Abstract
Background
Organic products of animals are getting more accepted by consumers. Using herbal additives may lead to more health animal products. In this research it is hypothesized that Lavandula angustifolia and/or Mentha spicata essential oils would be helpful to enhance production performance in laying hens.
Objectives
This experiment was conducted to evaluate the effects of Lavandula angustifolia and Mentha spicata essential oils on performance, egg traits and blood variables in laying hens.
Methods
144 Lohmann LSL‐Lite laying hens from 42 until 56 weeks of age were used in a completely randomized design in four treatments and six replicates (six birds per replicate). The treatments consisted of: (a) control group (basal diet), (b) basal diet supplemented with 250 mg/kg diet lavender essential oil (LEO), (c) basal diet supplemented with 250 mg/kg diet mint essential oil (MEO), and (d) basal diet supplemented with both LEO and MEO.
Results
Using LEO and/or MEO did not affect body weight changes, feed intake, egg weight, egg index, yolk index, Haugh unit, egg shell weight and egg shell thickness. Feeding LEO, individually or in combination with MEO, did not affect FCR compared with the control group (p < .05), however, feeding MEO individually increased feed conversation ratio (FCR) compared to LEO and the control group during 42–56 weeks (p < .05), as well as decreasing egg mass compared to LEO (p < .05). Feeding LEO increased egg production compared to MEO and combination of MEO and LEO (p < .05).
Conclusions
In conclusion, dietary supplemental MEO (250 mg/kg) may increase FCR, and LEO (250 mg/kg) is more effective than MEO (250 mg/kg) for egg production and egg mass purposes; besides MEO (250 mg/kg) negatively affected FCR compared with the control group. In addition, no specific beneficial effect of dietary supplemental MEO and/or LEO on the other measured variables was detected.
Keywords: blood variables, egg production, herbal additives, laying performance, morphometric egg traits, phytogenic feed additives
Consumer pressure related to the potential development of antibiotic‐resistant bacteria has resulted in the development of non‐antibiotic feed additives that may also improve poultry performance. It is known that most of their properties are due to the essential oils and other secondary plant metabolites. Essential oils enhance production of digestive secretions, stimulate blood circulation, exert antioxidant properties, reduce levels of pathogenic bacteria and may enhance immune status. This paper shows that dietary supplemental Lavandula angustifolia essential oil have a favourable effect on egg production of laying hens compared to Mentha spicata essential oil or combined form.

1. INTRODUCTION
The use of most antibiotic growth promoters has been banned in many countries, especially in the European Union since 2006 (Butaye et al., 2000). Herbal additives are given to animals or birds to improve their physiological and productive performance. The antimicrobial effect of some medicinal plants is reported (Valero & Salmeron, 2003). Medicinal plants, their extracts and essential oils have a wide range of activities, including inhibitory action on pathogens, effects on physio‐pathologies and activity in different body systems, e.g. endocrine and immune system (Francois, 2006). In layers and broilers, herbs and spices are not just appetite and digestion stimulants, but they can influence other physiological functions, help to sustain good health and welfare and also improve their performance (Frankic, Voljc, Salobir, & Rezar, 2009). The ajwain essential oil (Trachyspermum ammi L.) supplementation had a positive effect on the FCR, intestinal morphology and microbiota counts of breeder quails (Hajiaghapour & Rezaeipour, 2018).
The exact mechanisms by which plant components may alter blood profile are not fully understood; for instance, it is reported that peppermint (Mentha piperita) may prevent lipid peroxidation; besides, phenolic compounds and antioxidant activities found in peppermint tea may have hypoglycemic effects. It is also reported that peppermint (Mentha piperita) may interact with chromium resulting in insulin‐sensitive cell receptors or binding activity; in addition, some studies suggest that essential oils could increase insulin sensitivity‐receptors. The above‐mentioned mechanisms could lead to improve nutrient digestibility and modulate beneficial microbiota and also blood profile in laying hens' gut, which in turn may result in alter quantity and quality of their production. Nevertheless, exact mechanisms of action of phytogenic feed additives are still not fully understood.
Lavandula angustifolia (Common Lavender, English Lavender, French lavender, Garden Lavender, Lavender) is a flowering plant in the Lamiaceae family. Medicinal parts are usually roots, leaves or fruit of the plants, as well as essential oil from fresh flowers and/or the inflorescence; the flowers collected just before opening and dried, the fresh flowers and the dried flowers. Lavender oil has been reported to contain more than 100 components. The essential oil (1%–3%) of Lavandula is rich in linalool and linalyl acetate. Linalyl acetate is the major compound found in flowers. The plant contains also rosmarinic acid and coumarin (Lis‐Balchin, 2002). Carvacrol (26.2%), limonene (19.6%), 1,8‐cineole (11.8%), terpinen‐4‐ol (7.6%), spathulenol (4.9%), α‐pinene (4.2%), p‐cymene (4.2%), caryophyllene oxide (2.7%) and terpinolene (2.6%) were detected in Lavandula angustifolia essential oil by Bakhsha, Mazandarani, Aryaei, Jafari, & Bayate, 2014.
Mentha spicata (Spearmint), belongs to the Lamiaceae family. The plants of this family are a rich source of polyphenols, flavonoids and carvone, thus possessing strong antioxidant properties (Bimakr et al., 2011; Gulluce et al., 2007). The broiler chicks fed diet supplemented with of 2% spearmint was lower significantly in the serum total cholesterol compared with the control group and other groups (Abu Isha, El‐Hamid, Ziena, & Ahmed, 2018). It is stated that the improvement of FCR resulted from the increase in appetite due to the stimulation of salivary and gastric glands by spearmint oil, the decrease in pathogenic bacteria and better digestibility; besides, it is suggested that spearmint oil may stimulate salivary and gastric glands, and decrease bacteria which in turn improve digestibility and FCR (Abu Isha et al., 2018 qouted from Amal, 2012). Abu Isha et al., (2018) reported that, the addition of spearmint essential oil, to the diet increased significantly the feed intake of broiler chicks (Abu Isha et al., 2018). In addition, it is reported that feeding spearmint increases feed intake and consequently improves growth in broiler chickens (Saleh, Ijiri, & Ohtsuka, 2014). Galib, Al‐kassi, and Noor (2010) stated that the broiler chicks fed on peppermint (Mentha piperita) powder consumed significantly more feed consumption compared to the control group.
On this basis, we hypothesized that inclusion of Lavandula angustifolia and/or Mentha spicata into laying hens' diets would be helpful to enhance production performance, egg traits and blood biochemical variables; besides, assessing the probable synergistic interaction between dietary Lavandula angustifolia and Mentha spicata can be mentioned as novelty of the current study.
