Abstract
Aim: There is no randomized controlled trial to compare the effectiveness and safety of mechanical thrombectomy (MT) to intravenous thrombolysis in patients with posterior circulation occlusion (PCO). Hence, we firstly performed a meta-analysis to investigate the outcomes of MT in PCO and then compared these outcomes to anterior circulation occlusion (ACO) to provide fundamental data to further studies.
Methods: We searched the PubMed, EMBASE, and Cochrane Library from dates of inception to June 2019 for relevant studies. Outcomes including functional independence at 90 days, successful recanalization, mortality, symptomatic intracranial hemorrhage (sICH), and futile recanalization were extracted.
Results: Seven studies involving 474 patients with PCO thrombectomy were analyzed. There was a lower rate of functional independence at 90 days and a higher rate of mortality after thrombectomy in PCO versus ACO (odds ratios (OR) 0.72; 95% confidence interval (CI) 0.57–0.90; OR 2.03; 95% CI 1.30–3.18). Recanalization rates were comparable (OR 1.01; 95% CI 0.62–1.65), but a higher futile recanalization rate was found in basilar artery occlusion (BAO) (OR 1.75; 95% CI 1.30–2.37). There was a lower rate of sICH in MT for patients with PCO versus ACO (OR 0.54; 95% CI 0.29–0.99).
Conclusions: We found that the outcomes of MT for patients with PCO were poorer than with ACO. On the other hand, MT appears to have lower rates of sICH and to increase successful recanalization. Given the high recanalization rate, MT may serve as an adjunct to standard treatment. The key point to improve outcomes is recognizing reliable factors associated with futile recanalization and optimizing the results of MT. But in view of the different characteristics of posterior circulation stroke and anterior circulation stroke, the results are far from robust.
Keywords: Mechanical thrombectomy, Anterior circulation occlusion, Posterior circulation occlusion, Recanalization
Introduction
Posterior circulation stroke (PCS) accounts for about 20–25% of ischemic strokes and is usually associated with severe disability and high mortality rates1). Up to now, several randomized controlled trials on acute ischemic stroke due to large vessel occlusion in the anterior circulation demonstrated an overwhelming benefit of the new generations of mechanical thrombectomy (MT) devices compared to intravenous thrombolysis2–6), grounded on which American Heart Association/American Stroke Association guidelines published in 2015 and established stent retrievers as the standard of care in patient with large vessel occlusions in anterior circulation. Intravenous thrombolysis is the standard care in patients with basilar artery occlusion (BAO) and other large vessel occlusions of the posterior circulation. Yet, intravenous thrombolysis yields insufficient reperfusion rates and poor outcomes in patients with BAO7, 8), and MT appears to increase recanalization and to be more promising to help patients with posterior circulation occlusion (PCO) to improve clinical outcomes.
Recently, retrospective case series have demonstrated acceptable results of functional outcome for BAO or PCO treated with MT9–11). There is no doubt that the best way to identify the effect of MT in patients with PCO is through conducting a randomized controlled trial and comparing MT to intravenous thrombolysis alone, but such trial may not be feasible due to the lack of clinical equipoise that MT is performed for patients with PCO in many institutions in a real world. Up to now, there is no randomized controlled trial to prove the effectiveness and safety of MT over intravenous thrombolysis in patients with PCO (mainly basilar artery), and most data regarding safety and efficacy of MT in PCO are obtained from cohort studies, so evidence on thrombectomy in PCO is still weak. Several studies have reported on the outcomes for MT in patients with PCO versus anterior circulation occlusion (ACO), but the conclusions were inconsistent. Furthermore, reports were limited by the small number of patients.
Hence, we performed a meta-analysis to investigate the clinical outcomes of MT in PCO, including functional independence at 90 days (modified Rankin scale (mRS) 0–2), successful recanalization (modified thrombolysis in cerebral infarction (mTICI) 2b/3), mortality, symptomatic intracranial hemorrhage (sICH), and futile recanalization (poor outcome despite successful recanalization), and to compare these outcomes to ACO thrombectomy, providing a fundamental data to clinical practice and further studies as much as possible.
Methods
Search Strategy
We searched the PubMed, EMBASE, and Cochrane Library with no language restrictions from dates of inception to June 2019 and also manually searched reference lists of relevant articles. Our search strategy was as follows:
1. “Basilar artery” or “posterior circulation” or “vertebrobasilar artery” or “basilar artery (BA)”
2. “Endovascular” or “thrombectomy” or “recanalization” or “clot retrieval” or “intra-arterial”
3. 1 and 2
This meta-analysis was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines12). Two clinical research fellows reviewed each study for suitability. Studies from the reference lists of relevant articles were also manually searched to avoid omitting relevant trails. Reviews, case reports, comments, letters, studies in animals, and duplicates were removed from records. Articles were then screened based on title and abstract, and the remaining papers underwent full-text review. All disagreements were resolved by consensus.
Eligibility Criteria
We included published studies fulfilling the following criteria: (1) Study in which at least 20 patients were treated with MT due to PCO in acute ischemic stroke (PCO is defined as a documented large vessel occlusion in one or more segments of the BA and/or vertebral artery (VA) and/or posterior cerebral artery (PCA)); (2) reporting a measure of clinical outcome that could be extracted and compared with MT in ACO; and (3) the use of new generations of MT devices (e.g., Solitaire) in > 80% of cases.
Data Extraction
Data extraction was independently conducted by two authors. Demographic and baseline characteristics including first author, published time, number of participants, age, sex, initial National Institutes of Health Stroke Scale (NIHSS), treatment intervention devices, intravenous thrombolysis rate, number of passes, and onset to reperfusion time were extracted from all eligible studies. Outcomes were extracted from included studies as dichotomous variables. The primary outcome was a favorable outcome at 90 days (mRS 0–2). Secondary outcome included successful recanalization (mTICI 2b/3), mortality, sICH, and futile recanalization.
