Abstract
Objective
Motor and non-motor symptoms (NMS) negatively impact the health-related quality of life (HRQoL) for individuals with Parkinson’s disease (PD), as well as their caregivers. NMS can emerge decades prior to the manifestation of motor symptoms but often go unrecognized and therefore untreated. To guide clinical management, we surveyed differences and identified factors that influence HRQoL in a cohort of PD patients and family caregivers.
Methods
A total of 103 PD patients were compared with 81 caregivers. Outcome measures collected from validated questionnaires included generic and disease-specific HRQoL assessments, depression frequency and severity, constipation severity, upper and lower gastrointestinal symptoms, physical activity and motor symptom severity.
Results
PD patients reported significantly decreased physical and mental HRQoL compared to their caregivers (both p < 0.001). Unemployment, the need for social support services, rehabilitation use, REM sleep behavior disorder, impulse control disorders and features suggestive of increasing disease severity hallmarked by increasing PD duration, higher MDS UPDRS-III (Movement Disorder Society–Unified Parkinson’s Disease Rating Scale–Part III) scores, higher daily levodopa equivalence dose and motor fluctuations were consistent with a lower HRQoL in our PD cohort. Furthermore, decreased physical activity, chronic pain, depression, constipation and upper gastrointestinal dysfunction (particularly indigestion, excess fullness and bloating) suggested vulnerability to reduced HRQoL. Overall, PD patients perceived their health to decline by 12% more than their caregivers did over a 1-year period.
Conclusion
PD patients reported decreased HRQoL, with both motor symptoms and NMS negatively impacting HRQoL. Our findings support the routine clinical screening of HRQoL in PD patients to identify and address modifiable factors.
Keywords: Caregiver, Health care, Health related quality of life, Parkinson’s disease, Quality of life
Parkinson’s disease (PD) is an incurable multisystem disorder that contributes to significant morbidity and healthcare burden [1]. The physical, psychological and interpersonal impacts of PD are considerable. Health-related quality of life (HRQoL) is a subjective patient-reported outcome that complements clinical evaluation and provides information about disease activity and treatment effects [2]. In particular, a variety of motor symptoms and non-motor symptoms (NMS) are known to negatively impact the HRQoL for individuals with PD, as well as their families [3,4].
Motor symptoms arising during early clinical disease, including tremor, rigidity, bradykinesia and walking difficultly, classically reflect the majority of HRQoL impairment. For many patients, these symptoms may also include troublesome motor fluctuations, such as dystonic ‘off periods’ and dyskinesias, typically reflecting fluctuating levodopa responsiveness. Later in the disease course, cognitive and neuropsychiatric problems arise, with dementia commonly occurring in the advanced stages of PD [3].
NMS can present decades prior to the manifestation of motor symptoms, generally with an absence of observable disability. They are often under-recognized despite having a profoundly negative impact on HRQoL [3,5]. NMS associated with a decline in HRQoL include disrupted sleep architecture, constipation, hyposmia, anxiety, depression, fatigue, chronic pain, impaired speech and difficulty swallowing [2,3]. PD patients also attribute poorer HRQoL to other social and cultural impacts that indirectly influence their well-being, including an inability to work and the associated financial insecurity, as well as stigma and social avoidance due to visible tremor and dyskinesias. Possibly the most disruptive to patients and their families is the disengagement from family life and interpersonal relationships due to apathy and depression [6]. Interestingly, depressive symptoms and poorer HRQoL, more so than motor deficits, can be the strongest predictors for initiation of dopaminergic therapy [7]. Since many patients with PD go on to require a caregiver in their lives, many full-time, the impact of these NMS on the caregiver is also an important issue that impacts the overall care the patient receives. The HRQoL of the caregiver can also be assessed and used to direct patient care.
More recently, differences in HRQoL and depression in PD have been reported to reflect significant alterations in the composition of gastrointestinal microbiome profiles, potentially resulting from the imbalance of gut and central nervous system neurotransmitters that may disrupt communication between the gut-brain-axis [8-10]. Such novel mechanisms require ongoing evaluation with appropriate HRQoL scales that utilize both disease-oriented and validated instruments. These could include the Parkinson’s Disease Questionnaire (PDQ-39) [11] and the 36- item Short-Form Health Survey (SF-36), [12] which enable the assessment of health and functional ability in PD patients.
Despite the growing literature on HRQoL in PD patients [4,6,13-15], there is a lack of insight into the differences in HRQoL perceptions between PD patients and family caregivers, particularly in defining functional and psychological impacts that caregivers of PD patients face. Although HRQoL differences between PD and healthy unrelated control cohorts are well known, we took the approach to identify if caregivers were similarly susceptible to changes in their HRQoL from caring for an affected and dependent relative with PD. Here, we have 1) characterized important differences between HRQoL in PD patients and their caregivers, 2) quantified PD family caregiver HRQoL impacts and 3) identified notable demographic and clinical predictors for impaired HRQoL in PD. Additionally, we investigated the impact of other less-considered features, including gastrointestinal dysfunction, chronic pain, physical exercise and depression, to guide improved clinical management for PD patients and their caregivers.
MATERIALS & METHODS
Study setting and subjects
This study provides a secondary analysis of previously reported research [16]. Subjects were consecutively recruited from the movement disorder clinics at Royal North Shore Hospital, Sydney, Australia, June 2018 to June 2019. PD patient inclusion criteria were 1) > 18 years of age, 2) a clinical diagnosis of idiopathic PD according to the UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria [17], and 3) being managed by a specialist neurologist. The caregiver inclusion criteria were 1) > 18 years of age, 2) a spouse, sibling or child to their respective PD subject, 3) exhibited no clinical indication of PD on examination and 4) were subjectively “well” according to the caregiver. Approval from the local Human Research Ethics Committees was obtained, (HREC/18/HAWKE/109) and (NSPHEC 2018-LNR-009). All participants provided written informed consent to participate in the study.
