Abstract
We report on an innovative, fabric-based conformable, and easily fabricated electroceutical wound dressing that inhibits bacterial biofilm infections and shows significant promise for healing chronic wounds. Cyclic voltammetry demonstrates the ability of the electroceutical to produce reactive oxygen species, primarily HOCl that is responsible for bacterial inhibition. In vitro investigation with the lawn biofilm grown on a soft tissue mimic assay shows the efficacy of the dressing against both gram-positive and gram-negative bacteria in the biofilm form. In vivo, the printed electroceutical dressing was utilized as an intervention treatment for a canine subject with a non-healing wound due to a year-long persistent polymicrobial infection. The clinical case study with the canine subject exhibited the applicability in a clinical setting with the results showing infection inhibition within 11 days of initial treatment. This printed electroceutical dressing was integrated with a Bluetooth® enabled circuit allowing remote monitoring of the current flow within the wound bed. The potential to monitor wounds remotely in real-time with a Bluetooth® enabled circuit proposes a new physical biomarker for management of infected, chronic wounds.
Keywords: Electroceuticals, Dressing, Wound, Chronic, Biofilm, Treatment
I. INTRODUCTION
CHRONIC or non-healing wounds present a major healthcare burden on the US with an estimated $25 billion in cost for nearly 6.5 million human patients per year [1]. While there is limited data available for animal populations, chronic wound management in domestic and livestock animals are estimated to cost an additional $1.1 billion by 2021 with an expected 7% increase per year [2]. In human populations, 60% of chronic wounds have biofilm infections making them recalcitrant to state-of-art treatment, including antibiotics [3]–[5]. In the biofilm form, bacteria form ‘protective shields’ comprising of extracellular polymeric substances (EPS) generated by the bacterial cells which subsequently encapsulate the bacteria [6]. The EPS matrix promotes adhesion to both the wound surface and between bacterial cells, forming a 3D structure that protects the bacteria from antimicrobials and host immune defenses [6].
The EPS matrix also alters the nutrient and chemical gradients within the wound environment, providing additional defenses that make eradicating biofilms a difficult challenge, even for the current state-of-art treatment methods [7], [8].
Recently, electroceutical dressings that apply potential gradients to induce electric fields and may permit electrical current flow have become an emerging method for inhibition of bacterial biofilms [9], [10]. However, the commercially available electroceutical dressings lack a mechanistic understanding of the treatment outcomes. Commercially available and approved by the food and drug administration (FDA), passive dressings such as Arthrex and Procellera apply electric fields to the wound but have no current flow [9]. These dressings use the silver (Ag)-zinc (Zn) redox couple organized as electrically discontinuous dots. The redox couple is activated when a conducting fluid (e.g., saline or wound fluid) contacts the metal pattern on a polyester fabric to generate a measurable open circuit potential. In the presence of an adequate redox potential, reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) may be generated and act as antimicrobials for shallow or thin infection layers (< 0.25 mm) that may occur in wound beds [9].
On the other hand, direct current (DC) has proven to have inhibitory action against planktonic bacteria in systems that are both static and flowing [10], [11]. There have also been displays of the treatment efficacy of DC against bacterial biofilms of Pseudomonas aeruginosa [16] and Staphylococcus epidermidis [12], but many of these past studies used liquid media to study biofilms that is not representative of wounds, containing a soft tissue bed over which the biofilm forms. Our lawn biofilm grown on a soft tissue mimic assay provided a significant methodological advance in developing tools for analyzing the mechanistic actions of our printed electroceutical dressing (PED) [13]. In the present study we report the bacterial inhibitory mechanisms and efficacy of our device both in vitro and in vivo with a new physical biomarker proposed with the potential to integrate real-time, remote wound monitoring.
