Abstract
BACKGROUND: Alopecia areata and vitiligo vulgaris are common autoimmune diseases whose pathophysiology are not completely elucidated. Genetic susceptibility, immunological background, and stress have significant roles in their pathogenesis. Although macrophage migration inhibitory factor (MIF) is crucial for the maintenance of immune privilege in certain sites, it can upregulate different inflammatory cytokines and contribute to the pathogenesis of different autoimmune diseases. There is controversy about its role in alopecia and no adequate data about its role in vitiligo. OBJECTIVES: We sought to assess the serum level of MIF in alopecia areata and vitiligo and its relationship with different variables of both diseases. METHOD: Serum level of MIF was measured in 20 patients with vitiligo, 22 patients with alopecia areata, and 20 controls by ELISA. RESULTS: MIF was significantly higher in alopecia areata (8.477±4.1761ng/mL) and vitiligo vulgaris (3.930±2.7071ng/mL) compared to controls (0.725±0.5108 ng/mL) (P<0.01). In addition, MIF levels were positively correlated with the severity of alopecia areata and vitiligo. CONCLUSION: The MIF has an active role in the pathogenesis of alopecia areata and vitiligo and could be a target for the treatment of both diseases.
Keywords: Macrophage migration inhibitory factor, MIF, alopecia, vitiligo vulgaris, immunoregulatory cytokines
Vitiligo and alopecia areata (AA) are common autoimmune dermatological diseases, but the pathophysiology of both diseases is not completely understood. Genetic susceptibility, autoimmunity, and emotional stress have an important role in their pathogenesis.1,2
Vitiligo, an acquired depigmentation disorder, is mainly a result of autoimmune destruction of melanocytes. The common association of vitiligo with different autoimmune disorders, such as AA, autoimmune thyroiditis, and Addison’s disease supports the autoimmune theory of vitiligo.3
AA is characterized by recurrent episodes of nonscarring alopecia with an unexpected course. Loss of the immune privilege of anagen follicles is suggested to be the precipitating cause of AA.4,5 Hair follicles can conserve their immune privilege through decreased expression of major histocompatibility complexes (MHCs), which hinders their recognition by T-lymphocytes, and through inhibition of natural killer (NK) cells, which have the ability to detect and destroy MHCs negative cells through macrophage migration inhibitory factor (MIF) acting as NK cells.6,7
MIF is one of the many diverse immunoregulatory cytokines secreted by T-lymphocytes, macrophages, and the pituitary gland.8 MIF has various immunological functions. It has an essential anti-inflammatory effect in immune-privileged sites by inhibiting NK cells.9,10 However, MIF is thought to be involved in the pathogenesis of different autoimmune diseases, such as systemic lupus, pemphigus, and psoriasis via upregulation of different inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ) , interleukin (IL)-1, IL-8, IL-12, and IL-17, and by counteracting the effect of glucocorticoids.11,12
Until now, there has been controversy about the role of MIF in the pathogenesis of AA and no adequate data about its role in vitiligo. We designed this study to assess the serum MIF level in AA and vitiligo vulgaris compared to healthy controls and to evaluate its correlation with the severity of both diseases.
METHODS
Twenty-two patients with AA, 20 patients with vitiligo vulgaris and 20 age- and sex-matched healthy controls were recruited from the outpatient clinic of dermatology at Zagazig University Hospital from January 2017 to March 2018. Participants were excluded if they reported any history of systemic diseases, such as autoimmune diseases, malignancy, hypertension, and diabetes mellitus, family history of autoimmune diseases, or if they received systemic or topical therapy within four weeks before the collection of the blood sample. This study was approved by local ethics committee, Z.U.H Institutional Review Board (ZU-IRB). An informed written consent was obtained from every participant before starting the study.
A complete medical history was acquired from each patient and general and dermatological examinations were performed. Vitiligo area and severity index (VASI) and SALT (Severity of Alopecia Tool) scores were used to assess the severity of vitiligo and alopecia, respectively.
Three milliliters of venous blood samples were collected in sterile plane tubes and were allowed to clot for 20 minutes at room temperature, then centrifuged at 300g for five minutes. Sera was immediately separated and stored at -70C° until the time of analysis. MIF serum level was measured using enzyme linked immunosorbent Assay (ELISA) on the principle of double antibody sandwich technique.