Data collection
Participants completed self-administered questionnaires, in addition to providing socio-demographic, lifestyle and clinical management information. The SF-36 [12] was chosen as the common HRQoL tool and was administered on both PD patients and their caregivers to determine an individual’s perceived health status, with scales measuring each of the following eight health concepts: 1) physical functioning, 2) role limitations because of physical health problems, 3) bodily pain, 4) social functioning, 5) general mental health (psychological distress and psychological wellbeing), 6) role limitations because of emotional problems, 7) vitality (energy/fatigue) and 8) general health perceptions. Two aggregate summary scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS), are derived from the eight concepts. An additional question regarding the individual’s change in health status over the past year was asked to estimate changes in health. Each scale was scored from 0 (most disability) to 100 (least disability). PD patients completed the PDQ-39 [11], a validated measure of QoL in PD, that includes eight disease-specific scales of subjective health status: 1) mobility, 2) activities of daily living, 3) emotions, 4) social stigma, 5) social support, 6) cognition, 7) communication and 8) bodily discomfort. Each scale was scored from 0 (least affected) to 100 (most affected).
Depression was assessed by the Beck Depression Inventory (BDI), which contains 21 items on a 4-point scale from 0 (symptom absent) to 3 (severe symptoms), with a range of overall scores from 0–63 [18]. Depression was diagnosed in accordance with validated BDI cut-off criteria; > 13 for PD patients and > 9 for caregivers [19,20]. NMS were assessed by the Non-Motor Symptoms Scale (NMSS), scored between 0 (least affected) to 243 (most affected), from nine individual health domains [21]. Clinical motor assessments were performed by one neurologist during a patient’s ‘on’ state, as an objective measure of the prevailing motor function in accordance with the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale–Part III (MDS-UPDRS-III) criteria [22]. Medications were compared following standard methods for calculating daily levodopa equivalent dose (LED) [23], while rapid eye movement sleep behavior disorder (RBD) [24] and impulse control disorder (ICD) [25] were defined according to established diagnostic criteria. Other questionnaires included the Leeds Dyspepsia Questionnaire (LDQ) [26], assessing upper gastrointestinal symptoms; the Rome-IV criteria [27] and the Cleveland Constipation Scale (CCS) [28], to classify constipation severity; as well as the International Physical Activity Questionnaire (IPAQ) [29], to quantify physical activity, as described previously [16].
Statistical analysis
Two-sample, independent t-tests and chi-squared tests were used to analyze differences between continuous and categorical variables, respectively. Data normality was confirmed using the Shapiro-Wilk test. Logistic and linear regression models evaluated differences in HRQoL features between the PD and caregiver groups, as well as within the PD cohort, after controlling for demographic and clinical variables. Pearson correlations evaluated clinically relevant variables. Statistical significance was set at p < 0.05. Analysis was performed using SPSS, version 26 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographic and clinical characteristics
Demographic information pertaining to the cohort studied here has been reported previously [16]. Briefly, 103 PD patients and 81 caregivers were enrolled in the study (Table 1). Over half of the PD participants were male, and two-thirds of the caregivers were female. Of the combined cohort (PD and caregivers), approximately 80% were married and identified as Caucasian, 50% had completed tertiary studies, 70% reported a self-funded income and 10% were unemployed, with no significant differences observed between the groups. Overall, 32% of PD patients compared to 4.9% of caregivers reported utilizing any support services (p < 0.001), while 60% of PD patients versus 82% of caregivers reported interest in utilizing telemedicine for their own health (p < 0.001), which posed important considerations regarding the delivery of healthcare. Over one-third of all participants identified as being prior smokers, and over 90% of participants reported daily caffeine consumption, although PD patients reported lower daily intake [2.3 (SD 1.7) vs. 3.1 (SD 1.8) cups, p = 0.003]. PD patients also reported a lower alcohol consumption compared to caregivers (70% vs. 87.7%, p = 0.003, as reported previously [16]) (Table 1).
Table 1.
Cohort demographic and clinical characteristics
| Parkinson’s disease (n = 103) | Caregiver (n = 81) | Test statistic (df) | p value | |
|---|---|---|---|---|
| Age (years)* | 67.1 [12.2, 33–88] | 62.4 [15.6, 18–90] | t = 2.3 (182)† | 0.023 |
| Sex (%)* | χ2 = 10.7 (1)‡ | 0.001 | ||
| Male | 56.3 | 32.1 | ||
| Female | 43.7 | 67.9 | ||
| Ethnicity (%)* | χ2 = 2.3 (3)‡ | 0.506 | ||
| Caucasian | 78.6 | 79.0 | ||
| Asian | 3.9 | 6.2 | ||
| Middle Eastern | 6.8 | 2.5 | ||
| Other | 10.7 | 12.3 | ||
| Marital status (%)* | χ2 = 4.2 (3)‡ | 0.244 | ||
| Married/de facto | 76.7 | 85.1 | ||
| Single | 9.7 | 9.9 | ||
| Widowed | 5.8 | 1.2 | ||
| Other | 7.7 | 3.7 | ||
| Education status (%) | χ2 = 3.6 (3)‡ | 0.311 | ||
| Tertiary | 51.4 | 53.1 | ||
| Diploma | 32.0 | 22.2 | ||
| High school | 13.6 | 22.2 | ||
| Other | 2.9 | 2.5 | ||
| Employment (%) | χ2 = 5.9 (2)‡ | 0.052 | ||
| Working | 17.5 | 32.1 | ||
| Retired | 72.8 | 56.8 | ||
| Unemployed | 9.7 | 11.1 | ||
| Income (%) | χ2 = 1.0 (1)‡ | 0.321 | ||
| Pensioner | 40.0 | 27.2 | ||
| Self-funded | 66.0 | 72.8 | ||
| Support services (%) | χ2 = 20.9 (3)‡ | < 0.001 | ||
| None | 67.9 | 95.1 | ||
| Aged care package | 19.4 | 3.7 | ||
| National disability insurance scheme | 9.7 | 1.2 | ||
| Other | 2.9 | 0.0 | ||
