Abstract
Background and Purpose
Nonconvulsive status epilepticus (NCSE) is challenging to diagnose. This study aimed to describe and classify the clinical features and electroencephalography (EEG) findings of patients with de novo NCSE and to correlate them with clinical outcomes.
Methods
We retrospectively reviewed the medical and EEG records of patients admitted to our institution with altered mentation and EEG abnormalities from January 1, 2013 to December 31, 2018. We evaluated premorbid modified Rankin Scale (mRS) scores, underlying disorders, precipitating factors, clinical manifestations, laboratory tests, and outcomes after a 3-month follow-up. Patients who met the Salzburg Consensus Criteria for NCSE were categorized into good-outcome and poor-outcome groups. A good outcome was defined as 1) clinical and electrographic seizures ceasing after treatment, and 2) an mRS score of ≤2 or remaining unchanged during the 3-month follow-up. A poor outcome was defined as 1) death, 2) seizures continuing despite treatment, or 3) a follow-up mRS score of ≥3 in a patient with a premorbid mRS score of ≤2, or a follow-up mRS score that increased in a patient with a premorbid mRS score of ≥3.
Results
The 48 included patients comprised 37 categorized into the good-outcome group and 11 into the poor-outcome group. The presence of acute metabolic disturbances was significantly correlated with poor outcome (p=0.036), while the other analyzed variables were not significantly correlated with outcomes.
Conclusions
Acute metabolic disturbances in NCSE are associated with poor outcomes. Adequate treatment of underlying reversible disorders alongside controlling seizures is critical for patients with NCSE.
Keywords: nonconvulsive status epilepticus, de novo, modified Rankin Scale, metabolic
INTRODUCTION
Status epilepticus is a major medical and neurological emergency, and diagnosing nonconvulsive status epilepticus (NCSE) is often challenging.1,2 While its exact prevalence and annual incidence are unknown, NCSE is not a rare disorder and its prevalence may also be higher than reported since it is underdiagnosed and often mistaken for other disorders such as metabolic encephalopathy or psychiatric diseases, which can delay treatment.1,2,3,4 Electroencephalography (EEG) is mandatory for the diagnosis.5
NCSE typically presents in patients with pre-existing epilepsy, but de novo NCSE is often observed in the elderly and critically ill patients.6 Previous studies have found that the outcomes of patients with de novo status epilepticus are worse than those of patients with status epilepticus attributed to previous epilepsy.7,8 The poor prognosis may be attributed to potentially fatal cerebral disorders such as hypoxic-ischemic encephalopathy or severe stroke.8
The previous studies included patients with convulsive status epilepticus and NCSE, and the clinical characteristics and outcomes of patients with de novo NCSE have not previously been examined in detail. Therefore, this study aimed to describe and classify the clinical features and EEG findings of such patients and to correlate these with their clinical outcomes.
METHODS
Inclusion of patients
We retrospectively reviewed the medical records and EEG data of patients admitted with altered mentation and EEG abnormalities to Nowon Eulji Medical Center from January 1, 2013 to December 31, 2018. Premorbid states, underlying disorders, triggering factors, clinical manifestations, laboratory tests, and outcomes at discharge and after a 3-month follow-up were reviewed thoroughly, as were brain images obtained using computed tomography (CT) and magnetic resonance imaging (MRI).