II. FABRICATION
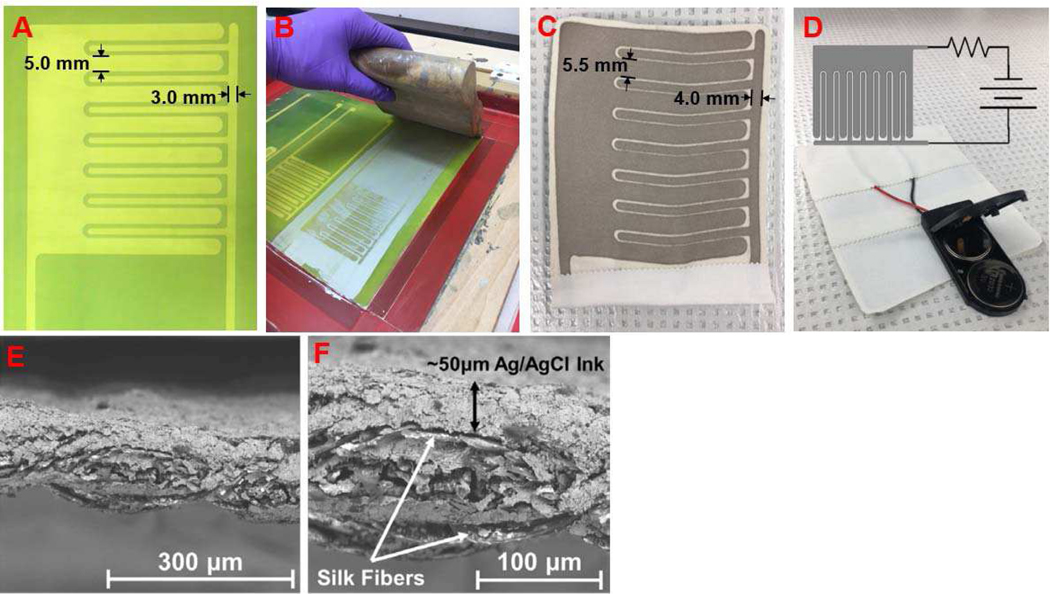
Our novel, printed electroceutical dressing was made by a facile silk-screen printing process for use on flexible substrates like fabric. The pattern screen was fabricated by coating a 196 amber polymer mesh with a photoactive polymer (Speedball Diazo Photo Emulsion). The screen was flood exposed using a 200 W clear incandescent bulb for 70 minutes showing the relatively simple infrastructure needed for PED fabrication. The photo-emulsion was then rinsed using regular, laboratory-grade filtered water, revealing the visible PED pattern (Fig. 1A). The nominal dimensions of the anode and cathode were 5 mm and 3 mm width with a 1 mm separation arranged in an interdigitated pattern to maximize electrode coverage over the 7.5 cm × 7.5 cm substrate. Next, the PED pattern was screen-printed by using a medically compatible Ag/AgCl ink (Creative Materials #113–09) onto habotai silk substrates (Fig. 1B). Silk-based materials are commonly used for wound dressings [14] and the habotai weave provides a breathable, lightweight fabric with accepted biocompatibility [15]. The screen-printing process is fast and enables high throughput [16] but also leads to differences in nominal vs. actual electrode sizes. The absorption of the ink onto a porous substrate combined with dispersion along the substrate lead to measured anode and cathode widths to be 5.5 mm and 4.0 mm (Fig. 1C) with an uneven (< 1 mm) spacing between the electrodes. Each electrode set is manually checked with a handheld multimeter before use to confirm that the entire electrical pattern is continuous without electrical shorts. The printed electrodes were then epoxied to the battery pack with two 3V batteries in series and a current limiting 1 k∖ resistor (Fig 1D). Medical tape was then used to cover the circuit connections and also provide a fluid and electrical isolation layer leaving the battery pack switch and the electrodes accessible for use. The microstructure of the printed Ag/AgCl ink is seen in the scanning electron microscopy (SEM) images in figures 1E and 1F.
Fig. 1.
