Women can synchronize their menstrual cycles with the Moon.
Abstract
Many species synchronize reproductive behavior with a particular phase of the lunar cycle to increase reproductive success. In humans, a lunar influence on reproductive behavior remains controversial, although the human menstrual cycle has a period close to that of the lunar cycle. Here, we analyzed long-term menstrual recordings of individual women with distinct methods for biological rhythm analysis. We show that women’s menstrual cycles with a period longer than 27 days were intermittently synchronous with the Moon’s luminance and/or gravimetric cycles. With age and upon exposure to artificial nocturnal light, menstrual cycles shortened and lost this synchrony. We hypothesize that in ancient times, human reproductive behavior was synchronous with the Moon but that our modern lifestyles have changed reproductive physiology and behavior.
INTRODUCTION
In many marine species (1–5) and some terrestrial species (6–9), reproductive behavior is synchronized with a particular phase of the lunar cycle (often full or new moon). This arrangement increases reproductive success by synchronizing the reproductive behavior of the individual members of a species. In light of this fact, it is of interest that the human menstrual cycle has a period close to that of the lunar cycle and that several older studies report a relation between the cycles. Women whose cycles approach the ~29.5-day period of the Moon have been reported to have the highest likelihood to become pregnant (10–12). In these studies, about 28% of reproductively mature women showed a cycle length of 29.5 ± 1 days. Among populations of women selected for a cycle length of 29.5 ± 1 days, a significant pattern of menses onset at full moon emerged (13–15). Each of these studies comprised >300 women, and the tests were performed in different years and seasons. However, no correlation between menses onset and the lunar cycle was found in other studies that did not select for a cycle length of 29.5 days [reviewed in (13, 14)]. Significant correlations also appear to exist between birth rate and moon phase. Two systematic large longitudinal studies carried out between 1948 and 1957 and between 1961 and 1963 and encompassing around 250,000 and 500,000 births, respectively, found that birth rates were elevated by 2 to 3% over the average at full moon and reduced by the same amount during new moon (16, 17). More recent findings indicate that the elevation of births at the full moon occurs during the night, whereas births at the new moon tend to occur during the daytime (18). Nevertheless, the scientific community generally remains skeptical of reports of lunar influence on human biology (19).
To address the question of lunar influence on human reproduction, we examined the course of menstrual cycles in 22 individuals who kept long-term records (up to 32 years) of menses onsets, on a case-by-case basis. This idiographic approach allowed for the possibilities that lunar influence might be present intermittently and in different forms over the females’ life. To our knowledge, this approach to the evaluation of such long-term data has not been used previously. Instead, previous studies included large numbers of women whose menstrual cycles were analyzed in aggregate, combining results of different women, ages, years, and seasons (14, 15, 20). Furthermore, in our study, we tested the Moon’s gravitational influences on menses onsets, in addition to its nocturnal light effects.
The Moon exhibits three different cycles that affect moonlight intensity and gravity on Earth and that are illustrated in Fig. 1 [see also figure 1 in the work by Andreatta and Tessmar-Raible (5)]. The full moon/new moon luminance cycle (the synodic month) repeats, on average, every 29.53 days as the Moon moves through its two points of alignment (syzygies) with the Earth and Sun (Fig. 1, A and B). Since the Moon and Earth do not move at constant speed in their elliptical orbits, the synodic period varies between 29.27 and 29.83 days (fig. S1C; see also undulating lines of full and new moon in the raster plots). The lunar standstill cycle (the tropical month) lasts 27.32 days and arises from the declination of the Moon’s orbit relative to the plane of Earth’s equator, which causes the Moon to move back and forth between its northernmost and southernmost positions in the sky (Fig. 1C). The perigee and apogee cycle (the anomalistic month) lasts, on average, 27.55 days and recurs as the Moon moves back and forth between the nearest (perigee) and farthest (apogee) extremes of its elliptical orbit (Fig. 1D). The anomalistic month shows even larger variations in period than the synodic month: It varies between 24.5 and 28.8 days (fig. S1D).
Fig. 1. Illuminance and gravimetric cycles that the Moon imposes on Earth.
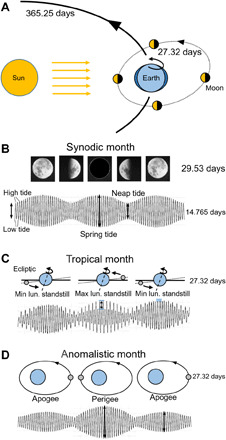
(A) Schematic of the movements of Moon and Earth around the Sun (sizes of Sun, Earth, and Moon and distances between them are not to scale). (B) The Sun illuminates the Moon, which causes a 29.53-day cycle (the synodic month) of changing nocturnal illuminance on Earth (full, half, and new moon). The synodic month is ~2.2 days longer than the orbital period of the Moon because Earth has moved in its orbit around the Sun and it takes 2.2 days longer until the Moon is again in its full moon position. The Moon also exerts gravitational effects on Earth that are most evident in the tides. High and low tides occur twice a day with a period of 12.4 hours. Spring tides happen every 14.765 days during full and new moons and neap tides during half-moons. (C) The Moon’s orbit around Earth is tilted with respect to the plane of Earth’s orbit around the Sun (the ecliptic). When the Moon is high in the northern sky, the difference in the amplitude between the two daily tides is maximal in the Northern Hemisphere of Earth. This happens every 27.32 days, and the corresponding cycle is called tropical month. (D) The orbit of the Moon around Earth is elliptic with Earth in one of the focal points of the ellipse. Therefore, the Moon-Earth distance varies cyclically: Every 27.55 days, the Moon is close to Earth (in its perigee) and exerts maximal gravitational forces on Earth, resulting in high tidal amplitudes, while it exerts minimal gravitational forces when it is far from Earth (in its apogee). This cycle is called anomalistic month.
The synodic month clearly affects moonlight intensity and gravity (visible in the tides), while the anomalistic and tropical months mainly affect gravity and, to a lesser degree, light intensity (21, 22). Furthermore, the three lunar cycles interact with each other. When the Moon is at its perigee and simultaneously in Moon-Sun-Earth syzygy (full or new moon), the gravitational forces on Earth are very high. This perigee-syzygy occurs every 206 days and can result in partial or full lunar or solar eclipses. Every 18 years and 10.33 days, the Moon passes exactly the same node at which its orbital plane intersects with the ecliptic Earth-Sun plane, and in addition, a full or new moon occurs (equal to a Saros series). If total eclipses and a close perigee distance characterize a certain Saros series, then the gravitational forces of the Moon on Earth are maximal.
We found that all three lunar cycles—the synodic, the anomalistic, and, to a lesser degree, the tropical month—affect menses onset. Nocturnal moonlight appears to be the strongest zeitgeber through which the Moon exerts its influence, but the gravitational forces of the Moon clearly contribute. We hypothesize that in ancient times, human reproductive behavior was synchronous with the Moon but that our modern lifestyle, notably our increasing exposure to artificial light, has changed this relation.
RESULTS
Our study comprised 22 women, who kept recordings of their menses onsets, on average, for 15 years (median: 14 years) (Table 1). Ten women’s recordings spanned both young (≤35 years) and older ages (>35 years). The remaining women’s recordings spanned only young or only older ages. Together, we had recordings of 15 women aged ≤35 and of 17 women aged >35 years (Table 1). As earlier studies have already shown (11, 23–26), we found a significant decrease in cycle length with age (Table 1 and Figs. 2 and 3). To determine whether menses onsets were ever synchronous with the lunar cycle, we analyzed the relation of menses onsets to the full moon and the new moon, to minimum and maximum lunar standstills, and to lunar perigees and apogees for each individual.
