Abstract
In recent years, epidemiological studies, genome-wide association studies, and Mendelian randomization studies have shown a strong association between increased levels of lipoproteins and increased risks of coronary heart disease and cardiovascular disease (CVD). Although lipoprotein(a) [Lp(a)] was an independent risk factor for ASCVD, the latest international clinical guidelines do not recommend direct reduction of plasma Lp(a) concentrations. The main reason was that there is no effective clinical medicine that directly lowers plasma Lp(a) concentrations. However, recent clinical trials have shown that proprotein convertase subtilisin/kexin-type 9 inhibitors (PCSK9) and second-generation antisense oligonucleotides can effectively reduce plasma Lp(a) levels. This review will present the structure, pathogenicity, prognostic evidences, and recent advances in therapeutic drugs for Lp(a).
Keywords: Atherosclerosis, Lipids, Cardiovascular Diseases
INTRODUCTION
One of standard therapies to reduce cardiovascular disease is statin therapy, but there are still residual risks after potent statin treatments. Several primary and secondary prevention clinical trials showed that statins significantly reduced the incidence of recurrent coronary heart disease events 1,2,3,4 and the Pravastatin or Atorvastatin Evaluation and Infection Therapy/Thrombolysis in Myocardial Infarction-22 (PROVE-IT/TIMI-22) study has shown that lower LDL levels can provide greater clinical benefits.5 However, in the recent Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) after a six-year follow-up period, the incidence of major adverse cardiac events (MACEs) in the simvastatin/ezetimibe group was lower.6 This result showed that aggressive cholesterol lowering was good for decreasing cardiac events. In addition, the LIPID and Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) studies have shown that lipoprotein(a) [Lp(a)] is an important determinant of residual risk for cardiovascular events.7
Lp(a) was discovered about 60 years ago and is an independent risk factor for cardiovascular diseases such as coronary artery disease, peripheral vascular disease and calcified aortic valve disease.8,9,10,11 Previous studies have shown that there are still residual risks after using statins to reduce low-density lipoprotein (LDL) cholesterol, and the plasma Lp(a) concentration is an important risk factor for the residual risk of coronary heart disease.7,12 However, most clinicians do not check Lp(a) in daily practice due to the lack of an effective drug treatment. This review will present forgotten biomarker Lp(a) which can be another target to decrease residual risks. We will present the structure, pathogenicity, prognostic evidence, and recent advances in therapeutic drugs for treating Lp(a).
STRUCTURE AND PATHOGENICITY OF Lp(a)
1. Structure
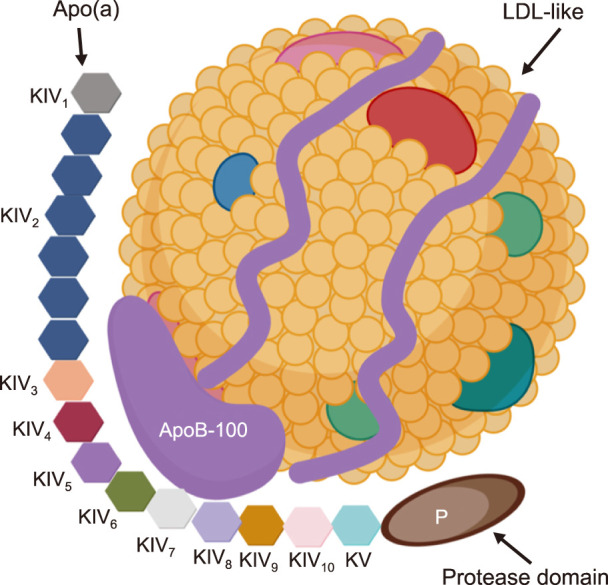
Lp(a) is composed of two parts: a low-density lipoprotein (LDL)-like particle with apolipoprotein B100 (apo-B100), which is shared with apolipoprotein(a) [apo(a)] through disulfide binding (Fig. 1).13,14,15,16 The existence of apo(a) determines the unique function of Lp(a). Apo(a) is composed of repeating kringle IV (KIV) and a protease-like domain.17 According to the amino acid sequence, the KIV domain in apo(a) can be divided into 10 types (KIV1 to KIV10). Only KIV2 is repeated in the apo(a) sequence and the number of KIV2 repetitions is determined by the Lp(a) gene.18,19 Genetic polymorphism of apo(a) by variable KIV2 repetition determines the level of plasma Lp(a).20,21,22,23 Both Kringle V and the protease-like domains in apo(a) are highly similar to the plasminogen.24,25
FIG. 1. Lipoprotein(a) [Lp(a)] structure. Lp(a) is composed of two parts: one part is low-density lipoprotein (LDL)-like particles with apolipoprotein B100 (apo-B100), and the other part is apolipoprotein a [apo(a)] covalently bound by disulfide bonds. Apo(a) contains 10 types of kringle IV (KIV) subtypes; one copy of both KIV1 and KIV3-10, and variable KIV2 repetition. In addition, apo(a) is composed of kringle V (KV) and an inactive protease-like domain (P). Created with BioRender.com.