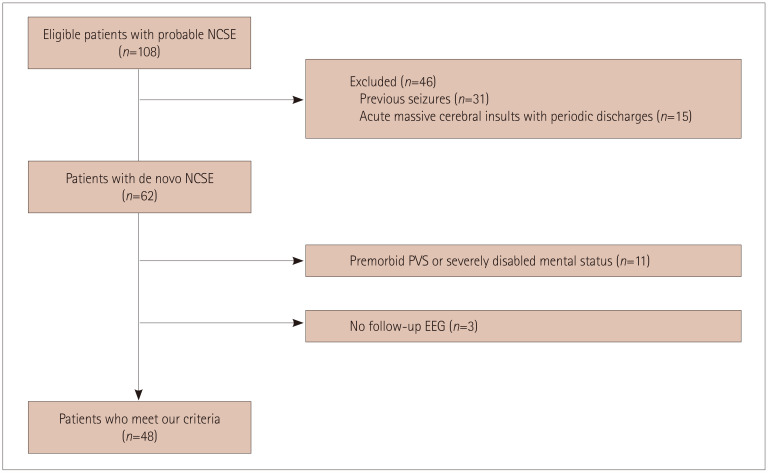
EEG findings were reviewed by two experienced epileptologists. Patients who met the Salzburg Consensus Criteria for NCSE were included.9 The following exclusion criteria were applied: history of seizures, in a coma due to acute massive cerebral insults (e.g., hypoxic-ischemic encephalopathy or acute severe stroke) with periodic epileptiform discharges (since it remains controversial whether these underlie NCSE),10 premorbid persistent vegetative state, or other type of severe mentation disability that would make the final outcomes challenging to assess. Patients who did not undergo follow-up EEG or who were not followed up within 3 months after their diagnosis were also excluded (Fig. 1). Every patient underwent continuous video-EEG monitoring or at least daily EEG monitoring until reasonable control of seizure activity was achieved.
Fig. 1. Flowchart of the enrollment of study participants. Of the 108 patients reviewed for enrolment, 46 were excluded because of a previous history of seizures (n=31) or acute massive cerebral insults with periodic discharges (n=15). The remaining 62 patients with de novo NCSE included 11 with a premorbid PVS or severe mentation disability and 3 who were lost to follow-up, and so 48 patients were finally enrolled. EEG: electroencephalography, NCSE: nonconvulsive status epilepticus, PVS: persistent vegetative state.
The modified Rankin Scale (mRS) is routinely evaluated in all patients admitted to our hospital. Premorbid mRS scores were estimated based on the patient history, and scores were re-evaluated during the follow-up period.
Definition of structural lesions
Patients with cortical or juxtacortical lesions of at least moderate size, or medial temporal lesions including hippocampal sclerosis in brain CT or MRI were classified into the lesional group. Patients with no visible or only small subcortical lesions were classified into the nonlesional group.
EEG patterns
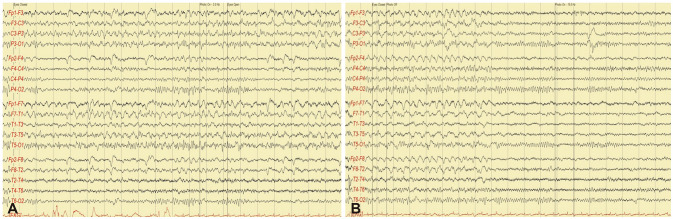
EEG patterns were defined as 1) focal discharges (FDs), comprising focal, regional rhythmic, or quasirhythmic discharges with spatiotemporal evolution (Fig. 2); or 2) rhythmic discharges without definite focal origin, comprising symmetric or asymmetric rhythmic discharges with fluctuation or evolution (Fig. 3). If definite spatiotemporal evolution was not observed, clinical and EEG improvements after administering intravenous benzodiazepine had to be detected for NCSE to be diagnosed.
Fig. 2. Example of focal discharges in EEG. A: EEG showing focal rhythmic discharges initiated from the left temporal area with evolution. B: The rhythmic activity ceased abruptly and was followed by normal background activity. EEG: electroencephalography.
Fig. 3. An example of asymmetric quasirhythmic discharges with fluctuation. electroencephalography seizure activity (A) disappeared (B) after an intravenous injection of 2 mg of lorazepam, which was associated with clinical improvement.
