Abstract
Background and Purpose
We aimed to determine the relationships of 33 biomarkers of inflammation, oxidation, and adipokines with the risk of progression of symptomatic intracranial atherosclerotic stenosis (ICAS).
Methods
Fifty-two of 409 patients who participated in the TOSS-2 (Trial of Cilostazol in Symptomatic Intracranial Stenosis-2) showed progression of symptomatic ICAS in magnetic resonance angiography at 7 months after an index stroke. We randomly selected 20 patients with progression as well as 40 age- and sex-matched control patients. We serially collected blood samples at baseline, 1 month, and 7 months after an index stroke. Multiplex analysis of biomarkers was then performed.
Results
Demographic features and risk factors such as hypertension, diabetes, and smoking history were comparable between the two groups. Univariate analyses revealed that the levels of platelet-derived growth factor (PDGF)-AA [median (interquartile range)=1.64 (0.76–4.57) vs. 0.77 (0.51–1.71) ng/mL], PDGF-AB/BB [10.31 (2.60–25.90) vs. 2.35 (0.74–6.70) ng/mL], and myeloperoxidase [10.5 (7.5–22.3) vs. 7.8 (5.5–12.2) ng/mL] at 7 months were higher in the progression group. In the multivariate analysis using logistic regression, the PDGF AB/BB level at 7 months was independently associated with the progression of ICAS (p=0.02).
Conclusions
The PDGF-AB/BB level is associated with the progression of ICAS, and so may play a significant role in the progression of human ICAS.
Keywords: platelet-derived growth factor, intracranial stenosis, ischemic stroke, magnetic resonance angiography
INTRODUCTION
Intracranial atherosclerotic stenosis (ICAS) is one of the main causes of ischemic stroke, especially among Asians, Hispanics, and Africans.1,2,3 In the WASID (Warfarin-Aspirin Symptomatic Intracranial Disease) trial, the rates of ischemic stroke in the territory of ICAS (50% to 99% stenosis) at 1 year were as high as 15% and 14% in the aspirin and warfarin arms, respectively.4 Since atherosclerotic plaques can change dynamically, ICASs may progress over time. Although the progression of ICAS is an important predictor of recurrent stroke,5,6 the mechanisms underlying this progression are still poorly understood.
Chronic inflammation in the arterial wall is a crucial feature associated with the development of atherosclerosis.7 Proatherogenic cytokines such as interleukin (IL)-1, IL-2, tumor necrosis factor (TNF)-α, and CD40 ligand (CD40L) were found to be positively associated with the progression of atherosclerosis in the cervical carotid arteries or coronary arteries, whereas antiatherogenic cytokines such as IL-1 receptor antagonist (IL-1ra), IL-6, and IL-10 had negative associations.8 A few studies have found associations between the progression of ICAS and cytokines including IL-6, C-reactive protein (CRP), and plasminogen activator inhibitor (PAI)-1.8,9
This prospective substudy of TOSS-2 (Trial of Cilostazol in Symptomatic Intracranial Stenosis-2)10 measured 33 biomarkers of inflammation, oxidation, and adipokines, with the aim of identifying their associations with the risk of progression of symptomatic ICAS.
METHODS
Initial TOSS-2 design
In TOSS-2, patients with symptomatic ICAS were randomly allocated to treatment with aspirin plus cilostazol or aspirin plus clopidogrel within 14 days of stroke onset.10 Follow-up magnetic resonance angiography (MRA) was performed 7 months after this allocation. The progression of symptomatic ICAS was indicated by changes in serial MRA findings. As reported previously, the severity of stenosis in both middle cerebral arteries (MCAs) was classified into five grades: normal, mild, moderate, severe, and occlusion.11 Progression was defined as worsening of stenosis by at least one grade in the follow-up MRA relative to the baseline MRA, whereas regression was defined as improvement of stenosis by at least one grade. The change in stenosis in MCAs was evaluated by gathering the raw data from magnetic resonance imaging and MRA data as DICOM files. Two raters blinded to clinical information and the location of symptomatic stenosis independently classified the degree of stenosis on MRA using Petaview, a noncommercial DICOM viewer program of Asan Medical Center. Discrepancies between the two reviewers were referred to the third rater and resolved by consensus among all three reviewers.
