Abstract
Rotator cuff tears continue to be at risk of retear or failure to heal after surgical repair, despite the use of various surgical techniques, which stimulate development of novel scaffolding strategies. They should be able to address the known causes of failure after the conventional rotator cuff repair: (1) failure to reproduce the normal tendon healing process, (2) resultant failure to reproduce four zones of the enthesis, and (3) failure to attain sufficient mechanical strength after repair. Nanofiber scaffolds are suited for this application because they can be engineered to mimic the ultrastructure and properties of the native rotator cuff tendon. Among various methods for tissue-engineered nanofibers, electrospinning has recently been highlighted in the rotator cuff field. Electrospinning can create fibrous and porous structures that resemble natural tendon's extracellular matrix. Other advantages include the ability to create relatively large surface-to-volume ratios, the ability to control fiber size from the micro to the nano scale, and the flexibility of material choices. In this review, we will discuss the anatomical and mechanical features of the rotator cuff tendon, their potential impacts on improper healing after repair, and the current knowledge of the use of electrospinning for rotator cuff tissue engineering.
Keywords: Rotator Cuff, Tissue Scaffolds, Nanofibers, Tissue Engineering
INTRODUCTION
Rotator cuff tears represent a major component of the family of tendon disorders. Over 4.8 million people each year visit hospitals for shoulder pain and rotator cuff pathologies which contribute most to shoulder pain.1 The prevalence of rotator cuff tears is 34% in the general population and reaches up to 54% in the elderly populations (those over 60 years of age).2,3 Accordingly, rotator cuff repair is one of the most common orthopedic procedures among athletes, workers, and even the adult population in general with over 300,000 rotator cuff repairs performed per year in the United States.4 Rotator cuff tears also have a similar impact on individual quality of life as diseases such as congestive heart failure, diabetes, and depression.5
The rotator cuff tendon plays a significant role in shoulder biomechanics and is predisposed to injury and degenerative changes because of its location and blood supply.6 Unfortunately, a natural history of rotator cuff tears shows that asymptomatic rotator cuff tears will become symptomatic and the size of the tear will progress with time.7 Furthermore, the possibility of spontaneous healing is low. Fukuda8 reported that histologic section of partial tears showed no evidence of active tissue repair. Safran et al.9 followed 51 full-thickness tears by ultrasound and found increases in tear size of 49% after mean 29-months. Maman et al.10 also showed tear progression is more likely to occur in patients with longer than 18 months of follow-up time. Therefore, small full-thickness tears can progress to large or massive tears over time. However, massive rotator cuff tears are known to alter glenohumeral kinematics leading to humeral head migration, pseudoparalysis of the shoulder, and cuff tear arthropathy as the end stage. The current strategy of surgical treatment is repairing the torn rotator cuff tendon. By open or arthroscopic surgery, a retracted torn end of the rotator cuff is brought back and repaired to the original footprint of the humeral head. However, surgical management is challenging especially in a massive cuff tear and the re-rupture rate has been reported in the literature as between 11%11 and 94%.12 Such procedures are technically demanding and require a steep learning curve.13,14 Even though the surgeon's skill continues to improve, successful repair of large-to-massive tears especially with retraction and immobility is still challenging, and retear could continue to occur. Recently, Lim et al.15 in their learning curve analysis demonstrated that as a surgeon's experience of rotator cuff repair accumulated, retear rate successfully decreased in small to medium-sized tears, but not in large-to-massive tears. This may reflect the fact that successful healing of large or massive rotator cuff tears might be associated with many factors including biologic aspects of tendon-bone healing, other than only surgical skill-dependent factors such as release, mobilization, and strong fixation.
To overcome this, the use of biological16,17 and synthetic scaffolds18,19 have been introduced and were encouraging in preclinical studies. however, the results were not replicated in human studies.18,19,20,21,22 In particular, Smith et al.23 compared the macro and nano/micro mechanical properties of 7 clinically approved and commercially available scaffolds (X-Repair, LARS ligament, Poly-Tape, BioFiber, GraftJacket, Permacol, and Conexa) to those of the human supraspinatus tendons. Unfortunately, none of the scaffolds tested adequately approximated the macro- and micro-mechanical properties of the human supraspinatus tendon. This finding may explain that the lack of their efficacy in clinical trials is in part due to insufficient mechanical properties and also present the need for alternative new technologies, such as nanofibers, nano coatings, and 3-dimensional printing to create more specialized scaffolds.24 In particular, nanofibrous scaffolds are suited for this application because they can be engineered to mimic the ultrastructure and properties of the native tendon. A variety of methods have been used to produce tissue-engineered nanofibers including self-assembly, drawing temperature-induced phase separation, and perhaps most commonly electrospinning.25 Therefore, the aim of this review is to discuss the current state of knowledge in the field of nanofiber scaffolds focusing on electrospinning for rotator cuff tissue engineering.
STRUCTURE, COMPOSITION, AND MECHANICAL PROPERTIES OF ROTATOR CUFF TENDON
Rotator cuff tendons consist of the supraspinatus, infraspinatus, teres minor and subscapularis. As a group, these tendons support the rotation of shoulder joint and provide stability of humeral head.26 Each tendon has a specific role in controlling the movement, stability, and rotation of the shoulder. The supraspinatus supports arm abduction. The subscapularis is an internal rotator of the humerus, while the infraspinatus and teres minor are external rotators. Dynamic stabilization through rotator cuff muscle contraction and the resulting compression of the articular surfaces is one of the most important factors in ensuring shoulder stability and function.27
The four rotator cuff tendons at the humeral insertion are not distinct structures. Clark and Harryman28 demonstrated in their anatomic study that all four tendons fuse to form the common insertion on the tuberosities. Fibers from the subscapularis anteriorly and the infraspinatus posteriorly interdigitate with those of the supraspinatus. The interdigitation of fibers occurs primarily in the deep layers. The tendinous portion of the cuff is also confluent with the capsule of the shoulder joint and with the coracohumeral and glenohumeral ligaments, depicting five microscopic layers with a blend of fibers in the oblique, transverse, and longitudinal orientations.28 The widely splayed and interdigitated humeral insertions of these multiple structures: rotator cuff tendons, ligaments, and capsule by means of a mixture of different orientations, horizontally and vertically improve their resistance to failure under load and stress distribution.