Acute metabolic disturbances
Patients were defined as having acute metabolic disturbances when they experienced severe systemic infection (sepsis), acute exacerbation of kidney function, hyponatremia (plasma sodium level <130 mEq/L), other electrolyte disturbances, acute elevation of liver enzymes, acute clinical aggravation of underlying chronic liver disease, hyperglycemia (>300 mg/dL), or hypoglycemia (<70 mg/dL). These abnormalities were considered as triggering factors of NCSE if they were present at admission or at the time of diagnosis. If they were observed after those periods, they were considered complications of NCSE or underlying disorders.
Patient outcomes
Patients were categorized into good-outcome and poor-outcome groups. A good outcome was defined as 1) clinical and electrographic seizures ceasing after treatment, and 2) an mRS score of ≤2 or remaining unchanged during the 3-month follow-up. A poor outcome was defined as 1) death, 2) seizures continuing despite treatment, or 3) a follow-up mRS score of ≥3 in a patient with a premorbid mRS score of ≤2, or a follow-up mRS score that increased in a patient with a premorbid mRS score of ≥3.
Statistical analyses
Outcomes were compared between groups using the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables. A p value of <0.05 was considered to indicate statistical significance.
This study was approved by the Institutional Review Board of Nowon Eulji Medical Center, Eulji University (IRB No. 2019-12-013).
RESULTS
Patient characteristics
The 48 patients included in this study comprised 18 males and 30 females with a mean age of 69.6 years (age range=41–93 years). Their general demographic data are summarized in Table 1. Underlying chronic cerebral lesions were present in 18 patients, 10 had acute cerebral lesions, 8 had chronic kidney disease, 17 had acute metabolic disturbances, and no definite cause was identified in 5 patients. The clinical symptoms at admission were diverse, ranging from mild confusion to comatose mentation. EEG was performed in all patients within 48 h of admission.
Table 1. Patient characteristics.
| Characteristic | Value |
|---|---|
| Age, years | 69.6±12.2 |
| Males:females | 18:30 |
| Underlying medical conditions | |
| Diabetes mellitus | 21 |
| Hypertension | 26 |
| Liver cirrhosis | 1 |
| Chronic kidney disease | 8 |
| Old cerebral lesions | 20 |
| Old strokes | 14 |
| Traumatic brain injuries | 4 |
| Meningioma (postoperative state) | 1 |
| Birth injury | 1 |
| Dementia | 7 |
| Chronic alcoholism | 1 |
| Triggering factors | |
| Acute metabolic disturbances | 17 |
| Sepsis | 7 |
| Acute kidney injury | 7 |
| Sepsis+acute kidney injury | 3 |
| Nonketotic hyperglycemia | 4 |
| Hypoglycemia | 1 |
| Hyponatremia | 1 |
| Drug intoxication | 3 |
| Acute cerebral lesions | |
| Acute strokes | 2 |
| Central nervous system infections | 3 |
| Autoimmune encephalitis | 5 |
| Cryptogenic | 5 |
Data are n or mean±standard-deviation values.
Antiepileptic drugs were administered to all patients and were maintained for at least 3 months in patients who survived that long. The choice of drugs was determined by the medical condition of each patient: intravenous levetiracetam (n=23), valproic acid (n=21), and phenytoin/fosphenytoin (n=10) were commonly used for the initial and add-on treatment; controlled-release forms of carbamazepine (n=16) and oxcarbazepine (n=4) were administered orally for the initial and add-on treatment; and oral pregabalin (n=5), topiramate (n=2), and zonisamide (n=1) were administered for add-on treatment. Monotherapy and polytherapy were applied in 20 and 28 patients, respectively. Five patients with poor seizure control underwent coma therapy, of whom three died and one experienced significant neurological sequelae.
Visible lesions were observed in neuroimaging in 24 patients, comprising underlying chronic lesions in 19 and acute cerebral lesions in 5. Concordance between neuroimaging and EEG findings was observed in 20 of these patients.