2. MATERIALS AND METHODS
2.1. Laboratory animals, experimental design and treatments
All experimental protocols were adhered and were approved by the guidelines of the Animal Ethics Committee of Razi University, Kermanshah, Iran (The animal use protocol number: Anim Sci, 116, Sep 2017). Hundred and forty four Lohmann LSL‐Lite laying hens in Phase II (42 weeks of age) were weighed individually and randomly assigned to four treatments with six replicates and six birds in each replicate in a completely randomized design. Four dietary isocaloric and isonitrogenous diets were used during three experimental periods, 42–46, 47–51 and 52–56 weeks. An empty cage was kept between treatments to eliminate cross‐feeding. The birds were placed in the cages and kept under 16 hr of light/8 hr dark cycle during the entire experimental period (14 weeks). The birds were housed under temperature 18–20°C for 14 weeks. Average ambient relative humidity inside the rearing house was 40%. The feed was offered on the basis of the recommendations (110 g/hen/day), and water (18 ± 4°C) was supplied ad‐libitum. To meet nutritional requirements of laying hens as recommended by the Lohmann LSL‐Lite catalogue (Lohmann LSL‐Classic International 2011), a corn‐soybean meal basal diet, 14.69% crude protein and 2,750 kilocalories of metabolizable energy per kg of feed was formulated, since we tried to maintain constant ME:CP ratio (Table 1).
TABLE 1.
Ingredients and nutrient composition of the basal diet (%, unless stated otherwise)
| Ingredients | Treatment 1 (control group) | Treatment 2 | Treatment 3 | Treatment 4 |
|---|---|---|---|---|
| Corn | 67.6 | 67.6 | 67.6 | 67.6 |
| Soybean meal (44% CP) | 21 | 21 | 21 | 21 |
| Wheat bran | 0.15 | 0.15 | 0.15 | 0.15 |
| Soybean oil | 0.07 | 0.07 | 0.07 | 0.07 |
| Lime stone | 3 | 3 | 3 | 3 |
| Oyster shells | 5.47 | 5.47 | 5.47 | 5.47 |
| Dicalcium phosphate | 1.64 | 1.64 | 1.64 | 1.64 |
| NaHCO3 | 0.18 | 0.18 | 0.18 | 0.18 |
| Common salt | 0.19 | 0.19 | 0.19 | 0.19 |
| Min. premix a | 0.25 | 0.25 | 0.25 | 0.25 |
| Vit. premix b | 0.25 | 0.25 | 0.25 | 0.25 |
| DL‐methionine | 0.15 | 0.15 | 0.15 | 0.15 |
| Lavender essential oil | 0 | 0.025 | 0 | 0.025 |
| Mint essential oil | 0 | 0 | 0.025 | 0.025 |
| Nutrient composition (as fed basis) | ||||
| 2,750 | ME (Kcal/kg) | |||
| 14.7 | Crude protein | |||
| 3.64 | Calcium | |||
| 0.37 | Available phosphorus | |||
| 0.15 | Sodium | |||
| 2.32 | Crude fiber | |||
| 207 | (Na + K)‐Cl (meg/kg) | |||
| 0.71 | Lysine | |||
| 0.37 | Methionine | |||
| 0.63 | Methionine + cystine | |||
| 0.54 | Threonine | |||
| 0.16 | Tryptophan | |||
Vitamin mixture per 2.5 kg of diet provides the following: vitamin A, 7,700,000 IU; vitamin D3, 3,300,000 IU; vitamin E, 6,600 mg; vitamin K3, 550 mg; thiamine, 2,200 mg; riboflavin, 4,400 mg; vitamin B6, 4,400 mg; capantothenate, 550 mg; nicotinic acid, 200 mg; folic acid, 110 mg; choline chloride, 275,000 mg; biotin, 55 mg; vitamin B12, 8. 8 mg.
Mineral mixture per 2.5 kg of diet provides the following: Mn, 66,000 mg; Zn, 66,000 mg; Fe, 33,000 mg; Cu, 8,800 mg; Se, 300 mg; I, 900 mg.
Supplemental Lavandula angustifolia and Mentha spicata essential oils were purchased from Barij Essence Pharmaceutical Co, Kashan, Iran; their active ingredients, according to the information received from the manufacture, are presented in the Table 2. Accordingly, 250 mg/kg diet LEO provided the followings per kg diet: 1 4.53 mg limonene, 69.75 mg 1,8‐cineole, 18.10 mg Camphor, 76.00 mg linalool, 3.15 mg linalyl acetate, and 1.05 Terpinen‐4‐ol; and 250 mg/kg diet MEO provided the followings per kg diet: 3.15 mg 1,8‐Cineole, 70.25 mg limonene, 5.60 mg menthol, 3.95 mg pulegone, 139.50 mg carvone, and 0.78 mg menthone. To minimize probable essential oil evaporation, the feeds were mixed weekly with the additives and kept in tied, double layered plastic bags in a dry, dark and well‐ventilated room at 25°C (Attia, Bakhashwain, & Bertu, 2017). The birds received one of the four experimental diets including: (a) the basal diet (control group), (b) basal diet supplemented with 250 mg/kg lavender essential oil (LEO), (c) basal diet supplemented with 250 mg/kg mint essential oil (MEO), and (d) basal diet supplemented with 250 mg/kg lavender +250 mg/kg mint essential oil. The levels of the essential oils used in this experiment were chosen based on previous results reported by Abd El‐Motaal, Ahmed, Bahakaim, and Fathi (2008), Hassan, El Sanhoury, Ali, and Ahmed (2011) and Akbari and Torki (2014).
TABLE 2.
The active ingredients of lavender and mint essential oils used in the current experiment
| Name | Compound | (%) |
|---|---|---|
| Lavender essential oil | Limonene | 1.81 |
| 1,8‐Cineole | 27.9 | |
| Campher | 7.24 | |
| Linalool | 30.4 | |
| Linalyl acetate | 1.26 | |
| Terpinen−4‐ol | 0.420 | |
| Mint essential oil | 1,8‐Cineole | 1.26 |
| Limonene | 28.1 | |
| Menthol | 2.24 | |
| Pulegone | 1.58 | |
| Carvone | 55.8 | |
| Menthone | 0.310 |
2.2. Productive performance and egg quality
The productive performance of the laying hens including hen‐day egg production, feed intake and egg weight were recorded daily, and feed conversation ratio (FCR) and egg mass were calculated. To evaluate egg quality characteristics including egg quality traits yolk colour, egg shell thickness, egg shell weight, egg index, yolk index and Haugh unit (HU), egg samples were collected twice on week 7 of experiment and each time all eggs during three frequent days were used. The eggs were marked according to the diet and replicate group.
