Abstract
Background:
The impact of nitrogen dioxide () and particulate matter with an aerodynamic diameter of less than or equal to 2.5. microns () exposures on lung function has been investigated mainly in children and less in adults. Furthermore, it is unclear whether short-term deviations of air pollutant concentration need to be considered in long-term exposure models.
Objectives:
The aims of this study were to investigate the association between short-term air pollution exposure and lung function and to assess whether short-term deviations of air pollutant concentration should be integrated into long-term exposure models.
Methods:
Short-term (daily averages 0–7 d prior) and long-term (1- and 4-y means) and concentrations were modeled using satellite, land use, and meteorological data calibrated on ground measurements. Forced expiratory volume within the first second (FEV1) of forced exhalation and forced vital capacity (FVC) were measured during a LuftiBus assessment (2003–2012) and linked to exposure information from the Swiss National Cohort for 36,085 adults (ages 18–95 y). We used multiple linear regression to estimate adjusted associations, and additionally adjusted models of long-term exposures for short-term deviations in air pollutant concentrations.
Results:
A increase in and on the day of the pulmonary function test was associated with lower FEV1 and FVC (: FEV1 [95% confidence interval: , ], FVC [, ]; : FEV1 [, ], FVC [, ]). A increase in 1-y mean was also associated with lower FEV1 (; , 0.5) and FVC (; , ), as was a increase in 1-y mean (FEV1: ; , ; FVC: ; , ). These associations were robust to adjustment for short-term deviations in the concentration of each air pollutant.
Conclusions:
Short- and long-term air pollution exposures were negatively associated with lung function, in particular long-term exposure with FVC. Our findings contribute substantially to the evidence of adverse associations between air pollution and lung function in adults. https://doi.org/10.1289/EHP7529
Introduction
Outdoor air pollution is one of the most important risk factors for respiratory and other chronic diseases, and was estimated to contribute to 3.3 million premature deaths worldwide in 2010 (Lelieveld et al. 2015; Sun and Zhu 2019). Monitoring and reducing the key sources of outdoor air pollution is a high priority for the World Health Organization (WHO 2016). Nitrogen dioxide () and fine particulate matter (, particulate matter with an aerodynamic diameter ) has been identified as an important pollutant that can penetrate into the lungs and trigger an inflammatory response (Dauchet et al. 2018; Weinmayr et al. 2010; WHO 2016).
Studies investigating the impact of outdoor air pollution on lung function have focused mainly on the effect of short-term exposures, for example, air pollutant concentrations up to 7 days prior to a pulmonary function test (PFT) (Dauchet et al. 2018; Panis et al. 2017; Rice et al. 2013; Schindler et al. 2001), or on long-term exposures, such as annual mean concentrations (Ackermann-Liebrich et al. 1997; Adam et al. 2015). Although the evidence for adverse long-term effects of air pollution on lung function is strong in children and adolescents, evidence is still weak for the adult general population (Götschi et al. 2008). Evidence from observational studies suggests negative associations between lung function in adults and short- and long-term air pollutant exposures (Ackermann-Liebrich et al. 1997; Adam et al. 2015; Dauchet et al. 2018; Edginton et al. 2019; Götschi et al. 2008; Panis et al. 2017; Rice et al. 2015, 2013; Schindler et al. 2001). Most of these studies have estimated associations with particulate matter with aerodynamic diameter less than or equal to 10 μm (), rather than with , but finer particles of can enter more deeply into the lungs than can and may represent a greater health risk (Ackermann-Liebrich et al. 1997; Dauchet et al. 2018; Panis et al. 2017). Furthermore, few previous studies have simultaneously estimated and compared associations with short- and long-term exposures.
Recent high-resolution spatiotemporal air pollution models suggest that and concentrations may vary substantially within a few days (de Hoogh et al. 2018, 2019). For example, the average value of daily mean concentrations measured across Switzerland on 8–14 February 2005 ranged from 11 to (de Hoogh et al. 2019). If lung function measures are influenced by recent air pollutant concentrations, daily variation in air pollution may distort estimated effects of long-term exposures. For example, the association between lung function and long-term air pollutant concentration may be underestimated if individuals who live in areas with low long-term air pollution complete a PFT in a place where there is high air pollution. We are aware of only two studies that have considered the potential influence of adjusting models of long-term air pollution exposures and lung function for recent air pollution exposures (on the previous day or day of lung function assessment) (Adar et al. 2015; Rice et al. 2015).
Therefore, the aims of this study were to estimate associations between lung function and recent (short-term) and exposures and to investigate whether models used to estimate association between long-term exposures and lung function should be adjusted for short-term deviations in and concentrations.