STATISTICAL ANALYSIS
Data were checked, entered, and analyzed using SPSS (version 19). Data were represented as mean ± SD for quantitative variables. Number and percentage were used for categorical variables. Chi-square (X2) or Fisher exact test were used when appropriate. P<0.05 was considered statistically significant. T-test was used to compare means. Kruskal Wallis test was used for the nonparametric variables.
RESULTS
Characteristics of the studied groups. This study included three groups of participants. The first group included 22 patients with AA, the second group included 20 patients with vitiligo vulgaris, and the control group included 22 healthy individuals. There were no statistically significant differences between the studied groups as regards age and sex. Demographic data of the three groups is summarized in Table 1.
TABLE 1.
Demographic data of participants in Group A (alopecia) Group V (vitiligo) and Group C (control)
| DEMOGRAPHIC | GROUP A (N-22) | GROUP V (N=20) | GROUP C (20) | TEST VALUE | P-VALUE |
|---|---|---|---|---|---|
| Age (mean ± ST) | 20.5 ± 12.7 | 29.2 ± 13.3 | 29.7 ± 10.6 | 1.341t | 0.270 |
| Sex | |||||
| Male | 7 (31.8%) | 12 (60.0%) | 7 (35.0%) | 4.000x2 | 0.135 |
| Female | 15 (68.2%) | 8(40.0%) | 13(65.0%) | ||
t: Independent sample t-test; X2: Chi-square test
The group of participants with AA were classified according to SALT score. Seven were classified as mild (<25% hair loss), eight as moderate (25–49% hair loss), and seven as severe (SALT score >50%). The mean duration of AA was 10 years±11.9, and three patients presented with active hair loss, indicated by positive hair pull test and presence of exclamation mark hairs, black dots, and broken hairs.
The patients with vitiligo vulgaris were classified according to VASI score. Seven patients were classified as mild (with VASI score <25), 10 as moderate ( VASI 25–50), and three were severe (VASI >50). Fourteen patients presented with active vitiligo, enlargement of the depigmenting area, presence of new depigmenting areas, or both within the previous month, while six patients were in the stable stage (inactive vitiligo). The mean duration of vitiligo was 24 years±20.6 (Table 2).
TABLE 2.
Onset and grading of severity of alopecia areata and vitiligo
| ALOPECIA AREATA | VALUE |
| Onset of disease (years) mean±SD | 10±11.9 |
| Disease grade (%) | |
| Mild | 7 (31.8%) |
| Moderate | 8 (36.4%) |
| Severe | 7 (31.8%) |
| VITILIGO VULGARIS | VALUE |
| Onset of disease (years) mean±SD | 24±(20.6) |
| Disease state (%) | |
| Stable | 6 (30%) |
| Progressive | 14 (70%) |
| Disease grade (%) | |
| Mild | 7 (35%) |
| Moderate | 10 (50%) |
| Severe | 3 (15%) |
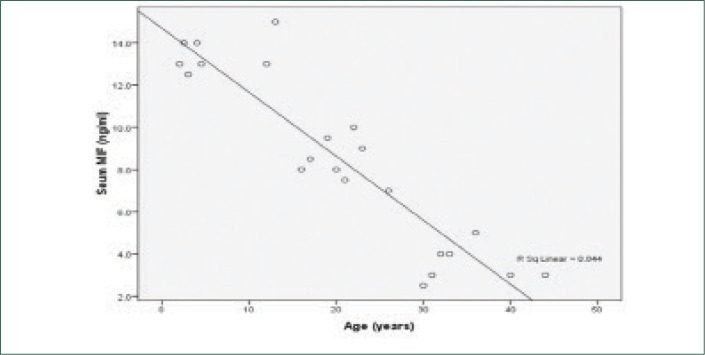
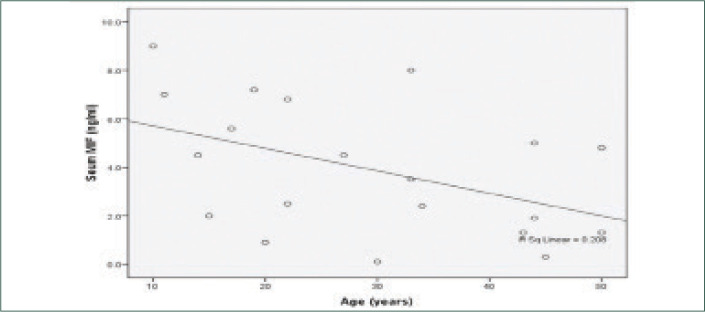
MIF levels in AA and vitiligo vulgaris were significantly higher than the control group (P<0.001) and significantly higher in AA than vitiligo vulgaris. The mean serum level of MIF of the control group was (0.725±0.5108ng/ ml), while it was (8.477±4.1761 ng/ml) in AA and (3.930±2.7071pg/ml) in vitiligo vulgaris (Table 3) (Figures 1 and 2). There was a highly significant positive correlation between MIF level and severity of AA and vitiligo, assessed by SALT and VASI scores, respectively. The relationship between serum MIF levels and the activity of AA was not statistically significant (P>0.05). MIF was significantly higher in active vitiligo than stable vitiligo (5.014 vs. 1.400ng/ml). There was significant negative correlation between serum MIF and age of patients of both diseases (Figures 3 and 4). However, the relation between the age of control group and MIF level was not significant. However, a positive correlation between the duration of alopecia and serum MIF levels was found. MIF was higher in patients with AA with a long disease duration. In contrast, the duration of vitiligo correlated negatively with MIF serum levels. The relation between MIF levels and the sex of patients and controls was insignificant.