(a) The screen with developed pattern for screen printing the PED electrodes. (b) The Ag/AgCl ink was spread over the screen mesh and manually printed using a squeegee. (c) The resultant printed design on the silk substrate with actual dimensions as marked due to ink spreading on porous substrate. (d) The printed electrodes were connected to the battery pack with two 3V batteries in series and a current limiting resistor. The open electrical connections were isolated by medical tape. Overlaid is a schematic for the working circuit diagram. (e) An scanning electron microscopy (SEM) image of the Ag/AgCl ink printed onto the silk fabric. (f) SEM image of the fabric-electrode cross-section shows the ink is absorbed within the fabric with a ∼50 μm ink layer on the silk fabric.
III. RESULTS
A. Soft Tissue Mimic Assay
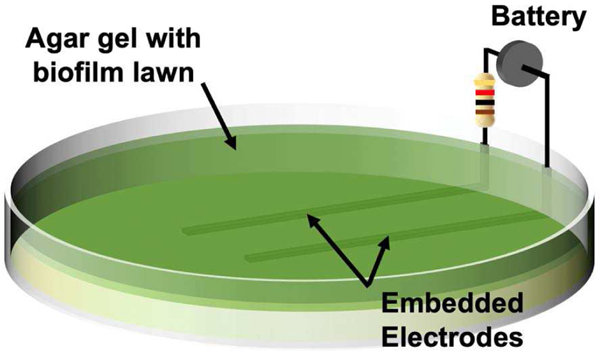
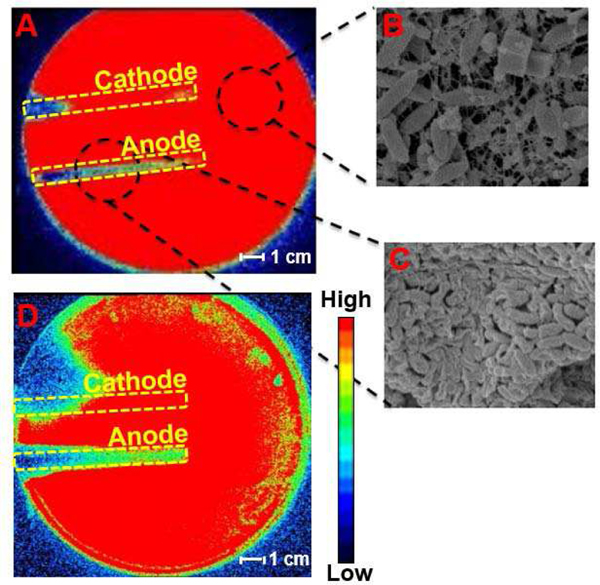
In vitro investigation displays biofilm inhibition as a result of PED operation using our lawn biofilm soft tissue assay [13]. For direct analysis of the antimicrobial activity of the PED, two geometrically simplified electrodes (3 mm wide × 8 cm long, separated by 3 cm) were embedded within agar on which 24 hour biofilm lawns of bioluminescent strains of Pseudomonas aeruginosa Xen-41 (PA) or Staphylococcus aureus SAP231 (SA) (two common pathogens found in infected wounds representing both gram-negative and gram-positive bacteria respectively) and connected to a PED equivalent circuit. The 6V battery with a 1 kΩ resistor had an active connection with the electrodes embedded in the agar for 24 h (Fig 2). In Vivo Imaging System (IVIS) images were used to evaluate the inactivation of the biofilm bacteria over the agar gel in which the electrodes were embedded. The bioluminescent biofilms of Pseudomonas aeruginosa (Fig. 3A) and Staphylococcus aureus (Fig. 3D) are indicated by red within the IVIS images where the bacteria are present and metabolically active. The blue (and other darker colors) indicate the absence of bacteria or metabolically inactive bacteria. For both biofilm species, the anode and cathode displayed different antimicrobial activity, with bacterial inhibition occurring primarily over the anode (Fig 3). Electrochemical reactions caused by the powered electrodes produce ROS, specifically HOCl [11] that is responsible for the damage to the bacterial cells over the anode. SEM images show the EPS present in the bacteria on areas away from the electrodes (Fig 3B) while the EPS is absent the regions over the anode (Fig 3C), indicating the breakdown of the biofilm. The bacteria over the cathode displayed less inhibition than over the anode. While the area of inhibition for Staphylococcus aureus appears fairly large, its inhibitory effects along the length of the cathode towards the center of the petri dish remain limited in comparison to the anode. The bacterial inhibition across both biofilms indicates the efficacy of the PED against both gram-positive and gram-negative bacteria with the specific differences in the treatment response being the subject of continued research.