Table 1. Summary menses of recorded subjects.
| Age at | Number of recorded |
Period length in days (mean ± SD/median)* |
Percent of time synchronized to the synodic month (f: full moon; n: new moon)† |
Percent of time synchronized to the anomalistic or tropical month (a: apogee; p: perigee; ma, mi: maximum, minimum lunar standstills)† |
Nocturnal light exposure |
|||||||
|
Record begin |
Record end |
Years | Menses | Births‡ | ≤35 years | >35 years | ≤35 years | >35 years | ≤35 years | >35 years | ||
| Subject 1§ | 21 | 53 | 32 | 337 | 3 | 32.2 ± 4.2/32.0 | 28.4 ± 2.1/28.0 | 31.8 (f + n) | 45.0 (f + n) | 0.0 | 14.6 (a, ma, and mi) |
Low |
| Subject 2§ | 19 | 50 | 31ǁ | 331 | 4 | 29.2 ± 2.1/29.0 | 26.8 ± 3.0/26.0 | 56.3 (f + n) | 14.3 (n) | 7.5 (mi) | 18.3 (a + mi) |
Low |
| Subject 3 | 24 | 51 | 27 | 360 | 0 | 30.4 ± 3.7/30.0 | 25.5 ± 2.3/25.0 | 41.7 (f) | 13.8 (f + n) | 3.4 (p) | 21.4 (a + p) |
Low |
| Subject 4 | 23 | 42 | 19 | 259 | 0 | 29.1 ± 3.7/29.0 | 25.8 ± 2.1/26.0 | 65.0 (f) | 0.0 | 28.1 (a, p, and ma) |
33.3 (a, p, and mi) |
Low |
| Subject 5 | 25 | 53 | 28 | 368 | 2 | 27.8 ± 1.8/28.0 | 26.7 ± 3.0/27.0 | 2.0 (n) | 25.7 (f + n) | 62.5 (a, p, ma, and mi) |
39.0 (a, p, ma, and mi) |
Medium |
| Subject 6 | 19 | 44 | 25ǁ | 226 | 2 | 26.0 ± 4.9/25.0 | 24.7 ± 2.4/25.0 | 0.0¶ | 0.0 | 7.1 (p) | 0.0 | High |
| Subject 7§ | 25 | 50 | 25 | 297 | 2 | 30.2 ± 2.9/30.0 | 26.4 ± 2.0/26.0 | 27.1 (f + n) | 19.4 (f + n) | 0.0 | 13.8 (p and ma) |
Low |
| Subject 8 | 31 | 51 | 20 | 195 | 3 | 31.5 ± 6.4/29.0 | 25.8 ± 3.0/26.0 | 0.0¶ | 0.0 | 11.8 (a and p) |
12.1 (p) | High |
| Subject 9 | 25 | 42 | 17 | 229 | 0 | 28.6 ± 2.2/29.0 | 27.2 ± 1.4/27.0 | 36.0 (f + n) | 37.5 (f) | 25.8 (a and ma) |
16.0 (a and p) |
Low |
| Subject 10 | 33 | 47 | 14 | 182 | 0 | 27.8 ± 4.8/27.0 | 26.9 ± 4.7/26.0 | 0.0 | 0.0 | 0.0 | 18.2 (p) | Medium |
| Subject 11 | 29 | 38 | 9 | 104 | 1 | 26.5 ± 1.6/26.0 | – | 0.0 | – | 17.6 (a and p) |
– | High |
| Subject 12§ | 12# | 18 | 6 | 85 | 0 | 28.5 ± 4.9/28.0 | – | 20.0 (f + n) | – | 28.5 (p) | – | Low |
| Subject 13 | 18 | 25 | 7 | 108 | 0 | 30.3 ± 6.2/31.0 | – | 32.3 (f + n) | – | 0.0 | – | Low |
| Subject 14 | 31 | 37 | 6 | 66 | 0 | 30.6 ± 4.9/30.0 | – | 14.3 (n) | – | 3.8 (ma) | – | Low |
| Subject 15§ | 12# | 17 | 5 | 49 | 0 | 32.4 ± 5.6/30.5 | – | 27.3 (f) | – | 0.0 | – | Low |
| Subject 16 | 45 | 54 | 9 | 114 | 0 | – | 27.1 ± 4.5/26.0 | – | 5.0 (n) | – | 16.7 (p) | Low |
| Subject 17 | 38 | 49 | 11 | 166 | 0 | – | 26.1 ± 3.7/26.0 | – | 0.0 | – | 29.7 (a and ma) |
Medium |
| Subject 18 | 36 | 49 | 13 | 180 | 0 | – | 26.5 ± 2.8/26.0 | – | 0.0 | – | 36.3 (a, p, and mi) |
High |
| Subject 19 | 45 | 51 | 6 | 81 | 0 | – | 25.7 ± 2.5/26.0 | – | 0.0 | – | 0.0 | Low |
| Subject 20 | 43 | 48 | 5 | 70 | 0 | – | 25.4 ± 6.6/24.0 | – | 0.0 | – | 11.3 (mi) | Medium |
| Subject 21§ | 40 | 46 | 5 | 109 | 0 | – | 26.3 ± 5.7/25.0 | – | 0.0 | – | 0.0 | Medium |
| Subject 22 | 36 | 50 | 14 | 196 | 0 | – | 26.0 ± 5.0/26.0 | – | 0.0 | – | 19.7 (a, ma, and mi) |
Medium |
| Summary | 4112 | 17 | 29.4 ± 1.9/28.9 | 26.3 ± 0.9/25.9 | 23.6% | 9.5% | 13.1% | 17.7% | ||||
*Period was determined by measuring the distances between the menses.
†Synchronization was determined on the actograms; only intervals at which menses occurred at least three times in a row at full (f) or new (n) moon were judged as synchronized.
‡Includes miscarriages; with the exception of subject 21, only the births occurring during the recorded years are included. For subject 21, the births of the two daughters (subjects 12 and 15) are included, although they happened long before she started the recording of her menses.
§Relations between subjects. Subject 8 is the mother of subjects 1 and 2; subject 21 is the mother of subjects 11 and 14. Actograms of subjects 19 to 21 are not shown in the figures.
ǁRecording was interrupted for several years.
¶Menses oscillated for five to six cycles with a period of 29.5 days and occurred during the waxing moon. These women were judged as not synchronized because the phase of menses onset was not at full or new moon.
#Recording started at menarche.
Fig. 2. Distribution of periods of the menstrual cycle relative to age.
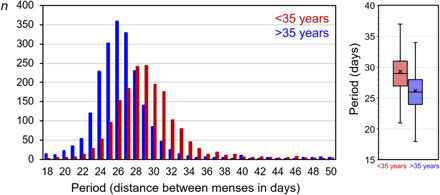
Periods were determined by calculating the distances between individual menses for all women for the ages ≤35 years (red) and >35 years (blue). In the box plots, the medians are indicated as horizontal lines and the means are indicated as crosses. A Kruskal-Wallis nonparametric test revealed that the period of the menstrual cycle was significantly shorter at ages >35 years than at younger ages (P < 0.001).