Haugh units were calculated from the records of albumen height and egg weight using the HU formula (Eisen, Bohren, & McKean, 1962). The shell thickness was a mean value of measurement at three locations on the egg (air cell, equator and sharp end) by using a dial and pipe gage. The yolk colour was scored with the aid of Roche Yolk Colour Fan. Yolk height and yolk diameter were measured by a tripod micrometer (Mitutoyo, 0.01 mm) and compass (Swordfish, 0.02 mm), respectively, then the yolk index was calculated. Egg length and width were individually recorded and used to calculate egg shape index.
2.3. Blood biochemical variables
At the end of the experiment, eight hens were randomly selected from each treatment and blood samples were collected from the brachial vein into a 5‐ml syringe. The collected blood samples were centrifuged at 805 g for 10 min, and the sera was frozen at −20°C until the analysis. Serum samples were thawed at room temperature and were analysed for glucose, triglyceride, albumin, uric acid and cholesterol according to the manufacture recommendations (Pars Azmun); besides, the enzyme activity of plasma glutathione peroxidase was measured according to the manufacture recommendations (Randox Laboratories kit).
2.4. Statistical analysis
All data obtained from the trials were subjected to the analysis of variance procedure of statistical analysis system (SAS, 2001, Version 8.02) according to completely randomized design. Means were separated by Duncan's new multiple range test. The level of significance was determined at <.05. The following model was considered for analysis: Yij = μ + (Ri) + (Cj) + (RCij) + (eij), where Yijk is the measured characteristic, μ is the overall mean, (Ri) is the main effect of LEO, (Cj) is the main effect of MEO, RCij is interaction between the effect of LEO and MEO, and (eij) is the residual error. The effects of the main factors were not considered, whenever the interaction was significant.(Figures 1, 2, 3).
FIGURE 1.
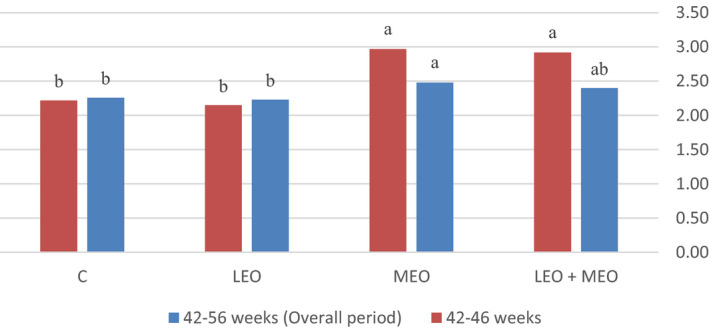
Effects of dietary treatments on feed conversion ratio (feed/egg) of the laying hens
FIGURE 2.
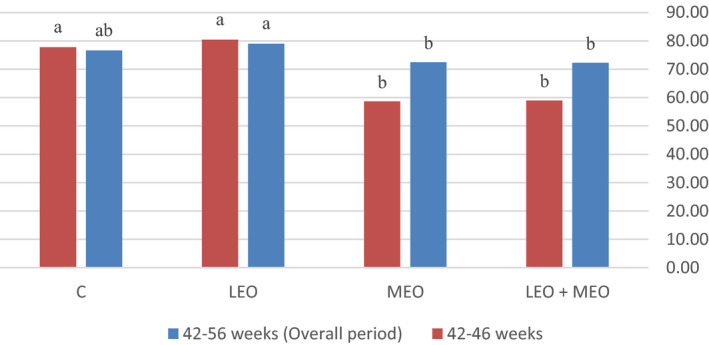
Effects of dietary treatments on hen‐day egg production (%) of the laying hens
FIGURE 3.
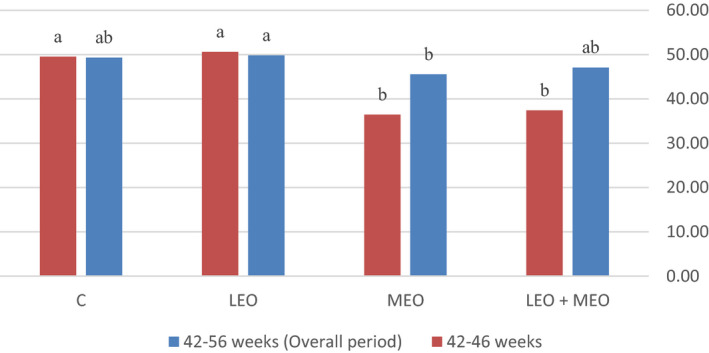
Effects of dietary treatments on egg mass (g hen−1 day−1) of the laying hens
3. RESULTS
3.1. Production performance
The productivity data of laying hens are summarized in Table 3. The result of the present study showed that adding LEO and/or MEO essential oils did not affect feed intake and body weight changes during the experimental period (p > .05). In this research feeding LEO, individually or in combination with MEO, did not affect FCR compared with the control group (p < .05), but feeding MEO individually increased FCR compared with LEO and the control group during 42–56 weeks (p < .05); besides, feeding MEO individually or in combination with MEO increased FCR compared with LEO and the control group during 42–46 weeks (p < .05). In the present study, supplementation of MEO individually or in combination with LEO significantly decreased egg production and also egg mass values during 42–46 weeks (p < .05). Egg production was significantly increased due to LEO feeding compared to MEO and LEO + MEO groups during 42–56 weeks (p < .05). LEO feeding also increased egg mass compared to MEO group during 42–56 weeks (p < .05).
TABLE 3.
Effects of dietary treatments on production performance of the laying hens
| Productive performance | Treatments | SEM a | p‐values | |||
|---|---|---|---|---|---|---|
| C | LEO | MEO | LEO + MEO | |||
| Body weight changes (g) | ||||||
| 52–56 weeks | 55.9 | 52.1 | 49.7 | 59.0 | 32.07 | .167 |
| Feed intake (g/hen/day) | ||||||
| 42–46 weeks | 110 | 108 | 108 | 109 | 0.627 | .362 |
| 47–51 weeks | 109 | 109 | 109 | 109 | 0.195 | .574 |
| 52–56 weeks | 109 | 109 | 109 | 108 | 0.510 | .609 |
| 42–56 weeks (overall period) | 109 | 109 | 109 | 109 | 0.350 | .576 |
| Feed conversion ratio (feed/egg) | ||||||
| 42–46 weeks | 2.22b | 2.15b | 2.97 a | 2.92 a | 0.050 | .001 |
| 47–51 weeks | 2.55 | 2.59 | 2.55 | 2.44 | 0.094 | .732 |
| 52–56 weeks | 2.01 | 1.93 | 1.93 | 1.85 | 0.072 | .471 |
| 42–56 weeks (overall period) | 2.26b | 2.23b | 2.48 a | 2.40 a | 0.059 | .022 |
| Hen‐day egg production (%) | ||||||
| 42–46 weeks | 77.8 a | 80.4 a | 58.7b | 59.0b | 1.43 | .001 |
| 47–51 weeks | 67.0 | 67.1 | 67.8 | 68.1 | 2.12 | .977 |
| 52–56 weeks | 85.0 | 89.6 | 91.1 | 89.9 | 2.92 | .491 |
| 42–56 weeks (overall period) | 76.6 a | 79.0 a | 72.5b | 72.3b | 1.79 | .040 |
| Egg mass (g/hen/day) | ||||||
| 42–46 weeks | 49.5 a | 50.61 a | 36.5b | 37.4b | 0.969 | .001 |
| 47–51 weeks | 43.5 | 42.40 | 43.4 | 45.0 | 1.48 | .678 |
| 52–56 weeks | 54.9 | 56.50 | 56.7 | 58.8 | 1.89 | .549 |
| 42–56 weeks (overall period) | 49.3 a | 49.8 a | 45.5b | 47.1 a | 1.24 | .077 |
Means within a row with different superscripts are significantly different (p < .05).
