Abstract
Aim: Synthetic vascular grafts are widely used in surgical revascularization, mainly for medium- to large-sized vessels. However, synthetic grafts smaller than 6 mm in diameter are associated with a high incidence of thrombosis. In this study, we evaluated silk fibroin, a major protein of silk, with high biocompatibility and biodegradability, as a useful material for extremely-small-diameter vascular grafts.
Methods: A small-sized (0.9 mm inner diameter) graft was braided from a silk fibroin thread. The right carotid arteries of 8- to 14-week-old male C57BL/6 mice were cut at the midpoint, and fibroin grafts (5- to 7-mm in length) were transplanted using a cuff technique with polyimide cuffs. The grafts were harvested at different time points and analyzed histologically.
Results: CD31+ endothelial cells had already started to proliferate at 2 weeks after implantation. At 4 weeks, neointima had formed with α-smooth muscle actin+ cells, and the luminal surface was covered with CD31+ endothelial cells. Mac3+ macrophages were accumulated in the grafts. Graft patency was confirmed at up to 6 months after implantation.
Conclusion: This mouse model of arterial graft implantation enables us to analyze the remodeling process and biocompatibility of extremely-small-diameter vascular grafts. Biodegradable silk fibroin might be applicable for further researches using genetically modified mice.
Keywords: Mouse, Fibroin, Vascular graft, Smooth muscle cells, Endothelial cells
Introduction
Artificial grafts are used for revascularization in peripheral arterial disease and pediatric diseases. However, in synthetic grafts, including polytetrafluoroethylene and polyethylene terephthalate (Dacron), thrombosis tends to occur in small-diameter grafts, especially in those less than 6-mm in diameter1). Moreover, physiological remodeling cannot be expected in synthetic grafts when implanted in children2). Silk fibroin, a major protein of silk, has excellent strength, biocompatibility, and biodegradability3, 4). Previously, we reported long-term patency and biodegradability of a small synthetic graft made of silk fibroin graft, 1.5 mm inner diameter, when implanted in the abdominal aorta of a wild-type rat5).
Many genetically modified mice have been developed and have greatly contributed to the elucidation of the pathophysiology of various human diseases. In order to apply synthetic grafts to humans, it is necessary to evaluate the biocompatibility and long-term patency in each disease model. Furthermore, gene knock-in mice expressing specific marker proteins enable us to trace the origin and fate of cells. Thus, basic studies using genetically modified mice would be considered to play an important role in the clinical application of synthetic grafts. However, few reports reported that extremely-small vascular grafts smaller than 1 mm in diameter could be implanted into mouse artery6, 7) because anastomosis of a small graft to mouse artery is technically very challenging.
In this study, we report that an artificial vascular graft could be implanted into mouse carotid arteries using a relatively easy method, which is the cuff technique that would be reproduced in many laboratories. This method is relatively easy compared with end-to-side or end-to-end anastomosis techniques with suture. We also show that silk fibroin-derived grafts with an extremely small inner diameter of 0.9 mm could be patent for as long as 6 months in mice. This method would be applicable for further researches using genetically modified mice to study the clinical utility of synthetic vascular grafts.
Materials and Methods
Preparation of Silk Fibroin Vascular Graft
Raw silk fibers with silk sericin obtained from cocoons were used as the starting materials to prepare a braided silk fibroin tube8). The tube with a 0.9-mm inner diameter was prepared with raw silk fibers by a braiding machine. The tubes cut to a length of 10 cm were put in an aqueous solution (100 ml) of a mixture of sodium carbonate (0.08 g) and Marseille soap (0.12 g) in a beaker and kept at 95°C for 120 min. Then, the tubes were rinsed several times with distilled water. This degummed process to remove silk sericin was repeated, and braided silk fibroin tubes were obtained.
Next, the tubes were coated with silk fibroin sponge, as follows. The raw silk fibers (1 g) were put in an aqueous solution of a mixture of sodium carbonate (0.08 g) and Marseille soap (0.12 g) in a beaker and kept at 95°C for 120 min while stirring carefully so that the fibers do not get tangled. Then, the fibers were rinsed several times with distilled water. This degummed process was repeated, and pure silk fibroin fiber was obtained. The obtained silk fibroins were dissolved in CaCl2–H2O–EtOH solution (molar ratio: 1:8:2) at a concentration of 10% (w/v) and then boiled at 70°C for 1 h9). This solution was dialyzed with a cellulose dialysis membrane (MWCO 14,000, Viskase Companies, Inc.) against distilled water at 4°C for three days, and an aqueous solution of silk fibroin was obtained for coating the silk fibroin tube. The preparation process of the silk fibroin graft coated with silk fibroin sponge was as follows9). A polyvinyl alcohol rod 0.9 mm in diameter was inserted into the braided silk fibroin tube with a 0.9-mm inner diameter. Then, the rod covered with the silk fibroin tube was immersed into a pipe filled with a mixed aqueous solution of 1:1 (w/w) ratio of silk fibroin and glycerin (porogen) for coating. The pipe was placed in a desiccator, the interior of which was maintained under reduced pressure of 100 hPa until no air bubbles appeared from the coated surface of the silk fibroin tube. After this treatment, the graft was frozen at −20°C overnight. Then, the graft was immersed in distilled water to remove the porogen. The graft was immersed in liquid nitrogen for 30 s and then freeze-dried. Then, the silk fibroin graft was pouched after immersion in an aqueous solution of 60% glycerin to prevent drying. The grafts were observed under a stereoscopic microscope and a scanning electron microscope (SEM, S-2600N, Hitachi High-Technologies, Tokyo, Japan). Microscope and SEM Images were analyzed using Image J software in order to measure the inner diameter and outer diameter under wet and dry conditions. All of the reagents used here are special grade chemicals from Wako Pure Chemical Industries, Ltd., Japan.
