Abstract
Background
Necrotizing soft-tissue infections (NSTI) are life-threatening conditions often caused by β-hemolytic streptococci, group A Streptococcus (GAS) in particular. Optimal treatment is contentious. The INFECT cohort includes the largest set of prospectively enrolled streptococcal NSTI cases to date.
Methods
From the INFECT cohort of 409 adults admitted with NSTI to 5 clinical centers in Scandinavia, patients culture-positive for GAS or Streptococcus dysgalactiae (SD) were selected. Risk factors were identified by comparison with a cohort of nonnecrotizing streptococcal cellulitis. The impact of baseline factors and treatment on 90-day mortality was explored using Lasso regression. Whole-genome sequencing of bacterial isolates was used for emm typing and virulence gene profiling.
Results
The 126 GAS NSTI cases and 27 cases caused by SD constituted 31% and 7% of the whole NSTI cohort, respectively. When comparing to nonnecrotizing streptococcal cellulitis, streptococcal NSTI was associated to blunt trauma, absence of preexisting skin lesions, and a lower body mass index. Septic shock was significantly more frequent in GAS (65%) compared to SD (41%) and polymicrobial, nonstreptococcal NSTI (46%). Age, male sex, septic shock, and no administration of intravenous immunoglobulin (IVIG) were among factors associated with 90-day mortality. Predominant emm types were emm1, emm3, and emm28 in GAS and stG62647 in SD.
Conclusions
Streptococcal NSTI was associated with several risk factors, including blunt trauma. Septic shock was more frequent in NSTI caused by GAS than in cases due to SD. Factors associated with mortality in GAS NSTI included age, septic shock, and no administration of IVIG.
Keywords: Streptococcus pyogenes, group A Streptococcus, Streptococcus dysgalactiae, necrotizing fasciitis, intravenous immunoglobulin G
This prospective study of streptococcal necrotizing soft-tissue infections comprised 126 Streptococcus pyogenes (GAS) cases and 27 Streptococcus dysgalactiae cases. Among the GAS cases, several factors were associated with mortality, including age, septic shock and no administration of intravenous immunoglobulin.
Necrotizing fasciitis and other necrotizing soft-tissue infections (NSTI) are rare, devastating conditions causing destruction primarily of subcutaneous tissue and/or muscle [1–3]. Historically, mortality is high, although a slight reduction has been reported [4]. Delayed diagnosis and controversies regarding optimal treatment are still major challenges and barriers toward improved survival [1–3]. The etiology is often polymicrobial, but since the upsurge of invasive Streptococcus pyogenes (group A Streptococcus [GAS]) infection in the industrialized world in the 1980s and 1990s, this pathogen has been a particularly feared cause of NSTI. Cases also among healthy individuals and high rates of shock, need for intensive care, and death were features highlighted by case series and surveillance studies at that time [5–9]. More recent surveillance studies on invasive GAS disease largely confirm this pattern [10, 11]. In addition, some reports have shown that Streptococcus dysgalactiae (SD), a species closely related to GAS and an emerging cause of invasive infections, also have the ability to cause NSTI [12, 13]. However, apart from 2 retrospective single-center studies, contemporary detailed data on streptococcal NSTI are scarce [13, 14]. Risk factors for streptococcal NSTI compared to more superficial streptococcal disease are still largely unknown. The predictive power of systemic toxicity and local findings in discerning streptococcal NSTI from nonstreptococcal cases is also uncertain. Furthermore, treatment controversies persist due to lack of randomized trials. Apart from early surgery and clindamycin treatment, which are measures strongly suggested by several observational studies, the optimal treatment of streptococcal NSTI is debated [1–3]. In particular, the role of intravenous polyspecific immunoglobulin G (IVIG) is controversial.
The present study is part of the INFECT project, which was initiated to advance the understanding of the pathophysiological mechanisms, diagnosis, and prognosis of NSTI [15]. The INFECT cohort, which consists of NSTI cases prospectively enrolled at 5 different Scandinavian hospitals, includes the largest clinical cohort of streptococcal NSTI cases to date. The purpose of focusing on the streptococcal cases of the cohort is to provide a comprehensive overview of the characteristics of NSTI unique to GAS and SD and explore the impact on mortality of baseline factors, early clindamycin treatment, early surgery, and IVIG.
METHODS
Study Design, Sites, and Participants
The INFECT study is a multicenter, prospective observational cohort study registered at ClinicalTrials.gov (NCT01790698). Design, study sites, inclusion criteria, and ethics of the INFECT cohort are previously described [16]. In short, patients (age ≥ 18 years) with NSTI confirmed by peroperative signs of wide destruction spreading along tissue planes, were enrolled in 5 Scandinavian referral centers for NSTI between February 2013 and June 2017. The method for assignment of microbial causes in each case of the cohort is described in the Supplementary Methods. The β-hemolytic streptococcal cohort was defined by cultures positive for GAS or SD in blood or tissue samples obtained before or within 48 hours of diagnosis. To identify factors predisposing to NSTI, the streptococcal NSTI cases were compared with a previously described Norwegian cohort of prospectively enrolled nonnecrotizing cellulitis cases with GAS or SD etiology confirmed by serology and/or culture [17]. Finally, patients with streptococcal NSTI were compared to patients with polymicrobial, nonstreptococcal NSTI.