| Telemedicine interest for own health (%) | 59.2 | 81.5 | χ2 = 10.5 (1)‡ | 0.001 |
| Smoking history (%)* | ||||
| Current smoker | 1.9 | 3.7 | χ2 = 0.6 (1)‡ | 0.457 |
| Prior smoker | 36.9 | 33.7 | χ2 = 0.2 (1)‡ | 0.659 |
| Pack year history | 13.3 (13.8) | 14.4 (14.6) | t = -0.3 (63)† | 0.758 |
| Alcohol consumption (%)* | 70.0 | 87.7 | χ2 = 8.7 (1)‡ | 0.003 |
| < Weekly | 23.5 | 27.2 | χ2 = 0.3 (1)‡ | 0.574 |
| Several times weekly | 31.1 | 33.3 | χ2 = 0.8 (1)‡ | 0.778 |
| Daily | 16.7 | 28.4 | χ2 = 3.6 (1)‡ | 0.057 |
| Caffeine consumption (coffee/tea) (%)* | 85.4 | 91.4 | χ2 = 1.5 (1)‡ | 0.219 |
| Number of daily cups | 2.3 (1.7) | 3.1 (1.8) | t = 3.0 (182)† | 0.003 |
| SF-36 (quality of life assessment) | ||||
| Physical functioning | 58.4 (29.5) | 86.7 (14.5) | t = -7.9 (182)† | < 0.001 |
| Role limitations due to physical health | 36.9 (41.9) | 82.9 (32.1) | t = -8.1 (180)† | < 0.001 |
| Bodily pain | 62.6 (26.5) | 79.7 (21.5) | t = -4.7 (182)† | < 0.001 |
| General health | 48.5 (21.6) | 72.5 (17.7) | t = -8.1 (182)† | < 0.001 |
| Vitality | 47.3 (21.1) | 68.5 (20.8) | t = -6.8 (181)† | < 0.001 |
| Social functioning | 64.9 (25.9) | 89.1 (17.3) | t = -7.2 (181)† | < 0.001 |
| Role limitations due to mental health | 65.3 (41.7) | 87.7 (28.3) | t = -4.1 (179)† | < 0.001 |
| Mental health | 68.7 (19.6) | 79.7 (14.7) | t = -4.2 (181)† | < 0.001 |
| Health change over last year | 38.8 (21.7) | 50.6 (16.3) | t = -4.0 (182)† | < 0.001 |
| Physical component summary | 51.6 (22.7) | 79.9 (17.7) | t = -9.3 (182)† | < 0.001 |
| Mental component summary | 60.9 (22.2) | 80.8 (17.4) | t = -6.6 (182)† | < 0.001 |
Data are presented as mean [standard deviation, range] or mean (standard deviation) unless otherwise indicated.
these data are partially reproduced from the previous study, [16]
independent sample t-test,
Pearson’s chi-squared test.
df: degrees of freedom, SF-36: 36-item Short-Form Health Survey.
Within the PD cohort, the mean age of symptom onset was 58.8 years (SD 13.6), and the mean duration of disease was 9.2 years (SD 6.5). A total of 10.7% were diagnosed as young onset (< 40 years), and 49.5% of patients reported late onset (> 60 years) disease (Table 2). Approximately one-third of all PD patients had either a tremor-dominant or akinetic rigid phenotype, and more than half identified troublesome motor fluctuations, particularly dyskinesias. Of the NMS, three quarters reported hyposmia, and half reported RBD [24]. Overall, 20% of PD patients displayed an ICD [25], and 16.5% of patients reported utilizing rehabilitation in the preceding 12 months, as previously reported [16]. Approximately 5% of the PD cohort was treatment-naïve, and more than 80% of patients reported medication ‘wearing off’ prior to their next dose. The utilization of standard and deviceassisted therapies, as well as the frequency and severity of chronic pain, depression, physical activity, gastrointestinal symptoms and other NMS in the PD cohort, are further outlined in Table 2.
Table 2.
Parkinson’s disease clinical characteristics
| Variables | Values |
|---|---|
| Age at diagnosis (years)* | 58.8 [13.6, 24–88] |
| Parkinson’s disease duration (years)* | 9.2 [6.5, 1–30] |
| Parkinson’s disease phenotype (%)* | |
| Tremor-dominant | 30.1 |
| Postural instability and gait impairment | 20.4 |
| Akinetic rigid | 38.9 |
| Young onset (< 40 years) | 10.7 |
| Late onset (> 60 years) | 49.5 |
| Disease complications (%)* | |
| Motor fluctuations | 58.3 |
| Dyskinesia | 58.3 |
| Wearing off | 81.6 |
| Impulse control disorder | 19.4 |
| REM sleep behavior disorder | 48.5 |
| PDQ-39 | |
| Mobility | 35.7 (28.7) |
| Activities of daily living | 32.2 (24.1) |
| Emotional well being | 26.9 (22.4) |
| Stigma | 21.6 (22.9) |
| Social support | 14.3 (19.3) |
| Cognitions | 33.0 (22.6) |
| Communication | 30.7 (26.1) |
| Bodily discomfort | 39.7 (26.8) |
| PDQ-39 summary index | 29.2 (17.3) |
| Parkinson’s disease therapy (%)* | |
| Treatment-naïve | 4.9 |
| Oral levodopa | 89.3 |
| Dopamine agonist | 35.0 |
| Monoamine oxidase B inhibitor | 18.4 |
| Anticholinergic | 12.6 |
| Catechol-O-methyl transferase inhibitor | 23.3 |
| Amantadine | 12.6 |
| Levodopa/carbidopa intestinal gel | 8.7 |
| Deep brain stimulation | 10.7 |
| Apomorphine (subcutaneous infusion) | 6.8 |
| Levodopa equivalent daily dose (mg)* | 834.8 [527.3, 0–2186] |
| MDS UPDRS-III (‘on’ state)* | 32.9 [17.7, 5–91] |
| Rehabilitation use in the last 12 months (%) | 16.5 |
| Gastrointestinal symptoms* | |
| Cleveland Constipation Score | 7.2 (4.7) |
| Rome-IV Criteria Constipation Score | 4.4 (3.5) |
| Functional constipation as per Rome-IV Criteria (%) | 78.6 |
| LDQ score* | 8.3 (7.7) |
| Most troublesome symptom (%) | |
| Indigestion | 18.4 |
| Heartburn | 7.8 |
| Regurgitation | 6.8 |
| Belching | 7.8 |
| Nausea | 15.6 |
| Vomiting | 1 |
| Excess fullness/bloating | 20.4 |
| None | 22.3 |
| Chronic pain over the last 3 months (%)* | 72.8 |
| Pain score (visual analogue scale) | 4.9 (2.5) |
| IPAQ score (MET-minutes/week)* | 1,823.6 (1,693.6) |
| IPAQ categorical score (%) | |
| Low | 35.2 |
| Moderate | 37.9 |
| High | 26.2 |
| Depression characteristics | |
| BDI total score | 11.9 (8.8) |
| BDI categories (%) | |
| Minimal depression (0–13) | 64.1 |
| Mild depression (14–19) | 19.4 |
| Moderate depression (20–28) | 10.7 |
| Severe depression (39–63) | 5.8 |
| Clinically depressed (> 13 for Parkinson’s disease) | 38.9 |
| MDS total NMSS | 62.7 (42.9) |
| NMSS-cardiovascular | 3.2 (3.5) |
| NMSS-sleep and fatigue | 11.6 (8.8) |
| NMSS-mood and cognition | 10.0 (11.9) |
| NMSS-perceptual problems | 2.7 (3.8) |
| NMSS-attention and memory | 6.2 (7.1) |
| NMSS-gastrointestinal symptoms | 6.1 (6.3) |
| NMSS-urinary | 8.8 (8.7) |
| NMSS-sexual | 5.4 (6.7) |
| NMSS-miscellaneous | 8.9 (7.5) |
| NMSS-total score | 62.7 (42.9) |
Data are presented as mean [standard deviation, range] or mean (standard deviation) unless otherwise indicated.