SEM
Grafts were air-dried for SEM observation. Dried grafts were cut in a radial direction and an axial direction and then placed on a stage and fixed with conductive carbon tapes. Samples were coated with sputtered gold. The cross-section, inner surface, and outer surface of samples were observed at ×50 and ×100 magnification, using SEM (Hitachi S-2700N), operated at 15 kV.
Animals
Eight- to 14-week-old male C57BL/6 mice were purchased from SLC (Shizuoka, Japan). Mice were fed normal chow and housed with a 12-hour light/dark cycle. All experimental procedures and protocols were approved by the Animal Care and Use Committee of the University of Tokyo (Approval No. P15-122).
Graft Implantation
Mice were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg body weight). A silk fibroin graft (5–7mm long, 0.9 mm inner diameter) was implanted into the right carotid artery with the cuff technique described elsewhere6, 10, 11). Briefly, the right common carotid artery was gently mobilized free from surrounding connective tissue. It was then ligated with 6-0 silk sutures at the midpoint and divided11). A polyimide tube (inner diameter 0.50 mm, wall thickness 0.05 mm, VASCULEX®, Furukawa Electric, Tokyo, Japan) was cut into small cuffs with a handle11). The polyimide cuffs were used to cover the proximal and distal ends of the artery. Then, both ends of the carotid artery were everted over the tubular body portion of the cuffs. The graft was sleeved over the artery cuffs and tied with 6-0 silk sutures.
Histological Analysis
The grafts were carefully removed from surrounding tissue, cut transversely at the midpoint into two pieces, fixed in 10% formalin or methanol, and embedded in paraffin. Paraffin-embedded sections (4 µm thickness) were processed for immunohistochemical staining. Immunohistochemical staining was performed, as previously reported12, 13). Sections were incubated with primary antibodies, including alkaline phosphatase-conjugated anti-α-smooth muscle actin (SMA; clone1A4, A 5691, Sigma-Aldrich, St. Louis, MO), rat anti-mouse CD31 (MEC 13.3, 550274, BD Pharmingen, San Jose, CA), or rabbit polyclonal anti-CD31(ab28364, Abcam, Cambridge, UK), rat antimouse Mac3 (M3/84, 550292, BD Pharmingen), or rat anti-mouse CD45 (30-F11, 550539, BD Pharmingen), followed by incubation with biotinylated rabbit anti-rat immunoglobulin G secondary antibody (BA-4001, Vector Laboratories, Burlingame, CA) or biotinylated polyclonal goat anti-rabbit immunoglobulins secondary antibody (E0432, Dako, Produktionsvej, Denmark), and subsequently stained using the avidin–biotin complex technique and a Vector Red substrate kit (Vector Laboratories). Antigen retrieval was performed by heating the sections in the microwave for 10 minutes in pH 6.0 citrate buffer for the anti-CD31 immunostaining. Sections incubated with horseradish peroxidase (HRP)-conjugated mouse anti-vimentin (V9, sc-6260 HRP, Santa Cruz Biotechnology, Dallas. TX) were stained using an Immpact DAB kit (Vector Laboratories). Nuclei were counterstained with hematoxylin. Collagen and elastin fibers were visualized by Elastica–Goldner staining.
Quantification of Neointima Formation and Endothelial Coverage
Using cross-sections stained for α-SMA or CD31, neointima formation and endothelial coverage were quantified using Image J software. The neointimal area was calculated by lumen area and outline area of neointima using the following formula (Supplemental Fig. 1).
Supplemental Fig. 1.
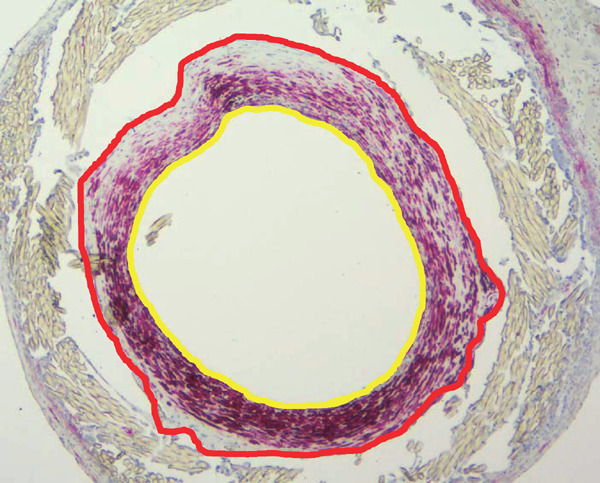
Calculation of the ratio of neointimal area to outline area
The Ratio of neointimal area to outline area was calculated using the following formula.