Clinical Parameters
The clinical and laboratory parameters collected are described elsewhere [16]. Data were registered prospectively using an electronic case report form and included demographics, comorbidities, preceding trauma or surgery, preoperative findings, antibiotic treatment, surgery, supportive care, hyperbaric oxygen therapy (HBOT), IVIG therapy, and details on outcome. Systemic severity was measured at inclusion using simplified acute physiology score (SAPS) II, sequential organ failure assessment (SOFA) score, and registering the absence or presence of septic shock. The most abnormal biochemistry values obtained prior to the first surgery were also registered, including measurements performed at referring hospitals. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score, a laboratory-based diagnostic tool for necrotizing fasciitis, was calculated based on preoperative measurements [18].
Microbiology
Identification of the bacteria and resistance testing was performed as part of the routine diagnostic service at each hospital. Streptococcal species identification was confirmed on all stored isolates by whole-genome-sequencing (WGS). Details on WGS and our use in emm-typing, MLST-typing and screening for virulence genes are provided in the Supplementary Methods. The genomic sequences were deposited in the European Nucleotide Archive (ENA database) under the BioProject PRJNA524111.
Statistical Analyses
Categorical data were analyzed using the χ 2 or Fisher exact test. Continuous data were compared using the Mann–Whitney U test. All reported statistical tests were 2-sided, and P values < .05 were considered statistically significant. A multivariate model of predictors of mortality was developed for GAS cases only, as the SD cases were considered to include a more heterogeneous, less clearly defined population. Factors previously found to be associated with death were included, representing predisposing conditions, acute disease severity, and major treatment modalities. HBOT is in general associated with a high risk of selection bias primarily due to limited accessibility. In this study, all study hospitals were referral centers for NSTI with HBOT available. Many patients were probably transferred in order to have this treatment option. Decisions not to provide HBOT to patients in these settings probably involved strong selection mechanisms related to low or high severity. HBOT was therefore included in the multivariate model primarily to control for confounding. Logistic lasso regression was used, due to a high number of independent variables compared to the number of deaths, and the accompanying risk of severe overfitting when using ordinary logistic models [19]. As the risk factors for streptococcal NSTI compared to cellulitis are largely undocumented, an explorative approach was used to include covariates in the adjusted logistic regression model of risk factors, selecting covariates with a P value < .10 in univariate analysis. Details concerning the statistical methods are provided in the Supplementary Methods.
RESULTS
Microbial Etiology
In the cohort of 409 NSTI patients there were 126 cases with GAS and 27 with SD, in total 37% of the cohort (Supplementary Figure 1). In addition, 1 case with S. equi [20] and 15 cases with S. agalactiae (group B Streptococcus [GBS]) were identified. Staphylococcus aureus was found together with GAS in 8 cases and with SD in a single case. Bacteremia was significantly more common in GAS than in SD cases (56% (69/126) versus 30% (8/27); P = .014). Relevant antimicrobial resistance was rare (Supplementary Table 1).
Risk Factors
Comorbidities and other possible risk factors were more common among SD compared to GAS cases, malignancy and blunt trauma in particular (Table 1). Among GAS cases, more than a third was without known comorbidity. In total, 57% (87/153) of GAS and SD cases had no preceding event in the form of surgery, trauma, wound, chronic skin disease, or intravenous drug use. When compared to nonnecrotizing streptococcal cellulitis, streptococcal NSTI was associated to a history of blunt trauma, absence of chronic wound and skin disease, and a lower body mass index (Table 2).
Table 1.
Demographics and Preexisting Factors in NSTI Caused by GAS or SD
| GAS N = 126 | SD N = 27 | P value | |
|---|---|---|---|
| Age (years) | 60 (46–69) | 63 (52–71) | .183 |
| Male sex | 67 (53) | 18 (67) | .200 |
| BMIa (kg/m2) | 26 (23–31) | 28 (24–34) | .239 |
| Currently smokingb | 25 (24) | 7 (30) | .491 |
| Excessive alcohol intakec | 13 (14) | 5 (23) | .333 |
| Comorbidities | |||
| Active malignancy | 8 (6) | 6 (22) | .019 |
| Peripheral vascular disease | 5 (4) | 2 (7) | .607 |
| Cardiovascular diseased | 48 (38) | 13 (48) | .333 |
| Immunodeficiency/immunosuppressione | 25 (20) | 4 (15) | .545 |
| No general comorbidityf | 47 (37) | 6 (22) | .135 |
| Preceding events or skin breachesg | |||
| Blunt traumah | 17 (14) | 9 (33) | .022 |
| Penetrating traumah | 12 (10) | 2 (7) | 1.000 |
| Surgeryh | 5 (4) | 4 (15) | .052 |
| Chronic wound or skin disease | 15 (12) | 2 (7) | .739 |
| Intravenous drug use | 3 (2) | 1 (4) | .544 |
| None of the factors listed above | 75 (60) | 12 (44) | .151 |
Data are presented as median (interquartile range) or no./No. evaluated. (%). Boldface indicates statistical significance (P < .05).
