Abstract
Purpose:
To identify factors associated with isolation yields of ATP-binding cassette (ABC) superfamily member B5 (ABCB5)-positive limbal stem cells (LSCs) from human cadaveric donor eyes.
Methods:
Whole eye globes were purchased from the Saving Sight eye bank, Kansas City, MO and the CorneaGen eye bank, Seattle, WA. ABCB5-positive LSCs were sorted by flow cytometry upon anti-ABCB5 monoclonal antibody staining within one week after donor death. The yields of live limbal epithelial cells in their entirety and of isolated pure ABCB5-positive LSC subsets were correlated with variables contained in the eye donors’ medical information.
Results:
The mean isolation yield of live limbal epithelial cells and ABCB5-positive LSCs per donor eye was (340,000 ± 160,000 and 2,608 ± 1,842 respectively, mean ± SD). Stepwise regression analysis showed that cardiac disease-related death was the strongest negative predictor of the ABCB5-positive LSC isolation yield (p=0.01). While we observed a trend for an age-related decline in the yield of ABCB5-positive LSCs, a statistically significant association could not be established (2% decrease / year, p=0.11). Additionally, despite a trend for decreased isolation yields of total live limbal epithelial cells isolated from single donors with a longer time between death and tissue processing (p=0.04), this did not affect the yields of purified ABCB5-positive LSC, which was independent of increasing time between death and tissue processing (p=0.50).
Conclusions:
Our study identifies cardiac disease-related death as a donor variable significantly associated with lower ABCB5-positive LSC isolation yields.
Keywords: Limbal stem cells, limbal stem cell deficiency, ABCB5, corneal blindness, limbal stem cell isolation
Introduction
Corneal epithelial maintenance and regeneration are sustained by a subpopulation of stem cells residing at the limbus, i.e. the border area between the cornea and conjunctiva. These stem cells, known as Limbal Stem Cells (LSCs), are essential for normal corneal epithelial homeostasis and wound healing through their ability to produce transient amplifying cells that migrate centripetally and give rise to differentiated corneal epithelial cells [1], [2].
Limbal stem cell deficiency (LSCD) due to either complete or partial loss or dysfunction of LSCs can be caused by diverse medical conditions, including, for example, chemical or thermal burns, long-term contact lens wear, autoimmune diseases such as Stevens-Johnson syndrome or ocular cicatricial pemphigoid, or genetic diseases such as aniridia [3]. Patients with LSCD cannot maintain the corneal epithelium, resulting in the loss of anti-angiogenic privilege and ingrowth of conjunctival epithelial cells. This leads to neovascularization of corneal stroma and corneal opacification and ultimately significant vision loss [4].
Currently, the cornea represents one of the few human tissues that can be successfully regenerated by stem cell transplantation. Several methods for LSC transplantation have been developed to date, including conjunctival limbal autografts (CLAU), keratolimbal allografts (KLAL), conjunctival limbal allografts (CLAL), cultivated limbal epithelial transplantation (CLET) and simple limbal epithelial transplantation (SLET) [5]. These methods employ transplantation of mixed limbal epithelial cell grafts, which contain variable numbers of LSCs. Rama et al. conducted an extensive study of the clinical effectiveness of autologous CLET grafts in patients with unilateral LSCD, finding that therapeutic success depended critically on the number of LSCs contained within grafts, determined retrospectively by the percentage of holoclone-forming p63-positive cells in ex vivo culture specimens [6]. These results highlighted the critical importance of LSC frequency determination in grafts selected for clinical transplantation.
Several studies investigated the demographic factors that might influence the numbers and functional characteristics of LSCs isolated from human donor limbal tissues. Notara et al. showed that the colony-forming efficiency of cultured limbal epithelial cells correlated negatively with donor age [7]. Nieto-Nicolau et al., observed increased p63 protein expression in cultured LSCs obtained from aged donors (> 60 years) compared to younger donors [8]. Additionally, several studies reported negative effects of prolonged tissue preservation times on the colony-forming efficiency or on stem cell marker expression levels in limbal tissue explants [9-11]. Here, we hypothesized that in addition to donor age and tissue processing time, other factors such as cause of death or other variables of the donor medical history might also influence LSC yields following isolation from limbal tissue.