Methods
Study Design and Population
This observational study is based on data from the “LuftiBus” cohort. LuftiBus is a health promotion campaign conducted by the Zurich Lung Association (Switzerland), a not-for-profit health organization (Zurich Lung Association 2020). The campaign included a bus that traveled throughout Switzerland (and all Swiss cantons) between 2002 and 2012 providing health information and offering free spirometry to the general population. Additionally, information on smoking and self-reported height and weight was collected during the LuftiBus assessment. These data were enriched with individual data from the Swiss National Cohort (SNC), and residential and data.
The SNC is a nationwide census-based cohort (covering all residents of Switzerland) that combines anonymized individual data from the 1990 and 2000 federal population censuses and yearly registry censuses since 2010 (Bopp et al. 2009; Egger et al. 2018). The SNC provides information on education, occupation, housing, and other sociodemographic characteristics on an individual level. Education was classified according to the International Standard Classification of Education 1997 (ISCED-97) as low/medium (ISCED levels 0–3) or high (ISCED levels 4–6) (UNESCO 1997). An area-based index of socioeconomic position (SEP) in Switzerland was derived from the 2000 census based on education, occupation, and housing conditions for of the nearest households, and—as a proxy for household income—median rent per square meter of the 50 nearest rented flats, mapped to a scale ranging from 0 (lowest SEP) to 100 (highest SEP) (Panczak et al. 2012).
The LuftiBus data set and the SNC database contain information on date of birth, residential postcode, and sex. Due to unavailability of a unique person identifier, we used this set of identifiers to deterministically link the respective SNC records to individuals in the LuftiBus data set. Of the 56,147 individuals in the LuftiBus data set, 42,149 were linked to the 2000 population census or the combined 2010–2012 registry censuses (see flow chart in Figure S1). Individuals without a suitable record identified in the SNC, or with several possible matching links, were excluded from the analysis (). Data on relative humidity and temperature at the location (using postcodes) and day of the PFT measurement were obtained from the nearest meteorological station and added to the LuftiBus–SNC cohort data. We excluded individuals () and those who completed lung function assessment in 2002 (before air pollution data was available) (). Another 88 individuals were excluded because of implausible lung function parameters (forced expiratory volume in the first second [FEV1] , forced vital capacity [FVC] or ), resulting in a final study population of 36,085 adults. Because we used existing data from 2003 to 2012 and it was impossible to obtain consent from the LuftiBus participants, the Ethics Committee of the Canton of Zurich (Switzerland) gave consent on behalf of the LuftiBus participants and approved this study (BASEC-Nr. 2017–01804).
Residential and Estimates
Residential and concentrations were estimated from fine scale prediction models with data from 2005 to 2016 and data from 2003 to 2013 in Switzerland (de Hoogh et al. 2018, 2019). The model integrates measurements from 67 to 108 monitoring sites depending on the year, with a minimum of 30 measurements per day and site. The model integrates data obtained from 10 measurement sites between 2003 and 2013 and measurements from 89 monitoring sites that were converted to concentrations using empirically derived conversion factors. In both models, satellite data, land use, and meteorological parameters were considered in a geostatistical framework. The final prediction models provide estimated daily averages of and concentrations (in micrograms per cubic meter) at a spatial resolution of . Validation of the and models was performed using a 10-fold cross-validation by dividing the monitoring data randomly into 10 groups of equal size. For each of the 10 validations, in turn, the model was trained on 90% of the data and predicted and on the 10% left out. The predicted and concentrations of all the test data were then regressed against the measured and concentrations. The 10-fold cross-validations of the and models were robust, predicting 57% () and 73% () of the variation in measured and concentrations at the level and another 73% () and 89% () of the variation in the residuals at a resolution (de Hoogh et al. 2018, 2019). Finally, residential coordinate information in the SNC was used to derive daily and concentrations for each individual in the LuftiBus–SNC cohort.
Pulmonary Function Test
During the LuftiBus assessment, participants completed a spirometry test using a computerized pneumotachograph (SensorMedics® Vmax Legacy 20c spirometer run by Vision 7-2b software; VIASYS) without prior use of bronchodilator. LuftiBus technicians calibrated the device daily and were trained at least twice a year. After receiving oral instructions from the technicians, participants performed the test while sitting with a straight back and with their neck in a neutral position, without use of a nose clip, according to ATS/European Respiratory Society guidelines (American Thoracic Society 1991; Miller et al. 2005). A minimum of two acceptable tests out of a maximum of eight performed tests was required. For the analyses, we used the highest FEV1 and FVC from the two acceptable tests. FEV1 measures how much air a person can exhale within the first second of forced exhalation, and FVC measures the total amount of exhaled air during the same test.