Fig. 3. Menstrual cycles in relation to the Moon’s synodic cycles.
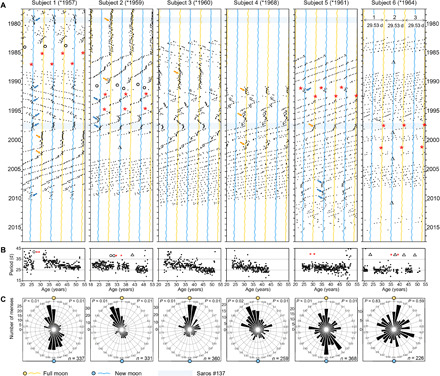
(A) Raster plots of menstrual cycles of six women (birth years in parenthesis) recorded between 1978 and 2015. The raster plots consist of 29.54-day segments of the time series of each individual’s record of menses onsets, which are shown consecutively beneath one another. The segments approximate the 29.53-day period of the synodic month. The plots are repeated three times, as indicated in the plot of subject 6, to facilitate visual inspection of the courses of the menstrual cycles. Onsets of menses are shown as black dots, while times of full moons and new moons are indicated as yellow and blue undulating lines, respectively. Black circles indicate miscarriages; red asterisks indicate births, and triangles indicate gaps in the record. In subjects 1 to 5, the menstrual cycle coupled temporarily to full moons (yellow arrows) or new moons (blue arrows). Light blue shaded areas indicate years of Saros #137, which were characterized by high luminance and high gravitational influence of the Moon during periods when Sun-Earth-Moon syzygies coincided with perigees in which the Moon was exceptionally close to Earth. (B) Periods of menstrual cycles over age. (C) Circular plots of the distribution of menses phases throughout the synodic cycle. The synodic cycle was divided into 30 equal segments each lasting for about 1 day. The number of menses onsets that occurred within a certain segment of the synodic month is plotted radially. Rayleigh tests to check for the presence of a uni- or bimodal distribution with the phases synchronized to the full/new moon (left P values) and for deviations from a uniform frequency distribution of any phase (right P values). Numbers in the lower left corner of each graph indicate the sum of all recorded menses.
Menstrual data were displayed as raster plots, together with lines depicting the courses of the full and the new moon (Figs. 3 and 4) or of minimum and maximum lunar standstills and perigees and apogees (Figs. 5 and 6 and fig. S1). The menstrual data were also subjected to time series analysis (27).
Fig. 4. Relation between timing of menses and the synodic month in the remaining eight women who kept records for several years before 35 years of age.
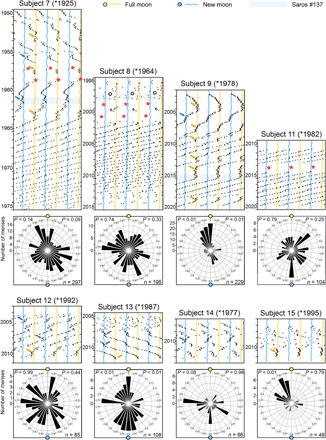
Two of the eight women (subjects 8 and 11) exhibited a rather short cycle length and did not show any synchronization to the synodic lunar month. The other six temporarily followed the full or new moon by visual judgement (see arrows in the raster plots). The Rayleigh tests for uni- or bimodal distribution with central tendency at full or new moon confirmed the latter judgement for three of these women (subjects 9, 13, and 15) and showed a tendency (P < 0.1) for two women (subjects 1 and 14) (left P values in the raster plots). In subject 1, this tendency became significant, when only the first 15 years were included in the analysis. For subject 12, the phase relation between menses onsets and the full or new moon was rather irregular; nevertheless, menses appear to have coupled loosely to the Moon. Labeling as in Fig. 1.
Fig. 5. Menstrual cycles temporarily follow the tropical and anomalistic cycles of the Moon.
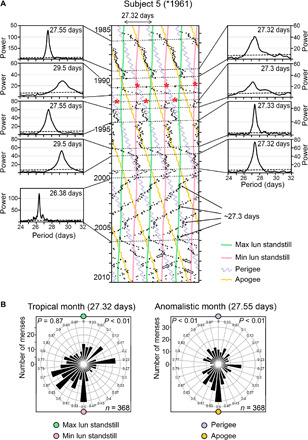
(A) Triple raster plot of subject 5 plotted with a period of 27.33 days, which approximates the orbital period of the Moon and the duration of the tropical month (27.32 days). Lunar phases are indicated as colored undulating lines. Time intervals demarcated by stippled lines in the raster plot were assessed by periodogram analyses. The respective periodograms are shown to the left or right of the raster plot. Stippled lines in the periodograms indicate the 5% significance level. The stronger the rhythm and the longer the duration of its presence, the higher the power. Menses temporarily followed the tropical month or the anomalistic cycles or exhibited phases of relative coordination to these cycles (long arrows). After age 46, when the rhythm displayed three instances of relative coordination with the new moon phase of the synodic month (Fig. 3A), sudden changes in period occurred when the cycle coincided with perigees or apogees (open arrows). (B) Circular plots showing the distribution of menses throughout the tropical and anomalistic month (labeling as in Fig. 1). The Rayleigh tests showed that menses distribution significantly deviates from a uniform distribution for both lunar cycles. During the anomalistic month, menses onsets were preferentially directed to apogees. During the tropical month, menses phase distribution was more variable.
Fig. 6. Relation of menses to the tropical and anomalistic months in five women who began recording onsets of their menses in their 30s.
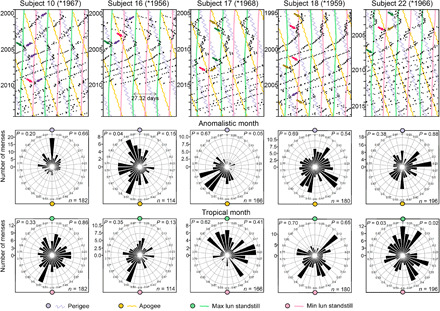
Subject 16 was synchronized with the synodic month during the first year of recording, while the others were never synchronized with the synodic month. Instead, by visual judgment, all five women temporarily followed the anomalistic and tropical months as indicated by colored arrows. Two colored arrows point to the times at which synchronization happened when the two types of cycles coincided. The circular statistics confirmed the alignment of menses onset with the anomalistic and tropical month only in a few cases. Labeling as in Fig. 5.
Relation of menses onset to the synodic lunar cycle
Figure 3A shows examples of raster plots that were plotted with the period of the synodic month for six women who kept long-term records between 1978 and 2014. All lived in South Germany (47.5° to 51° latitude and 6.5° to 12° longitude), and except for subjects 5 and 6, they inhabited rural areas or boundary areas of towns with less than 100,000 inhabitants. Subjects 1 and 2 were sisters but lived apart. Subject 5 lived in a town of ~500,000 inhabitants and was exposed to moderate nocturnal light coming from street lamps. None of these first five women led an extensive nightlife. In contrast, subject 6 mostly lived in large cities (>1.5 million inhabitants), was quite active during the night, and preferred to sleep with a night light; thus, she continuously exposed herself to high artificial light levels at night.
