Abstract
Background and Purpose
The study aimed to obtain optometric findings of amyotrophic lateral sclerosis (ALS) patients in different stages of the disease, and to determine the relation between ocular data and ALS-related features; that is, functional and cognitive impairment and staging.
Methods
The optometric protocol included tests of the ocular motility [broad-H test and Northeastern State University College of Optometry (NSUCO) test], near point of convergence (NPC), error refraction, best-corrected visual acuity, and binocular visual alignment, and an ocular symptoms questionnaire. The functional measures included the Amyotrophic Lateral Sclerosis Functional Rating Scale–revised (ALSFRS-r) and Milano-Torino staging (MiToS), and cognitive impairment was assessed using the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Demographic and clinical features were also collected, including whether the patients used an eye-tracking communication device (ETCD).
Results
Two-hundred consecutive ALS patients (median age of 64 years, 118 males and 82 females) in different stages of disease were recruited. Nearly 70% of patients reported at least one ocular symptom, and the use of an ETCD was found to be significantly related to the presence of most symptoms. Moreover, the severely symptomatic group was characterized by significantly lower ALSFRS-r total and subscale scores, and higher MiToS. Abnormal NPC values were significantly related to lower ALSFRS-r total and bulbar-subscale scores. Patients with acceptable NSUCO test values exhibited significantly higher ECAS scores.
Conclusions
The presence of ocular alteration in patients in different stages of ALS supports the idea that this is a multisystem disorder and emphasizes the importance of optometric evaluations in multidisciplinary assessments to address ocular impairment early in the disease process.
Keywords: amyotrophic lateral sclerosis, visual system, optometric analysis, ocular motility, Edinburgh Cognitive and Behavioural ALS Screen, Milano-Torino staging
INTRODUCTION
There has been considerable expansion in the knowledge of different aspects of amyotrophic lateral sclerosis (ALS) in recent decades, which emphasizes the concept that this is a multisystem disease that culminates in dysfunction of the motor system as its hallmark manifestation.1,2 Progressive weakness of the limbs and bulbar muscles often compromises the ability of patients to communicate, such that using an eye-tracking communication device (ETCD) becomes necessary.3,4 Therefore, early clinical evaluation of the visual function in ALS patients is important in the multidisciplinary care process.
Reports in the literature on ALS have focused mainly on ocular motility and changes in retinal tissue.5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 As far as ocular motility is concerned, there have been a few reports on saccade and pursuit impairments in ALS patients using orthoptic examination techniques and electrooculography in vivo.5,6,7,8 Increased antisaccade and delayed-saccade errors, gaze palsy, and nystagmus have also been found in ALS patients.9,10,11,12 Moreover, a longitudinal study of saccadic and cognitive tasks revealed that ALS patients exhibited impaired performance in executive and visual search tasks despite having normal basic saccadic function.13 A postmortem study of ALS patients found the remarkable preservation of extraocular muscles in comparison with the limb muscles.14
Regarding in vivo analyses of the retina, Simonett et al.15 found some abnormalities in ALS patients using fundus photography and optical coherence tomography (OCT), and in another study they showed that the macular retinal nerve fiber layer was significantly thinner in ALS patients than healthy controls.16 These results were confirmed by Ringelstein et al.,17 who used high-resolution spectral-domain OCT with retinal segmentation to reveal subtle thinning of the macula and retinal nerve fiber layer as well as marked thinning of the inner nuclear layer. In contrast with these studies, Roth et al.18 found no significant differences between ALS patients and healthy controls in any of the examined OCT measures, and no correlation with clinical measures of disease severity. Postmortem histopathologic and structural studies performed on donor retinal tissues revealed intraretinal inclusions.19,20
Few studies have been conducted to assess the anterior segment of the eye. Ferrari et al.21 demonstrated a corneal small-fiber sensory neuropathy in sporadic ALS patients using confocal microscopy, and found that bulbar function disability scores were significantly related to measures of anatomical damage to corneal nerve fibers. Recent visual acuity measurements by Moss et al.22 did not confirm previous observations of impaired visual acuity in ALS patients, and did not support the use of this particular measure of visual function in broad-scale assessments of visual pathway involvement in ALS.23
There have been few reports on the use of comprehensive functional vision assessments to clarify ocular involvement in ALS and how changes occur over time, according to the natural history of the disease. Therefore, this study applied comprehensive optometric screening to ALS patients with the overall aim of determining whether there are cross-sectional relationships between ocular findings and disease clinical features, namely functional and cognitive impairment and disease staging.
METHODS
This retrospective study formed part of the “augmented Environment for COntrol in Amyotrophic Lateral Sclerosis patients (ECO-ALS)” project funded by AriSLA (the Italian Research Foundation for ALS) on the use of an ETCD based on ocular functions in ALS.24 During this project we performed an extensive evaluation of ocular functions in ALS patients. We evaluated 240 Italian patients at the Neuromuscular Omnicentre from April 2016 to October 2019. Patients with a diagnosis of definite, probable, or laboratory-supported probable ALS according to the Revised El-Escorial Criteria25 were included.
Since the aim of the study was to collect optometric data in a large and heterogeneous cohort, we decided to include as many patients as possible aged at least 18 years with different clinical features. We excluded 21 people with a clinical condition that was considered too severe by the referring clinician, potentially interfering with the patient's compliance with the visual examination. We also excluded nine patients with severe cognitive dysfunction, behavioral disorders, or significant psychiatric disorders that could result in inadequate compliance with the evaluation. We excluded a further four patients with visual impairment caused by glaucoma and six patients affected by various types of retinopathies (one of them due to diabetes mellitus). Therefore, the final analysis was performed in 200 patients.
