A self-calibrated flexible epidermal biomicrofluidic device is proposed for wearable accurate blood glucose monitoring.
Abstract
This paper reports a flexible electronics-based epidermal biomicrofluidics technique for clinical continuous blood glucose monitoring, overcoming the drawback of the present wearables, unreliable measurements. A thermal activation method is proposed to improve the efficiency of transdermal interstitial fluid (ISF) extraction, enabling extraction with a low current density to notably reduce skin irritation. An Na+ sensor and a correction model are proposed to eliminate the effect of individual differences, which leads to fluctuations in the amount of ISF extraction. An electrochemical sensor with a 3D nanostructured working electrode surface is designed to enable precise in situ glucose measurement. A differential structure is proposed to eliminate the effect of passive perspiration, which leads to inaccurate blood glucose prediction. Fabrications of the epidermal biomicrofluidic device including formation of flexible electrodes, nanomaterial modification, and enzyme immobilization are fully realized by inkjet printing to enable facile manufacturing with low cost, which benefits practical production.
INTRODUCTION
Continuous blood glucose monitoring, which avoids missing information about important blood glucose changes, holds great significance for the diagnosis and treatment of diabetes (1). Especially, it has the potential to realize closed-loop treatment cooperating with an insulin pump (2). Present companies that elaborate on continuous blood glucose monitoring mainly include Dexcom (3), MiniMed (4), and Abbott (5). They enable minimally invasive continuous blood glucose monitoring using implantable enzymatic sensors to measure glucose concentration in interstitial fluid (ISF) because glucose concentration in ISF is closely related to that in blood (6, 7). However, the inherent in-body bioelectricity is easy to cause sensor signal drift, thus significantly affecting measurement precision (8, 9). In addition, biomacromolecule binding that causes foreign body reaction is hardly prevented. These inherent drawbacks make implantable products difficult to be widely promoted in clinics. The emerging wearable technology, especially for the fast-growing flexible electronics (10), brings opportunities to enable glucose monitoring outside the body, which is promising for practical application and large-scale clinical promotion.
The present research for blood glucose predication based on flexible electronics is mainly focused on sweat analysis. For example, Gao’s group (11) and Rogers’ group (12) tried to detect sweat glucose by wearable devices thus to indicate blood glucose. This kind of method is noninvasive. However, continuous sweat acquisition needs additional incentives such as sports or medicine activation (13). Most importantly, sweat glucose is not closely related to that in blood. Thus, blood glucose prediction based on sweat glucose monitoring is not reliable enough to be applied in clinics. Bandodkar et al. (14) and Chen et al. (15) tried to extract ISF by flexible electrodes and then detect the glucose in situ to enable wearable glucose monitoring. This concept is promising; however, the limited times of measurement and unreliable blood glucose predictions by the present wearables make them difficult to be used in clinics. The following problems of clinical wearable continuous blood glucose monitoring remain to be solved: (i) skin irritation caused by long-term ISF extraction, (ii) fluctuations in the amount of ISF extraction due to individual differences, (iii) precise capture of hypoglycemia, and (iv) effect of passive perspiration on the glucose monitoring.
Here, a flexible electronics-based epidermal biomicrofluidics technique is proposed to enable continuous blood glucose monitoring, overcoming the shortcomings of the present wearable devices, unreliable measurements, thereby satisfying the clinical diagnosis and treatment of diabetes. A thermal activation method, a precise in situ glucose measurement method, and a differential correction method are proposed to address the unreliable detection. In addition, the manufacturing of the biomicrofluidic device was also investigated. The device was fully fabricated by the direct-writing technique of inkjet printing (16), including formation of flexible electrodes, in situ modification of nanomaterials, and immobilization of enzyme molecules. The full printing process enables facile manufacturing with low cost, which is beneficial for practical production.
RESULTS AND DISCUSSIONS
Design of the epidermal biomicrofluidic device
A flexible epidermal biomicrofluidic device was developed to enable continuous blood glucose monitoring. As shown in Fig. 1A, the device can be tightly attached to the skin surface, similar to Band-Aid, to obtain information on the variation in the blood glucose concentration. The flexible device will not affect the normal activities of the human body, which is of great convenience for application. In addition, the flexible device can deform with the movement of the skin, which avoids discontinuity of the ISF extraction caused by the relative movement between the device and the skin, thereby facilitating transdermal ISF extraction. Figure 1B shows the structure of the epidermal biomicrofluidic device, which comprises two parts: the temperature control component and the glucose detection patch. Figure 1C shows the photo of the fabricated glucose detection patch. Figure 1D shows the structure of the temperature control component, which consists of two symmetrical heating wires and two symmetrical temperature sensors. As shown in Fig. 1B, the heating wire and temperature sensor would be attached to the back face of the ISF extraction electrode (EE) pair to enable thermal activation and temperature maintenance. Figure 1E shows the structure of the glucose detection patch, which consists of an ISF EE pair [EE and auxiliary electrode (AE); more details in Fig. 2 and fig. S1], a flexible electrochemical glucose sensor [working electrode (WE) and reference electrode/counter electrode (RE/CE); more details in Fig. 3], and a flexible differential Na+ sensor [Na+ WE (SWE) and Na+ RE (SRE); more details in Fig. 4]. Specifically, inkjet printing was used to enable facile micromanufacturing of multilayer structures on a flexible polyimide (PI) substrate to construct functional flexible electronics of the epidermal biomicrofluidic device for continuous blood glucose monitoring (more details in Materials and Methods). The full printing process enables fabrication with low cost, which benefits practical production. A four-path inkjet printing system was built to fabricate flexible electronics, as shown in fig. S2.
Fig. 1. Design of the flexible epidermal biomicrofluidic device for continuous blood glucose monitoring.
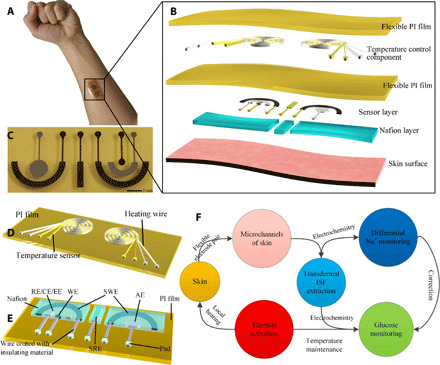
(A) Photo of the real application of the proposed device. (B) Detailed structure of the integrated epidermal biomicrofluidic device. (C) Photo of the fabricated flexible glucose detection patch. (D) Detailed structure of the temperature control component. (E) Detailed structure of the glucose detection patch consisting of an ISF extraction electrode pair (EE, extraction electrode; AE, auxiliary electrode), a glucose sensor (WE, working electrode; RE/CE, reference electrode/counter electrode), and a differential Na+ sensor (SWE, Na+ WE; SRE, Na+ RE). (F) Working mechanism of the integrated flexible epidermal biomicrofluidic device (photo credit: Zhihua Pu, Tianjin University).
Fig. 2. Thermal activation to improve the efficiency of transdermal ISF extraction.