these data are partially reproduced from the previous study. [16]
PDQ-39: Parkinson’s Disease Questionnaire, MDS UPDRS-IIII: Movement Disorder Society–Unified Parkinson’s Disease Rating Scale–Part III, LDQ: Leeds Dyspepsia Questionnaire, IPAQ: International Physical Activity Questionnaire, MET: metabolic equivalent of task, BDI: Beck Depression Inventory, NMSS: Non-Motor Symptoms Scale.
Quality of life differences between Parkinson’s disease patients and their caregivers
Patients with PD reported significantly decreased HRQoL across all eight health concepts assessed by the SF-36 (all p < 0.001) (Table 1). The mean PCS score in the PD cohort was 51.6 (SD 22.7) compared to 79.9 (SD 17.7; p < 0.001) for the caregiver group, while the mean MCS score was 60.9 (SD 22.2) vs. 80.8 (SD 17.4; p < 0.001), respectively (Table 1). Not surprisingly, PD patients also reported a significantly greater deterioration of their health over the year prior compared to their caregivers, 38.8 (SD 21.7) vs. 50.6 (SD 16.3, p < 0.001) (Table 1), which was inferred from a cross-sectionally acquired single SF-36 question about health perception over the preceding year. The self-perceived mean 12% reduction in health change within the PD cohort correlated most significantly with indicators of disease severity, including higher daily LED (r = -0.205, p = 0.038) and higher MDS-UPDRS-III score (r = -0.248, p = 0.012), as well as a lower IPAQ score (r = 0.310, p = 0.001), higher BDI (r = -0.390, p < 0.001), higher NMSS TS (r = -0.416, p < 0.001) and a higher LDQ score (r = -0.293, p = 0.003).
Logistic regression models evaluated the significance of HRQoL severity differences between the PD and caregiver groups. Statistical significance for both the PCS and MCS scores between the groups persisted after controlling for age, sex, support services, chronic pain, depression (BDI score), physical activity, constipation (Rome-IV criteria) and upper gastrointestinal dysfunction (Wald χ2 = 11.8, df = 7, p = 0.001 and Wald χ2 = 4.4, df = 7, p = 0.036, respectively).
Factors influencing quality of life in Parkinson’s disease patients
The PDQ-39 Summary Index (PDQ-39 SI) (composite score of the eight health domains) demonstrated overall moderate HRQoL impairment in the PD cohort (29.2, SD 17.3). Bodily discomfort had the highest impairment associations (39.7, SD 26.8), while the lowest impairment was associated with higher social support (14.3, SD 19.3) (Table 2). Analysis of NMS by the MDS NMSS Total Score (NMSS TS) showed a mean score of 62.7 (SD 42.9), with the individual nine domain scores provided in Table 2. PD patients who were employed rather than unemployed or retired reported a better HRQoL (β = 0.203, r2 = 0.084, p = 0.039), controlling for age, sex and PD duration. Not surprisingly, the 32% of individuals who required support services (of any type) reported a significantly worse HRQoL (higher PDQ-39 SI) compared to those who did not access services after controlling for age, sex and PD duration (β = 0.181, r2 = 0.294, p = 0.042). A moderate correlation between the increasing use of support services and the PDQ-39 SI was also shown (r = 0.344, p < 0.001). Subjects who required any form of rehabilitation in the preceding 12-months reported a poorer HRQoL than those who did not after controlling for age, sex and PD duration (β = -0.220, r2 = 0.089, p = 0.029). However, individuals with a longer disease duration reported a lower HRQoL (higher PDQ-39 SI) after controlling for age and sex (β = 0.500, r2 = 0.266, p < 0.001). Interestingly, individuals reporting RBD as well as an ICD had a worse HRQoL (higher PDQ-39 SI) after controlling for age, sex and PD duration (β = -0.305, r2 = 0.356, p < 0.001 and β = -0.194, r2 = 0.303, p = 0.027, respectively).
Treatment influences
Increased disease severity, reflected by a higher MDS-UPDRSIII score, was associated with a worse HRQoL (higher PDQ-39 SI) after controlling for age, sex and PD duration (β = 0.429, r2 = 0.423, p < 0.001), with a strong corresponding correlation (r = 0.509, p < 0.001) (Table 2). The MDS-UPDRS-III score also correlated with the NMSS TS (r = 0.414, p < 0.001). Motor control appeared to be an important predictor for HRQoL in PD, with individuals who reported dyskinesias, ‘on/off’ fluctuations and wearing off, having a lower HRQoL (higher PDQ-39 SI) after controlling for age, sex and disease duration (β = -0.267, r2 = 0.318, p = 0.008; β = -0.306, r2 = 0.126, p = 0.003; β = -0.250, r2 = 0.103, p = 0.012, respectively). Interestingly, dyskinesias were also associated with worse NMS (higher NMSS TS) after controlling for age, sex and PD duration (β = -0.428, r2 = 0.179, p < 0.001).
