strain were performed according to ASE guidelines, with image acquisition obtained between 60 and 80 frames per second (19). LA strain, LA strain rate, and LA volumes and phasic function were obtained and averaged from 4-chamber and 2-chamber views, using the R-wave as the reference point, according to previously described methodology and detailed in Supplemental Table 1 (21). Time to peak strain rate index (TPSRI) represents the time from QRS onset to peak SR during LV ejection (LA reservoir phase) indexed to the patient’s R-R interval. The Central Illustration graphically depicts LA strain, strain rate, and time-to-peak parameters. All patients were in sinus rhythm at the time of their echocardiograms.
Cardiac involvement of AL amyloidosis was deemed to be present if patients exhibited abnormal imaging findings (unexplained wall thickness ≥12 mm) or abnormal cardiac biomarkers including B-type natriuretic peptide (BNP) >100 pg/ml and/or elevated cardiac troponin. Supportive criteria of abnormal LV longitudinal strain and/or abnormal diastolic dysfunction were taken into consideration but were not mandatory criteria. Our institutional criteria was congruent with the updated consensus criteria established at the 2010 meeting of the International Society of Amyloidosis for cardiac involvement of AL amyloidosis (unexplained wall thickness ≥12 mm or elevated NT-proBNP >332 pg/ml in the absence of AF or renal failure) (22). BNP was used in our determination of cardiac involvement instead of NT-proBNP, as BNP was the more clinically available biomarker at Boston Medical Center before 2016. Cardiac magnetic resonance imaging and/or cardiac biopsy were performed in specific cases but were not required for the determination of cardiac involvement. Of note, 4 patients in this cohort underwent orthotopic cardiac transplantation (∼6 months before SCT) for cardiac involvement, and the baseline echocardiogram used in this study was within weeks of biopsy-confirmed absence of CA in the transplanted heart. Thus, all 4 of these patients were classified as being without CA for the purposes of this study.
Cardiac biomarker staging classification applied the 2019 Boston University scoring system for AL amyloidosis using BNP >81 pg/ml and cardiac troponin I >0.10 ng/ml as cutpoints for classification into 3 stages (23). Renal staging applied the 2014 staging system proposed by Palladini et al. (24), using estimated glomerular filtration rate <50 ml/min and/or proteinuria >5 g/24 h as cutpoints for staging (24).
Statistical analysis
Baseline clinical characteristics and echocardiographic data are reported as mean ± SD, median (25th and 75th percentiles [Q1–Q3]), or number (percentage) unless otherwise specified. The baseline data for both clinical and echocardiographic data are displayed for the total cohort (inclusive of patients with and without CA) and then divided by the presence of CA and development of AF to explore group differences in the context of smaller sample sizes in these subgroups. All continuous variables were tested for normality with Student's t-tests applied for normally distributed variables; Wilcoxon rank sum was applied for comparisons between non-normally distributed variables. Categorical variable comparisons used chi-square testing, and, for categorical groups fewer than 5, Fisher exact test was applied. Correlation testing among LA strain, LA strain rate, and LA volume parameters and clinical and echocardiographic parameters used Pearson correlation coefficient (r). Univariable logistic regression models used development of AF following HDM/SCT as the dependent variable with LA parameters or standard echocardiographic parameters as the independent variable. Adjusted means (accounting for age, history of AF, and diastolic dysfunction) of LA mechanics and relation to development of AF stratified by the presence or absence of CA were evaluated. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina). Inter-class correlation coefficients with 95% confidence limits were estimated to assess echocardiographic reliability and reproducibility (Supplemental Table 3).
Results
Clinical characteristics
A total of 91 patients comprised the total cohort (mean age 58 ± 9 years, 42% female) with 42 patients with cardiac involvement (46%) and 12 (13%) developing any AF during the peri-transplant period. Baseline clinical characteristics are shown in Table 1. No significant differences were noted in baseline cardiovascular risk factors (specifically, age, presence of hypertension, diabetes, presence of coronary artery disease), cardiac biomarkers (B-type natriuretic peptide or cardiac troponin I), or Boston University (BU) biomarker staging among the total cohort or within the subgroups with and without CA. However, the presence of a previous history of paroxysmal AF was significantly higher among patients who developed AF post-SCT (33%) versus those who did not develop AF (4%). Regarding timing in the peri-transplant period, 10 of the 12 subjects with development of AF (83%) developed AF after SCT; only 2 subjects developed AF during stem-cell mobilization.
Table 1.