Fig. 2.
Two geometrically simplified silver electrodes depicting the anodic and cathodic PED response are embedded in an agar gel with a 24 h PA lawn. The electrodes are connected to a 6V battery and 1 kΩ ballast resistor for 24 hr.
Fig. 3.
(a) The IVIS image of post-24 h treatment of the powered electrodes to PA 24 hr lawn. Red represents metabolically active/live and blue/black represents metabolically inactive or killed bacteria. (b) The SEM image of the PA biofilm on the area of the gel not directly above the electrodes acts as control for untreated area. (c) The SEM image of the PA biofilm on an area directly above the anode that was powered for 24 hr. The breakdown of EPS is visible. (d) The IVIS image of post-24 h treatment of the powered electrodes to SA 24 h lawn.
B. Real-Time Wound Monitoring
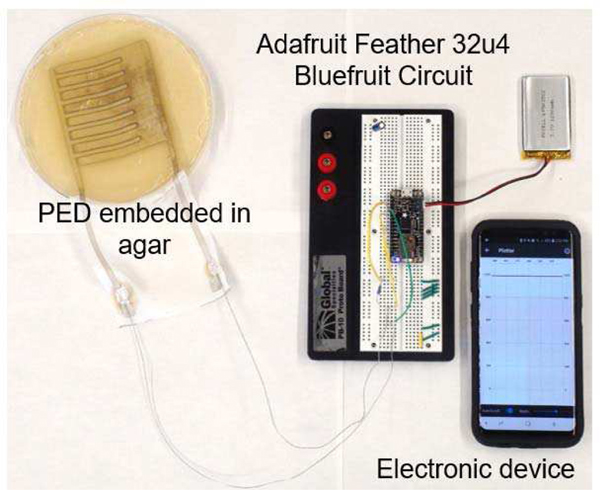
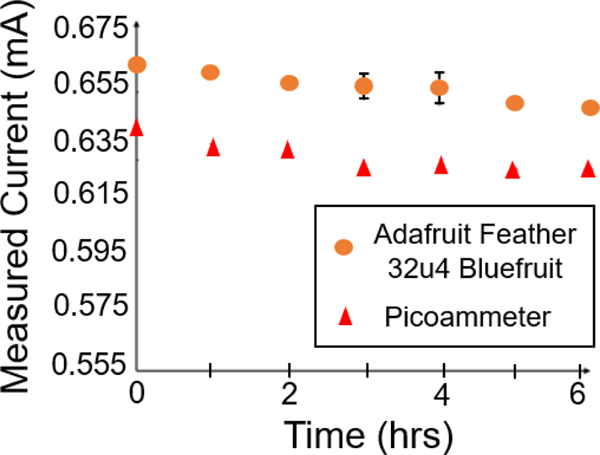
State of art clinical practice requires frequent visual inspections of the wound in order to determine healing outcomes including estimating infection clearance. The visual inspections are generally carried out by trained medical professionals adding significant work burden to the practice of wound management, especially for chronic wounds. In this work, we report on a potentially new way to assist clinicians by allowing remote monitoring of the wound site. Since the PED is an electroceutical dressing with active electrodes, the PED provides an opportunity to reduce the need for visual inspections and provide quantitative wound healing metrics. Therefore, we propose a new physical biomarker for healing. An initial in vitro test with the soft tissue assay demonstrated Bluetooth® integration of the PED with an Adafruit Feather 32u4 Bluefruit that records and transmits up to 20 ft real-time current monitoring (Fig 4). An Arduino IDE was used to measure the voltage drop across a known ballast resistor (10 kΩ). This measured voltage drop was pushed out via Bluetooth®, on the Adafruit Feather 32u4, to an Android application for a readout on a mobile phone. The reported current was post processed for a calculated current through the ballast resistor. The internal resistance of the Bluetooth® enabled monitoring circuit is lower than the resistance of the agar gel assay during a direct measurement leading to a higher recorded current than when measured directly (Fig 5) using an ammeter due to the direct connections with shorter, silver wires for the bluetooth enabled circuit. However, the trend over time is the same and may be sufficient for real-time wound monitoring.