Individuals using catechol-O-methyl transferase (COMT) inhibitors reported a worse HRQoL (higher PDQ-39 SI) after controlling for age, sex and PD duration (β = -0.202, r2 = 0.303, p = 0.028). COMT inhibitors and apomorphine infusions were also associated with higher NMSS TS after controlling for age, sex and PD duration (β = -0.250, r2 = 0.101, p = 0.017; β = -0.392, r2 = 0.195, p < 0.001, respectively). No associations between HRQoL and other standard and advanced therapies were observed. However, correlations were noted between individuals who required a higher LED, having worse HRQoL (higher PDQ-39 SI), higher NMSS TS and higher MDS-UPDRS-III scores (r = 0.329, p < 0.001; r = 0.295, p = 0.003; r = 0.344, p < 0.001, respectively), indicating that increased medication requirement was associated with worse HRQoL.
Lifestyle influences
Physical activity appeared to favorably predict HRQoL perceptions, with individuals who exercised more reporting a better HRQoL (lower PDQ-39 SI) and lower NMSS TS after controlling for age, sex and PD duration (β = -0.236, r2 = 0.313, p = 0.012; β = -0.287, r2 = 0.116, p = 0.007, respectively). Moderate correlations between the IPAQ score and PDQ-39 SI or NMSS TS were also identified (r = -0.390, p < 0.001; r = -0.260, p = 0.006, respectively). Individuals who reported chronic pain (> 3 months) were more likely to report a lower HRQoL (higher PDQ-39 SI) and higher NMSS TS after controlling for age, sex and PD duration (β = -0.310, r2 = 0.358, p < 0.001; β = -0.339, r2 = 0.156, p = 0.001, respectively). Pain severity scores, assessed by the Visual Analogue Scale, were also positively correlated with the PDQ-39 SI and NMSS TS (r = 0.408, p < 0.001; r = 0.346, p = 0.002, respectively). No associations with HRQoL were identified between smoking status or caffeine consumption.
Gastrointestinal influences
Individuals diagnosed with constipation according to the RomeIV criteria (score ≥ 2) or increased upper gastrointestinal dysfunction, namely indigestion and excess fullness and bloating, were noted to have a lower HRQoL (higher PDQ-39 SI) and higher NMSS TS after controlling for age, sex and PD duration (Rome IV: - β = -0.279, r2 = 0.337, p = 0.002; β = -0.300, r2 = 0.128, p = 0.003, respectively; LDQ: - β = 0.441, r2 = 0.438, p < 0.001; β = 0.443, r2 = 0.219, p < 0.001, respectively). Strong positive correlations between the PDQ-39 SI and the Rome-IV score, CCS score and LDQ score were noted (r = 0.408, p < 0.001; r = 0.487, p < 0.001; r = 0.524, p < 0.001, respectively) (Figure 1), as were relationships between the NMSS TS and the Rome-IV score, CCS score and LDQ score (r = 0.391, p < 0.001; r = 0.343, p < 0.001; r = 0.370, p < 0.001, respectively).
Figure 1.
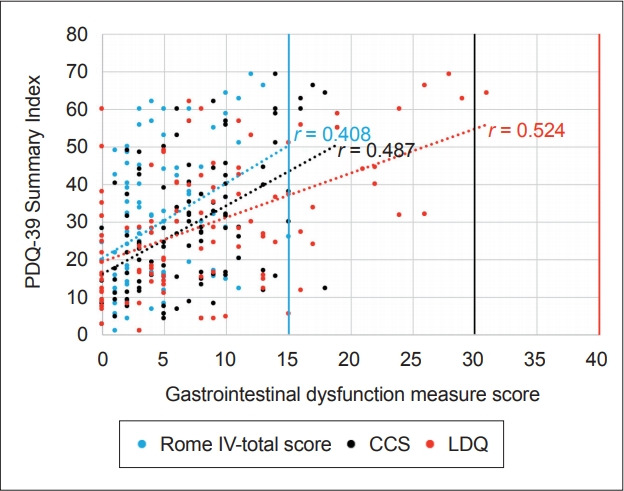
Associations between health-related quality of life and gastrointestinal dysfunction in Parkinson’s disease. Strong positive correlations between the PDQ-39 Summary Index and the RomeIV score, r = 0.408, p < 0.001; CCS score, r = 0.487, p < 0.001, and LDQ score r = 0.524, p < 0.001, were identified, suggesting that individuals with worse constipation severity (reflected by higher RomeIV scores and CCS scores) or upper gastrointestinal dysfunction (reflected by a higher LDQ score) had a higher overall PDQ-39 Summary Index, indicating a lower health-related quality of life. PDQ-39: Parkinson’s Disease Questionnaire, CCS: Cleveland Constipation Scale, LDQ: Leeds Dyspepsia Questionnaire.
Mood influences
PD patients diagnosed with depression reported a lower HRQoL (higher PDQ-39 SI) and higher NMS burden (higher NMSS TS) after controlling for age, sex and PD duration (β = 0.568, r2 = 0.585, p < 0.001; β = 0.527, r2 = 0.320, p < 0.001, respectively). Strong correlations between the BDI score, PDQ-39 SI and NMSS TS were identified (r = 0.693, p < 0.001; r = 0.632, p < 0.001, respectively) (Figure 2).
Figure 2.