On the other hand, rotator cuff tendons connect their muscles originating in the scapula to the humerus through tendon-bone insertions, so called, the enthesis. It consists of the transition of four distinct tissue zones: tendon, non-mineralized fibrocartilage, mineralized fibrocartilage, and bone. The tendon is composed of collagen type I, proteoglycan, decorin, and biglycan, which is filled with tendon cells aligned in the direction of tendon tension.29,30 The non-mineralized zone is filled with fibrochondrocytes, which produces the extracellular matrix (ECM) such as collagens type I and II, together with aggrecan.31 Mineralization and calcification begin in mineralized fibrous cartilage with a visible straight line called the tidemark that distinguishes the true transition between mineralized and non-mineralized tissues.32 Collagens type II and X and aggrecan are known to consist of mineralized fibrocartilage. The fourth zone corresponds to the bone and consists of a dense mineralized collagen type I matrix filled with bone cells (osteoblast, osteoclasts and osteocytes). The most important feature of this fibrocartilage transition region of the enthesis is a gradation in matrix mineralization increasing from tendon to bone. Such gradations along the interface are reflected in the cell phenotype, tissue organization, and mechanical properties.18,33,34 These natural gradations facilitate the effective transfer of load between the 2 materials by reducing the potentially damaging stress concentrations that would otherwise arise at more discrete interface.33 The collagen fibrils' mineral arrangement leads to a controlled and graded increase of stiffness, resulting in a smooth load transfer.35 As Derwin et al.36 conceptualize below, the mechanisms of stress transfer may be different according to fiber scales. On the millimeter scale, stresses can be reduced by optimizing the shape of the attachment.34,37 On the micrometer scale, interdigitation, fiber orientation, and a compliant band increase the toughness of the interface.38 On the nanometer scale, spatial gradients in mineralization stiffen the matrix.33 These multiscale features are consistent across fibrocartilaginous enthesis, including the rotator cuff, Achilles tendon, patellar tendon, flexor tendon, and meniscus root. However, the main problem is that none of these mechanisms are recreated after tendon-to-bone healing.39,40
LIMITATIONS OF THE CURRENT REPAIR STRATEGIES FOR ROTATOR CUFF HEALING
Limitations of the current repair strategies can be summarized into three categories: (1) failure to reproduce the normal tendon healing process, (2) resultant failure to reproduce the four zones of the enthesis, and (3) failure to attain sufficient mechanical strength after repair. Current rotator cuff repair techniques focus on securely attaching tendons to bones using sutures and bone anchors. The normal tendon healing process progresses in three overlapping stages41,42,43, including 1) inflammatory phase that collagen deposits and inflammatory cells migrate to the recovery site within 4-7 days. 2) a repair process when fibroblasts and tenocyte proliferate to form a temporary extracellular matrix composed of collagen type I and type III, and 3) as a final remodeling process that takes 6 to 8 weeks, forming oriented collagen type I. However, none of the current repair strategies replicates this normal healing process. The tissue formed after repair is of lower quality than the native rotator cuff tendon.42,44 Instead of four distinct zones, as in the native tendon, the repaired tendon is largely composed of fibrovascular scar tissue with a majority of type III collagen.45 During the remodeling stage, some of the type III collagen is remodeled to type I, resulting in a more organized matrix, but this remodeling often remains insufficient with conventional repair. The mechanical properties of fibrovascular scar tissue formed at the interface are not similar to those of normal tendon enthesis.36 Failure to recreate the structure and mechanics of the healthy enthesis may predispose the repair site to failure. The enthesis is a complex composite biomaterial junction that allows stress transfer between mechanically dissimilar materials29,36: the tensile modulus of tendon in the direction of muscle action is the order of 200 MPa, while that of bone is 20 GPa,33,46 resulting in the bone modulus being approximately 100 times more than that of tendon. Restoring hard–soft interfaces with such large mismatches in material properties can be clinically challenging. Initial mechanical strength after repair is another concern. As noted by Chainani and Little,47 to achieve adequate mechanical support immediately after repair, the constructs should aim to approximate the stiffness (~200 N/mm), modulus (~150 MPa) and ultimate load (~800 N) of the human rotator cuff combined with adequate suture retention properties.48,49 The scaffold properties should match those of the native tendon as closely as possible to consequently achieve long-term success.
ROTATOR CUFF TISSUE ENGINEERING
To overcome the high failure rate after rotator cuff repair and to enhance tendon-to-bone healing, tissue engineering has been investigated as a source of more effective repair. Tissue engineering is an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue formation.50 The ultimate goal of rotator cuff tissue engineering is to repair tissue defects by introducing an engineered tissue structure that can be recognized and used in the regeneration of the desired native rotator cuff tissue.51 Three main elements of tissue engineering field are cells, scaffolds, and signals.51 Scaffolds or extracellular matrix provides a “house52” or “mechanical platform51” for cells and can regulate the cellular function and behavior via cell–matrix interactions. The mechanical cues experienced by the cells are intimately related to the microstructure of the scaffold.52 The mechanical properties of this matrix scaffold influence stem cells differentiation into specific lineages (from neurogenic to osteogenic).53 Scaffolds facilitate cell penetration and provide an effective environment that supplies oxygen and nutrients to the cells, and so results in successful tissue regeneration. After the desired tissue is successfully regenerated, the scaffold is designed to break down naturally. Clinically, extracellular matrix scaffold allograft or xenograft patches have been used for mechanical augmentation of rotator cuff repair. These materials are expected to improve healing in the critical early postoperative period because they improve initial mechanical strength and contain bioactive molecules. However, they have limitations for various reasons, including poor cellularization, immunogenic responses, insufficient mechanical properties to support physiological loading postoperatively, and poor suture retention.17,23,54 Tissue engineering may overcome some of these limitations by offering a functional cell-based scaffold for rotator cuff repair. Specifically, nanofibrous scaffolds are suited for this application.
WHY ARE “NANOFIBERS” NEEDED?
