Abstract
The experimental objective was to characterize the impact of insoluble corn-based fiber, xylanase, and an arabinoxylan-oligosaccharide on ileal digesta and mucosa microbiome of pigs. Three replicates of 20 gilts were blocked by initial body weight, individually-housed, and assigned to 1 of 4 dietary treatments: a low-fiber control (LF), a 30% corn bran high-fiber control (HF), HF+100 mg/kg xylanase (HF+XY), and HF+50 mg/kg arabinoxylan oligosaccharide (HF+AX). Gilts were fed their respective treatments for 46 days. On day 46, pigs were euthanized and ileal digesta and mucosa were collected. The V4 region of the 16S rRNA was amplified and sequenced, generating a total of 2,413,572 and 1,739,013 high-quality sequences from the digesta and mucosa, respectively. Sequences were classified into 1,538 mucosa and 2,495 digesta operational taxonomic units (OTU). Hidden-state predictions of 25 enzymes were made using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUST2). Compared to LF, HF increased Erysipelotrichaceae_UCG-002, and Turicibacter in the digesta, Lachnospiraceae_unclassified in the mucosa, and decreased Actinobacillus in both (Q<0.05). Relative to HF, HF+XY increased 19 and 14 of the 100 most abundant OTUs characterized from digesta and mucosa, respectively (Q<0.05). Notably, HF+XY increased the OTU_23_Faecalibacterium by nearly 6 log2-fold change, compared to HF. Relative to HF, HF+XY increased genera Bifidobacterium, and Lactobacillus, and decreased Streptococcus and Turicibacter in digesta (Q<0.05), and increased Bifidobacterium and decreased Escherichia-Shigella in the mucosa (Q<0.05). Compared to HF, HF+AX increased 5 and 6 of the 100 most abundant OTUs characterized from digesta and mucosa, respectively, (Q<0.05), but HF+AX did not modulate similar taxa as HF+XY. The PICRUST2 predictions revealed HF+XY increased gene-predictions for enzymes associated with arabinoxylan degradation and xylose metabolism in the digesta, and increased enzymes related to short-chain fatty acid production in the mucosa. Collectively, these data suggest xylanase elicits a stimbiotic and prebiotic mechanism.
Introduction
Historically, there has been a discordant view about dietary fiber (DF) among monogastric nutritionists. In swine production, DF is often eschewed as much as possible, particularly insoluble DF sources, due to its antinutritive effects that result in reduced pig performance and carcass yield [1]. In contrast, companion animal and human nutritionists have concentrated on the health benefits of increasing DF, such as modulation of microbiota, glycemic and appetite control, laxation, disease prevention and improved intestinal health [2]. These different perspectives can largely be attributed to diet formulation objectives, and the sources of DF used in these different species. The inclusion of DF is more intentional in companion animal and human nutrition, as their aim for diet composition largely focuses on longevity and weight maintenance, and thus, the aforementioned benefits of providing DF favor its use. Pork production, on the other hand, tends to focus on optimizing growth and feed efficiency at low cost, production outcomes that may be compromised by increased DF levels. Dietary fiber is most commonly increased in swine diets through the use of industrial co-products when they are used to reduce feed cost [3]. There has been renewed interest in the benefits of microbial modulation via DF as numerous pork producers have moved towards antibiotic-free production [4]. Still, many of the economically practicable DF sources for swine diets are largely insoluble and poorly fermented, and thus, unable to modulate gut microbiota with the same efficacy as more fermentable DF sources [5,6]. Exogenous carbohydrases may bridge the gap between the economics of using insoluble DF sources (e.g. industrial co-products) and the health benefits of more rapidly fermentable DF [7].
Swine diets frequently contain substantial levels of arabinoxylans, a non-starch polysaccharide innate to cereal grains; often, they stem from corn and corn co-products [8]. It is well established that pigs do not produce the exogenous enzymes needed to hydrolyze arabinoxylans. Xylanase is a carbohydrase that hydrolyzes the β-(1–4) glycosidic bonds of arabinoxylans, and thus, has the potential to partially ameliorate the antinutritive effects of DF [9]. Moreover, an unexpected finding from utilizing xylanase in commercial pork production is its ability to reduce finishing pig mortality [10]. Likewise, supplementing xylanase in corn-based diets has been shown to modulate immune function [11], alter intestinal morphology [12], increase markers indicative of improved gut barrier integrity [13], and mitigate markers of oxidative stress [14].
It has been hypothesized that these modulations in gastrointestinal physiology are a provision of microbial modulation by the release products of xylanase, arabinoxylan oligosaccharides [AXOS; 15,16]. These AXOS can be rapidly fermented by resident microbiota, and potentially could act in a prebiotic and/or stimbiotic like manner [16,17]. Prebiotic is defined as ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’ [18], and a stimbiotic is defined as a ‘an additive that stimulates a fiber-degrading microbiome resulting in an increase in fiber fermentability even though the additive itself contributes little to SCFA production’ [16]. There is a paucity of research investigating the composition of distal ileal microbiota in pigs fed xylanase supplemented in a corn-based diet. Similarly, there is a dearth of research investigating the impact of supplementing AXOS on the ileal microbiome of pigs. Therefore, the experimental objective was to characterize the impact of insoluble fiber, xylanase, and directly supplemented AXOS on the ileal digesta and mucosa microbiome of pigs fed corn-based fiber. We hypothesized that the addition of xylanase or AXOS would modulate intestinal microbial communities that support fiber degradation (stimbiotic mechanism) or would be selectively utilized by communities that are known to confer a health benefit (prebiotic mechanism).
Materials and methods
All experimental protocols were approved by the Iowa State University Institutional Animal Care and Use Committee (#9-17-8613-S) and adhered to guidelines for the ethical and humane use of animals for research according to the Guide for the Care and Use of Agricultural Animals in Research and Teaching [19].
Experimental design
The experimental design, animal methods, and diets for this study were previously reported by Petry et al., [14] in a related study. In brief, a total of 60 growing gilts (progeny of Camborough sows × 337 sires; PIC Inc., Hendersonville, TN) were used in 3 replicates (20 gilts per replicate) of a 46-day trial. Gilts were individually housed in pens for 36 days, and subsequently moved to metabolism crates for the previously published 10-d metabolism study. Pigs were blocked by initial body weight (25.4 ± 0.9 kg) and randomly assigned within a block to 1 of 4 dietary treatments (n = 15; 5 pigs per treatment within a replicate): a low-fiber control (LF) with 7.5% neutral detergent fiber (NDF), a 30% corn bran without solubles higher-fiber control (HF; NDF = 21.9%), HF + 100 mg xylanase/kg (HF+XY; Econase XT 25P; AB Vista, Marlborough, UK) providing 16,000 birch xylan units per kg, and HF + 50 mg arabinoxylan-oligosaccharide/kg (HF+AX; 3–7 degrees of polymerization). The xylanase included in HF+XY was isolated from Trichoderma reesei using submerged fermentation. The AXOS supplemented in HF+AX was produced by hydrothermal treatment of corn cobs and subsequent xylanase treatment to produce oligosaccharides which range from 3–7 degrees of polymerization. Pigs were fed ad libitum for the 36-day adaptation period, and then limit fed 80% of the average daily feed intake among all treatments of the first replicate for the 10-day metabolism study. Pigs were given ad libitum access to water through the duration of the total study.
Sample collections, DNA extraction and sequencing
On day 46, pigs were fed half of their total daily feed allotment, and after consumption, were euthanized by captive bolt stunning and exsanguination. Pigs were necropsied and an approximately 24-cm section of ileum, 20 cm proximal to the ileocecal junction, was isolated. Ileal digesta was collected from this section, snap-frozen in liquid nitrogen, and stored at -80°C for later analysis. From this isolated section, a 12-cm portion was rinsed with sterile phosphate buffered saline, and mucosa scrapings were carefully collected, snap-frozen in liquid nitrogen, and stored at -80°C for later analysis. Total genomic DNA was extracted from ileal mucosa and digesta using a DNeasy PowerLyzer PowerSoil Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Extracted genomic DNA concentration and purity were evaluated using a ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE), and subsequently stored at -80°C for later sequencing. All samples had 260:280 nm ratios above 1.82. Extracted DNA was adjusted to a total well volume of 125 ng of DNA, and sequencing was conducted in the DNA facility at Iowa State University (Ames, IA).
A PCR-amplified 16S rRNA sequencing was conducted using a previously established protocol designed to amplify bacteria and archaea [20]. In brief, one replicate per sample of extracted genomic DNA was amplified using Platinum™ Taq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA). Collective 16S rRNA bacterial primers [515F (5′-GTGYCAGCMGCCGCGGTAA-3′; 21] and 806R [5′-GGACTACNVGGGTWTCTAAT-3′; 22] for the hypervariable region V4 were utilized as previously explained by Kozich et al., [23]. All samples were subjected to PCR with an initial denaturation step at 94°C for 3 min, followed by 35 PCR cycles (45s of denaturing at 94°C, 20s of annealing at 50°C, and 90s of extension at 72°C), and concluded with a 10 min extension at 72°C. Subsequentially, PCR products were purified with a QIA quick 96 PCR Purification Kit (Qiagen Sciences Inc, Germantown, MD) according to the manufacturer’s recommendations. The bar-coded amplicons were included at equal molar ratios and used for Illumina MiSeq paired-end sequencing with 150 bp read length and cluster generation with 10% PhiX control DNA on an Illumina MiSeq platform (Illumina Inc., San Diego, CA).