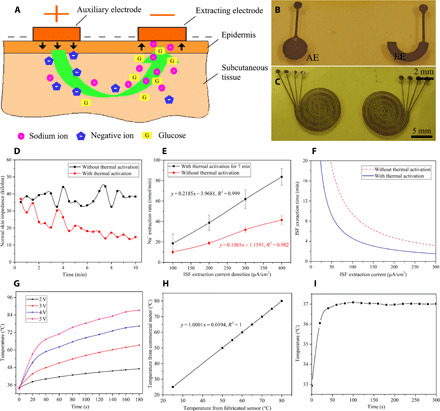
(A) Schematic diagram of the mechanism of transdermal ISF extraction and the formation of the epidermal biomicrofluidic system. (B) Photo of the fabricated flexile ISF EE pair. (C) Photo of the fabricated flexible heating wires and temperature sensors. (D) Normal skin impedance changes over time with and without thermal activation. (E) Correlations between the ISF extraction rate and extraction current density with and without thermal activation. (F) Correlations between the ISF extraction time and extraction current density with and without thermal activation when the desired amount of extracted Na+ is 100 nmol. (G) The temperature change with time under different applied voltages. (H) Temperature measurement results from the fabricated sensor and the commercial sensor. (I) Temperature test results from the temperature sensor when the fabricated epidermal temperature control component is in operation (photo credit: Zhihua Pu, Tianjin University).
Fig. 3. Characterization of the fabricated flexible epidermal electrochemical glucose sensor.
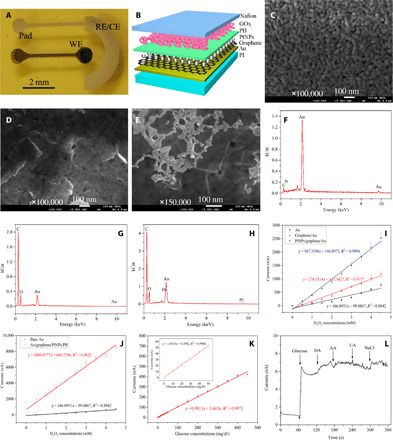
(A) Prototype of the fabricated flexible electrochemical glucose sensor including a WE and a CE (also serving as the RE). (B) Cross-sectional structure of the WE of the glucose sensor, including the flexible polyimide (PI) film substrate; gold electrode layer to transfer electrons to the detector; three-dimensional nanostructure of graphene and platinum nanoparticles (PtNPs) to enhance the electron transfer rate to improve the sensitivity; Prussian blue (PB) layer to reduce the working potential versus RE to 0 V to reduce the interference currents; GOx layer to enable selective detection of glucose; and Nafion layer to prevent GOx leakage, acting as an electrochemical reaction microtank, and make the device biocompatible with skin. (C) Field emission scanning electron microscopy (SEM) image of the Au WE surface. (D) SEM image of the graphene/Au WE surface. (E) SEM image of the PtNPs/graphene/Au WE surface. (F) Energy-dispersive spectroscopy (EDS) of the Au WE surface. (G) EDS of the graphene/Au WE surface. (H) EDS of the PtNPs/graphene/Au WE surface. (I) Amperometric responses to the addictive 0.5 mM H2O2 at an applied potential of −0.2 V versus RE for the different sensor configurations without modified PB layer (n = 5). (J) Amperometric responses of the sensor configuration with the PtNPs/graphene/Au WE before and after printing of the PB layer (n = 5). (K) Amperometric measurements of glucose solutions via the proposed flexible sensor (n = 6). (L) Selectivity of the glucose sensor [the addition of different analytes to 0.1 M PBS: 0.2 mM glucose, 0.1 mM dopamine (DA), 0.1 mM ascorbic acid (AA), and 0.1 mM uric acid (UA)] (photo credit: Zhihua Pu, Tianjin University).
Fig. 4. In vivo experiments using the proposed epidermal biomicrofluidic technique and the device.
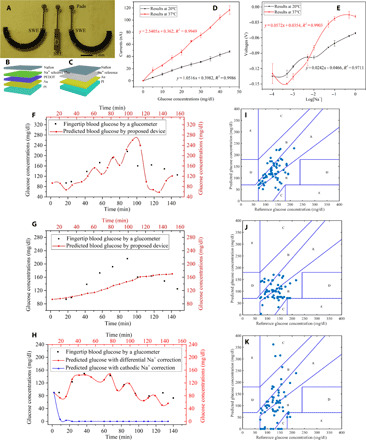
(A) Photo of the fabricated flexible differential Na+ sensor. (B) Structure of the WE of the Na+ sensor, from the bottom-up, includes a flexible PI substrate layer, a gold electrode layer, a poly(3,4-ethylenedioxythiophene) (PEDOT) layer, an Na+-selective membrane layer, and a Nafion layer. (C) Structure of the RE of the Na+ sensor, from the bottom-up, includes a flexible PI layer, a gold electrode layer, an Na+ reference membrane layer, and a Nafion layer. (D) Amperometric measurements in glucose solutions for the proposed flexible electrochemical glucose sensor at room temperature and 37°C (n = 6). (E) Test of the Na+, showing voltage changes as the Na+ concentration at room temperature and 37°C (n = 6). (F) Glucose measurement results from the fabricated epidermal biomicrofluidic device, and the fingertip blood glucose measured by a commercial glucometer (for one volunteer). The glucose concentrations obtained by the proposed system are delayed by approximately 10 min compared with those obtained by the glucometer due to the physiological delay between the glucose concentrations in ISF and in blood (36). (G) Glucose detection results without differential Na+ correction (for the same volunteer). (H) Glucose detection results with differential Na+ correction and with cathodic Na+ correction (for another volunteer who is sweaty). (I) Plot of predicted glucose concentration versus reference glucose concentration on the Clarke error grid with differential Na+ correction. (J) Plot of predicted glucose concentration versus reference glucose concentration on the Clarke error grid without correction. (K) Plot of predicted glucose concentration versus reference glucose concentration on the Clarke error grid with cathodic Na+ correction only (results from all seven volunteers) (photo credit: Zhihua Pu, Tianjin University).
Figure 1F shows the working mechanism of the epidermal biomicrofluidic device for continuous blood glucose monitoring: First, the temperature control component is activated to locally heat the skin and maintain the temperature to enable thermal activation, thus promoting skin permeability to facilitate the efficiency of transdermal ISF extraction. Second, a constant current is applied to the EE pair to transdermally extract ISF from subcutaneous tissue through the ion transport microchannels within the skin. Then, the flexible electrochemical glucose sensor is used to detect the glucose concentration in the extracted ISF (cathode) in situ, and the differential Na+ sensor is used to detect the Na+ concentrations in the cathode and anode in situ. Last, the corrected blood glucose concentration is obtained based on the correction model (details in the Supplementary Materials). Specifically, the temperature control component works all the time to maintain the temperature and thus facilitate electrochemical detection. The temperature maintenance makes the electrochemical measurements more reliable, avoiding temperature compensation (17), which is currently frequently used.