Baseline Clinical Characteristics
| Total Cohort (N = 91) | (–) Cardiac Amyloid (n = 49) |
(+) Cardiac Amyloid (n = 42) |
|||
|---|---|---|---|---|---|
| No AF (n = 44) | AF (n = 5) | No AF (n = 35) | AF (n = 7) | ||
| Age, yrs | 58 ± 9 | 59 ± 10 | 62 ± 4 | 57 ± 8 | 56 ± 10 |
| Female, % | 38 (42) | 18 (41) | 1 (20) | 14 (40) | 5 (71) |
| BMI, kg/m2 | 28 ± 5 | 28 ± 5 | 33 ± 5 | 27 ± 5 | 27 ± 7 |
| Hypertension | 37 (41) | 23 (52) | 3 (60) | 9 (26) | 2 (29) |
| Diabetes | 5 (5) | 3 (7) | 0 (0) | 1 (3) | 1 (14) |
| CVA | 5 (5) | 2 (5) | 0 (0) | 2 (6) | 1 (14) |
| Coronary artery disease | 1 (4) | 2 (5) | 0 (0) | 2 (6) | 0 (0) |
| Previous AF history | 7 (8) | 2 (5) | 1 (20) | 1 (3) | 3 (43) |
| NYHA functional class | |||||
| I | 50 (77) | 26 (93) | 3 (100) | 18 (62) | 3 (60) |
| II | 13 (20) | 2 (7) | 0 (0) | 9 (31) | 2 (40) |
| III | 2 (3) | 0 (0) | 0 (0) | 2 (7) | 0 (0) |
| Melphalan dose, mg/m2 | 181 ± 28 | 180 ± 29 | 176 ± 33 | 183 ± 28 | 183 ± 29 |
| Bortezomib before SCT | 46 (52) | 16 (37) | 4 (80) | 21 (64) | 5 (71) |
| BNP, pg/ml | 126 (49–294) | 70 (28–93) | 38 (22–83) | 295 (152–608) | 207 (171–529) |
| Troponin I, ng/ml | 0.02 (0.007–0.061) | 0.008 (0.006–0.022) | 0.012 (0.011–0.012) | 0.05 (0.029–0.120) | 0.045 (0.043–0.335) |
| BU cardiac biomarker stage | |||||
| 1 | 33 (36) | 27 (61) | 3 (60) | 3 (9) | 0 (0) |
| 2 | 42 (46) | 15 (34) | 2 (40) | 21 (60) | 4 (57) |
| 3 | 16 (18) | 2 (5) | 0 (0) | 11 (31) | 3 (43) |
| Renal involvement | 72 (80) | 39 (88) | 4 (80) | 26 (76) | 3 (43) |
| Proteinuria (g/24 h) | 5,702 ± 4,569 | 6,027 ± 3,892 | 9,072 ± 7,758 | 5,251 ± 4,513 | 3,492 ± 5,562 |
| Renal staging system | |||||
| 1 | 34 (37) | 11 (25) | 1 (20) | 18 (51) | 4 (57) |
| 2 | 41 (45) | 27 (61) | 2 (40) | 9 (26) | 3 (43) |
| 3 | 16 (18) | 6 (14) | 2 (40) | 8 (23) | 0 (0) |
| TSH, μIU/ml | 2.5 ± 1.5 | 2.7 ± 1.3 | 2.0 ± 1.4 | 2.6 ± 1.8 | 1.8 ± 0.9 |
| HR, beats/min | 79 ± 14 | 76 ± 13 | 77 ± 13 | 82 ± 15 | 84 ± 16 |
| eGFR, ml/min/1.73 m2 | 71 ± 30 | 71 ± 31 | 39 ± 26 | 73 ± 27 | 80 ± 31 |
| Beta-blocker/anti-arrhythmic use | 15 (17) | 6 (14) | 1 (20) | 4 (12) | 4 (57) |
Values are mean ± SD, n (%), or median (25th and 75th percentiles [Q1–Q3]).
AF = atrial fibrillation; BMI = body mass index; BNP = B-type natriuretic peptide; BU = Boston University; CVA = cerebrovascular accident; eGFR = estimated glomerular filtration rate; HR = heart rate; NYHA = New York Heart Association; SCT = stem-cell transplant; SD = standard deviation; TSH = thyroid stimulating hormone.
Standard echocardiographic characteristics
In the total cohort, cardiac wall thickness, LA diameter, diastolic grade, and LV systolic function did not differ among individuals who did and did not develop AF. However, in the CA subgroup (n = 42), there was a higher proportion of patients with more advanced diastolic dysfunction (grade 3) in those who developed AF compared with those without development of AF (43% vs. 24%, p = 0.010). LV ejection fraction (LVEF) remained preserved in the CA subgroup (with AF: 60 ± 9% vs. without AF: 60 ± 8%; p = 0.97), and global longitudinal strain was similarly reduced between those with AF (–12 ± 3%) and those without development of AF –12 ± 3%; p = 0.58).
Left atrial mechanics in individuals with and without development of AF
LA mechanics—specifically, LA strain, LA strain rate, LA volumetric analysis, and LA function—were evaluated (Table 2). In the total cohort, the LA reservoir strain (R) was reduced among patients with development of AF (23 ± 11%) compared with subjects without AF (30 ± 12%; p = 0.047). Although total and active stroke volumes (indexed to body surface area) were reduced among those who developed AF compared with those without AF, these differences were not statistically significant. However, TPSRI, the time to peak SR during atrial filling in the LA reservoir phase (indexed to R-R interval), was significantly prolonged among those who developed AF (32 ± 14 ms) compared with those without AF (21 ± 6 ms; p = 0.003).