Abbreviations: BMI, body mass index; GAS, group A Streptococcus; NSTI, necrotizing soft tissue infection; SD, Streptococcus dysgalactiae.
aData were missing for 3 patients. Obesity (BMI ≥ 30) was registered in 29% (44/150) overall.
bData were missing for 20 GAS and 4 SD patients.
cDefined as >14 units of alcohol/week for women and >21 units/week for men. Data were missing for 33 GAS and 5 SD patients.
dIncludes, but is not limited to, hypertension, myocardial infarction, angina pectoris, heart failure, apoplexia.
eInnate immunodeficiencies, human immunodeficiency virus, use of steroids or other immunosuppressant drugs, other acquired immunodeficiencies.
fNone of the following: active malignancy, chronic obstructive pulmonary disease or asthma, current or previous cardiovascular disease, diabetes mellitus, chronic kidney failure, chronic liver disease, rheumatoid disease, Immunodeficiency/ immunosuppression.
gNo cases of antecedent varicella was registered.
hIn a period of 4 weeks before NSTI diagnosis.
Table 2.
Risk Factors in Streptococcal Necrotizing Soft Tissue Infection versus Streptococcal Cellulitisa
| Univariate Model | Adjusted Modelb (n = 192) | |||||||
|---|---|---|---|---|---|---|---|---|
| NSTIc | Cellulitisc | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (years) | 42 (38–60) | 54 (43–67) | 1.008 | (.989, 1.026) | .410 | |||
| Sex (m) | 32/66 (49) | 92/146 (63) | 0.552 | (.307, .995) | .047 | 0.794 | (.344, 1.833) | .590 |
| BMI | 26 (23–30) | 28 (25–34) | 0.933d | (.886, .982) | .008 | 0.921 | (.851, .996) | .027 |
| Currently smoking | 12/61 (20) | 25/146 (17) | 1.185 | (.552, 2.545) | .663 | |||
| Excessive alcohol intakee | 7/50 (14) | 7/144 (5) | 3.186 | (1.058, 9.593) | .052 | 3.919 | (.987, 15.568) | .058 |
| Active malignancy | 3/66 (5) | 13/146 (9) | 0.487 | (.134, 1.771) | .401 | |||
| Peripheral vascular disease | 1/66 (2) | 7/146 (5) | 0.305 | (.037, 2.535) | .440 | |||
| Cardiovascular diseasef | 26/66 (39) | 60/146 (41) | 0.932 | (.515, 1.687) | .815 | |||
| Immunodeficiency/immunosuppressiong | 10/66 (15) | 12/146 (8) | 1.994 | (.815, 4.881) | .125 | |||
| Any comorbidityh | 41/66 (62) | 90/146 (62) | 1.020 | (.561, 1.857) | .947 | |||
| Blunt traumai | 11/66 (17) | 6/146 (4) | 4.667 | (1.645, 13.236) | .002 | 5.489 | (1.295, 23.269) | .016 |
| Penetrating traumai | 8/66 (12) | 20/146 (14) | 0.869 | (.362, 2.089) | .753 | |||
| Chronic wound or skin disease | 10/66 (15) | 60/146 (41) | 0.256 | (.121, .541) | <.005 | 0.377 | (.142, 1.006) | .041 |
| Intravenous drug use | 0/66 (0) | 4/144 (3) | 0.680 | (.059, 4.913) | .311 |
Boldface indicates statistical significance (P < .05).
Abbreviations: BMI, body mass index; CI, confidence interval; NSTI, necrotizing soft tissue infection; OR, odds ratio.
aNSTI caused by Streptococcus pyogenes or Streptococcus dysgalactiae vs cellulitis with these microbes confirmed by serology (anti-streptolysin O or anti-DNAse B) or culture in blood or normally sterile tissue. See ref. [17] for criteria. To analyze an NSTI population comparable with the cellulitis cohort, the following NSTI cases were excluded: polymicrobial cases with gram-negative or anaerobic bacteria, postoperative cases (cases with surgery the previous 4 weeks), and cases from Denmark (due to a substantially higher background level of alcohol consumption).
bFactors with a P value below .10 in the univariate analyses (performed also for chronic liver disease and diabetes mellitus) were included in the multivariable model. Adjustment for infection site was performed. A total of 49 NSTI patients and 143 cellulitis controls were included in the adjusted analysis.
cData are presented as median (interquartile range) or no./No. evaluated. (%).
dData were missing for 1 NSTI and 1 cellulitis patient.
eDefined as >14 units/week of alcohol for women and >21/week for men.
fIncludes hypertension and peripheral vascular disease.
gInnate immunodeficiencies, human immunodeficiency virus, use of steroids or other immunosuppressant drugs, other acquired immunodeficiencies.
hNone of the following: active malignancy, chronic obstructive pulmonary disease or asthma, current or previous cardiovascular disease, diabetes mellitus, chronic kidney failure, chronic liver disease, rheumatoid disease, immunodeficiency/ immunosuppression.
iIn a period of 4 weeks before diagnosis.
Clinical Features
As shown in Table 3, septic shock was more common in GAS disease, including the 12 cases involving copathogens of which 9 had septic shock. Among registered preoperative findings, severe pain was prevalent in both GAS and SD cases, but more than half of the patients did not receive opioid drugs before their first surgery. A LRINEC score <6, previously associated with low risk of NSTI [18], was found in 15% (19/128) of the GAS and SD cases overall.
Table 3.