Fifty-two (12.7%) of 409 patients who completed follow-up MRA showed progression of symptomatic ICAS in follow-up MRA.
Collection and storage of blood samples
We collected serum from all patients who were included in TOSS-2 to evaluate the vascular risk factors at baseline, 1 month, and 7 months after an index stroke. Blood samples were collected by venipuncture from the participants into EDTA-containing vacutainer tubes after an overnight fast. The freshly drawn blood was centrifuged at 3,000g for 20 min at 4℃. Plasma was then separated before being stored at −70℃ in small aliquots. All analyses were performed on frozen plasma samples. We randomly selected 20 patients with progressive ICAS as well as 40 age- and sex-matched controls without progressive ICAS, and analyzed their sera. The study protocol was approved by the Institutional Review Board of Dongguk University Ilsan Hospital. All participants or their legal representatives gave written informed consents.
Cytokine assay
Multianalyte profiling was performed on the Luminex-100 system and the XY Platform (Luminex Corporation, Austin, TX, USA). Calibration microspheres for classification and reporter readings as well as sheath fluid were also purchased from Luminex Corporation. The acquired fluorescence data were analyzed using the MasterPlex™ QT software (version 1.2, MiraiBio, Alameda, CA, USA). Plasma concentrations of fractalkine, IL-1a, IL-1b, IL-1ra, IL-2, IL-6, IL-8, IL-10, monocyte chemoattractant protein (MCP)-1, MCP-3, macrophage derived chemokine (MDC), sCD40L, TNF-α, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-AA, PDGF-AB/BB, serum amyloid A (SAA), matrix metalloproteinase (MMP)-9, E-selectin, intercellular adhesion molecule (ICAM)-1, vascular-cell adhesion molecule (VCAM)-1, and PAI-1 were determined by the Millipore 8-Plex panel (Millipore, Burlington, MA, USA). P-selectin, MMP-2, and MMP-3 were measured using the R&D 8-Plex panel (R&D Systems, Minneapolis, MN, USA). Myeloperoxidase (MPO) and S100 beta were analyzed using the Youngin Frontier 6-Plex panel (Youngin Frontier, Seoul, Korea). All analyses were performed in accordance with the manufacturers' protocols.
Statistical analysis
All statistical tests were two-sided, and the cutoff for significance was set at 0.05. Continuous variables are expressed as mean±standard-deviation or median (interquartile range) values, as appropriate, and categorical variables are described by numbers and percentages. Statistically significant intergroup differences was detected using the chi-square test for categorical variables and Student's t-test or the Mann-Whitney U test for continuous variables as appropriate.
Multivariate analyses were performed to examine the independent relationships between biomarkers and the progression of symptomatic ICAS. We included variables for which p<0.10 in the univariate analysis and covariates that were potentially associated with the progression of stenosis, including the degree of stenosis, statin use, and baseline low-density lipoprotein (LDL)-cholesterol level. We further divided patients into tertiles and then reran the analyses. All analyses were performed using the SAS statistical software package (version 9.1, SAS Institute, Cary, NC, USA).
RESULTS
The baseline characteristics of the subjects were similar in the progression and control groups (Table 1). The age at onset was 62.8±11.8 years in the progression group and 64.7±11.0 years in the control group. Vascular risk factors such as hypertension, diabetes, smoking, family history of stroke, and hyperlipidemia were comparable between the two groups. The severity of the symptomatic stenosis and the baseline neurological severity did not differ between the groups. There were also no intergroup differences in the baseline blood LDL-cholesterol, high-density lipoprotein (HDL)-cholesterol, glucose, CRP, and hemoglobin A1c (HbA1c) levels. Medication allocation to cilostazol versus clopidogrel occurred equally frequently in the two groups. In addition, the frequency of statin treatment was comparable between the two groups.