Table 2.
Cardiac Structure and Function
| Total Cohort (N = 91) | (–) Cardiac Amyloid (n = 49) |
(+) Cardiac Amyloid (n = 42) |
|||
|---|---|---|---|---|---|
| No AF (n = 44) | AF (n = 5) | No AF (n = 35) | AF (n = 7) | ||
| LA diameter, mm | 33 ± 6 | 32 ± 5 | 33 ± 7 | 35 ± 6 | 35 ± 8 |
| Interventricular septum, mm | 11 ± 3 | 10 ± 2 | 10 ± 2 | 13 ± 3 | 13 ± 3 |
| Diastolic grade | |||||
| 1 | 15 (18) | 7 (19) | 1 (20) | 5 (15) | 0 (0) |
| 2 | 13 (16) | 7 (19) | 0 (0) | 10 (29) | 2 (29) |
| 3 | 13 (16) | 1(3) | 1 (20) | 8 (24) | 3 (43) |
| Indeterminate | 30 (37) | 20 (56) | 4 (40) | 8 (24) | 0 (0) |
| Mitral E wave, cm/s | 78 ± 22 | 70 ± 13 | 72 ± 17 | 85 ± 27 | 100 ± 20 |
| Mitral A wave, cm/s | 69 ± 23 | 69 ± 18 | 73 ± 27 | 71 ± 27 | 54 ± 21 |
| Mitral E/A ratio | 1.3 ± 0.7 | 1.1 ± 0.4 | 1.3 ± 1.0 | 1.4 ± 0.9 | 2.1 ± 1.0 |
| Mean e’ velocity, cm/s | 7 ± 2 | 8 ± 2 | 8 ± 2 | 6 ± 2 | 6 ± 1 |
| LVM index, g/m2 | 88 ± 29 | 72 ± 19 | 82 ± 14 | 108 ± 31 | 98 ± 23 |
| Relative wall thickness | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| Mitral regurgitation severity | |||||
| No regurgitation | 33 (38) | 20 (49) | 2 (40) | 9 (26) | 2 (29) |
| Trace-mild | 55 (62) | 21 (51) | 3 (60) | 26 (74) | 5 (71) |
| Moderate-severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LV function | |||||
| LVEF, % | 62 ± 7 | 65 ± 5 | 64 ± 4 | 60 ± 8 | 60 ± 9 |
| GLS, % | –14 ± 4 | –16 ± 4 | –15 ± 2 | –12 ± 3 | –12 ± 3 |
| LA function | |||||
| LA strain | |||||
| R, % | 29 ± 12 | 35 ± 10 | 32 ± 11 | 24 ± 10 | 17 ± 7 |
| CT, % | 15 ± 8 | 18 ± 7 | 20 ± 12 | 12 ± 7 | 7 ± 5 |
| LA strain rate | |||||
| SR s’, 1/s | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.6 ± 0.3 |
| SR e’, 1/s | –0.7 ± 0.3 | –0.8 ± 0.3 | –0.7 ± 0.4 | –0.6 ± 0.3 | –0.3 ± 0.5 |
| SR a’, 1/s | –0.7 ± 0.4 | –0.9 ± 0.3 | –0.9 ± 0.4 | –0.6 ± 0.3 | –0.4 ± 0.4 |
| TPSRI, % | 22 ± 8 | 19 ± 5 | 26 ± 9 | 22 ± 6 | 35 ± 16 |
| Reservoir ratio (TPSRI/R) | 0.7 (0.5–1.1) | 0.6 (0.4–0.7) | 0.8 (0.6–1.3) | 1.0 (0.7–1.3) | 2.1 (1.1-5.0) |
| LA volume index | |||||
| Maximum, ml/m2 | 39 ± 12 | 35 ± 8 | 34 ± 13 | 44 ± 13 | 39 ± 12 |
| Minimum, ml/m2 | 19 ± 10 | 14 ± 5 | 13 ± 7 | 25 ± 11 | 25 ± 11 |
| Pre-p, ml/m2 | 29 ± 11 | 24 ± 8 | 25 ± 8 | 35 ± 12 | 30 ± 12 |
| LA stroke volume index | |||||
| Total, ml/m2 | 20 ± 7 | 21 ± 6 | 22 ± 8 | 20 ± 7 | 14 ± 4 |
| Passive, ml/m2 | 10 ± 5 | 11 ± 5 | 9 ± 7 | 10 ± 4 | 9 ± 5 |
| Active, ml/m2 | 10 ± 5 | 11 ± 5 | 13 ± 6 | 10 ± 5 | 5 ± 2 |
| LA phasic function | |||||
| Reservoir, % | 132 ± 69 | 164 ± 60 | 185 ± 82 | 99 ± 54 | 68 ± 39 |
| Conduit, % | 27 ± 12 | 30 ± 12 | 23 ± 15 | 23 ± 11 | 25 ± 12 |
| Booster, % | 36 ± 15 | 42 ± 11 | 49 ± 19 | 31 ± 13 | 18 ± 11 |
Values are mean ± SD, n (%), or median (25th and 75th percentiles [Q1–Q3]).