We have previously shown that ATP-binding cassette (ABC) superfamily member B5 (ABCB5) identifies LSCs and that this cell surface-expressed antigen can be employed to prospectively isolate LSC from limbal tissue with the unique capacity to fully restore the corneal epithelium, as opposed to ABCB5-negative limbal cells that were unable to do so [12, 13]. Furthermore, based on the recent emergence of ABCB5 as a novel biomarker for LSC dosage in human clinical trials (ClinicalTrials.gov Identifier NCT03549299: Allogeneic ABCB5-positive Limbal Stem Cells for Treatment of LSCD), we wished to investigate any potential associations between cadaveric eye donor characteristics and ABCB5-positive LSC isolation yields from such donors, as such associations could be relevant to optimal donor selection for more efficient LSC graft generation.
Methods
Sample collection
Whole human eye globes donated by 21 patients were purchased from the Saving Sight eye bank, Kansas City, MO and the CorneaGen eye bank, Seattle, WA. According to the records provided by the eye banks, enucleation was performed within 20 hours after death. All eye globes were preserved identically at 4°C in pots moistened with saline as storage medium, i.e. “moist chambers” [14], prior to LSC isolation, which was performed at variable time points within one week of donor death. Summaries of the donors’ medical records were provided by the eye banks (Supplemental Tables 1 & 2). The study adhered to the tenets of the Declaration of Helsinki and received Institutional Review Board (IRB) approval.
Tissue processing
Whole eye globes were kept in moist chambers at 4°C prior to tissue dissection. Globes were washed in phosphate-buffered saline (PBS) (GE Healthcare Life Sciences, Marlborough, MA) and incisions with 3mm margins were made around the limbal area of each eye. The lens and iris were removed from the anterior segment of each eye. Subsequently, the central cornea was excised from each eye with an 8 mm disposable biopsy punch (Integra Miltex, York, PA) and the corneal endothelium was detached mechanically by forceps. The remaining limbal tissues were incubated with PluriSTEM Dispase II Solution (MilliporeSigma, Burlington, MA) at 37°C for 1 hour each. Afterwards, limbal epithelial cells were scraped and incubated with TrypLE Express Enzyme (Thermo Fisher Scientific, Waltham, MA) at 37°C for 30 minutes. The dissociated limbal cells were resuspended in PBS supplemented with 2% fetal bovine serum (FBS) (Thermo Fisher Scientific), filtered using a 40μm cell strainer (BD biosciences, San Jose, CA) and kept on ice prior to antibody staining and flow cytometry.
Flow cytometric analyses
The limbal cell suspensions were stained with the following antibodies: 2.5μg/ml mouse anti-ABCB5 monoclonal antibody (mAb) (clone 3C2–1D12) [13, 15] conjugated with Alexa Fluor 647 (Thermo Fisher Scientific) or 2.5μg/ml Alexa Fluor 647 conjugated isotype-matched control mAb mouse IgG1 (clone MOPC-21, Thermo Fisher Scientific), 0.125μg/ml PE-conjugated anti-CD45 mAb (clone 2D1, BioLegend, San Diego, CA) or 0.125μg/ml PE-conjugated mouse IgG1, κ isotype control (FC) Antibody (clone MOPC-21, Biolegend), 3μg/ml PE-conjugated anti-CD11b mAb (clone REA592, Miltenyi Biotec, Bergisch Gladbach, Germany) or 3μg/ml PE-conjugated REA Control (S) antibody (clone REA293, Miltenyi Biotec). The cells were incubated with the respective antibodies for 30 min on ice, washed twice with PBS supplemented with 2% FBS and incubated with 30nM SYTOX Green Nucleic Acid Stain (Thermo Fisher Scientific). Subsequently, the cells were isolated using a FACSAria II cell sorter (BD Biosciences, San Jose, CA).
Quantification of live limbal epithelial cells and ABCB5-positive LSCs
After using forward scatter (FSC) and side scatter (SSC) to remove cell debris and doublets, SYTOX Green-negative, and CD45- and CD11b-negative cells were defined as live limbal epithelial cells. The total number of live limbal epithelial cells was calculated by multiplying the total number of epithelial cells counted manually after dissociation by the percentage of live epithelial cells determined by flow cytometry. The live ABCB5-positive LSC population was defined as the fraction of live limbal epithelial cells with the highest contrast against the isotype control-positive cells (Supplemental Figure 1). ABCB5-positive and ABCB5-negative LSCs were sorted by FACS and the absolute number of live ABCB5-positive LSCs was calculated by multiplying the number of ABCB5-positive sorted cells by the percentage of ABCB5-positive cells adjusted for isotype control antibody-positive cells. The data were analyzed using the BD FACSDiva and FlowJo (BD Biosciences) software programs. All experiments were performed by the same scientist (Y.S.).