Fig. 4.
The capability of the PED to be connected to a Bluetooth circuit for wound monitoring. The PED was embedded in the agar gel to measure the current flow via the Bluetooth circuit with the data read out displayed on the electronic device (cellular phone).
Fig. 5.
The Adafruit Feather 32u4 Bluefruit module with Arduino IDE and picoammeter current measurements recorded over time while the PED was embedded into the agar gel.
C. Cyclic Voltammetry
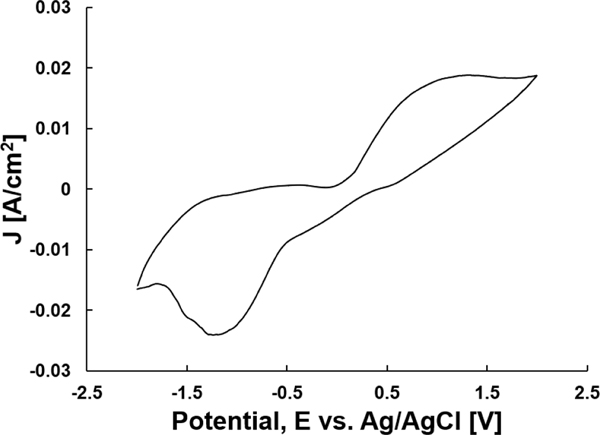
To further analyze the electrochemical reactions and ROS produced by the PED, cyclic voltammetry (CV) of the PED within the soft tissue agar assay was performed (Fig 6). The electric potential sweep started at −2 V and switched sweeping direction upon reaching +2 V at a scan rate of 200 mV/s. It has been previously reported that at an applied electrode potential of 1.38 V, HOCl may be generated as the primary ROS [11]. At an applied potential of −0.6 V, H2O2 is produced [15]. The flow of current present at these two potentials with our system shows that by altering the electrode potential, we can control the ROS produced.
Fig. 6.
Cyclic voltammogram of the PED embedded in the soft tissue agar gel to evaluate the products of the electrochemical products. The cycle had a starting potential of −2V and a switching potential of +2V at a scan rate of 200 mV/s.
The maximum and minimum current peaks present in Figure 6 are asymmetrical in their magnitudes and applied potential with respect to the zero-applied potential. This asymmetric shape to the CV profile indicates that the electroceutical treatment of biofilms grown on agar is electrochemically irreversible i.e., non-Nernstian [18]. The irreversibility indicates that the concentration of species needed to produce ROS are the limiting steps for the electrochemical reaction rates and not the transport of the species to and from the electrodes. Due to this non-Nernstian response, cyclic voltammetry is not an ideal technique to quantitatively evaluate HOCl concentrations even though the actively flowing current measured indicates the production of the ROS at their respective potentials [18].