Once sequencing was complete, corresponding paired-end reads were stitched to obtain an ultimate amplicon size of 255 bp. The sequencing data for each sample were screened for quality and paired-end reads were combined using mothur [v.1.40.4; 24]. Sequences that contained ambiguous bases, were shorter than 250bp, longer than 255bp, contained homopolymers > 8 bases in size, and potential chimeric sequences were removed. Remaining sequences were then clustered into operational taxonomic units (OTU) with a 99% sequence similarity based on a distance matrix generated in mothur. Consensus taxonomy for OTUs were assigned using the SILVA SSU database [version 132, 25]. Diversity indices, Shannon, Simpson, and Chao1, were computed as previously described [26].
The hidden-state predictions of gene families and their abundance within a given location (mucosa or digesta) were made using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 [PICRUSt2 v. 2.3.0b, 27], according to default parameters. Briefly, representative 16S rRNA amplicon sequences for each OTU were aligned and placed into a phylogenetic tree with relation to reference genomes from the Integrated Microbial Genomes database using HMMER [28], EPA-ng [29], and GAPPA [30]. Hidden-state gene predictions were determined by castor references [31] based on nearest-sequenced taxon index, with a threshold of 2 (OTUs with the nearest-sequenced taxon greater than 2 were removed). The hidden-state predictions of 2,064 enzymes from the Enzyme Commission (EC) database [32] were characterized. A total of 25 enzymes associated with arabinoxylan degradation, pentose metabolism, or short chain fatty acid (SCFA) production were selected (Table 1), normalized by predicted 16S rRNA gene copy number per OTU, and subsequentially analyzed using preplanned contrast.
Table 1. Enzymes associated with arabinoxylan degradation, pentose metabolism, or short chain fatty acid production that were selected for gene prediction-based analysis using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States.
| Enzyme Commission Number | Accepted Name |
|---|---|
| 1.1.1.9 | D-xylulose reductase |
| 1.1.1.27 | L-lactate dehydrogenase |
| 1.1.1.28 | D-lactate dehydrogenase |
| 2.3.1.8 | Phosphate acetyltransferase |
| 2.7.2.1 | Acetate kinase |
| 2.7.2.7 | Butyrate kinase |
| 2.7.2.15 | Propionate kinase |
| 2.7.1.17 | Xylulose kinase |
| 2.8.3.1 | Propionate CoA-transferase |
| 2.8.3.8 | Acetate CoA-transferase |
| 2.8.3.9 | Butyrate—acetoacetate CoA-transferase |
| 2.8.3.18 | Succinyl-CoA:acetate CoA-transferase |
| 3.1.1.73 | Ferulic acid esterase |
| 3.2.1.8 | Endo-1,4-β-xylanase |
| 3.2.1.22 | Alpha-galactosidase |
| 3.2.1.37 | Xylan 1,4-β-xylosidase |
| 3.2.1.55 | Non-reducing end α-L-arabinofuranosidase |
| 3.2.1.136 | Glucuronoarabinoxylan endo-1,4-β-xylanase |
| 3.2.1.139 | Alpha-glucosiduronase |
| 3.2.1.156 | Oligosaccharide reducing-end xylanase |
| 4.1.2.9 | Phosphoketolase |
| 5.3.1.5 | Xylose isomerase |
| 6.2.1.1 | Acetate—CoA ligase |
| 6.2.1.5 | Succinyl-CoA synthetase (ADP-forming) |
| 6.2.1.13 | Acetate—CoA ligase (ADP-forming) |
Statistics
Data were analyzed according to the following statistical model:
Where Yijkl is the observed value for lth experimental unit within the ith level of dietary treatment of the jth block for the lth pig in the kth replicate; μ is the general mean; τi is the fixed effect of the ith diet (i = 1 to 4); υj is the random effect of the jth block (j = 1 to 5); ρk is the random effect of the kth replicate (k = 1 to 3); and eijkl is the associated variance as described by the model for Yijkl (l = 1 through 60).
The absolute abundance of the 100 most abundant OTUs among samples, relative abundance of classified genera, and hidden-state predictions of the 25 enzymes of interest were analyzed using a negative binomial distribution in GLIMMIX procedure of SAS (Version 9.4, SAS Inst., Cary, NC), and they were offset by the total library count for a given sample. All P-values were corrected for false discovery rates using the MULTITEST procedure of SAS. Diversity indices and were analyzed using PROC MIXED procedure of SAS. Least square means were separated using Fisher’s Least Significant Difference test, and treatment differences were considered significant if P or Q values were < 0.05 and trends if 0.05 ≥ P or Q< 0.10. For the top 100 OTUs and genera classifications with a treatment Q value of < 0.05, the log2-fold change was calculated comparing LF vs HF, HF vs. HF+XY, and HF vs. HF+AX. Moreover, preplanned contrast comparing the hidden-state predictions for the 25 EC in Table 1 for HF vs. HF+XY, and HF vs. HF+AX was analyzed utilizing a negative binomial distribution in GLIMMIX procedure of SAS. The log2-fold change was calculated comparing HF vs. HF+XY, and HF vs. HF+AX for EC hidden-state predictions if Q values were < 0.10.
Results
General microbiota characterizations
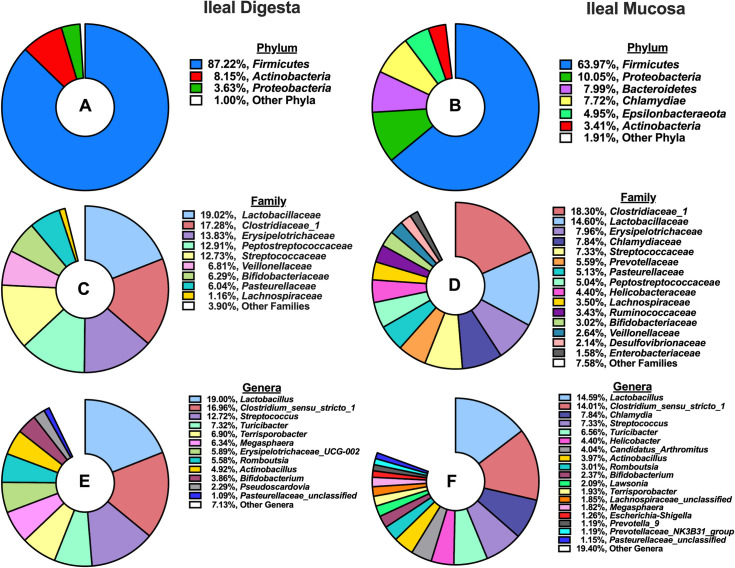
A total of 2,413,572 high-quality reads were obtained from ileal digesta samples after quality control, with a median of 40,958 sequences per sample. Subsequently, those sequences were classified into 1,538 OTUs (> 10 sequences per OTU), and taxonomically classified into 230 microbial genera. Characterization of phyla (Fig 1A) present in ileal digesta revealed microbiota stemming from the Firmicutes phylum dominated (87.22%), followed by smaller contributions from Actinobacteria (8.15%) and Proteobacteria (3.63%). When further characterized into families (Fig 1C), Lactobacillaceae (19.02%), Clostridiaceae_1 (17.28%), Erysipelotrichaceae (13.83%), Peptostreptococcaceae (12.91%), and Streptococcaceae (12.73%) were the 5 most abundant families. Microbial genera that accounted for >1% of all classified OTUs are depicted in Fig 1E, and Lactobacillus (19%), Clostridium_sensu_stricto_1 (16.96%), Streptococcus (12.72%), Turicibacter (7.32%), and Terrisporobacter (6.9%) were the 5 most abundant in ileal digesta.
Fig 1.
Summary of phylum, family, and genus-level microbial composition (>1% relative abundance) in the ileal digesta and mucosa among pigs (N = 60): A) Relative abundance of phyla present in the ileal digesta; B) Relative abundance of phyla present in the ileal mucosa; C) Relative abundance of families present in the ileal digesta; D) Relative abundance of families present in the ileal mucosa; E) Relative abundance of genera present in the ileal digesta; F) Relative abundance of genera present in the ileal mucosa.