Thermal activation to facilitate ISF extraction
As shown in Fig. 2A, when the two electrodes providing the electric field are implemented as flexible electrodes, the flexible electrode pair (Fig. 2B) for reverse iontophoresis in cooperation with the ion transport microchannels formed within the skin under the effect of a weak electric field constitutes a flexible epidermal biomicrofluidic system to enable transdermal ISF extraction (18) (more details in the Supplementary Materials). Here, a thermal activation method is proposed to promote skin permeability, which, in turn, increases the efficiency of transdermal ISF extraction. This method is realized by locally heating the skin to 37°C and maintaining this temperature (more details in Fig. 2C and Materials and Methods). The proposed method will reduce the extraction current density and shorten the extraction time while extracting enough ISF to meet the requirements of glucose monitoring, thereby effectively reducing the skin irritation caused by the extraction process.
The efficiency of transdermal ISF extraction is closely related to the skin permeability. Normal skin impedance was proven to be an important indicator of skin permeability (19). Therefore, normal skin impedance experiments were conducted firstly to verify the possibility of the proposed thermal activation method by a noninvasive three-electrode measurement method (20). The measurement method, setup, and data are shown in figs. S4 to S6 and tables S1 and S2. Figure 2D shows the normal skin impedance changes over time with and without thermal activation. As shown in Fig. 2D and tables S1 and S2, the normal skin impedance rapidly decreases in the first two and a half minutes and tends to stabilize approximately 7 min after starting heating. The impedance is reduced by 23 kilohms compared with that of approximately 38 kilohms at room temperature, which indicates that the skin permeability is significantly improved by the proposed thermal activation method. After that, the fabricated ISF extraction pair, as shown in Fig. 2B, was attached to the skin surface to enable transdermal ISF extraction. Na+ extraction rates were used to indicate the ISF extraction rate because the cation selectivity of the skin makes Na+ dominant in the ion flow (21). The average Na+ extraction rates using different current densities and different times with and without thermal activation are shown in Fig. 2E and tables S3 and S4. These results demonstrate that the ISF extraction rate with thermal activation for 7 min is approximately two times faster than that without thermal activation, which verifies the facilitation of transdermal ISF extraction by thermal activation. Specifically, we found that passive perspiration significantly affected the Na+ measurement, thus affecting the prediction of extracted ISF, as shown in table S5; therefore, a differential Na+ correction method was used to eliminate the effect of passive perspiration (the data used in Fig 2E have excluded the Na+ information of passive perspiration; more details are shown in the Supplementary Materials). If we want to extract the desired amount of Na+ (100 nmol here, enough for detection), various extraction currents and times can be used to achieve this goal; the correlations between them are shown in Fig. 2F. The results verify that the efficiency of transdermal ISF extraction can be significantly improved by thermal activation. Transdermal ISF extraction using low current densities for a short time can be enabled, thereby significantly reducing skin irritation, and shortening the single measurement period.
An epidermal temperature control component was designed to enable thermal activation and temperature maintenance. The design basis, simulation of heating and temperature maintaining, and proportional-integral-differential (PID) control circuit are shown in the Supplementary Materials in detail. Figure 2C shows the photo of the fabricated flexible heating wires and temperature sensors. Figure 2G shows the temperature change with time under different applied voltages, exhibiting the effectiveness of the designed and fabricated heating wire. Figure 2H shows the comparison of the temperature measurement results from the commercial temperature sensor and the fabricated temperature sensor. The experiments exhibit extraordinary compliance between the fabricated and commercial temperature sensors. Figure 2I shows the temperature test results from the temperature sensor when the fabricated epidermal temperature control component was in operation. This result demonstrates that the designed epidermal temperature control component can heat the epidermis to 37°C in 1.5 min and maintain the temperature with a fluctuation of less than 0.1°C.
Flexible epidermal electrochemical glucose sensing
An epidermal electrochemical glucose sensor that could be integrated with the ISF EE pair was designed to achieve in situ measurement, thus improving the detection accuracy. The detection mechanism and the reaction equations are shown in the Supplementary Materials. Figure 3A shows the photo of the fabricated glucose sensor, which is a two-electrode–based electrochemical sensor. Figure 3B shows the WE structure of the sensor. Because of the stability of Ag/AgCl electrode, the glucose sensor was integrated with the ISF EE pair by sharing the same Ag/AgCl electrode as the CR, RE, and EE to simplify the structure of the epidermal device.
Because the amount of extracted glucose molecules is small and difficult to accurately detect, we used graphene and platinum nanoparticles (PtNPs) to construct a three-dimensional (3D) nanostructure on the WE surface to improve the performance of the sensor, thus enabling the detection of low glucose concentrations. We used inkjet printing to construct the 3D nanostructure on the WE surface. This type of micromanufacturing technology enables maskless direct writing of nanomaterials at set positions and is thus beneficial for modification of tiny areas. The topographies of each layer of the printed WE surface (Au, graphene/Au, and PtNPs/graphene/Au) are shown in Fig. 3 (C, D, and E), respectively. The energy-dispersive spectroscopy (EDS) for each WE configuration is displayed in Fig. 3 (F, G, and H), respectively. These results indicate that the 3D nanostructure consisting of graphene and PtNPs was successfully constructed by inkjet printing. A nanometer-thick Prussian blue (PB) layer was also printed on the constructed 3D nanostructure to reduce the working potential and thus shielded the effect of interferents (15). Inkjet printing enables modification at low temperature; thus, glucose oxidase (GOx) was also deposited on the WE surface by this method, which maintains the activity of the GOx that is important for the glucose sensor.
H2O2 aqueous solutions were used to characterize the printed microelectrodes first before immobilizing GOx. The usability of the fabricated electrodes was verified by the cyclic voltammetry (CV) method first, as shown in fig. S14. After that, each configuration of the WE was tested using the amperometric technique. Figure 3I shows the amperometric responses for the various sensor configurations (without a deposited PB layer) to successive increments of 0.5 mM H2O2 at an applied potential of −0.2 V versus RE. All of the sensors demonstrate good linearity from 0 to 4.5 mM H2O2, while the PtNPs/graphene/Au WE exhibits the highest sensitivity. As shown in Fig. 3I, the sensor sensitivity increases by 3.42 times when the 3D nanostructure is constructed, similar to the results of other work (22, 23). Figure 3J shows the typical amperometric responses of the sensor configurations with the PtNPs/graphene/Au WE before and after printing of the PB layer to successive increments of 0.5 mM H2O2 at applied potentials of −0.2 and 0 V versus RE, respectively. As shown in Fig. 3J, the sensor sensitivity increases by 3.19 times when the PB layer is printed. Therefore, these experimental results exhibit the sensor sensitivity increases by 10.74 times when all the nanomaterials are printed.
The sensitivity and linearity of the fabricated flexible electrochemical sensor in response to changing glucose concentrations were determined after GOx immobilization. Figure 3K shows typical amperometric responses of the fabricated sensor to successive increments of glucose (5 mg/dl) using an applied potential of 0 V versus RE. As shown in Fig. 3K, the linear range of glucose measurements obtained using the fabricated glucose sensor is 0 to 400 mg/dl, and the detection limit is 0.52 mg/dl (S/N = 3). The results demonstrate that the proposed flexible electrochemical sensor can accurately detect glucose in ISF within the physiological range (24), also demonstrating the potential for hypoglycemia detection. The selectivity of the sensor was evaluated by adding dopamine (DA), ascorbic acid (AA), and uric acid (UA) to the analytical solutions (25), as shown in Fig. 3L.