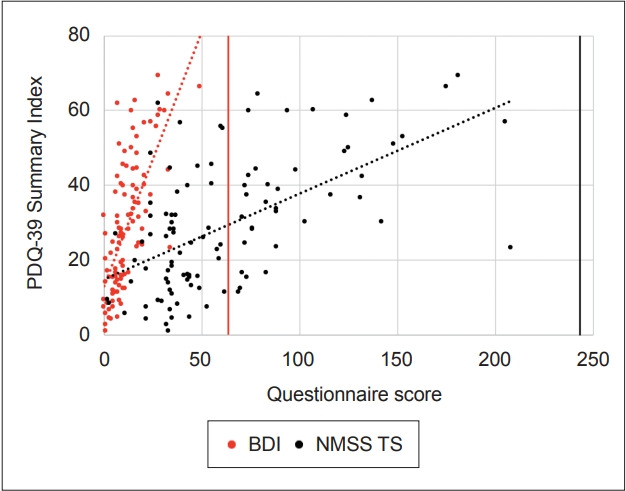
Associations between health-related quality of life (measured by the PDQ-39) and depression severity or non-motor symptoms in Parkinson’s disease. Strong positive correlations between the BDI score and the PDQ-39 Summary Index, r = 0.693, p < 0.001; and the NMSS TS and the PDQ-39 Summary Index, r = 0.632, p < 0.001, were identified, suggesting that individuals with worse depression severity, reflected by higher BDI scores as well as worse non-motor symptoms, suggested by a higher NMSS TS, had an overall higher PDQ-39 Summary Index, indicating a lower healthrelated quality of life. PDQ-39: Parkinson’s Disease Questionnaire, BDI: Beck Depression Inventory, NMSS TS: Non-Motor Symptoms Scale-Total Socre.
DISCUSSION
We identified numerous clinically significant HRQoL differences across a number of demographic and clinical characteristics in patients with PD (Figure 3). Additionally, this study is one of the first to assess HRQoL in an Australian cohort of people caring for family members with PD, with notable differences between PD patients and their caregivers. PD patients reported a significantly lower HRQoL across all health concepts, as well as the aggregate PCS and MCS scores assessed by the SF-36, in line with other international studies [30]. Surprisingly, an earlier Australian study showed no differences in perceived HRQoL between PD patients and their caregivers; however, the earlier study utilized a different assessment scale, which may have incurred methodological limitations, such as a ceiling effect [4]. Interestingly, the differences between the PD and caregiver groups in our study more notably identified increased physical limitations (> 8%) compared to differences in mental impairment, derived from the PCS and MCS aggregate scores, suggesting that individuals with PD are more significantly burdened by physical limitations, prompting appropriately increased provision of health services, particularly physical therapy. Furthermore, we showed that PD patients had a considerably accelerated decline (12%) in their perceived health status in the preceding year compared to their caregivers. This decline was most associated with markers of advancing PD severity, lower physical activity, higher depression severity and increased NMS, in particular upper gastrointestinal dysfunction. Again, this highlights the importance of timely provision of comprehensive medical and rehabilitative therapies to target the above associated factors that infer the greatest detrimental influence for QoL decline in PD.
Figure 3.
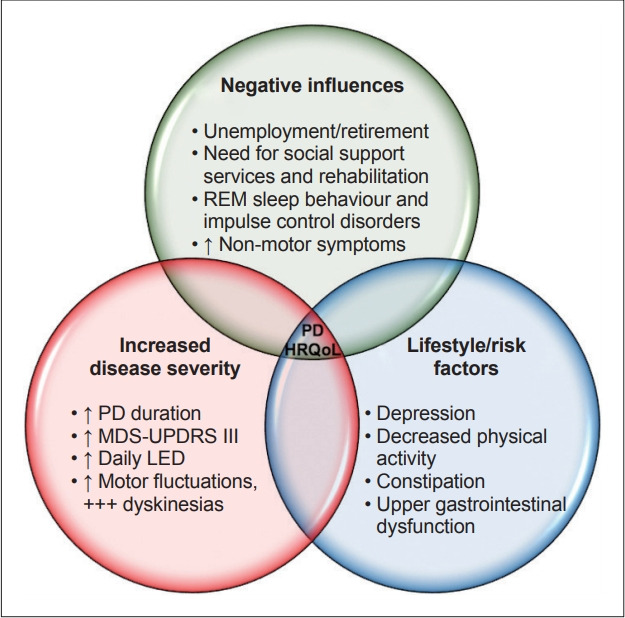
Summary of the influences leading to impaired HRQoL in PD. HRQoL: health-related quality of life, PD: Parkinson’s disease, MDS UPDRS-IIII: Movement Disorder Society–Unified Parkinson’s Disease Rating Scale–Part III, LED: levodopa equivalent dose.
The mean PDQ-39 SI of 29.2 for this PD cohort signified moderate HRQoL impairment severity and was comparable to other Australian and international studies [6,13,14,31,32]. The mean NMSS TS of 62.7 was slightly higher than that reported by the international NMSS validation study, which had a mean score of 56.5.21 This may be due to a longer disease duration of 2.8 years for the cohort studied here. Surprisingly, there is a lack of Australian studies reporting on NMSS in PD, particularly given that an earlier study showed a strong correlation (r2 = 0.7) between the NMSS and the PDQ-39 SI, supporting its validity for assessing HRQoL in PD [32]. Susceptibility to a lower HRQoL was identified in patients who were unemployed or retired, receiving any social support services or utilizing rehabilitation services, signifying a need to tailor improved social support structures for PD patients. We also showed that PD patients with RBD and ICD perceived a significantly lower HRQoL, as has been suggested previously [33]. These patients may be more vulnerable to worsening HRQoL due to potential confounded sleep and psychiatric disturbances that are observed in RBD and ICDs, although further studies examining such associations are required. Not surprisingly, clinical features suggestive of increasing disease severity were indicative of a lower HRQoL; advancing PD duration, increased MDS-UPDRS-III scores, increased daily LED, and motor complications hallmarked by the presence of ‘on/off’ fluctuations, ‘wearing off’ and particularly dyskinesias, all predicted a worse HRQoL in PD [34]. In our cohort, patients using COMT inhibitors and apomorphine infusions also reported a lower HRQoL, likely due to preferential use of these adjuvant therapies in advanced disease.