Colony-forming assay
Colony-forming assay was performed as previously described [16, 17]. Briefly, human limbal epithelial cells scraped from the donor limbal tissue were incubated with TrypLE Express Enzyme at 37°C for 30 minutes. The dissociated limbal epithelial cells were seeded on the mitomycin C (MilliporeSigma)-treated 3T3-J2 cells (Kerafast, Boston, MA) at 500 cells per well in 6-well plates. The cells were cultured in keratinocyte culture medium supplemented with 10 ng/ml keratinocyte growth factor (KGF) (PeproTech, Rocky Hill, NJ) and 10 μM Y-27632 (Tocris Bioscience, Bristol, UK) for 10 days. The colonies were fixed with 10% neutral buffered formalin (Thermo Fisher Scientific) and stained with rhodamine B (MilliporeSigma). The colony-forming efficiency (%) was calculated as a ratio of the colony number to the seeded cell number per plate.
Statistical analysis
Patient characteristics and data related to times of tissue processing were evaluated as candidate predictors of ABCB5-positive LSC recovery. These included age at death, gender, smoking status, cause of death, past medical history, time between death to ocular cooling, total ocular cooling time, time between death to enucleation of the whole eye, and time between death to FACS. The measured responses were the total number of live limbal epithelial cells and the number of ABCB5-positive LSCs, which were converted to natural logarithmic scales to achieve normality. Means and standard deviations (SD) were calculated for the continuous predictors, i.e. the total number of live limbal epithelial cells, number of ABCB5-positive LSCs, age, time between death to ocular cooling, total ocular cooling time, time between death to enucleation of the whole eye, time between death to FACS, and the colony-forming efficiency. Frequencies were calculated for the categorical predictors such as gender, smoking status, cause of death and past medical history. Linear regression analysis was performed to evaluate the association between the candidate predictors and responses. The percent change in responses was calculated as (eeffect estimate-1) × 100 for a 1-unit change in the predictor. Stepwise regression analysis using the Akaike information criterion was employed to identify the predictors of ABCB5-positive LSC isolation yield from a single donor [18]. Mediation analysis with 1,000 bootstrap samples was used to examine whether the effect of significant predictors on the number of ABCB5-positive cells was mediated by the effect of total number of live limbal epithelial cells [19, 20]. The differences in the colony-forming efficiency between the two groups were compared using an unpaired t-test. All statistical analyses were performed using R version 3.5.1.
Results
Patient characteristics
In this study, the donor ages ranged from 24 to 79 years with a mean age of 65.2 ± 16.5 (mean ± SD) years. Seventeen patients (81%) were male. Cardiac disease, including myocardial infarction and congestive heart failure, was listed as the most frequent cause of death (n=7). Other causes of death included gastrointestinal disease (n=3), pulmonary disease (n=3), sepsis (n=3), neurological disease (n=2), cancer (n=2) and multisystem organ failure (n=1). Due to the limited representation, the patients who died from causes other than cardiac disease were grouped together in the statistical analyses. Ocular cooling started at 3.6 ± 3.3 (mean ± SD) hours after death. All tissues were enucleated at 9.0 ± 5.0 (mean ± SD) hours and were preserved in moist chambers for up to 4.4 ± 1.0 (mean ± SD) days from the time of death. Patient demographic characteristics, cause of death, disease status and tissue processing times are summarized in Table 1 and Supplemental Table 1.
Table 1.