Differential Na+ correction and in vivo experiments
The skin permeability fluctuates for different individuals and different body parts, which leads to fluctuation in the amount of ISF extraction, and the passive perspiration has been proven to significantly affect blood glucose prediction. Thus, a correction method is needed to eliminate the effect of individual differences and passive perspiration. Because glucose molecules are extracted mainly based on the Na+ flow, a differential Na+ sensor is designed to overcome these challenges. The glucose measurement correction model based on Na+ monitoring is described in the Supplementary Materials in detail. The working principle of the Na+ sensor is shown in fig. S15. Figure 4A shows the photo of the fabricated flexible differential Na+ sensor. The sensor includes two duplicate WEs (Fig. 4B) and a shared RE (Fig. 4C). The fabricated Na+ sensor was characterized using NaCl aqueous solutions as shown in fig. S16. A pig skin simulation experiment was conducted to verify the effectiveness of the Na+ correction in the elimination of individual differences (more details in the Supplementary Materials).
Because the working temperature is maintained at 37°C and significantly affects the response of the electrochemical measurement, the glucose sensor and the Na+ sensor were characterized at 37°C before in vivo experiments. Figure 4D shows the amperometric responses of the glucose sensor to successive increments of glucose (5 mg/dl) at room temperature and 37°C. Figure 4E shows the voltage change with Na+ concentration variation at room temperature and 37°C. The new functions will be used in the real applications.
Last, in vivo glucose monitoring experiments were conducted to verify the proposed epidermal biomicrofluidic technique. Oral glucose tolerance test (OGTT) experiments (26) were used to characterize the proposed flexible epidermal biomicrofluidic device. The setup for the experiments is shown in fig. S19. Seven volunteers participated in the OGTT experiments. The measurement results of the proposed epidermal biomicrofluidic device and the commercial glucometer are shown in Fig. 4F (for one volunteer). This figure shows that the results obtained by the proposed epidermal biomicrofluidic device exhibit great compliance with those obtained by the commercial glucometer when the differential Na+ correction is used. However, the results without the differential Na+ correction show a large difference, as shown in Fig. 4G (for the same volunteer). To verify the effect of the differential correction, the glucose detection results with differential Na+ correction and with only cathodic Na+ correction are shown in Fig. 4H (for another volunteer who is sweaty), respectively. The results with only cathodic Na+ correction show a large difference from those obtained by the commercial glucometer. The results exhibit that passive perspiration significantly affects the measurements especially for the sweaty volunteers, and this effect can be eliminated by the differential Na+ correction.
Clarke error grid (27) was obtained to evaluate the prediction accuracy as shown in Fig. 4 (I to K) (the results were obtained from all seven volunteers). The Clarke error grid consists of five parts based on the probable treatment results according to the predicted glucose concentrations, and the definitions for the respective parts are as follows: A zone, clinically accepted accurate readings (no more than 20% deviation); B zone, moderate readings that are outside 20% but would not lead to inappropriate treatment; C zone, readings that would lead to unnecessary treatment; D zone, readings that would result in a potentially dangerous failure when used in blood glucose correction; and E zone, false readings that would cause confusion in treatment of hypoglycemia and hyperglycemia. As illustrated in Fig. 4I, all the predicted values fall into the A and B zones (61.54% in A and 38.46% in B), which indicates that the proposed epidermal biomicrofluidic device can robustly and accurately predict the blood glucose concentrations in the physiological range. To further indicate the effect of differential Na+ correction, Fig. 4 (J and K) shows the predicted results without differential Na+ correction and the predicted results with cathodic Na+ correction only. The values in Fig. 4J fall into the A, B, and E zones (33.85% in A, 61.54% in B, and 4.61% in E). The values in Fig. 4K fall into the A, B, C, and E zones (42.19% in A, 40.62% in B, 9.38% in C, and 7.81% in E). These results strongly verify that the differential Na+ correction is necessary for the glucose detection technique based on reverse iontophoresis. The effects of individual differences and passive perspiration are successfully eliminated by the differential Na+ correction.
In addition, the robustness and flexibility of the device were verified by the bending experiments; the results (figs. S26 and S27) and the bending videos are shown in the Supplementary Materials. Comparison of this work and the present commercial products is shown in table S7.
Limitations
The current experimental results exhibited the capability of the proposed methods and device for wearable accurate blood glucose monitoring. However, as shown in Fig. 4 (F and H), although data look robust overall after correction, there is still a small part of results exhibiting deviation, which may be caused by (i) sensor instability, (ii) temperature fluctuations caused by the local heater, (iii) mechanical motions between the sensor and subject, and (iv) the induction of Na+/glucose from sweat. Fortunately, the effect of all these factors can be restrained. The sensor instability can be restrained by optimization of the manufacturing procedures. The optimization of the PID control program can inhibit the temperature fluctuations caused by the local heater. The mechanical motions between the sensor and subject can be restricted by addition of a biocompatible adhesive layer to the sensor substrate. The effect of induction of Na+/glucose from sweat can be restrained by optimization of the differential Na+ correction model.
Here, a flexible electronics-based epidermal biomicrofluidics technique is proposed to enable continuous blood glucose monitoring, overcoming the shortcomings of the present wearable devices, unreliable measurements. A novel thermal activation method was proposed to enhance skin permeability to enable transdermal extraction of enough ISF in a short period of time under a small current density, thereby effectively reducing skin irritation caused by long-term extraction and shortening the single measurement time. The skin impedance detection experiment and the ISF extraction experiment verified the facilitation effect of the proposed thermal activation method. To enable thermal activation of human skin, a flexible epidermal temperature control component was designed and fabricated. The component also maintained the temperature at 37°C, thereby facilitating electrochemical detection and avoiding temperature compensation. A flexible epidermal electrochemical glucose sensor was designed for integration with the flexible ISF EE pair to enable glucose detection in situ. To overcome the challenge of detecting low glucose concentrations, a 3D nanostructure of graphene and PtNPs was constructed on the WE surface of the sensor to improve the sensitivity, thus enabling hypoglycemia detection. A flexible differential Na+ sensor was designed and fabricated to eliminate the effect of passive perspiration and individual differences, which leads to fluctuations in the amount of ISF extraction. According to the minor changes in the Na+ concentration in ISF, an Na+ correction model was constructed to eliminate the effect of individual differences due to the amount of extracted ISF that could be reflected by the Na+ sensor. The effect of passive perspiration was eliminated by the differential measurement of Na+. Last, an integrated flexible epidermal biomicrofluidic device was fabricated for continuous blood glucose monitoring. The device was fully fabricated by inkjet printing, enabling facile manufacturing with low cost, which benefits practical production. In vivo experimental results verify that the proposed technique and device have the potential to continuously monitor blood glucose even for hypoglycemia with significantly alleviated skin irritation.