Statistical Analysis
Our analysis was divided into two steps. In the first step, we estimated the impact of short-term and exposure on lung function. We defined “short-term” exposure as the daily mean air pollutant concentration on the day of the PFT (day 0) and each of the 7 d preceding the PFT. We estimated FEV1 and FVC values per increment of and using multiple linear regression models with and without adjustment for potential confounders identified using a directed acyclic graph (Figure S2), specifically, sex, age, height, weight, smoking status (never smoker/passive smoker, former smoker, current smoker), education (low vs. high), SEP index (continuous, as a measure of neighborhood socioeconomic status); the year, season (fall, winter, spring, summer), and time of the PFT (continuous); and relative humidity and temperature at the location and day of the PFT. We performed a sensitivity analysis excluding current smokers to determine whether associations were affected by smoking status.
In the second step, we examined the influence of additionally adjusting for short-term deviations in air pollutant concentrations when estimating associations between lung function and long-term air pollution exposures, including 1-y and 4-y means based on daily concentrations during the preceding 12 and 48 months, respectively. Short-term air pollution deviations were defined for each individual as the absolute difference between the air pollutant concentration on the day of PFT and their 1- or 4-y mean concentrations, respectively. For example, the short-term deviation in for models of 1-y mean was:
.
Long-term and data were limited to certain years. Therefore, LuftiBus participants were included in the long-term analyses if they completed the study assessment in 2006–2012 for 1-y mean , 2004–2012 for 1-y mean , 2009–2012 for 4-y mean , and 2007–2012 for 4-y mean . Missing values were present for smoking status (, 0.3%), education (, 9.1%), SEP index (, 1.9%), concentration ( participants, 3.5%), concentration (, 2.9%), temperature and relative humidity ( for each variable due to missing information on the location of the PFT). Therefore, we performed multiple imputation with 25 imputations, using all covariates and the outcomes as predictors in the imputation model (White et al. 2011), to impute missing covariate and exposure data. All analyses were conducted in R (version 3.6.1; R Development Core Team), and the “mice” package in R was used for multiple imputation (van Buuren and Groothuis-Oudshoorn 2011).
Results
Study Population
The mean age of the study population was 53 y old (maximum 95 y), and 51.6% were female (Table 1). Just over half of the participants were never smokers (56%), 24% were ex-smokers, and 20% were current smokers. The educational level was high, with 29% having completed higher education (ISCED levels 4–6). Most visits were in fall (40%); the number of visits in spring (28%) and summer (25%) were similar. The visits mainly took place between 1000 hours and 1600 hours (10 A.M. and 4 P.M.) (73%), with a peak time between 1000–1200 (26%) and 1400–1600 (28%). The mean FEV1 was (, range ), and mean FVC was (, range ).
Table 1.
Characteristics of the Swiss study population between 2003 and 2012 ().
| or (%) | ||
|---|---|---|
| Age (y) | 36,085 | |
| Women | 18,631 (51.6) | 36,085 |
| Smoking status | 35,977 | |
| Never smoker | 20,133 (56.0) | |
| Ex-smoker | 8,516 (23.7) | |
| Current smoker | 7,328 (20.4) | |
| Height (cm) | 36,085 | |
| Weight (kg) | 36,085 | |
| Higher education | 9,529 (29.1) | 32,803 |
| SEP index | 35,406 | |
| Season | 36,085 | |
| Fall | 14,565 (40.4) | |
| Winter | 2,365 (6.6) | |
| Spring | 10,131 (28.1) | |
| Summer | 9,024 (25.0) | |
| Temperature (°C) | 29,476 | |
| Relative humidity (%) | 29,476 | |
| Time of day (h) | 36,085 | |
| FEV1 (L) | 36,085 | |
| FVC (L) | 36,085 |
Note: The SEP index ranges from 0 (lowest SEP) to 100 (highest SEP). FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; SD, standard deviation; SEP, socioeconomic position.
Air Pollution Exposure
The mean residential concentration on the day of PFT was (range ), and the concentration was (range ) (Table 2). The 1- and 4-y mean concentrations were and , respectively, and 1- and 4-y mean concentrations were and , respectively. There were strong correlations between on the day of PFT and the 1- and 4-y mean concentrations (0.69 and 0.70, respectively) and weak to moderate correlations between on the day of PFT and the 1- and 4-y mean concentrations (0.29 and 0.35, respectively).
Table 2.