Summary of patient characteristics and tissue processing time.
| Patient characteristics | |
| Age, years (mean ± SD) | 65.2 ± 16.5 |
| Male, n (%) | 17 (81) |
| Ever smoking, n (%) | 4 (19) |
| Cause of death, n (%) | |
| Cardiac diseases | 7 (33) |
| Others | 14(67) |
| Past medical history, n (%) | |
| Hypertension | 15 (71) |
| Chronic obstructive pulmonary disease | 9 (43) |
| Cancer | 8 (38) |
| Congestive heart failure | 6 (29) |
| Sepsis before death | 5 (24) |
| Tissue processing time | |
| Time between death to ocular cooling, hours (mean ± SD) | 3.6 ± 3.3 |
| Total ocular cooling time, hours (mean ± SD) | 4.8 ± 4.3 |
| Time between death to enucleation, hours (mean ± SD) | 9.0 ± 5.0 |
| Time between death to FACS, days (mean ± SD) | 4.4 ± 1.0 |
Detection of ABCB5-positive LSCs by flow cytometry
The percentage of ABCB5-positive cells was determined by triple color flow cytometry. In 20 cases, a distinct ABCB5-positive LSC population was identified based on the set criteria described in the methods section (Figure 1A and Supplemental Figure 1). ABCB5-positive cells constituted on average 2.4 ± 0.9% (mean ± SD) of all live limbal epithelial cells. Overall, 2,608 ± 1,842 (mean ± SD) of ABCB5-positive LSCs were recovered from a single donor. Notably, in the patient with scleroderma, because of the high background staining of the control mAb, a distinct ABCB5-positive population could not be unequivocally established, and this case was excluded from further analyses (Figure 1B).
Figure 1. Detection of human ABCB5-positive LSCs.
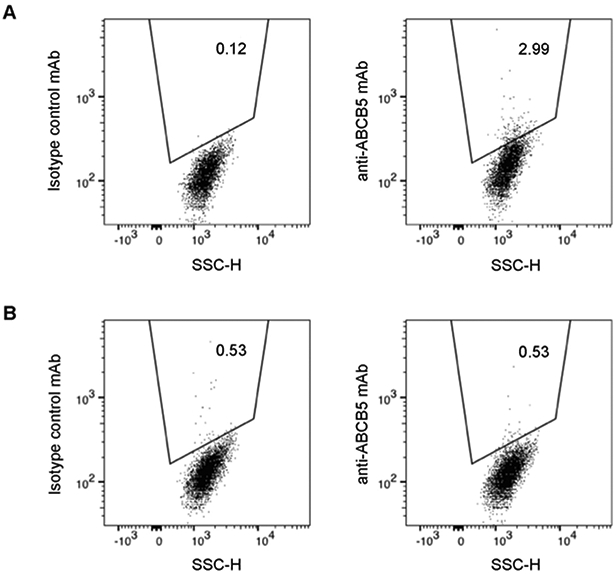
A representative flow cytometry analysis depicting an ABCB5-positive LSC population amongst live limbal epithelial cells isolated from a human donor (A). A flow cytometry analysis demonstrating lack of ABCB5-positive LSCs in a patient with scleroderma (B).
Factors associated with the preservation of live limbal epithelial cells in donor tissues
Flow cytometry analyses of dissociated limbal cell suspensions showed that on average 340,000 ± 160,000 (mean ± SD) live limbal epithelial cells could be isolated per case. Linear regression analysis revealed that female gender, history of congestive heart failure and time between death to FACS were associated with decreases in the number of live limbal epithelial cells (p=0.002, p=0.02 and p=0.04, respectively) (Table 2). Other factors that showed a trend to negatively influence the isolation yield of live limbal epithelial cells included cardiac disease-related death (p=0.08), total ocular cooling time (p=0.12) and time between death to enucleation (p=0.12) (Table 2).
Table 2.
Factors associated with the total number of live limbal epithelial cells recovered from human donors.
| Difference in the total number of live |
p-value | |
|---|---|---|
| Patient characteristics | ||
| Age, per year | −1% | 0.39 |
| Gender | ||
| Male | Ref | |
| Female | −64% | 0.002 |
| Smoking | ||
| Never | Ref | |
| Ever | 26% | 0.55 |
| Cause of death | ||
| Cardiac diseases | −42% | |
| Others | Ref | 0.08 |
| Past medical histories | ||
| Hypertension | −28% | 0.35 |
| Chronic obstructive pulmonary disease | 37% | 0.33 |
| Cancer | 11% | 0.74 |
| Congestive heart failure | −53% | 0.02 |
| Sepsis before death | 17% | 0.69 |
| Tissue processing time | ||
| Time between death to ocular cooling, per hour† | 7% | 0.16 |
| Total ocular cooling time, per hour‡ | −7% | 0.12 |
| Time between death to enucleation, per hour | −5% | 0.12 |
| Time between death to FACS, per day | −29% | 0.04* |
Data for time between death to ocular cooling was missing for n=2.