Study Quality Assessment and Reducing the Risk of Bias
The Newcastle–Ottawa Scale was employed to semiquantitatively assess the quality of the included studies. The maximum number of stars was 9. A study with ≥ 6 stars was of high quality; otherwise, it was of low quality. The quality of included studies was high (Table 1).
Table 1. Demographic and baseline characteristics of included studies.
| Demographics |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Design | Quality score | Sample size | Mean age | Male (%) | Stroke onset to reperfusion (min) | Initial NIHSS score | IV tPA (%) | No of passes | Secondary transfer | ||||||||||
| PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | Center | Device Type | ||||
| Singh 2017 | India | Cohort (R) | 7 | 25 | 112 | 56.4 ± 9.19 | 57.85 ± 12.52 | 16 (64) | 71 (63) | 326.4 ± 191.8a | 220.0 ± 80.6a | 19 ± 5.5 | 15.5 ± 4.32 | 7 (28.0) | 62 (55.4) | 1.6 ± 0.65 | 1.54 ± 0.78 | NR | NR | Multicenter | stent retriever |
| Serles 2016 | Austria | Cohort (R) | 8 | 43 | 258 | 72.3 (63–77) | 70.4 (60–77) | 24 (55.8) | 125 (48.4) | 291 (230–335) | 252 (195–305) | 19 (12.5–29.5) | 17 (13–20) | 31 (72.1) | 162 (62.8) | NR | NR | 20 (46.5) | 121 (46.9) | Multicenter | stent retriever |
| Hu 2017 | Korea | Cohort (R) | 7 | 24 | 137 | 65.7 (32–85) | 65.5 (22–87) | 13 (54.2) | 78 (56.9) | 390 (137–675) | 333 (90–870) | 14.2 (2–34) | 10.4 (3–26) | 9 (34.0) | 64 (46.7) | 2.0 (1–6) | 2.7 (1–10) | NR | NR | Single Center | stent retriever |
| Leciñana 2017 | Spain | Cohort (R) | 8 | 52 | 427 | 64 (50–74) | 70 (60–77) | 35 (67) | 213 (50) | 385 (320–540) | 315 (240–415) | 11 (6–23) | 18 (14–21) | 20 (38.5) | 220 (51.5) | 2 (1–4) | 2 (1–4) | NR | NR | Multicenter | NR |
| Meinel 2019 | Switzerland | Cohort (R) | 8 | 165 | 1574 | 70 (59–80) | 73 (61–82) | 96 (58.2) | 764 (48.5) | 300 (211–480)b | 225 (165–315)b | 18 (8–30) | 17 (12–20) | 71 (43.0) | 779 (49.5) | 1 (1–2) | 2 (1–3) | 80 (48.5) | 561 (35.7) | Multicenter | stent retrieve |
| Weber 2019 | Germany | Cohort (R) | 8 | 139 | 961 | 65.4 ± 15.8 | 69.0 ± 13.9 | 82 (59.0) | 498 (51.9) | 329 (249–543) | 274 (210–362) | 12 (6–21) | 15 (12–19) | 58 (41.7) | 505 (52.5) | 2 (1–3) | 2 (1–3) | 69 (50.4) | 419 (45.2) | Multicenter | stent retrieve and aspiration |
| Lefevre 2014 | France | NR | 6 | 26 | 36 | NR | NR | NR | NR | NR | NR | 19.9 | 19.7 | 1 (3.8) | 14 (38.9) | NR | NR | NR | NR | Single Center | stent retrieve |
Note: NR: Not reported
Door-to-needle time
Time from onset to groin puncture
Data Synthesis and Analysis
Unadjusted odds ratios (OR) and 95% confidence interval (CI) were calculated for the comparisons of outcomes of PCO versus ACO thrombectomy in all participants. RevMan5.3 software (Cochrane Information Management System) was used to conduct statistical analysis. Cochran Q test and I2 test were used to detect heterogeneity. Significant heterogeneity was defined as either Q values greater than the χ2 critical value at α = 0.1 level or I2 value greater than 50%. A random effects model (Mantel–Haenszel) was chosen to calculate pooled estimates. Sensitivity analysis was performed to detect the contribution of individual studies on the overall effect. Statistical significance was defined as P < 0.05.
Results
Seven studies involving 474 patients with PCO and 3,505 patients with ACO thrombectomy were analyzed13–19), and the selection flow was shown in Fig. 1. All but one, Lefevre (2014) (study design not mentioned), were cohort studies. The characteristics of the included studies and baseline data of patients were listed in Table 1. Outcomes of patients with PCO and ACO thrombectomy at 3 months were shown in supplementary data (Supplementary Table 1).
Fig. 1.
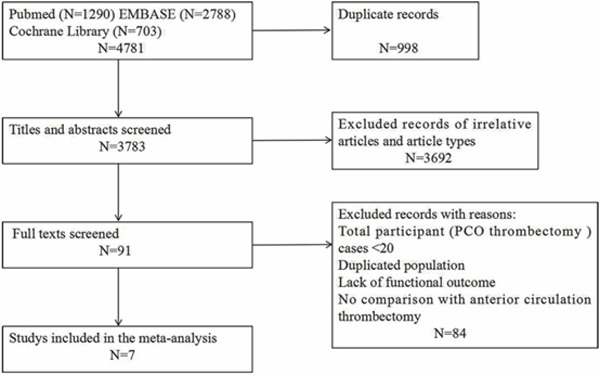
PRISMA flow chart for inclusion of eligible studies
Supplementary Table 1. Functional outcomes at 3 months.