Abbreviations: C, control group; LEO + MEO, lavender essential oil + mint essential oil; LEO, lavender essential oil; MEO, mint essential oil.
Standard error of mean.
3.2. Egg quality traits
Supplementation of the layer diet with LEO and/or MEO had no significant effects on egg weight, egg index, yolk index, HU, yolk colour, egg shell weight and egg shell thickness (p > .05) (Table 4).
TABLE 4.
Effects of dietary treatments on egg quality traits of the laying hens
| Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Egg weight (g) | Egg index (%) | Yolk index (%) | Haugh unit | Yolk colour | Egg shell weight (g) | Egg shell thickness (mm 10−2) | |
| Control | 65.5 | 73.7 | 40.5 | 77.3 | 6.83 | 5.91 | 37.3 | |
| LEO | 62.5 | 71.7 | 41.4 | 79.6 | 7.00 | 5.93 | 38.0 | |
| MEO | 62.5 | 72.7 | 40.0 | 77.6 | 6.58 | 5.72 | 37.7 | |
| LEO + MEO | 63.1 | 74.4 | 39.9 | 75.9 | 6.91 | 6.05 | 39.1 | |
| SEM a | 1.44 | 18.2 | 0.859 | 1.89 | 0.120 | 0.163 | 0.67 | |
| p‐values | .424 | .382 | .604 | .586 | .117 | .559 | .311 | |
Abbreviations: C, control group; LEO + MEO, lavender essential oil + mint essential oil; LEO, lavender essential oil; MEO, mint essential oil.
SEM.
3.3. Blood metabolites
Table 5 shows data obtained on the effect of experimental treatments on blood variables. No significant influence of experimental diets on albumin, uric acid, glucose, triglyceride and cholesterol was observed (p > .05).
TABLE 5.
Effects of dietary treatments on biochemical variables of the laying hens
| Variables | |||||
|---|---|---|---|---|---|
| Treatments | Albumin (g/dl) | Uric acid (mg/dl) | Glucose (mg/dl) | Triglycerides (mg/dl) | Cholesterol (mg/dl) |
| Control | 7.17 | 77.2 | 975 | 847 | 723 |
| LEO | 6.76 | 78.3 | 850 | 833 | 695 |
| MEO | 6.38 | 79.2 | 855 | 861 | 998 |
| LEO + MEO | 6.81 | 79.2 | 915 | 805 | 972 |
| SEM a | 0.443 | 23.4 | 86.1 | 30.1 | 242 |
| p‐values | .671 | .635 | .709 | .600 | .730 |
Abbreviations: C, control group; LEO + MEO, lavender essential oil + mint essential oil; LEO, lavender essential oil; MEO, mint essential oil.
SEM.
4. DISCUSSION
4.1. Production performance
Several medicinal plants (e.g., garlic, onion, thyme, spearmint, pepper, yucca, black cumin (black seed), ginger) have been used in animal and poultry (layers and broilers) production due to their health benefits, e.g., spearmint leaves is used as carminative, antispasmodic and choleretic in human reported by Al‐Kassie (2010). It is hypothesized that herb mixture (garlic, onion, thyme, spearmint, pepper, yucca, black cumin seed, and ginger) might have several vital benefits of such as antioxidant, antimicrobial and anti‐inflammatory, which raise its use as herbal feed additives as alternative to antibiotics in broilers diets (Saleh, Ebeid, & Abudabos, 2018). The ajwain essential oil (Trachyspermum ammi L.) supplementation did not affect egg weight, egg mass, egg production, and feed intake in quail breeders (Hajiaghapour & Rezaeipour, 2018). Spearmint is used as an anti‐spasmodic, choleretic and carminative (Al‐Kassie, 2010). It is reported that, addition of spearmint essential oils, to the diet increased significantly the feed intake of broiler chicks (Abu Isha et al., 2018), Feeding spearmint increases feed intake and consequently improves growth in broiler chicks (Saleh et al., 2014).
Some aromatic herbal essences like lavender contain phytoestrogens. According to Saleh, Ahmed, and Ebeid (2019) inclusion of mixed sources of phytoestrogens (combination of 1 g/kg flaxseeds and 1 g/kg fenugreek) in diets improve laying performance, egg quality, the antioxidative status, hormonal profile and steroidogenesis in aged laying hens. Generally it is assumed that essential oils of herbs and spices might improve the palatability of feed due to their flavourful characteristics, hence could promote feed intake when added to layers and broilers diets (Hertrampf, 2001; Williams & Losa, 2001).
In this study, the addition of LEO and MEO essential oils did not influence feed intake and body weight changes during the trial period. In agreement, Nasiri‐Moghaddam, Hassanabadi, and Bidar (2012) reported that there was no difference in feed intake values between broilers fed control and those fed lavender essence (350 mg/kg) at the period of 22–42 days age.
The feed conversion ratio (FCR) describes the hens’ overall efficiency in converting ingested feed mass into egg mass over a specific period of time. An important claim often made for phytogenic feed additives is improvement of the FCR and thereby enhancing the intestinal availability of essential nutrients for absorption (Langhout, 2000; Williams & Losa, 2001). In this research feeding LEO, individually or combined with MEO, did not influence FCR compared with the control, but feeding MEO individually significantly increased FCR compared with LEO and control group during 42–56 weeks. In contrast, Nasiri‐Moghaddam et al., (2012) reported that dietary lavender essential oil (at 350 mg/kg) significantly improved FCR in broilers. In addition, Salari et al reported that feeding Lavander stoechas essence (200, 400 and 600 ppm) caused insignificant improvement in FCR in laying hens (Salari, Taki, Bojarpour, Sari, & Taghizadeh, 2014).