Data for total ocular cooling time was missing for n=3.
Factors associated with ABCB5-positive LSC isolation yield from human donors
Linear regression analysis showed that cardiac disease-related death, the history of congestive heart failure and female gender were associated with 54% (p=0.01), 62% (p=0.003) and 62% (p=0.01) decreases in the total number of isolated ABCB5-positive LSCs, respectively (Figure 2A-C) (Table 3). Due to conflicting previous reports of the effect of patient age on functional LSC recovery [7, 8], we next evaluated the relationship between donor age and isolation yields of ABCB5-positive LSCs. While there was a tendency for an age-related decline in the yields of ABCB5-positive LSCs, a significant association could not be established (2% decrease / year, p=0.11) (Figure 2D) (Table 3). Despite a significant association between decreases in the total numbers of live limbal epithelial cells and longer times between death to FACS, time between death to FACS did not significantly affect the isolation yields of ABCB5-positive LSCs (p=0.50) (Figure 2E) (Table 3).
Figure 2. Factors associated with ABCB5-positive LSC isolation yield.
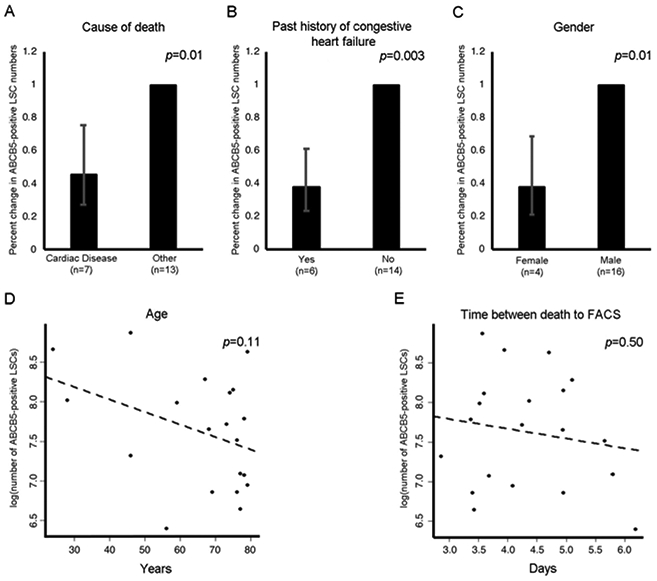
Bar graphs represent linear regression analysis of the factors negatively associated with the yield of ABCB5-positive LSCs: cause of death (A), past history of congestive heart failure (B) and gender (C). Scatter plots represent linear regression analysis of the factors that show a trend to be negatively associated with the yield of ABCB5-positive LSCs: donor age (D) and time between death to FACS (E). Error bars represent 95% confidence interval.
Table 3.
Factors associated with the number of ABCB5-positive LSCs recovered from human donors.
| Difference in the number of ABCB5- positive LSCs |
p-value | |
|---|---|---|
| Patient characteristics | ||
| Total number of live limbal epithelial cells, per 1,000 cells | 2% | 0.12 |
| Age, per year | −2% | 0.11 |
| Gender | ||
| Male | Ref | |
| Female | −62% | 0.01 |
| Smoking status | ||
| Never | Ref | |
| Ever | 75% | 0.17 |
| Cause of death | ||
| Cardiac diseases | −54% | 0.01 |
| Others | Ref | |
| Past medical history | ||
| Hypertension | −29% | 0.37 |
| Chronic obstructive pulmonary disease | 31% | 0.42 |
| Cancer | 75% | 0.09 |
| Congestive heart failure | −62% | 0.003 |
| Sepsis before death | 34% | 0.45 |
| Tissue processing time | ||
| Time between death to ocular cooling, per | 1% | 0.82 |
| Hour† | ||
| Total ocular cooling time, per hour‡ | −5% | 0.25 |
| Time between death to enucleation, per hour | −5% | 0.10 |
| Time between death to FACS, per day | −11% | 0.50 |
Data for time between death to ocular cooling was missing for n=2.
Data for total ocular cooling time was missing for n=3.