Detailed description of patient characteristics
Patients presented with diverse acute metabolic disturbances, including acute kidney injury with various precipitating factors (n=7), sepsis (n=7), both acute kidney injury and sepsis (n=3), nonketotic hyperglycemia (NKH; n=4), hypoglycemia (n=1), and liver cirrhosis and hyponatremia (n= 1). One patient with NKH also had acute cerebral infarction. The patients with NKH demonstrated FD in EEG and recovered without any sequelae. Two patients with underlying chronic kidney disease developed NCSE after the administration of cephalosporin for treating complicated pneumonia and sepsis. Both of these patients recovered after the resolution of pneumonia and the cessation of cephalosporin. Seven patients had poor outcomes: six had acute kidney injury, sepsis, or both, and two of them died. Metabolic disturbances were poorly controlled in four of the patients with poor outcomes, while the other patients with poor outcomes experienced poor seizure control despite their metabolic disturbances being relatively well controlled.
Some patients also experienced acute cerebral disorders, including meningitis (n=2), herpes encephalitis (n=1), acute cerebral infarction (n=2; one of them also had NKH), high titers of antithyroid antibodies (antithyroglobulin antibody or antimicrosomal antibody; n=3), and anti-leucinerich glioma inactivated-1 (LGI1) antibody (n=2). Regarding EEG, 66.7% (6/9) showed ictal EEG abnormalities that were concordant with MRI lesions. In the patient with herpes encephalitis, seizure activities in EEG were observed at the location of the cerebral MRI lesion. Two patients with autoimmune encephalopathies (one with antithyroid antibodies and the other with anti-LGI1 antibodies) had rhythmic ictal discharges that were localized to their acute focal cerebral lesions. One patient with antithyroid antibodies exhibited ictal discharges that originated from a previous cerebral lesion (an old cerebral infarct in the left middle cerebral artery territory). All patients with autoimmune encephalopathies were resistant to antiepileptic drugs. After immunological treatment including steroids and intravenous immunoglobulin, these patients became seizure-free and returned to their premorbid state. One patient with meningitis experienced persistent seizures during the follow-up and significant neurological sequelae.
Twenty-two patients had etiologies other than acute metabolic or acute cerebral disorders, which included chronic cerebral lesions, degenerative cerebral disorders, drug usage, chronic alcoholism, and cryptogenic disorders. One patient with a degenerative disorder and two patients with cryptogenic etiologies experienced poor outcomes.
Multiple etiologies were present in 13 patients. Ten of these patients with metabolic disturbances had underlying chronic structural lesions, degenerative cerebral disorders, and an acute cerebral disorder (acute small cerebral infarction with NKH). The remaining three patients comprised one who experienced a previous traumatic cerebral lesion and received medication (flumazenil), one with degenerative dementia and a previous stroke lesion, and one with autoimmune encephalopathy and a previous stroke lesion.
Outcomes
The good-outcome group had 37 patients (77.1%) and the poor-outcome group had 11 patients (22.9%), with no significant intergroup differences in age (68.5 and 73.3 years respectively, p=0.280) or sex (13 and 5 males respectively, p=0.724). Other demographic data and clinical findings are summarized in Table 2. Acute metabolic disturbances were significantly correlated with poor outcomes (p=0.036). Other variables such as premorbid mRS scores, presenting mentation, single or multiple etiologies, mono- or polytherapy, and EEG pattern did not differ significantly between the groups (Table 3). Three (6.3%) patients in the poor-outcome group died, while the others developed a persistent vegetative state. Four patients failed to achieve seizure control, two of whom had chronic kidney disease with acute kidney injury.