Optometric tests and clinical features
The optometric tests together with their corresponding investigated visual abilities are listed below:
• Ocular motility was evaluated using the broad-H test. The positions of gaze in which misalignment of the patient's eyes was observed or for which the patient reported doubling of the target were determined. Abnormal eye movements were recorded.26
• Saccades and pursuits were assessed using the Northeastern State University College of Optometry (NSUCO) oculomotor test, based on a 5-point scale.27,28 Ability, accuracy and head movement were evaluated for both saccades and pursuits. Since there are some limits related to the disease, particularly regarding lower limb motor impairment, we decided to perform the test while the patient was sitting. Therefore, the fourth NSUCO test item (“body movement”) was not evaluated. Moreover, the “head movement” score was recorded only for patients who could move their head. Scores were divided into two groups for the statistical analysis: 1) scores up to 3 were considered unacceptable, corresponding to abnormal movements, and 2) other scores were considered acceptable.
• Binocular visual alignment was assessed using the cover test in free space, with the presence of eventual near heterotropia (N-TR) or far heterotropia (F-TR) was recorded.28
• A Wolff Wand was used to measure the near point of convergence (NPC), which is the point where the visual axes intersect under the maximum effort of the convergence.28 Since our study involved a presbyopic population, an objective break point greater than 15 cm was considered unacceptable.29
• Monocular and binocular refractive errors (i.e., myopia, hyperopia, and astigmatism) were detected, with the right-eye and left-eye spherical equivalent refractive errors (RSE and LSE refractive errors, respectively) considered in the analysis.
• The binocular best-corrected visual acuity (BCVA) was measured, and expressed as the logarithm of the minimum angle of resolution (logMAR).30
• A questionnaire was administered that focused on the ocular history and related symptoms. All the patients were asked whether any of the following listed ocular symptoms was significantly present in their daily life: distance or near blurred vision, diplopia, eye strain, burning-eye sensation, watering eyes, ocular dryness, or photophobia. The patients were divided into three groups: asymptomatic (no symptoms reported), mildly symptomatic (only one symptom reported), and severely symptomatic (multiple symptoms reported).
Both the Amyotrophic Lateral Sclerosis Functional Rating Scale–revised (ALSFRS-r) and Milano-Torino staging (MiToS) were used to assess disease severity. The ALSFRS-r is a validated questionnaire-based scale that measures the physical function of ALS patients when they are carrying out the activities of daily living. It consists of 12 items that are divided into 3 main domains (bulbar, spinal and respiratory), and the total score ranges from 0 to 48.31,32,33 MiToS comprises six stages, with stage 0 being normal function and stage 5 being death.34
The relation between cognitive impairment and ocular function was evaluated by applying the Italian version of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS)35 to a subgroup of 71 patients. The ECAS assesses both ALS-specific cognitive functions (language, verbal fluency, and executive functioning) and non-ALS-specific functions (memory and visuospatial perception). The maximum total ECAS score is 136, and lower scores indicate worse cognitive performance. For the present analysis, we considered only the ECAS total score as a global summary index.
Regarding support received from ocular devices, information on the daily use of an ETCD was also collected, with an ETCD user considered to be a patient who used such a device for more than 4 hours/day.
Finally, a set of specific demographic and clinical variables was collected, including the patient's age at the evaluation, the disease duration expressed as the time from onset to the evaluation, the diagnostic delay, the disease progression rate calculated as [(48 minus ALSFRS-r total score at the evaluation)/disease duration], and the site of onset (limb or bulbar).
The study design was approved by the institutional ethics committee of Milano Area 3 (No. 353-072017), and all of the subjects included in this study signed an informed-consent form in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments.
Statistical analysis
The Shapiro-Wilk and Levene tests were used to assess the normality of the distribution and the homogeneity of the variance separately for each variable. Data were described using median and interquartile-range values or frequency and percentage values, as appropriate.
The associations between the ocular assessments, demographic and clinical features, functional status, and cognitive evaluations were first assessed at the univariate level using 1) the Mann-Whitney U test when the ocular features were dichotomous and the independent variables were continuous, 2) the chi-square test when both the ocular symptoms and the independent variables were categorical, and 3) Spearman's rho correlation coefficient when both the ocular symptoms and the independent variables were continuous.
Multivariable logistic regression models were finally used to confirm the significant associations between ocular assessments and both functional and cognitive features, while adjusting the analyses for the effects of sex, disease progression rate, disease duration, and diagnostic delay (these were the demographic and clinical features significantly related to the ocular assessments in the univariate analysis).
All tests were two-tailed and the statistical significance was set at p<0.05. All analyses were performed using SAS (version 9.3, SAS Institute, Cary, NC, USA).
RESULTS
The results of the descriptive analysis of the cohort are presented in Table 1.