Although all of the records overlapped in time, they displayed menstrual rhythms that differed greatly among individuals and that varied over time within individuals. For example, the cycles of all women were regular at some times and irregular (exhibiting phase jumps and period changes) at other times, but these variations happened during different years in the different individuals (Fig. 3A). In addition, the duration of menstrual cycles differed greatly among the individuals, including those of the two sisters (subjects 1 and 2). However, as expected, in all cases, the period of the menstrual cycle shortened with age (Table 1 and Fig. 3B). Notably, in all cases with the exception of subject 6, there were intervals during which menses onsets recurred at times of the full or the new moon, meaning that the ovulations that preceded the menses onsets would have happened around the times of the new or the full moon, respectively (17, 18). In the following, we describe the courses of menstrual cycles in the six women in more detail.
The menses of subject 1 occurred around the new moon from 1979 until the middle of 1980. Between 1990 and 1995, they coupled briefly and repeatedly to the new moon without remaining stably synchronized with it: a phenomenon called “relative coordination” (28). In 1997, the menses exhibited continuous synchrony with the new moon and, from August 1998 to May 2001, with the full moon phase. Last, beginning in 2008, they displayed weak synchrony with the new moon phase until menopause, 1.5 years later. Menses of subject 2 coincided precisely with the full moon for the first 7 years of recording. Between 1993 and 1998, she showed several instances of relative coordination with the new moon phase of the synodic month until she interrupted the recording. Menses of subject 3 coincided with the full moon for 4 years, beginning in 1988. Menses of subject 4 occurred around the full moon during three periods: 1 year at the beginning, ~3 years between 1995 and 1997, and another year in 2000. In the case of subject 5, menses onsets coincided with the new moon for about 1 year after the birth of her first child in 1991 and then with the full moon at age 36. Last, when the rhythm became more irregular after age 46, it displayed three instances of relative coordination with the new moon phase of the synodic month. In contrast to the other subjects, subject 6 never clearly synchronized to the synodic month. At younger ages, the average period of her menstrual cycle was 26.0 (±4.9) days, which is already rather short. With increasing age, it shortened further to an average period of 24.7 (±2.4) days (Table 1), which is far shorter than the lunar cycles. Nevertheless, in 1996–1997, before the birth of her first child, her menses oscillated in synchrony with the time of the waxing moon phase of the synodic month. Subsequently, her two children were born around the time of the full moon (Fig. 3A).
In summary, at certain intervals, the rhythm of these individuals’ menstrual cycles appeared to oscillate in synchrony with the synodic lunar month. Menses onsets occurred close to its full moon or its new moon phase, with the full moon phase predominating (Fig. 3, A and C). This was also true for the majority of the other women who had recorded their menses at ages ≤35 years (Fig. 4 and Table 1). Only subjects 8 and 11 failed to show any obvious synchrony with the full or new moon (Fig. 4). Similar to subject 6, these two women were “night-owls” who exposed themselves to much artificial light before going to bed, and their menses had rather short periods. Nevertheless, in 2000, between the births of her two children, the menses of subject 8 oscillated in synchrony with the waxing moon phase of the synodic month for six consecutive cycles, as occurred in subject 6 (Fig. 3A). Subject 10 was not included in Fig. 4 because she kept records from age 34 onward and thus had only 1 year of records for the ≤35 years period. During this 1 year, she was not synchronous with the synodic month (Table 1). Of the subjects >35 years old, the majority was not synchronous with the synodic cycle (Table 1). In summary, we found that the menses of women ≤35 years old synchronized with the full or new moon for an average of 23.6% of the recorded time, while the menses of women >35 years old showed this synchrony for an average of 9.5% of the time (Table 1). These results indicate that the probability of synchrony with the synodic cycle decreases with age. This probability also appears to decrease with the exposure to artificial light at night: All the younger women who had limited exposure to nocturnal light showed intermittent synchrony with the full or new moon, while the three young women who were exposed to high levels of nocturnal light failed to do so (Table 1).
When synchrony occurred, circular statistics showed that the phase distributions of the menses had a preferential direction and were not uniformly distributed (Figs. 3C and 4). A polar histogram of menses’ phases that included the menses of all 22 women regardless of age and synchrony with the synodic cycle confirmed the bimodal phase distribution (Fig. 7). Furthermore, a systematic test with different hypothetical lunar cycle lengths ranging from 27 to 32 days showed that the significant results for the a priori hypothesis of synchronization with the full/new moon did not emerge by chance (fig. S1). A maximum likelihood approach for the pooled data showed that the data are best described by a bimodal distribution with peaks slightly before the full and new moon (1.87 and 0.93 days, respectively) (Fig. 7 and table S2; see Material and Methods and the Supplementary Materials for details). Circular statistics did not reveal a significant synchrony of the recorded births with full or new moon, but the last menses before a pregnancy showed a tendency to be directed to full or new moon (Rayleigh statistics: P = 0.061).
Fig. 7. Menstrual cycle phases are aligned with the phases of full/new moon and perigee/apogee, and this alignment is stronger during winter.
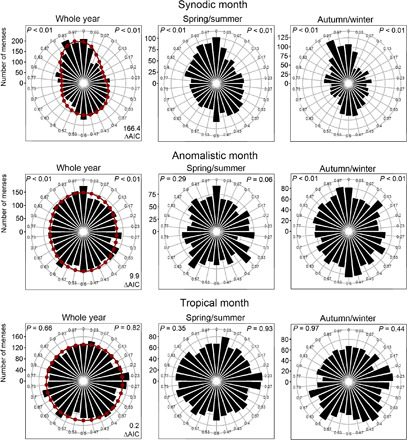
Left plots: Phase relation between menstrual cycles and the synodic, anomalistic, and tropical months. These plots combine all 4112 menses of the recorded 22 women. While menses onsets significantly align with the time of the full or the new moon and with the time of perigee or apogee, no systematic phase relation of menses to lunar standstills was observed (P values of Rayleigh tests are given at the top left and right corners). The best models of bimodal von Mises distributions are superimposed on the polar plots (red points and red curve). The parameters for these models have been estimated by a maximum likelihood approach (tables S1 to S4). The differences between the best model and a uniform distribution (null model) are expressed as ΔAIC values (right bottom corner in the plots). ΔAIC values >2 indicate peaked distributions, as found for the synodic and anomalistic months but not for the tropical month. For the full moon, the MLE (maximum likelihood model estimations) bootstrapping confidence interval for the central tendency (95% confidence interval between 0.96 and 0.98) was located left of “0,” meaning that most menses onsets occurred ~1 day before full moon (for details, see Material and Methods and the Supplementary Materials). Middle and right plots: Phase relation between menstrual cycles and the synodic, anomalistic, and tropical months separated for spring/summer (April to September) and autumn/winter (October to March). For the synodic month, the alignment with the Moon was highly significant for both times of the year but appeared stronger for autumn/winter. For the anomalistic month, the alignment was only significant for autumn/winter, while it reached no significance at any time for the tropical month.
Nevertheless, the synodic lunar cycle alone does not appear to be a strong zeitgeber because synchronization occurred only intermittently and, during any particular period, the women differed from each other in the courses of their menstrual cycles and in the timing of synodic synchronization. There were four episodes, in the second halves of 1961, 1979, 1997, and 2015, in which seven of nine women’s menstrual cycles oscillated in synchrony with the synodic month (blue shaded areas in Figs. 3A and 4). The 18+-year interval between these years corresponds to the period of a cycle of lunar eclipses that occur in September at those years, called Saros series #137. Saros #137 eclipses are distinguished by the contemporaneous occurrence of perigees that are unusually close to Earth. The conjunction of these two events may have augmented the Moon’s strength as a zeitgeber.