Isoprene derivatives, flavonoids, glucosinolates and other plant metabolites may affect the physiological and chemical function of the digestive tract. The stabilizing effect on intestinal microbiota may be associated with intermediate nutrient metabolism (Baratta, Dorman, Deans, Biondi, & Ruberto, 1998; Horton, Fennell, & Prasad, 1991; Jamroz et al., 2003). The pharmacological action of active plant substances or herbal extracts in humans is well‐known, but in animal nutrition the number of precise experiments is relatively low.
In this study, feed intake and body weight changes were not affected by supplemental MEO but FCR was affected by the supplementation of MEO compared to LEO and the control group. Body weight is not a common performance criteria in laying hens since hens are rather selected for laying performance and efficiency of feed conversion per unit of egg mass output. In a number of trials, laying rate, feed intake and FCR were not changed. Several studies confirmed the positive influence of herbs and their respective essential oils on body weight of hens with altered production performance (Bozkurt, Alcicek, Cabuk, Kucukyilmaz, & Catli, 2009).
In an experiment curcumin (0.5 g), capsaicin (0.015 g), piperine (0.02 g), ginger (0.05 g), cumin (1.25 g), asafoetida (0.25 g), ajwan (0.2 g), fennel (0.5 g), coriander (2.0 g), mint (1.0 g), garlic (0.5 g) and onion (2.0 g) could shorten the time of feed passage through digestive tract in rats (Platel & Srinivasan, 2001). Herbs with growth promoting activity increase the stability of feed and beneficially influence the gastrointestinal ecosystem mostly through growth inhibition of pathogenic microorganisms’ growth (Windisch, Schedle, Plitzner, & Kroismayr, 2008). Therefore, it might be possible that the increase in digestion and absorption of essential nutrients due to increasing the enzyme activity and/ or inhibition of pathogenic microorganism's growth could be the main reason of medicinal plants to accelerate the performance.
In a research, Al‐Ankari, Zaki and Al‐Sutan found that supplementation of Mentha piperita at the level of 1.5% for 35 days showed beneficial results on body weight, feed intake and FCR in broilers (Al‐Ankari, Zaki, & Al‐Sutan, 2004). Similarly, Nobakht, Norany & Safamehr reported that feeding 0.5% dried Mentha pulegium resulted in positive effects on performance in broilers at 42 days of age (Nobakht, Norany, & Safamehr, 2011). Whereas, Ocak et al. reported that supplemental Mentha piperita (0.2%) had no effects on broilers body weight and FCR at 42 days of age (Ocak et al., 2008). The controversy among these studies might be due to the differences in mint resources, which vary according to species, active ingredient, harvest time, etc (Brenes & Roura, 2010).
The phytogenic feed additives as performance enhancers for laying hens have primarily been supplemented to increase the utilization of the limit‐fed diet and, in turn, improve egg production (Bozkurt et al., 2009). In the current study, supplementing MEO individually or combined with LEO significantly decreased egg production and also egg mass values during 42–46 weeks; in addition, egg weights in MEO and LEO groups were not influenced compared to the control group. Egg production was significantly higher in LEO group compared to MEO and LEO + MEO groups during 42–56 weeks. LEO feeding also significantly increased egg mass compared to MEO group during 42–56 weeks. According to some authors, diets with higher inclusion levels of essential oils compounds had toxic effects (Krishan, & Narang, 2014). In this case, decline in egg production in treatment 4 (LEO + MEO), may be due to the accumulation of the essential oils and its probable toxicity; however, more adequate toxicological study must be carried out to clarify this assumption.
The results of this experiment are in accordance with the results of Nasiri‐Moghaddam, Hassanabadi & Bidar who did not observe any increases in egg weight, feed intake and egg production percentage by using lavender essence in the diets of layers (Nasiri‐Moghaddam et al., 2012). In addition, Bozkurt et al. showed that supplemented diets with mixture of essential oil (i.e., oregano oil, laurel leaf oil, sage leaf oil, myrtle leaf oil, fennel seed oil and citrus peel oil) (24 and 48 mg/kg of diet) did not affect egg production and egg weight of broiler breeders (Bozkurt et al., 2009). In another study (Botsoglou et al., 2005), the effects of dietary aromatic plant extracts (rosemary at 5 g/kg diet, oregano at 5 g/kg diet and saffron at 20 mg/kg diet) on the laying performance of hens from 32 to 40 weeks of age were investigated and the results showed no significant differences in egg production and egg weight among the treatment groups.
These observations partially support the hypothesis that herbs and their associated essential oils may favourably affect hen performance but the number of experimental studies conducted under standardized field conditions with larger numbers of hens and for longer durations, preferably over a whole laying cycle, is still limited (Bozkurt et al., 2009). In the current research, the essential oils did not increase egg production and egg mass, but as numerical, the highest egg production percentage and egg mass were found in LEO group.
Most of findings showed that improving FCR was related to increase in the amount of egg mass; consequently, increase in egg mass might lead to improved FCR (Nobakht et al., 2011; Sayedpiran, Nobakht, & Khodaei, 2011). In a study, the impact of feeding different amounts of eucalyptus (eucalyptus is similar to lavender due to containing cisteol) on productive performance, egg quality, blood variables and immune response in Japanese quails was investigated and it indicated enhanced daily weight gain, number of egg, egg weight, egg mass, improved FCR and quality of egg in the diets with eucalyptus compared to the control diet (Hassan et al., 2011). The results of our experiment are not in accordance with the results of Hassan, et al. that observed increase in egg weight, feed intake and production percentage by using eucalyptus powder in the diets of quails (Hassan et al., 2011). In another study, applying 3 g of eucalyptus per kilogram of diet improved the laying hens’ productive performance and immunocompetence; moreover, FCR was improved in the hens given 3 g of eucalyptus in the diet because of their health and vitality (Abd El‐Motaal et al., 2008). Different medicinal herbs contain different percentage of constituents and probably for this reason have different effects on productive performance.
4.2. Egg quality traits
Supplementation of the layer diet with Lavandula angustifolia and Mentha spicata essential oils had no significant effects on egg weight, egg index, yolk index, HU, yolk colour and egg quality variables (egg weight and egg shell thickness). The results of this study are in agreement with those reported by Salari et al. who stated that feeding lavender essence (200, 400 and 600 ppm) did not affect HU (Salari et al., 2014). It was observed from this study that feeding MEO did not increase egg weight. Increased egg weight might be due to better utilization of nutrients by mint, which in turn resulted in better egg weight (Merina Devi, Palod, Dar, & Shekhar, 2018). The results of the present study are not in line with the findings of Abdel‐Wareth and Lohakare, who reported that supplementation of peppermint leaves (5, 10, 15 or 20 g/kg) in laying hens improved egg weight due to the beneficial action of peppermint in the process of oviposition and imperative effect on the conversion of digested feed into eggs (Abdel‐Wareth & Lohakare, 2014). Dietary MEO supplementation did not influence HU which is not in agreement with findings were recorded by Sayedpiran et al., (2011) and Abdel‐Wareth and Lohakare, (2014). Different effects of supplemental mint on egg quality might stem from the amount and the source of mint extract.