Table 1. Description of the study cohort.
| Demographic and clinical characteristics | Values |
|---|---|
| Age at evaluation, years | 63.99 [55.26–70.71] |
| Disease duration, months | 42.90 [21.93–82.73] |
| Diagnostic delay, months | 12.17 [7.07–21.37] |
| Disease progression rate, ALSFRS-r score unit/months | 0.50 [0.26–0.89] |
| Sex, male | 118 (59) |
| C9ORF72 expansion | 5 (3) |
| Site of onset, bulbar | 38 (19) |
| EEC, definite | 34 (17) |
| NIV at evaluation | 92 (46) |
| IV at evaluation | 9 (5) |
| PEG at evaluation | 31 (16) |
| Ocular device used | |
| ETCD user | 23 (12) |
| Functional features | |
| MiToS | |
| 0 | 50 (34) |
| 1 | 34 (23) |
| 2 | 32 (21) |
| 3 | 15 (10) |
| 4 | 18 (12) |
| Missing | 51 |
| ALSFRS-r | |
| Total score | 29 [18–36] |
| Bulbar-subscale score | 10 [6–12] |
| Spinal-subscale score | 11 [4–15] |
| Respiratory-subscale score | 10 [3–12] |
| Cognitive assessment | |
| ECAS total score | 105.00 [92.00–115.00] |
Data are n (%) or median [interquartile range] values.
ALSFRS-r: Amyotrophic Lateral Sclerosis Functional Rating Scale–revised, ECAS: Edinburgh Cognitive and Behavioural ALS Screen, EEC: El-Escorial Criteria, ETCD: eye-tracking communication device, IV: invasive ventilation, MiToS: Milano-Torino staging, NIV: noninvasive ventilation, PEG: percutaneous endoscopic gastrostomy.
Ocular symptoms in ALS
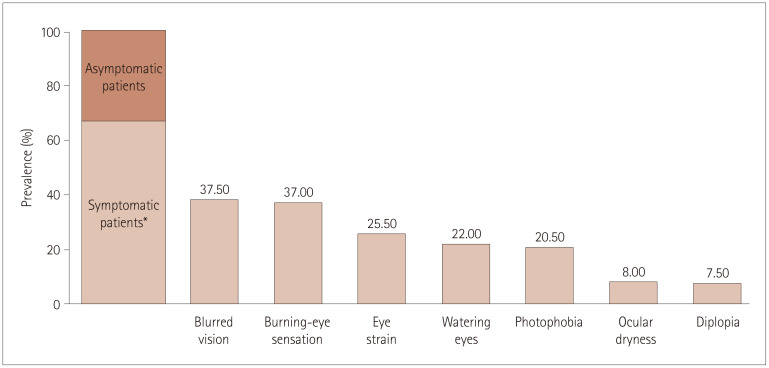
Sixty-six patients (33%) were asymptomatic, 61 (31%) were mildly symptomatic, and 73 (36%) were severely symptomatic. Fig. 1 shows the prevalence of each symptom considered reported as a percentage relative to the overall population. More than one-third of patients reported blurred vision and burning-eye sensation. Only symptoms reported by at least 10% of patients were considered in the analysis, and so diplopia and ocular dryness were excluded.
Fig. 1. Distribution of ocular symptoms. *Symptomatic patients include both mildly and severely symptomatic groups.
Optometric findings in ALS
Table 2 presents the results of the descriptive analysis of the optometric tests. A large percentage of patients obtained low scores on all items of the NSUCO test. Low saccade accuracies and abnormal head movements were found in 46% and 42% of the patients, respectively. Pursuits showed similarly poor results, with 61% and 37% of the patients having low scores for accuracy and head movements, respectively. In contrast, slowed eye movements in the broad-H test were recorded in only 15% of the cohort. In the NPC test, almost 30% of patients had a break point of convergence greater than 15 cm.
Table 2. Results of optometric tests.
| Optometric tests | Values |
|---|---|
| NSUCO oculomotor test | |
| Saccades accuracy | |
| Unacceptable values, 1–3 | 90 (46) |
| Acceptable values, 4 or 5 | 105 (54) |
| Pursuit accuracy | |
| Unacceptable values, 1–3 | 121 (61) |
| Acceptable values, 4 or 5 | 76 (39) |
| Saccade head movements | |
| Unacceptable values, 1–3 | 79 (42) |
| Acceptable values, 4 or 5 | 107 (58) |
| Pursuit head movements | |
| Unacceptable values, 1–3 | 69 (37) |
| Acceptable values, 4 or 5 | 119 (63) |
| Broad-H test | |
| Abnormal eye movements | 29 (15) |
| NPC | |
| Unacceptable values, break point ≥15 cm | 57 (29) |
| Acceptable values, break point <15 cm | 142 (71) |
| Cover test | |
| Far heterotropia | 12 (6) |
| Near heterotropia | 16 (8) |
| Refractive condition | |
| Right-eye spherical equivalent, diopters | 0.25 [-1.00 to 1.25] |
| Left-eye spherical equivalent, diopters | 0.12 [-0.75 to 1.12] |
| Best-corrected visual acuity, logMAR | 0.00 [0.00 to 0.00] |
Data are n (%) or median [interquartile range] values.
logMAR: logarithm of the minimum angle of resolution, NPC: near point of convergence, NSUCO: Northeastern State University College of Optometry.
The cover test revealed that F-TR and N-TR were present in 6% and 8% of patients, respectively. Considering the refractive condition of the cohort, the median RSE and LSE values were positive numbers close to zero. The median value of BCVA was 0 logMAR, corresponding to good visual acuity function when the refractive error is corrected using the appropriate lens.
Optometric findings and their correlations with ETCD use
The number of ETCD users differed significantly between the asymptomatic and symptomatic groups, being significantly higher in severely symptomatic patients than in both the mildly symptomatic and asymptomatic groups. Moreover, specific symptoms were reported significantly more by ETCD users. All of these significant relations were also confirmed when considering sex, disease progression rate, disease duration, and diagnostic delay as confounding effects (Table 3).