Table 2. Comparison between good-outcome and poor-outcome groups.
| Good-outcome group | Poor-outcome group | |
|---|---|---|
| Number of patients | 37 | 11 |
| Age, years | 68.5±12.1 | 73.3±12.6 |
| Males:females | 13:24 | 5:6 |
| Underlying metabolic disturbances | CKD (4) | CKD (4), liver cirrhosis (1) |
| Underlying cerebral lesions | Old TBI (3), old stroke lesion (11), postoperative meningioma (1), birth injury (1) | Old TBI (1), old stroke lesion (3) |
| Initial presentations | Coma (6), intermittent UR (9), drowsiness (9), aphasia (2), confusion (11) | Coma (5), intermittent UR (3), confusion (3) |
Data are n or mean±standard-deviation values.
CKD: chronic kidney disease, TBI: traumatic brain injury, UR: unresponsiveness.
Table 3. Correlations medical and neurological conditions with poor outcomes.
| Present | Absent | p | |
|---|---|---|---|
| Acute metabolic disturbances | 7/17 | 4/31 | 0.036 |
| Premorbid mRS score ≤2 | 7/35 | 4/13 | 0.458 |
| NCSE presenting with coma | 5/11 | 6/37 | 0.095 |
| Multiple etiologies | 5/13 | 6/35 | 0.134 |
| Monotherapy | 4/20 | 7/28 | 0.742 |
| FDs in EEG | 9/37 | 2/11 | 1.000 |
Data are n values.
EEG: electroencephalography, FDs: focal discharges, mRS: modified Rankin Scale, NCSE: nonconvulsive status epilepticus.
DISCUSSION
The results of this study show that de novo NCSE is associated with various etiologies. In the patients with acute metabolic disturbances and acute cerebral disorders, triggering factors or acute cerebral disorders played an important role in the development of NCSE. In the patients with chronic and degenerative disorders, underlying medical conditions themselves probably triggered seizures. Multiple factors were associated with seizure generation in 13 patients. Seizure activity in EEG was observed in 20 of 24 patients with visible focal cerebral lesions, indicating that the lesions were probably epileptogenic foci regardless of whether or not acute triggering factors were present. Acute metabolic disturbances or drugs may lower the threshold for seizure occurrence in epileptogenic lesions.
NCSE can manifest with various atypical symptoms that may result in misdiagnoses and delay proper treatment.11,12,13 While NCSE has been associated with various medical and neurological disorders such as metabolic disturbances, structural brain diseases, autoimmune diseases, drugs, and drug withdrawal,14,15,16,17,18 the exact causes of NCSE remain unclear in many cases.11
Many of our patients with kidney disorders, especially those with acute kidney injury, developed NCSE and experienced poor outcomes. The metabolic disturbances were poorly controlled in four of the six patients with acute kidney injury who showed poor outcomes. An accumulation of uremic toxins such as guanidine compounds, which stimulate excitatory N-methyl-D-aspartate (NMDA) receptors and concomitantly inhibit inhibitory gamma-aminobutyric acid (GABA) receptors, may result in seizures in patients with kidney disorders.19 Electrolyte imbalances associated with kidney disorders may also play a role in seizure generation. However, the precise mechanisms underlying NCSE in patients with kidney disease are not yet fully understood. Poor outcomes may be related to difficulties in seizure control due to problems with dose adjustment or the severity and complications of the kidney injury.
Acute cerebral disorders often cause seizures. However, NCSE caused by acute cerebral disorders is particularly challenging to diagnose due to the vague symptoms and the difficulty in differentiating whether the altered mentation is caused by seizures or by the cerebral damage itself. EEG is mandatory for confirming NCSE in these situations, and treatments should focus on the eradication of seizures and on addressing cerebral insults.
NKH often causes focal seizures, which are usually motor seizures20,21,22 although other types of focal seizure may also develop.23,24 Hyperosmolality and combined hyponatremia may result in seizures. The seizures in our patients were completely controlled and had good outcomes. Hyperglycemia may increase GABA metabolism, which lowers the seizure threshold in patients with NKH.25
Antibiotics such as cephalosporin often cause encephalopathy and seizures, especially in patients with kidney disorders. The competitive inhibition of GABA-A receptors is a likely epileptogenic mechanism.26 Two of the present patients with chronic kidney disease who developed NCSE with cephalosporin recovered well after achieving infection control and stopping antibiotics.