Lumen area = Yellow circle area
Outline area of the neointima = Red circle area
Neointimal area = Red circle area – Yellow circle area
Ratio of neointimal area to outline area = Neointimal area / Outline area of the neointima = (Red circle area – Yellow circle area)/ Red circle area
Neointimal area = Outline area of neointima − Lumen area
Ratio of neointimal area to outline area = Neointimal area/ Outline area of neointima
Endothelial coverage rate was calculated with luminal surface length and length of CD31+ surface using the following formula.
Endothelial coverage rate (%) =(length of CD31+ surface/ luminal surface length) × 100.
Statistical Analysis
Numerical values are expressed as mean ± SEM.
Results
Visual Characterization of Extremely-Small-Diameter Vascular Graft Made of Silk Fibroin
An extremely-small-diameter vascular graft, 0.9 mm inner diameter, was braided with fibroin fibers and coated with aqueous silk fibroin solution (Fig. 1A to E). The inner and outer diameters of the sample were 0.92 ± 0.02 mm and 1.42 ± 0.04 mm, respectively at wet conditions (Fig. 1A) and 1.01 ± 0.05 mm and 1.41 ± 0.07 mm, respectively, at dry conditions (Fig. 1C). To test the biocompatibility of the silk-fibroin-based extremely-small-diameter graft in vivo, the graft was implanted in the right carotid artery of a mouse (n = 117, 26.4 ± 0.2 g in body weight at graft implantation) (Fig. 1F). The implanted grafts were harvested at 1 week (n = 12, 25.2 ± 0.5 g in body weight at harvest), 2 weeks (n = 15, 25.8 ± 0.3 g), 4 weeks (n = 37, 27.3 ± 0.2 g), 3 months (n = 23, 30.3 ± 0.4 g), and 6 months (n = 30, 33.0 ± 0.3 g) (Fig. 1G). The harvested grafts were cut into two pieces, and graft patency was observed under a stereoscopic microscope. The numbers of evaluated grafts and patency rates are presented in Table 1.
Fig. 1.
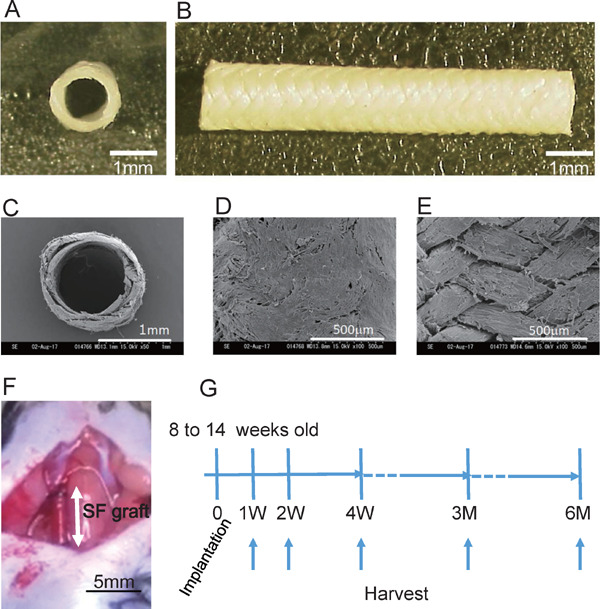
Preparation of silk fibroin graft and implantation into mouse carotid artery
A and B, Silk fibroin-based graft (0.9 mm in diameter). Cross-sectional image (A) and whole image (B) (scale bar, 1 mm). C to E, Scanning electron microscope (SEM) images of graft. Cross-sectional images (C), outside of graft (D) and inside of graft (E) (scale bar, 1 mm in C, 500 µm in D and E). F, The graft was implanted into the right carotid artery of a mouse with a cuff technique (SF, silk fibroin. scale bar, 5 mm). G, Study protocol of graft implantation. Grafts were implanted into 8- to 14-week-old male C57BL/6 mice and harvested at 1, 2, and 4 weeks and 3 and 6 months.
Table 1. Patency rates of silk fibroin grafts at each time point.
| Time point after implantation | 1 week | 2 weeks | 4 weeks | 3 months | 6 months |
|---|---|---|---|---|---|
| Total number of grafts | 12 | 15 | 37 | 23 | 30 |
| Number of patent grafts | 6 | 5 | 8 | 3 | 4 |
| Patency rate (%) | 50.0 | 33.3 | 21.6 | 13.0 | 13.3 |
Process of Neointimal Formation after Graft Implantation
At 1 week after implantation, thin neointima had formed on the luminal surface of the implanted silk fibroin graft. However, neither α-SMA+ cells (Fig. 2B) nor CD31+ endothelial cells (Fig. 2C) were detected (dotted line “b” to “a” in Fig. 2A). At 2 weeks, α -SMA+ cells had appeared in the neointima (Fig. 2D). CD31+ endothelial cells began to cover the luminal side of the neointima near the anastomosis (Fig. 2E).