From ileal mucosa samples, a total of 1,739,013 high-quality reads were obtained after quality control, with a median of 27,762 sequences per sample. A total of 2,495 OTUs (> 10 sequences per OTU) were classified and grouped into 317 genera. The 5 most abundant phyla, Firmicutes (63.97%), Proteobacteria (10.05%), Bacteroidetes (7.99%), Chlamydia (7.72%) and Epsilonbactereota (4.95%) accounted for 94.8% of all classified OTUs (Fig 1B). When further characterized into families (Fig 1D), Clostridiaceae_1 (18.2%), Lactobacillaceae (14.6%), Erysipelotrichaceae (7.96%), Chlamydiaceae (7.84%), and Streptococcaceae (7.33%) were the 5 most abundant in the ileal mucosa. At a genus level, 15 microbial genera accounted for >1% of all classified OTUs, with Lactobacillus (14.59%), Clostridium_sensu_stricto_1 (14.01%), Chlamydia (7.84%) Streptococcus (7.33%), and Turicibacter (6.56%) composing the top 5.
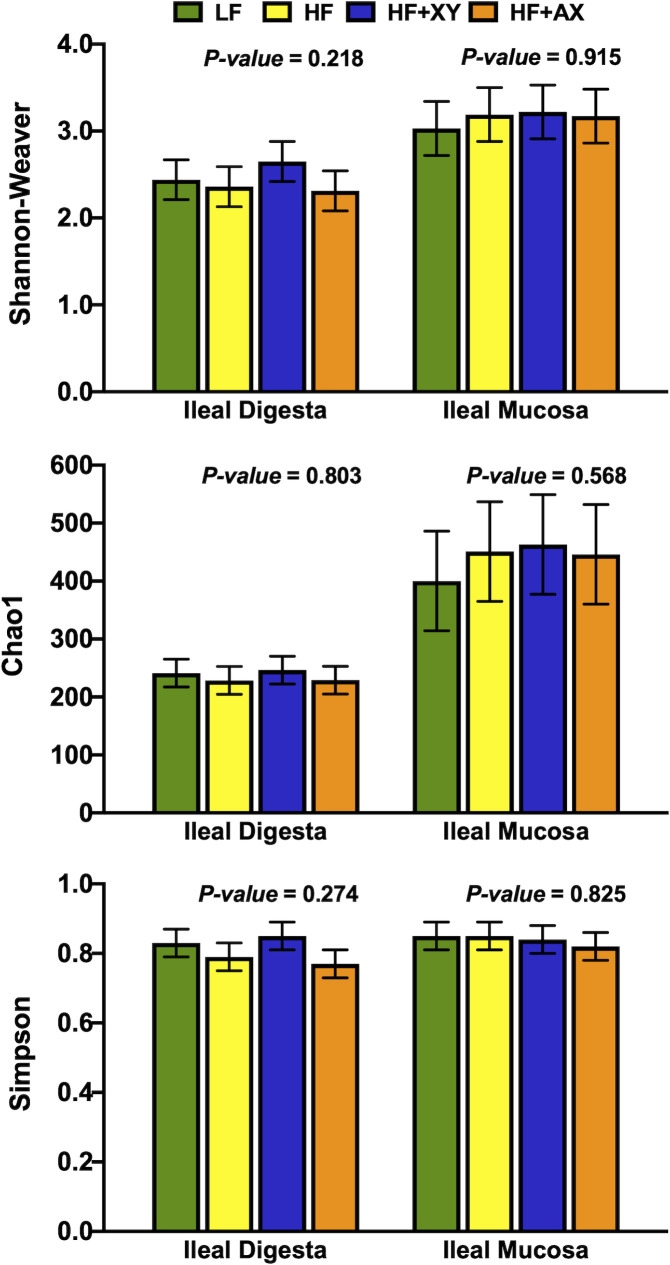
Shannon, Simpson, and Chao1 measures of diversity, evenness and richness are depicted in Fig 2. Numerically, ileal mucosa had a greater Shannon index relative to digesta (3.15 vs. 2.44), and similarly, the Chao1 index was greater for ileal digesta (440 vs. 236). However, the Shannon, Simpson, and Chao1 indices did not differ among treatments for both ileal digesta and mucosa (P > 0.22).
Fig 2. Impact of treatment on measures of α–diversity within the ileal digesta and mucosa.
Treatment modulation of microbial genera
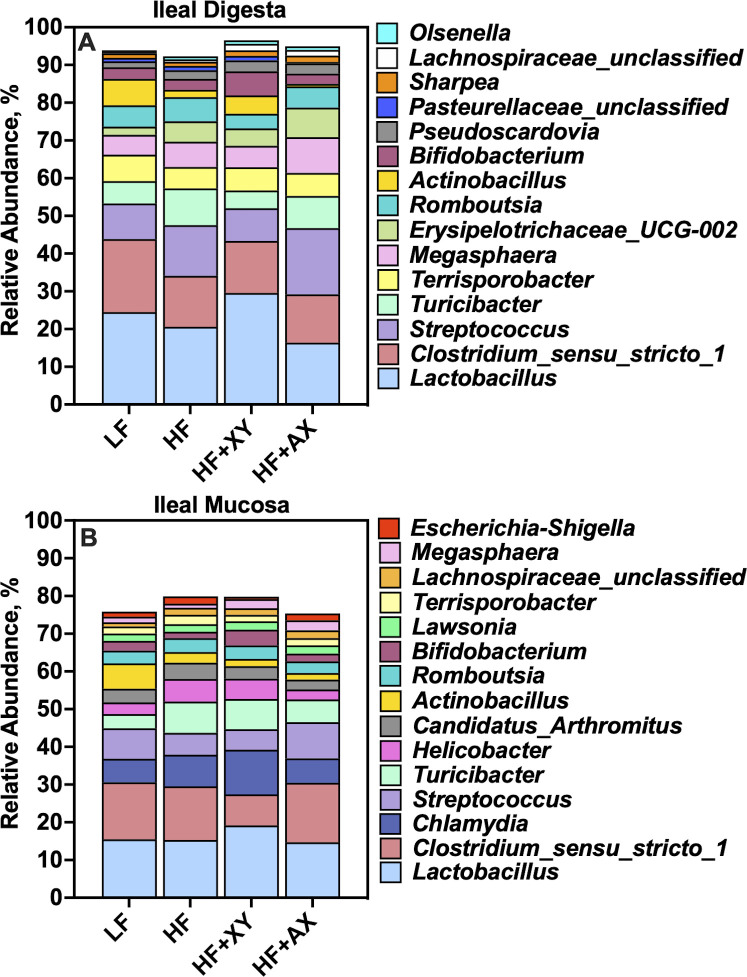
Fig 3 represents the relative abundance of the 15 most abundant genera among treatments present in the ileal digesta (Fig 3A) and mucosa (Fig 3B). The top 15 genera account for 93.7%, 91.9%, 95.5%, and 93.9% of the OTUs characterized in the ileal digesta from pigs fed LF, HF, HF+XY, and HF+AX, respectively. Likewise, the top 15 genera among microbiota characterized from ileal mucosa accounted for 75.1%, 79.0%, 79.1%, and 74.9% of the OTUs characterized in pigs fed LF, HF, HF+XY, and HF+AX, respectively.
Fig 3. The relative abundance of the 15 most abundant genera among treatments present in the ileal digesta and mucosa.
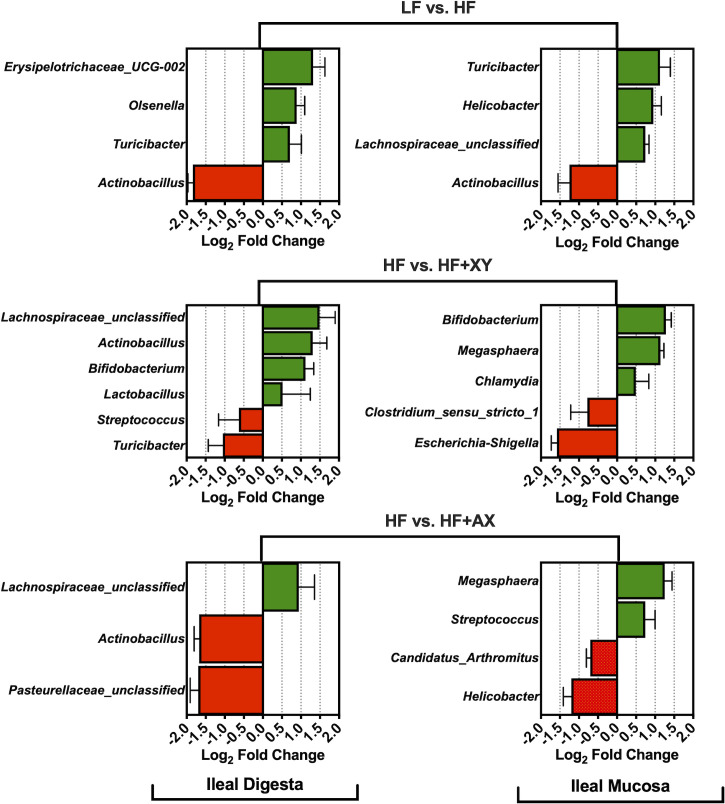
Relative to LF, HF increased the abundance of Erysipelotrichaceae_UCG-002, Olsenella, and Turicibacter, and reduced Actinobacillus presences in ileal digesta (Fig 4; Q < 0.05). Likewise, compared to LF, HF increased the absolute abundance of Turicibacter, Helicobacter, Lachnospiraceae_unclassified, and decreased Actinobacillus presences in the ileal mucosa (Q < 0.05). The addition of xylanase to HF increased the prevalence of Lachnospiraceae_unclassified. Actinobacillus, Bifidobacterium, and Lactobacillus, and decreased the presence of Streptococcus and Turicibacter in ileal digesta (Q < 0.05). Moreover, relative to HF, HF+XY increased Bifidobacterium, Megasphaera, and Chlamydia, while reducing the presence of Clostridium_sensu_stricto_1 and Escherichia-Shigella in the ileal mucosa (Q < 0.05). The direct supplementation of AXOS to HF increased the prevalence of Lachnospiraceae_unclassified, and decreased Actinobacillus and Pasteurellaceae_unclassified in the ileal digesta (Q < 0.05). Whereas, in the ileal mucosa, the addition of AXOS to HF increased the prevalence of Megasphaera and Streptococcus, but reduced Candidatus_arthromitus and Helicobacter (Q < 0.05).