Distribution of the estimated short- and long-term and concentrations (in micrograms per cubic meter) in the Swiss study population between 2003 and 2012.
| Range | 25th percentile | Median | 75th percentile | n | ||||
|---|---|---|---|---|---|---|---|---|
| Short-term concentration | Day | 0 | 0–86.1 | 11.0 | 17.8 | 27.1 | 29,315 | |
| 0–90.6 | 12.2 | 18.6 | 27.4 | 29,314 | ||||
| 0–81.9 | 11.4 | 17.9 | 26.7 | 29,314 | ||||
| 0–102.8 | 10.2 | 17.0 | 25.7 | 29,314 | ||||
| 0–104.0 | 10.3 | 16.4 | 25.0 | 29,314 | ||||
| 0–105.9 | 9.9 | 15.9 | 24.6 | 29,314 | ||||
| 0–100.3 | 9.2 | 16.1 | 24.8 | 29,314 | ||||
| 0–106.3 | 10.6 | 17.5 | 26.3 | 29,314 | ||||
| Short-term concentration | Day | 0 | 0–89.9 | 11.6 | 16.3 | 22.4 | 34,459 | |
| 0–78.2 | 11.6 | 16.2 | 22.3 | 34,459 | ||||
| 0–83.6 | 11.7 | 16.3 | 22.4 | 34,459 | ||||
| 0–90.9 | 11.2 | 15.9 | 22.3 | 34,459 | ||||
| 0–101.6 | 10.9 | 15.6 | 22.0 | 34,459 | ||||
| 0–106.8 | 11.0 | 15.5 | 21.8 | 34,459 | ||||
| 0–136.7 | 11.0 | 15.7 | 22.1 | 34,459 | ||||
| 0–111.0 | 11.4 | 16.3 | 22.7 | 34,459 | ||||
| Long-term concentration | 1-y mean | 2.2–63.4 | 15.8 | 20.1 | 25.0 | 24,794 | ||
| 4-y mean | 3.7–62.1 | 15.8 | 20.0 | 24.9 | 13,746 | |||
| Long-term concentration | 1-y mean | 0.4–40.4 | 15.8 | 17.8 | 20.5 | 31,555 | ||
| 4-y mean | 5.3–35.4 | 16.0 | 17.7 | 19.5 | 20,539 | |||
Note: Day from 0 to represents the number of days preceding the pulmonary function test (, .). ; ; SD, standard deviation.
Short-Term Exposure and Lung Function
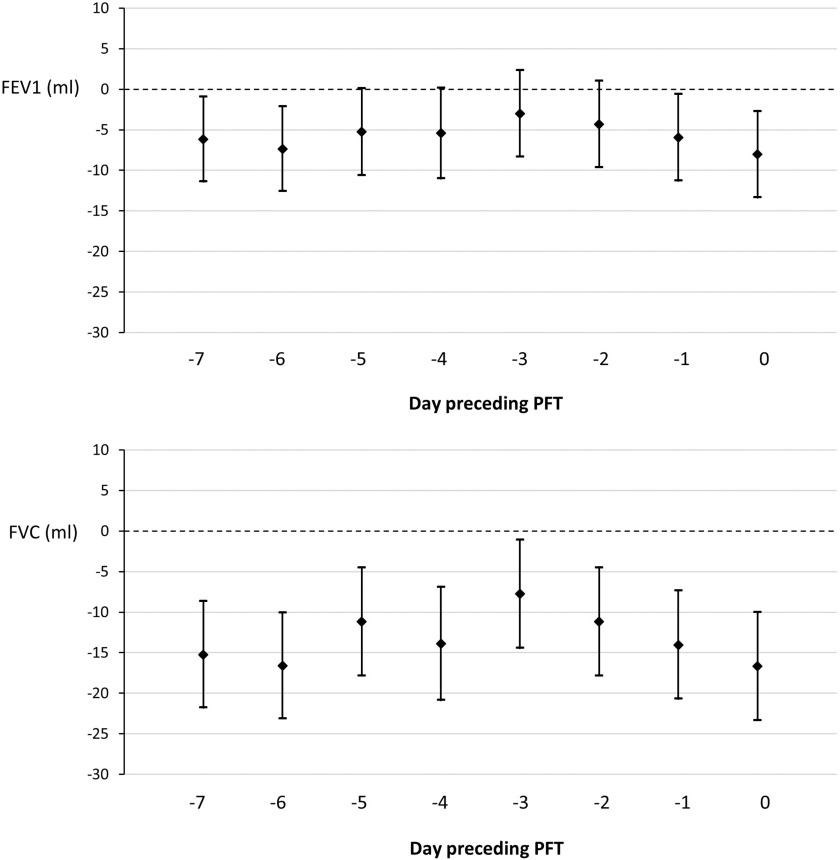
After adjustment for covariates, short-term concentrations were negatively associated with FEV1 and FVC (Figure 1; Tables S1 and S2). A increment in daily mean on the third day before the PFT ( preceding) was associated with a [95% confidence interval (CI): , 2.3] lower FEV1, whereas a increment of on the day of the PFT (0 d) was associated with a (95% CI: , ) lower FEV1. Adjusted associations between a increase in short-term and FVC ranged from a (95% CI: , ) lower FVC for daily mean 3 d before the PFT to a (95% CI: , ) lower FVC for on the day of PFT. The associations in the unadjusted models are less consistent and partially reverse in comparison with the associations in the adjusted models (Tables S1 and S2). The direction of the associations in the complete case analyses are similar but less negative (closer to zero) in comparison with the results using multiple imputation. The adjusted associations between short-term exposure and lung function did not change greatly in the sensitivity analysis with only nonsmokers.