To achieve the goal of engineering biomimetic, functional, and integrative scaffold systems for rotator cuff repair and augmentation, nanofiber scaffolds are promising. They can be manufactured by tissue engineering to mimic the ultrastructure and properties of the native tendon. Tendon structures are formed through aligned fiber bundles ranging in diameter from 20 nm for collagen fibrils to 3 mm for tertiary fiber bundles.55,56 Various fibrous structures constitute most ECM molecules in the nanoscale range that support cell adhesion and biological activity, and thus, fabricating scaffolds with an architecture that mimics that of ECM molecules in the nanoscale has been an active area of research in tissue engineering. The ideal scaffold for rotator cuff tendon recovery should be able to match the mechanical properties to meet the physiological needs of the native tendon. It needs to facilitate the host cell–mediated healing response by the ultrastructural organization similar to the native tendon. The scaffold must be able to integrate with the host tendon and surrounding bone tissue. In addition, the scaffold should be susceptible to remodelling so that new tissue can replace it gradually while physiologically relevant mechanical properties are maintained. Nanofibers are advantageous for tendon tissue engineering because of their superior biomimetic potential and physiological relevance. Currently, there are several methods to fabricate fiber-based structures, including wetspinning, microfluidic, and melt spinning. Among them, electrospinning is the most widely used strategy.55
ELECTROSPINNING
Electrospinning is a fiber production method used to draw a polymer solution or polymer melt down to a fiber diameter of some hundred nanometers using a strong electrical force. The process involves the application of a high voltage (tens of kilovolts) to an emitter (spinneret) containing a solution of the polymer to be electrospun.57 Above a certain voltage threshold, electrostatic charge breaks the surface tension of the released droplet, creating a liquid flow. The released stream quickly narrows due to evaporation of the solvent, forming fibers that are attracted to a grounded or counter electrode, which is used to collect the fibers. Electrospun fiber morphology and diameter can be controlled by adjusting several parameters that can be classified as ‘solution-related’, ‘process-related’ or related to ‘ambient conditions’. Solution-related parameters include the nature of the polymer and the solvent, the molecular weight of the polymer and the viscosity, as well as the surface tension and electroconductivity of the solution. Important parameters of the electrospinning process include the applied voltage, the flow rate of ejection of the polymer solution, the distance between the emitter and the collector, the angle of the emitter to the collector, and the type of collector used to harvest the fibers. The temperature and humidity of the electrospinning environment are also important. The size of the electrospun fiber can be nanoscale and the fiber can have a nanoscale surface texture, which can have different modes of interaction with other materials compared to macroscopic materials.58 The ultrafine fibers produced by electrospinning have two main properties: a very high surface-to-volume ratio and a relatively defect-free structure at the molecular level. The high surface area makes electrospun materials suitable for activities that require a high degree of physical contact, such as providing a site for chemical reactions or capturing particulate matter of small size by physical entanglement (filtration). Defect free fiber formation allows electrospun fibers to approach the theoretical maximum strength of the base material, opening up the possibility of creating composites exhibiting high mechanical performance.
The type of polymer can significantly affect the material properties of the electrospun scaffold: poly(lactic-coglycolic acid) and polylactic acid fibers generate very stiff scaffolds, polycaprolactone generates scaffolds that are less stiff, while collagen generates very compliant scaffolds.59,60 The stiffness of the electrospun fiber can be enhanced by cross-linking after electrospinning,60,61 or by incorporating other materials, such as nanodiamonds.62 Mechanical properties can also be altered by modulating fiber morphology and diameter and by controlling the alignment of the fibers. Electrospun fibers collected on a flat stationary collector form mats with randomly aligned fibers; while a rotating mandrel or drum collector generates mats with fibers whose extent of parallel alignment corresponds to the rate of rotation.63 These aligned fibers are strengthened anisotropically and become much stronger and stiffer in the direction of fiber alignment. Another benefit of controlling fiber alignment is that cells tend to attach, preferentially align, and deposit neomatrix along the fiber direction.63,64 Finally, generating fibers in the nanoscale range can enhance cell attachment and neomatrix formation, presumably due to fact that a single cell can attach to multiple fibers, which mimics cell attachment to natural biopolymers in native ECM.57
While the technology of electrospinning has been developed over several decades, its potential application for general tissue engineering was first reported in 2002.65 Since then, electrospun nanofibers have widely been tested as experimental constructs for tissue engineering research and have been proposed as scaffolds for not only the repair of tendons,66,67 but also bone,68 cartilage,69 meniscus,63 ligaments,70 liver,71 blood vessels,72 nerves,73 and for drug delivery.74
THE USE OF ELECTROSPINNING FOR ROTATOR CUFF TISSUE ENGINEERING
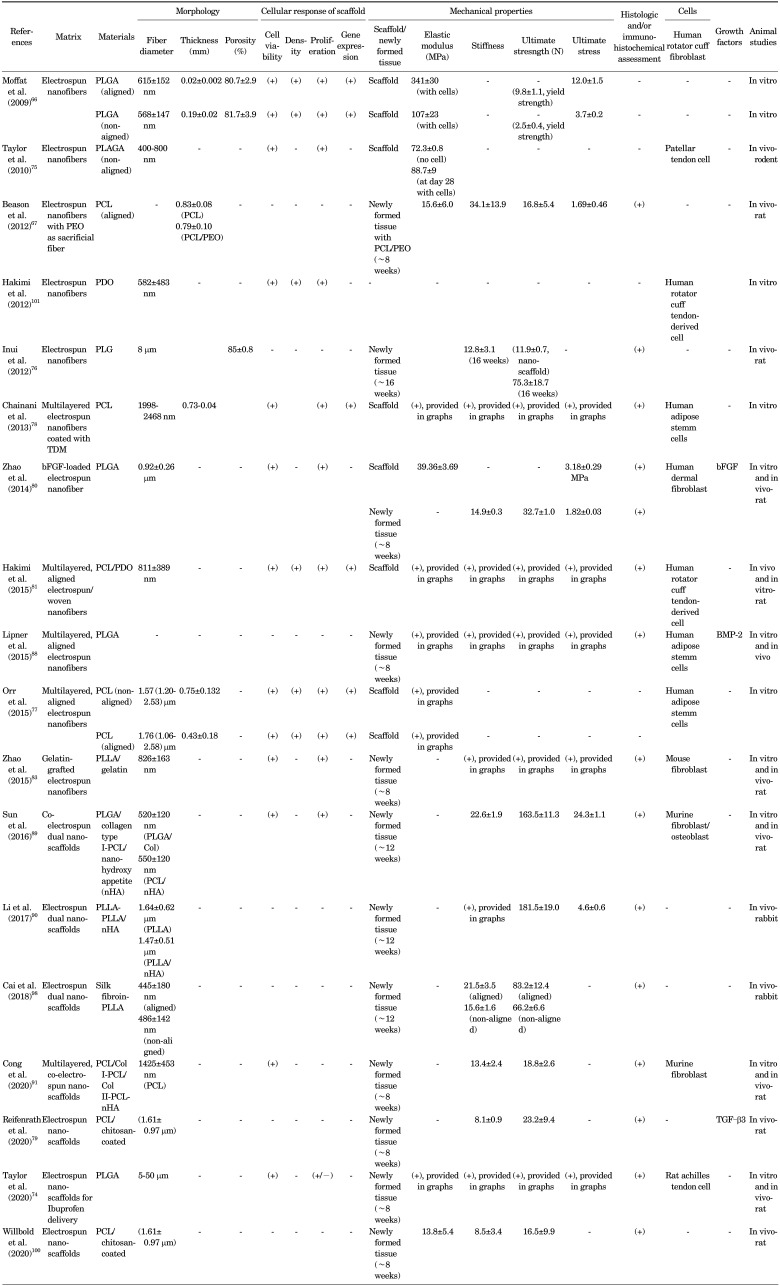
Electrospinning has recently been applied to the rotator cuff tissue engineering, as summarized in Table 1. In 2009, Moffat et al.66 first reported, to the best of our knowledge, the potential use of electrospun nanofiber-based scaffolds for rotator cuff tissue regeneration. They designed a poly (lactide-co-glycolide) (PLGA) nanofiber-based scaffold and then evaluated the attachment, alignment, gene expression, and matrix elaboration of human rotator cuff fibroblasts on the scaffolds. Taylor et al.75 later fabricated electrospun PLGA scaffolds and reported favorable in vitro results in terms of attachment, proliferation, and viability of human patellar tendon cells on the scaffolds. The rat supraspinatus tendons augmented with this scaffold in vivo also showed a higher Young's Modulus (48.6 MPa) than supraspinatus tendons repaired without scaffold (3.79 MPa). This novel material is a potential candidate as a scaffold for rotator cuff tendon healing, even with the use of nonaligned fibers.