D. Canine Case Study
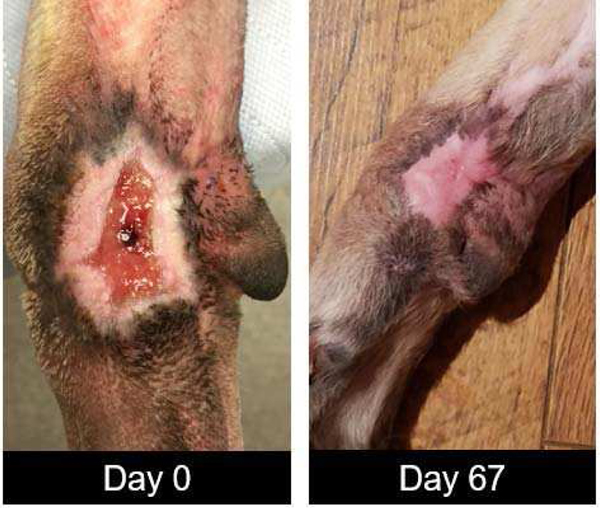
In vivo, the PED was used in a clinical case study as an experimental treatment for the therapeutic intervention on a non-healing and chronically infected wound. The wound was on the left front leg of a canine subject. Originally, the wound presented with a polymicrobial infection that was treated with open wound management for fifteen days followed by a skin graft and a negative pressure dressing as part of the state-of-art wound care at Ohio State’s small animal clinic in the College of Veterinary Medicine. Significant topical antibiotics were used to treat a persistent infection with the overall treatment and wound management lasting over a year. The wound was found to be non-healing with a continued polymicrobial infection. A tissue culture from punch biopsy revealed the infection contained primarily Staphylococcus pseudintermedius and Streptococcus canis. At this stage, as an experimental treatment, the PED (Fig. 1) was connected to a 6V battery with a ballast resistor limiting the peak current to 0.6 mA. After 11 days of PED treatment, a punch biopsy revealed no pathologically detected wound infection. The canine was released to in-home care with no additional treatment other than standard open wound care recommended with no topical or systemic antimicrobials. The next clinical inspection was possible (since the subject’s home city was hours away) only 67 days later when the canine subject was brought back to the clinic for visual wound inspection showing complete healing of the wound. (Fig 7).
Fig. 7.
Day 0, before the PED was applied with the polymicrobial infection. Day 67, following the beginning of PED treatment with a fully closed wound.
IV. DISCUSSION
The in vitro investigations of the PED reveal bacterial inhibition to be primarily over the anode for both gram-positive and gram-negative bacteria. This localized inhibition motivates the design of the electrodes to favor a large anode (Fig. 1). The positive potential held at the anode favors the production of HOCl [11] responsible for the bacterial clearance displayed in both Staphylococcus aureus and Pseudomonas aeruginosa biofilms [13]. The limited bacterial inhibition observed at the cathode may be due to the antimicrobial action of H2O2, a ROS that is favored at negative potentials [17], and also likely to be produced within our system. H2O2 has also been shown to be an effective agent against Pseudomonas aeruginosa [19]. However, Staphylococcus aureus recovers from the initial oxidative stress that is induced by H2O2 and the cells continue to grow as if they were untreated [20].
Persistent infection creates clinical complications in wound healing. For example, the polymicrobial infection present in a canine wound was not removed to allow natural healing processes to induce wound closure despite state-of-art wound care through open wound management, topical and systemic antimicrobial medications, a skin graft, and a negative pressure dressing. Following initial treatment and with mounting veterinary care costs, the canine was released to in-home care with the recommendation of open wound management. However, the presence of continued infection opposed the effectiveness of these treatments, evident in the non-healing state of the wound one year later. As noted in the results section, a punch biopsy revealed continuation of the polymicrobial infection with gram positive bacteria Staphylococcus pseudintermedius and Streptococcus canis present. The PED, which now is known to be safe for use in humans [21], demonstrated efficacy in removing persistent infection and leading to complete wound closure presents a new clinically viable method for infected, chronic wound management.