Clinical Features, Treatment, and Outcome in NSTI Caused by GAS or SD
| GAS N = 126 | SD N = 27 | P value | |
|---|---|---|---|
| Preoperative symptoms/signs | |||
| Pain treated with opioids | 55/122 (45) | 10/24 (42) | .758 |
| Skin bullae | 47/124 (38) | 6/26 (23) | .150 |
| Purple/black skin discoloration | 47/125 (38) | 12/26 (46) | .416 |
| Skin bruising | 78/124 (63) | 15/25 (60) | .785 |
| Skin anesthesia | 6/94 (6) | 1/23 (4) | 1.000 |
| Preoperative biochemistrya | |||
| CRP (mg/L)b | 295 (193–370) | 261 (148–339) | .131 |
| Leukocytes (x 109/L)c | 14.2 (8.2–22.2) | 16.9 (11.2–23.7) | .393 |
| Creatinine (μM)d | 178 (109–266) | 103 (73–181) | .001 |
| Severity | |||
| Septic shocke | 82/126 (65) | 11/27 (41) | .019 |
| SAPS II (0–163)f | 44 (34–57) | 44 (36–58) | .758 |
| SOFA score day 1 (0–20)g | 9 (7–12) | 8 (6–11) | .129 |
| LRINEC score (0–13)h | 8 (7–10) | 8 (6–9) | .148 |
| LRINEC score ≥ 6h | 90/104 (87) | 19/24 (79) | .352 |
| Muscle affectedi | 83/126 (66) | 14/27 (52) | .170 |
| Antibiotic treatment | |||
| Clindamycin before inclusionj | 89/126 (71) | 23/27 (85) | .121 |
| Betalactam + clindamycink | 122/126 (97) | 25/27 (93) | .286 |
| IVIGl | 95/126 (75) | 16/27 (59) | .088 |
| Hyperbaric oxygen at any time | 94/126 (75) | 21/27 (78) | .729 |
| Mechanical ventilationk,m | 116/126 (92) | 24/27 (89) | .702 |
| Surgeryn | |||
| Time from first admission to first surgery (hours) | 16 (6–29) | 22 (6–42) | .242 |
| Time from first admission to admission at referral hospital (hours) | 18 (6–36) | 14 (5–31) | .441 |
| Time between 1st and 2nd surgery (hours) | 11 (6–20) | 9 (5–14) | .165 |
| Number of operations | 4 (3–5) | 4 (3–5) | .636 |
| Outcome | |||
| Amputationn | 20/126 (16) | 3/27 (11) | .809 |
| 30-day CFR | 13/125 (10) | 5/27 (19) | .320 |
| 90-day CFR | 13/125 (10) | 6/27 (22) | .110 |
Data are presented as median (interquartile range) or no./No. evaluated (%). Boldface indicates statistical significance (P < .05).
Abbreviations: CFR, case-fatality-rate; GAS, group A Streptococcus, Streptococcus pyogenes; IVIG, intravenous polyspesific immunoglobulin G; LRINEC, laboratory risk indicator for necrotizing fasciitis; NSTI, necrotizing soft tissue infection; SAPS, simplified acute physiology score; SD, Streptococcus dysgalactiae; SOFA, sequential organ failure assessment.
aHighest values observed.
bData missing for 5 patients.
cData missing for 7 patients.
dData missing for 9 patients.
eDefined by use of vasopressor or inotropic agents and lactate >2 mmol/L.
fSAPS II is calculated the first 24 hours in the intensive care unit/high-dependency unit from 17 variables; scores range from 0 to 163, with higher scores indicating more severe disease. Data were missing for 16 patients.
gSOFA score includes subscores ranging from 0 to 4 for each of 5 components (circulation, lungs, liver, kidneys, and coagulation). Aggregated scores range from 0 to 20, with higher scores indicating more severe organ failure. The scoring was modified because cerebral failure was not assessed. Data were missing for 3 patients.
hData were missing for 25 patients.
iAssessed during surgery in the primary hospital or during first 7 days of stay at intensive care unit or high-dependency unit at specialized hospital.
jClindamycin given before admission to intensive care unit/high-dependency unit of the study hospital.
kDuring first 7 days of stay at intensive care unit or high-dependency unit.
lAny dosage. The median number of doses among those receiving IVIG was 3 (interquartile range 2–3).
mIntubated patient or continuous noninvasive ventilation.
nAt primary hospital or during first 7 days of stay at intensive care unit or high-dependency unit at study hospital. Any body part.
Selected clinical features of GAS NSTI were compared to polymicrobial nonstreptococcal NSTI. Among polymicrobial NSTI cases without β-hemolytic streptococci, general comorbidity (as defined in Table 1) was more frequent than in GAS cases (77% (128/166) versus 63% (79/126); P = .007), but the age distribution was similar (median 61 vs 60 years; P = .28). The rate of septic shock in the polymicrobial cases was 46% (77/166) compared to 65% (82/126) for GAS (P = .001), and median SOFA score day 1 was 8 versus 9 (P < .005). Regarding bacteremia, the proportions were 23% (38/166) for polymicrobial and 56% (70/126) for GAS cases (P < .005). A post hoc analysis of time between hospital admission and first surgery showed earlier surgery for the GAS cases (median 16 vs 18 hours, P = .013).