Autoimmune encephalitis often causes NCSE with encephalopathy, whose symptoms typically do not respond to any type of antiepileptic drug, with only immunological treatments being effective.27,28,29,30 Paraneoplastic autoantibodies (anti-Hu, anti-Yo, anti-Ri, anti-CV2/CRMP5, anti-Ma2, and anti-amphiphysin), antibodies targeting neuronal cell-surface antigens (e.g., LGI1, NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and GABA-B), and antithyroid antibodies should be assessed in patients with intractable NCSE. Autoimmune encephalopathy including Hashimoto's encephalopathy often mimics the symptoms of other disorders such as metabolic encephalopathy.31,32 A delayed diagnosis may result in irreversible brain damage, and hence appropriate clinical suspicion is crucial for ensuring proper and timely management.
Thirteen of the present patients had multiple etiological factors. Both underlying disorders and triggering factors may contribute to the development of NCSE. One patient had an underlying old stroke with FDs in an area concordant with the cerebral lesion. However, her seizures were controlled by steroids rather than any antiepileptic drug, since she had high titers of antithyroid antibodies. If seizures and mentation abnormalities are intractable to antiepileptic treatment, other causes of encephalopathy should be investigated.
Two previous studies found that the prognosis of de novo status epilepticus was poor due to the older onset age and fatal underlying conditions,7,8 with reported mortality rates of 18.4% and 55.4%, respectively. However, the prognosis of NCSE varies,4,14,33,34,35 and can be better in patients with NCSE and pre-existing epilepsy than in those with acute neurological or systemic disorders.14,35 The present study also found that acute metabolic disturbances, especially those due to acute kidney injury and sepsis, were significantly correlated with poor outcomes in patients with de novo NCSE. However, only 3 (6.3%) patients died while 11 (22.9%) had poor outcomes. These results may be associated with the criteria applied for patient selection, since we excluded patients with severe brain damage who presented with coma alongside periodic discharges in EEG. None of the patients with acute cerebral lesions died, and only one patient had a poor outcome.
This study aimed to elucidate the clinical characteristics and prognosis of patients who presented with de novo NCSE in comparison with other types of NCSE. Chronic underlying cerebral disorders as well as acute symptomatic disorders may contribute to the generation of seizures either independently or in combination.
This study was subject to several limitations. We did not examine how the duration of NCSE and the initiation of treatment were correlated with outcomes. The exact duration of seizures was unidentifiable in many cases due to the subtlety of symptoms. The premorbid status could only be assessed based on information provided by caregivers. The smallness of the sample, restriction to a single center, and retrospective study design are also limitations.
Despite these limitations, our study is clinically important. This is the first clinical study of de novo NCSE, and the results indicate that this condition is caused by diverse clinical disorders and that the outcomes vary with the underlying mechanisms. Acute metabolic disturbances such as acute kidney injury and sepsis were correlated with poor outcomes, and NCSE may be caused by single or multiple factors. Adequate treatment of underlying reversible disorders and seizure control are critical for patients with NCSE.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
- Conceptualization: Jung-Ju Lee, Kyung-Il Park.
- Data curation: Jung-Ju Lee, Byung-Kun Kim, Ohyun Kwon, Woong-Woo Lee.
- Formal analysis: Jung-Ju Lee, Jong-Moo Park, Kyusik Kang.
- Methodology: Kyung-Il Park.
- Validation: Jong-Moo Park, Kyusik Kang.
- Writing—original draft: Jung-Ju Lee, Kyung-Il, Ohyun Kwon.
