Abstract
Background
The incidence of rheumatic heart disease (RHD) among Indigenous Australians remains one of the highest in the world. Many studies have highlighted the relationship between the social determinants of health and RHD, but few have used registry data to link socioeconomic disadvantage to the delivery of patient care and long-term outcomes.
Methods
A retrospective study of individuals living with RHD in Far North Queensland (FNQ), Australia between 1997 and 2017. Patients were identified using the Queensland state RHD register. The Socio-Economic Indexes for Areas (SEIFA) Score–a measure of socioeconomic disadvantage–was correlated with RHD prevalence, disease severity and measures of RHD care.
Results
Of the 686 individuals, 622 (90.7%) were Indigenous Australians. RHD incidence increased in the region from 4.7/100,000/year in 1997 to 49.4/100,000/year in 2017 (p<0.001). In 2017, the prevalence of RHD was 12/1000 in the Indigenous population and 2/1000 in the non-Indigenous population (p<0.001). There was an inverse correlation between an area’s SEIFA score and its RHD prevalence (rho = -0.77, p = 0.005).
249 (36.2%) individuals in the cohort had 593 RHD-related hospitalisations; the number of RHD-related hospitalisations increased during the study period (p<0.001). In 2017, 293 (42.7%) patients met criteria for secondary prophylaxis, but only 73 (24.9%) had good adherence. Overall, 119/686 (17.3%) required valve surgery; the number of individuals having surgery increased over the study period (p = 0.02).
During the study 39/686 (5.7%) died. Non-Indigenous patients were more likely to die than Indigenous patients (9/64 (14%) versus 30/622 (5%), p = 0.002), but Indigenous patients died at a younger age (median (IQR): 52 (35–67) versus 73 (62–77) p = 0.013). RHD-related deaths occurred at a younger age in Indigenous individuals than non-Indigenous individuals (median (IQR) age: 29 (12–58) versus 77 (64–78), p = 0.007).
Conclusions
The incidence of RHD, RHD-related hospitalisations and RHD-related surgery continues to rise in FNQ. Whilst this is partly explained by increased disease recognition and improved delivery of care, the burden of RHD remains unacceptably high and is disproportionately borne by the socioeconomically disadvantaged Indigenous population.
Author summary
Rheumatic heart disease (RHD), a disease of poverty and disadvantage, is almost completely preventable. It is now extremely rare in wealthy countries, but in Far North Queensland in tropical Australia, the incidence of RHD, RHD-related hospitalisations and RHD-related surgery is continuing to rise, with the burden of disease borne almost entirely by the region’s Indigenous population. While the increasing incidence of RHD and its complications may be partly explained by improvements in local service delivery, the disease remains inextricably linked to socioeconomic disadvantage. In this study, not only were patients living in socioeconomically disadvantaged areas more likely to have RHD, but they were also paradoxically less likely to receive valve surgery. The current local model of care—which is centralised, medical and emphasises disease monitoring and secondary prophylaxis—appears to be having a limited impact on morbidity. Strategies must evolve—in partnership with Indigenous communities—to have a greater focus on disease prevention by addressing the personal, community and environmental factors that increase the risk of the disease. This is likely to not only reduce the incidence of RHD, but will also tend to reduce the burden of the many other diseases that result from socioeconomic disadvantage and that disproportionately affect Indigenous Australians.
Introduction
Rheumatic heart disease (RHD)—a chronic, debilitating and potentially fatal disease of social disadvantage—is almost entirely preventable [1–3]. Improvements in the standard of living and the development of more effective treatment of group A streptococcal (GAS) infections during the 20th century, resulted in the near elimination of acute rheumatic fever (ARF), which in turn, led to a marked reduction in the burden of RHD in high-income countries [4,5]. A similar trend has been observed in the non-Indigenous Australian population. However, the prevalence of RHD in some Indigenous Australian communities remains amongst the highest in the world, a source of continuing national shame [6,7].
“Indigenous Australians” is a frequently used term, but it does not account for the enormous variation in circumstances that exists for Indigenous individuals in different parts of the country [8]. Although socioeconomic disadvantage is more common among Indigenous Australians generally, there are distinct challenges faced by Indigenous people who live in rural and remote locations, where access to health care is limited and where other health conditions compete for finite resources [8–10]. It is also important to note that while Torres Strait Islander Australians are Indigenous Australians, they are ethnologically distinct to Aboriginal Australians and have their own unique cultural history. Although there is some overlap between the health challenges faced by the two peoples, there are also many differences [8].
RHD is estimated to kill over 300,000 people globally every year, but the disease remains profoundly neglected [11,12]. Although medical interventions can reduce the morbidity and mortality of established RHD, interventions that reduce the incidence of the inciting GAS infections–primordial prevention—are likely to be more cost-effective, and there is a growing recognition of the role of the socioeconomic factors in the development of RHD, even among clinicians [7]. However, while many studies have highlighted the relationship between the social determinants of health and the burden of RHD, most have been observational, cross-sectional and have not linked socioeconomic status to interventions or long-term outcomes [3]. This can be at least partly explained by the fact that RHD usually occurs in resource-poor settings where registry data—which provide more detail on delivery of care and longitudinal follow-up—are frequently lacking [11].
ARF has been a notifiable disease in the state of Queensland since 1999, while RHD has been notifiable since 2018. Affected patients’ demographic and clinical data are collected by staff employed by a dedicated RHD program, stored in a database—the RHD register—which is used to assist the coordination of medical care. Far North Queensland (FNQ), in tropical Australia is home to approximately 280,000 people who are dispersed across an area of 380,748 km2. No fewer than 17% of the local population identify as Indigenous Australians, almost half of whom report Torres Strait Islander heritage [13]. Evolving prosperity and public health interventions have seen the elimination of malaria and filariasis in the region, while the incidence of several others–including hepatitis B, strongyloidiasis and leprosy–is in steep decline [14–16]. However it is also a region which still contains 3 of the 10 most socio-economically disadvantaged local government areas in Australia, all 3 are communities with a predominantly Indigenous population [17]. When compared to other regions of Australia, there are relatively few studies that have examined the local prevalence of RHD and almost none that compare the relative burdens in Aboriginal and Torres Strait Islander Australians [18,19].
This study therefore used registry data to examine the temporospatial epidemiology of RHD in FNQ and the performance of the local RHD control program in addressing the disease. There was a focus on the relationship between measures of socioeconomic disadvantage and disease prevalence, severity, and treatment. The study sought to examine differences in disease burden between Aboriginal and Torres Strait Islander Australians and to evaluate the care of individuals living in rural and remote—rather than urban—locations. It was hoped that these data might be used to inform local strategies to address the disease in a more comprehensive manner.
Methods
Ethics statement
The Far North Queensland Human Research Ethics Committee provided ethical approval for the study (HREC/18/QCH/91–1261). As the data were retrospective and de-identified, the Committee waived the requirement for informed consent.