Figure 1.
Multiple linear regression models estimating FEV1 and FVC values per increase in short-term exposure of the preceding days () and the corresponding confidence interval in the Swiss population between 2005 and 2012 (). The models are adjusted for sex, age, height, weight, smoking status, education, socioeconomic position (SEP index), year, season, time, humidity, and temperature. Multiple imputation was used to deal with missing values in any variables of the analysis model. Note: CI, confidence interval; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PFT, pulmonary function test.
Short-Term Exposure and Lung Function
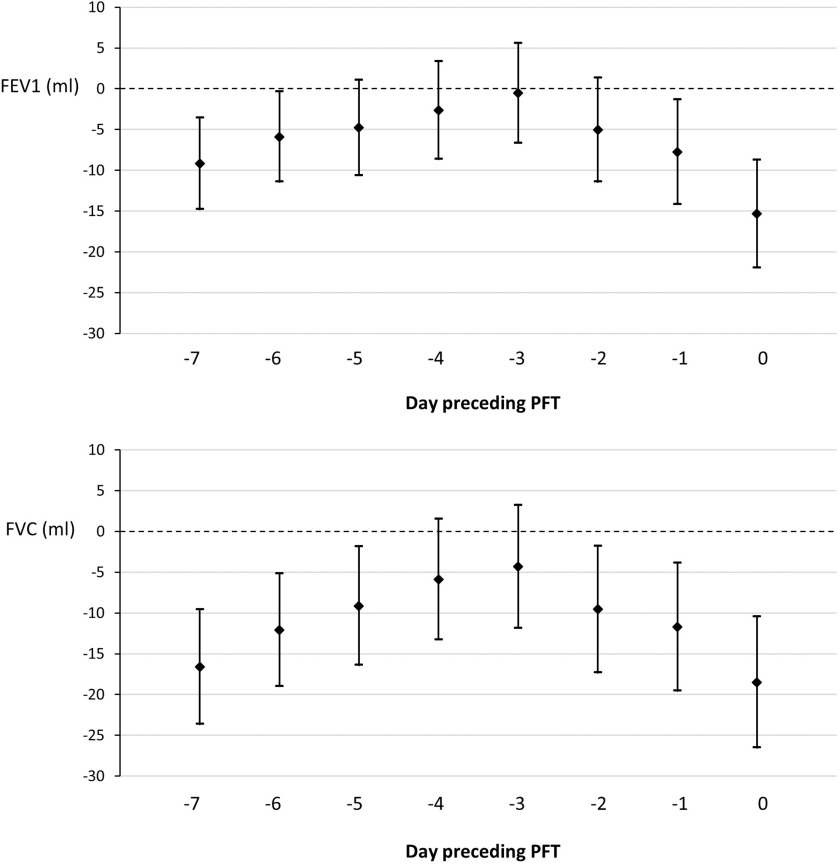
Associations between recent concentrations and lung function were all negative (Figure 2). A increment of 3 d before the PFT was associated with a (95% CI: , 5.6) lower FEV1, and a increment on the day of the PFT (0 d) was associated with a (95% CI: , ) lower FEV1. Associations between FVC and ranged from (95% CI: , 3.2) for 3 d before the PFT to (95% CI: , ) for on the day of the PFT. The unadjusted results were all negative and with one exception stronger than the adjusted results (Tables S1 and S2). The estimates were again less negative when including only the complete cases. The adjusted associations in the sensitivity analysis with only nonsmokers were consistent with the primary model estimates.
Figure 2.
Multiple linear regression models estimating FEV1 and FVC values per increase in short-term exposure of the preceding days () and the corresponding confidence interval in the Swiss population between 2003 and 2012 (). The models are adjusted for sex, age, height, weight, smoking status, education, socioeconomic position (SEP index), year, season, time, humidity, and temperature. Multiple imputation was used to deal with missing values in any variables of the analysis model. Note: CI, confidence interval; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PFT, pulmonary function test.
Long-Term and Exposure and Lung Function
A increment in 1-y mean concentration was associated with a (95% CI: , 0.5) lower FEV1 and a (95% CI: , ) lower FVC (Table 3, primary model). The 4-y mean concentration was associated with a (95% CI: , 10.1) lower FEV1 and a (95% CI: , 0.1) lower FVC. For , the 1-y mean concentration was associated with a (95% CI: , ) lower FEV1 and a (95% CI: , ) lower FVC. The 4-y mean concentration was associated with a (95% CI: , ) lower FEV1 and a (95% CI: , ) lower FVC. Unadjusted associations were more negative except for FEV1 and FVC of the 4-y mean concentration (Tables S1 and S2). The estimates in the complete-case analyses were inconsistent, showing partially more and less strong associations in comparison with the analysis with multiple imputation. The adjusted associations among nonsmokers were similar to estimates for the entire study population.