Fig. 2.
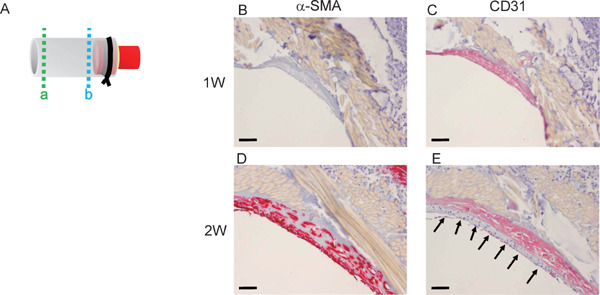
Early phase of graft implantation
A, Scheme of harvested graft. The graft was cut transversely in the middle into two pieces and then embedded in paraffin. The portion of dotted line “a” is near the midline. The portion of dotted line “b” is near the cuff. B and C, Section of the graft 1 week after implantation, near dotted line “b”. There were no α-SMA + (B) or CD31 + (C) cells (scale bar, 50µm). D and E, At 2 weeks after implantation, α-SMA+ cells (D) and CD31+ cells (E) appeared in the neointima. Arrows in E indicate CD31+ cells (scale bar, 50 µm).
Histologic Changes in Implanted Fibroin Graft at 4 Weeks after Implantation
Histological analysis of the patent grafts at 4 weeks (Fig. 3A) of sections from the midportion of the graft (dotted line “a” of Fig. 2A) showed the formation of neointima composed of α-SMA+ cells at the luminal surface of the graft (Fig. 3B and C). The neointima was totally covered by CD31 + endothelial cells (Fig. 3D). Mac3+ macrophages accumulated at the adventitial side of the silk fibroin grafts and infiltrated into the fibroin fibers (Fig. 3E). Collagen and elastin fibers, which are components of normal artery walls, were also detected in the neointima (Fig. 3F and G).
Fig. 3.
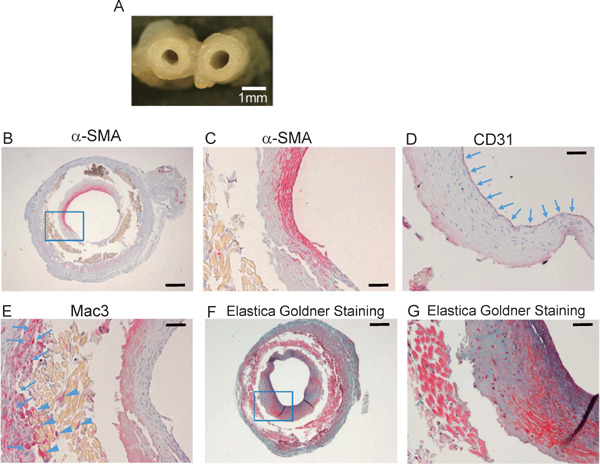
Histological analysis of grafts at 4 weeks after implantation
A, Cross-sectional image of harvested graft at 4 weeks (scale bar, 1 mm). B, Cross-section of graft at 4 weeks after implantation around dotted line “a” (Fig. 2A), stained with anti-α-SMA antibody (scale bar, 250 µm). C, High-power field view of blue square in B (scale bar, 50 µm). D and E, The luminal surface was covered by CD31+ cells (arrows in D). Mac3+ cells accumulated outside (arrows in E) and in the graft fibers (arrow heads in E) (scale bar, 50 µm). F, Elastica–Goldner staining of the section (scale bar, 250 µm). G, High-power field view of blue square in F. Green area indicates collagen fibers, and purple area indicates elastin fibers (scale bar, 50 µm).
Long-Term Patency of Implanted Grafts
At 3 or 6 months after implantation, patent grafts were examined histologically (Fig. 4A, B). The α-SMA+ cell layer was thickened (Fig. 4C, D, E, and F). The neointima was totally covered by CD31+ endothelial cells (Fig. 4G, H). Mac3+ macrophages accumulated at the adventitia and infiltrated into the graft (Fig. 4I, J). Part of the silk fibroin fibers was shown to be absorbed by macrophages.
Fig. 4.