2. Pathogenicity
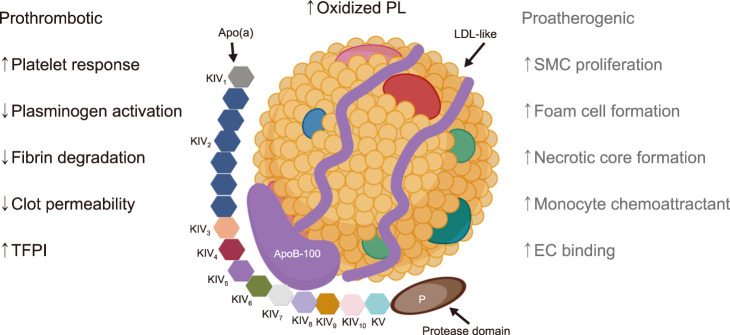
The plasma concentration of Lp(a) and individual differences are obvious. It is affected genetically, but hardly affected by environmental and dietary factors.26,27 The pathogenicity of Lp(a) can be roughly divided into three categories: atherosclerosis, inflammation and thrombosis (Fig. 2).
FIG. 2. Pathogenicity of Lp(a). The pathogenicity of Lp(a) can be roughly divided into three categories: atherosclerosis, inflammation and thrombosis. The main pathological mechanisms of each category are listed in this figure. TFPI: tissue factor pathway inhibitor, SMC: smooth muscle cell, EC: endothelial cell. Created with BioRender.com.
The most common method of measuring Lp(a) is to use a monoclonal anti-apo(a) antibody to determine the concentration of apo(a). Enzyme immunoassay (ELISA) is commonly used in clinical situations.28,29,30 However, due to the wide variation of the molecular weight of apo(a), the ratio of mass to molar concentration between individuals varies. Therefore, it is difficult to use a standardized method to determine the concentration of Lp(a). In addition, the abnormal level of Lp(a) in distinct risk and ethnic populations has not been completely determined clinically. Generally, the population average and the median level of Lp(a) vary with race/ethnicity, and are affected by certain diseases.
The concentration of Lp(a) can range from undetectable levels to >1,000 mg/dL, but numerous clinical studies have shown that an elevated plasma Lp(a) concentration (50 mg/dL) is an independent risk factor for cardiovascular diseases.
1) Atherosclerosis
The apo(a) in Lp(a) mediates the various atherosclerotic processes by accumulating Lp(a) during endothelial injury combined with several components of vascular endothelial cells and smooth muscle cells to interfere with normal endothelial function, and stimulating chemotactic activation of monocytes and macrophages. The strong lysine binding site in Apo(a) promotes Lp(a) accumulation in vascular tissues and enhances endothelial contraction and permeability through rhoa/rho kinase/mypt1-dependent pathways.31 Apo(a) can also mediate the concentration-dependent rejection of smooth muscle cells during migration assays through the action of integrin αvβ3 and rhoa/rho kinase.32 Lp(a) binds to macrophages through endocytosis by high-affinity VLDL-receptors to promote foam cell formation and cholesterol deposition.33,34
2) Thrombosis
Lp(a) mainly promotes thrombosis through a variety of mechanisms, the most important of which is to inhibit and interfere with fibrinolysis. Due to the homology of apo(a) and plasminogen, the formation of active plasmin can be inhibited, while Lp(a) competes with plasminogen to bind to fibrin, preventing plasmin-mediated thrombolysis.35,36,37 The apo(a) in Lp(a) can inhibit platelet aggregation by replacing fibrinogen to bind with integrin αIIbβ3,38 and through the effects of urokinase-type plasminogen activator inhibit plasminogen activation.39,40
3) Inflammation
Lp(a) is susceptible to oxidative modification and produce the oxidation specific epitope (OSE), which is an important mediator of inflammation and atherosclerotic formation. The OSE is mainly composed of oxidized LDL, apoptotic cells, and oxidized phospholipids and oxidized sterols.41,42
The lysine binding sites in certain KIV domains in apo(a) are associated with specific functions related to the pathogenicity of Lp(a).43 In particular, KIV10 also contains sites for covalent attachment of pro-inflammatory oxidized phospholipids (oxPLs).41,44 Lp(a) has a high proportion of OxPLs and is also one of the main carriers of oxPLs.45 The OxPLs have pro-inflammatory effects and also participate in the formation of atherosclerosis. Lp(a) can promote inflammation by inducing the inflammatory cytokines. Apo(a) can induce macrophages to release interleukin-8, tumor necrosis factor-α and monocyte chemotactic protein.44,46 Lp(a) can directly induce monocyte chemotaxis and attract monocytes by direct and indirect mechanism by vascular endothelial cells.47,48
EPIDEMIOLOGY AND CLINICAL CORRELATION
The plasma Lp(a) concentration is mainly determined by the LPA gene. In addition, more than 90% of the variability of plasma Lp(a) levels can be explained by the polymorphism of the apo(a) gene. However, race/ethnic factors also have an extremely important influence on the plasma Lp(a) concentration. The greater genetic difference in allele frequency produced the different average and median level of Lp(a).