Study design
This retrospective study was performed at Cairns Hospital, the sole tertiary referral hospital in FNQ. There was also significant contribution from the local Rheumatic Heart Disease Program which aims to improve the local care of ARF and RHD. The Queensland RHD register was used to identify patients, who were eligible for inclusion in the study if they had a diagnosis of RHD confirmed on echocardiogram that had been reported by a specialist physician, between January 1, 1997 and December 31, 2017. RHD was further categorised—based on the specialist physicians’ echocardiogram report—as mild, moderate or severe. Patients who were reported as having “borderline” disease (minor abnormalities identified by echocardiography that could represent normal variation) were not included in the study. Patients were said to having a history of ARF if they had had an episode of possible, probable or confirmed ARF reported to the RHD register; ARF was diagnosed by local clinicians using their clinical judgment, supported by the Jones criteria which were updated during the study period [7].
The patients’ demographics and their clinical course during the study period were collected from the database and their medical records. The patients’ Indigenous status was recorded; when individuals register with the public health system, they are routinely asked whether they identify as an Aboriginal Australian, a Torres Strait Islander Australian, both or neither. Australian Bureau of Statistics population data were used to calculate disease incidence and prevalence [13]. If an individual lived in the region’s administrative hub—Cairns—they were said to have an urban address, otherwise they were deemed to live in a rural or remote area. Socioeconomic status was quantified using the Socio-Economic Indexes for Areas (SEIFA) Score, a measure of socioeconomic disadvantage developed by the Australian Bureau of Statistics [20].
Complete secondary prophylaxis data were only available from 2007 and so adherence to secondary prophylaxis could only be assessed after this date. Adherence to parenteral penicillin was determined by dividing the number of doses of penicillin received by the number prescribed; ≥10 parenteral penicillin doses in 1 year was defined as good adherence. Hospitalisation data were recorded with the presentations classified using International Statistical Classification of Diseases and Related Health Problems (ICD-10) coding. Hospitalisations were defined as being RHD related if it could be explained by valvular pathology or its complications. All cardiac surgical interventions (valvuloplasty and valve replacement) were recorded. If a patient died, their cause of death was identified by review of the medical record where this was accessible.
Statistics
Data were collected from the RHD register or the medical record, de-identified, entered into an electronic database (Microsoft Excel 2016, Microsoft, Redmond, WA, USA) and analysed using statistical software (Stata version 14.2, StataCorp LLC, College Station, TX, USA). Groups were analysed using the Kruskal-Wallis and chi-squared tests, where appropriate. Correlation coefficients were determined using Spearman’s method. Trends over time were determined using an extension of the Wilcoxon rank-sum test [21]. Multivariate analysis was performed using backwards stepwise logistic regression. If individuals were missing data, they were not included in analyses which evaluated those variables.
Results
Demographics
There were 686 individuals diagnosed as living with RHD in the region during the study period. Their median (interquartile range (IQR)) age at the time of RHD diagnosis was 29 (17–44) years, 458 (66.7%) were female and 622 (90.7%) identified as Indigenous Australians. Among the 622 Indigenous patients, 347 (55.8%) identified as Aboriginal Australians, 205 (32.9%) identified as Torres Strait Islander Australians while 70 (11.3%) identified as both. Among the 64 non-Indigenous patients, 29 (45.3%) were born in Australia, while 25 (39%) were born in other countries from the Asia-Pacific region.
Indigenous patients were younger than non-Indigenous patients (median (IQR): 33 (23–47) versus 60 (27–74), p<0.001), more likely to live in a rural or remote location (333/622 (53.5%) versus 11/64 (17.2%), p<0.001) and more likely to live in a socioeconomically disadvantaged area (median (IQR) SEIFA: 870 (836–967) versus 967 (925–967), p<0.001.
Incidence of acute rheumatic fever
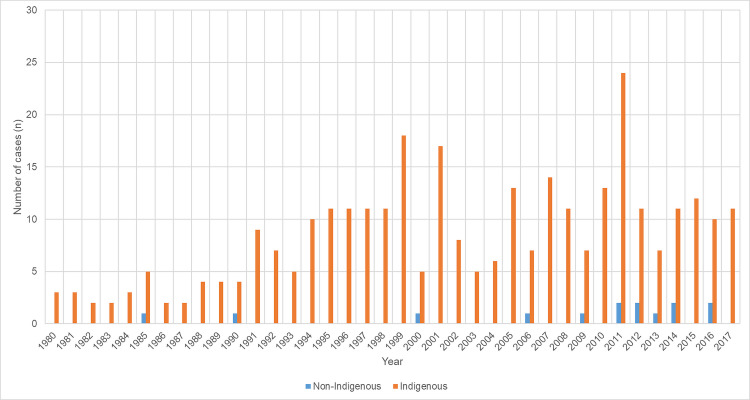
There was a documented history of ARF in 358/686 (52.2%); there was no reduction in new ARF diagnoses during the study period (p for trend = 0.46) (Fig 1).
Fig 1. Number of new cases of ARF diagnosed in the cohort annually after 1980, stratified by Indigenous status.
Incidence of RHD
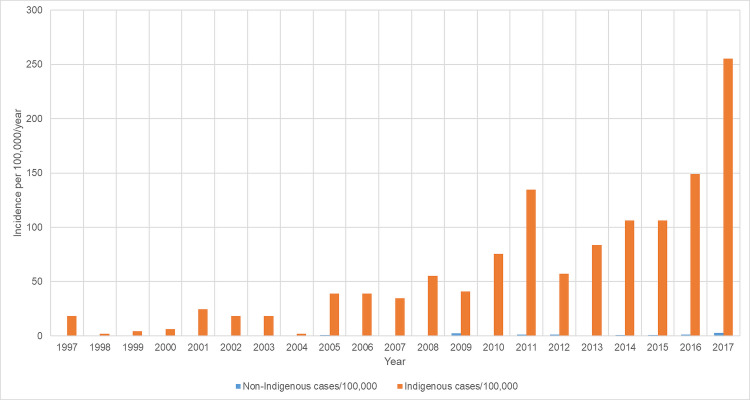
The incidence of new RHD diagnoses increased in the region from 4.7/100,000/year in 1997 to 49.4/100000/year in 2017 (p for trend<0.001). The incidence of RHD was higher in the Indigenous population than the non-Indigenous population throughout the study period (Fig 2). In 2017, the incidence of new RHD diagnoses was 255/100,000/year in Indigenous population compared with 3/100,000/year in the non-Indigenous population (p<0.0001).
Fig 2. Incidence of new RHD diagnosis during the study period, stratified by Indigenous status.
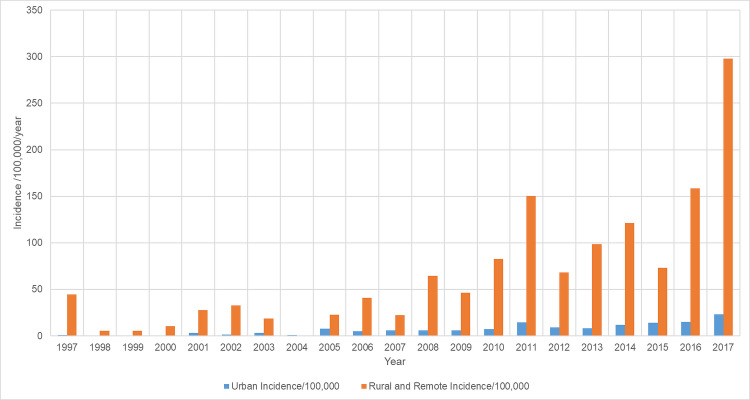
RHD incidence increased in both urban and rural and remote locations, but it was higher in rural and remote locations throughout the study (Fig 3). In 2017 the incidence was 298/100,000/year in rural and remote locations compared with 23/100,000/year in the urban population (p<0.001).