Table 1. Baseline characteristics of the study population.
| Parameter | Progression (n=20) | Controls (n=40) | p |
|---|---|---|---|
| Age, years | 62.8±11.8 | 64.7±11.0 | 0.53 |
| Sex, male | 10 (50) | 21 (52.5) | 0.86 |
| Hypertension | 12 (60) | 32 (80) | 0.10 |
| Diabetes | 8 (40) | 15 (37.5) | 0.85 |
| Smoking | 9 (45) | 18 (45) | >0.99 |
| Family history of stroke | 5 (25) | 8 (20) | 0.66 |
| Hyperlipidemia | 10 (50) | 20 (50) | >0.99 |
| NIHSS score | 3 (2–7) | 3 (2–6) | 0.29 |
| LDL-cholesterol, mg/dL | 120.9±30.8 | 117.6±48.7 | 0.79 |
| HDL-cholesterol, mg/dL | 42.6±13.4 | 42.6±11.0 | 0.99 |
| Glucose, mg/dL | 151.3±61.8 | 152.4±65.9 | 0.96 |
| CRP, mg/dL | 0.33±0.36 | 0.29±0.49 | 0.78 |
| HbA1c, % | 7.15±2.18 | 6.52±1.61 | 0.21 |
| Degree of stenosis | 0.56* | ||
| Mild | 6 (30) | 7 (18) | |
| Moderate | 11 (55) | 24 (60) | |
| Severe | 3 (15) | 9 (22) | |
| Medication: aspirin+cilostazol | 10 (50) | 20 (50) | >0.99 |
Data are mean±standard deviation, number (percentage), or median (IQR) values.
*Fisher's exact test was used.
HDL: high-density lipoprotein, IQR: interquartile range, LDL: low-density lipoprotein, NIHSS: National Institutes of Health Stroke Scale.
None of the analyzed cytokines differed at baseline or after 1 month. However, at 7 months the PDGF-AA, PDGF-AB/BB, and MPO levels were higher in the progression group than in control group (Table 2). Additional candidates for inclusion in the multivariate model, with p values of 0.05–0.10, were higher ICAM-1, PDGF-AB/BB, and P-selectin levels at baseline, and a higher P-selectin level at 7 months (Table 2).
Table 2. Cytokine levels at baseline, 1 months, and 7 months.
| Cytokine | Time point | Progression (n=20) | Control (n=40) | p |
|---|---|---|---|---|
| Fractalkine, pg/mL | Baseline | 1.86 (0–63.66) | 6.23 (0–41.03) | 0.93 |
| 1 month | 0 (0–22.73) | 13.89 (0–44.56) | 0.11 | |
| 7 months | 2.91 (0–15.32) | 18.04 (0–59.86) | 0.24 | |
| IL-1a, pg/mL | Baseline | 0 (0–14.89) | 0 (0–11.96) | 0.66 |
| 1 month | 0 | 0 (0–14.99) | 0.62 | |
| 7 months | 0 | 0 (0–15.83) | 0.30 | |
| IL-1b, pg/mL | Baseline | 1.36 (0–1.88) | 0.73 (0.34–2.15) | 0.96 |
| 1 month | 1.13 (0.93–2.10) | 0.94 (0.29–1.88) | 0.93 | |
| 7 months | 1.58 (0.50–2.27) | 1.0 (0–1.74) | 0.14 | |
| IL-1ra, pg/mL | Baseline | 7.58 (0–75.63) | 8.92 (0–37.18) | 0.76 |
| 1 month | 5.81 (0–28.67) | 6.28 (0–24.29) | 0.96 | |
| 7 months | 11.98 (0.17–48.43) | 5.63 (0–28.46) | 0.22 | |