4.3. Blood metabolites
No significant influence of experimental diets on albumin, uric acid, glucose, triglyceride and cholesterol was observed. There were some changes by addition of MEO, but the changes did not reach statistical significance. The results of this study revealed that LEO and MEO consumption could not change blood variables significantly. Most of spices and herbs enhance the synthesis and excretion of bile acids in the liver. As bile acids had beneficially effects on lipids’ digestion and absorption, it could improve the lipids’ digestion and absorption, which led to increase in the level of blood triglyceride (Srinivasan, 2005). Khursheed et al. mentioned that a variety of essential oil compounds, such as menthone, menthol and geraniol have been shown to suppress the hepatic 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase activity (Khursheed et al., 2017). However, in the present study, the effect of the essential oils on blood triglyceride was not significant.
Changes in blood biochemical variables in the present experiment are not supported by other researchers (Nobakht & Mehmannavaz, 2010; Sayedpiran et al., 2011), who reported increase in feed intake and decrease in blood triglyceride level by adding 2% of Lamiaceace menthapiperita. In a research, mixture of three medicinal plants (0.5% Malva silvestris, 1% Alhaji maurorum, 0.5% Mentha spicata) decreased blood glucose of broilers (Nobakht & Shahryar, 2010). In addition, using 0.5% of Mentha pulegium powder significantly improved the performance and reduced the blood glucose of broilers (Nobakht et al., 2011).
Akbari and Torki reported that peppermint extract has increased total protein and HDL and decreased blood serum concentrations of total cholesterol, triglycerides, LDL and glucose of broilers (Akbari & Torki, 2014). Fallah, Kiani, & Azarfar also reported that peppermint extract (200 meg/kg) has increased albumin, HDL and significantly reduced total cholesterol, triglycerides, LDL and glucose in broilers (Fallah, Kiani, & Azarfar, 2013). It seems as some components of peppermint including menthol and menthone have a potential to decrease blood lipids in broilers (Escop (European Scientific Cooperative on Phytotherapy), 2003). Mansoub also reported that peppermint extract (0.75%, 1%, 1.5%, and 2%) increases albumin, total protein and HDL and also significantly decreases glucose, triglycerides, total cholesterol and LDL in serum of broilers; he also suggested that the main reason for the reduction of serum lipids in broilers is due to the active ingredients of peppermint leaves such as tocopherol and menthol (Mansoub, 2011). Our observation in this experiment on blood biochemical variables is not in agreement with Hardari, Nobakht, and Safamehr (2010) report, who stated that using 1.5% of M. pulegium L. had positive effects on blood cholesterol level.
Definitely, several variables such as supplementation methods (in diet or drinking water), the supplemental level, its level in the basal diet, bioavailability, stress condition, degree of stress, as well as duration of usage could be, at least in part, probable reasons for contradictory results in miscellaneous experiments.
5. CONCLUSION
Based on the results of the present study, it can be concluded that dietary supplemental MEO (250 mg/kg) may increase FCR, and LEO (250 mg/kg) is more effective than LEO (250 mg/kg) for egg production and egg mass purposes; besides MEO (250 mg/kg) negatively affected FCR compared with the control group.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Mehran Torki: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing‐review & editing. Ahmad Mohebbifar: Data curation; Software. Hamed Mohammadi: Validation; Writing‐original draft.
ETHICAL STATEMENT
All the experimental proceedings in this experiment were approved by the Animal Ethics Committee of Razi University, Kermanshah, Iran.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.343.
Torki M, Mohebbifar A, Mohammadi H. Effects of supplementing hen diet with Lavandula angustifolia and/or Mentha spicata essential oils on production performance, egg quality and blood variables of laying hens. Vet Med Sci.2021;7:184–193. 10.1002/vms3.343
REFERENCES
- Abd El‐Motaal, A. M. , Ahmed, A. M. H. , Bahakaim, A. S. A. , & Fathi, M. M. (2008). Productive performance and immunocompetence of commercial laying hens given diets supplemented with eucalyptus. International Journal of Poultry Science, 7, 445–449. 10.3923/ijps.2008.445.449 [DOI] [Google Scholar]
- Abdel‐Wareth, A. A. , & Lohakare, J. D. (2014). Effect of dietary supplementation of peppermint on performance, egg quality, and serum metabolic profile of Hy‐Line Brown hens during the late laying period. Animal Feed Science Technology, 197, 114–120. 10.1016/j.anifeedsci.2014.07.007 [DOI] [Google Scholar]
- Abu Isha, A. , El‐Hamid, A. , Ziena, H. , & Ahmed, H. (2018). Effect of spearmint (mentha spicata) on productive and physiological parameters of broiler chicks. Egyptian Poultry Science Journal, 38(3), 815–829. 10.21608/epsj.2018.17106 [DOI] [Google Scholar]
- Akbari, M. , & Torki, M. (2014). Effects of dietary chromium picolinate and peppermint essential oil on growth performance and blood biochemical parameters of broiler chicks reared under heat stress conditions. International Journal of Biometeorology, 58, 1383–1391. 10.1007/s00484-013-0740-1 [DOI] [PubMed] [Google Scholar]
