Abstract
Purpose of review:
Activated protein C is a homeostatic coagulation protease with anticoagulant and cytoprotective activities. Focusing on APC’s effects in the brain, this review discusses three different scenarios that illustrate how APC functions are intimately affecting the physiology and pathophysiology of the brain.
Recent findings:
Cytoprotective APC therapy holds promise for treatment of ischemic stroke, and a recently completed trial suggested that cytoprotective-selective 3K3A-APC reduced bleeding in ischemic stroke patients. In contrast, APC’s anticoagulant activity contributes to brain bleeding as shown by the disproportional upregulation of APC generation in cerebral cavernous malformations (CCM) lesions in mice. However, too little APC generation also contributes to maladies of the brain, such as in case of cerebral malaria where the binding of infected erythrocytes to the endothelial protein C receptor (EPCR) may interfere with the EPCR-dependent functions of the protein C pathway. Furthermore, discoveries of new activities of APC such as the inhibition of the NLRP3-mediated inflammasome and of new applications of APC therapy such as in Alzheimer’s disease and graft-versus-host disease continue to advance our knowledge of this important proteolytic regulatory system.
Summary:
APC’s many activities or lack thereof are intimately involved in multiple neuropathologies, providing abundant opportunities for translational research.
The homeostatic protease APC:
Activated protein C (APC) is a coagulation protease with multiple biological functions regulating coagulation, inflammation, and cell survival that are important for maintaining the homeostatic balance during health and disease (Figure 1).1–7 Activation of protein C zymogen is mediated by the thrombin-thrombomodulin (TM) complex on the endothelial cell surface and facilitated by presentation of protein C by the endothelial protein C receptor (EPCR).2,8 Upon activation, the serine protease APC can engage with multiple substrates to mediate distinctly different functional effects that are broadly referred to as APC’s anticoagulant and cytoprotective activities. APC anticoagulant effects of involve the proteolytic inactivation of activated blood coagulation factors V and VIII aided by cofactors such as protein S, factor V itself, and various lipids, described in numerous reviews.9–11 Increased risks for thrombosis associated with impairments of APC’s anticoagulant pathway are well appreciated,12–14 but recent advances also highlight that disproportional APC generation contributes to bleeding in patients with severe trauma,15–17 hemophilia,18,19 or cerebral cavernous malformations20 (see Scenario 2 below). This information illustrates the importance of proper regulation of APC generation and activity, with both too little and too much APC contributing to pathologies. As such, recent interest in therapeutic modulation of APC activity focus on inhibition of APC anticoagulant activity to dampen bleeding.21–25 However, it is critical to consider potential effects on APC’s cytoprotective activities when evaluating such strategies.
Figure 1: Pathways of the protein C system.
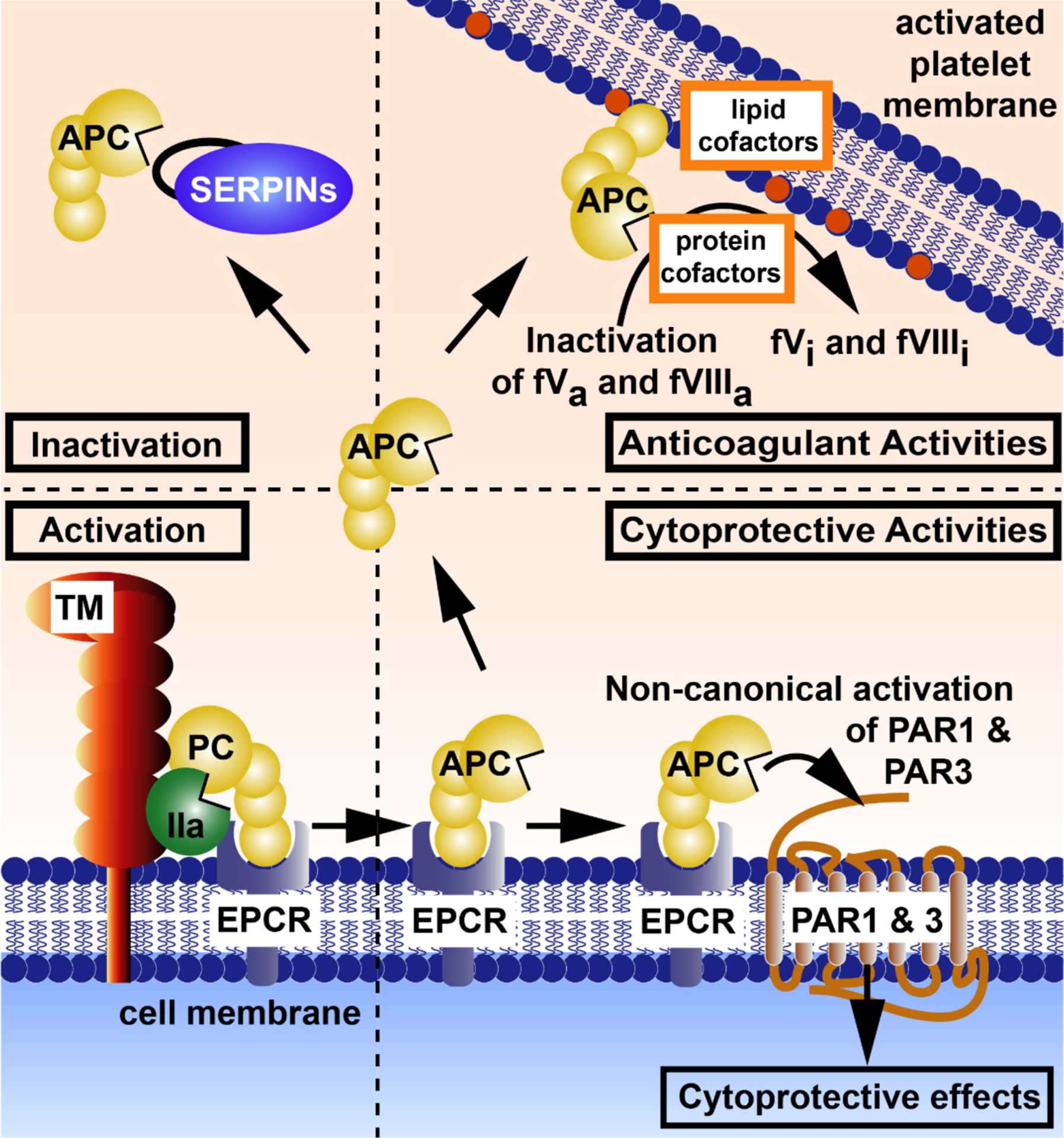
The four major pathways of the protein C system are: (1, bottom left) activation of protein C and generation of APC, (2, bottom right) the cytoprotective pathway, (3, top right) the anticoagulant pathway, and (4, top left) inactivation of APC by serine protease inhibitors (SERPINs). (1, bottom left) Activation of protein C is mediated by thrombomodulin (TM)-bound thrombin and facilitated by presentation of protein C bound to EPCR on the endothelial cell surface. (2, bottom right) After activation when APC is bound to EPCR, APC can induce cytoprotective activities via the non-canonical activation of PAR1 at Arg46 and of PAR3 at Arg41. Cytoprotective activities may include anti-apoptotic activities, anti-inflammatory activities including inhibition of NLRP3-mediated inflammasomes and inhibition of neutrophil extracellular trap formation, stabilization of endothelial barriers, and alterations of gene expression profiles. Not shown here is APC’s ability to inactivate extracellular histones, which does not require APC-mediated cell signaling but involves proteolytic degradation by APC. Also, not depicted, are other receptors required for some of APC’s cytoprotective effects, including Mac-1, S1P receptor 1, Tie2, and others. (3, top right) Dissociation of APC from EPCR and binding of APC to negatively charged lipid membranes such as on activated platelets permits anticoagulant activity of APC. Proteolytic inactivation of the activated clotting factors Va and VIIIa by APC is supported by various cofactors such as protein S, factor V, and various lipid cofactors. (4, top left) Inactivation of APC by SERPINs limits the activity of APC in plasma and is primarily responsible for the half-live of ~16 min of APC in vivo. Abbreviations: PC, protein C; APC, activated protein C; IIa, thrombin; TM, thrombomodulin; EPCR, endothelial protein C receptor; PAR, protease activated receptor; FVa, activated factor V; FVIIIa, activated factor VIII; SERPINs, serine protease inhibitors. This figure is modified from Griffin et al, ATVB, 2016.124
Mechanisms of action for APC cytoprotective effects:
Independent of APC’s anticoagulant activities are APC’s effects on cells that are collectively referred to as APC’s cytoprotective effects (Figure 1). Multiple receptors contribute to the cellular effects of APC on different cell types, including CD11b/CD18 (MAC-1), ApoER2, Tie2, protease activated receptor (PAR) 2, sphingosine-1-phosphate receptor 1 (S1P1), but most often a combination of EPCR and PAR1 and/or PAR3 are required.2–6,26 Beneficial effects of APC that require its cytoprotective activities have been reported in many different disease models of different organs including the brain, lung, and kidney that manifest as anti-apoptotic and anti-inflammatory effects, endothelial and epithelial cell barrier protective effects, and/or regenerative and wound healing effects.2–7,27 While signaling pathways induced by APC show some cell-type specificity, generally, APC is understood to induce PAR1-mediated biased signaling that encompasses β-Arrestin-2-mediated signal transduction in caveolin-1-enriched caveolae.28–31 In endothelial cells, PAR1-dependent APC signaling manifests as activation of the PI3K-Akt hub and Rac-1, after which downstream pathways provide anti-apoptotic and endothelial barrier protective effects. These signaling pathways contrast with those induced by thrombin-mediated PAR1 agonism on endothelial cells that instead involve the activation of ERK1/2 and Rho-A and manifest as proliferative and endothelial barrier disruptive responses.31
PARs do not rely on external ligands for activation but instead carry their own encrypted tethered ligand to be exposed after proteolytic cleavage, such as the canonical cleavage of PAR1 by thrombin at Arg41 exposing the classical “SFLLRN…” tethered-ligand that induces G-protein-dependent signaling.32–34 The notion that some proteases activate PAR1 at other sites, resulting in different tethered-ligands with different activities, provides an explanation for the conundrum how different proteases can use the same receptor to induce sometimes very different and contrasting effects (Table 1).3,33,34 In particular, APC induces biased agonism of PAR1 by activating PAR1 at non-canonical Arg46 thereby generating the tethered-ligand “NPNDKY…” that promotes PAR1 conformations associated with β-Arrestin-2-mediated biased signaling.31 Peptides (e.g. TR47) representing this N-terminal starting at Asn47 mimic APC-mediated PAR1 signaling.6,31,35 In addition, mice with PAR1 mutations at either canonical Arg41 or non-canonical Arg46, thereby rendering them resistant to activation by thrombin or APC, respectively, demonstrated that beneficial protective effects of APC in stroke and sepsis models require PAR1 activation at Arg46.36 Thus, providing in vivo proof for the non-canonical activation of PAR1 by APC and APC-induced biased signaling. Parmodulin 2, a small molecule binding to the cytoplasmic side of PAR1, also acts as a biased agonist, presumably by stabilizing PAR1 conformations associated with β-Arrestin-2-mediated signaling, and recapitulates PAR1-dependent cytoprotective signaling.37
Table 1:
Canonical agonist and biased agonist peptides of PAR1 and PAR3 generated by APC and thrombin.
| Peptide | PAR | Generated by | Sequence | Biological effect |
|---|---|---|---|---|
| TR42 (TRAP) | PAR1 | thrombin | 42-SFLLRNPNDKYEPFWEDEEKNESGL-66 | platelet activator, proinflammatory, endothelial barrier disruptive |
| TR47 | PAR1 | APC | 47-NPNDKYEPFWEDEEKNESGL-66 | cytoprotective, anti-inflammatory, endothelial barrier protective |
| P3K | PAR3 | thrombin | 39-TFRGAPPNSFEEFPFSALEGWTGATIT-65 | enhances PAR1-dependent ERK1/2 signaling by thrombin |
| P3R | PAR3 | APC | 42-GAPPNSFEEFPFSALEGWTGATIT-65 | endothelial barrier protective, activates Tie2 |
In addition, non-canonical activation of PAR3 at Arg41 by APC generates the tethered-ligand “GAPPNS…” with barrier protective properties.38 Similar to PAR1, PAR3 peptides (e.g., P3R), representing the non-canonical tethered-ligand generated by APC, mimic effects of APC (Table 1).38,39 While the APC generated PAR1 (TR47) and PAR3 (P3R) peptides share some functional outcomes, such as protection of endothelial barrier function, it is becoming increasingly clear that these peptides also can induce unique signaling pathways, such as the activation of Tie2 by P3R but not TR47 peptides (Figure 2).39 This suggests that APC uses PAR1 and PAR3 to diversify it signaling repertoire. New expansion of the repertoire of APC’s cytoprotective effects in diabetes and graft-versus-host disease adds to this notion.40,41
Figure 2: Functional selectivity of PAR1 and PAR3 signaling due to non-canonical activation by APC.
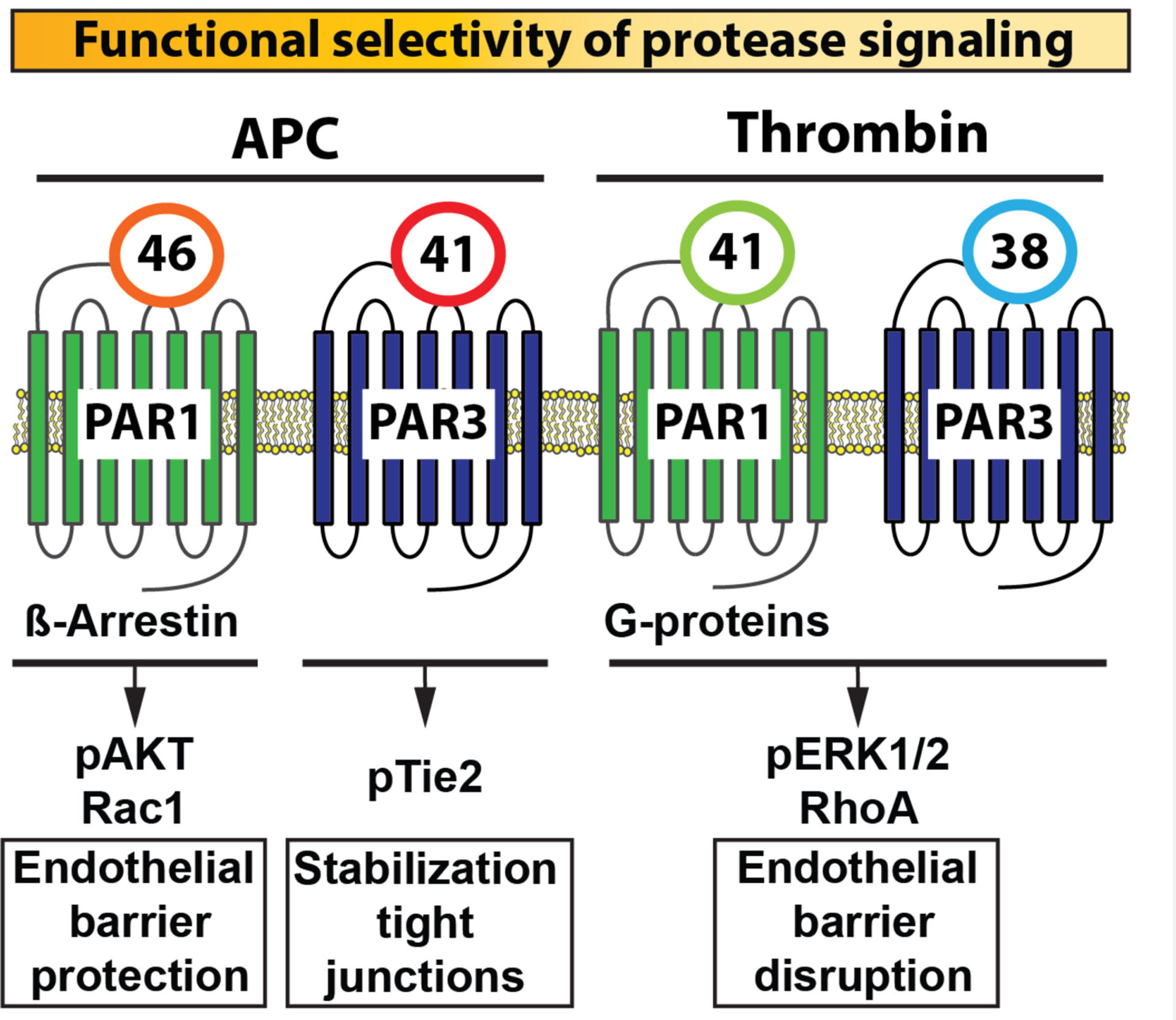
Schematic portrayal of how different PAR1 and PAR3 tethered-ligands are generated that start at either residue 42 or 47 for PAR1 or at residue 39 or 42 for PAR3 depending on whether activation occurred by thrombin or APC, respectively. These canonical and non-canonical tethered ligands induce PAR conformations that selectively promote the activation of distinctly different signaling pathways, thereby providing an explanation for the functional selectivity of thrombin’s and APC’s protease signaling. For instance, the thrombin generated PAR1 tethered-ligand starting at residue 42 promotes the association of conformational subsets of PAR1 with G-proteins, resulting in activation of ERK1/2 and RhoA that manifests in endothelial barrier disruption. The APC generated PAR1 tethered-ligand starting at residue 47 however, promotes association of PAR1 with β-Arrestin, resulting in biased signaling involving activation of Akt and Rac-1 that manifests in endothelial barrier protection. The PAR3 tethered-ligand sequence starting at residue 39 can form heterodimers with PAR1 to promote endothelial disruption via ERK1/2 activation.125 Alternatively, PAR3 can promote activation of Tie2 and vascular protective effects when the PAR3 tethered-ligand sequence starts at residue 42 due to cleavage by APC. Integration of PAR3 with PAR1 or PAR2 (not depicted here) signaling after activation by APC may also involve the formation of heterodimers but exact mechanisms remain to be elucidated. Thus, the PAR1-PAR3 activation profile of thrombin and APC represent the two ends of the spectrum for the functional selectivity of protease signaling with distinctly different cellular effects.