Streptococcal Virulence Profiles
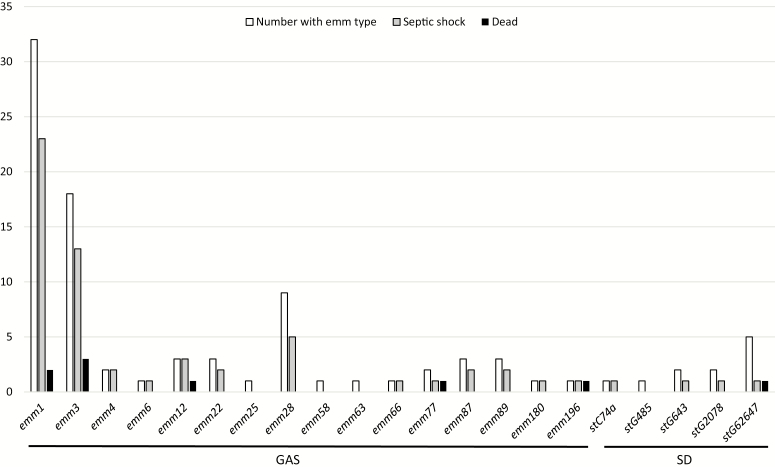
Among 82 available GAS isolates, the predominant emm type was emm1 (Figure 1). In total emm1, emm3 and emm28 comprised 72%. In SD, stG62647 was the predominant emm type among 11 available isolates. Virulence gene profiles of GAS were closely linked to emm types (Supplementary Table 2). In a few isolates, the recently characterized superantigen genes SpeQ and speR were identified [21]. In SD isolates, many had speG, but no other superantigen genes were detected.
Figure 1.
emm type distribution with rates of septic shock and 90-day mortality in 82 GAS and 11 SD cases of necrotizing soft-tissue infection. Forty-four GAS isolates and 16 SD isolates were not available for analysis. Abbreviations: GAS, group A Streptococcus, Streptococcus pyogenes; SD, Streptococcus dysgalactiae.
Treatment, Outcome, and Factors Associated With Mortality
Eighty-nine percent (136/153) of the GAS and SD cases were transferred from other hospitals. The great majority (96%) of the cases received standard antibiotic treatment with a betalactam antibiotic and clindamycin (Table 3). Three-quarters of the GAS cases received IVIG, and a similar proportion was treated with HBOT.
The frequency of amputations was 15% and particularly low among the SD cases (Table 3). The 90-day case-fatality rate of 10% for GAS was significantly lower compared to polymicrobial nonstreptococcal cases (20%; P = .02). Lasso regression analysis of the GAS cases identified increased age (odds ratio [OR] 1.15, per year), male sex (OR 5.09), septic shock (OR 1.96), and IVIG not administered (OR 3.15) as independent variables associated with mortality (Table 4). The variable with the highest OR was HBOT, but this was included in the model to control for confounding (see Methods section). The area under the curve (AUC) of the Lasso model was .97 after correcting for optimism. Due to the possible effect of IVIG on mortality, the groups receiving IVIG or no IVIG were compared in a post hoc analysis (Supplementary Table 3). No clear differences regarding severity or treatment that had not been adjusted for in the Lasso model were found, except more pain, fewer with mechanical ventilation, and longer intervals between surgeries in the non-IVIG group. These 3 parameters were not associated to mortality in an exploratory, univariate analysis in survivors versus nonsurvivors (Supplementary Table 4). Time to first surgery or clindamycin not given prior to admittance to the study hospital were not associated to increased 90-day mortality either, as opposed to high age, high SAPS and SOFA scores, and late clinical NSTI signs. No clear patterns relating emm types or other virulence genes to septic shock or mortality were observed (Figure 1, Supplementary Figures 2 and 3).
Table 4.
Factors Associated With 90-day Mortality in NSTI Caused by GAS
| Univariate Model (n = 125) | Adjusted Modela (n = 125) | Lasso Regressionb (n = 125) | |||
|---|---|---|---|---|---|
| Characteristic | OR | P value | OR | P value | OR |
| Age (years) | 1.06 | .015 | 1.32 | <.0005 | 1.15 |
| Sex (male) | 3.33 | .066 | 145.82 | .002 | 5.09 |
| CVD | 0.69 | .765 | 0.02 | .006 | 0.22 |
| Active malignancy | 0.89 | 1.000 | <0.01 | .008 | 0.04 |
| Septic shockc | 1.93 | .541 | 25.73 | .048 | 1.96 |
| Initial surgery > 24 hours after admissiond | 0.33 | .216 | 0.30 | .422 | 0.95 |
| No clindamycin before inclusione | 0.40 | .342 | 0.06 | .166 | 0.36 |
| IVIG not given | 2.98 | .086 | 7.60 | .125 | 3.15 |
| HBOT not given | 23.81 | <.0005 | 1220.47 | <.0005 | 78.80 |
Abbreviations: CVD, cardiovascular disease (including hypertension and peripheral vascular disease); GAS, group A Streptococcus, Streptococcus pyogenes; HBOT, hyperbaric oxygen treatment; IVIG, treatment with polyspecific immunoglobulin G (any dose); NSTI, necrotizing soft tissue infection; OR, odds ratio.
aLogistic regression analysis.
bLasso regression is a shrinkage method, which gives us a more reliable model by shrinking the coefficient estimates (compared to the logistic model). Variables with little or no predictive value will be shrunken to zero (an OR of 1). P values or confidence intervals can not be calculated (see also Supplementary Methods).
cSeptic shock the first 24 hours after admission to intensive care unit/high-dependency unit, defined as lactate >2 mmol/L and use of vasopressor or inotrope.
dInitial surgery performed >24 hours after the first admission to hospital.
eClindamycin not given before admission to intensive care unit/high-dependency unit of the study hospital.