Relation of menses onset to the gravimetric lunar cycles
To assess whether the gravimetric forces of the Moon, which express themselves in the cycles of the tropical and anomalistic month, also affected the menstrual cycles, we plotted onsets of menses in raster plots with a period of 27.32 days and delineated 27.32- and 27.55-day recurrences of lunar standstills and perigees/apogees, respectively (Figs. 5A and 6 and fig. S2). We found that menstrual cycles temporarily appeared to follow both types of cycles. This was confirmed by periodogram analyses (Fig. 5A) and was true for the majority of the women in both age clusters (Table 1). On average, menses were synchronized with the anomalistic or tropical month during 13.1% of the time in records of women ≤35 years old and 17.7% of the time in records of women >35 years old (Table 1).
As was true for the 29.53-day synodic month, we observed a systematic phase relation across individuals between menstrual cycles and the 27.55-day anomalistic month with a significant accumulation of menses around perigee and, to a lesser extent, around apogee (Fig. 7 and table S3). Only with the tropical month did we fail to detect a systematic phase relation between menses onset and maximum/minimum lunar standstills, indicating that the tropical month is the weakest zeitgeber of the three (Figs. 5 to 7 and table S4). The interaction between all three cycles might be the source of the sudden phase jumps and period changes that we observed in the behavior of the menstrual cycles (Figs. 3 to 6), as reported previously for perigee syzygies in bipolar mood cycles (29, 30).
To evaluate the importance of variations in the Moon’s luminescence in the synchronization of menstrual cycles, we tested menses distribution separately for summer (April to September) and winter (October to March). We hypothesized that the Moon’s influence would be smaller during the short nights of summer than on the long nights of winter if luminosity plays a major role. This was the case (Fig. 7). In addition, we found that the effects of the anomalistic cycle were only significant during the winter (Fig. 7).
DISCUSSION
We show here that menstrual cycles were intermittently synchronous with the luminescence and/or gravimetric cycles of the Moon, strongly suggesting that both cycles influence reproduction in humans. On its own, each type of lunar cycle appears to be a weak zeitgeber. However, both appear to cooperate, leading to the best synchrony between lunar and menstrual rhythms when the Moon was close to Earth.
While our findings do not prove causality, several of their features support the plausibility of a causal relation between the Moon’s cycle and women’s menstrual cycles. The Moon controlled not only the frequency of the menstrual cycle but also its phase. This was the case both across individuals and within individuals over time. The fact that menses slightly precede the full moon is notable because at that time, the Moon shines brightly during evening hours when women are still awake and, consequently, more likely to be exposed to it. In addition, they are more likely to be exposed to moonlight for a longer period at that time because sleep onset occurs at its latest times during those nights (31).
Our observation that gravitation works as a zeitgeber on humans may explain why menstrual cycles, mood cycles (30), and cycles in sleep onset and sleep length (31) coupled temporarily either close to the full moon or close to the new moon since at both phases, the Moon’s gravimetric influence on Earth is similar. Effects of gravity might also account for the fact that synchrony of sleep onset and sleep duration with the lunar cycle has been observed in college students living in the light-polluted city of Seattle, where the Moon’s luminance cycle is scarcely perceivable (31). Together, these observations raise a possibility that the human organism can respond not only to fast gravitational changes, like those detected by the vestibular system, but also to slow, periodically recurring gravitational changes. These changes might be sensed indirectly through changes in geophysical variables. For example, atmospheric pressure cycles are measurable during perigee-syzygy tides (32), and there are initial reports that terrestrial plants and animals can sense them (33, 34). In addition, the Moon affects Earth’s magnetotail, thereby creating oscillating electromagnetic fields on Earth’s surface that can be sensed by animals (35, 36). Perhaps humans can sense all these types of oscillations, as well (29, 31, 35). Our data revealed that synchronization with the synodic and anomalistic months was stronger during winter. This is intuitive for the synodic month because the full moon is more conspicuous in long nights but harder to understand for the anomalistic month since variations in night length do not affect gravitation. However, the atmospheric pressure and the electromagnetic fields on Earth’s surface show annual variations (37, 38), making it possible that the sensing of lunar rhythms may be facilitated during the winter.
Comparison with earlier studies
Our principal results are consistent with the results of earlier studies on menstrual, mood, and sleep-wake cycles, which revealed that humans are sensitive to the Moon’s luminance cycle and even synchronize with it (10, 13–15, 29, 39, 40). Earlier studies showed that about 22 to 32% of reproductive age women experienced menstrual cycles with 29.5-day cycle lengths and onsets of menses around the time of the full moon (11, 12, 14, 15, 20, 23). In our study, the younger women had an average cycle length of 29.4 (SD: ±1.9) days, but we found intermittent synchrony with the synodic month even in women who had longer and shorter cycle lengths (Table 1). Most likely, the high synchrony with the Moon escaped detection in previous studies because they were not based on long-term recordings. In addition, we found that menses onsets recurred in synchrony with the synodic month not only around the time of the full moon but also around the time of the new moon. Similar to the present findings, bipolar mood cycles also have been reported to oscillate in synchrony with either the full moon phase or the new moon phase of the Moon’s luminance cycle (30), and a very recent naturalistic study of human sleep in rural and urban environments showed that most individuals went to sleep later and slept the least on nights that preceded the full moon, while others did so on nights that preceded new moon (31). Together, these observations indicate that synchronization with the lunar cycle may have changed with the modernization of our lifestyle (see below).
Comparison with other species and putative impact on reproductive physiology
Duration of the interval between ovulation and menses onset shows little variability among individuals and over age (18–20). Therefore, the time of menses onset can be used as measure of the time of the ovulation that preceded it. According to a recent study that measured menstrual characteristics of more than 600,000 menstrual cycles, the time between ovulation and menses lasts, on average, 12.4 days (26). Our finding that menses onsets that were synchronous with lunar cycles tended mainly to cluster 1 to 2 days before the full moon suggests that, in former times, humans’ most fertile phase would have clustered around the time of the new moon. There are parallels in other species. For example, Platynereis dumerilii swarms (4) and badgers, Meles meles, mate mainly during the darkest Moon phases (8), times when it would be least likely to be seen by predators. Similarly, there might have been a selection for humans who avoided predation by staying in a secure refuge around the time of the new moon, and those humans might have used this time for reproduction. Gorillas and orangutans also have menstrual cycle lengths of about 30 days, and similar to humans, the length shortens with age (41). We are not aware of any study that investigated whether their fertile phases couple to the Moon, but one study in mountain gorillas suggests that this might be the case (fig. S3) (42). It will be most promising to investigate this in more detail in the future.
While former studies found onsets of women’s menses that occurred around the time of the full moon (11, 12, 14, 15, 20, 23), consistent with the hypothesis that ovulation happened predominantly at the new moon, we found that onsets of menses also occurred around the time of new moon. Thus, something may have changed over the decades that followed earlier studies. Certainly, light pollution has increased markedly during the past decades. In addition, human lifestyles have changed. In particular, in larger cities, where humans enjoy the nightlife, many expose themselves to artificial light at night. Several studies (43–45) including ours have shown that night light shortens the length of the menstrual cycle, which, in turn, reduces the probability of synchronization with the lunar cycle as ongoing synchronization is only possible if the length of the menstrual cycle is close to that of the lunar cycle. Since menstrual cycle length appears to be an age-dependent marker of female fertility (46), our findings may prove to be relevant not only to human physiology and behavior but also to fertility and contraception.