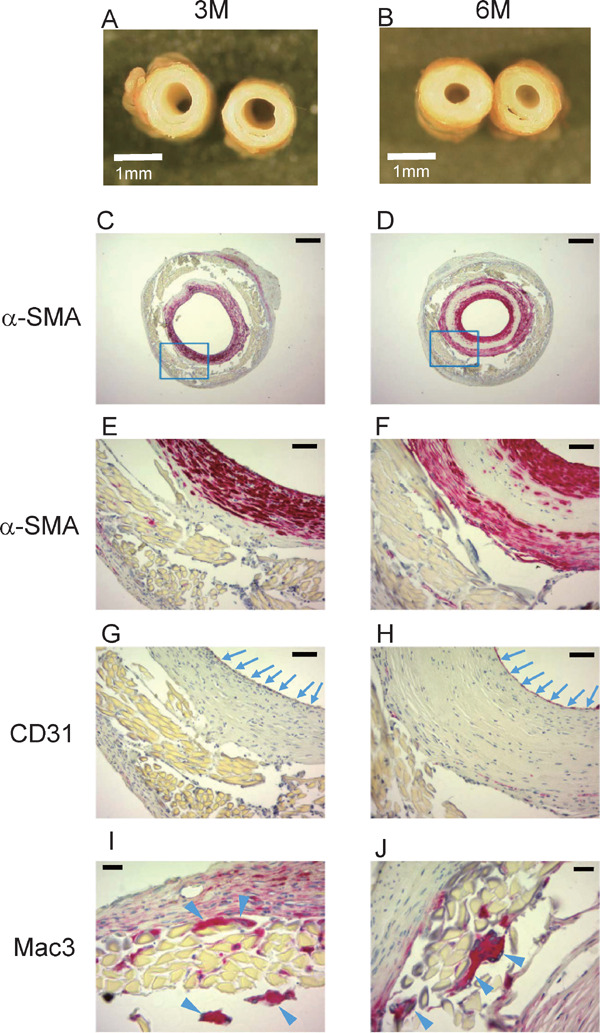
Sections of graft at 3 and 6 months after implantation
A and B, Cross-sectional images at 3 months (A) and 6 months (B) (scale bar, 1 mm). C to J, Sections at 3 months (C, E, G, and I) and 6 months (D, F, H, and J) with immunohistochemical staining. Sections were stained with anti-α-SMA antibody (C to F), anti-CD31 antibody (G and H), or anti-Mac3 antibody (I and J). E and F are high-power field views of blue squares in C and D, respectively. Arrows in G and H indicate CD31+ endothelial cells. Arrowheads in I and J indicate Mac3+ macrophages (scale bar, 250 µm in C and D, 50 µm in E to H, and 20 µm in I and J).
Neointima Formation and Endothelialization at Each Time Point
Neointima formation and endothelialization of the sections from the midportion of the grafts (dotted line “a” of Fig. 2A) at each time point were analyzed. Fig. 5A and 5B show the ratio of neointimal area to outline area and the luminal endothelial coverage rate at each time point. The value of mean endothelial coverage was high at 6 months (n = 4, 88.9 ± 5.6%) near the midline of the patent graft (“a” in Fig. 2A). At the sections that were 100 µm distal from the portion of “a”, the luminal surfaces of all 4 grafts were 100% covered by CD31+ cells.
Fig. 5.
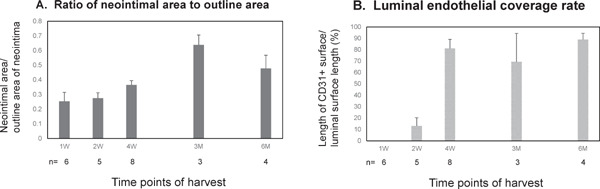
Ratio of neointimal area to outline area and luminal endothelial coverage rate
A. Ratio of neointimal area to outline area at each time point. B. Luminal endothelial coverage rate at each time point. n = Number of the patent grafts at each time point. All values are mean ± SEM.
Types of Cells Which Contribute to Neointima Formation
In Fig. 4F, a large amount of cells in the neo-intima of 6 months' graft was α-SMA+. However, there were some α-SMA negative cells. In the sections of the patent graft at 6 months, with the largest ratio of neointimal area to outline are (0.76, graft 1), these cells included vimentin+ cells (Fig. 6A). Vimentin is a general marker of cells originating from the mesenchyme and an established marker of fibroblasts. Some other cells were CD45+ (Fig. 6C), which is a marker of all cells of hematopoietic origin, except erythrocytes. Collagen and elastin fibers also increased (Fig. 6E) compared with the graft at 4 weeks (Fig. 3G). All of the patent grafts at 6 months (n = 4) showed a similar appearance. In the graft with the smallest ratio of neointimal area to outline area (0.29, graft 4), the amounts of these components were less (Fig. 6B, 6D, and 6F).
Fig. 6.
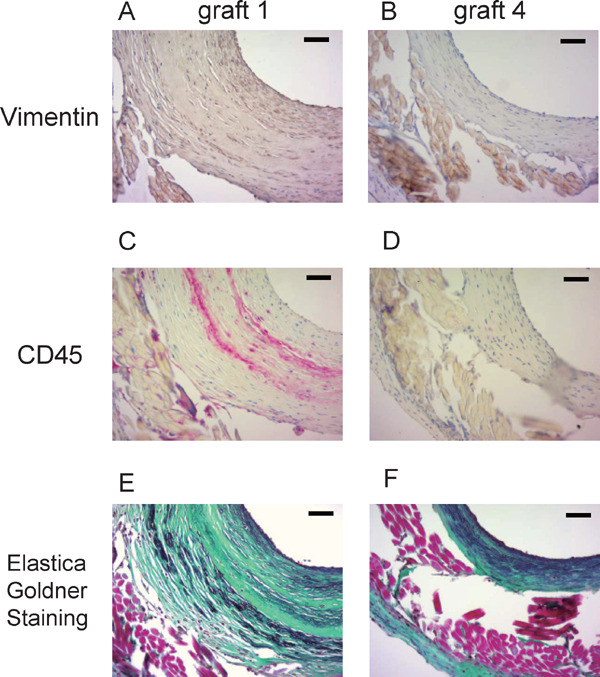
Types of cells which contribute to neointimal formation
The sections near the midline of the patent grafts harvested at 6 months with the largest ratio of neointimal area to outline area (graft 1) and the smallest ratio of neointimal area to outline area (graft 4) were stained with anti-vimentin antibody (A and B) and anti-CD45 antibody (C and D). Collagen and elastin fibers were stained by Elastica–Goldner staining (E and F). Green area indicates collagen fibers, and purple area indicates elastin fibers (scale bar, 50 µm).