DISCUSSION
In this study, the largest prospectively enrolled cohort of streptococcal NSTI to date is presented. The data provide a contemporary and detailed picture of risk factors, severity, and specific features of streptococcal NSTI. Important predictors of mortality are described.
GAS outnumbered SD by a factor of 4.7 and by 7.6 for the monomicrobial cases. Still, this study confirms that SD plays a significant role as a cause of NSTI. The rigorous procedure applied for review of all microbiology results, aiming at avoiding inclusion of probable contaminants and colonizers, may explain why the proportion of polymicrobial cases were lower than in some other studies.
Possible predisposing factors were frequent, particularly in NSTI caused by SD, but the ability of GAS to cause NSTI in previously healthy individuals was confirmed. Obesity has previously been identified as a risk factor for invasive GAS infection [22]. It was common also in this cohort, but it was even more frequent in streptococcal cellulitis and therefore not a risk factor specific to NSTI. Skin disease, antecedent surgery, or other wounds were rare, confirming streptococcal NSTI as a disease that often occurs without an obvious portal of entry [2]. Blunt trauma is recognized as an important preceding event in many streptococcal NSTI cases [2]. Our study affirms this association, by comparing with cellulitis.
Detailed data on clinical severity, including frequency of septic shock, are scarce, and the accuracy of such data from surveillance studies has been questioned [11]. The comparison of GAS and SD NSTI was limited by the number of SD cases, but when comparing with polymicrobial nonstreptococcal NSTI, GAS NSTI clearly stands out with high rates of septic shock, organ failure, and bacteremia. This accords with major differences in the pathogenesis of streptococcal versus polymicrobial infections, as recently demonstrated in a comparative, molecular study of selected cases from the INFECT cohort [23]. Septic shock was more frequent with higher age rather than younger, contrary to studies of invasive GAS linking streptococcal toxic shock syndrome (STSS) and younger age [9, 11, 24]. Lower mortality rate in GAS cases compared to SD and polymicrobial cases could be related to less comorbidity, as previously discussed [16]. Our finding that some comorbidities were inversely related to mortality illustrate the complexity of the host-pathogen-relationship, however. Earlier diagnosis and more aggressive management may have contributed to lower mortality in GAS disease, as suggested by the post hoc analysis revealing earlier surgery for this group. Systemic severity and the substantial number of patients that were previously healthy highlight the particular virulence potential of GAS. In addition to the strong association of streptococcal NSTI and extremity location [16], these characteristics may also give diagnostic clues on probable etiology even before results of any rapid test is available. With respect to the LRINEC score, it had suboptimal sensitivity among the streptococcal cases, as shown also for the whole cohort [16]. This underscores the questionable value of this score in our setting, in line with a recent systematic review [25].
The observed predominance of emm1 accords with a rise in this emm type in Scandinavia [26, 27]. Even though several other emm types where identified, the fact that >70% of the isolates belonged to only 3 emm types add to the evidence that some emm types have a particular ability to cause severe infections [11, 28, 29]. In SD, stG62747 was the predominant emm type, in accordance with a previous report from our region [30]. The virulence gene profiles of GAS largely reflected the emm type distribution, with a high proportion with speA and speG consistent with the predominance of emm1 and emm3 types. We did not find any clear association of these genes or emm types with severity, however, but the numbers for most emm types and virulence gene profiles were low.
As expected, systemic disease severity had an impact on mortality. Not receiving clindamycin before inclusion or undergoing surgery later than after 24 hours after admission was not associated with increased mortality in this cohort, possibly reflecting rapid identification and initiation of appropriate treatment for the most severely sick patients, as indicated by shorter time to surgery among patients with shock (Supplementary Table 3) and the relatively low mortality for GAS NSTI overall (10% at 30 days). Mortality was lower in IVIG-treated patients, and it was also associated with lower odds of death in the adjusted model. Previous observational studies addressing this issue have shown conflicting results. Several observational studies suggest an effect of IVIG in STSS [31–34], whereas others do not [35, 36]. The only randomized trial of IVIG in STSS was prematurely terminated due to low inclusion, but a favorable, nonsignificant effect was observed [37]. Some of the patients in our study were coenrolled in a recent randomized trial of IVIG in NSTI of all microbiological etiologies; in which no effect of IVIG was found on physical quality of life [38]. Possibly, the lack of effect in the latter study, as well as in the large observational study by Kadri et al [36], was the consequence of not restricting these studies to streptococcal NSTI. A propensity to offer IVIG as a last resort might also obscure beneficial effects. However, a recent meta-analysis of clindamycin-treated STSS patients from 1 randomized and 4 nonrandomized studies found that IVIG was significantly associated with increased survival [39]. Our study suggests such an association also in GAS NSTI. All of the observational studies performed are vulnerable to confounding, however. This includes our own study, but interestingly, the report by Bergsten et al [40] demonstrated a dose-related toxin inhibition in plasma of our patients, providing a mechanistic correlate that also supports a possible benefit of IVIG. Taken together, these studies encourage renewed efforts to conduct a sufficiently powered randomized clinical trial restricted to GAS NSTI.