Table 3. Significant associations between optometric findings and ETCD use.
| Optometric findings | Number of ETCD user,n (%) | p | |
|---|---|---|---|
| Univariate analysis | Multivariable analysis* | ||
| Symptoms | <0.0001 | 0.0006 | |
| Asymptomatic | 3 (5) | ||
| Mildly symptomatic | 1 (2) | ||
| Severely symptomatic | 19 (26) | ||
| Blurred vision | 0.0035 | 0.0029 | |
| No | 8 (6) | ||
| Yes | 15 (20) | ||
| Burning eye sensation | <0.0001 | 0.0005 | |
| No | 4 (3) | ||
| Yes | 19 (26) | ||
| Eye strain | <0.0001 | <0.0001 | |
| No | 6 (4) | ||
| Yes | 17 (33) | ||
| Watering eyes | <0.0001 | 0.0005 | |
| No | |||
| Yes | 12 (27) | ||
| Photophobia | <0.0001 | 0.0021 | |
| No | 1 (6) | ||
| Yes | 13 (32) | ||
Data are n (%) values.
*p value adjusted for sex, disease progression rate, disease duration, and diagnostic delay.
ETCD: eye-tracking communication device.
No associations were found between ETCD use and the findings for ocular motility tests, binocular alignment, and the refractive condition of the patients (i.e., RSE, LSE, and BCVA) (Supplementary Table 1 in the online-only Data Supplement).
Optometric findings and their correlations with functional status and staging
The median ALSFRS-r total and subscale scores were significantly lower in severely symptomatic patients than in the mildly symptomatic and asymptomatic groups. The ALSFRS-r total score and the scores on most ALSFRS-r subscales were also significantly lower in patients who reported specific symptoms. Similarly, significant intergroup differences were found in functional staging as assessed using MiToS: patients in the severely symptomatic group and who reported specific symptoms (except for blurred vision) were in more-advanced stages of disease. All of the significant relations were also confirmed in the multivariable model adjusted for the confounding effects of demographic and clinical variables. Table 4 summarizes all of the significant associations between symptoms and functional status and staging. Supplementary Fig. 1 (in the online-only Data Supplement) highlights that the percentage of severely symptomatic patients increased with the disease stage.
Table 4. Significant associations between ocular symptoms and functional and staging status.
| Functional features | Scores by symptoms | Univariate analysis | Multivariable analysis† | ||
|---|---|---|---|---|---|
| Asymptomatic patients | Mildly symptomatic patients | Severely symptomatic patients | p | p | |
| ALSFRS-r bulbar-subscale | 11 [7–12] | 10 [8–11] | 8 [3–10] | 0.0003 | 0.0016 |
| ALSFRS-r spinal-subscale | 12 [8–17] | 12 [8–16] | 7 [0–12] | 0.0001 | 0.0004 |
| ALSFRS-r respiratory-subscale | 11 [5–12] | 11 [3–12] | 6 [2–11] | 0.0007 | 0.0006 |
| ALSFRS-r total | 33 [24–38] | 31 [22–37] | 19 [9–31] | <0.0001 | <0.0001 |
| MiToS>* | 16 (33%) | 12 (27%) | 37 (66%) | 0.0054 | 0.0008 |
| No blurred vision | Blurred vision | p | p | ||
| ALSFRS-r bulbar-subscale | 10 [7–12] | 9 [3–11] | 0.0124 | 0.0143 | |
| ALSFRS-r total | 31 [22–36] | 26 [12–36] | 0.0351 | 0.0075 | |
| No eye strain | Eye strain | p | p | ||
| ALSFRS-r bulbar-subscale | 10 [7–12] | 8 [2–10] | 0.0002 | 0.0002 | |
| ALSFRS-r spinal-subscale | 12 [7–16] | 8 [0–11] | <0.0001 | 0.0003 | |
| ALSFRS-r respiratory-subscale | 11 [5–12] | 5 [2–10] | <0.0001 | 0.0002 | |
| ALSFRS-r total score | 31 [23–38] | 18 [9–27] | <0.0001 | <0.0001 | |
| MiToS>1* | 36 (33%) | 29 (67%) | 0.0002 | 0.0002 | |
| No burning-eye sensation | Burning-eye sensation | p | p | ||
| ALSFRS-r bulbar-subscale | 11 [8–12] | 8 [2–10] | <0.0001 | 0.0002 | |
| ALSFRS-r spinal-subscale | 12 [9–17] | 6 [0–12] | <0.0001 | <0.0001 | |
| ALSFRS-r respiratory-subscale | 11 [5–12] | 6 [2–11] | 0.0012 | 0.0027 | |
| ALSFRS-r total | 32 [24–38] | 19 [9–31] | <0.0001 | <0.0001 | |
| MiToS>1* | 29 (31%) | 36 (63%) | 0.0002 | <0.0001 | |
| No watering eyes | Watering eyes | p | p | ||
| ALSFRS-r spinal-subscale | 12 [8–16] | 9 [0–15] | 0.0038 | 0.0125 | |
| ALSFRS-r respiratory-subscale | 11 [3–16] | 6 [1–11] | 0.0021 | 0.0045 | |
| ALSFRS-r total | 31 [22–38] | 22 [8–32] | 0.0015 | 0.0036 | |
| MiToS>1* | 35 (33%) | 21 (64%) | 0.0020 | 0.0032 | |
| No photophobia | Photophobia | p | p | ||
| ALSFRS-r bulbar-subscale | 10 [7–12] | 6 [2–9] | <0.0001 | 0.0003 | |
| ALSFRS-r spinal-subscale | 12 [7–15] | 4 [0–12] | 0.0026 | 0.0405 | |
| ALSFRS-r respiratory-subscale | 10 [4–12] | 4 [1–10] | 0.0043 | 0.0037 | |
| ALSFRS-r total | 30 [22–37] | 17 [4–29] | 0.0001 | 0.0008 | |
| MiToS>1* | 45 (37%) | 20 (69%) | 0.0022 | 0.0056 | |
Data are n (%) or median [interquartile range] values.