AF = atrial fibrillation; LA = left atrial; LV = left ventricular; LVM = left-ventricular mass; LVEF = left-ventricular ejection fraction; GLS = global longitudinal strain; εR = left-atrial reservoir strain (peak left atrial longitudinal strain during ventricular systole); εCT = left atrial contractile strain (peak left atrial longitudinal strain during atrial contraction); SR = strain rate; TPSRI = time-to-peak strain rate indexed to R-R interval; Pre-p = left atrial volume measured just before P-wave on electrocardiogram tracing to quantify LA volume before atrial contraction.
In the CA subgroup, R was reduced (17 ± 7%) among patients who developed AF compared with those without development of AF (24 ± 10%; p = 0.088). Total LA stroke volume index (p = 0.033) and active stroke volume index (p = 0.013) were significantly reduced in patients with CA and development of AF compared with those without development of AF. LA booster function was significantly reduced in those developing AF compared with those without development of AF in the CA subgroup (18 ± 11% vs. 31 ± 13%, respectively; p = 0.026). As in the total cohort, among patients with CA, TPSRI was significantly prolonged (35 ± 16 ms) among patients developing AF compared with those without development of AF (22 ± 6 ms; p = 0.036).
Clinical and echocardiographic correlates of TPSRI
TPSRI was not correlated to clinical characteristics including age, body mass index, cardiac biomarkers, New York Heart Association functional calls, or renal function. Regarding standard echocardiographic parameters, diastolic dysfunction was not correlated with TPSRI (r = 0.07, p = 0.52) but was correlated with LA diameter (r = 0.27, p = 0.012); apical: basal left ventricular global longitudinal strain ratio (r = 0.31, p = 0.004); and LV longitudinal strain (r = 0.24, p = 0.028). In addition, TPSRI was inversely associated with R (r = –0.34, p = 0.001) and LA contraction parameters including CT (r = –0.25, p = 0.018), active LA stroke volume index (r = –0.23, p = 0.033), and LA booster function (r = –0.26, p = 0.018).
Associations of clinical and echocardiographic parameters and atrial fibrillation in the peri-transplant period
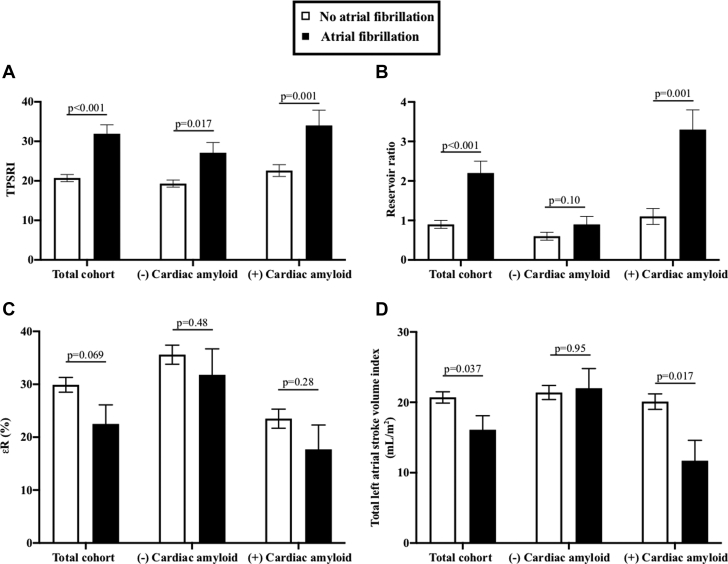
The association of both standard and LA mechanics parameters to development of AF in the peri-transplant period was evaluated with univariable analysis (Table 3). Of standard measures, only mitral E/A ratio showed an association with development of AF (p = 0.048). For LA mechanics parameters, R (p = 0.053), TPSRI (p = 0.001), reservoir ratio (ratio of TPSRI/R) (p = 0.006), were associated with development of AF in the total cohort in unadjusted analyses. Total LA stroke volume index was not statistically significant (p = 0.096). TPSRI was the only variable that was consistently associated in the total cohort as well as in subgroups with and without CA. Adjusted means (accounting for age, history of AF, and diastolic dysfunction) of LA mechanics according to development of AF stratified by the presence or absence of CA are shown in Figure 1. LA parameters were also compared according to previous AF history; these are shown in Supplemental Table 2.
Table 3.