MATERIALS AND METHODS
Fabrication of the flexible epidermal biomicrofluidic device
The epidermal biomicrofluidic device was fully fabricated by inkjet printing, which is an additive manufacturing technique, with the possibility of fabrication of various-shape substrates (28). Inkjet printing enables maskless direct writing, which avoids mask manipulation and enables multilayer structure patterning, especially for in situ fabrication and modification. In addition, this technique enables low-temperature fabrication, which is beneficial for maintaining the bioactivity of graphically deposited biomaterials, which is of importance to biosensors. Therefore, inkjet printing was used to enable facile micromanufacturing of multilayer microstructures on a PI substrate to construct functional flexible electronics of the epidermal biomicrofluidic device for continuous glucose monitoring. The flexible electrical components of the biomicrofluidic system include the ISF EE pair, glucose sensor, differential Na+ sensor, heating wire, and temperature sensor. Specifically, because of the small size of the WE and the temperature tolerance of the flexible substrate, traditional methods such as chemical vapor deposition (29), sputtering (30), and self-assembly (31) are difficult to be used to facilely modify nanomaterials onto the set small positions; therefore, we used inkjet printing to construct the 3D nanostructure on the WE surface. This type of micromanufacturing technology enables maskless direct writing of nanomaterials at set positions and is thus beneficial for modification of tiny areas.
Figure S20A shows the manufacturing process of the flexible PI substrate. First, a thin polydimethylsiloxane (PDMS) layer was spin coated on glass (300 rpm, 45 s) after treatment with O2 plasma, followed by heating for 30 min at 95°C. Second, after treating the PDMS surface with O2 plasma, the PI monomer (Sigma-Aldrich) was spin coated onto it (1000 rpm, 45 s), followed by heating for 2 hours at 250°C to produce PI film (~10 μm). Then, the PI film surface was treated with O2 plasma and maintained at room temperature for 48 hours. This pretreatment made the PI surface more hydrophilic, which was beneficial for the dispersion of inkjet droplets. This method avoids coalescence of adjacent droplets to form durable electrodes. Figure S21 (A and B) shows the topography of the PI surface before and after O2 plasma treatment. As demonstrated in the atomic force microscopy images, the treated PI surface is rougher at the nanoscale, which makes it more hydrophilic. As illustrated in fig. S21 (C and D), the diameter of the printed pattern on the treated PI film is larger than those on the untreated PI film. This phenomenon verifies that the PI film is more hydrophilic after O2 plasma treatment. Figure S21 (E and F) shows photos of the lines printed on the treated and untreated PI film, respectively. The two photos verify that the O2 plasma treatment method before printing avoids coalescence of adjacent droplets to help form electrodes with the designed shape.
For the manufacturing procedures of the proposed flexible biomicrofluidic device, the two parts (the temperature control component and the glucose detection patch) were fabricated separately. For the temperature control component, the gold wires were fabricated on the pretreated flexible PI film by inkjet printing, followed by heating for 2 hours at 200°C. For the glucose detection patch, according to the function of each component and the characteristics of the inkjet printing technology, the manufacturing procedure is as shown in fig. S22. First, the flexible PI film substrate was fabricated by spin coating. Second, the gold electrode layers of the WE, SWE, and SRE were inkjet printed onto the pretreated PI substrate (the width of the wires is ~200 μm), followed by heating for 2 hours at 200°C. Third, the silver electrode layers of the EE/RE/CE and AE were ejected onto the pretreated PI substrate, followed by heating for 1 hour at 200°C. Subsequently, the electrodes were immersed in a 50 mM FeCl3 aqueous solution for 20 s to obtain the stable Ag/AgCl EE/GRE/GCE and AE. Fourth, graphene was printed onto the WE surface, followed by heating for 2 hours at 200°C. Fifth, poly(styrenesulfonate) (PSS):poly(3,4-ethylenedioxythiophene) (PEDOT) conductive ink (Sigma-Aldrich) was inkjet printed onto the SWE surface, followed by heating for 1 hour at 100°C. Sixth, nanosilicon insulating ink (Ptdots Inc., USA) was inkjet printed to cover the wires connected to each electrode. Seventh, PtNP ink was ejected onto the WE graphene layer and naturally dried at room temperature to construct a 3D nanostructure. Eighth, a nanometer-thick PB layer was printed on the constructed 3D nanostructure and naturally dried at room temperature. Ninth, GOx ink was ejected onto the WE surface and naturally dried at room temperature. Tenth, an Na+-selective solution and a reference solution were dropped onto the SWE and SRE surfaces of the Na+ sensor, respectively, and naturally dried at room temperature. Last, 40 μl of Nafion solution was separately dropped on the WE and AE, and they naturally dispersed and were dried at room temperature. In addition, to be a microreaction environment and enzyme entrapper, the Nafion layer was also used as a biocompatible layer to improve electrical contact between the skin and the electrodes and thus reduce skin irritation. This fabrication process is completed by inkjet printing, which is an attempt to solve the processing challenge of implementing integrated flexible epidermal electronic devices with low cost, benefiting practical production.
The Na+-selective membrane (11) consists of the Na ionophore X (1% weight by weight, w/w), sodium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate (0.55%, w/w), high–molecular weight polyvinyl chloride (33%, w/w), and bis(2-ethylhexyl) sebacate (65.45%, w/w). One hundred milligrams of the membrane was dissolved in 660 μl of tetrahydrofuran. The ion-selective solution was sealed and stored at 4°C. The solution for the Na+ reference membrane (11) was prepared by dissolving 79.1 mg of polyvinyl butyral resin BUTVAR B-98 and 50 mg of NaCl into 1 ml of methanol. Then, 2-mg F127 and 0.2-mg multiwall carbon nanotubes were added into the reference solution to minimize the potential drift. All the chemicals for the Na+-selective and reference membranes were purchased from Sigma-Aldrich.
Thermal activation
As shown in fig. S3, the stratum corneum (SC), the outermost layer of the skin, plays an important role in preventing loss of nutrients, water, and electrolytes in the body and is responsible for the low permeability of the skin; therefore, the SC is considered to be the largest barrier to transdermal ISF extraction. To increase the amount of transdermally extracted ISF, several methods, such as electroporation (32), adjustment of the osmotic pressure (15), and ultrasonic pretreatment (33), were used to promote skin permeability. Electroporation uses a high-voltage pulse to pretreat the skin, thus generating micropores within the skin to promote skin permeability. However, the use of a high-voltage pulse is harmful to the human body and cannot be used frequently; therefore, this type of method cannot be used for long-term monitoring. Adjustment of the osmotic pressure is enabled by injecting a reagent into the subcutaneous tissue, which may introduce unpredictable harm to the human body. Ultrasonic pretreatment also promotes skin permeability by producing micropores within the skin. The generated micropores may be larger than those produced by electroporation and can remain open for more than 1 day. However, the open micropores enable invasion of harmful substances, such as bacteria, thus endangering human health. In addition, the surfactant used in the ultrasonic treatment easily causes skin irritation (22).
Here, a thermal activation method is proposed to promote skin permeability, which, in turn, increases the efficiency of transdermal ISF extraction. This method is realized by locally heating the skin to 37°C and maintaining this temperature. According to the theory of thermal expansion and contraction, the hair pores of the human body will open at a higher temperature (for example, 37°C, normal in-body temperature), and the sweat glands connected with the hair pores will also be activated. The opened hair pores significantly promote skin permeability. Because more than 95% of the ISF is extracted through the epidermal ion transport biomicrofluidic channels composed of sweat glands and hair pores, opened trichopores and active glands can greatly increase the transdermal ISF extraction rate (34). The proposed method will reduce the extraction current density and shorten the extraction time while extracting a sufficient amount of ISF to meet the requirements of glucose monitoring, thereby effectively reducing the polarization and stimulation effect of the extraction process on the skin. In addition, in contrast with the presented methods, such as electroporation, adjustment of the osmotic pressure, and ultrasonic pretreatment, the proposed method uses a natural physiological phenomenon that does not harm the skin and is easily realized by miniaturized and integrated flexible electronics.