*Comparison between patients who lost up to one function and patients who lost multiple functions, †p value adjusted for sex, disease progression rate, disease duration, and diagnostic delay.
ALSFRS-r: Amyotrophic Lateral Sclerosis Functional Rating Scale–revised, MiToS: Milano-Torino staging.
No associations were found between NSUCO data and the functional status as measured using either the ALSFRS-r total and subscale scores or the MiToS system, with the exception that the ALSFRS-r bulbar-subscale score was significantly lower in patients with unacceptable values for items related to head movements associated with saccades and pursuits, although this relationship was significant only at the univariate level. Similar results were found in patients with slowed eye movements in the broad-H test, who reported a significantly lower ALSFRS-r bulbar-subscale scores only in the univariate analysis.
Abnormal values in the NPC test were significantly related to lower ALSFRS-r bulbar-subscale and total scores in both the univariate and multivariable analyses (Table 5). The binocular alignment variables (i.e., N-TR and F-TR) and the refractive condition of the patients (i.e., RSE, LSE, and BCVA) were not correlated with either ALSFRS-r scores or MiToS (Supplementary Table 2 in the online-only Data Supplement).
Table 5. Significant associations between optometric findings and functional status.
| Functional features | Scores by optometric findings | Univariate analysis | Multivariable analysis* | |
|---|---|---|---|---|
| Abnormal saccade head movements | Normal saccade head movements | p | p | |
| ALSFRS-r bulbar-subscale | 9 [5–11] | 10 [8–12] | 0.0026 | ns |
| Abnormal pursuit head movements | Normal pursuit head movements | p | p | |
| ALSFRS-r bulbar-subscale | 9 [6–11] | 10 [8–12] | 0.0256 | ns |
| Abnormal eye movements (Broad-H test) | Normal eye movements (Broad-H test) | p | p | |
| ALSFRS-r bulbar-subscale | 7 [2–11] | 10 [7–12] | 0.0292 | ns |
| Abnormal NPC | Normal NPC | p | p | |
| ALSFRS-r bulbar-subscale | 8 [3–10] | 10 [7–12] | 0.0003 | 0.0040 |
| ALSFRS-r total | 25 [8–33] | 30 [21–37] | 0.0123 | 0.0389 |
Data are median [interquartile range] values.
*p value adjusted for sex, disease progression rate, disease duration, and diagnostic delay.
ALSFRS-r: Amyotrophic Lateral Sclerosis Functional Rating Scale–revised, NPC: near point of convergence, ns: not significant.
Optometric findings and their correlations with cognitive function
No significant associations were found between ocular symptoms and cognitive dysfunction as evaluated using the ECAS.
In the ocular motility tests, patients with abnormal saccade functions, abnormal pursuit functions, and slowed eye movements in the broad-H test obtained significantly lower ECAS total scores. These associations remained statistically significant also adjusting for the effects of sex, disease progression rate, disease duration, and diagnostic delay. The NPC, binocular alignment, and refractive condition of the patients were not significantly correlated with cognitive features (Supplementary Table 3 in the online-only Data Supplement).
All of the significant associations between optometric findings and cognitive function are reported in Table 6.
Table 6. Significant associations between optometric findings and cognitive status.
| Cognitive assessment | Scores by optometric findings | Univariate analysis | Multivariable analysis* | |
|---|---|---|---|---|
| Abnormal saccade accuracy | Normal saccade accuracy | p | p | |
| ECAS total | 95.0 [84.0–108.0] | 111.0 [103.0–117.0] | <0.0001 | 0.001 |
| Abnormal saccade head movements | Normal saccade head movements | p | p | |
| ECAS total | 96.0 [85.0–105.0] | 111.0 [102.0–116.0] | 0.0006 | 0.0105 |
| Abnormal pursuit accuracy | Normal pursuit accuracy | p | p | |
| ECAS total | 98.0 [85.0–111.0] | 111.0 [103.5–116.5] | 0.0027 | 0.0290 |
| Abnormal pursuit head movements | Normal pursuit head movements | p | p | |
| ECAS total | 96.0 [84.0–108.0] | 111.0 [102.0–116.0] | 0.0019 | 0.0202 |
| Abnormal eye movements (Broad-H test) | Normal eye movements (Broad-H test) | p | p | |
| ECAS total | 84.0 [69.0–99.0] | 108.0 [96.0–115.0] | 0.0246 | 0.0210 |
Data are median [interquartile range] values.
*p value adjusted for sex, disease progression rate, disease duration, and diagnostic delay.
ECAS: Edinburgh Cognitive and Behavioural ALS Screen.
DISCUSSION
To the best of our knowledge, this is the first study to have performed optometric tests in a large cohort of patients with ALS, and to have determined how these visual alterations are correlated with specific features of the disease.