Association of Left Atrial Parameters and Standard Echocardiographic Parameters on Development of Atrial Fibrillation
| Total Cohort (N = 91) |
(–) Cardiac Amyloid (n = 49) |
(+) Cardiac Amyloid (n = 42) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR∗ | 95% CI | p Value | OR∗ | 95% CI | p value | OR∗ | 95% CI | p Value | |
| Left atrial mechanics | |||||||||
| R | 0.94 | 0.89–1.00 | 0.053 | 0.96 | 0.87–1.06 | 0.43 | 0.92 | 0.83–1.02 | 0.11 |
| CT | 0.95 | 0.87–1.04 | 0.25 | 1.04 | 0.92–1.17 | 0.53 | 0.86 | 0.72–1.102 | 0.079 |
| TPSRI | 1.16 | 1.06–1.26 | 0.001 | 1.22 | 1.02–1.46 | 0.033 | 1.14 | 1.03–1.26 | 0.011 |
| Reservoir ratio (TPSRI/R) | 2.51 | 1.30–4.85 | 0.006 | 4.82 | 0.66–35.19 | 0.12 | 2.57 | 1.15–5.74 | 0.021 |
| LA total SV index | 0.91 | 0.82–1.02 | 0.096 | 1.02 | 0.87–1.18 | 0.84 | 0.79 | 0.64–0.98 | 0.036 |
| LA passive SV index | 0.95 | 0.83–1.08 | 0.41 | 0.94 | 0.78–1.14 | 0.52 | 0.96 | 0.79–1.16 | 0.65 |
| LA active SV index | 0.90 | 0.77–1.04 | 0.15 | 1.07 | 0.92–1.25 | 0.42 | 0.67 | 0.47–0.96 | 0.029 |
| Reservoir function | 1.00 | 0.99–1.01 | 0.39 | 1.01 | 0.99–1.02 | 0.48 | 0.99 | 0.97–1.01 | 0.17 |
| Conduit function | 0.98 | 0.93–1.03 | 0.45 | 0.96 | 0.89–1.03 | 0.25 | 1.01 | 0.94–1.09 | 0.70 |
| Booster function | 0.97 | 0.93–1.01 | 0.16 | 1.05 | 0.97–1.14 | 0.25 | 0.93 | 0.86–1.00 | 0.036 |
| Standard echocardiographic parameters | |||||||||
| Diastolic grade | 0.90 | 0.60–1.37 | 0.63 | 0.95 | 0.55–1.63 | 0.85 | 0.84 | 0.43–1.67 | 0.63 |
| Interventricular septum | 1.09 | 0.88–1.35 | 0.42 | 1.15 | 0.66–1.99 | 0.62 | 1.02 | 0.77–1.36 | 0.88 |
| Mitral regurgitation severity | 1.42 | 0.59–3.40 | 0.44 | 1.50 | 0.40–5.64 | 0.55 | 1.21 | 0.36–4.08 | 0.76 |
| Mitral E/A ratio | 2.09 | 1.01–4.34 | 0.048 | 2.06 | 0.38–11.10 | 0.40 | 2.06 | 0.83–5.12 | 0.12 |
| Mean e’ velocity | 0.94 | 0.70–1.26 | 0.68 | 0.89 | 0.55–1.45 | 0.66 | 1.08 | 0.69–1.70 | 0.73 |
| LA volume indexed to BSA | 0.98 | 0.93–1.04 | 0.57 | 0.99 | 0.89–1.11 | 0.67 | 0.97 | 0.90–1.04 | 0.33 |
CI = confidence interval; BSA = body surface area; εR = left atrial reservoir strain (peak left atrial longitudinal strain during ventricular systole); εCT = left atrial contractile strain (peak left-atrial longitudinal strain during atrial contraction); LA = left atrium; OR = odds ratio; SV = stroke volume; TPSRI = time-to-peak strain rate indexed to R-R interval.
Odds ratio reflects univariable logistic regression.
Figure 1.
Left Atrial Strain Measurements in Patients With and Without Development of Atrial Fibrillation Among the Total Cohort and Subgroups (With and Without Cardiac Amyloid Involvement)
(A) Differences in TPSRI; (B) Differences in reservoir ratio; (C) Differences in R; (D) Differences in total LA stroke volume index. Values represent adjusted mean and SE, after accounting for age, history of atrial fibrillation, and diastolic dysfunction. R = left atrial reservoir strain (peak left atrial longitudinal strain during ventricular systole); LA = left atrial; SE = standard error; TPSRI = time-to-peak strain rate indexed to R-R interval (reservoir ratio represents TPSRi/R).
Discussion
Among patients with AL amyloidosis undergoing HDM/SCT, the development of AF leads to significant morbidity in the peri-transplant period. Clinical predictors of AF, however, are highly prevalent in these patients (e.g., diastolic dysfunction, LA dilation) and lack specificity in identifying high-risk individuals. Our study found TPSRI, the time to peak SR during atrial filling in the LA reservoir phase (indexed to R-R interval), was significantly prolonged in patients developing AF in the peri-transplant period, with more pronounced delay in those with cardiac involvement.