Fig 3. Incidence of new RHD diagnosis during the study period, stratified by residential address.
Prevalence
At the end of the study period, the prevalence of RHD in the Indigenous population was 12/1000 compared with 2/1000 in the non-Indigenous population (p<0.001). The prevalence was also higher in individuals living in rural and remote regions in those with urban address (12/1000 versus 1/1000 p<0.001).
Spatial epidemiology of disease prevalence and association with socioeconomic status
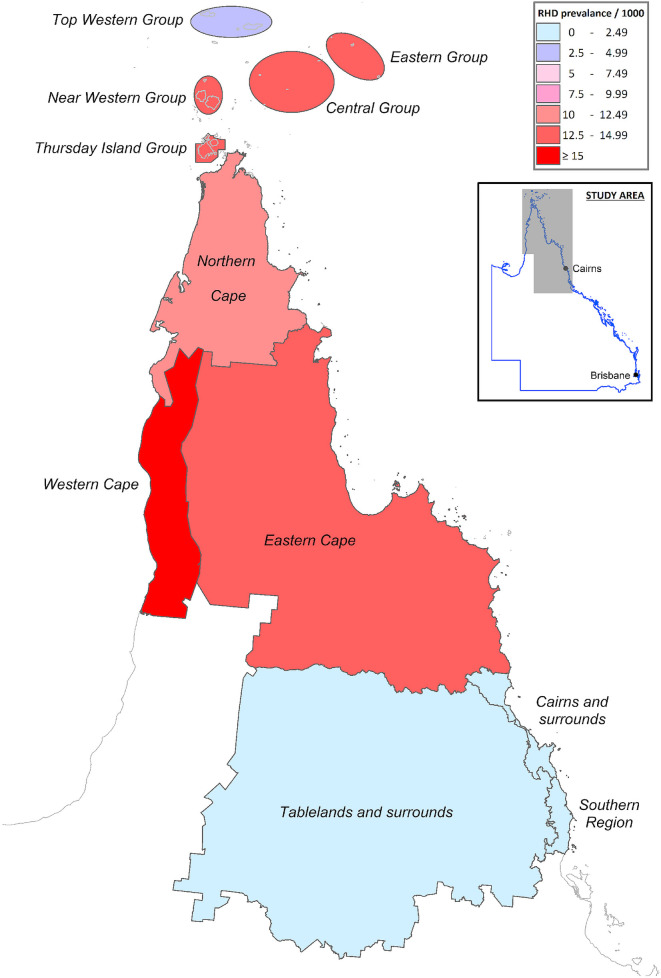
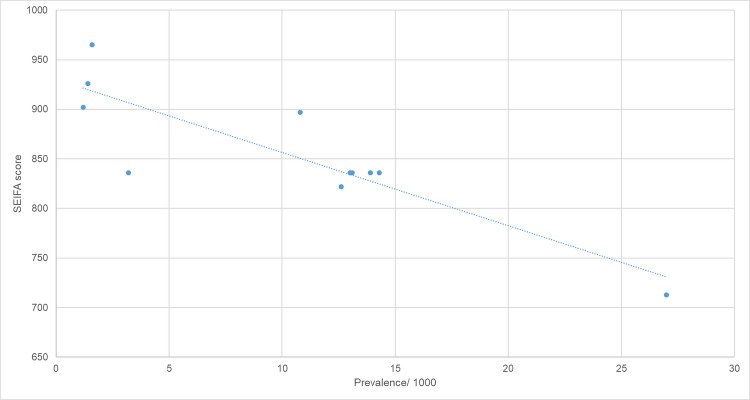
There was significant heterogeneity in the prevalence of RHD within the region (Fig 4). The prevalence varied from 1/1000 in the southern region to 27/1000 population in Western Cape York. There was an inverse correlation between an area’s SEIFA score and its RHD prevalence (Spearman’s rho = -0.77, p = 0.005) (Fig 5).
Fig 4. Regional prevalence of RHD in Far North Queensland.
The map was created using constructed using mapping software (MapInfo version 15.02, Connecticut, USA) using data provided by the State of Queensland (QSpatial). Queensland Place Names—State of Queensland (Department of Natural Resources, Mines and Energy) 2019, available under Creative Commons Attribution 4.0 International licence https://creativecommons.org/licenses/by/4.0/. ‘Coastline and state border–Queensland—State of Queensland (Department of Natural Resources, Mines and Energy) 2019, available under Creative Commons Attribution 4.0 International licence https://creativecommons.org/licenses/by/4.0/.
Fig 5. Prevalence of RHD by socioeconomic status (determined using SEIFA score [20]).
Disease severity
Indigenous patients, patients living in rural and remote locations and patients living in areas of greater socioeconomic disadvantage were less likely to have severe disease (Tables 1, 2 and 3). At the end of the study, 313/647 (48.4%) living patients had mild RHD, 176 (27.2%) had moderate and 158 (24.4%) had severe disease. Among the 592 living Indigenous patients, 138 (23.3%) had severe disease compared with 20/55 (36.4%) non-Indigenous patients (p = 0.03), however, Indigenous patients with severe disease were younger than non-Indigenous patients with severe disease (median (IQR): 41 (29–54) versus 62 (34–71) years, p = 0.005). In multivariate analysis that considered age, Indigenous status, SEIFA score and residence in a rural/remote location, only age (odds ratio (OR): 1.03 (95% Confidence interval (CI):1.02–1.04, p<0.001) and rural/remote residence were associated with disease severity (OR (95%CI): 0.63 (0.43–0.91), p = 0.01) at the end of the study period.
Table 1. Characteristics of the cohort stratified by Indigenous status.
| Variable | Indigenous n = 622 | Non-Indigenous n = 64 | p |
|---|---|---|---|
| Age at the end of the study | 33 (23–47) | 60 (27–74) | 0.0001 |
| Age at RHD diagnosis | 28 (17–43) | 54 (24–68) | 0.0001 |
| Female | 420 (67.5%) | 38 (59.4%) | 0.18 |
| Rural/remote residence | 333 (53.5%) | 11 (17.2%) | <0.0001 |
| Severe disease | 151 (24.3%) | 26 (40.6%) | 0.004 |
| Hospitalisations/person | 5 (2–10) | 6 (2–16) | 0.73 |
| RHD-related hospitalisations | 215 (35%) | 34 (62.5%) | 0.003 |
| Valve surgery or valvuloplasty | 98 (15.8%) | 21 (32.8%) | 0.001 |
| Died | 30 (4.8%) | 9 (14.1%) | 0.002 |
| Age at death | 54 (34–68) | 74 (62–77) | 0.01 |
| Adherence | 41 (23–58) | 46 (10–60) | 0.54 |
| Good adherence | 67/274 (24.5%) | 6/19 (31.6%) | 0.58 |
| Number of echocardiograms | 5 (3–8) | 4 (2–8) | 0.18 |
| RHD progression on serial echocardiograms | 121/575 (21%) | 10/54 (18.5%) | 0.66 |
| Specialist review | 585 (94.1%) | 58 (90.6%) | 0.28 |
| Dental review | 186 (29.9%) | 3 (4.7%) | <0.0001 |
All numbers are median (interquartile range) or the absolute number (%)
Table 2. Characteristics of the cohort stratified by residence in a rural/remote or an urban location.