The major strength of the study is its size and the prospective, multicenter design. The study was able to accurately stratify patients according to a wide range of clinical parameters, more so than retrospective studies and surveillance studies. However, because all study centers are academic referral hospitals for NSTI that offer HBOT, and transferred patients constituted the majority of cases, the present patient cohort may not represent streptococcal NSTI of all degrees of severity. Some patient categories are probably often not transferred, primarily those without a fair chance of survival as well as stable patients with smaller lesions. When analyzing factors associated to mortality, it is difficult to fully adjust for the lower propensity to provide costly treatment to patients with very high or low chance of survival. Furthermore, clinical details and the exact timing of initial treatment at the primary hospital were not registered, and incidence could not be calculated. In the study of risk factors there were differences regarding inclusion periods, study sites and microbiological methods for the cellulitis and NSTI cohorts, but the analyzed populations were well matched. The exploratory parts of the study, with many univariate comparisons between different groups and no correction for multiple testing, demands caution when interpreting low P-values.
In summary, this large prospective multicenter cohort study supports blunt trauma as an important risk factor for streptococcal NSTI. A high frequency of septic shock was observed, particularly in GAS disease. IVIG was among several factors associated with increased survival.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
INFECT study group. Torbjørn Nedrebø, Haraldsplass Deaconal Hospital, Bergen, Norway; Dag Harald Skutlaberg, Department of Microbiology, Haukeland University Hospital, Bergen, Norway; Morten Hedetoft, Marco B. Hansen, Peter Polzik, Hyperbaric Medicine Center, Department of Anesthesiology and Surgery, Head and Orthopedic Center, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark, Anders Rosén, Department of Anaesthesia and Intensive Care, Sahlgrenska University Hospital, Gothenburg; Mattias Svensson, Helena Bergsten, Center for Infectious Medicine, Karolinska Institutet, Stockholm, Sweden; Walter Israel Barrantes Bustinza, Helmholtz-Zentrum für Infektionsforschung GmbH, Braunschweig, Germany; Vitor A. P. Martins dos Santos, Laboratory of Systems and Synthetic Biology, Wageningen University and Research, Wageningen, The Netherlands and LifeGlimmer GmBH, Berlin, Germany.
Author contributions. A. N. T. is project coordinator of the INFECT study. T. B. and S. S. conceived the streptococcal part of the study. T. B. drafted the first paper. O. H., S. S., P. A., Y. K., and M. N. are national investigators and have contributed to study design and coordinated study conduct. M. B. M. is responsible for the database and contributed to patient inclusion and data collection, as did T. B., E. R., M. B. M., P. A., Y. K., M. N., O. O., and S. S. O. O., A. B., and A. I. performed streptococcal typing and virulence factor screening. T. B., E. R., M. B. M., O. O., F. B., O. H., A. N. T., and S. S. contributed to analysis or interpretation of data or both. All authors contributed to the writing of this paper and approved the final version.
Acknowledgments. The authors thank the clinical and research personnel at each of the including sites, the microbiological laboratories, patients and their relatives.
Financial support. The INFECT study was supported by the European Union’s Framework Programme 7 (grant number FP7/2007–2013 305340).
Potential conflicts of interest. M. B. M. has received a research grant from CSL Behring, Switzerland. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
INFECT Study Group:
Torbjørn Nedrebø, Dag Harald Skutlaberg, Morten Hedetoft, Marco B Hansen, Peter Polzik, Anders Rosén, Mattias Svensson, Helena Bergsten, Walter Israel Barrantes Bustinza, and Vitor A P Martins dos Santos
References
- 1. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 2. Stevens DL, Bryant AE. Necrotizing soft-tissue infections. N Engl J Med 2017; 377:2253–65. [DOI] [PubMed] [Google Scholar]
- 3. Peetermans M, de Prost N, Eckmann C, Norrby-Teglund A, Skrede S, De Waele JJ. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect 2020; 26:8-17. [DOI] [PubMed] [Google Scholar]
- 4. May AK. Skin and soft tissue infections. Surg Clin North Am 2009; 89:403–20, viii. [DOI] [PubMed] [Google Scholar]
- 5. Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989; 321:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Chelsom J, Halstensen A, Haga T, Høiby EA. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet 1994; 344:1111–5. [DOI] [PubMed] [Google Scholar]
- 7. Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med 1997; 103:18–24. [DOI] [PubMed] [Google Scholar]
- 8. Sharkawy A, Low DE, Saginur R, et al. Severe group A streptococcal soft-tissue infections in Ontario: 1992–1996. Clin Infect Dis 2002; 34:454–60. [DOI] [PubMed] [Google Scholar]
- 9. Hoge CW, Schwartz B, Talkington DF, Breiman RF, MacNeill EM, Englender SJ. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA 1993; 269:384–9. [PubMed] [Google Scholar]
- 10. Lamagni TL, Darenberg J, Luca-Harari B, et al. ; Strep-EURO Study Group Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 2008; 46:2359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekelund K, Skinhoj P, Madsen J, Konradsen HB. Invasive group A, B, C and G streptococcal infections in Denmark 1999–2002: epidemiological and clinical aspects. Clin Microbiol Infect 2005; 11:569–76. [DOI] [PubMed] [Google Scholar]
- 13. Bruun T, Kittang BR, de Hoog BJ, et al. Necrotizing soft tissue infections caused by Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis of groups C and G in western Norway. Clin Microbiol Infect 2013; 19:E545–50. [DOI] [PubMed] [Google Scholar]
- 14. Lin JN, Chang LL, Lai CH, Lin HH, Chen YH. Group A streptococcal necrotizing fasciitis in the emergency department. J Emerg Med 2013; 45:781–8. [DOI] [PubMed] [Google Scholar]
- 15. INFECT: Improving Outcome of Necrotizing Fasciitis: Elucidation of complex host and pathogen signatures that dictate severity of tissue infection Available at: https://cordis.europa.eu/project/id/305340. Accessed 18 August 2019.