Limitations of our study are the relatively low numbers of investigated women. It will be most interesting to investigate the temporal relation of menstrual cycles and the Moon and especially the influence of artificial light on menses cycle length in a large number of women all over the world. The availability of modern cell phone apps makes this endeavor possible. In view of the differences that we observed between responses to lunar influence in women living in different lighting environments, it will be important to investigate menstrual cycle rhythms in native populations that are not exposed to artificial light.
MATERIALS AND METHODS
Data collection and subjects
Menses data were collected over the past 15 years from healthy women who were free of contraceptive medications and had recorded the onsets of their menses out of personal interest. The women provided their records to the first and second authors after they learned of their interest in diaries of menstrual cycles. The study was not funded by third parties and had no official or specific recruiting process. All records that comprised 5 years or longer were included in the study. No other selection took place. All 22 datasets were from women who originated from the Northern Hemisphere (latitudes ranging between 41°N and 51°N) and started recording the first day of their menses at ages between 14 and 45. They all agreed to our use of their data in a scientific study. In most cases, the women sent us the data as copies of calendars, in which the onsets of menses were noted as a cross (example in fig. S4). Others sent Word files with lists of their menses onsets. We had no or only little information about special life events except for the births of children. However, we asked the women about their lifestyle and exposure to nocturnal light. Women, who lived in rural areas or boundary areas of towns with less than 100,000 inhabitants and additionally led no distinctive nightlife, were judged as exposed to low nocturnal light levels. Women without distinctive nightlife but living in middle-sized towns (~500,000 inhabitants) or in villages with few street lamps close to their bedroom were judged as exposed to medium nocturnal light levels. Women living in large cities (>1 million inhabitants) and night-active women were judged as exposed to high nocturnal light levels. Table 1 summarizes the data for all women.
Data handling
Moon phases (full and new moons, apogees and perigees, and minimal and maximal lunar standstills) were downloaded for each year between 1950 and 2020 from www.fourmilab.ch/earthview/pacalc.html and http://astropixels.com/ephemeris/moon/moondec1901.html or http://astropixels.com/ephemeris/moon/moondec2001.html and copy-pasted to Notepad++. Only the relevant information such as date (year, month, and day) and time (in full hours) was transformed into a CSV file. The CSV file was then imported into a common Microsoft SQL Server database. A spreadsheet was generated, in which the first column contained date/time (from 1 January 1950, 0000 until 31 December 2018, 2300) in 1-hour steps in consecutive lines. The next six columns contained the dates/times of lunar events (perigee, apogee, full moon, new moon, and maximal and minimal lunar standstill). The time points of lunar events were approximated to full hours and set to “1” at the relevant date/time. All other values in the columns were set to “0.” The spreadsheet with the lunar data was opened in Excel, and the onsets of menses for all women were entered manually (as “1”) in consecutive columns, respectively. Since we had no information about the exact times of menses onset, we entered “1” for each hour of the respective day. The file was then exported as a txt file and uploaded in ActogramJ (27) to display the data of each woman as raster plot. The raster plots were plotted with periods close to the synodic and tropical months together with the lunar data. This facilitated the visual detection of any correlations of lunar events with menses onsets. To display these plots in composite figures, they had to be greatly reduced in size, which made the following modifications necessary. (i) The horizontal lines indicating months in the original raster plots were removed; (ii) the dots indicating the menses onsets were manually enlarged, and (iii) phases of the lunar cycles were colored. We made all of these modifications manually in Microsoft Paint or Corel Photo-Paint.
Determination of phase jumps and menses period
All raster plots were visually inspected, and phase jumps were determined by eye. For recorded intervals at which no phase jumps occurred and the menstrual period appeared stable by visual inspection, we calculated the period length with χ2 periodogram analysis software embedded in ActogramJ (27). In addition, we determined the number of days between consecutive menses for all women and plotted these as histograms and boxplots, separately for the two age intervals ≤35 and > 35 years (Fig. 2). For each woman, we then calculated means and medians of these values during the two age intervals (Table 1).
Circular plots and Rayleigh test
For determining the phase distribution of the menses onsets in relation to the lunar phases, we plotted the data in circular plots and tested for deviation from a uniform distribution with the Rayleigh test using the statistics software R [R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: www.R-project.org/]. To do so, we had to convert the dates of menses onsets to cyclic data within the different moon cycles. This was slightly complicated by the fact that the latter is not strictly constant but varies cyclically around a certain mean (29.53 days for the synodic, 27.55 days for the anomalistic, and 27.32 days for the tropical month). Furthermore, menstrual data were provided only in full dates, i.e., with lower precision than the data for the Moon cycles. To reduce rounding artifacts and artificial clumping of data, we thus created hourly menstrual dates by assuming that they occurred in the middle of the day at 1200. These “hour-accurate” menstrual dates were then normalized to the exact length of the time interval between the previous and next full moon (or perigee or maximal lunar standstill, respectively); resulting values were then converted to radians by multiplying them by 2π. For the graphical presentation in circular plots, we split the range 0 to 2π into 30 equally sized segments (one segment is ~1 day for the synodic and ~22 hours for the anomalistic and tropical cycles). We created separate circular plots for each woman in relation to the relevant phases of the lunar cycles (full/new moon, apogee/perigee, maximum/minimum lunar standstills) (Figs. 3 to 6 and fig. S1). We also combined all data into a single graph (separately for the synodic, anomalistic, and tropical months), as well as separately for the summer and winter term (Fig. 7).
The standard Rayleigh test can discriminate between a uniform (null hypothesis) and a unimodal frequency distribution. As menstrual cycles could be locked either on the full or the new moon and thus be axial bimodal, we applied phase duplication to the data so that both data close to the full moon or close to the new moon will fall into the same residual class (thus transforming an axial bimodal into a unimodal distribution) (47). Two separate Rayleigh tests, (i) for any deviations from a uniform frequency distribution of any phase and (ii) for the particular presence of a uni- or bimodal distribution with the phases synchronized to a central parameter 0 (e.g., the full/new moon, perigee/apogee or maximum/minimum lunar standstill, respectively), were then carried out for each woman using the R package “circular” from C. Agostinelli and U. Lund: Circular Statistics (version 0.4-93) (available at https://cran.r-project.org/web/packages/circular/citation.html).
Maximum likelihood approach and model selection
In addition, we confirmed our results by a maximum likelihood approach independent of symmetry assumption for the distribution using R package CircMLE (47). To directly assess the potential bimodal distribution of menses phases and the location of parameters for the pooled data, we applied a maximum likelihood approach combined with model selection based on the Akaike information criterion (AIC) (table S1) (47). Calculations were performed with R package “CircMLE” (48).