| Variable | Rural/Remote n = 344 | Urban n = 342 | p |
|---|---|---|---|
| Age at the end of the study | 35 (24–50) | 34 (22–50) | 0.23 |
| Age at RHD diagnosis | 29 (18–45) | 28 (16–44) | 0.16 |
| Female | 224 (65.1%) | 234 (68.4%) | 0.36 |
| Indigenous Australian | 333 (96.8%) | 289 (84.5%) | <0.0001 |
| Severe disease | 76 (22.1%) | 101 (29.5%) | 0.03 |
| Hospitalisations/person | 5 (2–9) | 5 (3–12) | 0.11 |
| RHD-related hospitalisations | 104 (30.2%) | 145 (42.4%) | 0.001 |
| Valve surgery or valvuloplasty | 42 (12.2%) | 77 (22.5%) | <0.0001 |
| Died | 17 (4.9%) | 22 (6.4%) | 0.40 |
| Age at death | 55 (30–70) | 61 (43–71) | 0.60 |
| Adherence | 45 (28–62) | 38 (19–53) | 0.02 |
| Good adherence | 38/139 (27%) | 35/154 (22.7%) | 0.36 |
| Number of echocardiograms | 5 (3–9) | 5 (3–8) | 0.32 |
| RHD progression on serial echocardiograms | 66/319 (20.7%) | 65/310 (21.0%) | 0.93 |
| Specialist review | 333 (96.8%) | 310 (90.6%) | 0.001 |
| Dental review | 134 (39.0%) | 55/342 (16.1%) | <0.0001 |
All numbers are median (interquartile range) or the absolute number (%)
Table 3. Association of socioeconomic status and demographic characteristics, and the features, management, and clinical course of the RHD.
| Variable | Yes (n) | No (n) | SEIFA if yes Median (IQR) | SEIFA if no Median (IQR) | p |
|---|---|---|---|---|---|
| Age <40 years | 410 | 276 | 870 (836–967) | 870 (771–967) | 0.26 |
| Female | 458 | 228 | 870 (836–967) | 870 (836–967) | 0.21 |
| Indigenous Australian | 622 | 64 | 870 (836–967) | 967 (924–967) | 0.0001 |
| Remote/rural residence | 344 | 342 | 836 (713–853) | 967 (914–967) | 0.0001 |
| Severe disease | 177 | 509 | 914 (836–967) | 870 (836–967) | 0.02 |
| >10 hospitalisations | 190 | 496 | 922 (836–967) | 870 (836–967) | 0.004 |
| RHD related hospitalisation | 249 | 437 | 914 (836–967) | 859 (771–967) | 0.0001 |
| Surgical intervention | 119 | 567 | 958 (859–967) | 870 (836–967) | 0.0001 |
| Died | 39 | 647 | 931 (836–967) | 870 (836–967) | 0.17 |
| Good Adherence to secondary prophylaxis | 73 | 220 | 870 (836–967) | 896 (836–967) | 0.85 |
| ≥ 5 echocardiograms | 404 | 282 | 870 (836–967) | 914 (836–967) | 0.14 |
| RHD progression on serial echocardiograms | 131 | 498 | 870 (771–967) | 870 (836–967) | 0.54 |
| Specialist review | 643 | 43 | 870 (836–967) | 967 (836–967) | 0.0003 |
| Dental review | 189 | 497 | 859 (771–958) | 896 (836–967) | 0.0001 |
IQR: interquartile range; RHD: Rheumatic heart disease
Service delivery and retention in care
Patients living in rural and remote locations and patients living in areas of greater socioeconomic disadvantage were more likely to receive specialist review (Tables 2 and 3). A greater proportion of Indigenous patients received specialist review than non-Indigenous patients, however the difference failed to reach statistical significance (Table 1)
Patients had a median (IQR) of 5 (3–8) echocardiograms over median (IQR) follow-up time of 9 (5–16) years. Among the 629 (91.6%) patients who had more than one echocardiogram performed during the study period, RHD progressed in 131 (20.8%), was stable in 443 (79.4%) and improved in 55 (8.7%) over a median (IQR) of 8 (4–14) years. The risk of disease progression was not influenced by Indigenous status, rural/remote residence, or SEIFA score (Tables 1, 2 and 3). Only 189/686 (27.6%) had documented dental review in the public health system.
Secondary prophylaxis
From 2007—the year when data were available—a total of 388 (57%) patients were prescribed secondary prophylaxis, 367 (95%) received parenteral penicillin while 21 (5%) received oral therapy. During this time, the median (IQR) adherence for parenteral prophylaxis was 49% (34–63). There was no difference in adherence between Indigenous and non-Indigenous patients (median (IQR): 41 (23–58) versus 46 (10–60), p = 0.90). However, patients living in a rural or remote location had greater adherence than urban patients (median (IQR): 48% (25–62) versus 38% (19–53), p = 0.0001) and SEIFA score was inversely associated with adherence (Spearman’s rho = -0.13, p = 0.002). Patients with disease progression on sequential echocardiograms were likely to have lower adherence to secondary prophylaxis (median (IQR): 35% (19–58) versus 44% (26–60), p = 0.03). At the end of the study period, 293/686 (42.7%) met criteria for secondary prophylaxis, 79 (24.9%) of whom had good adherence.
Hospitalisations
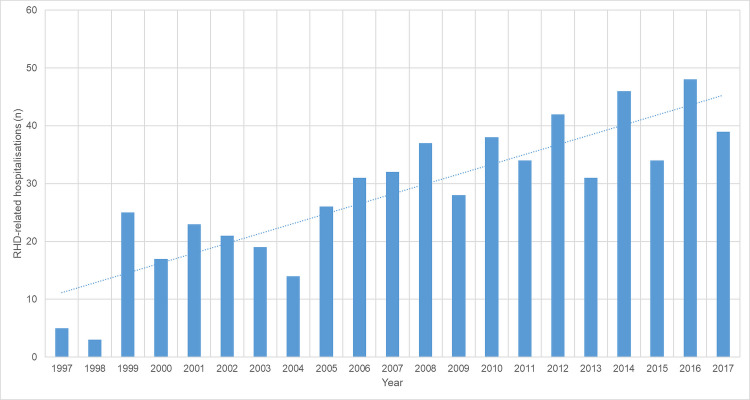
During the study 627/686 (91.4%) were hospitalised, in 249 (39.7%) at least one of the hospitalisations was RHD-related. There were 593 RHD-related hospitalisations among these 249 patients; the number of RHD-related hospitalisations increased over the study period (p<0.001) (Fig 6). 218/249 (87.6%) hospitalisations were related to cardiac manifestations alone, 12/249 (4.8%) had only neurological hospitalisations, while 19/249 had both cardiac and neurological hospitalisations. There were 50 strokes (38 (76%) ischaemic and 12 (24%) haemorrhagic) among the 31 patients with a neurological admission.
Fig 6. RHD-related hospitalisations by year.