Table 3.
Associations between lung function and long-term and exposure and the estimated impact of short-term deviations in the Swiss study population (estimated effects per increment).
| FEV1 in mL (95% CI) | -Value | FVC in mL (95% CI) | -Value | ||
|---|---|---|---|---|---|
| : 1-y mean | |||||
| Primary model | 25,528 | (, 0.5) | 0.07 | (, ) | |
| (, 0.5) | 0.07 | (, ) | |||
| : 4-y mean | |||||
| Primary model | 13,964 | (, 10.1) | 0.89 | (, 0.1) | 0.05 |
| (, 9.6) | 0.82 | (, ) | 0.04 | ||
| : 1-year mean | |||||
| Primary model | 32,826 | (, ) | (, ) | ||
| (, ) | (, ) | ||||
| : 4-year mean | |||||
| Primary model | 21,058 | (, ) | 0.02 | (, ) | |
| (, ) | 0.02 | (, ) | |||
Note: This table represents the association between lung function (FEV1, FVC) and long-term and exposure with and without considering short-term deviations in air pollutant concentrations using multiple linear regression. The short-term deviation is defined by the absolute difference between the air pollutant concentration on the day of pulmonary function test and the long-term concentration. All models are adjusted for sex, age, height, weight, smoking status, education, socioeconomic position (SEP index), year, season, time, humidity, and temperature. Multiple imputation was used to deal with missing values in any variables of the analysis model. CI, confidence interval; dev., deviation; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; ; .
Short-Term Deviation in Long-Term Exposure Models
There was little change in effect estimates for long-term air pollutant exposures after adjustment for short-term deviations, i.e., the absolute difference between the air pollutant concentration on the day of the PFT and its 1- or 4-y mean concentration (Table 3). For example, the estimated difference in FEV1 with a increase in 4-y mean concentration was (95% CI: , ) based on the primary model, compared with (95% CI: , ) after adjustment for short-term deviation in .
Discussion
In Swiss adults who participated in the LuftiBus health campaign, higher short- and long-term exposures to and were associated with lower FEV1 and FVC, with stronger associations for FVC than for FEV1. Associations between and lung function were stronger for 1- and 4-y mean concentrations compared with concentrations on the same day or up to 7 d before lung function was measured, whereas associations with long- and short-term concentrations were similar in magnitude. Adjusting for short-term deviations in air pollutant concentrations had little effect on estimated associations with long-term exposures.
We made two important observations in our study. First, long-term exposure was more strongly associated with lower FEV1 and FVC than short-term exposure. However, we could not find such a clear pattern for the exposure. In the meta-analysis of the multicenter European Study of Cohorts for Air Pollution Effects (ESCAPE) with 7,613 participants, associations between lung function and increments in 1-y exposure were similar in magnitude to our estimates (FEV1 ; 95% CI: , 28.2; FVC ; 95% CI: , 21.0) (Adam et al. 2015).
Second, we estimated stronger associations with FVC than with FEV1 for long- and short-term exposures to both pollutants. Studies providing lung function changes in percentage instead of milliliters are inconsistent. The multicenter Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA, 9,651 participants) reported slightly stronger inverse associations with FVC (; 95% CI: , ) than with FEV1 (; 95% CI: , ) per increase in annual concentration (Ackermann-Liebrich et al. 1997). However, a longitudinal cohort study from Taiwan with 285,046 participants reported a larger percent difference in FEV1 (; 95% CI: , ) than FVC (; 95% CI: , ) per increase in 2-y average from a fully adjusted model (Guo et al. 2018).
To our knowledge, adjustment for recent air pollutant concentrations has been considered by only two studies of long-term air pollution exposures and lung function (Adar et al. 2015; Rice et al. 2015). In a prospective cohort study with 2,222 participants, a increase in long-term exposure was associated with a lower FEV1 (95% CI: , ) (Rice et al. 2015). After additionally adjusting for the previous-day concentration, the association increased to (95% CI: , ). The Multi-Ethnic Study of Atherosclerosis (MESA), with 3,791 participants ages 45–84 y, reported only that negative associations between lung function (FEV1, FVC) and 1-y average , , and concentrations were robust to adjustment for 1-d average concentrations (Adar et al. 2015). We adjusted for short-term deviations in and concentrations instead of previous- or same-day concentrations and found that adjusting for short-term deviation did not influence or alter conclusions about potential associations between lung function and long-term air pollution exposures. This finding suggests that it may not be necessary to adjust for short-term deviations in air pollution when estimating effects of long-term ambient air pollution exposures on lung function.