| Study | mTICI 2b/3 |
Mortality |
sICH |
mRS 0–2 |
FR |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | PCO | ACO | |
| Singh 2017 | 21 (84.0) | 104 (92.9) | 2 (8.0) | 7 (6.3) | 0 (0) | 11 (9.8) | 12 (48.0) | 71 (63.4) | NR | NR |
| Serles 2016 | 35 (81.4) | 207 (80.2) | 10 (23.3) | 24 (9.3) | 0 (0) | 18 (7) | NR | NR | NR | NR |
| Hu 2017 | 19 (79.1) | 110 (80.2) | 4 (16.6) | 8 (5.8) | 1 (4.1) | 12 (8.7) | 4.2 (0–6) | 3.0 (0–6) | NR | NR |
| Leciñana 2017 | 39 (75) | 359 (84) | 17 (33) | 48 (12) | 1 (2) | 23 (5) | 21 (40) | 237 (58) | 21 (50) | 119 (35) |
| Meinel 2019 | 149 (90.3) | 1299 (82.7) | 55 (36.2) | 344 (24.4) | 8 (4.8) | 98 (6.3) | 55 (36.2) | 604 (42.9) | 70 (47) | 442 (34) |
| Weber 2019 | 96 (71.6) | 719 (76.0) | 31 (33.7) | 203 (30.8) | 0 (0) | 29 (3.0) | 35 (38.0) | 281 (42.6) | NR | NR |
| Lefevre 2014 | 23 (88.5) | 23 (63.9) | NR | NR | NR | NR | 14 (53.8) | 11 (30.5) | NR | NR |
Note: NR: Not reported; PCO: Posterior circulation occlusion; ACO: Anterior circulation occlusion; mTICI: Modified thrombolysis in cerebral infarction; sICH: Symptomatic intracranial hemorrhage; mRS: Modified Rankin scale; FR: Futile recanalization
Baseline Characteristics of PCO versus ACO
Patients with MT in PCO were younger compared to patients in ACO (Meinel 2019, Leciñana 2017, Weber 2019). All studies consistently showed that time from onset to recanalization for PCO thrombectomy was much longer than ACO. Initial NIHSS scores varied widely. Some showed a higher NIHSS scores in PCO than in ACO, while some were just the opposite. The rate of intravenous thrombolysis in PCO was lower than in ACO, with an OR of 0.66 (95% CI 0.46–0.94; P = 0.02) (Supplementary Fig. 1).
Supplementary Fig. 1.
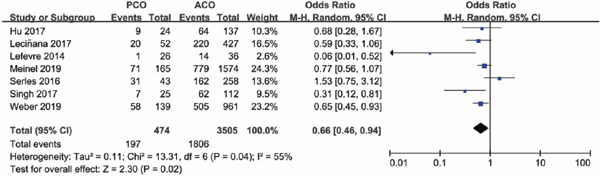
Forest plot for intravenous thrombolysis of PCO versus ACO
PCO versus ACO Thrombectomy Outcomes
Functional independence at 90 days was seen in 137/394 (34.8%) of patients with PCO intervention and 1204/2945(40.9%) of patients with ACO intervention. There was no significant difference in patients with mRS 0–2 between PCO and ACO thrombectomy (OR 0.77; 95%CI 0.55–1.09; P = 0.15) with moderate heterogeneity (I2 = 46%; P = 0.11) (Fig. 2A). However, sensitivity analysis showed a lower rate of functional independence rate after thrombectomy in PCO 123/368 (33.4%) versus ACO 1193/2909 (41.0%) on exclusion of Lefevre's study (OR 0.72; 95% CI 0.57–0.90; P = 0.005), and heterogeneity decreased remarkably (I2 = 0%; P = 0.62) (Fig. 3A).
Fig. 2.
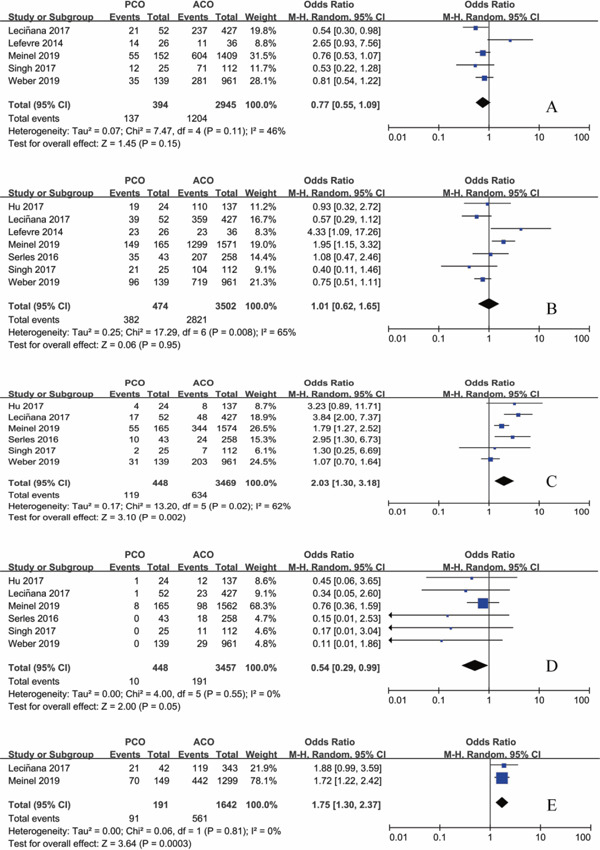
Forest plot for the main outcomes of PCO versus ACO thrombectomy
A: Functional independence at 90 days. B: Successful recanalization. C: Mortality. D: Symptomatic intracranial hemorrhage. E: Futile recanalization.
PCO: posterior circulation occlusion. ACO: anterior circulation occlusion.
Fig. 3.
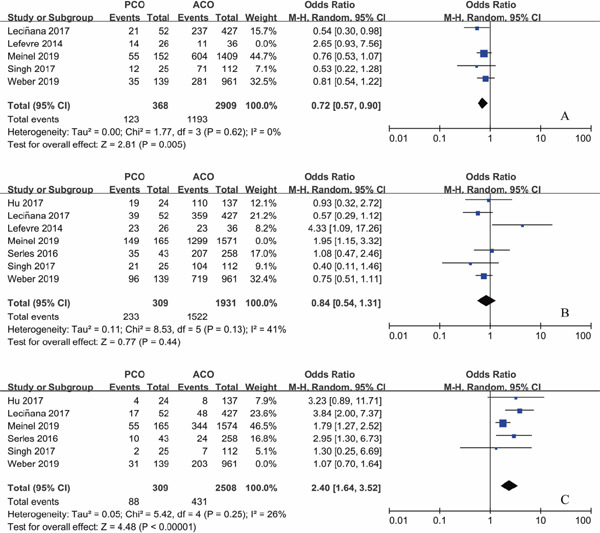
Forest plot for the main outcomes of PCO versus ACO thrombectomy after sensitivity analysis
A: Functional independence at 90 days. B: Successful recanalization. C: Mortality.