| IL-2, pg/mL | Baseline | 0.03 (0–3.3) | 0.91 (0–4.91) | 0.38 |
| 1 month | 0 (0–1.53) | 0.81 (0–4.47) | 0.13 | |
| 7 months | 0.28 (0–2.75) | 0.75 (0–3.99) | 0.51 | |
| IL-6, pg/mL | Baseline | 0 (0–26.13) | 0.46 (0–11.22) | 0.71 |
| 1 month | 0 (0–0) | 0 (0–14.07) | 0.13 | |
| 7 months | 0 (0–4.23) | 0.24 (0–8.37) | 0.56 | |
| IL-8, pg/mL | Baseline | 7.65 (4.92–14.18) | 6.25 (4.75–9.39) | 0.33 |
| 1 month | 5.5 (4.71–10.82) | 6.06 (4.7–10.45) | 0.98 | |
| 7 months | 7.11 (4.89–12.53) | 6.13 (5.02–10.29) | 0.57 | |
| IL-10, pg/mL | Baseline | 2.34 (0–5.25) | 1.71 (0.43–4.91) | 0.83 |
| 1 month | 1.93 (0–5.20) | 1.37 (0–3.78) | 0.75 | |
| 7 months | 2.04 (0.10–10.6) | 1.75 (0.27–4.43) | 0.56 | |
| MCP-1, pg/mL | Baseline | 290.1 (224.8–346.8) | 275.2 (239.23–338.0) | 0.93 |
| 1 month | 288.3 (202.3–364.0) | 240 (182.2–324.8) | 0.22 | |
| 7 months | 271.4 (220.1–327.4) | 285.1 (228.8–340.8) | 0.68 | |
| MCP-2, pg/mL | Baseline | 6.62 (6.09–8.08) | 6.67 (6.21–7.10) | 0.93 |
| 1 month | 6.35 (6.17–7.24) | 6.55 (6.16–7.04) | 0.41 | |
| 7 months | 6.60 (6.26–6.94) | 6.61 (5.96–7.34) | 0.88 | |
| MDC, pg/mL | Baseline | 1559.5 (812–2183) | 1335.7 (938–1629) | 0.24 |
| 1 month | 1045 (787–1640) | 1330.9 (916–1715) | 0.65 | |
| 7 months | 1365 (928–1877) | 1400 (1058–2018) | 0.34 | |
| sCD40L, ng/mL | Baseline | 2.07 (0.84–20.35) | 1.84 (0.54–567.7) | 0.26 |
| 1 month | 1.95 (0.53–5.59) | 1.79 (0.88–4.36) | 0.86 | |
| 7 months | 3.00 (0.69–27.62) | 1.52 (0.72–6.04) | 0.24 | |
| TNF-α, pg/mL | Baseline | 4.38 (2.77–5.46) | 3.82 (2.76–4.73) | 0.26 |
| 1 month | 4.53 (2.66–5.89) | 3.91 (3.07–4.99) | 0.68 | |
| 7 months | 4.24 (3.08–5.45) | 4.0 (3.13–5.07) | 0.78 | |
| VEGF, pg/mL | Baseline | 15.58 (0–105.8) | 9.47 (0–42.4) | 0.50 |
| 1 month | 0 (0–28.9) | 0 (0–26.8) | 0.96 | |
| 7 months | 13.1 (0–66.3) | 0 (0–28.7) | 0.27 | |
| PDGF-AA, ng/mL | Baseline | 1.76 (0.75–4.16) | 1.204 (0.66–2.39) | 0.29 |
| 1 month | 1.04 (0.69–1.99) | 0.88 (0.48–1.61) | 0.28 | |
| 7 months | 1.64 (0.76–4.57) | 0.77 (0.51–1.71) | 0.006* | |
| PDGF-AB/BB, ng/mL | Baseline | 7.0 (2.40–2.22) | 3.40 (1.62–9.30) | 0.09 |
| 1 month | 2.58 (1.51–7.72) | 2.50 (1.14–5.90) | 0.54 | |
| 7 months | 10.31 (2.60–25.90) | 2.35 (0.74–6.70) | 0.004* | |
| SAA, ng/mL | Baseline | 2982.0 (1001.2–8596.2) | 3130.5 (1190–7218.5) | 0.89 |
| 1 month | 1875.0 (140.0–6298.7) | 2242.0 (762.5–5281.7) | 0.42 | |
| 7 months | 2664.5 (412.0–4371.3) | 2052.5 (796.3–7062.5) | 0.70 | |