In a new finding, we developed and reported on the use of electrical current as a potential parameter to monitor wound healing. The implementation of this technology was demonstrated in vitro with the PED embedded in the agar gel assay without the lawn-biofilm. Damaged or broken skin allows a DC potential to be generated by the ‘skin battery’ due to the lowered resistance created by the presence of the electrically conducting wound-fluids in comparison to intact skin [22]. Moreover, dry and unbroken skin behaves as insulator high resistance construct to flow of electrical current with reported resistance ranging from 10 kΩ to 1 MΩ [23], [24]. The integration of Bluetooth-enabled current monitoring circuit with the PED allows additional wound evaluation methods to be incorporated. Such a method may be complementary to measurements of trans-epidermal water loss (TEWL) in quantifying the barrier function of healed skin. TEWL is a quantitative evaluation of wound closure and the restoration of skin barrier function [25]. In our initial in vitro tests, we were able to measure current flow in the gel-plate representative of tissue up to 20 feet away from the gel-plate. The current profile was recorded on a mobile phone app and is then easily transmitted to clinicians. The Adafruit Feather 32u4 Bluefruit circuit requires the addition of a resistor and battery to transmit current/voltage data to the mobile device to which it’s paired. Given such components presently compose the circuit of the PED, Bluetooth monitoring of the wound is a viable option. If such a technology can be developed and validated for clinical use, the use of a simple physical parameter based on direct measurement of electrical current within the wound bed can be an easily monitored parameter for state of wound healing without active visual inspections by trained medical professionals. Clearly, such validation is beyond the scope of this paper and is part of our on-going efforts.
V. CONCLUSION
We report on a novel, printed electroceutical dressing that inhibits biofilms and has a potential to provide physical metrics for real-time wound monitoring. Screen printing Ag/AgCl ink onto silk substrates generates a fast and inexpensive fabrication process for high throughput. Through the connection of a 6V battery and current limiting resistor, the PED showed efficacy in the soft tissue biofilm assay due to the production of ROS. HOCl is the main contributor to the antimicrobial effect we observe primarily over the anode. The PED was used to treat a chronic wound in vivo in a canine subject.
ACKNOWLEDGMENT
We thank The Ohio State University Public Health Preparedness for Infectious Disease (PHPID) Transdisciplinary Team Grant, Departments of Microbial Infection and Immunity, Mechanical and Aerospace Engineering. We acknowledge supports from the staff at campus microscopy and imaging facility (CMIF) this facility is supported in part by grant P30 CA016058, National Cancer Institute, Bethesda, MD, and center for electron microscopy and analysis (CEMAS) at The Ohio State University for assistance in various imaging and experimental aspects of this research effort.
Partial personnel support is acknowledged from the National Institute of Health for grants R01HL141941 (SP) and R01GM124436 (PS).
REFERENCES
- [1].Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, and Longaker MT, “Human skin wounds: a major and snowballing threat to public health and the economy.” Wound Repair and Regeneration. vol. 17, no. 6, pp 763–771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. https://www.marketsandmarkets.com/Market-reports/animal-wound-care-market-253831778.html.
- [3].James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costeron JW, and Stewart PS, “Biofilms in chronic wounds.” Wound Repair and Regeneration. vol. 16, no. 1, pp. 37–44, 2008. [DOI] [PubMed] [Google Scholar]
- [4].Ammons MC, “Anti-biofilm strategies and the need for innovations in wound care.” Recent Patents on Anti-Infective Drug Discovery. vol. 5, no. 1, pp. 10–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Attinger C. and Wolcott R, “Clinically addressing biofilm in chronic wounds.” Advances in Wound Care. vol. 1, no. 3, pp. 127–132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.[] dummy reference [Google Scholar]