Inhibition of inflammasome, a new framework for APC’s anti-inflammatory activity:
Anti-inflammatory effects of APC have long been appreciated, and include inhibition of NFkB activation, changes in gene expression profiles, down regulation of adhesion molecules on endothelial cells, inhibition of neutrophil NETosis, and cleavage of extracellular histones.42–47
The recent discovery that APC inhibits activation of the NLRP3-mediated inflammasome puts a new framework on APC’s anti-inflammatory activities.48 Inflammasomes are intracellular sensors for infectious and sterile stressors to execute the release of inflammatory mediators.49,50 Especially, the NOD-like receptor NLRP3-mediated inflammasome responds to both endogenous stimuli, e.g., generated by ischemia-reperfusion injury as well as infection-generated danger signals such as LPS, to activate caspase 1 and mediate the proteolytic activation and release of IL1β, and IL-18 from cells in a two-step process.51,52 In the first priming step, activation of pattern recognition receptors upregulate inflammasome components in reactions that involve NFκB-dependent gene expression pathways.52,53 Mechanisms for NLRP3 activation in the second step, that involve the formation of the caspase-1 activating macromolecular complex of NLRP3 with adaptor and effector proteins, remain to be fully elucidated but may result from cellular electrolyte imbalance, and/or metabolic, mitochondrial or lysosomal dysfunction.51,52 Early data suggests that APC has potential to inhibit both the priming step, potentially by inhibition of NFκB activation, and the NLRP3 activation step.48,53–55 Mechanisms for inhibition of NLRP3 activation by APC remain to be elucidated but may involve dampening of mitochondrial and/or metabolic dysfunction as these are activities of APC that have been previously reported.48,56–58
Accumulating evidence implicates that inflammasome activation contributes to tissue damage in many cardiovascular diseases, including ischemia-reperfusion injury, atherosclerosis, diabetes and stroke.50–52 This notion is further supported by the realization that in addition to innate immune cells also many other tissue resident cell types have functional inflammasomes, including endothelial cells, fibroblasts and even platelets. Based on the initial report that APC inhibits the NLRP3-mediated inflammasome,48 an obvious question is whether protective effects of endogenously generated APC or pharmacologically administered APC may involve inflammasome inhibition in other cardiovascular diseases where the inflammasome is implicated to contribute to pathology? Research in this area is likely to see considerable developments in the next few years.
Neuroprotective effects of APC:
APC therapy provides beneficial effects in multiple rodent models of brain disease that include ischemic stroke, amyotrophic lateral sclerosis, multiple sclerosis, and recently, Alzheimer’s disease.2,59–62 EPCR-dependent translocation of APC across the blood-brain-barrier indicates that APC effects in the brain are not limited to the vascular compartment but can also involve cellular targets within the brain.63 APC’s neuroprotective effects primarily involve its cytoprotective activities that require PAR1, PAR3 and EPCR and manifest as protection of blood-brain-barrier function, inhibition of neuroinflammation, inhibition of neuronal apoptosis, and regenerative effects targeting neuronal stem cells.2,64–66
Bleeding in the brain and hemorrhagic conversion after thrombolytic therapy is a serious concern in ischemic stroke patients,67,68 and lessons learned from APC therapy (Xigris, Eli Lilly) in sepsis patients in the early 2000’s indicated that wild type (wt)-APC therapy is limited by its anticoagulant activity and associated bleeding risk.69–71 Since substrates for APC anticoagulant activity (factor Va and VIIIa) differ from APC cytoprotective activity (PAR1 and PAR3), mutation of exosite residues in APC important for interactions with specific substrates permitted the generation of activity-selective APC variants.9,72 The cytoprotective-selective APC variant 3K3A-APC with 3 Lys residues replaced with Ala in loop 37 has full cytoprotective activity yet <10% anticoagulant activity (Figure 3) and has been extensively tested in ischemic stroke and other brain disease models demonstrating beneficial effects.2,72
Figure 3: Cytoprotective-selective 3K3A-APC.
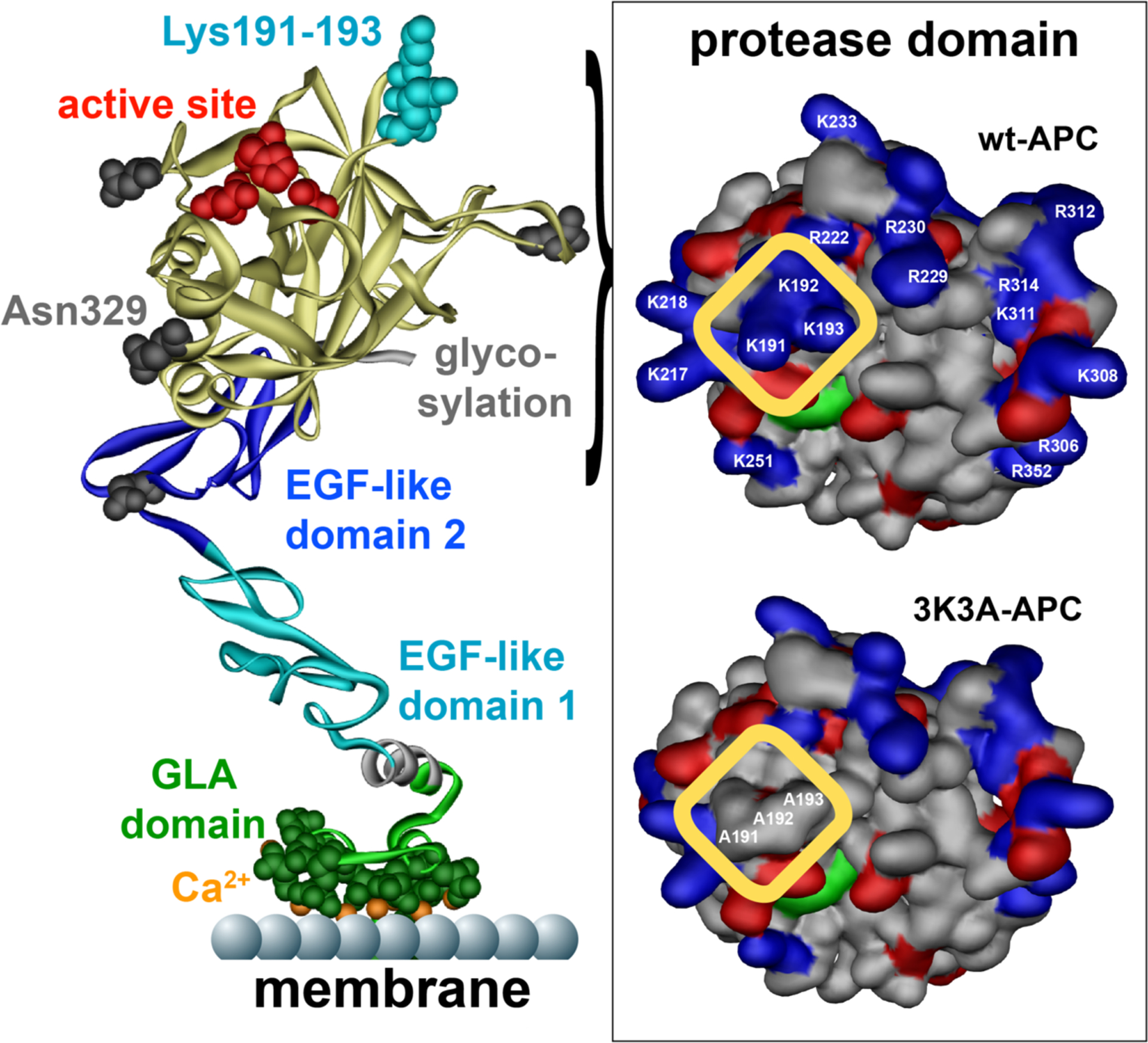
APC, denoted on the left by a ribbon structure, is comprised of an N-terminal GLA domain (green) that binds to negatively charged phospholipids and EPCR, two EGF-like domains (blue), and the protease domain (yellow) containing the active site (red). Four glycosylation attachment sites are indicated in gray. Interaction of APC with its multiple substrates is often mediated by charged residues in surface exposed loops on APC. These so called binding exosites on APC vary for different substrates. Three positively charged lysine residues (KKK191–193) in the 37-loop of APC form a positively charged patch (see highlighted yellow box top right) important for APC’s recognition of factors Va and VIIIa. Mutation of these 3 lysine residues to alanine (3K3A-APC) eliminates this binding exosite for factors Va and VIIIa on the surface of APC (see highlighted yellow box bottom right) and results in a reduction of APC’s anticoagulant activity by 90%. In contrast, the cytoprotective substrates, PAR1, or its other known cell signaling receptors do not require these 3 lysine residues for interaction with APC and consequently 3K3A-APC retains normal cytoprotective activities. While surfaces on APC interacting with PAR1 remain to be fully defined, a potential binding exosite for PAR1 is located in the protease domain of APC (yellow) around the glycosylation attachment site at Asn329 (bottom left). Elimination of the glycosylation at Asn329 increases APC’s cytoprotective activities, whereas mutation of two nearby negatively charged glutamic acid residues at 330 and 333 to alanine eliminates APC’s cytoprotective activities.126,127 This suggests that exosites for APC’s anticoagulant and cytoprotective substrates are located on different and opposite sides of the protease domain thereby providing an explanation why 3K3A-APC is cytoprotective-selective. This figure is modified from Griffin et al, Blood, 2018.2