| E-selectin, ng/mL | Baseline | 40.4 (34.6–48.7) | 41.4 (37.1–47.1) | 0.94 |
| 1 month | 41.7 (36.1–49.5) | 41.5 (36.7–47.2) | 0.94 | |
| 7 months | 42.3 (39.2–50.9) | 41. 3 (38.2–48.26) | 0.42 | |
| ICAM-1, ng/mL | Baseline | 92.1 (65.3–114.2) | 103.6 (84.1–143.8) | 0.08 |
| 1 month | 90.0 (68.5–112.7) | 99.3 (64.0–136.2) | 0.83 | |
| 7 months | 93.5 (64.5–114.2) | 99.7 (67.3–144.8) | 0.63 | |
| VCAM-1, ng/mL | Baseline | 551.4 (481.3–680.8) | 582.2 (478.6–766.8) | 0.49 |
| 1 month | 585.1 (518.3–792.7) | 603.0 (514.0–714.0) | 0.66 | |
| 7 months | 627.1 (524.6–682.9) | 599.4 (515.1–673.7) | 0.85 | |
| PAI-1, ng/mL | Baseline | 17.0 (12.5–27.2) | 16.9 (11.8–24.9) | 0.89 |
| 1 month | 13.6 (9.8–20.8) | 15.3 (10.3–21.5) | 0.60 | |
| 7 months | 19.9 (13.0–32.4) | 18.3 (11.3–27.4) | 0.43 | |
| P-selectin, pg/mL | Baseline | 41697.7 (28153.4–73949.5) | 32490.8 (24769.8–38011.8) | 0.06 |
| 1 month | 32620.0 (20263.9–45428.2) | 30852.6 (24503.3–38460.2) | >0.99 | |
| 7 months | 49919.0 (28343.0–85196.9) | 33103.0 (24532.9–42906.6) | 0.06 | |
| MMP–2, ng/mL | Baseline | 124.4 (100.6–226.7) | 130.8 (110.4–275.1) | 0.57 |
| 1 month | 145.1 (112.9–305.3) | 134.3 (112.7–213.8) | 0.80 | |
| 7 months | 133.5 (107.8–269.8) | 169.5 (118.4–352.4) | 0.27 | |
| MMP-3, ng/mL | Baseline | 4.8 (3.3–8.3) | 5.9 (4.0–8.4) | 0.41 |
| 1 month | 5.2 (3.5–10.2) | 6.0 (4.0–8.3) | 0.55 | |
| 7 months | 5.6 (3.1–8.7) | 6.9 (4.3–9.2) | 0.29 | |
| MPO, ng/mL | Baseline | 10.5 (6.7–37.3) | 7.8 (5.4–13.4) | 0.13 |
| 1 month | 11.7 (5.2–17.0) | 9.3 (5.2–12.6) | 0.42 | |
| 7 months | 10.5 (7.5–22.3) | 7.8 (5.5–12.2) | 0.014* | |
| MMP-9, ng/mL | Baseline | 63.1 (28.4–117.2) | 44.1 (27.2–84.5) | 0.29 |
| 1 month | 41.8 (27.0–60.4) | 43.7 (31.6–56.9) | 0.94 | |
| 7 months | 63.3 (36.0–111.9) | 41.5 (25.8–67.4) | 0.09 | |
| S100 beta, ng/mL | Baseline | 4.1 (1.1–10.3) | 1.3 (0.8–9.4) | 0.24 |
| 1 month | 2.1 (1.0–6.9) | 1.3 (0.6–3.1) | 0.15 | |
| 7 months | 2.0 (0.9–7.9) | 1.2 (0.6–2.2) | 0.15 |
Data are median (IQR) values.
*p<0.05.
CD40L: CD40 ligand, ICAM: intercellular adhesion molecule, IL: interleukin, MCP: monocyte chemoattractant protein, MDC: macrophage derived chemokine, MMP: matrix metalloproteinase, MPP: myeloperoxidase, PAI: plasminogen activator inhibitor, PDGF: platelet-derived growth factor, SAA: serum amyloid A, TNF: tumor necrosis factor, VCAM: vascular-cell adhesion molecule, VEGF: vascular endothelial growth factor.