Surgical management
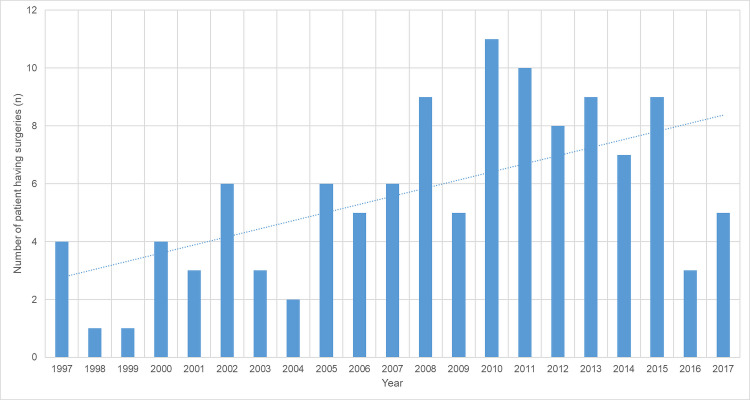
During the study period 119 (17.4%) individuals had 150 episodes of surgery during which 215 individual procedures were performed; 152/215 (70.7%) procedures were valve replacements and 63 (29.3%) were valvuloplasties (Table 4). The number of individuals having surgery increased during the study period (p for trend = 0.02). (Fig 7)
Table 4. Description of valvular interventions.
| Procedures | Mitral valve | Aortic valve | Tricuspid valve | Pulmonary valve |
|---|---|---|---|---|
| Metallic valve replacement | 61 | 40 | 0 | 0 |
| Bioprosthetic valve replacement | 26 | 21 | 3 | 0 |
| Valvuloplasty | 39 | 6 | 17 | 1 |
| Total procedures | 126 | 67 | 21 | 1 |
Absolute numbers are presented.
Fig 7. Number of individual patients undergoing surgical procedures over time.
Surgical procedures were performed in 98/622 (15.8%) Indigenous patients and 21/64 (32.8%) non-Indigenous patients (p = 0.001) and in 42/344 (12.2%) of patients living in remote locations compared with 77/342 (22.5%) with an urban address (p<0.001). The median (IQR) SEIFA score was higher in patients receiving surgery (958 (859–967)) than those that did not (870 (836–967)), p<0.001. In multivariate analysis that considered disease severity, Indigenous status, SEIFA score and residence in a remote/rural location, only disease severity (p<0.001) and SEIFA score (p<0.001) remained significantly associated with surgery.
Indigenous patients had surgery at a younger age than non-Indigenous patients (median (IQR) age at the time of first surgical intervention: 33 (19–47) versus 54 (35–64), (p = 0.001). There were 51 women of child-bearing age (13–50) who had surgery during the study period, 49 (96%) of whom were Indigenous.
Mortality
Non-Indigenous patients were more likely to die than Indigenous patients (9/64 (14%) versus 30/622 (5%), p = 0.002), but Indigenous patients died at a younger age (median (IQR): 52 (35–67) versus 73 (62–77) p = 0.01). In multivariate analysis that considered age, disease severity, Indigenous status, SEIFA score and residence in a remote/rural location, only age (OR (95%CI): 1.06 (1.04–1.08), p<0.001) was significantly associated with death.
Of the 39 deaths, 15 (38%) were linked directly to RHD: 9 from heart failure, 2 from infective endocarditis, 2 from a stroke, 1 from bleeding complications of warfarin therapy and 1 from complications of valve surgery. Of the 15 RHD-related deaths, 9 (60%) occurred in Indigenous patients. The median (IQR) age of Indigenous patients dying from RHD was 29 (12–58) years compared with 77 (64–78) among non-Indigenous patients dying from RHD (p = 0.007). Of the remaining 24 deaths, 17 were not RHD-related, while in 7 the patient’s medical record could not be accessed. Of the 17 non-RHD related deaths, 14 (82%) occurred in Indigenous patients. Of these 14 deaths, 5 were related to cancer, 4 were from sepsis, 2 were from end stage renal disease, 1 was from ischaemic heart disease, 1 was from trauma and 1 was from dementia. The median (IQR) age of Indigenous patients dying from non-RHD causes was 59 (46–69) years compared with 62 (61–68) among non-Indigenous patients (p = 0.57).
Comparison of Aboriginal and Torres Strait Islander Australians
Torres Strait islander Australians were more likely to live in a remote location than Aboriginal Australians but were less likely to have disease progression on serial echocardiograms and less likely to require hospitalisation. However, there were few other differences between the two populations (Table 5).
Table 5. Characteristics of the Indigenous Australians in the cohort stratified by whether the patient identified as an Aboriginal Australian or a Torres Strait Islander Australian.
| Variable | Aboriginal Australian n = 347 | Torres Strait Islander Australian n = 205 | p |
|---|---|---|---|
| Age at the end of the study | 36 (23–51) | 31 (23–45) | 0.054 |
| Age at RHD diagnosis | 30 (19–46) | 24 (16–39) | 0.005 |
| Female | 233 (67.2%) | 141 (68.8%) | 0.69 |
| Rural/remote residence | 165 (47.6%) | 124 (60.5%) | 0.003 |
| Severe disease | 90 (25.9%) | 41 (20%) | 0.11 |
| Hospitalisations/person | 6 (3–12) | 4 (2–8) | 0.0001 |
| RHD-related hospitalisations | 0 (0–1) | 0 (0–1) | 0.36 |
| Valve surgery or valvuloplasty | 56 (16.1%) | 26 (12.7%) | 0.27 |
| Died | 17 (4.9%) | 13 (6.3%) | 0.47 |
| Age at death | 57 (37–68) | 51 (29–69) | 0.59 |
| Adherence | 39% (23–56) | 42% (25–61) | 0.24 |
| Good adherence | 35/149 (23.5%) | 24/90 (26.7%) | 0.58 |
| Number of echocardiograms | 6 (3–9) | 5 (3–7) | 0.0007 |
| RHD progression on serial echocardiograms | 87/328 (26.5%) | 24/181 (13.3%) | 0.001 |
| Specialist review | 329 (94.8%) | 188 (91.7%) | 0.15 |
| Dental revie | 105 (30.3%) | 60 (29.3%) | 0.81 |
| SEIFA score of residential address | 870 (713–960) | 836 (836–967) | 0.17 |
All numbers are median (interquartile range) or the absolute number (%)
Discussion
The incidence of new RHD diagnoses is rising in FNQ and Indigenous Australians living in the region’s rural and remote locations continue to bear the greatest burden of disease. The number of RHD-related hospitalisations is also increasing, as is the number of patients requiring valve surgery. Whilst this is likely to be partly explained by greater disease recognition and enhanced service delivery, the strong association between socioeconomic disadvantage and RHD prevalence in the study and the absence of any diminution in new ARF diagnoses, suggests that a greater focus on primordial and primary prevention is necessary if we are to reduce the significant impact of RHD in the region.
Although Australia has a well-resourced, universal healthcare system, the prevalence of RHD varies considerably between–and even within–regions in Australia [6]. This is a result of the complex interplay of local socioeconomic and sociocultural factors, differences in health seeking behaviour and heterogeneous trans-jurisdictional strategies that influence the delivery of care to Australia’s geographically dispersed population [7]. All too predictably, Aboriginal and Torres Strait Islander Australians were over-represented in this cohort. Despite Indigenous Australians comprising 17% of the local population, they accounted for over 90% of the people living with RHD in the region. The prevalence of 27/1000 in Western Cape York, a region home to several Indigenous communities, is comparable to that seen in some of the poorest countries in the world [12,22–27]. The median age of Australian born non-Indigenous individuals living with RHD was 60, highlighting the fact that the disease was successfully countered with improvements in socioeconomic conditions and interventions that occurred in the 20th Century [7]. The median age of Indigenous Australians living with RHD was, by comparison, 33.