TABLE 1. Summary of published studies on electrospun nanofibers for rotator cuff tissue engineering.
Inui et al.76 observed the restoration of rotator cuff enthesis using electrospun nanofibers without cells or growth factors. They fabricated a poly(D,L-lactide-co-glycolide) (PLG) scaffold and applied it in a rabbit rotator cuff defect model. It was quite promising that cells migrated into the “cell-free” PLG scaffold and deposited type II collagen and proteoglycan at the scaffold-bone junction at 16 weeks post operation, a promising result. The ultimate failure load and stiffness in the scaffold group were comparable to those in the reattachment group at each time point. However, although the distributions of types I, II, and III collagen and proteoglycan indicated that the scaffold could regenerate the tendon-bone insertion with fibrocartilage; it was not identical to the normal enthesis. The proteoglycan along the long axis of the tendon was not seen in both scaffold-augmented and reattachment group. This scaffold was also significantly weaker than an intact tendon at each time point. The disorganized distribution of the proteoglycan might be related to the use of non-aligned nanofibers in this study. Aligned fibers may enhance the organization of the proteoglycan to mimic the enthesis. Several aligned scaffolds have been shown to direct cell orientation as well as affect the expression of matrix proteins.66,67,77
As a distinct advantage, nanofiber organization and alignment can be modulated during fabrication,66,67,77 which allows for the structural and material properties of the scaffold to meet the functional demands of the rotator cuff tendons. In normal tendons, collagen bundles are oriented along the long axis of the tendon. Novel scaffolds, created predominantly by electrospinning, can mimic this orientation of collagen fibrils in the tendon, and in some cases the transition from aligned to random orientation at the tendon-to-bone insertion site.66,67 Moffat et al.66 specifically compared the outcomes between aligned and nonaligned fibers and showed that nanofiber organization has a significant effect on human rotator cuff fibroblast response. The structural anisotropy of the aligned and the isotropy of the unaligned scaffold directly guided cell attachment, integrin expression, and matrix deposition. This control of cell response resulted in a biomimetic matrix for rotator cuff repair on the aligned nanofiber scaffold, and physiologically relevant scaffold mechanical properties were maintained in vitro.66 This suggests that the aligned nanofiber scaffold has significant potential for tendon regeneration and represents a functional tissue-engineering solution for rotator cuff repair. To enhance alignment of electrospun fibers, Orr et al.77 combined a multilayered electrospinning technique with a hybrid of several electrospinning alignment techniques and fabricated multilayered aligned scaffolds with PCL polymers. They showed that both aligned and nonaligned multilayered scaffolds demonstrated cell infiltration and ECM deposition through the full thickness of the scaffold after only 28 days of culture. However, aligned scaffolds displayed significantly increased expression of tenomodulin compared to nonaligned scaffolds and exhibited aligned collagen fibrils throughout the full thickness, the presence of which may account for the increased yield stress and Young's modulus of cell-seeded aligned scaffolds along the axis of fiber alignment.77
Cell infiltration through the full thickness of the nanofibrous scaffold as well as control of cell growth and differentiation can be challenging. Chainani et al.78 addressed this issue with the use of a multilayered (nonaligned) electrospun scaffold with PCL and then coated it with tendon- derived extracellular matrix (TDM). Their products could permit cellular infiltration and formation of a tendon-like extracellular matrix by human adipose stem cells. Coating each side of the scaffolds with TDM enhanced the synthesis and accumulation of collagen. Therefore, nonaligned multilayered electrospun scaffolds allowed tenogenic differentiation by human adipose stem cells and the authors postulated that TDM might promote some aspects of this differentiation.
Strategies have been explored for enhancing the mechanical and tendon matrix properties of electrospun scaffolds as well as restoring the enthesis of the rotator cuff by a combination of natural polymers,24 growth factors,79,80 or woven fiber.81 Gelatin is a derivative of collagen obtained via controlled hydrolysis and is the major component of skin, bones, and connective tissues. As a natural polymer, it can provide biological signaling and cell adhesion, can be degraded by cells, and can be remodeled. However, it lacks adequate mechanical properties and tunable structure, limiting its applications.82 Therefore, Zhao et al.83 fabricated gelatin-grafted poly(L lactide) (PLLA) fibrous membranes using electrospinning. PLLA showed excellent biocompatibility and biodegradability and significantly increased the area of glycosaminoglycan and improved collagen organization at the tendon-bone interface. Biomechanical testing also revealed that the gelatin-PLLA group had a greater ultimate load to failure and stiffness at 4 and 8 weeks.