Activation of the NLRP3 inflammasome has been implicated to promote disease progression of numerous brain maladies, including neuroinflammation and post-ischemic reperfusion injury after ischemic stroke.50,73 Inflammasomes have been described in microglia, astrocytes and neurons and some of APC’s effect in the brain may involve inhibition of inflammasome activation. 3K3A-APC efficiently inhibited NLRP3 inflammasome in the heart and kidney and 3K3A-APC therapy reduced brain bleeding associated with thrombolytic therapy in a rodent stroke model,48,74 similar to attenuation of NLRP3.75 Encouraging initial results for NLRP3 inhibition demonstrating neuroprotection in rodent stroke models highlight the potential important contribution of inflammasome activation in the pathogenesis of ischemic stroke and potential for therapeutic approaches.76,77 However, it is not known whether inflammasome inhibition by APC contributes to the repertoire of its neuroprotective effects.
Three scenarios for APC effects in the brain:
As the activities of APC are involved in multiple reactions that govern homeostasis, its intimate involvement in brain pathophysiology is therefore not surprising. For better or for worse, the next three scenarios highlight recent new insights into the contributions of the protein C pathway in the brain.
Scenario 1: Cytoprotective-selective APC therapy for ischemic stroke.
Clinical observations support a potential protective effect of protein C in stroke. Prospective epidemiologic studies found an inverse association of protein C levels with the incidence of stroke and circulating APC levels are decreased in stroke patients.78–80 These clinical associations and multiple studies demonstrating neuroprotective effects of APC in rodent stroke models prompted the translation of the cytoprotective-selective 3K3A-APC variant for therapy in ischemic stroke patients. Preclinical studies in Cynomolgus monkey confirmed an approximately 10-fold difference in anticoagulant activity between 3K3A-APC and wild type-APC (Xigris) in vivo.81 Initial clinical studies in healthy subjects indicated that 3K3A-APC at multiple high doses (up to 540 μg/kg every 12 hours for 5 doses) was well tolerated with minimal prolongation of APTT clotting times.82 Administration of 3K3A-APC at 540 μg/kg in a single iv bolus resulted in peak plasma levels of ~4300 ng/ml with an elimination half-life of ~16 min. The reduced anticoagulant activity of 3K3A-APC, permitting high transient levels of cytoprotective-selective APC in the circulation to reset signaling pathways in stressed cell, highlights the key conceptual difference between 3K3A-APC therapy and previous wild type-APC (Xigris) therapy that was targeting anticoagulation.
The recently completed NeuroNEXT (Rhapsody) trial, a phase 2A randomized, controlled, blinded, dose-escalation safety trial for 3K3A-APC in ischemic stroke patients, reported encouraging results.83 When 3K3A-APC (120–540 μg/kg) was administered in a 15 min bolus starting 30–120 min following tPA or mechanical thrombectomy every 12 hr for 5 doses, exploratory analysis suggested that 3K3A-APC reduced intracranial hemorrhage rates in the combined treatment arms compared to placebo from 86.5% to 67.4% (p=0.046) and total hemorrhagic volume from 2.1 ml to 0.8 ml (p = 0.066). While this trend is encouraging, a larger trial is needed to confirm these results.
Scenario 2: Bleeding in the brain due to excessive APC generation.
Organotypic vascular bed heterogeneity is determined by function and multiple humoral and biophysical factors.84,85 The vascular bed of the brain is programmed to be prohemostatic, presumably to avert hemorrhage, with relatively low endothelial expression of TM and EPCR.86,87 Recent insights indicate that this status quo is disrupted in vascular malformations in the brain.
Cerebral cavernous malformations (CCM) are hemorrhagic brain lesions due to clusters of low-flow dilated capillaries that lack a proper vessel architecture. Familial forms of CCM are due to loss-of-function mutations in one of three genes: KRIT1 (CCM1), CCM2 or PDCD10 (CCM3), and patients with CCM are at high risk of developing hemorrhagic stroke, seizures and neurological deficits.88,89 Comprehensive transcriptome analyses of CCM lesions have provided new mechanistic insights.90–92 Lopez-Ramirez et al. noted that transcripts for TM and EPCR were increased upon loss of KRIT1 or PDCD10 and demonstrated abnormally high endothelial TM and EPCR expression in CCM lesions, suggesting that disproportional APC generation in CCM lesions may promote hemorrhaging.20,90 Indeed, deletion of endothelial TM and blocking antibodies to TM and EPCR significantly reduced CCM-associated bleeding in mice. These and other studies, e.g., post-ischemic bleeding induced by anticoagulant-selective E149A-APC,93 indicate that a stressed blood-brain-barrier is particularly susceptible to anticoagulant APC-facilitated bleeding.
On the other hand, enhanced generation of APC at the site of lesions may serve to restore vascular integrity via its cytoprotective activity that require PAR1 and PAR3, especially since endothelial tight-junctions have deteriorated in CCM lesions.90 The enhanced expression of TM was induced by Krüppel-like transcription factors KLF2 and KL4,20,94 which are known to be upregulated in CCM and implicated in the pathogenesis.90,95 These flow-regulated transcription factors normally induce an anti-inflammatory, anti-thrombotic, quiescence state of the endothelium and promote endothelial barrier stabilization,96–98 but in their quest for endothelial quiescence also downregulate expression of PAR1, PAR2 and PAR3, presumably to minimize proinflammatory effects of thrombin and tissue factor-mediated signaling.90,94,99–101 Whether the KLF2/4-mediated downregulation of PAR1 and PAR3 diminishes APC’s ability to induce cytoprotective effects remains to be determined, but it is possible that upregulation of KLF2/4 in CCM lesions facilitate an (unintended) shift in the balance between anticoagulant and cytoprotective effects of APC.
Scenario 3: Cerebral malaria due to an acquired protein C pathway defect.