- Writing—review & editing: Jung-Ju Lee, Kyung-Il Park, Ohyun Kwon.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Kaplan PW. Assessing the outcomes in patients with nonconvulsive status epilepticus: nonconvulsive status epilepticus is underdiagnosed, potentially overtreated, and confounded by comorbidity. J Clin Neurophysiol. 1999;16:341–352. doi: 10.1097/00004691-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Shorvon S. What is nonconvulsive status epilepticus, and what are its subtypes? Epilepsia. 2007;48 Suppl 8:35–38. doi: 10.1111/j.1528-1167.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 3.Walker M, Cross H, Smith S, Young C, Aicardi J, Appleton R, et al. Nonconvulsive status epilepticus: Epilepsy Research Foundation workshop reports. Epileptic Disord. 2005;7:253–296. [PubMed] [Google Scholar]
- 4.Towne AR, Waterhouse EJ, Boggs JG, Garnett LK, Brown AJ, Smith JR, Jr, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 5.Bearden S, Eisenschenk S, Uthman B. Diagnosis of nonconvulsive status epilepticus (NCSE) in adults with altered mental status: clinico-electroencephalographic considerations. Am J Electroneurodiagnostic Technol. 2008;48:11–37. [PubMed] [Google Scholar]
- 6.Bottaro FJ, Martinez OA, Pardal MM, Bruetman JE, Reisin RC. Nonconvulsive status epilepticus in the elderly: a case-control study. Epilepsia. 2007;48:966–972. doi: 10.1111/j.1528-1167.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 7.Delanty N, French JA, Labar DR, Pedley TA, Rowan AJ. Status epilepticus arising de novo in hospitalized patients: an analysis of 41 patients. Seizure. 2001;10:116–119. doi: 10.1053/seiz.2000.0482. [DOI] [PubMed] [Google Scholar]
- 8.Tsai MH, Chuang YC, Chang HW, Chang WN, Lai SL, Huang CR, et al. Factors predictive of outcome in patients with de novo status epilepticus. QJM. 2009;102:57–62. doi: 10.1093/qjmed/hcn149. [DOI] [PubMed] [Google Scholar]
- 9.Leitinger M, Beniczky S, Rohracher A, Gardella E, Kalss G, Qerama E, et al. Salzburg consensus criteria for non-convulsive status epilepticus--approach to clinical application. Epilepsy Behav. 2015;49:158–163. doi: 10.1016/j.yebeh.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Sutter R, Kaplan PW. Electroencephalographic criteria for nonconvulsive status epilepticus: synopsis and comprehensive survey. Epilepsia. 2012;53 Suppl 3:1–51. doi: 10.1111/j.1528-1167.2012.03593.x. [DOI] [PubMed] [Google Scholar]
- 11.Tomson T, Lindbom U, Nilsson BY. Nonconvulsive status epilepticus in adults: thirty-two consecutive patients from a general hospital population. Epilepsia. 1992;33:829–835. doi: 10.1111/j.1528-1157.1992.tb02190.x. [DOI] [PubMed] [Google Scholar]
- 12.Jordan KG. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 1999;16:332–340. doi: 10.1097/00004691-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia. 1996;37:643–650. doi: 10.1111/j.1528-1157.1996.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 14.Kang BS, Jhang Y, Kim YS, Moon J, Shin JW, Moon HJ, et al. Etiology and prognosis of non-convulsive status epilepticus. J Clin Neurosci. 2014;21:1915–1919. doi: 10.1016/j.jocn.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Torre JL, Martínez-Martínez M, González-Rato J, Maestro I, Alonso I, Rodrigo E, et al. Cephalosporin-induced nonconvulsive status epilepticus: clinical and electroencephalographic features. Epilepsia. 2005;46:1550–1552. doi: 10.1111/j.1528-1167.2005.16305.x. [DOI] [PubMed] [Google Scholar]