The significant variation in RHD prevalence across the region was linked strongly to socioeconomic disadvantage. Furthermore, patients living in socioeconomically disadvantaged areas were not only more likely to have RHD, but they were also less likely to receive surgery, the only intervention that can definitively address established, advanced disease [7]. This paradoxical situation is not limited to RHD. The local Indigenous population, particularly those living in remote, socioeconomically disadvantaged locations bear a disproportionate burden of both infectious and non-communicable diseases, but also has less access to sophisticated healthcare [14,16,28–33]. And this captures the essence of the challenges in addressing the life expectancy gap between Indigenous and non-Indigenous Australians. Between one third and one half of this “gap” is explained by differences in the social determinants of health, while up to 43% of the difference in life expectancy can be explained by poorer access to health services [8]. Successive state and federal governments have attempted to address this complex issue, however it is generally agreed there has been very limited success [34].
In the case of RHD, there is uncertainty about where it is best to start. Although the relationship between socioeconomic disadvantage and RHD is clearly established, it is less certain which social and environmental factors are the most significant in pathogenesis, nor is it clear how best to address these factors cost-effectively [3,22]. Crowding (household or other settings), income, dwelling characteristics, education level, employment, and nutrition have all been examined, with crowding consistently identified as not only one of the most important, but also one of the most amenable to improvement [3]. Crowded households have 1.7–2.8 times the risk of GAS infections, ARF, and RHD than uncrowded households, and also have higher rates of respiratory, gastrointestinal, eye and ear diseases [3,35]. Crowding also facilitates the potentiation of scabies infection which is a major driving force of streptococcal pyoderma in many Indigenous Australian communities [36,37].
Over 30% of Indigenous Australians in remote Queensland locations are living in overcrowded households [38]. In 2001, the Queensland Government implemented the Aboriginal and Torres Strait Islander Environmental Health Plan, a suite of environmental health interventions that aimed to improve the health and wellbeing of Indigenous communities. Greater construction and improved maintenance of housing is one of the key initiatives of this program which also addresses sanitation, vector control, food hygiene and animal management [39]. There are some data to suggest that these environmental interventions are beginning to have an impact with the rates of leprosy and strongyloidiasis declining sharply in the region during this period [14,15]. However, these infections are arguably far easier to address than GAS which are part of the normal human skin flora. It will be necessary to follow ARF notifications closely and to consider active surveillance which could be used to expedite more targeted action [40].
It is important to note that the rising incidence of RHD and continuing identification of ARF in the region is likely to be partly due to increased recognition from an expanded RHD program [41]. ARF was declared a notifiable disease in the state of Queensland in 1999, a time when education of local healthcare workers and enhanced surveillance for ARF was also commenced [42]. Echocardiography services have also increased: in 2011 a paediatric cardiologist began outreach services to the region, and since 2014 there has been an augmented adult specialist physician outreach service. New RHD diagnoses in the rural and remote communities served by these programs increased significantly, particularly among young people, after this expansion (Fig 3). The recent boosting of cardiology outreach services are also expected to improve medical and surgical management [43]. This strengthening of the medical effector arm of the RHD program is welcome as the cohort’s median age is 34 and it would be expected that a significant proportion will develop complicated disease in the decades ahead [44].
Improving patients’ access to medical care will reduce the morbidity and mortality attributable to RHD [45,46]. Indeed, there is some early evidence that the RHD program is reaching its most vulnerable patients. Patients in rural and remote locations are more likely to have received specialist review than patients living in urban areas, as are those in socioeconomically disadvantaged regions when compared to locations of greater affluence. Indeed, the expanded adult and paediatric specialist outreach programs and the resulting lower threshold for echocardiography is likely to explain why the incidence of RHD appears to be rising significantly despite the incidence of ARF remaining stable. It is also likely to explain why mild-moderate RHD was identified more commonly in remote, socioeconomically disadvantaged communities. This earlier recognition of RHD allows prompt prescription of secondary prophylaxis to reduce the risk of disease evolution [47,48]. Indeed, adherence to secondary prophylaxis in rural and remote communities and socioeconomically disadvantaged areas was also higher in this cohort. These data suggest that the local RHD program’s efforts to educate health workers and provide care to patients in regions with the highest disease burden have been at least somewhat successful.
However, the fact that young people are still dying from RHD and that cases of ARF are still being diagnosed in 21st Century Australia, emphasises that the current approach needs to evolve. Even with an expanded RHD program, only 24.9% of the patients in the cohort prescribed secondary prophylaxis had good adherence at the end of the study period, meanwhile less than 30% had received a documented dental review, increasing their risk of infective complications. Practical guidelines have been developed for the recognition and management of ARF and RHD in Australia, however the challenge is now to translate these guidelines into practice that result in improvements in clinically meaningful endpoints [7,49]. To achieve this end it is essential to have adequately trained and supported Aboriginal and Torres Strait Islander workforce as they are more likely to have an insight into the personal, community, organisational and environmental factors that may influence engagement with care, particularly early presentation with GAS infection or long-term adherence to secondary prophylaxis [7,50]. The high rates of RHD among young women also highlight the need for a skilled female health workforce, including Aboriginal and Torres Strait Islander Health Workers, nurses, midwives and doctors [18].
This study was performed in the only part of Australia which has the homelands of both Aboriginal and Torres Strait Islander Australians, peoples that are frequently conflated but who have very different cultural histories. It is one of the largest to examine the burden of RHD in Torres Strait Islander Australians and to compare their longitudinal care and clinical course with that of Aboriginal Australians. It is essential to recognise that there is great diversity within these two broadly described populations, but it is notable that their burden of RHD was similar. Despite these similarities, prospective examination of the barriers and enablers of RHD prevention and treatment in the ethnologically distinct Torres Strait Islander population is also needed if we are to address RHD in this population adequately.
This retrospective study has several limitations and almost certainly underestimates the local RHD burden. A determination of the true RHD prevalence would require systematic community screening [51], but this has never been performed in adults in the region. Although registry data were used to identify patients for this study, RHD was only declared a notifiable disease in September 2018, meaning that cases are likely to have been missed. Even though ARF was declared a notifiable disease in 1999—early in the study period—the addition of a patient to the register requires the treating clinician to contact the local public health unit; this is an imperfect process which will again underestimate disease incidence. Although this study aimed to examine the impact of socioeconomic status on disease burden, severity and access to care, registry data have a clinical focus and do not capture the environmental, economic, social or behavioural data which would influence these endpoints. Although the SEIFA score is determined by the Australian Bureau of Statistics using census data, it is calculated for entire regions rather than for individual residents. This may explain why there was greater correlation between the SEIFA score and the prevalence of RHD in different communities than the clinical endpoints in individual patients. Some data that were collected in private health settings–particularly dental review–were not accessible. This will tend to underestimate the amount of dental care patients were able to receive, although as private dental services are unavailable in most of the region, it is almost certainly still the case that local dental services also need to be improved, particularly for those with RHD. Finally, there are inherent limitations in using ICD coding to define the underlying cause of a patient’s hospitalisation [52,53].