Normal skin impedance measurement
Normal skin impedances were measured by the three-electrode method as shown in fig. S4. The commercial Ag/AgCl wet electrode patches (ZTEMG-4001) commonly used in biophysical therapy were used to enable the resistance measurement. This type of patch can minimize the effect of electrode-to-skin contact on skin impedance measurements. For the normal skin impedance measurement without thermal activation, as shown in fig. S5, an LCR meter (Agilent Inc., USA) was used to record the skin impedances between any two electrodes of the three electrodes attached on the volunteer’s arm (a healthy male volunteer 25 years of age). Then, the normal skin impedance at a single site (site B) could be calculated. The recording parameters used for the measurement were 100 mV and 10 Hz. The impedances between any two electrodes were recorded every 30 s. After the normal skin impedance was measured at room temperature, a water bath was used to locally heat the skin and adjust its temperature to 37°C. The heating and skin impedance measurement system is shown in fig. S6. A plastic film was used to cover the volunteer’s arm where the three Ag/AgCl wet electrode patches were attached to prevent direct contact between the skin with electrodes and water. The wet electrode patches were connected to the LCR meter by copper wires. The impedances between any two electrodes were also recorded every 30 s.
Transdermal ISF extraction
The fabricated ISF extraction pair was attached to the skin surface to enable transdermal ISF extraction as shown in fig. S23. A constant current source (ZF-9, Shanghai Zheng Fang Dian Zi Dian Qi Ltd., China) was used to supply the ISF extraction current. First, ISF was extracted using different current densities and different times without thermal activation. After each extraction, the Nafion membrane on the cathode was stripped and thrown into a centrifuge tube within 4 ml of deionized (DI) water. After centrifugation, the Na+ concentration in the mixture was measured by a commercial sodium ion densimeter (Shanghai INESA Scientific Instrument Co. Ltd., China, DWS-51), and the amount of extracted Na+ could be calculated to indicate the ISF extraction rate. Second, ISF extraction experiments with thermal activation were conducted. The volunteer’s arm was first immersed in the water bath for thermal activation for 7 min. Then, the arm was removed from the water bath and quickly dried using facial tissue (a few seconds of processing rarely affected the thermal activation). Afterward, the flexible ISF EE pair was attached to the ventral aspect of the forearm, and the arm was placed on a hot plate (IKA Inc., Germany, C-MAG HS 7 C S025) (approximately 2 cm from the surface of the hot plate) to maintain the temperature of the extraction position at 37°C (the temperature of the hot plate was set as 65°C, the temperature at the position of 2 cm from the surface of hot plate would be approximately 37°C after heat transfer through air). A platinum temperature sensor was attached on the skin surface adjacent to the ISF extraction position to verify the temperature. Specifically, we found that passive perspiration significantly affected the Na+ measurement by affecting the extracted ISF; therefore, another Nafion membrane without EEs was positioned adjacent to the EEs to determine the passive perspiration effect. Then, a differential Na+ correction method was used to eliminate the effect of passive perspiration (tactual amount of extracted Na+ was obtained by using the amount of Na+ at the cathode minus that at the additional Nafion membrane).
In particular, the skin irritation caused by the ISF extraction was evaluated by the volunteers; we found that there was a clear sense of pain when extracted more than 1 min under the current density of above 400 μA/cm2, and it became unbearable when extracted more than 15 min. For the current density of 300 μA/cm2, when extracted more than 10 min, there would be a clear sense of pain, and it became unbearable when extracted more than 20 min. For the current density of 200 μA/cm2, only when extracted more than 60 min, there would be a slight itching sense, while there would be no obvious itching when the current density was below 150 μA/cm2 even if extracted to 4 hours. Therefore, 150 μA/cm2 was chosen as the extraction current density in this work.
Characterization of the temperature control component
The heating performance of the fabricated Archimedes spiral structure of gold heating wire was characterized by applying different voltages and a commercial temperature detection device (a platinum resistance temperature sensor). The fabricated temperature sensor was characterized by using a commercial temperature detection device (a platinum resistance temperature sensor), a hot plate (IKA Inc., Germany, C-MAG HS 7 C S025), and an LCR meter (Agilent Inc., USA). For the experiment, the fabricated flexible temperature sensor and commercial temperature sensor were both attached to the hot plate, which changed the temperature during the test. The commercial temperature detection device recorded the temperature from the commercial temperature sensor, while the LCR meter recorded the resistance change from the fabricated sensor to calculate the temperature change according to equation S53. The fabricated epidermal temperature control component was characterized on the skin. The PI film with the fabricated heating wire and temperature sensor, which were connected to the temperature control circuit, was placed on the skin surface.
Characterization of flexible electrochemical glucose sensor
Figure S24 shows the measurement system for in vitro experiments. The fabricated electrochemical sensor was characterized by immersing it into electrolyte solutions containing 0.1 M phosphate buffer solution (PBS) (pH 7.4, Tianjin Xiqing Jinyuanmao Biological Reagents Business Department, China) and different concentrations of glucose or H2O2 (Tianjin Jiangtian Huagong Co., China). All experiments were conducted at room temperature. During electrochemical glucose sensing, wherein glucose is catalytically decomposed by GOx, the generated H2O2 is subsequently oxidized at the electrode surface, producing a measurable current signal. Therefore, an H2O2 aqueous solution was used to characterize the printed microelectrodes first before immobilizing GOx. First, 5 mM K3[Fe(CN)6] aqueous solution containing 1 M NaCl and 0.1 M PBS with 1 mM H2O2 was used to characterize the electron transfer rate of the fabricated WEs with different configurations using the CV method. The working potential was scanned from −0.3 to 0.5 V with the scan rate of 0.05 V/s. Each configuration of the WE was also tested using the amperometric technique. The various sensor configurations (without deposited PB layer) were characterized by successively increasing 0.5 mM H2O2 to the determinand at an applied potential of −0.2 V versus RE. The WE with deposited PB layer was characterized by successively increasing 0.5 mM H2O2 to the determinand at an applied potential of 0 V versus RE. After immobilizing GOx, the sensor with the WE configuration of GOx/PB/PtNPs/graphene/Au was tested using amperometry. The glucose sensor was characterized by successively increasing glucose (5 mg/dl) to the 0.1 M PBS at an applied potential of 0 V versus RE. All the amperometric electrochemical experiments were conducted several times to verify the repeatability of the fabricated sensors. The selectivity of the glucose sensor was evaluated using the amperometric technique by adding DA, AA, and UA to the 0.1 M PBS, respectively.
In vivo experiments
The in vivo experiments were approved by the Ethical Committee of Tianjin University, China; the identification number (ID) of the certification is TJVE-2020-172. The certification and the related recruiting information are provided as the Supplementary Materials.