TABLE 3.
Comparison between three groups as regarding serum MIF levels
| GROUPS | SERUM MIF (NG\ML) MEAN± SD |
|---|---|
| Group A (n=22) | 8.477 ± 4.1761 |
| Group V (n=20) | 3.930 ± 2.7071 |
| Group C (n= 20) | 0.725 ± 0.5108 |
| F | 36.960ANOVA |
| P-value | <0.001 |
MIF: macrophage migration inhibitory factor; ANOVA: analysis of variant test
P<0.001: statistically highly significant
FIGURE 1.
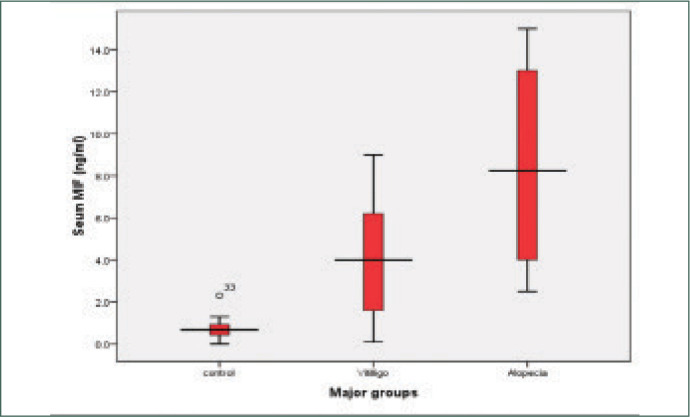
Serum MIF levels in the studied groups
FIGURE 2.
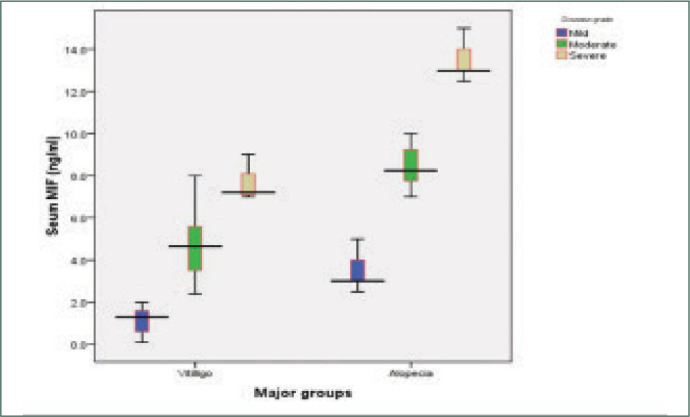
Shows serum MIF levels in different disease grades in both groups
FIGURE 3.
Shows a significant negative correlation between the age of patients and serum MIF levels in alopecia
FIGURE 4.
Shows a significant negative correlation between serum level of MIF and the age of patients in vitiligo
DISCUSSION
Alopecia areata and vitiligo vulgaris are common dermatological diseases that could be sources of significant psychological sequelae. The pathogenesis of both diseases are not completely elucidated. Different theories are thought to be involved in their pathogenesis. The autoimmune theory is the most accepted one.12–15
MIF is a proinflammatory cytokine that has been found to be involved in the pathogenesis of many inflammatory and autoimmune skin diseases, such as psoriasis, atopic dermatitis, systemic sclerosis, and bullous pemphigoid. Different types of cells produce MIF-like T-cells, macrophages, eosinophil, B-cells, glandular and surface epithelium.16,17
This study assessed serum levels of MIF in AA and vitiligo vulgaris using ELISA technique. Significantly higher serum levels of MIF were found in patients with AA and vitiligo vulgaris compared to the control group. MIF levels were higher in AA than vitiligo vulgaris.