Zhao et al.80 used emulsion electrospinning to prepare bFGF-loaded PLGA electrospun fibrous membranes with a core-sheath structure. Electrospun fibers have the potential to increase the encapsulation efficiency, dalay the initial burst release and maintain bFGF bioactivity. Basic fibroblast growth factor (bFGF) can induce fibroblast collagenase production and capillary endothelial cell proliferation, which are required for neovascularization.84,85 After RC injury, bFGF is expressed by fibroblasts and inflammatory cells at the site of tendon repair and is associated with cell proliferation, cell migration, collagen production, and angiogenesis.32,86 At 2, 4, and 8 weeks after in vivo RCT repair surgery, electrospun fibrous membranes significantly increased the area of glycosaminoglycan staining at the tendon–bone interface compared with the control group, and bFGF–PLGA significantly improved collagen organization, as measured by birefringence under polarized light at the healing enthesis compared with the control and PLGA groups.80 The bFGF–PLGA membranes had the highest ultimate stress and stiffness of the healing enthesis, and their superiority compared to PLGA alone was significant.80
As a result of limitations in the electrospinning technique, conventional electrospun mats have inadequate thickness (related to the electrospinning technique) and low strength. Therefore, Hakimi et al.81 developed a non-destructive technique to stack and bond electrospun and non-electrospun layers, to enable assembly of more robust scaffolds. Using this technique, they fabricated a scaffold prototype made of electrospun and woven polydioxanone layers for the augmentation of rotator cuff repairs. The resulting scaffold presents a maximum suture pull out strength of 167 N, closely matching that of human rotator cuff tendons.81
Therapeutic applications are limited by poor healing between the rotator cuff tendon and bone. Two key features of this suboptimal healing are mineral loss in the bone adjacent to the tendon attachment and poorly organized collagen deposition at the repair site.39,87 Lipner et al.88 designed an aligned nanofibrous poly lactic co-glycolic acid scaffold with a gradient in mineral content which was seeded with adipose-derived stromal cells (ASCs) and implanted at the repair site of a rat rotator cuff model. In one group, cells were transduced with the osteogenic factor BMP2. However, histology showed that the healing interface was dominated by a fibrovascular scar response in all groups. When examining bone morphology parameters, bone loss was evident in the Cellular+BMP2 group compared to other groups at 28 days. When examining repair-site mechanical properties, strength and modulus were decreased in the Cellular+BMP2 groups as compared to other groups at 28 and 56 days. Unfortunately, tendon-to-bone healing in this animal model was dominated by scar formation, preventing any positive effects of the implanted biomimetic scaffold. Furthermore, cells transduced with the osteogenic factor BMP2 led to impaired healing. The authors concluded that this growth factor should not be used in the tendon-to-bone repair setting.88
Sun et al.89 fabricated a dual nanofibrous matrix for healing massive rotator cuff tears in rabbits for the enthesis regeneration. Their goal was to develop an ideal matrix that could support tendon and bone tissue regeneration simultaneously and bridge a massive rotator cuff tear. A coelectrospun dual nanofibrous matrix of cell-laden poly (lactic-coglycolic acid)/collagen I-polycaprolactone/nanohydroxyapatite (PLGA/Col-PCL/nHA) was farbricated. The PCL/nHA portion was inserted into bone tunnel while the PLGA/Col portion was wrapped around the tendon stump and bridged the gap between the stump and the bone. The study found that PLGA/Col-PCL/nHA matrices could support cell growth, spreading, viability, along with collagen secretion and osteoblast mineralization. After 12 weeks, tendon-like tissue and newly formed bone were observed in the matrix. The biocompatibility and biomechanical properties of the dual nanofibrous matrix, enhanced potential for tendon rotator cuff regeneration. A similar concept was used by Li et al.90 for rotator cuff enthesis regeneration in a rabbit model. A dual layer of nano hydroxyapatite-poly-L-lactic acid (nHA/PLLA) and poly-L lactic acid (PLLA) bipolar fibrous matrix was fabricated by electrospinning to mimic mineralized and non-mineralized fibrocartilage in the enthesis, respectively. At 8 and 12 weeks after implantation, the cartilage regeneration and collagen organization significantly improved at the enthesis compared to the control groups. Implantation of bipolar fibrous matrices significantly increased BMP-2 expression and induced bone formation. After 12 weeks, the biomechanical properties of the treated group were statistically higher than the control groups. Cong et al.91 advanced this concept with a triple-layered “enthesis-mimicking” scaffold structure, which consisted of aligned PCL/collagen type I for top layer and tendon portion, non-aligned PCL/collagen type II for the middle layer, and non-aligned PCL/nanohydroxyapatite crystal for the bottom layer. As a result, the enthesismimicking scaffold group had a higher graft-bone healing score and significantly more newly formed fibrocartilage than the control group. An enthesis-like structure with transitional layers was observed in the EM group at 8 weeks. The ultimate failure load and stiffness of newly formed tissues at the tendon-graft-bone interface were significantly higher in the EM group than in the control group at 8 weeks.
Another key factor for a successful incorporation of tissue- engineered constructs is a rapid ingrowth of cells and tissues to regenerate the tendon-bone-transition.92 Ingrowth of cells and tissues strongly depend on a rapid vascularization of the implant material for transporting nutrients, growth factors, and supporting gas exchange and removal of waste materials. Electrospun nanofiber scaffolds can be potential candidates to fulfill these criteria. Gniesmer et al.92 tested chitosan-graft-PCL coated electrospun PCL (CS-g-PCL) fiber mats in vivo. Previously, aligned chitosan-PCL nanofibers showed promising results. Human bone marrow stem cells in vitro showed a tenogenic commitment when cultured on aligned chitosan–PCL nanofibres93 and, an increase in inflammation, cellularity, vascularity, and cell proliferation, in vivo together led to significant improvements in structural properties.94 This study used the dorsal skinfold chamber in mice for characterization of the early vascularization of electrospun PCL implant materials. This in vivo model allows the repetitive observation of the vascularization of different implant materials by means of intravital fluorescence microscopy.95 As results, vascularization was significantly increased in CS-g-PCL fiber mats at day 14 compared to the porous polymer patch and uncoated PCL fiber mats. Furthermore CS-g-PCL fiber mats also showed a reduced activation of immune cells.92 CS-g-PCL being able to improve the formation of vascularized tissue and the ingrowth of cells into electrospun PCL scaffolds is clinically meaningful. The same group further investigated the usefulness of CS-g-PCL nanofiber mats in combination of transforming growth factor beta 3 (TGF–β3) for rotator cuff healing.79 With respect to the tendon-to-bone environment and especially to the enthesis, members of the transforming growth factor β (TGF–β) superfamily are of particular interest because these proteins are known to stimulate chondrogenic differentiation of mesenchymal stromal cells.96,97 However, the biomechanical analysis revealed that tendon–bone constructs with unloaded scaffolds had significantly lower values for maximum force compared to native tendons.79 Tendon-bone constructs with TGF–β3-loaded fiber scaffolds showed only slightly lower values, but still were inferior to native tendons. In histological evaluation, minor differences could be observed.79 In conclusion, TGF–β3-loading of electrospun PCL fiber scaffolds resulted in more robust constructs without causing significant advantages on a cellular level.79