The Swiss federal government has introduced limits for annual and daily and concentrations to protect public health (Federal Office for the Environment 2019b). In our study population, 16.2% of the annual concentrations in 2011 exceeded the statutory annual limit of , and one of the 4,349 estimated daily concentrations exceeded the daily limit of . In contrast, annual concentrations in 2011 were above the statutory limit of for all participants. The government has not defined a daily limit for . Hence, whereas air quality in Switzerland has improved substantially since 1990, air pollutant concentrations in our study population frequently exceeded statutory limits, particularly for (Federal Office for the Environment 2019a). At first glance, the estimated effect sizes for short-term exposures seem small, but given recent evidence that concentrations in Switzerland can vary up to within a 7-d period, the small estimated differences in lung function with a increase in exposure may indicate a nonnegligible public health impact.
Nevertheless, we acknowledge some limitations to our study. First, our study population may differ from the general Swiss population by being sicker or healthier (Bopp et al. 2014). Therefore, generalizability of the study results may be limited. Because the Zurich Lung Association is located in the city of Zurich, the health promotion campaign called LuftiBus was most active in the canton of Zurich (62% participants). Hence, measurements from the French-speaking parts of Switzerland are less represented. Second, we enhanced the LuftiBus data set by external data using deterministic record linkage. We cannot completely rule out that there may have been exposure misclassifications for some individuals and that some participants may have moved in the years preceding the PFT. Third, we could not account for exposures at locations other than residence, including exposure to ambient air pollution at workplaces. Because people spend a lot of time at work, it would be appropriate to take into account the air pollutant concentration at the workplace. The fact that we could not consider air pollution exposure at the workplace may have led to an under- or overestimation of the associations. Fourth, we cannot distinguish between various pollutant types; therefore, it may be that pollutants other than or may have caused reduced lung function. If so, is more likely to represent primary air pollution close to combustion sources like road traffic and cement plants, whereas is a surrogate for the spatial distribution of secondary background air pollutants (Manisalidis et al. 2020).
This study benefits from a long exposure time, the linkage with air pollutant concentrations based on fine scale prediction models, a large number of observations, and participants from the general population. Furthermore, we were able to adjust for smoking status, as well as relative humidity and temperature at the day of PFT, which may be important confounders for association with short-term exposures. In conclusion, our findings contribute to the evidence of adverse associations between residential short- and long-term and exposure and lung function in adults. We also provide evidence that short-term deviation of air pollutant concentration does not have significant impact in long-term exposure models. Our findings indicate that controlling air pollution emission is of great importance to protect public health, especially in regard to the lungs.
Supplementary Material
Acknowledgments
This study was funded by the Swiss National Science Foundation and by a Zurich Lung Association grant. The authors thank the Swiss Federal Statistical Office for providing mortality and census data and for the support, which made the SNC and this study possible. The SNC was supported by the Swiss National Science Foundation (grants 3347CO-108806, 33CS30_134273, and 33CS30_148415). The members of the Swiss National Cohort Study Group are M. Egger (Chairman of the Executive Board), A. Spoerri, and M. Zwahlen (all Bern); M.A.P. (Chairman of the Scientific Board) and M.B. (both Zurich); M.R. (Basel); M. Bochud (Lausanne); and M. Oris (Geneva). The data of this study are available from the corresponding author (miloalan.puhan@uzh.ch) on reasonable request.
References
- Ackermann-Liebrich U, Leuenberger P, Schwartz J, Schindler C, Monn C, Bolognini G, et al. 1997. Lung function and long term exposure to air pollutants in Switzerland. Am J Respir Crit Care Med 155(1):122–129, PMID: 9001300, 10.1164/ajrccm.155.1.9001300. [DOI] [PubMed] [Google Scholar]
- Adam M, Schikowski T, Carsin AE, Cai Y, Jacquemin B, Sanchez M, et al. 2015. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J 45(1):38–50, PMID: 25193994, 10.1183/09031936.00130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar SD, Kaufman JD, Diez-Roux AV, Hoffman EA, D’Souza J, Stukovsky KH, et al. 2015. Air pollution and percent emphysema identified by computed tomography in the Multi-Ethnic study of Atherosclerosis. Environ Health Perspect 123(2):144–151, PMID: 25302408, 10.1289/ehp.1307951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society. 1991. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 144:1202–1218, PMID: 1952453, 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- Bopp M, Braun J, Faeh D, Swiss National Cohort Study Group. 2014. Variation in mortality patterns among the general population, study participants, and different types of nonparticipants: evidence from 25 years of follow-up. Am J Epidemiol 180(10):1028–1035, PMID: 25344298, 10.1093/aje/kwu226. [DOI] [PubMed] [Google Scholar]