Fig 4. The log2-fold change difference between LF vs. HF, HF vs. HF+XY, HF vs. HF+AX for the 15 most abundant microbial genera present in the ileal digesta and mucosa with a Q-value < 0.05.
Treatment modulation of the 100 most abundant OTUs
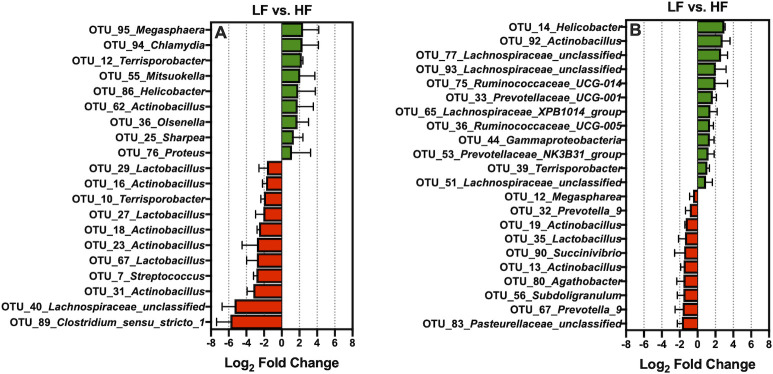
Relative to LF, HF increased the prevalence of 9 of the 100 most abundant OTUs characterized from ileal digesta: OTU_95_Megasphaera, OTU_94_Chlamydia, OTU_12_Terrisporobacter, OTU_55_Mitsuokella, OTU_86_Helicobacter, OTU_62_Actinobacillus, OTU_36_Olsenella, OTU_25_Sharpea, and OTU_76_Proteus (Fig 5A; Q < 0.05). In contrast, compared to the LF, HF decreased 11 of the 100 most abundant OTUs found in ileal digesta: OTU_29_Lactobacillus, OTU_16_Actinobacillus, OTU_10_Terrisporobacter, OTU_27_Lactobacillus, OTU_18_Actinobacillus, OTU_23_Actinobacillus, OTU_67_Lactobacillus, OTU_7_Streptococcus, OTU_31_Actinobacillus, OTU_40_Lachnospiraceae_unclassified and, OTU_89_Clostridium_sensu_stricto_1 (Fig 5A; Q < 0.05). Moreover, relative to LF, HF increased the abundance of 12 of the 100 most abundant OTUs characterized from ileal mucosa: OTU_14_Helicobacter, OTU_92_Actinobacillus, OTU_77_Lachnospiraceae_unclassified, OTU_93_Lachnospiraceae_unclassified, OTU_75_Ruminococcaceae_UCG-014, OTU_33_Prevotellaceae_UCG-001, OTU_65_Lachnospiraceae_XPB1014_group, OTU_36_Ruminococcaceae_UCG-005, OTU_44_Gammaproteobacteria, OTU_53_Prevotellaceae_NK3B31_group, OTU_39_Terrisporobacter, and OTU_51_Lachnospiraceae_unclassified (Fig 5B; Q < 0.05). Conversely, in relation to LF, HF decreased the prevalence of 10 of the 100 most abundant OTUs found in the ileal mucosa: OTU_83_Pasteurellaceae_unclassified, OTU_67_Prevotella_9, OTU_56_Subdoligranulum, OTU_80_Agathobacter, OTU_13_Actinobacillus, OTU_90_Succinivibrio, OTU_35_Lactobacillus, OTU_19_Actinobacillus, OTU_32_Prevotella_9, and OTU_12_Megaspharea (Fig 5B; Q < 0.05).
Fig 5.
The log2-fold change difference between LF vs. HF for the significant OTUs from the 100 most abundant OTUs among treatments present in the ileal digesta (A) and mucosa (B).
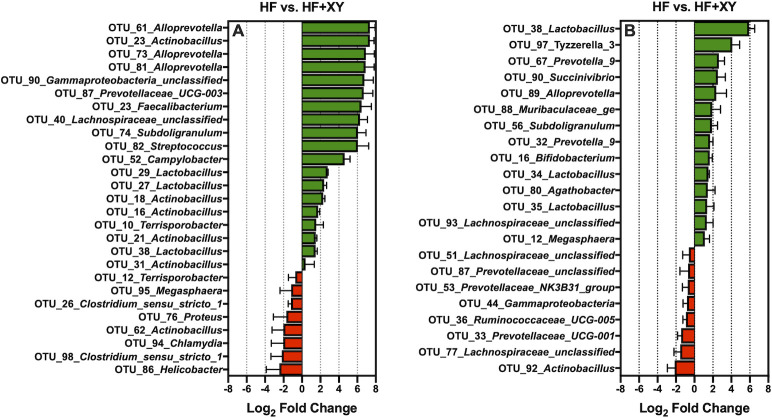
The addition of xylanase to HF (HF+XY), increased 19 of the 100 most abundant OTUs (Fig 6A; Q < 0.05). Notably, HF+XY increased the prevalence of 3 OTUs (OTU # 27, 29, and 38) stemming from the Lactobacillus genus, 3 OTUs (OTU # 61, 73, and 83) from the Alloprevotella genus, and 5 OTUs (OTU # 16, 18, 21, 23, 29, and 31) from the Actinobacillus genus in ileal digesta (Q < 0.05). Interestingly, relative to HF, HF+XY increased the abundance of OTU_23_Faecalibacterium, OTU_40_Lachnospiraceae_unclassified, OTU_74_Subdoligranulum, OTU_82_Streptococcus, OTU_87_Prevotellaceae_UCG-003.
Fig 6.
The log2-fold change differences between HF vs. HF+XY for the significant OTUs from the 100 most abundant OTUs among treatments present in the ileal digesta (A) and mucosa (B).
OTU_90_Gammaproteobacteria_unclassified, each by over 6 log2-fold (Q < 0.05). In contrast, the addition of xylanase to HF decreased 8 of the 100 most abundant OTUs, and notably, this included OTU_86_Helicobacter, OTU_94_Chlamydia, and OTU_95_Megasphaera (Fig 6A; Q < 0.05). With regards to the ileal mucosa, HF+XY increased the abundance of 14, and decreased 8 of the 100 most abundant OTUs (Fig 6B; Q < 0.05). Notably, in the ileal mucosa, HF+XY increased the prevalence of 3 OTUs (OTU # 34, 35, and 38) stemming from the Lactobacillus genus, and also increased OTU_16_Bifidobacterium by nearly 2 log2-fold. On the other hand, pigs fed HF+XY had decreased prevalence of OTU_92_Actinobacillus, OTU_77_Lachnospiraceae_unclassified, OTU_33_Prevotellaceae_UCG-001, OTU_36_Ruminococcaceae_UCG-005, OTU_44_Gammaproteobacteria, OTU_53_Prevotellaceae_NK3B31_group, OTU_87_Prevotellaceae_unclassified, and OTU_51_Lachnospiraceae_unclassified, in the ileal mucosa (Fig 6B; Q < 0.05).