- 16. Madsen MB, Skrede S, Perner A, et al. ; INFECT study group Patient’s characteristics and outcomes in necrotising soft-tissue infections: results from a Scandinavian, multicentre, prospective cohort study. Intensive Care Med 2019; 45:1241–51. [DOI] [PubMed] [Google Scholar]
- 17. Bruun T, Oppegaard O, Kittang BR, Mylvaganam H, Langeland N, Skrede S. Etiology of cellulitis and clinical prediction of streptococcal disease: a prospective study. Open Forum Infect Dis 2016; 3:ofv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–41. [DOI] [PubMed] [Google Scholar]
- 19. Pavlou M, Ambler G, Seaman SR, et al. How to develop a more accurate risk prediction model when there are few events. BMJ 2015; 351:h3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kittang BR, Pettersen VK, Oppegaard O, et al. Zoonotic necrotizing myositis caused by Streptococcus equi subsp. zooepidemicus in a farmer. BMC Infect Dis 2017; 17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reglinski M, Sriskandan S, Turner CE. Identification of two new core chromosome-encoded superantigens in Streptococcus pyogenes; speQ and speR. J Infect 2019; 78:358–63. [DOI] [PubMed] [Google Scholar]
- 22. Langley G, Hao Y, Pondo T, et al. The impact of obesity and diabetes on the risk of disease and death due to invasive group A Streptococcus infections in adults. Clin Infect Dis 2016; 62:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thänert R, Itzek A, Hoßmann J, et al. ; INFECT study group Molecular profiling of tissue biopsies reveals unique signatures associated with streptococcal necrotizing soft tissue infections. Nat Commun 2019; 10:3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamagni TL, Neal S, Keshishian C, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg Infect Dis 2008; 14: 202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernando SM, Tran A, Cheng W, et al. Necrotizing soft tissue infection: diagnostic accuracy of physical examination, imaging, and LRINEC Score: a systematic review and meta-analysis. Ann Surg 2019; 269:58–65. [DOI] [PubMed] [Google Scholar]
- 26. Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012–February 2013. Euro Surveill 2013; 18:20443. [DOI] [PubMed] [Google Scholar]
- 27. Naseer U, Steinbakk M, Blystad H, Caugant DA. Epidemiology of invasive group A streptococcal infections in Norway 2010–2014: a retrospective cohort study. Eur J Microbiol Infect Dis 2016; 35:1639–48. [DOI] [PubMed] [Google Scholar]
- 28. Luca-Harari B, Darenberg J, Neal S, et al. ; Strep-EURO Study Group Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 2009; 47:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ikebe T, Okuno R, Sasaki M, et al. ; Working Group for Beta-Hemolytic Streptococci in Japan Molecular characterization and antibiotic resistance of Streptococcus dysgalactiae subspecies equisimilis isolated from patients with streptococcal toxic shock syndrome. J Infect Chemother 2018; 24:117–22. [DOI] [PubMed] [Google Scholar]
- 30. Oppegaard O, Mylvaganam H, Skrede S, Lindemann PC, Kittang BR. Emergence of a Streptococcus dysgalactiae subspecies equisimilis stG62647-lineage associated with severe clinical manifestations. Sci Rep 2017; 7:7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norrby-Teglund A, Muller MP, Mcgeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis 2005; 37:166–72. [DOI] [PubMed] [Google Scholar]
- 32. Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome: a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999; 28:800–7. [DOI] [PubMed] [Google Scholar]
- 33. Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 2014; 59:358–65. [DOI] [PubMed] [Google Scholar]
- 34. Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 2014; 59:851–7. [DOI] [PubMed] [Google Scholar]
- 35. Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis 2009; 49:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kadri SS, Swihart BJ, Bonne SL, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with vasopressor-dependent shock: a propensity score-matched analysis from 130 US hospitals. Clin Infect Dis 2017; 64:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Darenberg J, Ihendyane N, Sjölin J, et al. ; StreptIg Study Group Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003; 37:333–40. [DOI] [PubMed] [Google Scholar]
- 38. Madsen MB, Hjortrup PB, Hansen MB, et al. Immunoglobulin G for patients with necrotising soft tissue infection (INSTINCT): a randomised, blinded, placebo-controlled trial. Intensive Care Med 2017; 43:1585–93. [DOI] [PubMed] [Google Scholar]
- 39. Parks T, Wilson C, Curtis N, Norrby-Teglund A, Sriskandan S. Polyspecific Intravenous Immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:1434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bergsten H, Madsen MB, Bergey F, et al. Correlation between immunoglobulin dose administered and plasma neutralization of streptococcal superantigens in patients with necrotizing soft tissue infections. Clin Infect Dis [Preprint]. 2020. doi:10.1093/cid/ciaa022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.