- Bopp M, Spoerri A, Zwahlen M, Gutzwiller F, Paccaud F, Braun-Fahrländer C, et al. 2009. Cohort profile: the Swiss National Cohort—a longitudinal study of 6.8 million people. Int J Epidemiol 38(2):379–384, PMID: 18326512, 10.1093/ije/dyn042. [DOI] [PubMed] [Google Scholar]
- Dauchet L, Hulo S, Cherot-Kornobis N, Matran R, Amouyel P, Edmé J-L, et al. 2018. Short-term exposure to air pollution: associations with lung function and inflammatory markers in non-smoking, healthy adults. Environ Int 121:610–619, PMID: 30312964, 10.1016/j.envint.2018.09.036. [DOI] [PubMed] [Google Scholar]
- de Hoogh K, Héritier H, Stafoggia M, Künzli N, Kloog I. 2018. Modelling daily PM2.5 concentrations at high spatio-temporal resolution across Switzerland. Environ Pollut 233:1147–1154, PMID: 29037492, 10.1016/j.envpol.2017.10.025. [DOI] [PubMed] [Google Scholar]
- de Hoogh K, Saucy A, Shtein A, Schwartz J, West EA, Strassmann A, et al. 2019. Predicting fine-scale daily NO2 for 2005–2016 incorporating OMI satellite data across Switzerland. Environ Sci Technol 53(17):10279–10287, PMID: 31415154, 10.1021/acs.est.9b03107. [DOI] [PubMed] [Google Scholar]
- Edginton S, O’Sullivan DE, King W, Lougheed MD. 2019. Effect of outdoor particulate air pollution on FEV1 in healthy adults: a systematic review and meta-analysis. Occup Environ Med 76(8):583–591, PMID: 31189694, 10.1136/oemed-2018-105420. [DOI] [PubMed] [Google Scholar]
- Egger M, Spoerri A, Zwahlen M. 2018. The Swiss National Cohort. https://www.swissnationalcohort.ch [accessed 25 May 2020].
- Federal Office for the Environment. 2019a. Grafiken Jahreswerte NABEL.
- Federal Office for the Environment. 2019b. Luftqualität 2018. Messresultate des Nationalen Beobachtungsnetzes für Luftfremdstoffe (NABEL). Bern, Switzerland: BAFU. [Google Scholar]
- Götschi T, Heinrich J, Sunyer J, Künzli N. 2008. Long-term effects of ambient air pollution on lung function: a review. Epidemiology 19(5):690–701, PMID: 18703932, 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- Guo C, Zhang Z, Lau AKH, Lin CQ, Chuang YC, Chan J, et al. 2018. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health 2(3):e114–25–e125, PMID: 29615226, 10.1016/S2542-5196(18)30028-7. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525(7569):367–371, PMID: 26381985, 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. 2020. Environmental and health impacts of air pollution: a review. Front Public Health 8:1–13, PMID: 32154200, 10.3389/fpubh.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. 2005. Standardisation of spirometry. Eur Respir J 26(2):319–338, PMID: 16055882, 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Panczak R, Galobardes B, Voorpostel M, Spoerri A, Zwahlen M, Egger M, et al. 2012. A Swiss neighbourhood index of socioeconomic position: development and association with mortality. J Epidemiol Community Health 66(12):1129–1136, PMID: 22717282, 10.1136/jech-2011-200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panis LI, Provost EB, Cox B, et al. 2017. Short-term air pollution exposure decreases lung function: a repeated measures study in healthy adults. Environ Health 16:60, PMID: 28615020, 10.1186/s12940-017-0271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, et al. 2013. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med 188(11):1351–1357, PMID: 24200465, 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. 2015. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am J Respir Crit Care Med 191(6):656–664, PMID: 25590631, 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Künzli N, Bongard JP, Leuenberger P, Karrer W, Rapp R, et al. 2001. Short-term variation in air pollution and in average lung function among never-smokers: the Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). Am J Respir Crit Care Med 163(2):356–361, PMID: 11179106, 10.1164/ajrccm.163.2.9911116. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhu D. 2019. Exposure to outdoor air pollution and its human health outcomes: a scoping review. PLoS One 14(5):e0216550, PMID: 31095592, 10.1371/journal.pone.0216550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNESCO (United Nations Educational, Scientific and Cultural Organization). 1997. International Standard Classification of Education - ISCED 1997. Paris, France: UNESCO. [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. 2011. mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- Weinmayr G, Romeo E, Sario MD, Weiland SK, Forastiere F. 2010. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review. Environ Health Perspect 118(4):449–457, PMID: 20064785, 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30(4):377–399, PMID: 21225900, 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. Ambient air pollution: a global assessment of exposure and burden of disease. Clean Air J 26(2), 10.17159/2410-972X/2016/v26n2a4. [DOI] [Google Scholar]
- Zurich Lung Association. 2020. LuftiBus. https://www.lunge-zuerich.ch/de/projekte/luftibus [accessed 25 May 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.