However, notwithstanding these deficiencies, the study uses almost over two decades of longitudinal data collected in both community and hospital settings to provide an overview of the scale of the problem of RHD in this unique part of Australia. It identifies many of the challenges that clinicians, public health physicians and governments will have in addressing a disease that persists so stubbornly in 21st century Australia.
Conclusions
The incidence of RHD and RHD-related hospitalisations and surgery continues to rise in FNQ. Although this is likely to be partly explained by increased disease recognition and enhanced service delivery, the incidence of the disease remains unacceptably high and is almost entirely borne by the socioeconomically disadvantaged, Indigenous population. The current model of RHD care must evolve—in partnership with Indigenous communities—to have a greater focus on primordial prevention, and to improve access to care. This is not only likely to reduce the burden of RHD, but also the burden of many other diseases that are disproportionately borne by Indigenous Australians.
Supporting information
(DOCX)
Acknowledgments
The authors would like to acknowledge the work of all the healthcare workers involved in the care of patients living with RHD in Far North Queensland during the study period.
Data Availability
Data cannot be shared publicly because of the Queensland Public Health Act 2005. Data are available from the Far North Queensland Human Research Ethics Committee (contact via email Cairns_Ethics@health.qld.gov.au) for researchers who meet the criteria for access to confidential data. This is because the data are obtained from the Queensland State RHD register and these data are not available publicly.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM, World Heart F. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10(5):284–92. Epub 2013/04/03. 10.1038/nrcardio.2013.34 . [DOI] [PubMed] [Google Scholar]
- 2.Carapetis J SA, Mulholland E. The Current Evidence for the Burden of Group A Streptococcal Diseases. Geneva: 2005.
- 3.Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: A systematic review. PLoS Negl Trop Dis. 2018;12(6):e0006577 Epub 2018/06/14. 10.1371/journal.pntd.0006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordis L. The virtual disappearance of rheumatic fever in the United States: lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation. 1985;72(6):1155–62. Epub 1985/12/01. 10.1161/01.cir.72.6.1155 . [DOI] [PubMed] [Google Scholar]
- 5.Massell BF, Chute CG, Walker AM, Kurland GS. Penicillin and the marked decrease in morbidity and mortality from rheumatic fever in the United States. N Engl J Med. 1988;318(5):280–6. Epub 1988/02/04. 10.1056/NEJM198802043180504 . [DOI] [PubMed] [Google Scholar]
- 6.Roberts KV, Maguire GP, Brown A, Atkinson DN, Remenyi B, Wheaton G, et al. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust. 2015;203(5):221 e1–7. Epub 2016/02/08. 10.5694/mja15.00139 . [DOI] [PubMed] [Google Scholar]
- 7.The 2020 Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease (3rd edition). 2020. [DOI] [PubMed]
- 8.The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples 2015. Australian Institute of Health and Welfare; Canberra: 2015. [Google Scholar]
- 9.Australia’s health 2014: Australian Institute of Health and Welfare 2014 [cited 2020 24 February]. Australia’s health series no. 14. Cat. no. AUS 178]. Available from: https://www.aihw.gov.au/getmedia/3fae0eb7-b2be-4ffc-9903-a414388af557/7_7-indigenous-health-remoteness.pdf.aspx.
- 10.Fisher D, Huffam S. Management of Chronic Hepatitis B Virus Infection in remote-dwelling Aboriginal and Torres Strait Islanders: an update for primary healthcare providers. Med J Aust. 2003;178:82–5. 10.5694/j.1326-5377.2003.tb05070.x [DOI] [PubMed] [Google Scholar]
- 11.Macleod CK, Bright P, Steer AC, Kim J, Mabey D, Parks T. Neglecting the neglected: the objective evidence of underfunding in rheumatic heart disease. Trans R Soc Trop Med Hyg. 2019;113(5):287–90. Epub 2019/03/31. 10.1093/trstmh/trz014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med. 2017;377(8):713–22. Epub 2017/08/24. 10.1056/NEJMoa1603693 . [DOI] [PubMed] [Google Scholar]
- 13.2016 Australian Census: Australian Bureau of Statistics; 2016. Available from: https://www.abs.gov.au/websitedbs/D3310114.nsf/Home/2016%20search%20by%20geography.
- 14.Paltridge M, Smith S, Traves A, McDermott R, Fang X, Blake C, et al. Rapid Progress toward Elimination of Strongyloidiasis in North Queensland, Tropical Australia, 2000–2018. Am J Trop Med Hyg. 2020;102(2):339–45. Epub 2019/12/06. 10.4269/ajtmh.19-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hempenstall A, Smith S, Hanson J. Leprosy in Far North Queensland: almost gone, but not to be forgotten. Med J Aust. 2019;211(4):182–3. Epub 2019/06/25. 10.5694/mja2.50243 . [DOI] [PubMed] [Google Scholar]
- 16.Hanson J, Fox M, Anderson A, Fox P, Webster K, Williams C, et al. Chronic hepatitis B in remote, tropical Australia; successes and challenges. PLoS One. 2020;15(9):e0238719 Epub 2020/09/04. 10.1371/journal.pone.0238719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2071.0—Census of Population and Housing: Reflecting Australia—Stories from the Census, 2016 Canberra, Australia: Australian Bureau of Statistics; 2018 [cited 2019 24 November]. Available from: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2071.0~2016~Main%20Features~Socio-Economic%20Advantage%20and%20Disadvantage~123.
- 18.McLean A, Waters M, Spencer E, Hadfield C. Experience with cardiac valve operations in Cape York Peninsula and the Torres Strait Islands, Australia. Med J Aust. 2007;186(11):560–3. Epub 2007/06/06. 10.5694/j.1326-5377.2007.tb01053.x . [DOI] [PubMed] [Google Scholar]
- 19.Remond MG, Severin KL, Hodder Y, Martin J, Nelson C, Atkinson D, et al. Variability in disease burden and management of rheumatic fever and rheumatic heart disease in two regions of tropical Australia. Intern Med J. 2013;43(4):386–93. Epub 2012/06/01. 10.1111/j.1445-5994.2012.02838.x . [DOI] [PubMed] [Google Scholar]
- 20.Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2011 Canberra: Australian Bureau of Statistics; 2013 [cited 2020 1 March]. Available from: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/2033.0.55.001main+features42011.