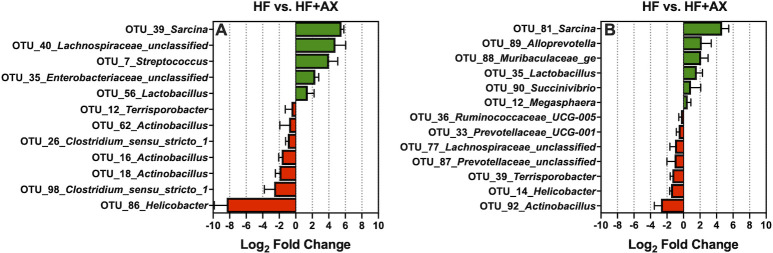
The addition of AXOS to HF (HF+AX), increased 5 of the 100 most abundant OTUs found in ileal digesta: OTU_39_Sarcina, OTU_40_Lachnospiraceae_unclassified, OTU_7_Streptococcus, OTU_35_Enterobacteriaceae_unclassified, and OTU_56_Lactobacillus (Fig 7A; Q < 0.05). In contrast, relative to HF, HF+AX decreased 7 of the 100 most abundant OTUs found in ileal digesta: OTU_86_Helicobacter, OTU_26 and OTU_98 (both:Clostridium_sensu_stricto_1), OTU_16, OTU_18, and OTU_62 (all: Actinobacillus), and OTU_12_Terrisporobacter (Fig 7A; Q < 0.05). For the mucosa, relative to HF, HF+AX increased the abundance of 6 of the 100 most abundant OTUs: OTU_81_Sarcina, OTU_89_Alloprevotella, OTU_88_Muribaculaceae_ge, OTU_35_Lactobacillus, OTU_90_Succinivibrio, and OTU_12_Megasphaera (Fig 7B; Q < 0.05). Additionally, relative to HF, HF+AX decreased the prevalence of 7 of the 100 most abundant OTUs: OTU_92_Actinobacillus, OTU_14_Helicobacter, OTU_39_Terrisporobacter, OTU_87_Prevotellaceae_unclassified, OTU_77_Lachnospiraceae_unclassified, OTU_33_Prevotellaceae_UCG-001, and OTU_36_Ruminococcaceae_UCG-005 (Fig 7B; Q < 0.05).
Fig 7.
The log2-fold change differences between HF vs. HF+AX for the significant OTUs from the 100 most abundant OTUs among treatments present in the ileal digesta (A) and mucosa (B).
PICRUST2 targeted gene predictions
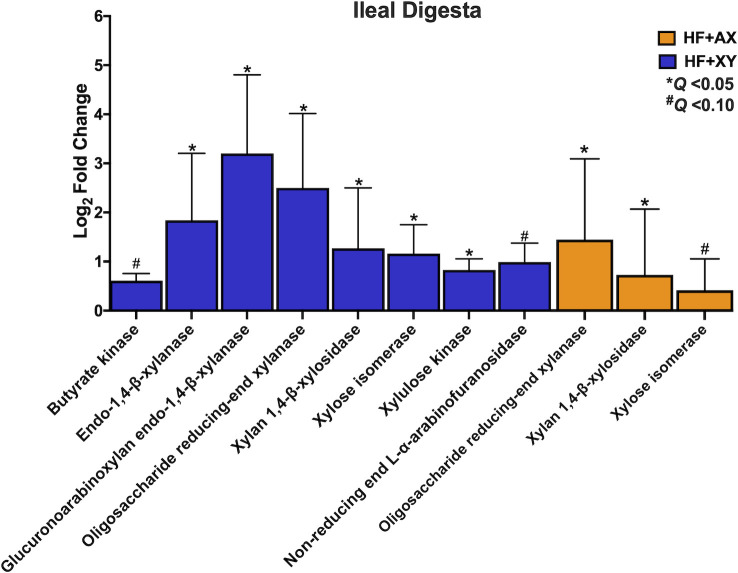
In the ileal digesta, relative to HF, HF+XY tended to upregulate butyrate kinase and non-reducing end α-L-arabinofuranosidase (Fig 8; Q < 0.10). Moreover, the addition of xylanase to HF, increased the predicted gene counts for endo-1,4-β-xylanase, glucuronoarabinoxylan endo-1,4-β-xylanase, oligosaccharide reducing-end xylanase, xylan 1,4-β-xylosidase, xylose isomerase, and xylulose kinase in the ileal digesta (Fig 8; Q < 0.05). Relative to HF, HF+AX increased oligosaccharide reducing-end xylanase and xylan 1,4-β-xylosidase (Fig 9; Q < 0.10) and tended to increase xylose isomerase (Fig 8; Q < 0.05).
Fig 8. The log2-fold change between HF vs. HF+XY or HF vs. HF+AX for the PICRUST2 predicted gene counts for enzymes associated with bacterial arabinoxylan degradation, xylose metabolism, or short chain fatty acid metabolism in the ileal digesta that tended or significantly differed.
Fig 9. The log2-fold change between HF vs. HF+XY for the PICRUST2 predicted gene counts for enzymes associated with bacterial arabinoxylan degradation, xylose metabolism, or short chain fatty acid metabolism in the ileal mucosa that tended or significantly differed.
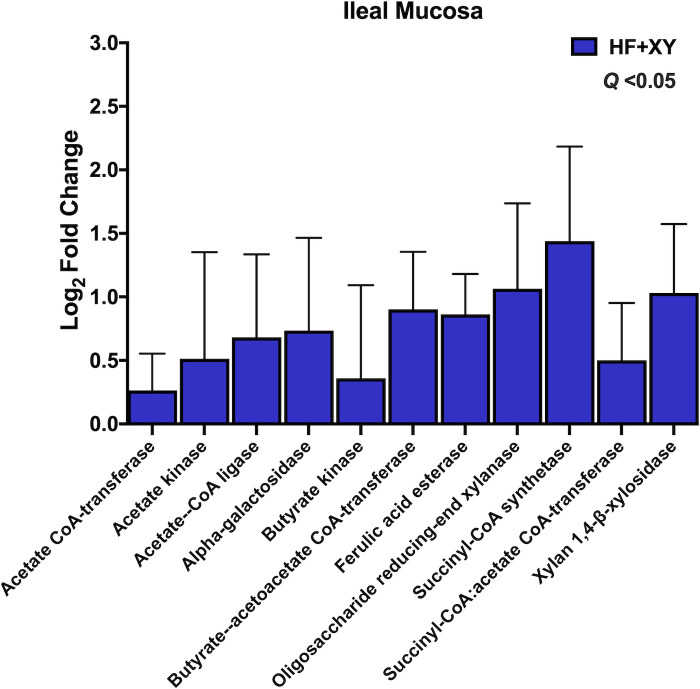
In the ileal mucosa, HF+AX did not differ from HF for any of the chosen enzymes in Table 1 (Q > 0.10). However, compared to HF, HF+XY increased the predicted gene counts for acetate CoA-transferase, acetate kinase, acetate—CoA ligase, alpha-galactosidase, butyrate kinase, butyrate—acetoacetate CoA-transferase, ferulic acid esterase, oligosaccharide reducing-end xylanase, succinyl-CoA synthetase, succinyl-CoA:acetate CoA-transferase, and xylan 1,4-β-xylosidase in the ileal mucosa (Fig 9; Q < 0.05).
Discussion
In a healthy state, the pig and gastrointestinal microbiota share a symbiotic relationship that contributes to the nutrition and health of the pig, and in turn, the pig provides substrate for microbiota largely in the form of DF. However, all DF is not created equal and its efficacy to modulate microbial taxa, diversity, and composition is dependent on DF composition, solubility, and concentration [33,34]. In general, soluble DF and non-digestible oligosaccharides are fermented at a faster rate compared to insoluble DF [35,36] and are associated with reducing pathogenetic microbes, increasing microbial diversity, and improving the proliferation of ‘beneficial’ microbes [37,38]. In contrast, swine diets are largely composed of poorly fermentable insoluble DF emanating from cereal grains and industrial co-products [8,39]. Globally, corn is the most abundant cereal grain, and thus, swine diets often contain considerable amounts of insoluble corn-based fiber, particularly arabinoxylans, that are poorly fermented by the pig’s gastrointestinal microbiota [3,5]. Xylanase, a carbohydrase that targets arabinoxylans, may modulate the gastrointestinal microbial ecology of pigs fed corn-based diets by hydrolyzing arabinoxylans into smaller and more fermentable fragments [i.e. AXOS; 15]. Indeed, it is often postulated that the unexpected health benefits observed with xylanase supplementation are a result of enteric microbiota modulation [16,17]. Still, the impact of xylanase, particularly its mode of action, on gastrointestinal microbiota in pigs fed corn-based fiber is largely unknown. Thus, the experimental objective was to characterize the impact of increased insoluble fiber, xylanase, and directly supplemented AXOS on the ileal digesta and mucosa microbiome of pigs fed corn-based fiber.
The composition of microbial phyla summarized in Fig 1 and is comparable with other reports of ileal digesta and mucosa microbiota composition in pigs under commercial conditions [40,41]. Similar to herein, a meta-analysis of 16S rRNA bacterial gene sequences across the gastrointestinal tract of the pig identified Clostridium and Lactobacillus as the most prevalent genera in the ileal digesta and mucosa [40]. However, there were some differences among the most abundant genera, compared to Holman et al., [40]; this is likely driven by differences in diet, host genetics, and relative environment. Moreover, Zhang et al., [42] also observed numerically higher OTU counts, Shannon index values, and Chao1 index values in the ileal mucosa relative to ileal digesta in pigs fed a corn-based diet (Fig 2). The increased richness and diversity of mucosal-associated microbiota is not unanticipated as we observed a greater sequence count and OTU abundance in the mucosa, relative to the digesta. These differences in microbial communities are probably a result of differing nutrient availability and solubility, associated commensal microbiota, and environmental differences, particularly the presence of oxygen found in the epithelial mucosa versus the lumen of the ileum [43].