Vascular sequestration of infected erythrocytes (IEs) is the hallmark of cerebral malaria.102–104 Sequestration is mediated by the P. falciparum erythrocyte membrane protein 1 (PfEMP1), a parasite-encoded protein family expressed on IEs that mediates the binding to various vascular receptors thereby providing a protected environment for the parasitic biomass to multiply without splenic clearance. An unexpected link between severe malaria and the protein C pathway came from the discovery that some PfEMP1 variants bind to EPCR and that these infections were associated with the most severe forms of malaria, including cerebral malaria.105–111 Increased awareness that vascular dysfunction underlies the symptomatic manifestations of severe malaria were fueled by observations that expression of TM and EPCR is deceased at sites of IE sequestration in the brain and that brain edema is associated with mortality in children with cerebral malaria suggesting a deterioration of blood-brain-barrier function.106,112 It is important to note that expression of EPCR-binding PfEMP1 on IEs is restricted to P. falciparum parasites. Other Plasmodium strains that infect humans or mice do not express PfEMP1 molecules and therefore do not encompass the binding of IEs to EPCR in their pathogenesis.113
The PfEMP1 domains that are responsible for binding to EPCR (i.e., Cysteine-rich interdomain regions (CIDRα1)) block protein C and APC binding to EPCR and inhibit EPCR-facilitated protein C activation, activation of PAR1 and PAR3 by APC, and APC’s ability to induce EPCR-dependent cytoprotective effects, including endothelial barrier protection.105,114,115 EPCR has important functions in the protein C system and the activities of APC are important to maintain homeostasis in the brain.2,8,116 Human protein C deficiency, when left untreated, results in neurological defects,117,118 and the brain is very susceptible to endotoxemia-induced vascular permeability when the expression of EPCR is compromised.119 Furthermore, transcripts of a EPCR-binding CIDRα1 domain that inhibited APC’s barrier protective effects were highly enriched in children with cerebral malaria and brain swelling.120 Thus, there are multiple mechanistic and pathologic indications supporting a defective protein C system in severe malaria and that this may contribute to the pathogenesis of cerebral malaria.116,121–123
Conclusion:
These scenarios illustrate that the multiple activities of APC, for better or for worse, are intimately involved in the normal physiology and pathophysiology of the brain. This provides many opportunities for relevant translational research focusing either on APC’s anticoagulant activity to mitigate bleeding or on APC’s cytoprotective activity to restore normal brain function. The continuous discovery of new molecular mechanisms for APC’s cytoprotective activities, new applications for APC therapy, and preliminary beneficial effects of APC therapy in ischemic stroke patients, are encouraging that cytoprotective-selective APC therapy may become ultimately successful and may expand to other diseases of the brain in addition to ischemic stroke.
Key points:
APC is a homeostatic coagulation protease with anticoagulant and cytoprotective activities that are intimate involved in multiple neuropathologies.
APC therapy with the cytoprotective-selective 3K3A-APC variant showed encouraging results in ischemic stroke patients.
Disproportional upregulation of APC anticoagulant activity can contribute to bleeding associated with cerebral cavernous malformations.
A dysfunction of the protein C pathway due to targeting of the endothelial protein C receptor (EPCR) by infected erythrocytes likely contributes to the pathogenesis of cerebral malaria.
Acknowledgements:
The author apologizes to colleagues whose work was not cited due limitations on text size and number of references.
This study was funded by National Institutes of Health grants HL142975, HL104165, HL130678.
Footnotes
Conflict of interest:
L.O.M. is an inventor on patents that are the property of the Scripps Research Institute related to some uses of APC mutants and PAR1 peptides.
Reference section:
- 1.O’Donnell JS, O’Sullivan JM, Preston RJS. Advances in understanding the molecular mechanisms that maintain normal haemostasis. Br J Haematol 2019. [DOI] [PubMed] [Google Scholar]; * Timely review on the coagulation pathway.
- 2.Griffin JH, Zlokovic BV, Mosnier LO. Activated protein C, protease activated receptor 1, and neuroprotection. Blood 2018;132(2):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Comprehensive review on the effects of APC therapy in the brain.
- 3.Isermann B Homeostatic effects of coagulation protease-dependent signaling and protease activated receptors. J Thromb Haemost 2017;15(7):1273–1284. [DOI] [PubMed] [Google Scholar]; ** Detailed review of PAR signaling by coagulation proteases.
- 4.Shahzad K, Kohli S, Al-Dabet MM, Isermann B. Cell biology of activated protein C. Curr Opin Hematol 2019;26(1):41–50. [DOI] [PubMed] [Google Scholar]; ** Excellent review of mechanisms for APC-induced cell signaling pathways.
- 5.Zhao R, Lin H, Bereza-Malcolm L, Clarke E, Jackson CJ, Xue M. Activated Protein C in Cutaneous Wound Healing: From Bench to Bedside. Int J Mol Sci 2019;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Timely review on the role of the protein C pathway in wound healing.
- 6.Gorbacheva LR, Kiseleva EV, Savinkova IG, Strukova SM. A New Concept of Action of Hemostatic Proteases on Inflammation, Neurotoxicity, and Tissue Regeneration. Biochemistry (Mosc) 2017;82(7):778–790. [DOI] [PubMed] [Google Scholar]; * Interesting review foccusing on APC-mediated PAR1 signaling.
- 7.Ren D, Giri H, Li J, Rezaie AR. The Cardioprotective Signaling Activity of Activated Protein C in Heart Failure and Ischemic Heart Diseases. Int J Mol Sci 2019;20(7). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Detailed review of APC’s effects in the heart and related APC-induced signaling mechanisms and pathways, including the phenomenom of “EPCR occupancy”.
- 8.Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood 2014;124(10):1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent review on EPCR and related functions with a focus on the factor VIIa-EPCR interaction.
- 9.Wildhagen KC, Lutgens E, Loubele ST, ten Cate H, Nicolaes GA. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb Haemost 2011;106(6):1034–1045. [DOI] [PubMed] [Google Scholar]; ** Outstanding review on APC structure-function with a summary of activity-selective APC variants and their effects.
- 10.Griffin JH, Fernandez JA, Gale AJ, Mosnier LO. Activated protein C. J Thromb Haemost 2007;5 Suppl 1:73–80. [DOI] [PubMed] [Google Scholar]
- 11.Dahlback B Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol 2016;38 Suppl 1:4–11. [DOI] [PubMed] [Google Scholar]; * Interesting review summarizing factor V biology and other functions of factor V.
- 12.Lipe B, Ornstein DL. Deficiencies of natural anticoagulants, protein C, protein S, and antithrombin. Circulation 2011;124(14):e365–368. [DOI] [PubMed] [Google Scholar]
- 13.Manco-Johnson MJ, Bomgaars L, Palascak J, et al. Efficacy and safety of protein C concentrate to treat purpura fulminans and thromboembolic events in severe congenital protein C deficiency. Thromb Haemost 2016;116(1):58–68. [DOI] [PubMed] [Google Scholar]
- 14.Mosnier LO, Griffin JH. Protein C, protein S, thrombomodulin and the endothelial protein C receptor pathways In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC, Colman RW, eds. Hemostasis and Thrombosis: Basic Principles and Clinical Practice 6th ed Philadelphia: Lippincott Williams & Wilkins; 2013:300–313. [Google Scholar]
- 15.Davenport RA, Guerreiro M, Frith D, et al. Activated Protein C Drives the Hyperfibrinolysis of Acute Traumatic Coagulopathy. Anesthesiology 2017;126(1):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper demonstrating the deleterious effects of disproportional APC generation in acute traumatic coagulopathy.
- 16.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock 2009;32(6):659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: The past, present, and future. J Thromb Haemost 2019;17(6):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Up-to-date review of the proposed mechanisms contributing to acute traumatic coagulopathy, including the role of disproportional APC generation.
- 18.Polderdijk SG, Adams TE, Ivanciu L, Camire RM, Baglin TP, Huntington JA. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood 2017;129(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Description of an engineered SERPIN with high specificity for APC to mitigate APC-induced bleeding.
- 19.Prince R, Bologna L, Manetti M, et al. Targeting anticoagulant protein S to improve hemostasis in hemophilia. Blood 2018;131(12):1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper establishing a role for protein S and by extension APC anticoagulant activity mediating bleeding in hemophilia.
- 20.Lopez-Ramirez MA, Pham A, Girard R, et al. Cerebral cavernous malformations form an anticoagulant vascular domain in humans and mice. Blood 2019;133(3):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]; * New discovery that TM and EPCR are upregulated in CCM vascular lesions and that disproportional APC generation contributes to bleeding.