LIMITATIONS OF ELECTROSPUN NANOFIBER SCAFFOLDS
Despite several advantages and encouraging outcomes of electrospun scaffolds for rotator cuff tendon healing, they have limitations. First, the mechanical properties are still lower than desired. The healthy tendon can effectively withstand physiologic loading around the shoulder joint; therefore, the mechanical properties of the engineered tissues should be adequate to survive after implantation. The major forces on the rotator cuff are tensile stresses in the longitudinal direction, while compressive stresses to withstand superior migration of the humeral head have been recently recognized especially for superior capsular reconstructions in massive, irreparable rotator cuff tears. The mechanical strength of integration with host tissue (scaffold-to-bone interface in most repairs and tendon-to-scaffold in the bridging repair model) is also critical to assess potential for repair.57 Several methods could help to improve tensile properties, such as the choice of polymers with higher mechanical properties, the use of cell-seeded scaffolds,75 the use of aligned fibers,63,77 a multilayered approach,78 adding woven fibers,81,98 scaling up by stacked or braided nanofiber mats,99 or post-fabrication treatments81 (cross-linking, bonding and annealing). However, those methods have yet to improve mechanical properties to a sufficient degree or resulted in adverse effects (decreased cell attachment or proliferation and more complex fabricating process). Insufficient mechanical properties of electrospun nanofibers may also contribute to their poor suture retention and handling properties, which is important for surgical application, however this factor is rarely evaluated in studies.75,81 Additionally, native tendon often presents a nonlinear elastic behavior which means that the slope of the stress–strain curve alters at different strains, typically in the toe region. Such nonlinear, elastic behavior of biological tissues is probably absent in electrospun scaffolds.52 Thus, it is important to choose the Young's modulus within ranges of physiological strain to fabricate biomimetic scaffolds.52,78 Even chitosan-graft-PCL coated electrospun PCL scaffolds, recently reported in rat models,92,100 failed to achieve the Young's Modulus of intact control infraspinatus tendon (9.3±4.4 MPa in suture-fixed tendons, 13.8±5.4 MPa in the CS-g-PCL-patched group, and 20.6±10.3 MPa in intact control group).79 Viscoelastic measurements are also not commonly used for mechanical analysis of rotator cuff scaffolds, probably due to the lack of attention to viscoelastic behavior or the thin sheet geometry (typically about tens or hundreds of µm thickness) that is insufficient for dynamic mechanical analysis testing (with the thickness of several mm).52 With viscoelastic properties, tendons at low strain rates tend to absorb more mechanical energy but are less effective in carrying mechanical loads. However, tendons become stiffer and more effective in transmitting large muscular loads to bone at high strain rates. To reproduce this tendon behavior, nanoscaffolds should be enhanced to accommodate dynamic in vivo muscle and tendon loads and consider dynamic mechanical properties. Second, degradation products of the polymers used for fabricating nanofibers are an important concern, as emphasized by Hakimi et al.81 High concentrations of lactic acid and glycolic acid were found to markedly inhibit growth of tendon-derived cells.101 Therefore, the rate of degradation and the accumulation of acidic degradation products are important to the safety of synthetic scaffolds. Finally, clear, consistent, and universally accepted performance criteria are lacking. As shown in the studies reviewed, the assessment of tenogenic performance and potential for repair generally includes cell viability, density, proliferation and migration; expression of genes or proteins of interest; histologic and immunochemical assessment of newly formed tissue; and mechanical properties of the cell–scaffold construct. Similar to the application of nanofibrous scaffolds for meniscus tissue engineering,57 there is, as yet, no clear consensus on a criteria to rank the performance of different cell types, scaffold materials, or culture conditions in the rotator cuff field. Furthermore, the wide variation in testing protocols and reporting of outcome measures preclude the validation and comparison of results among different studies. Therefore, comprehensive performance criteria should be developed to effectively compare the matrix components and mechanical properties of the engineered tissue to that of native rotator cuff tendon to quantify the potential for clinical translation.
CONCLUSION
The rotator cuff tendon is crucial to maintain the stability and function of the shoulder joint and consistent healing of rotator cuff tears is one of the unsolved problems facing orthopedic surgeons. Over the last decade, nanofiber scaffolds by electrospinning for rotator cuff tissue engineering have shown promising in vitro and in vivo results. Nanofibrous scaffolds can mimic the native tendon structure and have the potential to provide biomimetic mechanical and physiological properties that warrant further improvement. The complex regional inhomogeneity of the rotator cuff tendon (as shown in macroscopic and microscopic anatomy), the nonlinearity of the mechanical properties of tendon itself, gradations in biocompositions and mechanical properties at the enthesis, and the multiaxial loading environment surrounding the shoulder joint indicate that greater sophistication in scaffold design is needed. While some limitations are apparent in the present state-of-the-art and clinical trials in human are not yet available, overcoming these obstacles with the development of biomimetic nanofiber scaffolds will eventually improve clinical uses and long-term success in humans.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227–233. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 3.Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995;77:10–15. doi: 10.2106/00004623-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Jain NB, Higgins LD, Losina E, Collins J, Blazar PE, Katz JN. Epidemiology of musculoskeletal upper extremity ambulatory surgery in the United States. BMC Musculoskelet Disord. 2014;15:4. doi: 10.1186/1471-2474-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gartsman GM, Brinker MR, Khan M, Karahan M. Self-assessment of general health status in patients with five common shoulder conditions. J Shoulder Elbow Surg. 1998;7:228–237. doi: 10.1016/s1058-2746(98)90050-7. [DOI] [PubMed] [Google Scholar]
- 6.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;(254):35–38. [PubMed] [Google Scholar]
- 7.Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10:199–203. doi: 10.1067/mse.2001.113086. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda H. Partial-thickness rotator cuff tears: a modern view on Codman's classic. J Shoulder Elbow Surg. 2000;9:163–168. [PubMed] [Google Scholar]
- 9.Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710–714. doi: 10.1177/0363546510393944. [DOI] [PubMed] [Google Scholar]