Patency Rate at Each Time Point after Graft Implantation
The number of patent grafts, total implanted grafts, and patency rate (patent/total implanted grafts) at each time point is presented in Table 1. The patency rate was 50.0%, 33.3%, 21.6%, 13.0%, and 13.3% at 1, 2, and 4 weeks and 3 and 6 months, respectively.
Cellular Composition of The Occluded Grafts
To investigate the causes of graft occlusion, we analyzed the occluded grafts harvested at 1 week, 4 weeks, 3 months, and 6 months after implantation (Supplemental Fig. 2A to D). The cross-sections of the occluded lumens looked red at 1 week and 4 weeks, while the lumen looked white at 3 months and 6 months. Immunostaining of the occluded grafts harvested at 6 months revealed that the occluded lumen was composed of α-SMA+ cells (Supplemental Fig. 2E and F), CD31+ microvessels (Supplemental Fig. 2G), and Mac3+ macrophages (Supplemental Fig. 2H). These results suggest that the cause of graft occlusion was thrombosis, followed by organization and recanalization.
Supplemental Fig. 2.
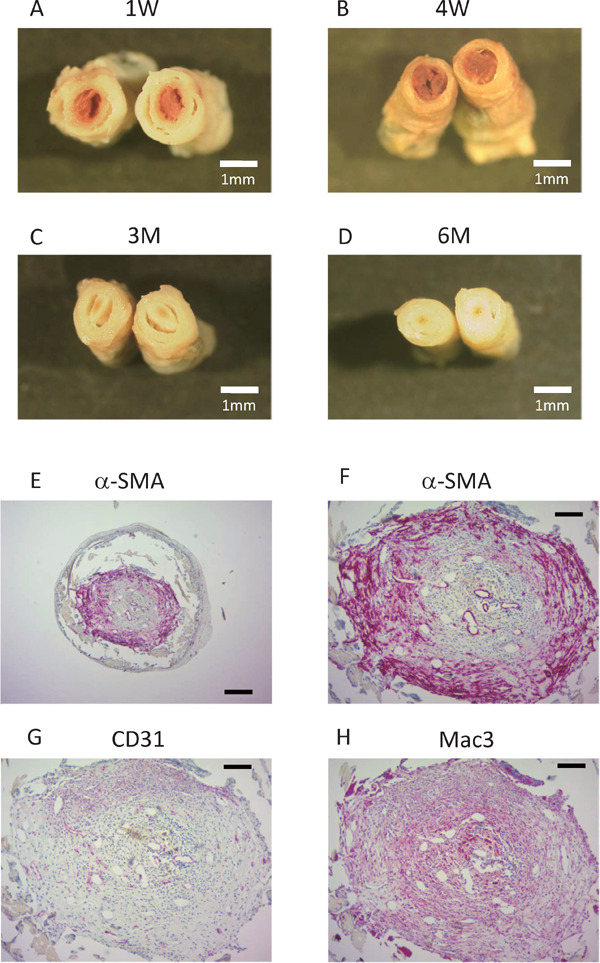
Cellular composition of the occluded grafts
A to D, Cross-sectional images of the occluded graft at 1 week (A), 4 weeks (B), 3 months (C) and 6 months (D) after implantation. (scale bar, 1mm). E to H, Immunohistochemical staining on the sections of the occluded graft harvested at 6 months after implantation. Sections were stained with an anti-α -SMA antibody (E and F), an anti-CD31 antibody (G), or an anti-Mac3 antibody (H).
scale bar, 250µm in E, 100µm in F to H
Discussion
In this study, an extremely-small-diameter synthetic vascular graft made of silk fibroin was implanted into the mouse carotid artery using a cuff technique, and histological changes of the implanted grafts were analyzed. The silk fibroin-derived grafts could be patent for as long as 6 months in mice.
Small-diameter artificial vascular grafts would be useful to manage various vascular diseases in pediatric cases and adults for bypass treatment in coronary stenosis or critical limb ischemia below the knee. A small-diameter vascular graft is defined as being less than 6 mm in internal diameter. In clinical usage, these small-diameter grafts have low patency rates in humans due to thrombosis and intimal hyperplasia. Both of these processes, at least in part, likely originate due to the lack of development of an endothelial layer on the luminal surface of the graft and/or abnormal endothelial and smooth muscle cell direct and indirect communication1).