- 21.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. Epub 1985/01/01. 10.1002/sim.4780040112 . [DOI] [PubMed] [Google Scholar]
- 22.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084 Epub 2016/05/18. 10.1038/nrdp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abouzeid M, Katzenellenbogen J, Wyber R, Watkins D, Johnson TD, Carapetis J. Rheumatic heart disease across the Western Pacific: not just a Pacific Island problem. Heart Asia. 2017;9(2):e010948 Epub 2018/02/07. 10.1136/heartasia-2017-010948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carapetis JR. Rheumatic heart disease in Asia. Circulation. 2008;118(25):2748–53. Epub 2008/12/25. 10.1161/CIRCULATIONAHA.108.774307 . [DOI] [PubMed] [Google Scholar]
- 25.Davis K, Remenyi B, Draper AD, Dos Santos J, Bayley N, Paratz E, et al. Rheumatic heart disease in Timor-Leste school students: an echocardiography-based prevalence study. Med J Aust. 2018;208(7):303–7. Epub 2018/04/13. 10.5694/mja17.00666 . [DOI] [PubMed] [Google Scholar]
- 26.Myint N, Aung NM, Win MS, Htut TY, Ralph AP, Cooper DA, et al. The clinical characteristics of adults with rheumatic heart disease in Yangon, Myanmar: An observational study. PLoS One. 2018;13(2):e0192880 Epub 2018/02/22. 10.1371/journal.pone.0192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negi PC, Sondhi S, Asotra S, Mahajan K, Mehta A. Current status of rheumatic heart disease in India. Indian Heart J. 2019;71(1):85–90. Epub 2019/04/20. 10.1016/j.ihj.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nofz L, Koppen J, De Alwis N, Smith S, Hanson J. The microbiology of ear cultures in a high-burden setting in tropical Australia: Implications for clinicians. Clin Otolaryngol. 2019;44(6):1195–200. Epub 2019/10/02. 10.1111/coa.13451 . [DOI] [PubMed] [Google Scholar]
- 29.Guthridge I, Smith S, Horne P, Hanson J. Increasing prevalence of methicillin-resistant Staphylococcus aureus in remote Australian communities: implications for patients and clinicians. Pathology. 2019;51(4):428–31. Epub 2019/04/20. 10.1016/j.pathol.2018.11.015 . [DOI] [PubMed] [Google Scholar]
- 30.Smith S, Russell D, Horne P, Hanson J. HTLV-1 is rare in Far North Queensland despite a significant burden of classically associated diseases. Pathology. 2019;51(1):91–4. Epub 2018/12/07. 10.1016/j.pathol.2018.10.010 . [DOI] [PubMed] [Google Scholar]
- 31.Hanson J, Smith S. High Rates of Premature and Potentially Preventable Death among Patients Surviving Melioidosis in Tropical Australia. Am J Trop Med Hyg. 2019;101(2):328–31. Epub 2019/07/03. 10.4269/ajtmh.19-0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson J, Smith S, Brooks J, Groch T, Sivalingam S, Curnow V, et al. The applicability of commonly used predictive scoring systems in Indigenous Australians with sepsis: An observational study. PLoS One. 2020;15(7):e0236339 Epub 2020/07/23. 10.1371/journal.pone.0236339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Health of Queenslanders. Report of the Chief Health Officer. Queensland Health, State of Queensland Brisbane 2018. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0032/732794/cho-report-2018-full.pdf.
- 34.Closing the Gap Report 2020. Commonwealth of Australia, Department of the Prime Minister and Cabinet. Canberra: 2020.
- 35.Ali SH, Foster T, Hall NL. The Relationship between Infectious Diseases and Housing Maintenance in Indigenous Australian Households. Int J Environ Res Public Health. 2018;15(12). Epub 2018/12/14. 10.3390/ijerph15122827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitehall J, Kuzulugil D, Sheldrick K, Wood A. Burden of paediatric pyoderma and scabies in North West Queensland. J Paediatr Child Health. 2013;49(2):141–3. Epub 2013/01/26. 10.1111/jpc.12095 . [DOI] [PubMed] [Google Scholar]
- 37.Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20(2):268–79. Epub 2007/04/13. 10.1128/CMR.00042-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report. Australian Health Ministers’ Advisory Council. Canberra: 2017.
- 39.Queensland Aboriginal and Torres Strait Islander Environmental Health Plan 2019–2022. Department of Health, Queensland Government. Brisbane: 2019.
- 40.May PJ, Bowen AC, Carapetis JR. The inequitable burden of group A streptococcal diseases in Indigenous Australians. Med J Aust. 2016;205(5):201–3. Epub 2016/09/02. 10.5694/mja16.00400 . [DOI] [PubMed] [Google Scholar]
- 41.Hanna JN, Clark MF. Acute rheumatic fever in Indigenous people in North Queensland: some good news at last? Med J Aust. 2010;192(10):581–4. Epub 2010/05/19. 10.5694/j.1326-5377.2010.tb03641.x . [DOI] [PubMed] [Google Scholar]
- 42.Hanna JN, Heazlewood RJ. The epidemiology of acute rheumatic fever in Indigenous people in north Queensland. Aust N Z J Public Health. 2005;29(4):313–7. Epub 2005/10/15. 10.1111/j.1467-842x.2005.tb00199.x . [DOI] [PubMed] [Google Scholar]
- 43.Looking after the hearts of North Queenslanders. Department of Health. Queensland Government. Brisbane. 2019.
- 44.He VY, Condon JR, Ralph AP, Zhao Y, Roberts K, de Dassel JL, et al. Long-Term Outcomes From Acute Rheumatic Fever and Rheumatic Heart Disease: A Data-Linkage and Survival Analysis Approach. Circulation. 2016;134(3):222–32. Epub 2016/07/14. 10.1161/CIRCULATIONAHA.115.020966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordis L. Effectiveness of comprehensive-care programs in preventing rheumatic fever. N Engl J Med. 1973;289(7):331–5. Epub 1973/08/16. 10.1056/NEJM197308162890701 . [DOI] [PubMed] [Google Scholar]
- 46.Nordet P, Lopez R, Duenas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986-1996-2002). Cardiovasc J Afr. 2008;19(3):135–40. Epub 2008/06/24. [PMC free article] [PubMed] [Google Scholar]
- 47.Stollerman GH, Rusoff JH, Hirschfeld I. Prophylaxis against group A streptococci in rheumatic fever; the use of single monthly injections of benzathine penicillin G. N Engl J Med. 1955;252(19):787–92. Epub 1955/05/12. 10.1056/NEJM195505122521901 . [DOI] [PubMed] [Google Scholar]
- 48.de Dassel JL, de Klerk N, Carapetis JR, Ralph AP. How Many Doses Make a Difference? An Analysis of Secondary Prevention of Rheumatic Fever and Rheumatic Heart Disease. J Am Heart Assoc. 2018;7(24):e010223 Epub 2018/12/19. 10.1161/JAHA.118.010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mickan S, Burls A, Glasziou P. Patterns of 'leakage' in the utilisation of clinical guidelines: a systematic review. Postgrad Med J. 2011;87(1032):670–9. Epub 2011/07/01. 10.1136/pgmj.2010.116012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevat PM, Reeves BM, Ruben AR, Gunnarsson R. Adherence to Secondary Prophylaxis for Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review. Curr Cardiol Rev. 2017;13(2):155–66. Epub 2017/01/18. 10.2174/1573403X13666170116120828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357(5):470–6. Epub 2007/08/03. 10.1056/NEJMoa065085 . [DOI] [PubMed] [Google Scholar]
- 52.Katzenellenbogen JM, Nedkoff L, Cannon J, Kruger D, Pretty F, Carapetis JR, et al. Low positive predictive value of International Classification of Diseases, 10th Revision codes in relation to rheumatic heart disease: a challenge for global surveillance. Intern Med J. 2019;49(3):400–3. Epub 2019/03/22. 10.1111/imj.14221 . [DOI] [PubMed] [Google Scholar]
- 53.O'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5 Pt 2):1620–39. Epub 2005/09/24. 10.1111/j.1475-6773.2005.00444.x [DOI] [PMC free article] [PubMed] [Google Scholar]