When comparing HF to LF, HF increased the abundance of Erysipelotrichaceae_UCG-002, Olsenella, and Turicibacter in the ileal digesta (Fig 4A). While the role of genera stemming from Erysipelotrichaceae is largely unknown in the pig, in humans they are associated with subjects who consume high-fat western diets and may increase gut inflammation through immunogenic properties [44]. However, the acid ether-extract content of LF and HF were relatively similar [14], and certainly, the fiber level in HF is not typical of a human western diet. Potentially, the increased endogenous fat losses associated with diets high in insoluble fiber favored the metabolism of Erysipelotrichaceae [45], but this is purely speculative. Moreover, an increase of OTUs stemming from Prevotella, Ruminococcaceae, and Lachnospiraceae observed in Fig 5B suggest a greater abundance of mucins and sloughed cells in HF as these microbiota digest and metabolize mucins and sloughed epithelial cells through cooperative metabolism [46,47]. An increase in Turicibacter was observed in both mucosa and digesta of pigs fed HF, relative to LF; this is in agreement with Le Sciellour [48] who observed a greater abundance of Turicibacter in feces from pigs fed a diet with 20% NDF compared to a diet with 10% NDF. However, an increased abundance of Turicibacter in feces has been positively correlated with increased body weight in grow-finish pigs [49], but this is counterintuitive to what was observed in a related study, as pigs fed HF weighed significantly less than LF [14]. Pigs fed HF had a lower abundance of Actinobacillus present in both ileal digesta and mucosa (Fig 4A and 4B). Little is known about the Actinobacillus genus with the exception of species known to cause oral and respiratory diseases [50]. It is unclear if Actinobacillus stemming from the gastrointestinal tract are disease causing or commensal. Notably, work by Gottschalk et al., [51] discovered two non-pathogenic Actinobacillus species in healthy pigs that were antigenically and biochemically similar to A. pleuropneumoniae; research characterizing ileal associated Actinobacillus in the pig is thus warranted.
In this study, the addition of xylanase increased the prevalence of several genera, and OTUs, generally recognized as beneficial, Bifidobacterium, Lactobacillus, Lachnospiraceae_unclassified, Faecalibacterium, and Alloprevotella, and decreased two genera known to contain potentially disease-causing species, Streptococcus and Escherichia-Shigella (Figs 4 and 6). The upregulation of Bifidobacterium, Lachnospiraceae_unclassified, and Lactobacillus by xylanase is in agreement with xylanase supplementation in poultry and turkey diets [52,53]. However, the modulation by xylanase in this study is in contrast to work from Zhang et al., [54] who evaluated the ileal microbiota from pigs fed five mono-component xylanases in corn distiller‘s dried grain with solubles (DDGS)-based diets. This is potentially a result of utilizing corn-bran without solubles herein as the main source of corn-based fiber instead of DDGS as corn-bran appears to be more susceptible to xylanase hydrolysis [14]. Indeed, related work from the same animal study observed HF+XY increased the apparent ileal digestibility of insoluble arabinoxylans by 54% compared to HF [55].
The increased abundance of Bifidobacterium, Lachnospiraceae_unclassified, Lactobacillus, and Faecalibacterium suggest xylanase promotes a symbiotic cooperation for cross-feeding of the potential release products of xylanase that likely increases SCFA production, particularly acetate and butyrate; this cooperation would coincide with a prebiotic-like mechanism [56]. Moreover, the increase in predicted gene counts of enzymes associated with AXOS degradation and SCFA production also support this hypothesis (Figs 8 and 9). Theoretically, the AXOS released by xylanase could be metabolized by Lactobacillus, Bifidobacterium, and Lachnospiraceae_unclassified associated microbiota as several strains from these genera produce many of the accessory enzymes needed to hydrolyze xylo-oligosaccharides and AXOS [57–59]. However, depending on the degrees of polymerization of the AXOS produced by xylanase, these genera likely utilize AXOS with varying efficiencies [60]. Bifidobacterium preferentially favor the uptake of short AXOS into the cell, where they are further degraded to arabinose and xylose; these pentoses are shuttled into the bifid shunt by their conversion into xylulose 5-phosphate, and ultimately the production of acetate [61]. Indeed, xylanase supplementation favored ileal digesta microbiota with a higher predicted gene count for xylose isomerase and xylulose kinase (Fig 8), and acetate kinase producing bacteria in the ileal mucosa (Fig 9). Several strains of Lactobacillus are known to degrade higher molecular weight AXOS [58,59], and there is genomic and in vitro evidence to suggest species deriving from Lachnospiraceae can hydrolyze arabinoxylan and AXOS, and metabolize xylose [62,63]. Finally, the upregulation of Faecalibacterium by HF+XY, whose solely known species is Faecalibacterium prausnitzii, is most likely an indirect result of cross-feeding on the acetate produced by Lactobacillus and Bifidobacterium as Faecalibacterium cannot metabolize pentose monomers [64]. This is further supported by the upregulation of butyryl-CoA:acetate CoA transferase and acetate kinase predicted genes by HF+XY, two key enzymes in the conversion of acetate to butyrate by Faecalibacterium prausnitzii, [65,66]. Thus, the increased modulation of Bifidobacterium, Lachnospiraceae_unclassified, Lactobacillus, and Faecalibacterium by HF+XY suggest there is likely a direct upregulation of acetate and butyrate in the ileum via AXOS and acetate metabolism, respectively.
It is well established that increased butyrate will enhance gut barrier integrity through mediation of the nuclear NF-kB pathway and histone deacetylase inhibition, aid in the control of enteric pathogens, and reduce inflammation and oxidative stress [67,68]. Similarly, acetate can modulate intestinal epithelial integrity and homeostasis via G protein coupled FFAR2 receptors that activate the NLRP3 inflammasome pathway [69]. Indeed, research by Petry et al. [14] from the same animal study discovered that HF+XY increased total antioxidant capacity of the ileum, reduced serum malondialdehyde concentrations, and increased ileal mRNA of claudin 4 and occludin tight junction proteins. Interestingly, xylanase also increased predicted gene counts for ferulic acid esterase; ferulic acid is a potent antioxidant capable of scavenging free radicals and inhibiting free radical production [70]. Corn arabinoxylan is highly substituted with ferulic acid, but its bioavailability is reduced due to esterification within arabinoxylan [71]. It is plausible that hydrolysis of arabinoxylan by HF+XY released feruloylated AXOS that could be further degraded by microbial ferulic acid esterase to improve ferulic acid bioavailability, and possible contribute to the total antioxidant capacity of the pig [72]. Indeed, supplementation of corn-based feruloylated oligosaccharides in rats increased Lactobacillus abundance, and decreased Streptococcus and Clostridium, which is similar to what was found in HF+XY herein [73].
Overall, the improved oxidative status and gut barrier integrity observed by Petry et al., [14], and the upregulation of beneficial acetate and butyrate producing bacteria observed herein, favors a prebiotic like mechanism. However, the increased modulation of predicted genes associated with arabinoxylan degradation in the ileal digesta favor the definition of a stimbiotic mechanism [16]. A holistic view of these data suggests both of these mechanisms are likely occurring synergistically in that xylanase acts as a stimbiotic in the digesta increasing arabinoxylan solubilization and AXOS production, and then in turn, the released AXOS acts in a prebiotic-like manner with mucosal-associated bacteria. Further research is warranted to determine the composition of AXOS produced in situ by xylanase in the presence of corn-based fiber. However, due to the chemical complexities of corn-based arabinoxylans, determining the composition of in situ produced AXOS from corn-based arabinoxylans is an analytically challenging pursuit that has yet to be mastered [74].
Directly supplementing AXOS (HF+AX) did not modulate ileal microbiota to the same degree as xylanase (Fig 7); this is in contrast to what has been reported in poultry [75,76]. Potentially, supplementing xylanase directly may have produced a greater ileal AXOS concentration than what was supplemented in HF+AX, and increasing the concentration within the diet may improve its efficacy. Moreover, the in situ produced AXOS from corn-based fiber may differ in composition to what was supplemented (i.e. DP and substitution pattern). It is also plausible that the directly supplemented AXOS may have been fermented prior to reaching the ileum of the pig. However, when compared to HF, HF+AX did increase the abundance of OTU_40_Lachnospiraceae_unclassified, and OTU_56_Lactobacillus and this is likely through fermentation of AXOS as previously described. This is also supported by the increased abundance of the predicted genes for oligosaccharide reducing-end xylanase, xylan 1,4-β-xylosidase, and xylose isomerase by HF+AX in the ileal digesta (Fig 8). Interestingly, HF+AX greatly increased OTU_39_Sarcina and OTU_81_Sarcina, relative to HF, in the digesta and mucosa, respectively. There is a dearth of information about Sarcina in the pig but has been associated with gastrointestinal disease and delayed gastric emptying in humans [77].