- [7].Koo H, Allan RN, Howlin RP, Stoodley P, and Hall-Stoodley L, “Targeting microbial biofilms: current and prospective therapeutic strategies.” Nat Rev Microbiol. vol. 15, no. 12, pp. 740–755, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lebeaux D, Ghigo JM, and Beloin C, “Biofilm related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotic.” Microbiology and Molecular Biology Reviews. vol. 78, no. 3, pp. 510–543, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Banerjee J, Ghatak PD, Roy S, Khanna S, Sequin EK, Bellman K, Dickinson BC, Suri P, Subramaniam VV, Chang CJ, and Sen CK, “Improvement of Human Keratinocyte Migration by a Redox Active Bioelectric Dressing”, PLoS One, vol. 9, no. 3, p. e89239, March 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sultana ST, Babauta JT, and Beyenal H. “Electrochemical biofilm control: a review.” Biofouling vol. 31, no. 9–10, pp. 745–758, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kiamco MM, Zmuda HM, Mohamed A, Call DR, Raval YS, Patel R, and Beyenal H. 2019. “Hypochlorous-acid-generating electrochemical scaffold for treatment of wound biofilms.” Scientific Reports. vol 9, no. 1, pp. 2683, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sandvik EL, McLeod BR, Parker AE, and Stewart PS, “Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid.” PloS ONE. vol. 8, no. 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dusane DH, Lochab V, Jones T, Peters CW, Sindeldecker D, Das A, Roy S, Sen CK, Subramaniam VV, Wozniak DJ, Prakash S, and Stoodley P, “Electroceutical Treatment of Pseudomonas aeruginosa Biofilms.” Scientific Reports. vol. 9, no. 1, pp. 1–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farokhi M, Mottaghoitalab F, Fatahi Y, Khademhosseini A, and Kaplan D, “Overview of silk fibroin use in wound dressings.” Trends in Biotechnology vol. 36 no. 9, pp. 907–922, 2018 [DOI] [PubMed] [Google Scholar]
- [15].Bennett MM. “Design, fabrication, and characterization of electroceutical bandages for treatment of chronically infected wounds.” M.S. Thesis. 2016. The Ohio State University, Columbus [Google Scholar]
- [16].Dominguez Renedo O, Alonso-Lomillo MA, and Arcos Martinez MJ, “Recent developments in the field of screen-printed electrodes and their related applications.” Talanta. vol. 73, no. 2, pp. 202–219, 2007. [DOI] [PubMed] [Google Scholar]
- [17].Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR, and Beyenal H, “Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms” Scientific Reports. vol. 5, pp. 14908, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, and Dempsey JL, “A practical beginner’s guide to cyclic voltammetry.” Journal o f Chemical Education vol. 95, no. 2, pp. 197–206, 2018. [Google Scholar]
- [19].Sultana ST, Call DR, and Beyenal H, “Eradication of Pseudomonas aeruginosa biofilms and persister cells using an electrochemical scaffold and enhanced antibiotic susceptibility.” Biofilms Microbiomes vol. 2, no. 2, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang W, Small D, Toghrol F, and Bentley W, “Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide.” Journal of Bacteriology. vol. 188, no. 4, pp. 1648–1659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roy S, Prakash S, Mathew-Steiner SS, Ghatak PD, Lochab V, Jones TH, Mohana Sundaram P, Gordillo GM, Subramaniam VV, and Sen CK. “Disposable patterned electroceutical dressing (PED-10) is safe for treatment of open clinical chronic wounds.” Advances in Wound Care. vol. 8, no. 4, pp. 149–159, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Foulds IS, Barker AT. “Human skin battery potentials and their possible role in wound healing.” British Journal of Dermatology. vol. 109, no. 5, pp. 515–522, 1983. [DOI] [PubMed] [Google Scholar]
- [23].McAdams E, “Bioelectrodes,” in Encyclopedia of Medical Devices and Instrumentation, 2nd ed., Ed Webster John G, Wiley InterScience, pp. 120–166, 2006. [Google Scholar]
- [24].Rosell J, Colominas J, Riu P, Pallas-Arney R, Webster JG. “Skin impedance from 1 Hz to MHz.” IEEE Transactions on Biomedical Engineering. vol. 35, no. 8, pp. 649–651, 1988. [DOI] [PubMed] [Google Scholar]
- [25].Roy S, Elgharalby H, Sinha M, Ganesh K, Chaney S, Mann E, Miller C, Khanna S, Bergdall V, Powell HM, Cook CH, Gordillo GM, Wozniak DJ, and Sen CK. “Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function.” Journal of Pathology. vol. 233, no. 4, pp. 331–343, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]