The mean MIF serum level in AA and vitiligo vulgaris was not affected by sex. However, a significant negative correlation between age of the patients and serum levels of MIF was found. These findings are supported by the findings of Shimizu et al18 and Salem et al,19 who also reported significant negative correlation between the age of patients and MIF levels in AA.
In regards to AA, there is controversy about the role of MIF in the pathogenesis of alopecia areata. The results of immunohistochemical studies regarding MIF are inconsistent. Shimizu et al18 Salem et al19 and Kang et al20 reported higher MIF expression in lesions of AA lesions compared to healthy controls. Moreover, previous studies reported higher serum levels of MIF in patients with AA compared to healthy controls in accordance with the results of our study.19,21 On the contrary, Ito et al7 reported lower MIF in alopecic lesions compared to healthy controls.
The elevated serum level of MIF and its positive correlation with disease severity and duration suggests a possible role of MIF in the pathogenesis of AA. This comes in agreement with Salem et al19 and Younan et al.21 Shimizu et al18 reported the presence of MIF in lymphocytes in the perifollicular area of telogen hair follicles in patients with AA, suggesting that activated T-cells to be a source of MIF in the serum.18
Regarding vitiligo vulgaris, the mean MIF serum level was significantly higher than the control group, in accordance with Serarslan et al,22 Ma et al,23 and Farag et al.24 Also, MIF level was positively correlated with VASI and it was higher in active vitiligo than stable vitiligo. This finding agrees with Ma et al and Farag et al who also reported presence of positive correlation between MIF serum level and the severity of vitiligo assessed by VASI, but disagrees with Serarslan et al, who reported no relationship between MIF levels and the severity of vitiligo assessed by body surface area (BSA).22–24 This difference may be due to assessment by different severity scoring tools, BSA versus VASI score in our study.
However, the correlation between MIF serum levels and the duration of vitiligo was negative in contrast to Farag et al,24 who reported a positive correlation between duration of vitiligo and MIF level. This can be explained by the presence of other important factors that can affect MIF level besides the duration and activity of vitiligo, as the recent cases of vitiligo presented with active state more than long-standing vitiligo that is mainly in stable state.
MIF is a proinflammatory mediator that plays an important role in the effector phases of adaptive cellular and humoral immune responses, including antigen-dependent T-cell activation, type 4 hypersensitivity reactions, and antibody production. These mechanisms are involved in the development of AA and Vitiligo vulgaris.6,8,11 MIF participates in the initiation of T-cell mediated immunity, by stimulation the secretion of inflammatory cytokines, such as TNF- α, IFNγ, IL-1b, IL-6 and IL-8.25 TNF-α and IL-1 inhibit the proliferation of cells of the hair follicle, leading to hair growth inhibition.6 Similarly, TNF- α and IL-6 have an inhibitory effect on pigmentation. The elevated IL-6 levels in vitiligo have been shown to have cytotoxic effect on melanocyte with subsequent inhibition of melanin production.26
Previous studies have demonstrated a marked macrophage infiltration in the vitiliginous and perilesional skin. These macrophages play an important role in the inhibition of melanocytes and secretion of MIF.27 MIF, in turn, leads to further macrophage accumulation and activation at the site of inflammation, leading to sustained inflammation, so positive feedback between MIF and macrophage might have an important role in the pathogenesis of vitiligo.16
CONCLUSION
Results from our study suggest that MIF might play an important role in the pathogenesis of vitiligo vulgaris and AA and could be used as a marker of their severity and a marker of the activity of vitiligo. Accordingly, inhibition of its action might be a promising line of treatment of vitiligo vulgaris and AA.