Next, we performed stepwise regression analysis to determine factors independently associated with ABCB5-positive LSC isolation yields. The total number of live epithelial cells, patient characteristics including age, gender, smoking status, cause of death and tissue processing times, such as time between death to ocular cooling, total ocular cooling time, time between death to enucleation and time between death to FACS, were used as explanatory variables to be examined. As a result, cause of death was identified as the strongest factor in the final model (p=0.01). Since cardiac disease-related death showed a significant association with both the yield of all live limbal epithelial cells and also the yield of ABCB5-positive LSCs, we performed a mediation analysis to examine whether the latter effect was direct or whether it was mediated by the reduction of total numbers of live limbal epithelial cells. Mediation analysis showed that 76% of the effect of cardiac disease-related death on the yield of ABCB5-positive LSCs was direct (p=0.048). The analysis showed that this effect was not significantly mediated through the total number of live limbal epithelial cells (p=0.21).
Colony-forming efficiency of human donor-derived limbal epithelial cells
In addition to quantifying ABCB5-expressing LSC in donor specimens, we analyzed the relative in vitro colony-forming capacity of unsegregated limbal epithelial cells isolated from donors with cardiac disease (n=3) or donors with non-cardiac causes of death (n=4) (donor characteristics shown in Supplemental Table 2). A slightly lower colony-forming capacity was observed by un-selected limbal epithelial cells isolated from cardiac disease donors’ corneas compared to non-cardiac disease donors (cardiac disease vs. other causes: 5.97 ± 2.48% vs. 7.60 ± 4.65%, mean ± SD) (Supplemental Figure 2), but the observed difference was not statistically significant (p=0.61). This result is consistent with previous findings of a lack of predictor function of colony-forming capacity as a surrogate assay for the stem cell criterion graft success [6], although we cannot entirely exclude the possibility that a positive correlation with ABCB5 stem cell numbers could have emerged upon increasing the numbers of studied specimens.
Discussion
A retrospective study by Rama et al. examined 10-year clinical outcomes of mixed limbal grafts in patients with LSCD and demonstrated that the LSC frequency within grafts is the major determinant of successful clinical outcome [4, 6]. When autologous limbal grafts contained at least 3% of LSC in limbal epithelial cell cultures, the success of long-term outcomes was significantly improved. For this reason, and for ongoing and future clinical trials that employ purified LSC, it is therefore critical to understand the factors that might affect LSC yield from donor tissues. Our current study suggests that graft donor cardiac disease-related death represents an important negative factor associated with ABCB5-positive LSC isolation yield. Additionally, our study revealed that as long as limbal epithelial cells were isolated within 7 days from the time of donor death, LSC yields were not significantly affected by the tissue processing time.
Our finding that donor death from cardiac disease is associated with lower LSC yield is intriguing and warrants further examination in a larger clinical study. As both myocardial infarction caused by systemic atherosclerosis and congestive heart failure lead to a reduction of peripheral blood flow [21, 22], it is plausible that donors who died from cardiac diseases may have suffered from chronic vascular insufficiency and abnormal limbal blood circulation, resulting in an impaired LSC niche and leading to reduced LSC viability. Medication records of eye donors were not available. Therefore, we cannot exclude the possibility that cardiac disease-specific medications might have contributed to the identified negative association of ABCB5-positive cell yield and cardiac disease.
It has been shown previously that stem cell functionality declines with age in diverse tissues [23]. While some studies of human LSCs suggested age-related functional impairment evidenced by reduced clonogenicity [7], the numbers of p63-positive LSCs appeared to be preserved [7, 8]. Our study suggested a trend whereby the number of ABCB5-positive LSCs appeared to decline with age, even though this observation did not reach the statistical significance threshold. However, such an effect could not be fully separated from the cause of death effect, as the cases who died from cardiac disease tended to be older than the cases who died from other causes (71.6 years old vs 61.9 years old). Similarly, as all female cases died from cardiac causes, it is not possible to attribute decreases in LSC yields to female gender.