- 10.Maman E, Harris C, White L, Tomlinson G, Shashank M, Boynton E. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898–1906. doi: 10.2106/JBJS.G.01335. [DOI] [PubMed] [Google Scholar]
- 11.Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89:1533–1541. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 12.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21:394–400. doi: 10.1016/j.arthro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18:136–144. doi: 10.1053/jars.2002.30443. [DOI] [PubMed] [Google Scholar]
- 15.Lim TK, Bae KH, Choi YS, Kim JH, Yoo JC. Clinical outcome and repair integrity after arthroscopic rotator cuff repair significantly improved during the surgeon's learning curve. J Shoulder Elbow Surg. 2020 doi: 10.1016/j.jse.2020.10.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Cook JL, Fox DB, Kuroki K, Jayo M, De Deyne PG. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–156. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 17.Shea KP, McCarthy MB, Ledgard F, Arciero C, Chowaniec D, Mazzocca AD. Human tendon cell response to 7 commercially available extracellular matrix materials: an in vitro study. Arthroscopy. 2010;26:1181–1188. doi: 10.1016/j.arthro.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91:1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16:21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907–911. doi: 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- 21.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 22.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg. 2004;13:538–541. doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Smith RDJ, Zargar N, Brown CP, Nagra NS, Dakin SG, Snelling SJB, et al. Characterizing the macro and micro mechanical properties of scaffolds for rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2038–2046. doi: 10.1016/j.jse.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad T, Shin HJ, Lee J, Shin YM, Perikamana SKM, Park SY, et al. Fabrication of in vitro 3D mineralized tissue by fusion of composite spheroids incorporating biomineral-coated nanofibers and human adipose-derived stem cells. Acta Biomater. 2018;74:464–477. doi: 10.1016/j.actbio.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59:1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Harryman DT, 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA., 3rd Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72:1334–1343. [PubMed] [Google Scholar]
- 27.Bouaicha S, Ernstbrunner L, Jud L, Meyer DC, Snedeker JG, Bachmann E. The lever arm ratio of the rotator cuff to deltoid muscle explains and predicts pseudoparalysis of the shoulder: the Shoulder Abduction Moment index. Bone Joint J. 2018;100-B:1600–1608. doi: 10.1302/0301-620X.100B12.BJJ-2018-0493.R1. [DOI] [PubMed] [Google Scholar]
- 28.Clark JM, Harryman DT., 2nd Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Joint Surg Am. 1992;74:713–725. [PubMed] [Google Scholar]
- 29.Rossetti L, Kuntz LA, Kunold E, Schock J, Müller KW, Grabmayr H, et al. The microstructure and micromechanics of the tendon-bone insertion. Nat Mater. 2017;16:664–670. doi: 10.1038/nmat4863. [DOI] [PubMed] [Google Scholar]
- 30.Kuntz LA, Rossetti L, Kunold E, Schmitt A, von Eisenhart-Rothe R, Bausch AR, et al. Biomarkers for tissue engineering of the tendon-bone interface. PLoS One. 2018;13:e0189668. doi: 10.1371/journal.pone.0189668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007;25:1621–1628. doi: 10.1002/jor.20441. [DOI] [PubMed] [Google Scholar]
- 32.Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech. 2006;39:1842–1851. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976–985. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Birman V, Chen C, Thomopoulos S, Genin GM. Mechanisms of bimaterial attachment at the interface of tendon to bone. J Eng Mater Technol. 2011;133:011006. doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Thomopoulos S, Chen C, Birman V, Buehler MJ, Genin GM. Modelling the mechanics of partially mineralized collagen fibrils, fibres and tissue. J R Soc Interface. 2013;11:20130835. doi: 10.1098/rsif.2013.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derwin KA, Galatz LM, Ratcliffe A, Thomopoulos S. Enthesis repair: challenges and opportunities for effective tendon-tobone healing. J Bone Joint Surg Am. 2018;100:e109. doi: 10.2106/JBJS.18.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deymier-Black AC, Pasteris JD, Genin GM, Thomopoulos S. Allometry of the tendon enthesis: mechanisms of load transfer between tendon and bone. J Biomech Eng. 2015;137:111005. doi: 10.1115/1.4031571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Birman V, Deymier-Black A, Schwartz AG, Thomopoulos S, Genin GM. Stochastic interdigitation as a toughening mechanism at the interface between tendon and bone. Biophys J. 2015;108:431–437. doi: 10.1016/j.bpj.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 40.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am. 2003;85:539–550. doi: 10.2106/00004623-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 43.Edwards SL, Lynch TS, Saltzman MD, Terry MA, Nuber GW. Biologic and pharmacologic augmentation of rotator cuff repairs. J Am Acad Orthop Surg. 2011;19:583–589. doi: 10.5435/00124635-201110000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 46.Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chainani A, Little D. Current status of tissue-engineered scaffolds for rotator cuff repair. Tech Orthop. 2016;31:91–97. doi: 10.1097/BTO.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21:251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 51.Rim NG, Shin CS, Shin H. Current approaches to electrospun nanofibers for tissue engineering. Biomed Mater. 2013;8:014102. doi: 10.1088/1748-6041/8/1/014102. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen-Truong M, Li YV, Wang Z. Mechanical considerations of electrospun scaffolds for myocardial tissue and regenerative engineering. Bioengineering (Basel) 2020;7:122. doi: 10.3390/bioengineering7040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res. 2010;468:1476–1484. doi: 10.1007/s11999-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saveh-Shemshaki N, S Nair L, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater. 2019;94:64–81. doi: 10.1016/j.actbio.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y, Han Y, Wong YS, Fuh JYH. Fibre-based scaffolding techniques for tendon tissue engineering. J Tissue Eng Regen Med. 2018;12:1798–1821. doi: 10.1002/term.2701. [DOI] [PubMed] [Google Scholar]
- 57.Grogan SP, Baek J, D'Lima DD. Meniscal tissue repair with nanofibers: future perspectives. Nanomedicine (Lond) 2020;15:2517–2538. doi: 10.2217/nnm-2020-0183. [DOI] [PubMed] [Google Scholar]
- 58.Reznik SN, Yarin AL, Theron A, Zussman E. Transient and steady shapes of droplets attached to a surface in a strong electric field. J Fluid Mech. 2004;516:349–377. [Google Scholar]