A novelty of this study is the evaluation of features beyond the PD-specific factors examined in earlier studies [4,6,13-15], with a number of important associations between physical activity, chronic pain, pain severity, gastrointestinal dysfunction and mood identified. We showed that PD patients reporting lower physical activity were more likely to perceive a lower HRQoL, with correlations to NMS severity. This finding reveals important management implications, as a low outcome expectation from exercise, a lack of time to exercise and a fear of falling are important perceived barriers to PD patients engaging in exercise [35], despite the positive impact exercise has on the global NMS burden [36]. Physical activity has also been suggested to moderate the deterioration of motor skills and depression and increase the HRQoL of PD patients, with aerobic training being most beneficial [37]. Another important modifiable risk factor for improving HRQoL in PD is chronic pain. We found a correlation between chronic pain, the severity of NMS and HRQoL, which has not been reported for Australian PD patients. However, the associations between chronic pain in PD, as well as increasing pain severity and a lower HRQoL, have been reported in other cohorts [38], with an estimated 40–80% of PD patients living with chronic pain [39]. Furthermore, the comparatively lower willingness for PD patients to utilize telemedicine for their health compared to their caregivers is rather surprising and identifies an important gap to address in the provision of quality healthcare via an online medium. The possibilities for not engaging with telemedicine include PD patients being resistant to engage with clinicians via technology, a presumed decrease in the quality of care obtained, lack of trust or confidentiality reasons, caregivers being overly enthusiastic to use telemedicine, reduced travel requirements and reduction of their own social isolation, or caregiver-related stress due to caring for their spouse. Exploring these mechanisms further is vital to developing an adaptive and integrative future telemedicine model of care for PD patients. Despite this, other literature has reported that approximately 60% of PD individuals desire to utilize telemedicine [14].
Between 60–80% of PD patients report constipation, which is known to impact a patient’s HRQoL considerably [40], although this has not previously been evaluated in an Australian cohort. We found that upper and lower gastrointestinal dysfunction was associated with a lower HRQoL and increased NMS in our PD cohort [16]. In addition to constipation, indigestion, excess fullness and bloating were also related to a lower HRQoL and higher NMS burden. These findings represent important clinical implications in the management of gastrointestinal dysfunction in PD [16].
Lastly, the negative impacts of depression on HRQoL have been extensively reported in other PD populations [41]. In this study, unsurprisingly, we confirmed that individuals with increased NMS and a lower HRQoL were more likely to be depressed. Correlations between depression and its severity with a higher NMS burden and lower HRQoL highlight the importance of considering the modification of a patient’s mood as a practical means of improving their HRQoL. However, it is uncertain whether impairment of HRQoL results in vulnerability to depression or whether depression affects the perception of HRQoL. We would suggest that both are likely contributory, given that depressed patients are more likely to report poor disability status as a reflection of their poor perception of disability [42]. Perhaps co-treatment of mood for more immediate results and other features for longer-term sustainability are needed.
While our data do not fully address some potential confounding factors, including other comorbidities and medication effects, several important known and novel HRQoL-related differences in PD patients and their caregivers were identified. The findings of this study should be interpreted in light of its limitations, which include selection bias from specialist PD clinic recruitment (i.e., over-representation of PD patients with lower HRQoL presenting for treatment) [16] and bias from the self-reporting data collected. Another consideration is that the caregivers in this study were related to patients with PD. Due to potential caregiver-related fatigue or distress, if compared to a healthy control group, the perceptible decrease in caregiver HRQoL is likely overrepresented and/or indirectly influenced. However, this was a considered limitation, given our intention to examine HRQoL changes in PD patient caregivers. Understanding what PD-related modifiable symptoms correlate with lower HRQoL for caregivers may help to target these factors and improve the lives of not only the patients, but their caregivers as well. Furthermore, our sample population was drawn from a single metropolitan area. While it has been described that PD patients from urban centers report a lower HRQoL, individuals from regional areas were more satisfied with their treatment, despite reporting a poorer understanding of their illness [14]. Significant gender-related differences have also been reported in PD, with men experiencing lower HRQoL, specifically with activities of daily living, cognition and communication compared to women [13].
In our cohort of PD patients and their caregivers, we demonstrate that individuals with PD have a significantly lower HRQoL than their caregivers, with the largest differences noted in the physical rather than mental limitations. We have also identified potential factors to target in future studies to improve the lives of patients with PD, as well as their caregivers. Unemployment or retirement, need for social support services, rehabilitation use, RBD and ICDs were all associated with a lower HRQoL in our PD cohort. Increasing disease severity, hallmarked by increasing PD duration, higher MDS-UPDRS-III, higher daily LED, motor fluctuations and in particular dyskinesias, were also reflective of a lower HRQoL. In addition, our findings suggest that PD patients with decreased physical activity, chronic pain, depression, constipation and upper gastrointestinal dysfunction are more vulnerable to a lower HRQoL. We recommend routinely screening for and optimizing the HRQoL of PD patients by treating depression and chronic pain, promoting physical activity, as well as recognizing and treating constipation and upper gastrointestinal dysfunction as part of routine clinical practice.
Acknowledgments
We thank Parkinson’s New South Wales for a Research Seed Grant to perform microbiome studies. We would also like to thank all our participants and Dr. Carly Oboudiyat for critically reviewing the manuscript. Not industry sponsored. All authors report no relevant disclosures.
Footnotes
Conflicts of Interest
Not industry sponsored. All authors report no relevant disclosures. ML is the recipient of a RACP Research Entry Scholarship. RLD was a New South Wales Health Early-Mid Career Research Fellow. CMS is the recipient of a NHMRC Practitioner Fellowship (APP1136800).
Ethical Standard
Ethics approval was obtained from the Northern Sydney Local Health District (HREC/18/HAWKE/109) and Ramsay Health for North Shore Private Hospital (NSPHEC 2018-LNR-009) Human Research Ethics Committees. All participants provided written informed consent to participate in the study.