LA reservoir function impairment in AL amyloidosis
LA function facilitates LV filling by functioning as a reservoir during LV systole from the pulmonary veins, a direct conduit from pulmonary veins to LV during early ventricular diastole, and serving as an atrial booster pump for LV filling during late diastole (LA contraction phase) (25). With the advent of LA strain deformation technology, impairments in these 3 phases have lent insight into aberrations of atrial function in cardiovascular disease, particularly in AF. LA mechanics, assessed by LA strain and strain rate, recently have been shown to associate with paroxysmal and persistent AF and with AF burden (13,26).
LA reservoir strain (R), in particular, has shown strength in AF studies in terms of prediction of development of recurrence of AF and providing incremental risk prediction of cardiovascular outcomes over CHADS2 risk assessment (14, 15, 16,27). Expert consensus guidelines regarding patients with AF suggest that R <30% indicates significantly impaired reservoir function and is associated with poor outcomes (28). Of note, in our whole cohort, the mean R was reduced at 29%, indicating higher risk among all patients; in patients with development of AF, the mean R was even further reduced at 23 ± 11%. Furthermore, R was lowest among patients with CA and development of AF peri-transplant at 17 ± 7%. However, this metric of reservoir function was not significantly associated with development of AF in this amyloidosis cohort. In assessing reservoir ratio (a ratio of TPSRI/R) to assess strain and SR information (mechanical dyssynchrony) from the reservoir phase, this ratio helped differentiate between development of AF and non-AF development in both the total cohort and cardiac cohort.
Mechanical dyssynchrony in AL amyloidosis
TPSRI was consistently prolonged in patients with development of AF compared with those without development of AF, and this relationship persisted in subgroups with and without CA involvement. Several studies have implicated time to peak of strain as an index of LA mechanical dyssynchrony in paroxysmal AF in a variety of cohorts (29, 30, 31, 32). Mechanical dyssynchrony in paroxysmal AF is associated with low-voltage zones by electrophysiological mapping, which has corresponded to LA fibrosis (26,32,33). It is intriguing that our results suggest this prolongation in TPSRI and relationship to development of AF persists even among the subset of patients without known CA, thus providing useful information about the mechanical properties of the LA and gaining insight to all patients with higher risk for development of AF in AL amyloidosis.
Importantly, the patients with the greatest prolongation in TPSRI were among the patients with CA, particularly those with development of AF. An elegant electrophysiological study by Li et al. (34), in 1999, evaluated the LA substrate preceding development of AF in dogs with heart failure (HF) compared with controls and found that dogs with HF exhibited increased heterogeneity in atrial conduction. Histologically, these dogs with HF had evidence of extensive atrial interstitial fibrosis, cell loss, and hypertrophy with less tightly packed atrial myofibrils (34). Amyloid cardiomyopathy has also been shown histologically to have heterogeneous infiltration in the LV with a small proportion of fibrosis present (35,36). Thus, the underlying mechanism of more extensive mechanical atrial dyssynchrony in CA (extensive infiltration of amyloid protein in the atrium, fibrosis, or other pathologic explanation) is not currently well understood and requires dedicated histologic correlation in future investigations.
Clinical implications
Our center and other groups have previously reported that development of AF in the peri-transplant period for AL amyloidosis leads to more hypotension, with more patients with AF requiring vasopressors and admission to intensive care units (6,17). Clinical characteristics such as older age, previous history of AF, and diastolic dysfunction are associated with AF following HDM/SCT, but these features are highly prevalent in these patients, particularly those with CA, and lack specificity in identifying which patients may be at highest risk for AF and subsequent morbidity in the peri-transplant period. In addition, diastolic function is often normal in patients with AL amyloidosis without cardiac involvement and, thus, it is not an effective predictor of development of AF in patients without CA undergoing SCT. Not surprisingly, a consistent principal risk factor for development of AF has been previous history of AF; however, previous history of AF was only present in 33% of patients developing AF in the peri-transplant period (6). In this study, standard echocardiographic parameters, such as LVEF, wall thickness, and LA size, were not associated with development of AF. Our current study underscores the potential predictive strength of atrial mechanics—specifically, LA mechanical dyssynchrony using TPSRI—in associating with development of AF in patients with AL amyloidosis in the peri-transplant period. The addition of this parameter to risk assessment pre-transplant may lend to early identification of high-risk potential patients to consider preventative therapeutics for mitigation of risk.
Importantly, along with other markers of LA mechanical dyssynchrony consistently noted in several studies of prediction of AF among different cohorts (29, 30, 31), TPSRI may prove clinically useful as an independent predictor for development of AF in other high-risk cohorts. In CA, it may allow early insight regarding atrial electromechanical disassociation, an entity characterized by reduced or absent atrial mechanical function in the context of normal sinus rhythm, which often leads to devastating clinical outcomes of intracardiac thrombus or thromboembolism.