- 16.Maganti R, Jolin D, Rishi D, Biswas A. Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function. Epilepsy Behav. 2006;8:312–314. doi: 10.1016/j.yebeh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Koussa SF, Chahine SL, Samaha EI, Riachi MA. Generalized status epilepticus possibly induced by gatifloxacin. Eur J Neurol. 2006;13:671–672. doi: 10.1111/j.1468-1331.2006.01250.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan PW, Birbeck G. Lithium-induced confusional states: nonconvulsive status epilepticus or triphasic encephalopathy? Epilepsia. 2006;47:2071–2074. doi: 10.1111/j.1528-1167.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 19.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 20.Singh BM, Gupta DR, Strobos RJ. Nonketotic hyperglycemia and epilepsia partialis continua. Arch Neurol. 1973;29:187–190. doi: 10.1001/archneur.1973.00490270069011. [DOI] [PubMed] [Google Scholar]
- 21.Singh BM, Strobos RJ. Epilepsia partialis continua associated with nonketotic hyperglycemia: clinical and biochemical profile of 21 patients. Ann Neurol. 1980;8:155–160. doi: 10.1002/ana.410080205. [DOI] [PubMed] [Google Scholar]
- 22.Maccario M, Messis CP, Vastola EF. Focal seizures as a manifestation of hyperglycemia without ketoacidosis. a report of seven cases with review of the literature. Neurology. 1965;15:195–206. doi: 10.1212/wnl.15.3.195. [DOI] [PubMed] [Google Scholar]
- 23.Harden CL, Rosenbaum DH, Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia. 1991;32:215–220. doi: 10.1111/j.1528-1157.1991.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 24.Pro S, Randi F, Pulitano P, Vicenzini E, Mecarelli O. Non-convulsive status epilepticus characterised exclusively by a language disorder induced by non-ketotic hyperglycaemia. Epileptic Disord. 2011;13:193–196. doi: 10.1684/epd.2011.0425. [DOI] [PubMed] [Google Scholar]
- 25.Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism. 1975;24:665–679. doi: 10.1016/0026-0495(75)90146-8. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016;86:963–971. doi: 10.1212/WNL.0000000000002455. [DOI] [PubMed] [Google Scholar]
- 27.Tüzün E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271. doi: 10.1097/NRL.0b013e31813e34a5. [DOI] [PubMed] [Google Scholar]
- 28.Asztely F, Kumlien E. The diagnosis and treatment of limbic encephalitis. Acta Neurol Scand. 2012;126:365–375. doi: 10.1111/j.1600-0404.2012.01691.x. [DOI] [PubMed] [Google Scholar]
- 29.Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 30.Machado S, Pinto AN, Irani SR. What should you know about limbic encephalitis? Arq Neuropsiquiatr. 2012;70:817–822. doi: 10.1590/s0004-282x2012001000012. [DOI] [PubMed] [Google Scholar]
- 31.Schäuble B, Castillo PR, Boeve BF, Westmoreland BF. EEG findings in steroid-responsive encephalopathy associated with autoimmune thyroiditis. Clin Neurophysiol. 2003;114:32–37. doi: 10.1016/s1388-2457(02)00343-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Shin H, Kang K, Kwon O, Park JM, Kim BK, et al. Hashimoto's encephalopathy: South Korean experiences. Acta Neurol Belg. 2014;114:209–216. doi: 10.1007/s13760-013-0268-5. [DOI] [PubMed] [Google Scholar]
- 33.Litt B, Wityk RJ, Hertz SH, Mullen PD, Weiss H, Ryan DD, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998;39:1194–1202. doi: 10.1111/j.1528-1157.1998.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 34.Lowenstein DH, Aminoff MJ. Clinical and EEG features of status epilepticus in comatose patients. Neurology. 1992;42:100–104. doi: 10.1212/wnl.42.1.100. [DOI] [PubMed] [Google Scholar]
- 35.Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066–1073. doi: 10.1212/01.wnl.0000082653.40257.0b. [DOI] [PubMed] [Google Scholar]