- 21.Polderdijk SGI, Huntington JA. Identification of serpins specific for activated protein C using a lysate-based screening assay. Sci Rep 2018;8(1):8793. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Drug screening method to identify engineered SERPINs with specificity for APC to inhibit APC-mediated bleeding.
- 22.Sperandio O, Wildhagen KC, Schrijver R, Wielders S, Villoutreix BO, Nicolaes GA. Identification of novel small molecule inhibitors of activated protein C. Thromb Res 2014;133(6):1105–1114. [DOI] [PubMed] [Google Scholar]; * Drug screening to identify small molecule inhibitors that specifically disrupt the interaction of APC with factor Va with the goal to obtain anticoagulant-selective APC inhibitors.
- 23.Bach P, Knerr L, Fjellstrom O, Hansson K, Mattsson C, Gustafsson D. Design, synthesis, and SAR of a series of activated protein C (APC) inhibitors with selectivity against thrombin for the treatment of haemophilia. Bioorg Med Chem Lett 2014;24(3):821–827. [DOI] [PubMed] [Google Scholar]
- 24.Zhao XY, Yegneswaran S, Bauzon M, et al. Targeted Inhibition of Activated Protein C anticoagulant activity by monoclonal antibody HAPC1573 for treatment of hemophilia. Blood 2016;128(22):80. [Google Scholar]
- 25.von Drygalski A, Bhat V, Gale AJ, et al. An engineered factor Va prevents bleeding induced by anticoagulant wt activated protein C. PLoS One 2014;9(8):e104304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezaie AR. The occupancy of endothelial protein C receptor by its ligand modulates the PAR-1 dependent signaling specificity of coagulation proteases. IUBMB Life 2011;63(6):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood 2010;115(6):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Little older review, but with an excellent summary of diseases and models where APC therapy demonstrated effects.
- 28.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci U S A 2007;104(8):2867–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper describing that caveolin-1-containing caveolae are required for APC-mediated cytoprotective signaling.
- 29.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci U S A 2009;106(15):6393–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper describing that caveolin-1-containing caveolae are required for APC-mediated cytoprotective signaling.
- 30.Soh UJK, Trejo J. Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through beta-arrestin and dishevelled-2 scaffolds. Proc Natl Acad Sci U S A 2011;108(50):E1372–E1380. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that cytoprotective APC signaling requires β-arrestin-mediated signaling, thereby providing one of the critical pieces for the current APC biased signaling paradigm.
- 31.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012;120(26):5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Benchmark paper demonstrating that APC activates PAR1 predominantly at the non-canonical Arg46 site, which is different for the typical activation of PAR1 by thrombin at Arg41.
- 32.Coughlin SR. How the protease thrombin talks to cells. Proc Natl Acad Sci U S A 1999;96(20):11023–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieman MT. Protease-activated receptors in hemostasis. Blood 2016;128(2):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent review of the biology of the protease activated receptors.
- 34.Flaumenhaft R, De Ceunynck K. Targeting PAR1: Now What? Trends Pharmacol Sci 2017;38(8):701–716. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Very comprehensive review of PAR1 activators, agonists, and antagonists in relation to the biology of PAR1 signaling.
- 35.de Oliveira AS, de Almeida VH, Gomes FG, Rezaie AR, Monteiro RQ. TR47, a PAR1-based peptide, inhibits melanoma cell migration in vitro and metastasis in vivo. Biochem Biophys Res Commun 2018;495(1):1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha RK, Wang Y, Zhao Z, et al. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood 2018;131(11):1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First paper to establish that PAR1 activation at Arg46 in vivo is required for protective therapeutic effects of APC.
- 37.De Ceunynck K, Peters CG, Jain A, et al. PAR1 agonists stimulate APC-like endothelial cytoprotection and confer resistance to thromboinflammatory injury. Proc Natl Acad Sci U S A 2018;115(5):E982–E991. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Demonstration that Parmodulin 2 is a biased agonist of PAR1 that can induce cytoprotective signaling.
- 38.Burnier L, Mosnier LO. Novel mechanisms for activated protein C cytoprotective activities involving noncanonical activation of protease-activated receptor 3. Blood 2013;122(5):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Discovery that APC activates PAR3 at non-canonical Arg41 which is different from typical PAR3 activation by thrombin at Lys38.
- 39.Stavenuiter F, Mosnier LO. Noncanonical PAR3 activation by factor Xa identifies a novel pathway for Tie2 activation and stabilization of vascular integrity. Blood 2014;124(23):3480–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Discovery that non-canonical PAR3 activation by APC or factor X and by the P3R peptide result in activation of Tie2.
- 40.Shahzad K, Gadi I, Nazir S, et al. Activated protein C reverses epigenetically sustained p66(Shc) expression in plaque-associated macrophages in diabetes. Commun Biol 2018;1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper demonstrating that APC can modify epigenetically programmed gene expression.
- 41.Ranjan S, Goihl A, Kohli S, et al. Activated protein C protects from GvHD via PAR2/PAR3 signalling in regulatory T-cells. Nat Commun 2017;8(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper demonstrates that APC protects against the effects of acute graft-versus-host disease thereby expanding the indications for potential APC therapy.
- 42.Healy LD, Rigg RA, Griffin JH, McCarty OJT. Regulation of immune cell signaling by activated protein C. J Leukoc Biol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Timely review of APC effects in immune cells with a focus on the inhibition of neutrophil extracellular trap formation by APC.
- 43.Healy LD, Puy C, Fernandez JA, et al. Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem 2017;292(21):8616–8629. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Discovery that APC inhibits neutrophil extracellular trap formation, providing yet another mechanism for anti-inflammatory effects of APC.
- 44.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009;15(11):1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper demonstrating that proteolytical inactivation of extracellular histones by APC contributes to mortality reduction in sepsis.
- 45.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem 2005;280(20):19808–19814. [DOI] [PubMed] [Google Scholar]; * Older paper that provides a detailed analysis of APC- versus thrombin-induced changes in gene expression in endothelial cells.
- 46.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 2001;276(14):11199–11203. [DOI] [PubMed] [Google Scholar]; ** Benchmark paper demonstrating for the first time that APC can convey cytoprotective effects on cells.
- 47.Liang HP, Kerschen EJ, Basu S, et al. Coagulation factor V mediates inhibition of tissue factor signaling by activated protein C in mice. Blood 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper that proposes additional mechanisms for APC’s anti-inflammatory activities involving factor V but not factor Va.
- 48.Nazir S, Gadi I, Al-Dabet MM, et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia-reperfusion injury via mTORC1 inhibition. Blood 2017;130(24):2664–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper demonstrating for the first time that APC can inhibit NLRP3-mediated inflammasome.
- 49.Mathur A, Hayward JA, Man SM. Molecular mechanisms of inflammasome signaling. J Leukoc Biol 2018;103(2):233–257. [DOI] [PubMed] [Google Scholar]
- 50.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 2018;19(10):610–621. [DOI] [PubMed] [Google Scholar]; ** Excellent review summarizing the current knowledge on the involvement of inflammasomes in the brain.
- 51.Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 2018;17(8):588–606. [DOI] [PubMed] [Google Scholar]; ** Very informative review on the participation of the NLRP3 inflammasome in various diseases and the state-of-the-art on inflammasome inhibitors.
- 52.Patel MN, Carroll RG, Galvan-Pena S, et al. Inflammasome Priming in Sterile Inflammatory Disease. Trends Mol Med 2017;23(2):165–180. [DOI] [PubMed] [Google Scholar]; ** Educational review on the molecular mechanisms of inflammasomes.
- 53.Fann DY, Lim YA, Cheng YL, et al. Evidence that NF-kappaB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol Neurobiol 2018;55(2):1082–1096. [DOI] [PubMed] [Google Scholar]
- 54.Guitton C, Cottereau A, Gerard N, et al. Protective crosstalk between Activated Protein C and TNF signaling in vascular endothelial cells: Implication of EPCR, non canonical NF59B and ERK1/2 MAPKinases. Am. J Physiol. Cell Physiol 2011. [DOI] [PubMed] [Google Scholar]
- 55.Bae JS, Rezaie AR. Thrombin inhibits nuclear factor kappaB and RhoA pathways in cytokine-stimulated vascular endothelial cells when EPCR is occupied by protein C. Thromb. Haemost 2009;101(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- 56.Madhusudhan T, Wang H, Ghosh S, et al. Signal integration at the PI3K-p85-XBP1 hub endows coagulation protease activated protein C with insulin-like function. Blood 2017;130(12):1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper demonstrating that APC can correct metabolic dysfunction which is potentially a mechanism by which APC can inhibit one of the second hits required for inflammasome activation.