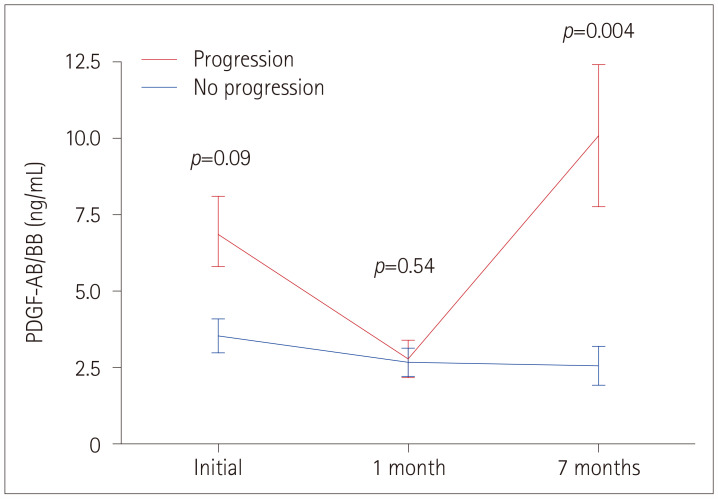
Multivariate analysis using logistic regression identified the PDGF-AB/BB level at 7 months as the single cytokine factor that was independently associated with the progression of ICAS (p=0.02) (Table 3). Considering the ranges of PDGF-AB/BB levels, patients in the highest tertile at 7 months had a substantially higher probability of progression than those in the lowest tertile, with an adjusted odds ratio of 6.93 (95% confidence interval=1.53–31.38, p=0.01). The evolutions of PDGF-AB/BB levels over time in progressors and control subjects are shown in Fig. 1.
Table 3. Results from the multivariate analysis of the progression of symptomatic intracranial atherosclerotic stenosis.
| Parameter | Value† | p* |
|---|---|---|
| Continuous PDGF-AB/BB at 7 months, per 1-ng/mL increase | 1.07 (1.02–1.13) | 0.02 |
| Tertiles of PDGF-AB/BB at 7 months | ||
| First (<1.63 ng/mL) | Reference | |
| Second (1.63–7.16 ng/mL) | 2.43 (0.51–11.51) | 0.26 |
| Third (>7.16 ng/mL) | 6.93 (1.53–31.38) | 0.01 |
Data are adjusted odds ratio (95% confidence interval) values.
*Adjusted for P-selectin and MPO at 7 months, †Odds ratio was calculated in separate models for continuous and categorical PDGF-AB/BB.
MPO: myeloperoxidase, PDGF: platelet derived growth factor.
Fig. 1. Comparison of PDGF-AB/BB levels between the progression and control groups. Data are mean and standard-deviation values. PDGF: platelet derived growth factor.
DISCUSSION
This study found an independent association between the PDGF-AB/BB level at 7 months after an index stroke and the progression of ICAS. Patients in the highest tertile of PDGF-AB/BB levels at 7 months had a sevenfold higher probability of progression of ICAS compared with the lowest tertile. Other atherogenic cytokines (e.g., IL-1, IL-2, IL-6, TNF-α, fractalkine, MCP-1, and CD40L) and antiatherogenic cytokines (e.g., IL-1ra, and IL-10) were not associated with the progression of ICAS. To our knowledge, this is the first study to investigate and demonstrate an association between PDGF-AB/BB and ICAS progression.
PDGF plays a significant role in the formation of blood vessels (angiogenesis) and their growth. PDGF is a potent mitogen for cells of mesenchymal origin, including smoothmuscle cells, and is produced by platelets, smooth-muscle cells, activated macrophages, and endothelial cells.12 PDGF exists as a disulfide-linked dimer and comprises two chains: A and B.13 Two receptors for PDGF, called α and β, have been identified.14 Binding of PDGF to the receptor induces receptor dimerization and activation of the kinase activity. The PDGF-α receptor binds both PDGF-A and -B chains, whereas the PDGF-β receptor binds only the PDGF-B chain.15 Blockade of the PDGF-β receptor (but not the PDGF-α receptor) prevents the accumulation of vascular smooth-muscle cells in apolipoprotein-E-knockout mice.16 These results indicate that the PDGF-β receptor plays an important role in the development of fibrous atherosclerotic lesions and that the regulation of signal transduction via the PDGF-β receptor could affect atherosclerosis in mice.16 Additional support for this mechanism comes from a study finding that a PDGF inhibitor, imatinib, attenuates diabetes-associated atherosclerosis.17 In addition to direct atherogenic effects, several studies have suggested that PDGF is an important mediator of arterial remodeling,18,19,20,21 which may indirectly contribute to the progression of stenosis.