- Al‐Ankari, A. S. , Zaki, M. M. , & Al‐Sutan, S. I. (2004). Use of habek mint (Mentha longifolia) in broiler chicken diets. International Journal of Poultry Science, 3, 629–634. 10.3923/ijps.2004.629.634 [DOI] [Google Scholar]
- Al‐Kassie, G. A. M. (2010). The role of peppermint (Mentha piperita) on performance in broiler diets. Agricultural and Biological Journal of North America, 15, 1009–1013. 10.5251/abjna.2010.1.5.1009.1013 [DOI] [Google Scholar]
- Attia, Y. A. , Bakhashwain, A. A. , & Bertu, N. K. (2017). Thyme oil (Thyme vulgaris L.) as a natural growth promoter for broiler chickens reared under hot climate. Italian Journal of Animal Science, 16(2), 275–282. 10.1080/1828051X.2016.1245594 [DOI] [Google Scholar]
- Aydin, E. , Turkez, H. , & Sait Keles, M. (2015). Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicology and Industrial Health, 31(8), 764–772. 10.1177/0748233713484660 [DOI] [PubMed] [Google Scholar]
- Bakhsha, F. , Mazandarani, M. , Aryaei, M. , Jafari, S. Y. , & Bayate, H. (2014). Phytochemical and anti‐oxidant activity of Lavandula Angustifolia Mill. essential oil on preoperative anxiety in patients undergoing diagnostic curettage. International Journal of Women’s Health Reproduction Science, 2, 268–271. 10.15296/ijwhr.2014.42 [DOI] [Google Scholar]
- Baratta, M. T. , Dorman, H. J. D. , Deans, S. G. , Biondi, D. M. , & Ruberto, G. (1998). Chemical composition, antimicrobial and antioxidative activity of Laurel, sage, rosemary, oregano and coriander essentials oils. Journal of Essential Oil Research, 10, 618–627. 10.1080/10412905.1998.9700989 [DOI] [Google Scholar]
- Bimakr, M. , Rahman, R. A. , Taip, F. S. , Ganjloo, A. , Salleh, L. M. , Selamat, J. , … Zaidul, I. S. M. (2011). Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food and Bioproducts Processing, 89, 67–72. 10.1016/j.fbp.2010.03.002 [DOI] [Google Scholar]
- Botsoglou, N. A. , Florou‐Paneri, P. , Botsoglou, E. , Datos, V. , Giannenas, I. , Koidis, A. , & Mitrakos, P. (2005). The effect of feeding rosemary, oregano, saffron and α‐tocopheryl acetate on hen performance and oxidative stability of eggs. South African Journal ofAnimal Science, 35, 143–151. 10.2141/jpsa.43.143 [DOI] [Google Scholar]
- Bozkurt, M. , Alcicek, A. , Cabuk, M. , Kucukyilmaz, K. , & Catli, A. U. (2009). Effect of an herbal essential oil mixture on growth, laying traits, and egg hatching characteristics of broiler breeders. Poultry Science, 88, 2368–2374. 10.3382/ps.2009-00048 [DOI] [PubMed] [Google Scholar]
- Bozkurt, M. , Hippenstiel, F. , Abdel‐Wareth, A. A. A. , Kehraus, S. , Kuçukyılmaz, K. , & Sudekum, K. H. (2014). Effects of selected herbs and essential oils on performance, egg quality and some metabolic activites in laying hens‐ a review. European Poultry Science, 78(1–15), 78 10.1399/eps.2014.49 [DOI] [Google Scholar]
- Brenes, A. , & Roura, E. (2010). Essential oils in poultry nutrition: Main effects and modes of action. Animal Feed Science and Technology, 158, 1–14. 10.1016/j.anifeedsci.2010.03.007 [DOI] [Google Scholar]
- Butaye, P. , van Damme, K. , Devriese, L. A. , van Damme, L. , Bael, M. , Lauwers, S. , & Haesebrouck, F. (2000). In vitro susceptibility of Enterococcus faecium isolated from food to growth‐promoting and therapeutic antibiotics. International Journal of Food Microbiology, 54, 181–187. 10.1016/S0168-1605(99)00198-1 [DOI] [PubMed] [Google Scholar]
- Eisen, E. J. , Bohren, B. B. , & McKean, H. E. (1962). The Haugh unit as a measure of egg albumen quality. Poultry Science, 41, 1461–1468. 10.3382/ps.0411461 [DOI] [Google Scholar]
- Escop (European Scientific Cooperative on Phytotherapy) . (2003). ESCOP monographs. 2nd ed. The scientific foundation for medicinal products. pp. 554 UK: Thieme. [Google Scholar]
- Fallah, R. , Kiani, A. , & Azarfar, A. (2013). Effect of artichoke leaves meal and mentha extract (Mentha piperita) on immune cells and blood biochemical parameters of broilers. Global Veterinaria, 10, 99–102. 10.5829/idosi.gv.2013.10.1.71206 [DOI] [Google Scholar]
- Francois, R. (2006). Active plant extracts show promise in poultry production. Poultry International, 20, 28–31. [Google Scholar]
- Frankic, T. , Voljc, M. , Salobir, J. , & Rezar, V. (2009). Use of herbs and spices and their extracts in animal nutrition. Acta Agriculturae Slovenica, 94, 95–102. [Google Scholar]
- Galib, M. , Al‐kassi, M. W. , & Noor, A. (2010). A comparative study on diet supplementation with a mixture of herbal plants and dandelion as a source of probiotics on the performance of broilers. Pakistan Journal of Nutrition, 9(1), 67–71. [Google Scholar]
- Gulluce, M. , Sahin, F. , Sokmen, M. , Ozer, H. , Daferera, D. , Sokmen, A. , … Ozkan, H. (2007). Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia . Food Chemistry, 103, 1449–1456. 10.1016/j.foodchem.2006.10.061 [DOI] [Google Scholar]
- Hajiaghapour, M. , & Rezaeipour, V. (2018). Comparison of two herbal essential oils, probiotic, and mannan‐oligosaccharides on egg production, hatchability, serum metabolites, intestinal morphology, and microbiota activity of quail breeders. Livestock Science, 210, 93–98. 10.1016/j.livsci.2018.02.007 [DOI] [Google Scholar]
- Hardari, A. , Nobakht, A. , & Safamehr, A. (2010). Investigation the effects using Nettle (Urtica dioica), Menta pulagum (Oreganum valgare) and Zizaphora (Thymyus vulgaris) medicinal plants and there mixtures on biochemical and immunity parameters of broilers. In 4th Congresson Animal Science. September 2010, Karaj, Iran. pp. 214–217.