A large proportion of the included patients complained of various ocular discomforts, emphasizing how they are a common feature in ALS, especially in patients with severe functional impairment and in the more-advanced stages of the disease. It may therefore be worthwhile to routinely perform eye examinations in ALS patients within the context of multidisciplinary care, so as to address any ocular problems they may have with the aim of maintaining the quality of life in these patients and their families. Particular attention should be paid in the advanced stage of the disease, as highlighted by the data in Supplementary Fig. 1 (in the online-only Data Supplement), and since troublesome ocular symptoms are more common when functional impairment occurs.
When looking at each symptom, the use of an ETCD plays an important role in the perception of symptoms. In accordance with the recent optometric review by Olsen amd Denton,36 the high visual demands required when using an ETCD mean that ALS patients tend to exhibit a partial or reduced blink rate, resulting in ocular discomfort. It is also well known that the high cognitive demands of a reading task reduce the spontaneous blink rate,37 which can result in an inadequate tear distribution as well as tear thinning over the exposed ocular and resulting eye pain. This characteristic is likely to be combined with the complex clinical impairment that often occurs in the final stage of the disease, when the patient is forced to use their eyes to communicate with the outside world.
It is important to consider that the alterations of the tear film in ALS patients could also be related to the drugs used to manage different ALS-related symptoms (e.g., drooling and mood disturbances) that influence secretion from the tear glands. However, since most of the reported symptoms are also characteristic of computer vision syndrome, it would have been interesting to evaluate whether the duration of using an ETCD is correlated with the frequency of ocular symptoms. Unfortunately, the small number of patients using such a device meant that such stratification was not possible.
Simple tests of ocular motility may reveal relevant information about specific clinical features related to ALS, in particular to bulbar function defined by the ALSFRS-r. Indeed, patients who had lower ALSFRS-r bulbar-subscale and total scores showed ocular convergence deficit, as expressed by the NPC—to the best of our knowledge this is the first report of such an outcome, and this deserves further investigation.
The relationship between ocular motility (specifically, head movements associated with saccades and pursuits) and bulbar function has been discussed previously,10,38 focusing on the type of onset rather than the level of impairment. Kang et al.38 found that abnormal smooth pursuits and saccadic dysmetria were more common in ALS patients with bulbar onset than in those with spinal onset. Donaghy et al.10 similarly reported slower saccades in bulbar-onset than spinalonset motor neuron disease. They ascribed these findings to increased brainstem pathology in bulbar-onset disease that involves burst cell neurons, which are responsible for saccadic movements.39
The present study found that bulbar impairment—as assessed using the ALSFRS-r bulbar-subscale score—was significantly related to head-movement compensations in both saccade and pursuit tests, but only at the univariate level, and so no causal relationship emerged between the variables. The same result was found for the relationship between bulbar impairment and slowed eye movements, in which the rate of disease progression rate was a significant independent factor in the multivariable model. The most plausible explanation is that patients with bulbar impairment had a faster disease progression, which is consistent with the literature.40
Our results suggest that ALS oculomotor functions and cognitive impairment worsen in parallel. Indeed, the ECAS total score was significantly lower in patients who performed worse in the ocular motility tests, independently of the stage of the disease and its aggressiveness. We consider this to be a particularly interesting result, since it was obtained using tests that are easy to implement in clinical practice. This relationship was already discussed by Gorges et al.,12 who in assessments of ocular motility using a video-oculography device showed that the ECAS total score was strongly correlated with delayed-saccade and antisaccade errors and the number of voluntary gaze shifts. The refractive condition of the patients does not seem to add significant clinical information to ALS disease, which is consistent with the recent findings of Moss et al.22
While the present cohort was large and the applied tests covered a broad spectrum of potential eye problems in ALS, this study was subject to some limitations. Firstly, the study lacked an age- and sex-matched control group to determine whether the findings were specific to ALS or related to aging. Secondly, the visual examinations that we performed did not include a fundus oculi examination, which is an important parameter. Thirdly, the smallness of the group of ETCD users prevented us from investigating how the level of usage of such a device influences the worsening of ocular symptoms over time or whether there is a correlation between the level of ocular disturbances and specific technical features (e.g., screen brightness, white or black background, or use of corrective lenses). Fourthly, we did not perform specific autonomic tests, and although the optometric tests would be little influenced by dysautonomia, the questionnaire focused on the ocular history and symptoms, which can be greatly affected by this factor.
In conclusion, we have performed baseline assessments in a large cohort of consecutive patients. How ocular involvement progresses over time and how this impacts the activities of daily living and the perceptions of patients is currently being explored. This approach might make it possible to identify patients at a higher risk of ocular involvement and provide more-tailored intervention and monitoring. The findings of this preliminary study support the idea that ALS is a multisystem disorder and emphasize the importance of applying optometric evaluations during the multidisciplinary care of ALS patients. The clinical implications of this study are in the need to detect possible ocular alterations early during the disease process and provide patients and their families with ocular care throughout, and especially as the disease progresses.
Acknowledgements
We thank AriSLA-Italian Research Foundation for ALS for the financial support of this study and we gratefully acknowledge all the patients and caregivers involved.
This work was supported by the Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica (AriSLA, 2015 grant “Ice Bucket Call for Assistive Technology Projects”).
Footnotes
- Conceptualization: all authors.
- Data curation: Federica Cozza, Andrea Lizio, Lucia Catherine Greco.
- Formal analysis: Andrea Lizio.
- Funding acquisition: Christian Lunetta.
- Investigation: Federica Cozza, Lucia Catherine Greco.