- 57.Wang J, Yang L, Rezaie AR, Li J. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost 2011;9(7):1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Paper demonstrating that APC promotes AMPK activation which has been implicated as a mechanism by which APC inhibits inflammasome activation.
- 58.Wolter J, Schild L, Bock F, et al. Thrombomodulin-dependent protein C activation is required for mitochondrial function and myelination in the central nervous system. J Thromb. Haemost 2016;14(11):2212–2226. [DOI] [PubMed] [Google Scholar]; * Paper demonstrating that APC can correct mitochondrial dysfunction which is potentially a mechanism by which APC can inhibit one of the second hits required for inflammasome activation.
- 59.Lazic D, Sagare AP, Nikolakopoulou AM, Griffin JH, Vassar R, Zlokovic BV. 3K3A-activated protein C blocks amyloidogenic BACE1 pathway and improves functional outcome in mice. J Exp Med 2019;216(2):279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that APC may inhibit disease progression in Alzheimer’s disease.
- 60.Shibata M, Kumar SR, Amar A, et al. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 2001;103(13):1799–1805. [DOI] [PubMed] [Google Scholar]; * Benchmark paper demonstrating for the first time that APC improves outcomes after ischemic stroke in mice.
- 61.Zhong Z, Ilieva H, Hallagan L, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest 2009;119(11):3437–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Discovery that APC may delay disease progression in ALS.
- 62.Han MH, Hwang SI, Roy DB, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008;451(7182):1076–1081. [DOI] [PubMed] [Google Scholar]; * Discovery that APC may delay disease progression in MS.
- 63.Deane R, LaRue B, Sagare AP, Castellino FJ, Zhong Z, Zlokovic BV. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab 2009;29(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Zhao Z, Rege SV, et al. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat Med 2016;22(9):1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that APC can improve the outcome of neuronal stem cell transplantation.
- 65.Wang Y, Zhao Z, Chow N, Ali T, Griffin JH, Zlokovic BV. Activated protein C analog promotes neurogenesis and improves neurological outcome after focal ischemic stroke in mice via protease activated receptor 1. Brain Res 2013;1507:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper demonstrating the regenerating capacity of the brain after ischemic stroke and that regeneration is promoted by APC.
- 66.Guo H, Liu D, Gelbard H, et al. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 2004;41(4):563–572. [DOI] [PubMed] [Google Scholar]
- 67.Stone JA, Willey JZ, Keyrouz S, et al. Therapies for Hemorrhagic Transformation in Acute Ischemic Stroke. Curr Treat Options Neurol 2017;19(1):1. [DOI] [PubMed] [Google Scholar]; * Educational clinical review on bleeding associated with ischemic stroke.
- 68.Ho WM, Reis C, Akyol O, et al. Pharmacological Management Options to Prevent and Reduce Ischemic Hemorrhagic Transformation. Curr Drug Targets 2017;18(12):1441–1459. [DOI] [PubMed] [Google Scholar]; * Educational review focusing on potential mechanisms contributing to bleeding associated with ischemic stroke.
- 69.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344(10):699–709. [DOI] [PubMed] [Google Scholar]; * Benchmark study of wt-APC (Xigris) therapy in severe sepsis patients on which FDA approval was based.
- 70.Bernard GR, Macias WL, Joyce DE, Williams MD, Bailey J, Vincent JL. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Crit Care 2003;7(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin Alfa (Activated) in Adults with Septic Shock. N. Engl. J Med 2012;366(22):2055–2064. [DOI] [PubMed] [Google Scholar]; * Benchmark study demonstrating a lack of efficacy of wt-APC (Xigris) in severe sepsis patients a decade after the successful first study. This resulted in the withdrawal of wt-APC (Xigris).
- 72.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood 2004;104(6):1740–1744. [DOI] [PubMed] [Google Scholar]; * Discovery that 3K3A-APC, which is now in clinical development for ischemic stroke, retained normal cytoprotective activities despite severely reduced anticoagulant activities.
- 73.Barrington J, Lemarchand E, Allan SM. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol 2017;27(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Educational review on the role of inflammasomes in the pathology of stroke.
- 74.Cheng T, Petraglia AL, Li Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med 2006;12(11):1278–1285. [DOI] [PubMed] [Google Scholar]
- 75.Guo Z, Yu S, Chen X, et al. Suppression of NLRP3 attenuates hemorrhagic transformation after delayed rtPA treatment in thromboembolic stroke rats: Involvement of neutrophil recruitment. Brain Res Bull 2018;137:229–240. [DOI] [PubMed] [Google Scholar]; * Interesting observation that inhibition of the NLRP3 inflammasome reduced bleeding after thrombolytic therapy in a rat model of ischemic stroke.
- 76.Alishahi M, Farzaneh M, Ghaedrahmati F, Nejabatdoust A, Sarkaki A, Khoshnam SE. NLRP3 inflammasome in ischemic stroke: As possible therapeutic target. Int J Stroke 2019:1747493019841242. [DOI] [PubMed] [Google Scholar]; ** Excellent review summarizing what is known to data about the NLRP3 inflammasome in ischemic stroke.
- 77.Ismael S, Zhao L, Nasoohi S, Ishrat T. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 2018;8(1):5971. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper demonstrating that inhibition of the NLRP3 inflammasome with MCC950 improves outcomes after ischemic stroke in mice.
- 78.Folsom AR, Ohira T, Yamagishi K, Cushman M. Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Thromb Haemost 2009;7(11):1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Folsom AR, Rosamond WD, Shahar E, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation 1999;100(7):736–742. [DOI] [PubMed] [Google Scholar]
- 80.Macko RF, Ameriso SF, Gruber A, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke 1996;27(11):2005–2011. [DOI] [PubMed] [Google Scholar]
- 81.Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des 2012;18(27):4215–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyden P, Levy H, Weymer S, et al. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des 2013;19(42):7479–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Paper detailing the first administration of 3K3A-APC in human healthy subjects.
- 83.Lyden P, Pryor KE, Coffey CS, et al. Final Results of the RHAPSODY Trial: A Multi-Center, Phase 2 Trial Using a Continual Reassessment Method to Determine the Safety and Tolerability of 3K3A-APC, A Recombinant Variant of Human Activated Protein C, in Combination with Tissue Plasminogen Activator, Mechanical Thrombectomy or both in Moderate to Severe Acute Ischemic Stroke. Ann Neurol 2019;85(1):125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Results of the phase 2 trial of 3K3A-APC in ischemic stroke patients, suggesting that 3K3A-APC may reduce bleeding.
- 84.Augustin HG, Koh GY. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017;357(6353). [DOI] [PubMed] [Google Scholar]; ** Educational review of the heterogeneity of vascular beds in different organs.
- 85.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100(2):174–190. [DOI] [PubMed] [Google Scholar]
- 86.Wong VL, Hofman FM, Ishii H, Fisher M. Regional distribution of thrombomodulin in human brain. Brain Res 1991;556(1):1–5. [DOI] [PubMed] [Google Scholar]
- 87.Laszik Z, Mitro A, Taylor FB Jr., Ferrell G, Esmon CT. Human protein C receptor is present primarily on endothelium of large blood vessels. Implications for the control of the protein C pathway. Circulation 1997;96(10):3633–3640. [DOI] [PubMed] [Google Scholar]; * Benchmark paper demonstrating that the relative ratio of expression of TM and EPCR differ in different vascular beds.
- 88.Stapleton CJ, Barker FG 2nd. Cranial Cavernous Malformations: Natural History and Treatment. Stroke 2018;49(4):1029–1035. [DOI] [PubMed] [Google Scholar]; * Educational recent clinical review on CCM.
- 89.Zafar A, Quadri SA, Farooqui M, et al. Familial Cerebral Cavernous Malformations. Stroke 2019;50(5):1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Comprehensive review of familial CCM, great starting point to know more about CCM.
- 90.Lopez-Ramirez MA, Fonseca G, Zeineddine HA, et al. Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J Exp Med 2017;214(11):3331–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent paper linking changes in endothelial gene expression due to Krit1 deletion to mechanisms of vascular malformation development in CCM.