PCO: posterior circulation occlusion. ACO: anterior circulation occlusion.
Modified TICI 2b/3 was seen in 382/474 (80.6%) of patients with PCO intervention and 2821/3502 (80.6%) of patients with ACO intervention. There was no significant difference in patients with mTICI 2b/3 recanalization between PCO and ACO thrombectomy (OR 1.01; 95% CI 0.62–1.65; P = 0.95) with significant heterogeneity (I2 = 65%; P = 0.008) (Fig. 2B). On exclusion of Meinel's study, heterogeneity decreased notably (I2 = 41%; P = 0.13), with a pooled OR of successful recanalization of 0.84 (95% CI 0.54–1.31; P = 0.44) (Fig. 3B), while this study was of relatively high quality and large sample size.
Mortality occurred in 119/448 (26.6%) of patients with PCO intervention and 634/3469 (18.3%) of patients with ACO intervention. There was a higher rate of mortality after thrombectomy in PCO versus ACO (OR 2.03; 95% CI 1.30–3.18; P = 0.002), with significant heterogeneity (I2 = 62%; P = 0.02) (Fig. 2C). A sensitivity analysis showed that the study reported by Weber was a contributor to heterogeneity. On exclusion of this study, heterogeneity decreased (I2 = 26%; P = 0.25), with pooled OR of 2.40 (95% CI 1.64–3.52; P < 0.001) (Fig. 3C), while this study was of relatively high quality and large sample size.
sICH was significantly lower in patients with PCO intervention 10/448 (2.23%) compared with ACO intervention 191/3457 (5.53%) with a pooled OR of 0.54 (95%CI 0.29–0.99; P = 0.05). No significant heterogeneity was seen across the studies (I2 = 0%; P = 0.55) (Fig. 2D).
Two studies reported the futile recanalization of MT in large vessel occlusion, both of which the occlusion site of posterior circulation was BA alone. Futile recanalization occurred in 91/191 (47.6%) of patients with BAO intervention and 561/1642 (34.2%) of patients with ACO intervention. There was a significant higher futile recanalization rate of BAO than ACO (OR 1.75; 95% CI 1.30–2.37; P = 0.003), with no heterogeneity (I2 = 0%; P = 0.81) (Fig. 2E).
Subgroup Analysis
Subgroup Analysis according to Sample Size
As sample size is a powerful impact factor of the outcome, subgroup analysis was conducted based on the sample size of included studies. Considering the distribution of sample for enrolled studies, we set the cutoff of the sample “n” as “50,” after referring to other studies. Subgroup analysis illustrated that there was no significant difference in functional independence at 90 days for PCO versus ACO intervention with sample size of PCO groups containing less than 50 patients (OR 1.16; 95%CI 0.24–5.57; P = 0.86); however, with PCO groups containing more than 50 patients, functional independence rate at 90 days was significantly lower than ACO (OR 0.73; 95%CI 0.58–0.93; P = 0.01) (Supplementary Fig. 2). The results for successful recanalization rate between PCO and ACO intervention were consistent when grouped by sample size, OR 1.11 with PCO groups containing less than 50 patients (95%CI 0.49–2.48; P = 0.80) and OR 0.95 with PCO groups containing more than 50 patients (95%CI 0.47–1.92; P = 0.89) (Supplementary Fig. 3). There was significant higher mortality for PCO versus ACO intervention in studies with sample size of PCO groups containing less than 50 patients (OR 2.66; 95%CI 1.41–5.05; P = 0.003); in addition, the results persisted in the studies with PCO groups containing more than 50 patients (OR 1.86; 95%CI 1.01–3.42; P = 0.05) (Supplementary Fig. 4). There was no statistically significant difference in sICH rate for PCO versus ACO intervention in studies with PCO groups containing less than 50 patients (OR 0.26; 95%CI 0.06–1.13; P = 0.07), and the results also persisted in the studies with sample size of PCO groups containing more than 50 patients (OR 0.57; 95%CI 0.25–1.32; P = 0.19) (Supplementary Fig. 5), both with a lower tendency of sICH rate for PCO thrombectomy.
Supplementary Fig. 2.
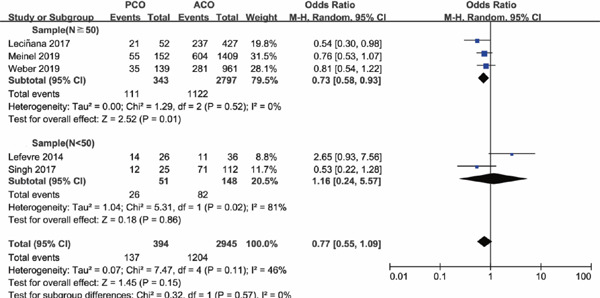
Forest plot for functional independence at 90 days of PCO versus ACO thrombectomy subgroup by sample size
Supplementary Fig. 3.
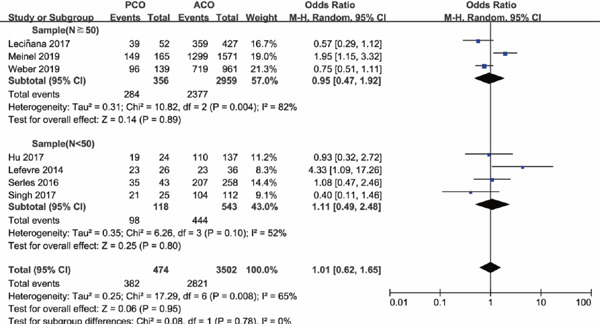
Forest plot for successful recanalization of PCO versus ACO thrombectomy subgroup by sample size
Supplementary Fig. 4.