In our previous study, we implanted a small-sized vascular graft derived from silk fibroin with an inner diameter of 1.5 mm into the rat abdominal aorta5). Endothelial cells and vascular smooth muscle cells infiltrated into the implanted graft early after implantation and replaced the graft5). In this study, a silk fibroin graft with an inner diameter of 0.9 mm was implanted in the mouse carotid artery using a novel cuff method. Neointima composed of endothelial cells and vascular smooth muscle cells formed from the anastomosis side at 2 weeks after implantation. Although the implanted silk fibroin graft was not fully replaced by native tissue at 6 months, the fibroin fibers appeared to be gradually biodegraded with macrophage infiltration.
A silk fibroin-derived graft is composed of a base and a silk coating. The silk coating is biodegraded within 1 to 3 months, whereas the base of the graft needs more than one year to be biodegraded5, 14). The strength of the graft would be preserved for a while because the base structure of the graft is maintained for more than one year. The biodegradable period of the silk coating could be adjusted by the density of the silk coating solution and the degree of the insolubility of the coating14). In this study, an extremely-small-size graft was observed up to 6 months, during which a part of the graft started to be degraded.
Few types of extremely-small-diameter vascular grafts with inner diameters less than 1 mm have been reported. Wu et al. implanted a graft with an inner diameter of 720 µm, composed of a porous tube made of poly(glycerol sebacate) and a sheath made of polycaprolactone, into the rat abdominal aorta15). Native cells infiltrated into the small holes of the graft within 3 days after implantation. α-SMA+ cells had spread to the inner surface of the graft by 14 days, and endothelial cells were observed at the border between the lumen and the layer of α-SMA+ cells of the graft. At 90 days, the implanted graft material was almost absorbed and replaced with neo-artery consisting of vascular smooth muscle cells, endothelial cells, collagen, and elastin15). Sugiura et al. generated an extremely-small-size graft with an inner diameter of 600 µm with a biodegradable porous sponge type scaffold made of 50:50 poly(1-lactide-co-ε-caprolactone) and poly(1-lactic acid) by electrospinning and implanted it into the mouse infrarenal abdominal aorta using microsurgical techniques7). The mice were treated with aspirin for 3 days before and after implantation. At 8 weeks after implantation, the implanted graft was harvested and evaluated histologically. Favorable cells had infiltrated into the graft. Neointima composed of endothelial cells and smooth muscle cells was formed7). Ishii et al. implanted a “MicroBiotube” with an inner diameter of 0.6 mm into the abdominal aorta of a rat with an end-to-end anastomosis technique16). The “MicroBiotube” was made as follows. A stainless wire covered with a silicon tube was implanted into the dorsal subcutaneous space of a rat. Two months later, the stainless wire was harvested with surrounding capsular tissue. The stainless wire was removed, and the tube made of connective tissue was used as a graft16). Blood flow imaging by magnetic resonance angiography (MRA) demonstrated patency of the implanted graft at 12 months. Histological analysis showed the formation of neointima resembling the native artery, which consisted of smooth muscle cells covered by an endothelial layer. Elastic fibers were also detected in the neointima16, 17). Implantation of an artificial graft into the mouse aorta would be technically challenging. The “MicroBiotube” would take a long time to produce. Silk fibroin would be a favorable material for an extremely-small-size synthetic graft because it is biocompatible so that endothelial cells and smooth muscle cells easily adhere, migrate, and proliferate on and within the graft. Moreover, we can adjust the strength and the biodegradable period by changing the compositions of a base and a silk coating of a silk fibroin-derived graft. Although the long-term patency rate is not high, silk fibroin-derived grafts with an extremely small inner diameter of 0.9 mm could be patent for as long as 6 months in mice. An artificial vascular graft could be implanted into mouse carotid arteries using a relatively easy method, the cuff technique. Implantation of a silk fibroin small graft into the mouse carotid artery using our technique would be useful.
There is a discrepancy in the total number of grafts at each time point (Table 1). At the beginning of this research, we harvested most of the grafts at 4 weeks after implantation to check the patency of the grafts. When we were preparing the manuscript, we realized that we should check the histological time course of the grafts. Then, we tried to harvest the grafts at different time points. Thus, the number of 4 weeks is the largest. We harvested only 12 grafts at one week because we focused on the long patency of the grafts.
In the present study, the patency rate of the grafts was not high enough. Histological analyses of the occluded grafts suggested that the cause of occlusion was thrombosis, followed by organization and recanalization. One possible reason for the high incidence of thrombosis might be the lack of use of antiplatelet agents. In addition, the cuff technique, with which we implanted the fibroin graft into the mouse carotid artery, is technically easy but might be associated with occasional thrombosis of arterial grafts. The cuff technique was originally developed as a mouse vein graft model10). We observed long-term patency of up to 6 months using a mouse arterial graft model in this study, although the patency rate was not high. Moreover, in this study, graft patency was evaluated mainly by histological analysis and not by ultrasonography or MRA. Because ultra-thin fibroin fibers were easily detached from glass slides in histological sample preparation, it was very difficult to observe complete grafts histologically.