Finally, subclinical or uncertain cardiac involvement in patients with AL amyloidosis with normal or only mildly increased wall thickness, preserved ejection fraction, borderline or mildly abnormal global longitudinal LV strain, and mildly elevated cardiac biomarkers, and/or abnormal electrocardiographic findings are often seen clinically. Our study reveals that all patients with AL amyloidosis, irrespective of whether they have known CA, with LA dyssynchrony discerned by a prolonged TPSRI are at increased risk for development of AF and carry higher risk. Whether these LA mechanical changes perhaps reflect subclinical and early AL cardiac involvement warrants additional evaluation.
Study limitations
Limitations of this study include the retrospective nature of the analysis and, importantly, the small sample size from a single institution. Nearly one-half of the patients with AL amyloidosis who were deemed to have cardiac involvement were determined by criteria consistent with the International Society of Amyloidosis consensus guidelines for cardiac involvement. However, not all patients underwent cardiac biopsy for diagnosis; in some cases, patients classified into noncardiac AL disease could not be absolutely ruled out for subclinical cardiac involvement of their AL amyloidosis. We did not have histological samples to correlate pathology with the findings of LA mechanical dyssynchrony and the underlying pathophysiological etiology of the dyssynchrony noted in these patients; thus, further investigation for the pathophysiology is warranted. Finally, we acknowledge that our number of AF events is small (n = 12), limiting our abilities to build multivariable models and adjust for potential confounders. As a result, our logistic regression analyses were limited to univariable models only. However, our cohort of patients with AL amyloidosis undergoing HDM/SCT (in sinus rhythm) is relatively large for the field, and our AF rate is comparable with other studies evaluating supraventricular arrhythmias following SCT (2). These findings provide motivation for larger, multicenter studies.
Conclusions
LA mechanics—specifically, TPSRI—is associated with development of AF in the peri-transplant period for treatment of AL amyloidosis in both patients with and without CA. We postulate that TPSRI may also serve as an important metric in assessing LA dyssynchrony not only in AL amyloidosis and cardiac amyloidosis but in other high-risk cohorts for development of paroxysmal AF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study highlights that >10% of individuals undergoing high-dose melphalan and stem-cell transplantation for light-chain amyloidosis develop paroxysmal AF. Although standard clinical and echocardiographic parameters did not differ between patients who did and did not develop AF, left atrial strain parameters, such as reservoir ratio (ratio of TPSRI/R) and time-to-peak strain rate (indexed to R-R interval) (TPSRI), did associate with patients who developed AF. TPSRI was prolonged not only the total cohort but also in the subgroups with and without CA who developed AF.
TRANSLATIONAL OUTLOOK: Additional research is warranted to validate our initial findings in larger multicenter cohorts of AL amyloidosis post-stem cell transplantation as well as to extend the role of left atrial mechanics in prediction of AF in both light-chain and transthyretin cardiac amyloidosis. With further study, development of predictive clinical tools and strategies to mitigate risk in these patients can contribute to the improvement of cardiovascular outcomes and quality of life in patients with amyloidosis.
Author Disclosures
This study was supported by grants from the American Heart Association (FTF 17FTF33670369, to Dr. Gopal) and the National Center for Advancing Translational Sciences, National Institutes of Health (1UL1TR001430, Boston University CTSI). Dr. Ruberg has received research grants from Pfizer, Eidos Therapeutics, and Akcea Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Hidalgo J.D., Krone R., Rich M.W. Supraventricular tachyarrhythmias after hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant. 2004;34:615–619. doi: 10.1038/sj.bmt.1704623. [DOI] [PubMed] [Google Scholar]
- 2.Singla A., Hogan W.J., Ansell S.M. Incidence of supraventricular arrhythmias during autologous peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1233–1237. doi: 10.1016/j.bbmt.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sureddi R.K., Amani F., Hebbar P. Atrial fibrillation following autologous stem cell transplantation in patients with multiple myeloma: incidence and risk factors. Ther Adv Cardiovasc Dis. 2012;6:229–236. doi: 10.1177/1753944712464102. [DOI] [PubMed] [Google Scholar]
- 4.Cibeira M.T., Sanchorawala V., Seldin D.C. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–4352. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchorawala V., Sun F., Quillen K., Sloan J.M., Berk J.L., Seldin D.C. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126:2345–2347. doi: 10.1182/blood-2015-08-662726. [DOI] [PubMed] [Google Scholar]
- 6.Arun M., Brauneis D., Doros G. The incidence of atrial fibrillation among patients with AL amyloidosis undergoing high-dose melphalan and stem cell transplantation: experience at a single institution. Bone Marrow Transplant. 2017;52:1349–1351. doi: 10.1038/bmt.2017.148. [DOI] [PubMed] [Google Scholar]
- 7.Fatema K., Gertz M.A., Barnes M.E. Acute weight gain and diastolic dysfunction as a potent risk complex for post stem cell transplant atrial fibrillation. Am J Hematol. 2009;84:499–503. doi: 10.1002/ajh.21459. [DOI] [PubMed] [Google Scholar]