- Methodology: Christian Lunetta, Federica Cozza, Andrea Lizio.
- Project administration: Christian Lunetta.
- Resources: Christian Lunetta, Federica Cozza.
- Supervision: Christian Lunetta.
- Validation: Christian Lunetta, Federica Cozza.
- Visualization: all authors.
- Writing—original draft: Christian Lunetta, Federica Cozza, Andrea Lizio.
- Writing—review & editing: all authors.
Conflicts of Interest: Dr. Lunetta received funds for scientific consultation from Neuraltus, Italfarmaco, Mitsubishi Tanabe Europe and Cytokinetics and has received funds from Agenzia Italiana per la Ricerca sulla SLA (ARISLA) and Ministry of Health (CCM2011). The other authors have no conflicts of interest to declare.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.1.96.
Descriptive statistics of the not-significant associations between optometric findings and use of an ETCD
Distribution of asymptomatic, mildly symptomatic, and severely symptomatic groups across MiToS. MiToS: Milano-Torino staging.
Descriptive statistics of the nonsignificant associations between optometric findings and functional and staging status
Descriptive statistics of the not-significant associations between optometric findings and cognitive status
References
- 1.Strong MJ. Revisiting the concept of amyotrophic lateral sclerosis as a multisystems disorder of limited phenotypic expression. Curr Opin Neurol. 2017;30:599–607. doi: 10.1097/WCO.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 2.Günther R, Richter N, Sauerbier A, Chaudhuri KR, Martinez-Martin P, Storch A, et al. Non-motor symptoms in patients suffering from motor neuron diseases. Front Neurol. 2016;7:117. doi: 10.3389/fneur.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neto LL, Constantini AC, Chun RYS. Communication vulnerable in patients with amyotrophic lateral sclerosis: a systematic review. NeuroRehabilitation. 2017;40:561–568. doi: 10.3233/NRE-171443. [DOI] [PubMed] [Google Scholar]
- 4.Caligari M, Godi M, Guglielmetti S, Franchignoni F, Nardone A. Eye tracking communication devices in amyotrophic lateral sclerosis: impact on disability and quality of life. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:546–552. doi: 10.3109/21678421.2013.803576. [DOI] [PubMed] [Google Scholar]
- 5.Ohki M, Kanayama R, Nakamura T, Okuyama T, Kimura Y, Koike Y. Ocular abnormalities in amyotrophic lateral sclerosis. Acta Otolaryngol Suppl. 1994;511:138–142. doi: 10.3109/00016489409128318. [DOI] [PubMed] [Google Scholar]
- 6.Palmowski A, Jost WH, Prudlo J, Osterhage J, Käsmann B, Schimrigk K, et al. Eye movement in amyotrophic lateral sclerosis: a longitudinal study. Ger J Ophthalmol. 1995;4:355–362. [PubMed] [Google Scholar]
- 7.Shaunak S, Orrell RW, O'Sullivan E, Hawken MB, Lane RJ, Henderson L, et al. Oculomotor function in amyotrophic lateral sclerosis: evidence for frontal impairment. Ann Neurol. 1995;38:38–44. doi: 10.1002/ana.410380109. [DOI] [PubMed] [Google Scholar]
- 8.Averbuch-Heller L, Helmchen C, Horn AK, Leigh RJ, Büttner-Ennerver JA. Slow vertical saccades in motor neuron disease: correlation of structure and function. Ann Neurol. 1998;44:641–648. doi: 10.1002/ana.410440410. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy C, Thurtell MJ, Pioro EP, Gibson JM, Leigh RJ. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry. 2011;82:110–116. doi: 10.1136/jnnp.2010.212407. [DOI] [PubMed] [Google Scholar]
- 10.Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010;257:1134–1140. doi: 10.1007/s00415-010-5478-7. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Hicks S, Berna CM, Kennard C, Talbot K, Turner MR. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch Neurol. 2011;68:857–861. doi: 10.1001/archneurol.2011.130. [DOI] [PubMed] [Google Scholar]
- 12.Gorges M, Müller HP, Lulé D, Del Tredici K, Brettschneider J, Keller J, et al. Eye movement deficits are consistent with a staging model of pTDP-43 pathology in amyotrophic lateral sclerosis. PLoS One. 2015;10:e0142546. doi: 10.1371/journal.pone.0142546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proudfoot M, Menke RA, Sharma R, Berna CM, Hicks SL, Kennard C, et al. Eye-tracking in amyotrophic lateral sclerosis: a longitudinal study of saccadic and cognitive tasks. Amyotroph Lateral Scler Frontotemporal Degener. 2015;17:101–111. doi: 10.3109/21678421.2015.1054292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmadi M, Liu JX, Brännström T, Andersen PM, Stål P, Pedrosa-Domellöf F. Human extraocular muscles in ALS. Invest Ophthalmol Vis Sci. 2010;51:3494–3501. doi: 10.1167/iovs.09-5030. [DOI] [PubMed] [Google Scholar]
- 15.Simonett J, Chou J, Siddique N, Armstrong J, Fawzi A, Siddique T, et al. Ocular manifestations and optic nerve changes in patients with amyotrophic lateral sclerosis (ALS) Invest Ophthalmol Vis Sci. 2013;54:4382 [Google Scholar]