In conclusion, we found that an extremely-small-diameter silk fibroin graft can be implanted into the mouse carotid artery with long-term patency. It was observed that neointima composed of endothelial cells and vascular smooth muscle cells was formed at an early phase after implantation. Silk fibroin might be a useful material to develop ultra-small vascular grafts for mice. The method used in this study might be applicable for further researches using genetically modified mice to study the clinical utility of synthetic vascular grafts.
Acknowledgements
The authors thank Hiromi Kato and Yumi Sugawara (The University of Tokyo) and Ikue Suzuki (Tokyo University of Agriculture and Technology), Etsuko Uematsu and Shintaro Okamoto (Tokushima University) for their technical assistance.
Competing Interests
T.S. is an employee of The Japan Wool Textile Co., Ltd.
Sources of Funding
This work was partially supported by Japan Society for the Promotion of Science Kakenhi Grants (19H03654, 26248050 and 19K05609), Takeda Science Foundation, and the Vehicle Racing Commemorative Foundation. The funders had no role in the study design, data collection, and analysis, or preparation of the manuscript.
References
- 1). Pawlowski KJ, Rittgers SE, Schmidt SP, Bowlin GL: Endothelial cell seeding of polymeric vascular grafts. Front Biosci, 2004; 9: 1412-1421 [DOI] [PubMed] [Google Scholar]
- 2). Mirensky TL, Hibino N, Sawh-Martinez RF, Yi T, Villalona G, Shinoka T, Breuer CK: Tissue-engineered vascular grafts: does cell seeding matter? J Pediatr Surg, 2010; 45: 1299-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL: Silk-based biomaterials. Biomaterials, 2003; 24: 401-416 [DOI] [PubMed] [Google Scholar]
- 4). Asakura T, Tanaka T, Tanaka R: Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk vascular Grafts. ACS Biomater Sci Eng, 2019; 5: 5561-5577 [DOI] [PubMed] [Google Scholar]
- 5). Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M: Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J Vasc Surg, 2010; 51: 155-164 [DOI] [PubMed] [Google Scholar]
- 6). Wong MM, Hong X, Karamariti E, Hu Y, Xu Q: Generation and grafting of tissue-engineered vessels in a mouse model. J Vis Exp, 2015; 97: e52565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Sugiura T, Tara S, Nakayama H, Kurobe H, Yi T, Lee YU, Lee AY, Breuer CK, Shinoka T: Novel Bioresorbable Vascular Graft With Sponge-Type Scaffold as a Small-Diameter Arterial Graft. Ann Thorac Surg, 2016; 102: 720-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Saotome T, Hayashi H, Tanaka R, Kinugasa A, Uesugi S, Tatematsu K, Sezutsu H, Kuwabara N, Asakura T: Introduction of VEGF or RGD Sequences Improves Revascularization Properties of Bombyx Mori Silk Fibroin Produced by Transgenic Silkworm. J Mater Chem B, 2015; 3: 7109-7116 [DOI] [PubMed] [Google Scholar]
- 9). Tanaka T, Uemura A, Tanaka R, Tasei Y, Asakura T: Comparison of the knitted silk vascular grafts coated with fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J Biomater Appl, 2018; 32: 1239-1252 [DOI] [PubMed] [Google Scholar]
- 10). Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q: Mouse model of venous bypass graft arteriosclerosis. Am J Pathol, 1998; 153: 1301-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Yu P, Nguyen BT, Tao M, Campagna C, Ozaki CK: Rationale and practical techniques for mouse models of early vein graft adaptations. J Vasc Surg, 2010; 52: 444-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Tanaka K, Sata M, Hirata Y, Nagai R: Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res, 2003; 93: 783-790 [DOI] [PubMed] [Google Scholar]
- 13). Tanaka K, Nagata D, Hirata Y, Tabata Y, Nagai R, Sata M: Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis, 2011; 215: 366-373 [DOI] [PubMed] [Google Scholar]
- 14). Fukayama T, Ozai Y, Shimokawadoko H, Aytemiz D, Tanaka R, Machida N, Asakura T: Effect of fibroin sponge coating on in vivo performance of knitted silk small diameter vascular grafts. Organogenesis, 2015; 11: 137-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Wu W, Allen RA, Wang Y: Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med, 2012; 18: 1148-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ishii D, Enmi J, Moriwaki T, Ishibashi-Ueda H, Kobayashi M, Iwana S, Iida H, Satow T, Takahashi JC, Kurisu K, Nakayama Y: Development of in vivo tissue-engineered microvascular grafts with an ultra small diameter of 0.6 mm (MicroBiotubes): acute phase evaluation by optical coherence tomography and magnetic resonance angiography. J Artif Organs, 2016; 19: 262-269 [DOI] [PubMed] [Google Scholar]
- 17). Ishii D, Enmi JI, Iwai R, Kurisu K, Tatsumi E, Nakayama Y: One year Rat Study of iBTA-induced “Microbiotube” Microvascular Grafts With an Ultra-Small Diameter of 0.6 mm. Eur J Vasc Endovasc Surg, 2018; 55: 882-887 [DOI] [PubMed] [Google Scholar]