- 91.Koskimaki J, Girard R, Li Y, et al. Comprehensive transcriptome analysis of cerebral cavernous malformation across multiple species and genotypes. JCI Insight 2019;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Interesting paper comparing changes in gene expression due to CCM between different models in different species.
- 92.Kar S, Bali KK, Baisantry A, Geffers R, Samii A, Bertalanffy H. Genome-Wide Sequencing Reveals MicroRNAs Downregulated in Cerebral Cavernous Malformations. J Mol Neurosci 2017;61(2):178–188. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Sinha RK, Mosnier LO, Griffin JH, Zlokovic BV. Neurotoxicity of the anticoagulant-selective E149A-activated protein C variant after focal ischemic stroke in mice. Blood Cells Mol Dis 2013;51(2):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 2005;96(5):e48–57. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Z, Tang AT, Wong WY, et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature 2016;532(7597):122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that upregulation of the KLF2 and KLF4 transcription factors in CCM are driving forces for the development of vascular malformations.
- 96.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res 2007;100(12):1686–1695. [DOI] [PubMed] [Google Scholar]; * Educational review to learn more about the biology and function of Kruppel-like transcription factors.
- 97.Shi H, Sheng B, Zhang F, et al. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am J Physiol Heart Circ Physiol 2013;304(6):H796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Interesting paper demonstrating the importance of KLF2 for the blood-brain-barrier.
- 98.Ma J, Wang P, Liu Y, Zhao L, Li Z, Xue Y. Kruppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J Cell Physiol 2014;229(7):916–926. [DOI] [PubMed] [Google Scholar]
- 99.Lin Z, Hamik A, Jain R, Kumar A, Jain MK. Kruppel-like factor 2 inhibits protease activated receptor-1 expression and thrombin-mediated endothelial activation. Arterioscler Thromb Vasc Biol 2006;26(5):1185–1189. [DOI] [PubMed] [Google Scholar]; * Little older paper that convincingly describes the inhibition of PAR1 expression by KLF2 and the effects on thrombin signaling.
- 100.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 2006;116(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper describes that PAR2 is down regulated by KLF2.
- 101.Ruf W Flow perturbation is linked to endothelial par signaling. Arterioscler Thromb Vasc Biol 2006;26(5):962–964. [DOI] [PubMed] [Google Scholar]; * Intriguing editorial review discussing how downregulation of PAR1 and PAR2 by KLF2 may affect proinflammatory thrombin and tissue factor-dependent signaling.
- 102.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014;383(9918):723–735. [DOI] [PubMed] [Google Scholar]; ** Excellent clinically oriented review of to learn more about malaria.
- 103.Deitsch KW, Chitnis CE. Molecular basis of severe malaria. Proc Natl Acad Sci U S A 2012;109(26):10130–10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med 2013;19(2):156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Comprehensive review with detailed mechanisms involved in malaria.
- 105.Turner L, Lavstsen T, Berger SS, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013;498(7455):502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that certain domains of PfEMP1 bind to EPCR.
- 106.Moxon CA, Wassmer SC, Milner DA Jr., et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 2013;122(5):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discovery that expression of TM and EPCR is severely reduced at sites of IE sequestration in cerebral malaria.
- 107.Lavstsen T, Turner L, Saguti F, et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 2012;109(26):E1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Key paper linking infections with PfEMP1 transcripts encoding for with EPCR-binding domains to more severe clinical manifestations of malaria.
- 108.Bertin GI, Lavstsen T, Guillonneau F, et al. Expression of the Domain Cassette 8 Plasmodium falciparum Erythrocyte Membrane Protein 1 Is Associated with Cerebral Malaria in Benin. PLoS One 2013;8(7):e68368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernabeu M, Danziger SA, Avril M, et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc Natl Acad Sci U S A 2016;113(23):E3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Interesting paper describing heterogeneity among different EPCR binding domains in isolates with different var gene recombinations that encode the PfEMP1 family of adhesion molecules.
- 110.Bernabeu M, Gunnarsson C, Vishnyakova M, et al. Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1. MBio 2019;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Intriguing study in a 3D human brain microvessel model to determine the cooperation of EPCR and ICAM-1 binding domains for sequestration of IEs under different conditions.
- 111.Storm J, Jespersen JS, Seydel KB, et al. Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol Med 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Comprehensive paper with detailed analysis confirming the association of EPCR-binding IEs with cerebral malaria.
- 112.Seydel KB, Kampondeni SD, Valim C, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med 2015;372(12):1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Benchmark paper demonstrating the association of brain edema with mortality in children with cerebral malaria.
- 113.Craig AG, Grau GE, Janse C, et al. The role of animal models for research on severe malaria. PLoS Pathog 2012;8(2):e1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Little older but comprehensive review of the limitation of animal models for malaria and the difficulties associated with models for P. falciparum.
- 114.Lau CK, Turner L, Jespersen JS, et al. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 2015;17(1):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Key paper for understanding the molecular interaction of CIDRα1 domains with EPCR, concluding that the interaction is mediated by a shape-fit type of binding rather that interactions between individual residues based on the extensive sequence diversity among EPCR-binding CIDRα1 domains.
- 115.Petersen JE, Bouwens EA, Tamayo I, et al. Protein C system defects inflicted by the malaria parasite protein PfEMP1 can be overcome by a soluble EPCR variant. Thromb Haemost 2015;114(5):1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Detailed report that EPCR-binding CIDRα1 domains inhibit EPCR function and APC cytoprotective activities.
- 116.Mosnier LO, Lavstsen T. The role of EPCR in the pathogenesis of severe malaria. Thromb. Res 2016;141 Suppl 2:S46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia 2008;14(6):1214–1221. [DOI] [PubMed] [Google Scholar]; * One of the more recent reviews dedicated to protein C deficiency.
- 118.Griffin JH, Evatt B, Zimmerman TS, Kleiss AJ, Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest 1981;68(5):1370–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.von Drygalski A, Furlan-Freguia C, Ruf W, Griffin JH, Mosnier LO. Organ-Specific Protection Against Lipopolysaccharide-Induced Vascular Leak Is Dependent on the Endothelial Protein C Receptor. Arterioscler. Thromb. Vasc. Biol 2013;33(4):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kessler A, Dankwa S, Bernabeu M, et al. Linking EPCR-Binding PfEMP1 to Brain Swelling in Pediatric Cerebral Malaria. Cell Host Microbe 2017;22(5):601–614 e605. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Key paper, providing one of the most direct evidence available to date for the involvement of EPCR inactivation by IEs causing worse outcome in cerebral malaria.
- 121.Aird WC, Mosnier LO, Fairhurst RM. Plasmodium falciparum picks (on) EPCR. Blood 2014;123(2):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernabeu M, Smith JD. EPCR and Malaria Severity: The Center of a Perfect Storm. Trends Parasitol 2017;33(4):295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent review focused on the pathophysiology of malaria.
- 123.O’Sullivan JM, Preston RJ, O’Regan N, O’Donnell JS. Emerging roles for haemostatic dysfunction in malaria pathogenesis. Blood 2016;127(19):2281–2288. [DOI] [PubMed] [Google Scholar]; * Comprehensive review of the interactions and involvement of hemostatic pathways in malaria.
- 124.Griffin JH, Mosnier LO, Fernandez JA, Zlokovic BV. 2016 Scientific Sessions Sol Sherry Distinguished Lecturer in Thrombosis: Thrombotic Stroke: Neuroprotective Therapy by Recombinant-Activated Protein C. Arterioscler Thromb Vasc Biol 2016;36(11):2143–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A 2007;104(13):5662–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Key PAR3 paper that demonstrates that the formation of PAR1-PAR3 heterodimer is associated with different G protein selectivity.
- 126.Gleeson EM, Dichiara MG, Salicio A, et al. Activated protein C beta-glycoform promotes enhanced noncanonical PAR1 proteolysis and superior resistance to ischemic injury. Blood 2015;126(7):915–919. [DOI] [PubMed] [Google Scholar]; ** This paper demonstrates that β-protein C, a natural protein C glycoform circulating in plasma, has enhanced cytoprotective activities.
- 127.Yang L, Bae JS, Manithody C, Rezaie AR. Identification of a specific exosite on activated protein C for interaction with protease activated receptor 1. J Biol Chem 2007;282(35):25493–25500. [DOI] [PubMed] [Google Scholar]; ** Identification of an exosite for PAR1 on the surface of the APC protease domain involving E330 and E333.