As candidate models, we considered the model set suggested by Schnute and Groot (48), implemented in CircMLE by Fitak and Johnsen (47) and based on bimodal von Mises distributions (tables S2 to S4). These models can represent one of three types: (i) a uniform model (M1) with random onset of menses, which is the null model of the set; (ii) unimodal models (M2A, M2B, and M2C) with a single preferred phase; and (iii) bimodal models (M3A, M3B, M4A, M4B, M5A, and M5B) with two preferred phases. In addition, bimodal models can either be characterized as axial (M3A, M3B, M4A, and M4B) where preferred phases point into opposite direction or nonaxial (M5A and M5B). Models are specified by up to five parameters: mean direction (ϕ1) and concentration (κ1) for the first mode (e.g., full moon, perigee, or maximum lunar standstill), mean direction (ϕ2) and concentration (κ2) for the second mode (e.g., new moon, apogee, or minimum lunar standstill), and the relative weight of the first distribution (0 ≤ λ ≤ 1; with the second distribution fixed at fraction 1 − λ). We calculated models for menses distribution during the synodic lunar cycle (table S2), the anomalistic lunar cycle (table S3), and the tropical lunar cycle (table S4), always presenting the best to least fitting models in descending order. The best fitting models and the models assuming a uniform distribution (M1, the null model) are highlighted in bold letters, respectively. In general, models with an AIC deviation (ΔAIC) less than 2 from the best model are well supported, models with ΔAIC between 2 and 7 should rarely be dismissed, and models with ΔAIC >9 have little support (49, 50). In tables S2 to S4, all models with a ΔAIC >9 (difference from the best model) are shown in gray letters. The differences in AIC values between the best model and M1 (uniform distribution) are of specific interest because they indicate a peaked distribution when ΔAIC > 2. These ΔAIC values are given in Fig. 7, and they indicate a peaked distribution of menses during the synodic and anomalistic lunar cycles (tables S2 and S3) but not for the tropical lunar cycle (table S4).
Assessment of menses phase distance to the phases of the Moon
To assess mean phase distance of menses onset to the full/new moon, perigee and apogee, and lunar standstills, we estimated 95% confidence intervals (CI) of the central tendency by bootstrapping the maximum likelihood model estimations (from R package CircMLE) with 200 replications. The phases of new moon, apogee, and minimum lunar standstill are at 0.5 (50% of the full circle representing the oscillation period of the different lunar events), respectively, and that of full moon, perigee, and maximum lunar standstill are at 0/1 (0/100% of the full circle), respectively [mean direction (full moon): lower CI = 0.95 (28.2 days) and upper CI = 0.99 (29.1 days); mean direction (new moon): lower CI = 0.45 (13.4 days) and upper CI = 0.49 (14.4 days); mean direction (perigee): lower CI = 0.95 (26.1 days) and upper CI = 1.04 (28.6 days); mean direction (apogee): lower CI = 0.45 (12.4 days) and upper CI = 0.54 (14.8 days); and mean direction (lunar standstills): lower CI = 0.01 (0.30 days) and upper CI = 0.91 (24.7 days)]
For the synodic and the anomalistic cycles, the average phases of menses onset lie before full moon and perigee, respectively. However, the 95% CI of mean menses onset does exclude the 0/1 direction only for the synodic cycle. This provides strong evidence that mean onset of menses is, in fact, slightly earlier than full/new moon in this case. While the 95% CI is small for the synodic lunar cycle and of intermediate size for the anomalistic cycle, it is very large for the tropical lunar cycle. Menses onsets do not happen at a specific phase in the tropical cycle, as already shown in the polar plot and by modeling.
Rayleigh tests to exclude accidental significances
To test whether the significant bidirectionality (synchronization) detected for the synodic and anomalistic lunar cycles did not occur just by chance, we performed additional Rayleigh tests based on the empirical menstrual cycle data but under the assumption that the lunar cycle would have fixed lengths different from the empirical means (29.53 and 27.55 days). For this purpose, we created artificial Moon cycle data with alternative lengths in the range from 27 to 32 days; we covered this range in 100 steps of 0.05 days, i.e., 72 min. We added two further cycles with the exact mean length of the tropical (27.32 days) and anomalistic (27.55 days) lunar cycle, respectively. Following the same procedures as described in our main text (cf. Fig. 7), we then carried out Rayleigh tests for unidirectionality using the phase-duplicated empirical data for the menstrual cycles but in relation to these artificial lunar cycles. As we tested particularly for the hypothesis of synchronization with the full and/or new moon and with perigee and/or apogee (the hypothesis is specific on the phase of synchronization), the exact onset of the lunar cycle in relation to the menstrual cycle data may also be relevant. We thus replicated each test five times assuming different starting moments (after 0, 23, 46, 69, and 92% of the time spanning the first period) for the onset of the lunar time series. In addition, we tested for uni-/bimodality without specifying a particular direction.
Acknowledgments
We thank all the women who contributed their data as well as K. Adelmann, I. Wenzel, B. Helfrich, and C. Förster for help with data input, spreadsheet organization, and calculations; T. Yoshii for help with chronobiological data analysis; K. Monecke for help with geophysical parameters; and J. Ache, R. Costa, T. Cambras, H. de la Iglesia, C. Valeggia, W. Engelmann, B. Helm, E. Klerman, S. Montagnese, and D. Skene for discussions and comments on the manuscript. In addition, we are very grateful to S. Habumuremyi and T. Deschner for sharing data of progesterone levels measured in the feces of mountain gorillas (shown in fig. S3). Funding: We acknowledge that we received no funding in support of this research. The publication of this article was supported by the Open Access Publication Fund of the Julius-Maximilians University of Würzburg. Author contributions: C.H.-F. initiated the study, and S.M. and C.H.-F. collected the data. C.H.-F., O.M., T.H., and I.S. analyzed the data and composed the figures. O.M. and T.H. performed the circular statistics and the modeling. S.M. did a comprehensive literature research on menstrual cycles. All authors interpreted and discussed the data, and T.A.W., C.H.-F., and S.M. designed the manuscript. T.A.W. and C.H.-F. wrote the paper with contributions from S.M., O.M., and T.H. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. IRB guidelines: We assure that we met the IRB guidelines in our study.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/5/eabe1358/DC1
REFERENCES AND NOTES
- 1.Fox H. M., Lunar periodicity in reproduction. Proc. R. Soc. B 95, 523–550 (1924). [Google Scholar]
- 2.E. Naylor, Chronobiology of Marine Organisms (Cambridge Core, 2010), doi:10.1017/CBO9780511803567. [Google Scholar]
- 3.Kronfeld-Schor N., Dominoni D., de la Iglesia H., Levy O., Herzog E. D., Dayan T., Helfrich-Förster C., Chronobiology by moonlight. Proc. R. Soc. B 280, 20123088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raible F., Takekata H., Tessmar-Raible K., An overview of monthly rhythms and clocks. Front. Neurol. 8, 189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreatta G., Tessmar-Raible K., The still dark side of the moon: Molecular mechanisms of lunar-controlled rhythms and clocks. J. Mol. Biol. 432, 3525–3546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinclair A. R. E., Lunar cycle and timing of mating season in Serengeti wildebeest. Nature 267, 832–833 (1977). [Google Scholar]