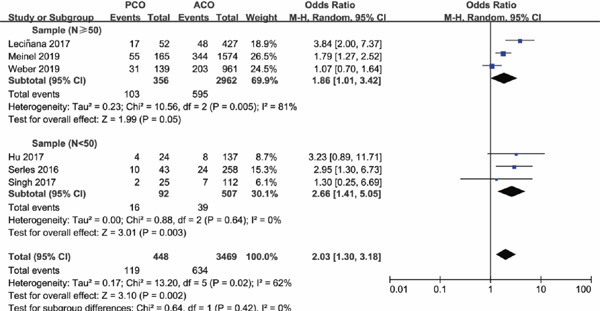
Forest plot for mortality of PCO versus ACO thrombectomy subgroup by sample size
Supplementary Fig. 5.
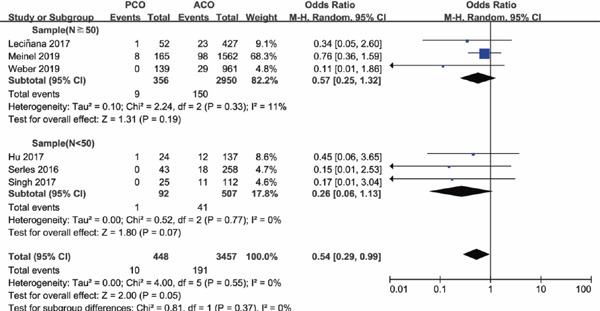
Forest plot for sICH of PCO versus ACO thrombectomy subgroup by sample size
Subgroup Analysis according to Site of Vessel Occlusion of Posterior Circulation
Four of the seven studies included PCO thrombectomy involving BA alone (BA subgroup), other three studies involving vessels of BA and VA, BA and PCA, and vertebrobasilar artery and PCA (BA + (VA and/or PCA subgroup)), respectively. The occlusion sites and the number of each site of the study were shown in Supplementary Table 2. Subgroup analysis was conducted based on whether occlusion site of posterior circulation involved BA alone or not. Subgroup analysis illustrated that there was a lower functional independence rate at 90 days for PCO versus ACO intervention in the BA subgroup (OR 0.67; 95%CI 0.51–0.90; P = 0.006), but in the BA + (VA and/or PCA) subgroup versus ACO, functional independence rate was comparable (OR 1.33; 95%CI 0.42–4.14; P = 0.63) (Supplementary Fig. 6). The results of successful recanalization rate between PCO and ACO intervention were consistent in the two subgroups, with OR of the BA subgroup of 0.87 (95%CI 0.40–1.91; P = 0.74) and OR of the BA + (VA and/or PCA) subgroup of 1.21 (95%CI 0.54–2.70; P = 0.64) (Supplementary Fig. 7). There was a significant higher mortality rate for PCO versus ACO intervention in the BA subgroup (OR 2.36; 95%CI 1.45–3.85; P = 0.0006), but in the BA + (VA and/or PCA) subgroup, mortality rates were comparable (OR 1.67; 95%CI 0.62–4.49; P = 0.31) (Supplementary Fig. 8). There was no significant difference in sICH rate for PCO versus ACO intervention in the BA subgroup (OR 0.62; 95%CI 0.33–1.18; P = 0.15), and in the BA + (VA and/or PCA) subgroup, sICH rate was significantly lower than ACO thrombectomy (OR 0.13; 95%CI 0.02–0.95; P = 0.04) (Supplementary Fig. 9).
Supplementary Table 2. Site of vessel occlusion of included studies.
| Study | Singh 2017 | Serles 2016 | Hu 2017 | Lecinana 2017 | Meinel 2019 | Weber 2019 | Lefevre 2014 | |
|---|---|---|---|---|---|---|---|---|
| PCO | BA | 25 (18.3%) | 40 (13.3%) | 24 (14.9%) | 52 (10.9%) | 165 (9.5%) | 118 (10.7%) | 25 (40.3%) |
| VA | 3 (1.0%) | 15 (1.4%) | ||||||
| PCA | 6 (0.5%) | 1 (1.6%) | ||||||
| ACO | ICA | 51 (37.2%) | 60 (19.9%) | 42 (26.1%) | 100 (20.9%) | 1574 (90.5%) | 961 (87.4) | 9 (14.5%) |
| M1 | 61 (44.5%) | 161 (53.5%) | 94 (58.4%) | 244 (50.9%) | 16 (25.8%) | |||
| M2 | 28 (9.3%) | 40 (8.3%) | ||||||
| ACA | 1 (0.6%) | |||||||
| others | 9 (3.0%)# | 43 (9.0%)* | 11 (17.8%)* |
Note:
Represents others
Represents tandem occlusion; BA: Basilar artery; VA: Vertebral artery; PCA: Posterior cerebral artery; ICA: Internal carotid artery; M1: 1st segment of middle cerebral artery; M2: 2nd segment of middle cerebral artery; ACA: Anterior cerebral artery
Supplementary Fig. 6.
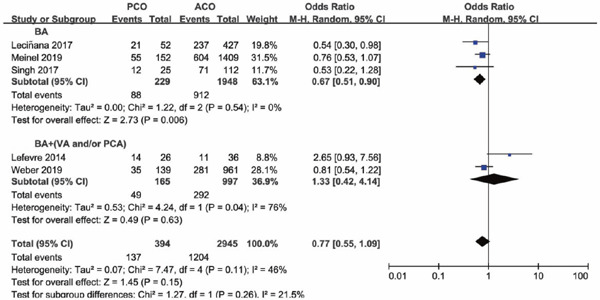
Forest plot for functional independence at 90 days of PCO versus ACO thrombectomy subgroup by site of vessel occlusion
Supplementary Fig. 7.