In addition to identifying that PDGF-AA/BB is independently associated with ICAS progression, this study is notable for not finding a strong link between other candidate cytokines and further vessel narrowing. Cytokines such as TNF-α, IL-2, IL-3, IL-6, IL-8, IL-10, and interferon-γ are expressed in human atherosclerotic plaques and have been reported to play roles in the progression of atherosclerosis.7 With respect to the extracranial carotid artery, there are several reports of atherosclerosis being associated with cytokine profiles. Plasma CRP and TNF-α levels are independently associated with the risk of cardiovascular events in patients with atherosclerotic occlusive disease,22 and serum levels of IL-8, IL-6, and MCP-1 have been found to be related to the severity of carotid atherosclerosis.23,24,25,26 Furthermore, experimental studies have shown that mitigating these inflammatory cytokines impedes the progression of atherosclerosis.23,24 There is also evidence of associations between cytokines and coronary artery disease.27,28,29 Compared with extracranial arteries of similar sizes, intracranial arteries have a thinner tunica muscularis, tunica adventitia, and internal elastic lamina.3
Clinical studies have indicated that the risk factors, progression rate, and outcomes may differ between coronary/carotid arteries and intracranial arteries. Thus, the aforementioned cytokines may influence the progression of ICAS in different ways; alternatively, the number of patients and the follow-up period in the present study might have been insufficient to reveal the true relationships.
This study had several limitations. First, the sample was small and the follow-up was short, and so the obtained results need to be confirmed in larger patient cohorts. Second, although our results showed an association of the PDGF-AB/BB level with progression of symptomatic intracranial large-artery atherosclerosis, the findings should be interpreted with caution since the PDGF-AB/BB level was found to be lower at 1 month after study initiation. Different inflammatory responses occur during the acute, subacute, and chronic periods of stroke, and so the secretion of cytokines may have been affected accordingly.30 Further research is needed into this. Third, we were unable to acquire blood samples from all of the TOSS-2 participants. Although we randomly selected the study population, this limitation might have resulted in selection bias. Fourth, there are other subtypes of PDGF, including PDGF-CC and PDGF-DD; however, this study only analyzed the AA and BB subtypes that have been reported to be associated with atherosclerosis.
In conclusion, the PDGF-AB/BB level is associated with the progression of symptomatic ICAS, suggesting that PDGF-AB/BB plays a role in the progression of human ICAS. Although the causal relationship remains to be elucidated, this result may have clinical implications, and it should be investigated in further studies of PDGF-AB/BB.
Acknowledgements
Korea Otsuka Pharmaceutical (KOP) Company, Korea Otsuka International Asia, and Arab Co, Ltd. provided financial support for the TOSS-2 study. However, these organizations played no role in protocol development, data collection, analysis, or manuscript preparation for the current study.
This work was also supported by the Dongguk University Research Fund of 2015.
Footnotes
- Conceptualization: Sang Wuk Jeong, Wi-Sun Ryu.
- Data curation: Kyeong Joon Kim, Wi-Sun Ryu.
- Formal analysis: Wi-Sun Ryu.
- Funding acquisition: Sang Wuk Jeong.
- Investigation: Sang Wuk Jeong, Wi-Sun Ryu.
- Methodology: Sang Wuk Jeong, Wi-Sun Ryu.
- Project administration: Sang Wuk Jeong.
- Supervision: Sun U. Kwon.
- Writing—original draft: Kyeong Joon Kim, Wi-Sun Ryu.