- 7.Canivenc R., Bonnin M., Delayed implantation is under environmental control in the badger (Meles meles L.). Nature 278, 849–850 (1979). [DOI] [PubMed] [Google Scholar]
- 8.Dixon D. R., Dixon L. R. J., Bishop J. D., Pettifor R. A., Lunar-related reproductive behaviour in the badger (Meles meles). Acta Ethol. 9, 59–63 (2006). [Google Scholar]
- 9.Yonezawa T., Uchida M., Tomioka M., Matsuki N., Lunar cycle influences spontaneous delivery in cows. PLOS ONE 11, e0161735 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vollman R. F., The menstrual cycle. Major Probl. Obstet. Gynecol. 7, 1–193 (1977). [PubMed] [Google Scholar]
- 11.Treloar A. E., Boynton R. E., Behn B. G., Brown B. W., Variation of the human menstrual cycle through reproductive life. Int. J. Fertil. 12, 77–126 (1967). [PubMed] [Google Scholar]
- 12.Treloar A. E., Menstrual cyclicity and the pre-menopause. Maturitas 3, 249–264 (1981). [DOI] [PubMed] [Google Scholar]
- 13.Cutler W. B., Lunar and menstrual phase locking. Am. J. Obstet. Gynecol. 137, 834–839 (1980). [DOI] [PubMed] [Google Scholar]
- 14.Cutler W. B., Schleidt W. M., Friedmann E., Preti G., Stine R., Lunar influences on the reproductive cycle in women. Hum. Biol. 59, 959–972 (1987). [PubMed] [Google Scholar]
- 15.Friedmann E., Menstrual and lunar cycles. Am. J. Obstet. Gynecol. 140, 350 (1981). [DOI] [PubMed] [Google Scholar]
- 16.Menaker W., Menaker A., Lunar periodicity in human reproduction: A likely unit of biological time. Am. J. Obstet. Gynecol. 77, 905–914 (1959). [DOI] [PubMed] [Google Scholar]
- 17.Menaker W., Lunar periodicity with reference to live births. Am. J. Obstet. Gynecol. 98, 1002–1004 (1967). [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S.-i., Shirahashi K., Novel perspectives on the influence of the lunar cycle on the timing of full-term human births. Chronobiol. Int. 37, 1082–1089 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Foster R. G., Roenneberg T., Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 18, R784–R794 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Cutler W. B., Garcia C. R., Krieger A. M., Sexual behavior frequency and menstrual cycle length in mature premenopausal women. Psychoneuroendocrinology 4, 297–309 (1979). [DOI] [PubMed] [Google Scholar]
- 21.Coughenour C. L., Archer A. W., Lacovara K. J., Tides, tidalites, and secular changes in the Earth–Moon system. Earth Sci. Rev. 97, 59–79 (2009). [Google Scholar]
- 22.Archer A. W., Reliability of lunar orbital periods extracted from ancient cyclic tidal rhythmites. Earth Planet. Sci. Lett. 141, 1–10 (1996). [Google Scholar]
- 23.Vollman R. F., The degree of variability of the length of the menstrual cycle in correlation with age of woman. Gynaecologia 142, 310–314 (1956). [DOI] [PubMed] [Google Scholar]
- 24.Sherman B. M., Korenman S. G., Hormonal characteristics of the human menstrual cycle throughout reproductive life. J. Clin. Invest. 55, 699–706 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenton E. A., Landgren B. M., Sexton L., Harper R., Normal variation in the length of the follicular phase of the menstrual cycle: Effect of chronological age. Br. J. Obstet. Gynaecol. 91, 681–684 (1984). [DOI] [PubMed] [Google Scholar]
- 26.Bull J. R., Rowland S. P., Scherwitzl E. B., Scherwitzl R., Danielsson K. G., Harper J., Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2, 83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid B., Helfrich-Förster C., Yoshii T., A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J. Biol. Rhythms 26, 464–467 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Roenneberg T., Merrow M., The circadian clock and human health. Curr. Biol. 26, R432–R443 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Wehr T. A., Bipolar mood cycles associated with lunar entrainment of a circadian rhythm. Transl. Psychiatry 8, 151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehr T. A., Bipolar mood cycles and lunar tidal cycles. Mol. Psychiatry 23, 923–931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casiraghi L., Spiousas I., Dunster G., McGlothlen K., Fernández-Duque E., Valeggia C., de la Iglesia H., Moonstruck sleep: Synchronization of human sleep timing with the moon cycle under natural conditions. Sci. Adv. 7, eabe0465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.N. Sidorenkov, Y. Perevedenzev, Perygee-syzygy tides in the atmosphere. Curr. Trends Civil Struct. Engin. 2, (2019); https://irispublishers.com/ctcse/fulltext/perygee-syzygy-tides-in-the-atmosphere.ID.000540.php.
- 33.Barlow P. W., Leaf movements and their relationship with the lunisolar gravitational force. Ann. Bot. 117, 215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portugal S. J., White C. R., Frappell P. B., Green J. A., Butler P. J., Impacts of “supermoon” events on the physiology of a wild bird. Ecol. Evol. 9, 7974–7984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bevington M., Lunar biological effects and the magnetosphere. Pathophysiology 22, 211–222 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Cresci A., Durif C. M., Paris C. B., Thompson C. R. S., Shema S., Skiftesvik A. B., Browman H. I., The relationship between the moon cycle and the orientation of glass eels (Anguilla anguilla) at sea. R. Soc. Open Sci. 6, 190812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jónsson T., Miles M. W., Anomalies in the seasonal cycle of sea level pressure in Iceland and the North Atlantic Oscillation. Geophys. Res. Lett. 28, 4231–4234 (2001). [Google Scholar]
- 38.Malin S. R. C., Winch D. E., Annual variation of the geomagnetic field. Geophys. J. Int. 124, 170–174 (1996). [Google Scholar]
- 39.Cajochen C., Altanay-Ekici S., Münch M., Frey S., Knoblauch V., Wirz-Justice A., Evidence that the lunar cycle influences human sleep. Curr. Biol. 23, 1485–1488 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Della Monica C., Atzori G., Dijk D.-J., Effects of lunar phase on sleep in men and women in Surrey. J. Sleep Res. 24, 687–694 (2015). [DOI] [PubMed] [Google Scholar]
- 41.C. E. Graham, Reproductive Biology of the Great Apes (Elsevier, 1981); https://linkinghub.elsevier.com/retrieve/pii/B9780122950209X50018.
- 42.Habumuremyi S., Stephens C., Fawcett K. A., Deschner T., Robbins M. M., Endocrine assessment of ovarian cycle activity in wild female mountain gorillas (Gorilla beringei beringei). Physiol. Behav. 157, 185–195 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Lin M. C., Kripke D. F., Parry B. L., Berga S. L., Night light alters menstrual cycles. Psychiatry Res. 33, 135–138 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Rex K. M., Kripke D. F., Cole R. J., Klauber M. R., Nocturnal light effects on menstrual cycle length. J. Altern. Complement Med. 3, 387–390 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Danilenko K. V., Shortening of the menstrual cycle following light therapy in seasonal affective disorder. Psychiatry Res. 153, 93–95 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Brodin T., Bergh T., Berglund L., Hadziosmanovic N., Holte J., Menstrual cycle length is an age-independent marker of female fertility: Results from 6271 treatment cycles of in vitro fertilization. Fertil. Steril. 90, 1656–1661 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Fitak R. R., Johnsen S., Bringing the analysis of animal orientation data full circle: Model-based approaches with maximum likelihood. J. Exp. Biol. 220, 3878–3882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnute J. T., Groot K., Statistical analysis of animal orientation data. Animal Behav. 43, 15–33 (1992). [Google Scholar]
- 49.Burnham K. P., Anderson D. R., Multimodel Inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004). [Google Scholar]
- 50.Burnham K. P., Anderson D. R., Huyvaert K. P., AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/5/eabe1358/DC1