- 16.Simonett JM, Huang R, Siddique N, Farsiu S, Siddique T, Volpe NJ, et al. Macular sub-layer thinning and association with pulmonary function tests in amyotrophic lateral sclerosis. Sci Rep. 2016;6:29187. doi: 10.1038/srep29187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringelstein M, Albrecht P, Südmeyer M, Harmel J, Müller AK, Keser N, et al. Subtle retinal pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2014;1:290–297. doi: 10.1002/acn3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth NM, Saidha S, Zimmermann H, Brandt AU, Oberwahrenbrock T, Maragakis NJ, et al. Optical coherence tomography does not support optic nerve involvement in amyotrophic lateral sclerosis. Eur J Neurol. 2013;20:1170–1176. doi: 10.1111/ene.12146. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi AA, Simonett JM, Purta P, Moss HE, Lowry JL, Deng HX, et al. Clinicopathologic report of ocular involvement in ALS patients with C9orf72 mutation. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:569–580. doi: 10.3109/21678421.2014.951941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpe NJ, Simonett J, Fawzi AA, Siddique T. Ophthalmic manifestations of amyotrophic lateral sclerosis (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2015;113:T12. [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari G, Grisan E, Scarpa F, Fazio R, Comola M, Quattrini A, et al. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front Aging Neurosci. 2014;6:278. doi: 10.3389/fnagi.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss HE, Samelson M, Mohan G, Jiang QL. High and low contrast visual acuity are not affected in amyotrophic lateral sclerosis. PLoS One. 2016;11:e0168714. doi: 10.1371/journal.pone.0168714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss HE, McCluskey L, Elman L, Hoskins K, Talman L, Grossman M, et al. Cross-sectional evaluation of clinical neuro-ophthalmic abnormalities in an amyotrophic lateral sclerosis population. J Neurol Sci. 2012;314:97–101. doi: 10.1016/j.jns.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bona S, Donvito G, Cozza F, Malberti I, Vaccari P, Lizio A, et al. The development of an augmented reality device for the autonomous management of the electric bed and the electric wheelchair for patients with amyotrophic lateral sclerosis: a pilot study. Disabil Rehabil Assist Technol. 2019 Nov 05; doi: 10.1080/17483107.2019.1683237. [Epub]. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 26.Elliott DB. Clinical Procedures in Primary Eye Care. 3rd ed. Oxford: Butterworth-Heinemann; 2008. [Google Scholar]
- 27.Maples WC, Atchley J, Ficklin T. Northeastern State University College of Optometry's oculomotor norms. J Behav Optom. 1992;3:143–150. [Google Scholar]
- 28.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. 4th ed. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 29.Ostadimoghaddam H, Hashemi H, Nabovati P, Yekta A, Khabazkhoob M. The distribution of near point of convergence and its association with age, gender and refractive error: a population-based study. Clin Exp Optom. 2017;100:255–259. doi: 10.1111/cxo.12471. [DOI] [PubMed] [Google Scholar]
- 30.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 32.Franchignoni F, Mora G, Giordano A, Volanti P, Chiò A. Evidence of multidimensionality in the ALSFRS-R scale: a critical appraisal on its measurement properties using Rasch analysis. J Neurol Neurosurg Psychiatry. 2013;84:1340–1345. doi: 10.1136/jnnp-2012-304701. [DOI] [PubMed] [Google Scholar]
- 33.Franchignoni F, Mandrioli J, Giordano A, Ferro S ERRALS Group. A further Rasch study confirms that ALSFRS-R does not conform to fundamental measurement requirements. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:331–337. doi: 10.3109/21678421.2015.1026829. [DOI] [PubMed] [Google Scholar]
- 34.Chiò A, Hammond ER, Mora G, Bonito V, Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:38–44. doi: 10.1136/jnnp-2013-306589. [DOI] [PubMed] [Google Scholar]
- 35.Poletti B, Solca F, Carelli L, Madotto F, Lafronza A, Faini A, et al. The validation of the Italian Edinburgh Cognitive and Behavioural ALS Screen (ECAS) Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:489–498. doi: 10.1080/21678421.2016.1183679. [DOI] [PubMed] [Google Scholar]
- 36.Olsen ZL, Denton WJ. ALS: beyond the bucket. The ice bucket challenge was a sobering reminder of the deleterious effects of amyotrophic lateral sclerosis [Internet] Newtown Square (PA): Review of Optometry; [2014 Nov 15]. Available from: https://www.reviewofoptometry.com/article/als-beyond-the-bucket. [Google Scholar]
- 37.Argilés M, Cardona G, Pérez-Cabré E, Rodríguez M. Blink rate and incomplete blinks in six different controlled hard-copy and electronic reading conditions. Invest Ophthalmol Vis Sci. 2015;56:6679–6685. doi: 10.1167/iovs.15-16967. [DOI] [PubMed] [Google Scholar]
- 38.Kang BH, Kim JI, Lim YM, Kim KK. Abnormal oculomotor functions in amyotrophic lateral sclerosis. J Clin Neurol. 2018;14:464–471. doi: 10.3988/jcn.2018.14.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- 40.Yunusova Y, Plowman EK, Green JR, Barnett C, Bede P. Clinical measures of bulbar dysfunction in ALS. Front Neurol. 2019;10:106. doi: 10.3389/fneur.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptive statistics of the not-significant associations between optometric findings and use of an ETCD
Distribution of asymptomatic, mildly symptomatic, and severely symptomatic groups across MiToS. MiToS: Milano-Torino staging.
Descriptive statistics of the nonsignificant associations between optometric findings and functional and staging status
Descriptive statistics of the not-significant associations between optometric findings and cognitive status