REFERENCES
- Li S, Zhu G, Yang Y et al. Oxidative stress drives CD8+ T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J Allergy Clin Immunol. 2017;1(140):177–89. doi: 10.1016/j.jaci.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Mir A, Zlotogorski A, Gordon D et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet. 2007;80(2):316–328. doi: 10.1086/511442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingordo V, Gentile C, Iannazzone SS et al. Vitiligo and autoimmunity: an epidemiological study in a representative sample of young Italian males. J Eur Acad Dermatol Venereol. 2011;25(1):105–109. doi: 10.1111/j.1468-3083.2010.03696.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A. Collapse of immune privilege in alopecia areata: coincidental or substantial?. J Invest Dermatol. 2010;130(11):2535–2537. doi: 10.1038/jid.2010.260. [DOI] [PubMed] [Google Scholar]
- Ito T, Ito N, Bettermann A et al. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164(2):623–634. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KC, Klatte JE, Dinh HV et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159(5):1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Ito N, Saatoff M et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Stojanovic I, Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimmun Rev. 2009;8(3):244–249. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. British Journal of Dermatology. 2018;179(5):1033–48. doi: 10.1111/bjd.16808. Nov. [DOI] [PubMed] [Google Scholar]
- Apte RS, Sinha D, Mayhew E et al. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160(12):5693–5696. [PubMed] [Google Scholar]
- Denkinger CM, Metz C, Fingerle-Rowson G et al. Macrophage migration inhibitory factor and its role in autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2004;52(6):389–400. [PubMed] [Google Scholar]
- Rajabi F, Amoli MM, Robati RM et al. Macrophage migration inhibitory factor polymorphism (rs755622) in alopecia areata: a possible role in disease prevention. Arch Dermatol Res. 2019;311(8):589–594. doi: 10.1007/s00403-019-01934-9. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Sanders DA, Bowen J et al. Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 1996;135(6):942–948. doi: 10.1046/j.1365-2133.1996.d01-1099.x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Andersen G, Yorgov D et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet. 2016;48(11):1418–1424. doi: 10.1038/ng.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert R, Speeckaert M, De Schepper S et al. Biomarkers of disease activity in vitiligo: A systematic review. Autoimmun Rev. 2017;16(9):937–945. doi: 10.1016/j.autrev.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Role of macrophage migration inhibitory factor (MIF) in the skin. J Dermatol Sci. 2005;37(2):65–73. doi: 10.1016/j.jdermsci.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Pazyar N, Feily A, Yaghoobi R. Macrophage migration inhibitory factor as an incriminating agent in dermatological disorders. Indian J Dermatol. 2013;58(2):157. doi: 10.4103/0019-5154.108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hizawa N, Honda A et al. Promoter region polymorphism of macrophage migration inhibitory factor is strong risk factor for young onset of extensive alopecia areata. Genes Immun. 2005;6(4):285–289. doi: 10.1038/sj.gene.6364191. [DOI] [PubMed] [Google Scholar]
- Oh HA, Kwak J, Kim BJ et al. Migration Inhibitory Factor in Conditioned Medium from Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Stimulates Hair Growth. Cells. 2020;9(6):1344. doi: 10.3390/cells9061344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Wu WY, Lo BK et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2010;130(11):2677–2680. doi: 10.1038/jid.2010.180. [DOI] [PubMed] [Google Scholar]
- Younan DN, Agamia N, Elshafei A et al. Serum level of macrophage migration inhibitory factor (MIF) in Egyptians with alopecia areata and its relation to the clinical severity of the disease. J Clin Lab Anal. 2015;29(1):74–79. doi: 10.1002/jcla.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serarslan G, Yönden Z, Söğüt S et al. Macrophage migration inhibitory factor in patients with vitiligo and relationship between duration and clinical type of disease. Clin Exp Dermatol. 2010;35(5):487–90. doi: 10.1111/j.1365-2230.2009.03617.x. [DOI] [PubMed] [Google Scholar]
- Ma L, Xue HB, Guan XH et al. Relationship of macrophage migration inhibitory factor levels in PBMCs, lesional skin and serum with disease severity and activity in vitiligo vulgaris. Braz J Med Biol Res. 2013;46(5):460–4. doi: 10.1590/S0100-879X2012007500152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag A, Habib M, Kamh M et al. Macrophage migration inhibitory factor as an incriminating agent in vitiligo. Anais Brasileiros de Dermatologia. 2018;93(2):191–196. doi: 10.1590/abd1806-4841.20186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R, Lolis E. (Macrophage migration inhibitory factor: a critical component of autoimmune inflammatory diseases. Drug News Perspect. 2005;18(7):417–26. doi: 10.1358/dnp.2005.18.7.939345. [DOI] [PubMed] [Google Scholar]
- Moretti S, Fabbri P, Baroni G et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009;24(7):849–857. doi: 10.14670/HH-24.849. [DOI] [PubMed] [Google Scholar]
- Van Den Wijngaard R, Wankowicz-Kalinska A, Le Poole C et al. Local immune response in skin of generalized vitiligo patients. Lab Invest. 2000;80(8):1299. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]