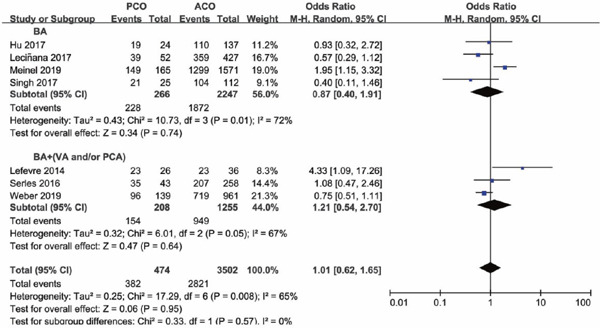
Forest plot for successful recanalization of PCO versus ACO thrombectomy subgroup by site of vessel occlusion
Supplementary Fig. 8.
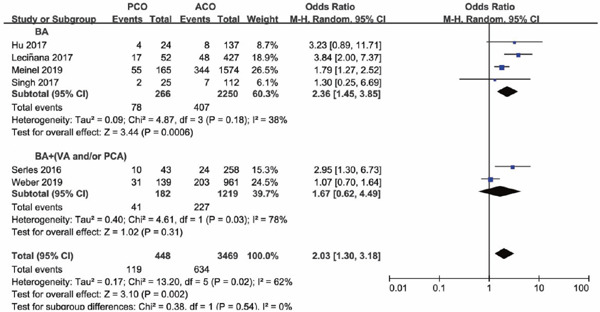
Forest plot for mortality of PCO versus ACO thrombectomy subgroup by site of vessel occlusion
Supplementary Fig. 9.
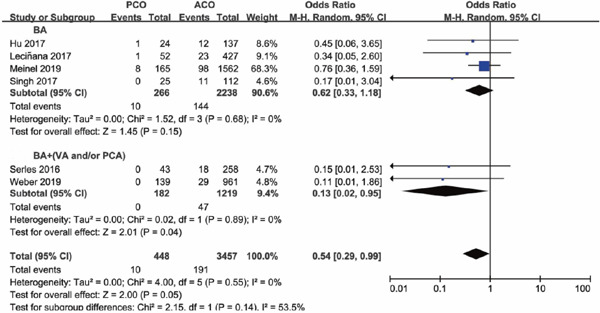
Forest plot for sICH of PCO versus ACO thrombectomy subgroup by site of vessel occlusion
Discussion
A valuable picture of the efficacy and safety of modern-device MT for PCO patients was displayed in this meta-analysis. Seven studies involving 474 patients were identified, and outcomes were compared with ACO. With regard to functional independence at 90 days, Lefevre's study was excluded due to the smallest sample size and the lowest quality in all of the studies. In addition, the incidence of PCS (41.9%) of this study was too high, indicating an obvious selection bias. Due to a fewer number of studies included, publication bias cannot be effectively presented by a funnel plot. In our meta-analysis, most of the studies included were retrospective and observational; thus withdraw bias existed. One of the studies was sponsored by the drug company, leading to the possibility of bias. Furthermore, the sample size of some studies was small, and baseline characteristics were different, all of which resulting in the possibility of publications bias.
Overall, our meta-analysis illustrated that compared to ACO, patients with PCO thrombectomy achieved similar revascularization rate and lower rate of sICH but possibly lower rate of functional independence and much higher mortality, providing a fundamental data to clinical practice and further studies as much as possible. However, due to the heterogeneity of baseline characteristics of posterior and anterior circulation, the results might be shaky.
In agreement with previous studies comparing intravenous thrombolysis in anterior circulation stroke and in PCS, patients with PCO intervention were younger20). The lower rate of intravenous thrombolysis in PCO may be explained by a delayed or missed diagnosis of PCS and beyond the time window of intravenous thrombolysis. It seems to be more time-consuming from onset to recanalization in PCO than in ACO, probably because of longer time taken from onset to initiation of treatment. Additionally, interventions of PCO usually require general anesthesia, which was more laborious than local anesthesia21). Two studies reported the way of anesthesia in our meta-analysis: the rate of general anesthesia was 64.0% and 88.2% in PCO thrombectomy versus 58.9% and 54.9% in ACO thrombectomy, respectively13, 17). The high variance of NIHSS scores of PCS could be partly explained by the fact that NIHSS has limitations in the assessment of neurological deficits in patients with PCO, such as unsteady gait, dysphagia, or oculomotor disorders, which are not represented on this scale22). On the other hand, once PCO leads to disturbance of consciousness, NIHSS score will increase significantly.
Previous studies have demonstrated that in contrast to anterior circulation stroke, PCS is usually associated with poor outcomes. Morbidity and mortality rates have been reported to be up to 90% of acute BAO without active treatment23). The devastating clinical outcome of PCO limited the possibility of conducting a randomized controlled trial that in most cases MT is performed to improve the clinical prognosis.
Our meta-analysis showed that 80.6% of patients achieved successful recanalization, along with lower rates of sICH (2.23%), moderate rates of functional independence (34.8%), but also relatively high rate of mortality (26.6%) in patients with PCO intervention, in keeping with two previous meta-analysis evaluating the outcomes of stent retriever thrombectomy in case series of BAO, in which the recanalization rates were 81% and 80.0%, rates of favorable outcome at 90 days were 42% and 42.8%, rates of sICH were 4% and 6.8%, and rates of mortality were 30% and 29.4%, respectively24, 25).
Even with comparable successful recanalization rates, patients with PCO thrombectomy had relatively worse outcomes than ACO, as was shown by a lower rate of favorable outcome and a higher rate of mortality in our analysis. This may be explained by the higher (OR, 1.75) chance of futile recanalization, which was defined as mRS score 4 to 6 despite successful recanalization, in PCO (47.6%) than in ACO (34.2%) intervention17). Due to the inability to extract data from the two studies referring to futile recanalization, analysis of related risk factors could not be conducted. Of the two included studies, predictors for futile recanalization were age, stroke severity, maneuver count, intracranial stenting, and duration of the procedure. Other factors such as baseline NIHSS score, lower initial posterior circulation Alberta stroke program early CT score, and onset to start of endovascular treatment time were also identified by previous literature24, 26). Our study showed comparable rates of successful recanalization in patients with PCO thrombectomy to patients with ACO. Despite the higher rate of futile recanalization, achievement of recanalization was associated with a higher probability of functional independence in patients with PCO, as was elucidated in the included studies of Hu (2017), Singh (2017), and Meinel (2019). Recognition of reliable predictors associated with futile recanalization in PCO might help increase the efficacy of MT. Patients with PCO thrombectomy had a lower rate of sICH compared with ACO, which was consisted with previous study on risk of bleeding in intravenous thrombolysis for PCS and anterior circulation stroke27). Compared to ACO, PCO typically causes smaller ischemic lesion volumes, which may reduce the rate of hemorrhagic transformation after revascularization therapy. A study found that pretreatment permeability abnormality, an indicator of blood-brain barrier derangements, was an infrequent finding in acute PCS (7%) but occurred in 22% of patients with anterior circulation stroke28).