- 59.Baek J, Sovani S, Glembotski NE, Du J, Jin S, Grogan SP, et al. Repair of avascular meniscus tears with electrospun collagen scaffolds seeded with human cells. Tissue Eng Part A. 2016;22:436–448. doi: 10.1089/ten.tea.2015.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baek J, Sovani S, Choi W, Jin S, Grogan SP, D'Lima DD. Meniscal tissue engineering using aligned collagen fibrous scaffolds: comparison of different human cell sources. Tissue Eng Part A. 2018;24:81–93. doi: 10.1089/ten.tea.2016.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song KH, Heo SJ, Peredo AP, Davidson MD, Mauck RL, Burdick JA. Influence of fiber stiffness on meniscal cell migration into dense fibrous networks. Adv Healthc Mater. 2020;9:e1901228. doi: 10.1002/adhm.201901228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Şelaru A, Drăgușin DM, Olăreț E, Serafim A, Steinmüller-Nethl D, Vasile E, et al. Fabrication and biocompatibility evaluation of nanodiamonds-gelatin electrospun materials designed for prospective tissue regeneration applications. Materials (Basel) 2019;12:2933. doi: 10.3390/ma12182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baek J, Chen X, Sovani S, Jin S, Grogan SP, D'Lima DD. Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. J Orthop Res. 2015;33:572–583. doi: 10.1002/jor.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 66.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15:115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, et al. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J Shoulder Elbow Surg. 2012;21:245–250. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 69.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 70.Sahoo S, Ouyang H, Goh JC, Tay TE, Toh SL. Characterization of a novel polymeric scaffold for potential application in tendon/ ligament tissue engineering. Tissue Eng. 2006;12:91–99. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 71.Kazemnejad S, Allameh A, Soleimani M, Gharehbaghian A, Mohammadi Y, Amirizadeh N, et al. Biochemical and molecular characterization of hepatocyte-like cells derived from human bone marrow mesenchymal stem cells on a novel three-dimensional biocompatible nanofibrous scaffold. J Gastroenterol Hepatol. 2009;24:278–287. doi: 10.1111/j.1440-1746.2008.05530.x. [DOI] [PubMed] [Google Scholar]
- 72.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–1432. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 73.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Taylor BL, Kim DH, Huegel J, Raja HA, Burkholder SJ, Weiss SN, et al. Localized delivery of ibuprofen via a bilayer delivery system (BiLDS) for supraspinatus tendon healing in a rat model. J Orthop Res. 2020;38:2339–2349. doi: 10.1002/jor.24670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor ED, Nair LS, Nukavarapu SP, McLaughlin S, Laurencin CT. Novel nanostructured scaffolds as therapeutic replacement options for rotator cuff disease. J Bone Joint Surg Am. 2010;92 Suppl 2:170–179. doi: 10.2106/JBJS.J.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inui A, Kokubu T, Mifune Y, Sakata R, Nishimoto H, Nishida K, et al. Regeneration of rotator cuff tear using electrospun poly (d,l-Lactide-Co-Glycolide) scaffolds in a rabbit model. Arthroscopy. 2012;28:1790–1799. doi: 10.1016/j.arthro.2012.05.887. [DOI] [PubMed] [Google Scholar]
- 77.Orr SB, Chainani A, Hippensteel KJ, Kishan A, Gilchrist C, Garrigues NW, et al. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015;24:117–126. doi: 10.1016/j.actbio.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chainani A, Hippensteel KJ, Kishan A, Garrigues NW, Ruch DS, Guilak F, et al. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng Part A. 2013;19:2594–2604. doi: 10.1089/ten.tea.2013.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reifenrath J, Wellmann M, Kempfert M, Angrisani N, Welke B, Gniesmer S, et al. TGF-b3 loaded electrospun polycaprolacton fibre scaffolds for rotator cuff tear repair: an in vivo study in rats. Int J Mol Sci. 2020;21:1046. doi: 10.3390/ijms21031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao S, Zhao J, Dong S, Huangfu X, Li B, Yang H, et al. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int J Nanomedicine. 2014;9:2373–2385. doi: 10.2147/IJN.S59536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hakimi O, Mouthuy PA, Zargar N, Lostis E, Morrey M, Carr A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater. 2015;26:124–135. doi: 10.1016/j.actbio.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Shanmugasundaram N, Ravichandran P, Reddy PN, Ramamurty N, Pal S, Rao KP. Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials. 2001;22:1943–1951. doi: 10.1016/s0142-9612(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 83.Zhao S, Xie X, Pan G, Shen P, Zhao J, Cui W. Healing improvement after rotator cuff repair using gelatin-grafted poly(L-lactide) electrospun fibrous membranes. J Surg Res. 2015;193:33–42. doi: 10.1016/j.jss.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. The effects of fibroblast growth factor-2 on rotator cuff reconstruction with acellular dermal matrix grafts. Arthroscopy. 2009;25:608–616. doi: 10.1016/j.arthro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 85.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J Hand Surg Am. 1998;23:1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- 87.Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441–1447. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 88.Lipner J, Shen H, Cavinatto L, Liu W, Havlioglu N, Xia Y, et al. In vivo evaluation of adipose-derived stromal cells delivered with a nanofiber scaffold for tendon-to-bone repair. Tissue Eng Part A. 2015;21:2766–2774. doi: 10.1089/ten.tea.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun Y, Han F, Zhang P, Zhi Y, Yang J, Yao X, et al. A synthetic bridging patch of modified co-electrospun dual nano-scaffolds for massive rotator cuff tear. J Mater Chem B. 2016;4:7259–7269. doi: 10.1039/c6tb01674j. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Cheng R, Sun Z, Su W, Pan G, Zhao S, et al. Flexible bipolar nanofibrous membranes for improving gradient microstructure in tendon-to-bone healing. Acta Biomater. 2017;61:204–216. doi: 10.1016/j.actbio.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 91.Cong S, Sun Y, Lin J, Liu S, Chen J. A synthetic graft with multilayered co-electrospinning nanoscaffolds for bridging massive rotator cuff tear in a rat model. Am J Sports Med. 2020;48:1826–1836. doi: 10.1177/0363546520917684. [DOI] [PubMed] [Google Scholar]
- 92.Gniesmer S, Brehm R, Hoffmann A, de Cassan D, Menzel H, Hoheisel AL, et al. Vascularization and biocompatibility of poly(e-caprolactone) fiber mats for rotator cuff tear repair. PLoS One. 2020;15:e02275633. doi: 10.1371/journal.pone.0227563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leung M, Jana S, Tsao CT, Zhang M. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan-poly-caprolactone nanofibers and TGF-b3. J Mater Chem B. 2013;1:6516–6524. doi: 10.1039/c3tb20825g. [DOI] [PubMed] [Google Scholar]
- 94.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29:1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 95.Rücker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mülhaupt R, et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27:5027–5038. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 96.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujio K, Komai T, Inoue M, Morita K, Okamura T, Yamamoto K. Revisiting the regulatory roles of the TGF-b family of cytokines. Autoimmun Rev. 2016;15:917–922. doi: 10.1016/j.autrev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Cai J, Wang J, Ye K, Li D, Ai C, Sheng D, et al. Dual-layer alignedrandom nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int J Nanomedicine. 2018;13:3481–3492. doi: 10.2147/IJN.S165633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V, Tuan RS. Braided and stacked electrospun nanofibrous scaffolds for tendon and ligament tissue engineering. Tissue Eng Part A. 2017;23:378–389. doi: 10.1089/ten.tea.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Willbold E, Wellmann M, Welke B, Angrisani N, Gniesmer S, Kampmann A, et al. Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair. J Tissue Eng Regen Med. 2020;14:186–197. doi: 10.1002/term.2985. [DOI] [PubMed] [Google Scholar]
- 101.Hakimi O, Murphy R, Stachewicz U, Hislop S, Carr AJ. An electrospun polydioxanone patch for the localisation of biological therapies during tendon repair. Eur Cell Mater. 2012;24:344–357. doi: 10.22203/ecm.v024a25. discussion 357. [DOI] [PubMed] [Google Scholar]