- 8.Mathur P., Paydak H., Thanendrarajan S., van Rhee F. Atrial fibrillation in hematologic malignancies, especially after autologous hematopoietic stem cell transplantation: review of risk factors, current management, and future directions. Clin Lymphoma Myeloma Leuk. 2016;16:70–75. doi: 10.1016/j.clml.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Steuter J.A., Villanueva M.L., Loberiza F.R. Factors affecting the development of atrial fibrillation and atrial flutter (AF/AFL) following autologous hematopoietic SCT (auto-HSCT) Bone Marrow Transplant. 2013;48:963–965. doi: 10.1038/bmt.2012.253. [DOI] [PubMed] [Google Scholar]
- 10.Feliz V., Saiyad S., Ramarao S.M., Khan H., Leonelli F., Guglin M. Melphalan-Induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34:356–359. doi: 10.1002/clc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debonnaire P., Joyce E., Hiemstra Y. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004052. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki A., Yuda S., Oi Y. Assessment of left atrial deformation and synchrony by three-dimensional speckle-tracking echocardiography: comparative studies in healthy subjects and patients with atrial fibrillation. J Am Soc Echocardiogr. 2013;26:165–174. doi: 10.1016/j.echo.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Schaaf M., Andre P., Altman M. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2017;18:46–53. doi: 10.1093/ehjci/jew028. [DOI] [PubMed] [Google Scholar]
- 14.Obokata M., Negishi K., Kurosawa K. Left atrial strain provides incremental value for embolism risk stratification over CHA(2)DS(2)-VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr. 2014;27:709–716.e4. doi: 10.1016/j.echo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Pathan F., Sivaraj E., Negishi K. Use of atrial strain to predict atrial fibrillation after cerebral ischemia. J Am Coll Cardiol Img. 2018;11:1557–1565. doi: 10.1016/j.jcmg.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Saha S.K., Anderson P.L., Caracciolo G. Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr. 2011;24:506–512. doi: 10.1016/j.echo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith Y.B., Liu J., Chou J., Hoffman J., Comenzo R.L., Steingart R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;104:990–994. doi: 10.1016/j.amjcard.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Nochioka K., Quarta C.C., Claggett B. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18:1128–1137. doi: 10.1093/ehjci/jex097. [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Saraiva R.M., Demirkol S., Buakhamsri A. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–180. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 22.From Molecular Mechanisms Toward the Cure of Systemic Amyloidoses. Abstracts of the XII International Symposium on Amyloidosis. April 18–21, 2010. Rome, Italy. Amyloid. 2010;17(Suppl 1):48–49. doi: 10.3109/13506121003737385. [DOI] [PubMed] [Google Scholar]
- 23.Lilleness B., Ruberg F.L., Mussinelli R., Doros G., Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133:215–223. doi: 10.1182/blood-2018-06-858951. [DOI] [PubMed] [Google Scholar]
- 24.Palladini G., Hegenbart U., Milani P. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 25.Hoit B.D. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 26.Kuppahally S.S., Akoum N., Burgon N.S. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 27.Motoki H., Negishi K., Kusunose K. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr. 2014;27:1184–1192. doi: 10.1016/j.echo.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donal E., Lip G.Y., Galderisi M. EACVI/EHRA expert consensus document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17:355–383. doi: 10.1093/ehjci/jev354. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa A., Ishii K., Hyodo E. Three-dimensional speckle tracking imaging for assessing left atrial function in hypertensive patients with paroxysmal atrial fibrillation. Int Heart J. 2016;57:705–711. doi: 10.1536/ihj.16-121. [DOI] [PubMed] [Google Scholar]
- 30.Loghin C., Karimzadehnajar K., Ekeruo I.A., Mukerji S.S., Memon N.B., Kantharia B.K. Outcome of pulmonary vein isolation ablation for paroxysmal atrial fibrillation: predictive role of left atrial mechanical dyssynchrony by speckle tracking echocardiography. J Interv Card Electrophysiol. 2014;39:7–15. doi: 10.1007/s10840-013-9841-3. [DOI] [PubMed] [Google Scholar]
- 31.Shang Z., Su D., Cong T. Assessment of left atrial mechanical function and synchrony in paroxysmal atrial fibrillation with two-dimensional speckle tracking echocardiography. Echocardiography. 2017;34:176–183. doi: 10.1111/echo.13434. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe Y., Nakano Y., Hidaka T. Mechanical and substrate abnormalities of the left atrium assessed by 3-dimensional speckle-tracking echocardiography and electroanatomic mapping system in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2015;12:490–497. doi: 10.1016/j.hrthm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Oakes R.S., Badger T.J., Kholmovski E.G. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D., Fareh S., Leung T.K., Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 35.Leone O., Longhi S., Quarta C.C. New pathological insights into cardiac amyloidosis: implications for non-invasive diagnosis. Amyloid. 2012;19:99–105. doi: 10.3109/13506129.2012.684810. [DOI] [PubMed] [Google Scholar]
- 36.Maceira A.M., Joshi J., Prasad S.K. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.