Subgroup analysis according to sample size gave rise to different functional outcomes. The reason why there was no significant difference in functional independence at 90 days between the two groups with sample size of PCO groups containing less than 50 patients could be explained by a bias due to small sample size. The reason why the BA subgroup had a lower functional independence rate and higher mortality rate than the BA + (VA and/or PCA) subgroup may be that stroke owing to BA occlusion is particularly severe.
Our meta-analysis has strengths. Firstly, to the best of our knowledge, this is the first meta-analysis that compared the outcomes between PCO and ACO thrombectomy, which correlates closely with clinical practice and provides a fundamental data to clinical practice and further studies as much as possible. Secondly, subgroup and sensitivity analyses were also conducted. Limitations of our meta-analysis should be addressed here. Firstly, all studies included were observational and retrospective design with heterogeneous baseline characteristics, leading to the shaky results. Secondly, there were fewer studies included, and the sample size of some studies was small, which might cause the possibility of bias.
Conclusions
In terms of the disagreements of previous reports on outcomes of MT in PCO versus ACO and to explore the efficacy and safety of MT in PCO, we conducted this meta-analysis. Our meta-analysis suggests that outcomes of MT in PCO were far from being ideal, with a slightly but statistically significant lower rate of functional independence and a higher rate of mortality compared to ACO. On the other hand, MT appears to have lower rates of sICH and to increase successful recanalization. Given the high recanalization rate provided by MT, our study supports the recommendation to treat patients of PCO with MT as an adjunct to standard treatment. The key point to improve outcomes of MT in PCO patients is recognizing the factors associated with futile recanalization and optimizing the results of MT. However, with regard to the different characteristics of PCS and anterior circulation stroke, our results are far from robust. Large-scale randomized controlled trials are needed to warrant the efficacy and safety of MT in PCO in the future.
Contributors
Wang F for the study design, data acquisition and analysis, manuscript preparation, and editing. Wang J for data acquisition and analysis, manuscript preparation, and editing. He Q data for analysis, manuscript preparation, and editing. Wang L and Cao Y for data acquisition and analysis. Zhang H for manuscript preparation and editing. Xu Z designed the study, monitored the study inclusion and data extraction, and revised the manuscript. He is the guarantor.
Funding
This work was funded by the National Natural Science Foundation of China (81671159) and the National Natural Science Foundation of China (31700927).
COI
All authors declare that they have no conflict of interest.
References
- 1). Merwick A, Werring D: Posterior circulation ischaemic stroke. BMJ, 2014; 348: g3175. [DOI] [PubMed] [Google Scholar]
- 2). Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, ESCAPE Trial Investigators : Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med, 2015; 372: 1019-1030 [DOI] [PubMed] [Google Scholar]
- 3). Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à, Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, MR CLEAN Investigators : A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med, 2015; 372: 11-20 [DOI] [PubMed] [Google Scholar]
- 4). Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, SWIFT PRIME Investigators : Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med, 2015; 372: 2285-2295 [DOI] [PubMed] [Google Scholar]
- 5). Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, EXTEND-IA Investigators : Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med, 2015; 372: 1009-1018 [DOI] [PubMed] [Google Scholar]
- 6). Jovin TG, Chamorro A, Cobo E, deMiquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A, REVASCAT Trial Investigators : Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med, 2015; 372: 2296-2306 [DOI] [PubMed] [Google Scholar]
- 7). Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, Engelter ST, Tanne D, Muir KW, Molina CA, Thijs V, Audebert H, Pfefferkorn T, Szabo K, Lindsberg PJ, de Freitas G, Kappelle LJ, Algra A, BASICS study group : Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol, 2009; 8: 724-730 [DOI] [PubMed] [Google Scholar]
- 8). Ritvonen J, Strbian D, Silvennoinen H, Virtanen P, Salonen O, Lindsberg PJ, Sairanen T: Thrombolysis and adjunct anticoagulation in patients with acute basilar artery occlusion. Eur J Neurol, 2019; 26: 128-135 [DOI] [PubMed] [Google Scholar]
- 9). Gilberti N, Gamba M, Premi E, Costa A, Vergani V, Delrio I, Spezi R, Mardighian D, Frigerio M, Gasparotti R, Padovani A, Magoni M: Endovascular mechanical thrombectomy in basilar artery occlusion: variables affecting recanalization and outcome. J Neurol, 2016; 263: 707-713 [DOI] [PubMed] [Google Scholar]
- 10). Du S, Mao G, Li D, Qiu M, Nie Q, Zhu H, Yang Y, Zhang Y, Li Y, Wu Z: Mechanical thrombectomy with the solitaire AB stent for treatment of acute basilar artery occlusion: A single center experience. J Clin Neurosci, 2016; 32: 67-71 [DOI] [PubMed] [Google Scholar]