- Hassan, M. S. H. , El Sanhoury, M. H. , Ali, W. A. H. , & Ahmed, A. M. H. (2011). Effect of using Eucalyptus levels as natural additives on productive, physiological, immunological and histological performance of laying Japanese quail. Egyptian Poultry Science, 31, 305–329. [Google Scholar]
- Hertrampf, J. W. (2001). Alternative antibacterial performance promoters. International Journal of Poultry Science, 40, 50–52. [Google Scholar]
- Horton, G. M. J. , Fennell, M. J. , & Prasad, B. M. (1991). Effect of dietary garlic (Allium sativum) on performance, carcass composition and blood chemistry changes in broiler chickens. Canadian Journal of Animal Science, 71, 939–942. 10.4141/cjas91-113 [DOI] [Google Scholar]
- Jamroz, D. , Orda, J. , Kamel, C. , Williczkiewicz, A. , Wertelecki, T. , & Skorupin’Ska, J. (2003). The influence of phytogenic extract on performance, nutrients digestibility, carcass characteristic and gut microbial status in broiler chickens. Journal of Animal and Feed Science, 12, 583–596. 10.22358/jafs/67752/2003. [DOI] [Google Scholar]
- Kamel, C. (2001). Tracing modes of action and the roles of plant extracts in non‐ruminants In Garnsworthy P. C. & Wiseman J. (Eds.), Recent advances in animal nutrition (pp. 135–150). Nottingham: Nottingham University Press. [Google Scholar]
- Khursheed, A. , Banday, M. T. , Khan, A. A. , Adil, S. , Ganai, A. M. , Sheikh, I. U. , & Sofi, A. H. (2017). Effect of mint leaves with or without enzyme supplementation on blood biochemistry, carcass characteristics and sensory attributes of broiler chicken. Advances in Animal and Veterinary Sciences, 5(11), 449–455. 10.17582/journal.aavs/2017/5.11.449.455 [DOI] [Google Scholar]
- Krishan, G. , & Narang, A. (2014). Use of essential oils in poultry nutrition: A new approach. Journal of Advanced Veterinary and Animal Research, 1, 156–162. 10.5455/javar.2014.a36 [DOI] [Google Scholar]
- Langhout, P. (2000). New additives for broiler chickens. World Poultry, 16, 22–27. [Google Scholar]
- Lis‐Balchin, M. (2002). Lavander. The Genus Lavandula. Medicinal and Aromatic Plants– Industrial Profiles (1st ed.). London: CRC Press. [Google Scholar]
- Mansoub, N. H. (2011). The evaluation of different levels of Menthe pulagum on performance, and blood parameters of broilers. Journal of American Science, 7, 388–391. [Google Scholar]
- Mellor, S. (2000). Nutraceuticals–alternatives to antibiotics. World Poultry, 16, 30–33. [Google Scholar]
- Merina Devi, K. , Palod, J. , Dar, A. H. , & Shekhar, S. (2018). Effect of feeding graded levels of Pudina (Mentha arvensis) leaf powder on egg quality traits in laying hens. International Journal of Current Microbiology and Applied Sciences, 7, 756–761. 10.20546/ijcmas.2018.703.088 [DOI] [Google Scholar]
- Nasiri‐Moghaddam, H. , Hassanabadi, A. , & Bidar, N. (2012). Effects of increasing levels of lavender essential oil (Lavandula angustifolia) on performance and hematological traits of broilers. Iranian Journal of Animal Science Research, 4, 115–121. (In Persian, with English abstract). [Google Scholar]
- Nobakht, A. , & Mehmannavaz, Y. (2010). Investigation the effects of using of Thymus vulgaris, Lamiaceae menthapiperita, Oreganum valgare medicinal plants on performance, egg quality, blood and immunity parameters of laying hens. Iranian Journal of Animal Science, 41, 129–136. [Google Scholar]
- Nobakht, A. , Norany, J. , & Safamehr, A. R. (2011). The effects of different amounts of Mentha pulegium L. (pennyroyal) on performance, carcass traits, hematological and blood biochemical parameters of broilers. Journal of Medical Plants Research, 5, 3763–3768. [Google Scholar]
- Nobakht, A. , & Shahryar, H. A. (2010). The effects mixture of Malva silvestris, Alhaji mauroum and Mentha spicata on performance, carcass traits and blood metabolits of broilers. Journal of Animal Science, 3, 51–63. [Google Scholar]
- Ocak, N. , Erener, G. , Burak, F. , Ak, M. , Sungu, A. , Altop, A. , & Ozmen, A. (2008). Performance of broilers feed diets supplemented with dry peppermint (Mentha piperita L.) or thyme (Thymus vulgaris L.) leaves as growth promoter source. Czech Journal of Animal Science, 4, 169–175. 10.17221/373-CJAS. [DOI] [Google Scholar]
- Platel, K. , & Srinivasan, K. (2001). Studies on the influence of dietary spices on food transit time in experimental rats. Nutrition Research, 21, 1309–1314. 10.1016/S0271-5317(01)00331-1 [DOI] [Google Scholar]
- Salari, S. , Taki, A. , Bojarpour, M. , Sari, M. , & Taghizadeh, M. (2014). Effect of different levels of lavandula stoechas essence on production performance and egg quality of laying hens. In Proceedings of the International Symposium on Animal Science (pp. 295‐299) September 2014, Belgrade‐Zemun.
- Saleh, A. A. (2012). The effect of feeding aspergillus oryzae on growth performance, carcass parameters and some biochemical traits of broilers. Egyptian Poultry Science Journal, 32, 749–761. [Google Scholar]
- Saleh, A. A. , Ahmed, E. A. M. , & Ebeid, T. A. (2019). The impact of phytoestrogen source supplementation on reproductive performance, plasma profile, yolk fatty acids and antioxidative status in aged laying hens. Reproduction in Domestic Animals, 54(6), 846–854. 10.1111/rda.13432 [DOI] [PubMed] [Google Scholar]
- Saleh, A. A. , Ebeid, T. A. , & Abudabos, A. M. (2018). Effect of dietary phytogenics (herbal mixture) supplementation on growth performance, nutrient utilization, antioxidative properties, and immune response in broilers. Environmental Science and Pollution Research, 25, 14606–14613. 10.1007/s11356-018-1685-z [DOI] [PubMed] [Google Scholar]
- Saleh, A. A. , Ijiri, D. , & Ohtsuka, A. (2014). Effects of summer shield supplementation on growth performance, nutrient utilisation, and plasma lipid profiles in broiler chickens. Veterinarni Medicina, 59(11), 536–542. 10.17221/7818-VETMED. [DOI] [Google Scholar]
- SAS (2001). SAS User’s Guide, Version 8.02 Ed. Cary, NC, USA: SAS Institute Inc. [Google Scholar]
- Sayedpiran, S. A. , Nobakht, A. , & Khodaei, S. (2011). The effects of using of probiotic, organic acid and blends of some medicinal herbs on performance, egg quality, blood biochemical an immunity parameters of laying hens. Veterinary Clinical Pathology (Veterinary Journal Tabriz), 5, 1111–1122. [Google Scholar]
- Srinivasan, K. (2005). Role of spices beyond food flavouring: Nutraceuticals with multiple health effects. Food Reviews International, 21, 167–188. 10.1081/FRI-200051872 [DOI] [Google Scholar]
- Valero, M. , & Salmeron, M. C. (2003). Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. International Journal of Food Microbiology, 85, 73–81. 10.1016/S0168-1605(02)00484-1 [DOI] [PubMed] [Google Scholar]
- Wenk, C. (2000). Recent advances in animal feed additives such as metabolic modifiers, antimicrobial agents, probiotics, enzymes and highly available minerals. Review. Asian‐australasian Journal of Animal Sciences, 13, 86–95. 10.5713/ajas.2000.86 [DOI] [Google Scholar]
- Williams, P. , & Losa, R. (2001). The use of essential oils and their compounds in poultry nutrition. World Poultry, 17, 14–15. [Google Scholar]
- Windisch, W. , Schedle, K. , Plitzner, C. , & Kroismayr, A. (2008). Use of phytogenic products as feed additives for swine and poultry. Journal of Animal Science, 86, 140–148. 10.2527/jas.2007-0459 [DOI] [PubMed] [Google Scholar]