- Writing—review & editing: all authors.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Gorelick P, Han J, Huang Y, Wong KS. Epidemiology. In: Kim JS, Caplan LR, Wong KS, editors. Intracranial Atherosclerosis. West Sussex, UK: Wiley-Blackwell; 2008. pp. 33–44. [Google Scholar]
- 2.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 3.Moossy J. Morphology, sites and epidemiology of cerebral atherosclerosis. Res Publ Assoc Res Nerv Ment Dis. 1966;41:1–22. [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 5.Arenillas JF, Molina CA, Montaner J, Abilleira S, Gonzalez-Sanchez MA, Alvarez-Sabin J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898–2904. doi: 10.1161/hs1201.099652. [DOI] [PubMed] [Google Scholar]
- 6.Wong KS, Li H, Lam WW, Chan YL, Kay R. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke. 2002;33:532–536. doi: 10.1161/hs0202.102602. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 8.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K, Shimomura K, Tokuyama Y, Sakurai K, Isahaya K, Takaishi S, et al. Association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:211–217. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Kwon SU, Hong KS, Kang DW, Park JM, Lee JH, Cho YJ, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42:2883–2890. doi: 10.1161/STROKEAHA.110.609370. [DOI] [PubMed] [Google Scholar]
- 11.Kwon SU, Cho YJ, Koo JS, Bae HJ, Lee YS, Hong KS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–786. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 12.Heldin CH, Westermark B. Platelet-derived growth factor: three isoforms and two receptor types. Trends Genet. 1989;5:108–111. doi: 10.1016/0168-9525(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 13.Heldin CH, Westermark B, Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979;76:3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 15.Hart CE, Forstrom JW, Kelly JD, Seifert RA, Smith RA, Ross R, et al. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 16.Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, et al. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103:2955–2960. doi: 10.1161/01.cir.103.24.2955. [DOI] [PubMed] [Google Scholar]
- 17.Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:935–942. doi: 10.1161/01.ATV.0000124105.39900.db. [DOI] [PubMed] [Google Scholar]
- 18.Sakao S, Tatsumi K. Vascular remodeling in pulmonary arterial hypertension: multiple cancer-like pathways and possible treatment modalities. Int J Cardiol. 2011;147:4–12. doi: 10.1016/j.ijcard.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Hannink M, Donoghue DJ. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta. 1989;989:1–10. doi: 10.1016/0304-419x(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 21.Hyacinth HI, Gee BE, Adamkiewicz TV, Adams RJ, Kutlar A, Stiles JK, et al. Plasma BDNF and PDGF-AA levels are associated with high TCD velocity and stroke in children with sickle cell anemia. Cytokine. 2012;60:302–308. doi: 10.1016/j.cyto.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kablak-Ziembicka A, Przewlocki T, Sokołowski A, Tracz W, Podolec P. Carotid intima-media thickness, hs-CRP and TNF-α are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis. 2011;214:185–190. doi: 10.1016/j.atherosclerosis.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Hu ZJ, Liao Y, Hu LY, Wang JL, Li J, Lu WM, et al. Effects of antiatherosclerosis in carotid artery by RNAi-mediated silencing of MCP-1 expression. Ann Vasc Surg. 2009;23:652–662. doi: 10.1016/j.avsg.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Ge S, Zhou G, Cheng S, Liu D, Xu J, Xu G, et al. Anti-atherogenic effects of montelukast associated with reduced MCP-1 expression in a rabbit carotid balloon injury model. Atherosclerosis. 2009;205:74–79. doi: 10.1016/j.atherosclerosis.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Jeong MH, Bae HR, Jeong SJ, Jang JY, Lim YJ, et al. Circulating levels of interleukin-8 and vascular endothelial growth factor in patients with carotid stenosis. J Korean Med Sci. 2001;16:198–203. doi: 10.3346/jkms.2001.16.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauramaa R, Vaisanen SB, Luong LA, Schmidt-Trucksass A, Penttila IM, Bouchard C, et al. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2657–2662. doi: 10.1161/01.atv.20.12.2657. [DOI] [PubMed] [Google Scholar]
- 27.Christenson RH, deFilippi CP, Kreutzer D. Biomarkers of ischemia in patients with known coronary artery disease: do interleukin-6 and tissue factor measurements during dobutamine stress echocardiography give additional insight? Circulation. 2005;112:3215–3217. doi: 10.1161/CIRCULATIONAHA.105.581918. [DOI] [PubMed] [Google Scholar]
- 28.Ikonomidis I, Athanassopoulos G, Lekakis J, Venetsanou K, Marinou M, Stamatelopoulos K, et al. Myocardial ischemia induces interleukin-6 and tissue factor production in patients with coronary artery disease: a dobutamine stress echocardiography study. Circulation. 2005;112:3272–3279. doi: 10.1161/CIRCULATIONAHA.104.532259. [DOI] [PubMed] [Google Scholar]
- 29.Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619–628. doi: 10.1161/CIRCIMAGING.113.001193. [DOI] [PubMed] [Google Scholar]
- 30.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]