- 11). Huo X, Gao F, Sun X, Ma N, Song L, Mo D, Liu L, Wang B, Zhang X, Miao Z: Endovascular mechanical thrombectomy with the solitaire device for the treatment of acute basilar artery occlusion. World Neurosurg, 2016; 89: 301-308 [DOI] [PubMed] [Google Scholar]
- 12). Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group : Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ, 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 13). Singh RK, Chafale VA, Lalla RS, Panchal KC, Karapurkar AP, Khadilkar SV, Ojha PK, Godge Y, Singh RK, Benny R: Acute ischemic stroke treatment using mechanical thrombectomy: a study of 137 patients. Ann Indian Acad Neurol, 2017; 20: 211-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Serles W, Gattringer T, Mutzenbach S, Seyfang L, Trenkler J, Killer-Oberpfalzer M, Deutschmann H, Niederkorn K, Wolf F, Gruber A, Hausegger K, Weber J, Thurnher S, Gizewski E, Willeit J, Karaic R, Fertl E, Našel C, Brainin M, Erian J, Oberndorfer S, Karnel F, Grisold W, Auff E, Fazekas F, Haring HP, Lang W, Austrian Stroke Unit Registry Collaborators : Endovascular stroke therapy in Austria: a nationwide 1-year experience. Eur J Neurol, 2016; 23: 906-911 [DOI] [PubMed] [Google Scholar]
- 15). Hu SY, Yi HJ, Lee DH, Hong JT, Sung JH, Lee SW: Effectiveness and safety of mechanical thrombectomy with stent retrievers in basilar artery occlusion: comparison with anterior circulation occlusions. J Korean Neurosurg Soc, 2017; 60: 635-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Alonso de Leciñana M, Kawiorski MM, Ximénez-Carrillo Á, Cruz-Culebras A, García-Pastor A, Martínez-Sánchez P, Fernández-Prieto A, Caniego JL, Méndez JC, Zapata-Wainberg G, De Felipe-Mimbrera A, Díaz-Otero F, Ruiz-Ares G, Frutos R, Bárcena-Ruiz E, Fandiño E, Marín B, Vivancos J, Masjuan J, Gil-Nuñez A, Díez-Tejedor E, Fuentes B, Madrid Stroke Network : Mechanical thrombectomy for basilar artery thrombosis: a comparison ofoutcomes with anterior circulation occlusions. J Neurointerv Surg, 2017; 9: 1173-1178 [DOI] [PubMed] [Google Scholar]
- 17). Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Mosimann PJ, Michel P, Hajdu S, Ribo M, Requena M, Maegerlein C, Friedrich B, Costalat V, Benali A, Pierot L, Gawlitza M, Schaafsma J, Pereira VM, Gralla J, Fischer U: Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futilerecanalization in comparison with the anterior circulation. J Neurointerv Surg, 2019; 11: 1174-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Weber R, Minnerup J, Nordmeyer H, Eyding J, Krogias C, Hadisurya J, Berger K, REVASK investigators : Thrombectomy in posterior circulation stroke: differences in procedures andoutcome compared to anterior circulation stroke in the prospectivemulticentre REVASK registry. Eur J Neurol, 2019; 26: 299-305 [DOI] [PubMed] [Google Scholar]
- 19). Lefevre PH, Lainay C, Thouant P, Chavent A, Kazemi A, Ricolfi F: Solitaire FR as a first-line device in acute intracerebral occlusion: a single-centre retrospective analysis. J Neuroradiol, 2014; 41: 80-86 [DOI] [PubMed] [Google Scholar]
- 20). Sarikaya H, Arnold M, Engelter ST, Lyrer PA, Mattle HP, Georgiadis D, Bonati LH, Fluri F, Fischer U, Findling O, Ballinari P, Baumgartner RW: Outcomes of intravenous thrombolysis in posterior versus anterior circulation stroke. Stroke, 2011; 42: 2498-2502 [DOI] [PubMed] [Google Scholar]
- 21). Sarraj A, Medrek S, Albright K, Martin-Schild S, Bibars W, Vahidy F, Grotta JC, Savitz SI: Posterior circulation stroke is associated with prolonged door-to-needle time. Int J Stroke, 2015; 10: 672-678 [DOI] [PubMed] [Google Scholar]
- 22). Kasner SE: Clinical interpretation and use of stroke scales. Lancet Neurology, 2006; 5: 603-612 [DOI] [PubMed] [Google Scholar]
- 23). Mortimer AM, Bradley M, Renowden SA: Endovascular therapy for acute basilar artery occlusion: a review of the literature. J Neurointerv Surg, 2012; 4: 266-273 [DOI] [PubMed] [Google Scholar]
- 24). Gory B, Eldesouky I, Sivan-Hoffmann R, Rabilloud M, Ong E, Riva R, Gherasim DN, Turjman A. Nighoghossian N, Turjman F: Outcomes of stent retriever thrombectomy in basilar artery occlusion: an observational study and systematic review. J Neurol Neurosurg Psychiatry, 2016; 87: 520-525 [DOI] [PubMed] [Google Scholar]
- 25). Phan K, Phan S, Huo YR, Jia F, Mortimer A: Outcomes of endovascular treatment of basilar artery occlusion in the stent retriever era: a systematic review and meta-analysis. J Neurointerv Surg, 2016; 8: 1107-1115 [DOI] [PubMed] [Google Scholar]
- 26). Hussein HM, Saleem MA, Qureshi AI: Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology, 2018; 60: 557-563 [DOI] [PubMed] [Google Scholar]
- 27). Tong X, Liao X, Pan Y, Cao Y, Wang C, Liu L, Zheng H, Zhao X, Wang C, Wang Y, Wang Y. Thrombolysis Implementation, Monitor of Acute Ischemic Stroke in China (TIMS-China) Investigators: Intravenous thrombolysis is more safe and effective for posterior circulation stroke: Data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China). Medicine (Baltimore), 2016; 95: e3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Lee M, Saver JL, Alger JR, Hao Q, Starkman S, Ali LK, Kim D, Ovbiagele B, Vespa PM, Froehler MT, Tenser MS, Salamon N, Villablanca JP, Jahan R, Duckwiler GR, Tateshima S, Gonzalez N, Vinuela F, Liebeskind DS: Blood-brain barrier permeability derangements in posterior circulation ischemic stroke: frequency and relation to hemorrhagic transformation. J Neurol Sci, 2012; 313: 142-146 [DOI] [PMC free article] [PubMed] [Google Scholar]