Conclusions
Increasing insoluble corn-based DF through the addition of corn bran without solubles modulated microbial communities associated with mucin and epithelial cell degradation and fat metabolism, while decreasing the abundance of Actinobacillus that is often associated with disease causing species. The direct supplementation of AXOS did not modulate ileal microbiota in a similar fashion or to same degree as xylanase but did favor bacteria with increased genes for oligosaccharide reducing-end xylanase, xylan 1,4-β-xylosidase, and xylose isomerase in the digesta. Directly-supplemented AXOS may differ in composition from what is produced in situ by xylanase’s degradation of corn-based arabinoxylan, or xylanase may increase AXOS production to a greater concentration than what was supplemented in HF+AX. However, supplementing xylanase to HF modulated ileal microbiota to favor beneficial microbial communities that are associated with the symbiotic cross-feeding of oligosaccharides derived from AXOS. The addition of xylanase also upregulated predicted gene counts for enzymes associated with arabinoxylan and AXOS degradation, pentose metabolism, and SCFA production. These data, in combination with the unexpected health benefits often reported in the literature, suggest xylanase favors a prebiotic-like mechanism likely through the release of AXOS that modulate the cross-feeding metabolism of certain beneficial microbial communities. However, the increased predicted gene counts for enzymes associated with arabinoxylan and AXOS degradation in the ileal digesta favors a stimbiotic mechanism. Potentially, xylanase could act as a stimbiotic in the digesta increasing arabinoxylan solubilization and AXOS production, and then in turn, the released AXOS act in a prebiotic-like manner with mucosal associated bacteria.
Acknowledgments
The authors would like to thank Iowa Corn Processors, DSM and Ajinomoto Heartland, Inc for providing in-kind contributions to the Applied Swine Nutrition program at Iowa State University.
Data Availability
All relevant data and 16S rRNA gene sequences are available at the National Center for Biotechnology Information Sequence Read Archive under the BioProject ID: PRJNA664606 or through this link: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA664606.
Funding Statement
Amy Petry (AP) is a recipient of a United States Department of Agriculture National Institute of Food and Agriculture (USDA‐AFRI; https://nifa.usda.gov) National Needs Fellowship for Ph.D. Studies (Grant # 2016‐38420‐25496). USDA‐AFRI provided support in the form of salaries for AP only, but the funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was supported by AB Vista, and they provided the xylanase and AXOS used in this trial. MR Bedford is an employee of AB Vista. As one of the authors, MB had influence over the study design. However, MB had a smaller role in the decision to publish or preparation of the manuscript, and there was no point at which conclusions or interpretations put forward, that were suggested by MB, without full agreement of the other authors. AB Vista was involved in the conceptualization of the project and study design. The specific roles of all authors are articulated in the ‘author contributions’ section.
References
- 1.Weber EK, Patience JF, Stalder KJ. Wean-to-finish feeder space availability effects on nursery and finishing pig performance and total tract digestibility in a commercial setting when feeding dried distillers grains with solubles. J Anim Sci. 2015;93:1905–1915. 10.2527/jas.2014-8136 [DOI] [PubMed] [Google Scholar]
- 2.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. 10.3390/nu5041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta JA, Stein HH, Patience JF. Impact of increasing the levels of insoluble fiber and the method of diet formulation on measures of energy and nutrient digestibility in growing pigs. J Anim Sci. 2020. 10.1093/jas/skaa130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lekagul A, Tangcharoensathien V, Yeung S. Patterns of antibiotic use in global pig production: a systematic review. Vet Anim Sci. 2019;7:100058 10.1016/j.vas.2019.100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutierrez NA, Kerr BJ, Patience JF. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J Anim Sci. 2013;91:5314–5325. 10.2527/jas.2013-6328 [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Peng X, Burrough ER, Sahin O, Gould SA, Gabler NK, et al. Dietary soluble and insoluble fiber with or without enzymes altered the intestinal microbiota in weaned pigs challenged with enterotoxigenic E. coli F18. Front Microbiol. 2020;11:1110–16. 10.3389/fmicb.2020.01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeola O, Cowieson AJ. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. 2010;89:3189–3218. 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- 8.Jaworski NW, Lærke HN, Back Knudsen KE, Stein HH. Carbohydrate composition and in vitro digestibility of dry matter and non-starch polysaccharides in corn, sorghum, and wheat, and coproducts from these grains. J Anim Sci. 2015;93:1103–1113. 10.2527/jas.2014-8147 [DOI] [PubMed] [Google Scholar]
- 9.Dodd D, Cann IKO. Enzymatic deconstruction of xylan for biofuel production. Glob Change Biol Bioenergy. 2009;1:2–17. 10.1111/j.1757-1707.2009.01004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zier-Rush CE, Groom C, Tillman M, Remus J, Boyd RD. The feed enzyme xylanase improves finish pig viability and carcass feed efficiency. J Anim Sci. 2016;94:115 10.2527/msasas2016-244 [DOI] [Google Scholar]
- 11.Chen H, Zhang S, Kim SW. Effects of supplemental xylanase on health of the small intestine in nursery pigs fed diets with corn distillers' dried grains with solubles. J Anim Sci. 2020;98(6):skaa185 10.1093/jas/skaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte ME, Zhou FX, Dutra WM Jr, Kim SW. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim Nutri. 2019;5(4):351–8. 10.1016/j.aninu.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari UP, Chen H, Kim SW, Jha R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim Feed Sci Technol. 2018;245:77–90. 10.1016/j.anifeedsci.2018.07.002 [DOI] [Google Scholar]
- 14.Petry AL, Huntley NF, Bedford MR, Patience JF. Xylanase increased the energetic contribution of fiber and improved the oxidative status, gut barrier integrity, and growth performance of growing pigs fed insoluble corn-based fiber. J Anim Sci. 2020;98(7):skaa233 10.1093/jas/skaa233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen MB, Dalsgaard S, Arent S, Lorentsen R, Knudsen KE, Yu S, et al. Xylanase and protease increase solubilization of non-starch polysaccharides and nutrient release of corn-and wheat distillers dried grains with solubles. Biochem Eng J. 2015;98:99–106. 10.1016/j.bej.2015.02.036 [DOI] [Google Scholar]
- 16.González-Ortiz G, Gomes GA, Dos Santos TT, Bedford MR. New strategies influencing gut functionality and animal performance In: Gonzalez-Ortiz G, Bedford MR, Bach Knudsen KE, Courtin C, Classen HL, editors. The value of fibre. Engaging the second brain for animal nutrition. Wageningen: Wageningen Academic Press; 2019. pp.233–253. [Google Scholar]
- 17.Bedford MR. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Brit Poult Sci. 2018;59:486–493. 10.1080/00071668.2018.1484074 [DOI] [PubMed] [Google Scholar]
- 18.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- 19.FASS. Guide for the Care and Use of Agricultural Animals in Research and Teaching: Third Edition Champaign, IL: Federation of Animal Science Societies; 2010; 169 pp. [Google Scholar]
- 20.Caporaso J, Ackermann G, Apprill A, Bauer M, Berg-Lyons D, Betley J, et al. EMP 16S Illumina Amplicon Protocol: Earth Microbiome Project; 2018. [Cited 2019 November 18]. Available from: http://press.igsb.anl.gov/earthmicrobiome/protocols-and-standards/16s/. [Google Scholar]
- 21.Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18(5):1403–14. 10.1111/1462-2920.13023 [DOI] [PubMed] [Google Scholar]
- 22.Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aqua Microb Ecol. 2015;75(2):129–37. 10.3354/ame01753 [DOI] [Google Scholar]
- 23.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–20. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35(21):7188 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim BR, Shin J, Guevarra R, Lee JH, Kim DW, Seol KH, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27(12): 2089–93. 10.4014/jmb.1709.09027 [DOI] [PubMed] [Google Scholar]
- 27.Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;1: 1–5. 10.1038/s41587-020-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bengtsson-Palme J, Metaxa FA, Parsers FA, Metaxa FA. Tag: HMMER. Update. 2020. June 3. [Google Scholar]
- 29.Barbera P, Kozlov AM, Czech L, Morel B, Darriba D, Flouri T, et al. EPA-ng: massively parallel evolutionary placement of genetic sequences. Syst Bio. 2019;68(2): 65–9. 10.1093/sysbio/syy054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czech L, Barbera P, Stamatakis A. Genesis and Gappa: processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics. 2020;36(10):3263–5. 10.1093/bioinformatics/btaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louca S, Doebeli M. Efficient comparative phylogenetics on large trees. Bioinformatics. 2018;34(6):1053–5. 10.1093/bioinformatics/btx701 [DOI] [PubMed] [Google Scholar]