First, the flexible electrochemical glucose sensor and the flexible electrochemical Na+ sensor were characterized at 37°C before in vivo experiments. Amperometry was used to characterize the response of the fabricated flexible electrochemical sensor to changing glucose concentrations at 37°C. A water bath box was used to set the target solution temperature to 37°C. The glucose sensor was characterized by successively increasing glucose (5 mg/dl) to 0.1 M PBS under an applied potential of 0 V versus RE at 37°C. Similarly, the Na+ sensor was characterized by the open-circuit method at 37°C.
The OGTT is a glucose tolerance test used to understand the function of islet β cells and the body’s ability to regulate blood glucose (35). The OGTT is a diagnostic test for diabetes and is widely used in clinical practice. Therefore, OGTT experiments are used to characterize the proposed flexible epidermal biomicrofluidic device. The setup for the OGTT experiments is shown in fig. S19. The integrated epidermal biomicrofluidic device was attached to the skin surface of the ventral aspect of the forearm. The temperature control component was set on the skin near the epidermal biomicrofluidic device. A constant current source (ZF-9, Shanghai Zheng Fang Dian Zi Dian Qi Ltd., China) was connected with the ISF extraction pair to enable transdermal ISF extraction. A CHI workstation was used to enable glucose measurement and Na+ detection. Seven volunteers participated in the OGTT experiments. For the OGTT experiments, the volunteer should fast for 8 hours before the test. After 30 min of monitoring, the volunteer should drink a cup of glucose aqueous solution (75 g of glucose in 250 ml of water) to raise the blood glucose concentration. Then, detection occurred for approximately 2 hours. Specifically, all the devices used for OGTT experiments were first fabricated and stored in the refrigerator at 4°C, and then used one by one for each experiment. The OGTT experiments took approximately 2 weeks, and the devices were kept available. Thus, we think the shelf time of the fabricated devices is more than 2 weeks at 4°C. In addition, during the in vivo experiments, the airtight PI film accumulated fluid, which significantly affected the detection results, especially for long-term monitoring. Thus, some micropores were formed in the PI film by manual needling to make the film air permeable. For the OGTT experiments, two commercial glucometers were used to detect the fingertip blood glucose for comparison. The fingertip blood samples were collected every ~15 min. For each fingertip blood glucose detection, two commercial glucometers were both used, and the average recording was regarded as the real blood glucose. For the correction process, the calibration coefficient K1 was obtained from the first set of detection values for each individual. Different calibration coefficients were used for different people. Thus, for the real application of the proposed blood glucose monitoring technique, a single-point calibration (only one calibration for each biomicrofluidic device) based on fingertip blood glucose detection was required.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China (nos. 2018YFE0205000 and 2017YFA0205103) and the 111 Project of China (no. B07014). Author contributions: Z.P., H.Y., and D.L. designed the research. Z.P. wrote the manuscript, and all the authors commented. Z.P. and X.Z. fabricated the epidermal biomicrofluidic device and conducted the in vitro electrochemical characterization experiments. Z.P., J.T., H.C., and Y.L. performed the epidermal temperature control experiments. Z.P. derived the differential correction model and preformed the pig skin simulation experiments. Z.P. performed the in vivo experiments. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/5/eabd0199/DC1
REFERENCES AND NOTES
- 1.I. D. Federation, IDF Diabetes Atlas, (International Diabetes Federation, ed. 9, Brussels, Belgium, 2019).
- 2.Tauschmann M., Hovorka R., Technology in the management of type 1 diabetes mellitus - current status and future prospects. Nat. Rev. Endocrinol. 14, 464–475 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Larose S., Rabasa-Lhoret R., Roy-Fleming A., Suppère C., Tagougui S., Messier V., Taleb N., Changes in accuracy of continuous glucose monitoring using dexcom G4 platinum over the course of moderate intensity aerobic exercise in type 1 diabetes. Diabetes Technol. Ther. 21, 364–369 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Jendle J., Pöhlmann J., de Portu S., Smith-Palmer J., Roze S., Cost-effectiveness analysis of the MiniMed 670G hybrid closed-loop system versus continuous subcutaneous insulin infusion for treatment of type 1 diabetes. Diabetes Technol. Ther. 21, 110–118 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Zaharieva D. P., Turksoy K., McGaugh S. M., Pooni R., Vienneau T., Ly T., Riddell M. C., Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol. Ther. 21, 313–321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heikenfeld J., Jajack A., Feldman B., Granger S. W., Gaitonde S., Begtrup G., Katchman B. A., Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Cobelli C., Schiavon M., Man C. D., Basu A., Basu R., Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: Insight from an In Silico study. Diabetes Technol. Ther. 18, 505–511 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu Z., Wang R., Wu J., Yu H., Xu K., Li D., A flexible electrochemical glucose sensor with composite nanostructured surface of the working electrode. Sens. Actuators B 230, 801–809 (2016). [Google Scholar]
- 9.Novak M. T., Reichert W. M., Modeling the physiological factors affecting glucose sensor function in Vivo. J. Diabetes Sci. Technol. 9, 993–998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D.-H., Lu N., Ma R., Kim Y.-S., Kim R.-H., Wang S., Wu J., Won S. M., Tao H., Islam A., Yu K. J., Kim T.-i., Chowdhury R., Ying M., Xu L., Li M., Chung H.-J., Keum H., Cormick M. M., Liu P., Zhang Y.-W., Omenetto F. G., Huang Y., Coleman T., Rogers J. A., Epidermal electronics. Science 333, 838–843 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Gao W., Emaminejad S., Nyein H. Y. Y., Challa S., Chen K., Peck A., Fahad H. M., Ota H., Shiraki H., Kiriya D., Lien D.-H., Brooks G. A., Davis R. W., Javey A., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandodkar A. J., Gutruf P., Choi J., Lee K. H., Sekine Y., Reeder J. T., Jeang W. J., Aranyosi A. J., Lee S. P., Model J. B., Ghaffari R., Su C.-J., Leshock J. P., Ray T., Verrillo A., Thomas K., Krishnamurthi V., Han S., Kim J., Krishnan S., Hang T., Rogers J. A., Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeder J. T., Xue Y., Franklin D., Deng Y., Choi J., Prado O., Kim R., Liu C., Hanson J., Ciraldo J., Bandodkar A. J., Krishnan S., Johnson A., Patnaude E., Avila R., Huang Y., Rogers J. A., Resettable skin interfaced microfluidic sweat collection devices with chemesthetic hydration feedback. Nat. Commun. 10, 5513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandodkar A. J., Jia W., Yardımcı C., Wang X., Ramirez J., Wang J., Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 87, 394–398 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Lu S., Zhang S., Li Y., Qu Z., Chen Y., Lu B., Wang X., Feng X., Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 3, e1701629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D., Lai W.-Y., Zhang Y.-Z., Huang W., Printable transparent conductive films for flexible electronics. Adv. Mater. 30, 1704738 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kim K. B., Choi H., Jung H. J., Oh Y.-J., Cho C.-H., Min J. H., Yoon S., Kim J., Cho S. J., Cha H. J., Mussel-inspired enzyme immobilization and dual real-time compensation algorithms for durable and accurate continuous glucose monitoring. Biosens. Bioelectron. 