Author Contributions
Conceptualization: all authors. Data curation: all authors. Formal analysis: Michal Lubomski, Ryan L. Davis. Funding acquisition: all authors. Investigation: all authors. Methodology: all authors. Project administration: all authors. Resources: all authors. Software: Michal Lubomski, Ryan L. Davis. Supervision: all authors. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
REFERENCES
- 1.Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry. 2015;86:324–330. doi: 10.1136/jnnp-2014-307822. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson’s disease and movement disorders. Mov Disord. 2017;32:382–392. doi: 10.1002/mds.26885. [DOI] [PubMed] [Google Scholar]
- 3.Opara JA, Brola W, Leonardi M, Błaszczyk B. Quality of life in Parkinson’s disease. J Med Life. 2012;5:375–381. [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly DH, McGinley JL, Huxham FE, Menz HB, Watts JJ, Iansek R, et al. Health-related quality of life and strain in caregivers of Australians with Parkinson’s disease: an observational study. BMC Neurol. 2012;12:57. doi: 10.1186/1471-2377-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 6.Soh SE, McGinley JL, Watts JJ, Iansek R, Morris ME. Health-related quality of life of australians with Parkinson disease: a comparison with international studies. Physiother Can. 2012;64:338–346. doi: 10.3138/ptc.2011-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69:342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubomski M, Tan AH, Lim SY, Holmes AJ, Davis RL, Sue CM. Parkinson’s disease and the gastrointestinal microbiome. J Neurol. 2020;267:2507–2523. doi: 10.1007/s00415-019-09320-1. [DOI] [PubMed] [Google Scholar]
- 9.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 10.Lubomski M, Davis RL, Sue CM. The gut microbiota: a novel therapeutic target in Parkinson’s disease? Parkinsonism Relat Disord. 2019;66:265–266. doi: 10.1016/j.parkreldis.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26:353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 13.Lubomski M, Louise Rushworth R, Lee W, Bertram KL, Williams DR. Sex differences in Parkinson’s disease. J Clin Neurosci. 2014;21:1503–1506. doi: 10.1016/j.jocn.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Lubomski M, Rushworth RL, Lee W, Bertram K, Williams DR. A cross-sectional study of clinical management, and provision of health services and their utilisation, by patients with Parkinson’s disease in urban and regional Victoria. J Clin Neurosci. 2013;20:102–106. doi: 10.1016/j.jocn.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Bucks RS, Cruise KE, Skinner TC, Loftus AM, Barker RA, Thomas MG. Coping processes and health-related quality of life in Parkinson’s disease. Int J Geriatr Psychiatry. 2011;26:247–255. doi: 10.1002/gps.2520. [DOI] [PubMed] [Google Scholar]
- 16.Lubomski M, Davis RL, Sue CM. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol. 2020;267:1377–1388. doi: 10.1007/s00415-020-09723-5. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Visser M, Leentjens AF, Marinus J, Stiggelbout AM, van Hilten JJ. Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Mov Disord. 2006;21:668–672. doi: 10.1002/mds.20792. [DOI] [PubMed] [Google Scholar]
- 20.Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22:1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 22.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 24.Sateia MJ. International Classification of Sleep Disorders-third edition: highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 26.Moayyedi P, Duffett S, Braunholtz D, Mason S, Richards ID, Dowell AC, et al. The Leeds Dyspepsia Questionnaire: a valid tool for measuring the presence and severity of dyspepsia. Aliment Pharmacol Ther. 1998;12:1257–1262. doi: 10.1046/j.1365-2036.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- 27.Sood R, Ford AC. Diagnosis: Rome IV criteria for FGIDs - an improvement or more of the same? Nat Rev Gastroenterol Hepatol. 2016;13:501–502. doi: 10.1038/nrgastro.2016.110. [DOI] [PubMed] [Google Scholar]
- 28.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- 29.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 30.Winter Y, von Campenhausen S, Arend M, Longo K, Boetzel K, Eggert K, et al. Health-related quality of life and its determinants in Parkinson’s disease: results of an Italian cohort study. Parkinsonism Relat Disord. 2011;17:265–269. doi: 10.1016/j.parkreldis.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Soh SE, Morris ME, Watts JJ, McGinley JL, Iansek R. Health-related quality of life in people with Parkinson’s disease receiving comprehensive care. Aust Health Rev. 2016;40:613–618. doi: 10.1071/AH15113. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, NMSS Validation Group The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26:399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 33.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23:1665–1672. doi: 10.1002/mds.22099. [DOI] [PubMed] [Google Scholar]
- 34.Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123:2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- 35.Ellis T, Boudreau JK, DeAngelis TR, Brown LE, Cavanaugh JT, Earhart GM, et al. Barriers to exercise in people with Parkinson disease. Phys Ther. 2013;93:628–636. doi: 10.2522/ptj.20120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cusso ME, Donald KJ, Khoo TK. The impact of physical activity on nonmotor symptoms in Parkinson’s disease: a systematic review. Front Med (Lausanne) 2016;3:35. doi: 10.3389/fmed.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson’s disease: a systematic review. PLoS One. 2017;12:e0181515. doi: 10.1371/journal.pone.0181515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SM, Kim BC, Jung HJ, Yoon GJ, Kang KW, Choi KH, et al. Impact of pain and pain subtypes on the quality of life of patients with Parkinson’s disease. J Clin Neurosci. 2017;45:105–109. doi: 10.1016/j.jocn.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Mylius V, Ciampi de Andrade D, Cury RG, Teepker M, Ehrt U, Eggert KM, et al. Pain in Parkinson’s disease: current concepts and a new diagnostic algorithm. Mov Disord Clin Pract. 2015;2:357–364. doi: 10.1002/mdc3.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poirier AA, Aubé B, Côté M, Morin N, Di Paolo T, Soulet D. Gastrointestinal dysfunctions in Parkinson’s disease: symptoms and treatments. Parkinsons Dis. 2016;2016:6762528. doi: 10.1155/2016/6762528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pachana NA, Egan SJ, Laidlaw K, Dissanayaka N, Byrne GJ, Brockman S, et al. Clinical issues in the treatment of anxiety and depression in older adults with Parkinson’s disease. Mov Disord. 2013;28:1930–1934. doi: 10.1002/mds.25689. [DOI] [PubMed] [Google Scholar]
- 42.Schrag A, Jahanshahi M, Quinn NP. What contributes to depression in Parkinson’s disease? Psychol Med. 2001;31:65–73. doi: 10.1017/s0033291799003141. [DOI] [PubMed] [Google Scholar]