It might appear intuitive that shorter tissue preservation times would be associated with higher LSC yield and/or functionality. Several studies demonstrated an association of reduced p63 expression and colony-forming efficiency with prolonged tissue preservation time [9-11]. In our study, while the total number of live limbal epithelial cells correlated negatively with tissue processing time, the isolation yield of ABCB5-positive LSCs was unaffected. This apparent discrepancy between the current study and previous studies might be explained by technical differences in tissue preservation and/or the methods of LSC evaluation. The herein reported refrigeration of eyes in moist chambers might have resulted in improved tissue preservation compared to the storage techniques used in prior reports. Additionally, in the current study we focused on ABCB5-positivity as an established marker for LSC determination, rather than the surrogate functional marker of cellular colony-forming efficiency.
In the case of autologous transplants, grafting of mixed limbal epithelial cell cultures containing as little as 3% of p63-positive LSCs successfully regenerated the cornea in the setting of LSCD. However, transplantation of mixed allogeneic grafts containing LSCs and other limbal cell populations frequently results in immune rejection due to the presence of immunogenic cells residing in the limbal stem cell niche, or in immunosuppression-related adverse events [24]. Therefore, a further significance of our study is our demonstrated ability, through use of the ABCB5 cell surface marker, to reliably purify LSCs from mixed limbal epithelial cell suspensions from a variety of human donors. Transplantation of purified ABCB5-positive human LSCs to allogeneic recipients with LSCD, in a currently ongoing FDA-approved clinical trial (ClinicalTrials.gov Identifier: NCT03549299) or future clinical trials, might reduce the occurrence of immune-mediated graft rejection by eliminating immunogenic cell contaminants [25]. Thus, our novel results contribute to an improved understanding of the factors associated with ABCB5-positive LSC recovery from human donors and hence might lead to improvements of patient outcomes.
Supplementary Material
Supplemental Figure 1. Detection of live ABCB5-positive LSCs by flow cytometry. First, cellular debris are gated out based on the established forward scatter (FSC) and side scatter (SSC) parameters (A). Then cell doublets are gated out based on SSC-W vs. SSC-H (B) and FSC-W vs. FSC-H (C) profiles. This is followed by gating out dead cells, which are defined by positivity for the SYTOX Green Nucleic Acid Stain (D). Hematopoietic cells are gated out based on CD45 and CD11b antibody positivity (E). Live ABCB5-positive LSCs are isolated based on ABCB5 mAb staining (F).
Supplemental Figure 2. Colony-forming efficiency of human donor-derived limbal epithelial cells. The bar graph depicts the comparative quantitative analyses of the colony-forming efficiency. Data were analyzed using the unpaired t-test. Error bars indicate S.D. p=0.61 (A). Images depicting the colony formation (Rhodamine B, pink) in wells seeded with cells isolated from donors with cardiac disease-related death (upper panel) or other causes of death (lower panel) (B).
Acknowledgements:
We would like to thank the patients for their generous donation that enabled this research.
Financial Support:
This work was supported by NIH/NEI grants 1RO1EY025794 and R24EY028767 to N.Y.F., B.R.K and M.H.F, NIH/NEI Schepens Core grant P30EY003790 to B.R.K., VA RR&D Merit Review Award 1I01RX000989 and a Harvard Stem Cell Institute seed grant award to N.Y.F., and by support of the Kanae Foundation for the Promotion of Medical Science (Tokyo, Japan), Alcon Japan Ltd. (Tokyo, Japan) and the Japan Eye Bank Association (Tokyo, Japan) to Y.S..