- 32.Caspi R, Billington R, Keseler IM, Kothari A, Krummenacker M, Midford PE, et al. The MetaCyc database of metabolic pathways and enzymes-a 2019 update. Nucleic Acids Res. 2020;48(D1):D445–53. 10.1093/nar/gkz862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Chen D, Tian G, Zheng P, Mao X, Yu J, et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. BioMed Res Int. 2019;23:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha R, Berrocoso JD. Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9(9):1441–52. 10.1017/S1751731115000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jørgensen H, Serena A, Hedemann MS, Knudsen KE. The fermentative capacity of growing pigs and adult sows fed diets with contrasting type and level of dietary fibre. Livest Sci. 2007;109(1):111–4. [Google Scholar]
- 36.Tiwari UP, Singh AK, Jha R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim Nutr. 2019;5(3):217–26. 10.1016/j.aninu.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaacson R, Kim HB. The intestinal microbiome of the pig. Anim Health Res Revs. 2012;13: 100–9. 10.1017/S1466252312000084 [DOI] [PubMed] [Google Scholar]
- 38.Fouhse JM, Zijlstra RT, Willing BP. The role of gut microbiota in the health and disease of pigs. Anim Front. 2016;6:30–6. 10.2527/af.2016-0031 [DOI] [Google Scholar]
- 39.Gutierrez NA, Serão NV, Kerr BJ, Zijlstra RT, Patience JF. Relationships among dietary fiber components and the digestibility of energy, dietary fiber, and amino acids and energy content of nine corn coproducts fed to growing pigs. J Anim Sci. 2014;92(10):4505–17. 10.2527/jas.2013-7265 [DOI] [PubMed] [Google Scholar]
- 40.Holman DB, Brunelle BW, Trachsel J, Allen H K. Meta-analysis to define a core microbiota in the swine gut. mSystems. 2017;2(3): e00004–17. 10.1128/mSystems.00004-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rodas B, Youmans BP, Danzeisen JL, Tran H, Johnson TJ. Microbiome profiling of commercial pigs from farrow to finish. J Anim Sci. 2018;96(5):1778–94. 10.1093/jas/sky109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Wu W, Lee YK, Xie J, Zhang H. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. 2018; 26(9): 48 10.3389/fmicb.2018.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Abbeele P, Van de Wiele T, Verstraete W, Possemiers S. The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS Microbiol Rev. 2011;35(4): 681–704. 10.1111/j.1574-6976.2011.00270.x [DOI] [PubMed] [Google Scholar]
- 44.Kaakoush NO. Insights into the role of Erysipelotrichaceae in the human host. Front Cell Infect Microbiol. 2015;20(5):84 10.3389/fcimb.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Wang Z, Ding J, Ming D, Wang W, Jiang Z, et al. Effects of dietary fiber content and different fiber-rich ingredients on endogenous loss of fat and fatty acids in growing pigs. J Anim Sci Biotechnol. 2019;10(1):42 10.1186/s40104-019-0348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10(4): 336–347. 10.1016/j.chom.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran TH, Boudry C, Everaert N, Théwis A, Portetelle D, Daube G, et al. Adding mucins to an in vitro batch fermentation model of the large intestine induces changes in microbial population isolated from porcine feces depending on the substrate. FEMS Microbiol Ecol. 2016;92(2):fiv165 10.1093/femsec/fiv165 [DOI] [PubMed] [Google Scholar]
- 48.Le Sciellour M, Labussière E, Zemb O, Renaudeau D. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS One. 2018. October 24;13(10):e0206159 10.1371/journal.pone.0206159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Tsai T, Deng F, Wei X, Chai J, Knapp J, et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome. 2019;7(1):109 10.1186/s40168-019-0721-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J. 2000;159(1):18–36. 10.1053/tvjl.1999.0403 [DOI] [PubMed] [Google Scholar]
- 51.Gottschalk M, Broes A, Mittal KR, Kobisch M, Kuhnert P, Lebrun A, et al. Non-pathogenic Actinobacillus isolates antigenically and biochemically similar to Actinobacillus pleuropneumoniae: a novel species? Vet Microbiol. 2003;92(1–2):87–101. 10.1016/s0378-1135(02)00341-3 [DOI] [PubMed] [Google Scholar]
- 52.González-Ortiz G, Olukosi OA, Jurgens G, Apajalahti J, Bedford MR. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult Sci. 2020;99(4):2068–77. 10.1016/j.psj.2019.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vahjen W, Gläser K, Schäfer K, Simon O. Influence of xylanase-supplemented feed on the development of selected bacterial groups in the intestinal tract of broiler chicks. J Agric Sci. 1998;130(4):489–500. 10.1017/S0021859698005498 [DOI] [Google Scholar]
- 54.Zhang Z, Tun HM, Li R, Gonzalez BJ, Keenes HC, Nyachoti CM, et al. Impact of xylanases on gut microbiota of growing pigs fed corn-or wheat-based diets. Anim Nutri. 2018;4(4):339–50. 10.1016/j.aninu.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petry AL, Huntley NF, Bedford MR, Zijlstra RT, Patience JF. Supplementing xylanase increased the digestibility of non-starch polysaccharides, particularly arabinoxylan, in diets high in insoluble corn fiber fed to swine with a 36-d dietary adaptation period. J Anim Sci. 2020; 98(3):52 10.1093/jas/skaa054.095 [DOI] [Google Scholar]
- 56.Zeybek N, Rastall RA, Büyükkileci AO. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr Polym. 2020; 236(15):116076 10.1016/j.carbpol.2020.116076 [DOI] [PubMed] [Google Scholar]
- 57.Stackebrandt E. The family Lachnospiraceae In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Berlin: Springer; 2014. pp. 197–201. [Google Scholar]
- 58.Grootaert C, Delcour JA, Courtin CM, Broekaert WF, Verstraete W, Van de Wiele T. Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine. Trends Food Sci Technol. 2007;18(2):64–71. 10.1016/j.tifs.2006.08.004 [DOI] [Google Scholar]
- 59.McLaughlin HP, Motherway MO, Lakshminarayanan B, Stanton C, Ross RP, Brulc J, et al. Carbohydrate catabolic diversity of bifidobacteria and lactobacilli of human origin. Int J Food Microbiol. 2015;203:109–21. 10.1016/j.ijfoodmicro.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 60.Feng G, Flanagan BM, Mikkelsen D, Williams BA, Yu W, Gilbert RG, et al. Mechanisms of utilisation of arabinoxylans by a porcine faecal inoculum: competition and co-operation. Sci Rep. 2018;8(1):1–1. 10.1038/s41598-017-17765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979 10.3389/fmicb.2016.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5(3):627–40. 10.3390/d5030627 [DOI] [Google Scholar]
- 63.Rombouts JL, Mos G, Weissbrodt DG, Kleerebezem R, Van Loosdrecht MC. Diversity and metabolism of xylose and glucose fermenting microbial communities in sequencing batch or continuous culturing. FEMS Microbiol Ecol. 2019;95(2):fiy233 10.1093/femsec/fiy233 [DOI] [PubMed] [Google Scholar]
- 64.Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72(12):7835–41. 10.1128/AEM.01296-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miquel S, Martín R, Bridonneau C, Robert V, Sokol H, Bermúdez-Humarán LG, et al. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5(2):146–51. 10.4161/gmic.27651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–61. 10.1016/j.mib.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 67.Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Nielsen G, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;(10):1499 10.3390/nu10101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018; 4(2):151–159. 10.1016/j.aninu.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Com. 2015;1(6):6734 10.1038/ncomms7734 [DOI] [PubMed] [Google Scholar]
- 70.Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, et al. Radical scavenging activity and cytotoxicity of ferulic acid. Anticanc Res. 2002;22(5):2711–7. doi: 10.12529986 [PubMed] [Google Scholar]
- 71.Anson NM, van den Berg R, Havenaar R, Bast A, Haenen GR. Bioavailability of ferulic acid is determined by its bioaccessibility. J Cereal Sci. 2009;49(2):296–300. 10.1016/j.jcs.2008.12.001 [DOI] [Google Scholar]
- 72.Mathew S, Abraham TE. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol. 2004;24(2–3):59–83. 10.1080/07388550490491467 [DOI] [PubMed] [Google Scholar]
- 73.Ou JY, Huang JQ, Song Y, Yao SW, Peng XC, Wang MF, et al. Feruloylated oligosaccharides from maize bran modulated the gut microbiota in rats. Plant Foods Hum Nutr. 2016;71:123–8. 10.1007/s11130-016-0547-4 [DOI] [PubMed] [Google Scholar]
- 74.Alyassin M, Campbell GM. Challenges and constraints in analysis of oligosaccharides and other fibre components In: González-Ortiz G, Bedford MR, Bach Knudsen KE, Courtin CM, Classen HL editors. The value of fibre. Engaging the second brain for animal nutrition. Wageningen: Wageningen Academic Press; 2019. pp.343–49. [Google Scholar]
- 75.Bautil A, Verspreet J, Buyse J, Goos P, Bedford MR, Courtin CM. Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler. Poult Sci. 2020;99(5). 10.1016/j.psj.2019.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pourabedin M, Guan L, Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3(1):1–2. 10.1186/s40168-014-0066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lam-Himlin D, Tsiatis AC, Montgomery E, Pai RK, Brown JA, Razavi M, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35(11):1700 10.1097/PAS.0b013e31822911e6 [DOI] [PMC free article] [PubMed] [Google Scholar]