143, 111622 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Leboulanger B., Guy R. H., Delgado-Charro M. B., Reverse iontophoresis for non-invasive transdermal monitoring. Physiol. Meas. 25, R35–R50 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Karande P., Jain A., Mitragotri S., Relationships between skin’s electrical impedance and permeability in the presence of chemical enhancers. J. Control. Release 110, 307–313 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Li D., Wang R., Yu H., Li G., Sun Y., Liang W., Xu K., A method for measuring the volume of transdermally extracted interstitial fluid by a three-electrode skin resistance sensor. Sensors 14, 7084–7095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudry B., Guy R. H., Delgado-Charro M. B., Transport numbers in transdermal iontophoresis. Biophys. J. 90, 2822–2830 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu Z., Zou C., Wang R., Lai X., Yu H., Xu K., Li D., A continuous glucose monitoring device by graphene modified electrochemical sensor in microfluidic system. Biomicrofluidics 10, 011910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pu Z., Tu J., Han R., Zhang X., Wu J., Fang C., Wu H., Zhang X., Yu H., Li D., A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab Chip 18, 3570–3577 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Marathe C. S., Rayner C. K., Bound M., Checklin H., Standfield S., Wishart J., Lange K., Jones K. L., Horowitz M., Small intestinal glucose exposure determines the magnitude of the incretin effect in health and type 2 diabetes. Diabetes 63, 2668–2675 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Liu M., Liu R., Chen W., Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 45, 206–212 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S., Ghormli L. E., Kim J. Y., Bacha F., Chan C., Ismail H. M., Katz L. E. L., Levitsky L., Tryggestad J. B., White N. H.; TODAY Study Group , The shape of the glucose response curve during an oral glucose tolerance test: Forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 42, 164–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke W. L., Anderson S., Farhy L., Breton M., Gonder-Frederick L., Cox D., Kovatchev B., Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose–error grid analysis. Diabetes Care 28, 2412–2417 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Kang H., Lee G.-H., Jung H., Lee J. W., Nam Y., Inkjet-printed biofunctional thermo-plasmonic interfaces for patterned neuromodulation. ACS Nano 12, 1128–1138 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Wang M., Wang X., Moni P., Liu A., Kim D. H., Jo W. J., Sojoudi H., Gleason K. K., CVD polymers for devices and device fabrication. Adv. Mater. 29, 1604606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner J. H., Rümmeli M. H., Bachmatiuk A., Büchner B., Atomic resolution imaging and topography of boron nitride sheets produced by chemical exfoliation. ACS Nano 4, 1299–1304 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Packwood D. M., Hitosugi T., Materials informatics for self-assembly of functionalized organic precursors on metal surfaces. Nat. Commun. 9, 2469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao R. J., Wang C., Lu F., Du L., Fang Z., Guo X., Liu J.-T., Chen C.-J., Zhao Z., A flexible interdigital electrode used in skin penetration promotion and evaluation with electroporation and reverse iontophoresis synergistically. Sensors 18, 1431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H., Li D., Roberts R. C., Xu K., Tien N. C., An interstitial fluid transdermal extraction system for continuous glucose monitoring. J. Microelectromech. Syst. 21, 917–925 (2012). [Google Scholar]
- 34.Delgado-Charro M. B., Recent advances on transdermal iontophoretic drug delivery and non-invasive sampling. J. Drug Deliv. Sci. Tec. 19, 75–88 (2009). [Google Scholar]
- 35.Lee I.-T., Chen C.-H., Wang J.-S., Fu C.-P., Lee W.-J., Liang K.-W., Lin S.-Y., Sheu W. H.-H., The association between brain-derived neurotrophic factor and central pulse pressure after an oral glucose tolerance test. Clin. Chim. Acta 476, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Rebrin K., Sheppard N. F. Jr., Steil G. M., Use of subcutaneous interstitial fluid glucose to estimate blood glucose: Revisiting delay and sensor offset. J. Diabetes Sci. Technol. 4, 1087–1098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pishko M. V., Analysis: Glucose monitoring by reverse iontophoresis. Diabetes Technol. Ther. 2, 209–210 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Willems P., Weekx S., Meskal A., Schouwers S., Biological variation of chloride and sodium in sweat obtained by pilocarpine iontophoresis in adults: How sure are you about sweat test results? Lung 195, 241–246 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Kasha P. C., Banga A. K., A review of patent literature for iontophoretic delivery and devices. Recent. Pat. Drug Deliv. Formul. 2, 41–50 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Xu P.-G., Lei X.-F., Ren B.-D., Lv S.-Y., Zhang J.-L., Diclofenac transdermal patch versus the sustained release tablet: A randomized clinical trial in rheumatoid arthritic patients. Trop. J. Pharm. Res. 16, 477–482 (2017). [Google Scholar]
- 41.Lu F., Wang C., Zhao R., Du L., Fang Z., Guo X., Zhao Z., Review of stratum corneum impedance measurement in non-invasive penetration application. Biosensors 8, 31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventrelli L., Marsilio Strambini L., Barillaro G., Microneedles for transdermal biosensing: Current picture and future direction. Adv. Healthc. Mater. 4, 2606–2640 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T., Yamamoto Y., Electrical properties of the epidermal stratum corneum. Med. Biol. Eng. 14, 151–158 (1976). [DOI] [PubMed] [Google Scholar]
- 44.Baroni A., Buommino E., De Gregorio V., Ruocco E., Ruocco V., Wolf R., Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 30, 257–262 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Korhonen I., Ahola J., Estimating the specific heat capacity and heating of electronic sensors and devices. IEEE Instrum. Meas. Mag. 21, 54–62 (2018). [Google Scholar]
- 46.R. J. Lopez, Newton’s Law of Cooling, in Maple via Calculus (Birkhäuser, Boston, MA, 1994), pp. 83–84.
- 47.Garjonyte R., Yigzaw Y., Meskys R., Malinauskas A., Gorton L., Prussian Blue- and lactate oxidase-based amperometric biosensor for lactic acid. Sensor Actuat. B Chem. 79, 33–38 (2001). [Google Scholar]
- 48.Chi Q., Dong S., Amperometric biosensors based on the immobilization of oxidases in a Prussian blue film by electrochemical codeposition. Anal. Chim. Acta 310, 429–436 (1995). [Google Scholar]
- 49.Li S. K., Higuchi W. I., Zhu H., Kern S. E., Miller D. J., Hastings M. S., In vitro and in vivo comparisons of constant resistance AC iontophoresis and DC iontophoresis. J. Control. Release 91, 327–343 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Sieg A., Guy R. H., Delgado-Charro M. B., Noninvasive glucose monitoring by reverse iontophoresis in vivo: Application of the internal standard concept. Clin. Chem. 50, 1383–1390 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi K., Hirota Y., Hashimoto N., Ogawa W., Hamaguchi T., Matsuo T., Miyagawa J.-i., Namba M., Sato T., Okada S., Tomita K., Matsuhisa M., Kaneto H., Kosugi K., Maegawa H., Nakajima H., Kashiwagi A., Evaluation of a minimally invasive system for measuring glucose area under the curve during oral glucose tolerance tests: Usefulness of sweat monitoring for precise measurement. J. Diabetes Sci. Technol. 7, 678–688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/5/eabd0199/DC1