Abbreviations:
- ABCB5
ATP-binding cassette (ABC) superfamily member B5
- LSC
limbal stem cell
- FACS
fluorescence activated cell sorting
- SD
standard deviation
- LSCD
limbal stem cell deficiency
- CLAU
conjunctival limbal autograft
- KLAL
keratolimbal allografts
- CLAL
conjunctival limbal allografts
- CLET
cultivated limbal epithelial transplantation
- SLET
simple limbal epithelial transplantation
- IRB
Institutional Review Board
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- FSC
forward scatter
- SSC
side scatter
- H
height
- A
area
- W
width
- KGF
keratinocyte growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest Statement:
M.H.F., B.R.K. and N.Y.F. are inventors or co-inventors of US and international patents assigned to Brigham and Women’s Hospital, Boston Children's Hospital, the Massachusetts Eye and Ear Infirmary and/or the VA Boston Healthcare System, Boston, MA, licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG
References
- [1].Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–3. [PubMed] [Google Scholar]
- [3].Ahmad S Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tseng SC. Concept and application of limbal stem cells. Eye (Lond). 1989;3 ( Pt 2):141–57. [DOI] [PubMed] [Google Scholar]
- [5].Sasamoto Y, Ksander BR, Frank MH, Frank NY. Repairing the corneal epithelium using limbal stem cells or alternative cell-based therapies. Expert Opin Biol Ther. 2018;18:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. [DOI] [PubMed] [Google Scholar]
- [7].Notara M, Shortt AJ, O'Callaghan AR, Daniels JT. The impact of age on the physical and cellular properties of the human limbal stem cell niche. Age (Dordr). 2013;35:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nieto-Nicolau N, Martinez-Conesa EM, Casaroli-Marano RP. Limbal Stem Cells from Aged Donors Are a Suitable Source for Clinical Application. Stem Cells Int. 2016;2016:3032128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li C, Dong N, Wu H, Dong F, Xu Y, Du H, et al. A novel method for preservation of human corneal limbal tissue. Invest Ophthalmol Vis Sci. 2013;54:4041–7. [DOI] [PubMed] [Google Scholar]
- [10].Liu T, Wang Y, Duan HY, Qu ML, Yang LL, Xu YY, et al. Effects of preservation time on proliferative potential of human limbal stem/progenitor cells. Int J Ophthalmol. 2012;5:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mason SL, Stewart RM, Sheridan CM, Keshtkar F, Rooney P, Austin E, et al. Yield and Viability of Human Limbal Stem Cells From Fresh and Stored Tissue. Invest Ophthalmol Vis Sci. 2016;57:3708–13. [DOI] [PubMed] [Google Scholar]
- [12].Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip Rev Dev Biol. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol. 2008;28:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem. 2003;278:47156–65. [DOI] [PubMed] [Google Scholar]
- [16].Hayashi R, Ishikawa Y, Sasamoto Y, Katori R, Nomura N, Ichikawa T, et al. Coordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–80. [DOI] [PubMed] [Google Scholar]
- [17].Hayashi R, Ishikawa Y, Katori R, Sasamoto Y, Taniwaki Y, Takayanagi H, et al. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat Protoc. 2017;12:683–96. [DOI] [PubMed] [Google Scholar]
- [18].Akaike H A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–23. [Google Scholar]
- [19].Tingley D, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. Journal of Statistical Software. 2014;59:1–38.26917999 [Google Scholar]
- [20].Imai K, Keele L, Yamamoto T. Identification, Inference and Sensitivity Analysis for Causal Mediation Effects. Statistical Science. 2010;25:51–71. [Google Scholar]
- [21].Leithe ME, Margorien RD, Hermiller JB, Unverferth DV, Leier CV. Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation. 1984;69:57–64. [DOI] [PubMed] [Google Scholar]
- [22].Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25:1197–207. [DOI] [PubMed] [Google Scholar]
- [23].Goodell MA, Rando TA. Stem cells and healthy aging. Science. 2015;350:1199–204. [DOI] [PubMed] [Google Scholar]
- [24].Haagdorens M, Van Acker SI, Van Gerwen V, Ni Dhubhghaill S, Koppen C, Tassignon MJ, et al. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016;2016:9798374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frank MH, Frank NY. Restoring the cornea from limbal stem cells. Regen Med. 2015;10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Detection of live ABCB5-positive LSCs by flow cytometry. First, cellular debris are gated out based on the established forward scatter (FSC) and side scatter (SSC) parameters (A). Then cell doublets are gated out based on SSC-W vs. SSC-H (B) and FSC-W vs. FSC-H (C) profiles. This is followed by gating out dead cells, which are defined by positivity for the SYTOX Green Nucleic Acid Stain (D). Hematopoietic cells are gated out based on CD45 and CD11b antibody positivity (E). Live ABCB5-positive LSCs are isolated based on ABCB5 mAb staining (F).
Supplemental Figure 2. Colony-forming efficiency of human donor-derived limbal epithelial cells. The bar graph depicts the comparative quantitative analyses of the colony-forming efficiency. Data were analyzed using the unpaired t-test. Error bars indicate S.D. p=0.61 (A). Images depicting the colony formation (Rhodamine B, pink) in wells seeded with cells isolated from donors with cardiac disease-related death (upper panel) or other causes of death (lower panel) (B).