In the Copenhagen General Population and Copenhagen City Heart Study, it was proved that the increased risk of myocardial infarction in the general population was associated with increased plasma Lp(a) levels.15 Secondly, in the Copenhagen General Population study, the increased Lp(a) levels were related to decreased KIV-2 repeats. The number of KIV-2 repeats explained 27% of the change in plasma Lp(a) concentration.
In the Genetic Variants Associated study, gene chip detection technology is used to investigate the relationship between genetic variation and the risk of coronary heart disease (CHD).14 They found two variants in the LPA gene (rs10455872 and rs3798220), which explained 36% of the total variation in plasma Lp(a) levels, and two variations were associated with increased risk of CHD. In addition, it has been found that the LPA gene in the 6q26-27 region and its surrounding genetic variants are closely related to plasma Lp(a) levels and the risk of CHD.49,50,51
In the Emerging Risk Factors Collaboration study, 126,634 participants from 36 prospective studies were collected. After adjusting for age and gender, Lp(a) has been continuously associated with the risk of coronary heart disease, which is represented by the RR for CHD was 1.16 (95% CI, 1.11–1.22) for every 3.5-fold increase in Lp(a) levels. There is a continuous, independent, and moderate correlation between Lp(a) concentration and the risk of CHD, and this correlation seems to be only related to vascular outcomes.9
Some prospective studies also support that Lp(a) is indeed an independent risk factor for cardiovascular disease.29,52 Mehta et al.53 reviewed the results of a long-term cohort study of a total of 15,000 patients based on two asymptomatic populations in the community. The study found that the increase in Lp(a) can independently predict long-term atherosclerotic cardiovascular disease (ASCVD) and CHD risks in asymptomatic populations of the community. Increased Lp(a) combined with family history were effective at predicting the risk of ASCVD and CHD.
In the existing prospective analysis and meta-analysis, it is generally believed that the Lp(a) level in people of African descent is two to three times higher than that of Caucasians followed by Hispanics and East Asians.29,52,54 In addition, some studies have shown that Chinese Lp(a) levels are lower than those of Caucasians.55 A total of 4,593 participants were included in the MESA study, including Caucasian, Black, Hispanic, and Chinese Americans.56 After adjusting for race/ethnicity and other CHD risk factors, the plasma Lp(a) levels in the Black and White populations are significantly correlated with the incidence of CHD. However, there is no significant correlation among Chinese Americans and Hispanics. In addition, a higher Lp(a) level (≥50 mg/dL) was associated with higher risks of coronary heart disease in all races except Chinese Americans.
A total of 6,086 first myocardial infarction and 6,857 control patients were included in the INTERHEART study, including Africans, Chinese, Arabs, Europeans, Latin Americans, south Asians and southeast Asians.57 After adjusting for age, gender, apo(B) and apoA1, Lp(a)>50 mg/dL was associated with an increased risk of myocardial infarction (odds ratio 1.48; 95% CI 1.32–1.67). In the Korean population, Lp(a) has been identified as an independent risk factor for 6,252 patients with CHD, and associated with poorer prognosis.58 However, Lee et al.59 showed a close relationship between Lp(a) (carries of OxPLs) and MACE despite significant ethnic differences. LPA SNPs, apo(a) isoforms, Lp(a), and OxPLs levels were measured in 1792 Black, 1030 White, and 597 Hispanic subjects in Dallas Heart Study. The prevalence and association of LPA SNPs with size of apo(a) isoforms, Lp(a), and OxPLs levels are highly variable and ethnicity-specific. The relationship to MACE is best explained by elevated plasma Lp(a) or OxPLs levels, despite significant ethnic differences in LPA genetic markers.
The prospective study enrolled 77,680 patients from the Copenhagen City Heart Study and the Copenhagen General Population Study showed the relationship between increased levels of Lp(a) and severe aortic valve stenosis in the Caucasian population.60 In addition, the relationship between rs10455872 genotype and aortic stenosis risk was observed. Another genome-wide association study was performed by standard CT scanning on 6,942 patients to analyze the relationship between aortic valve calcification and Lp(a).61 The study showed that the rs10455872 genotype was closely related to the aortic valve calcification in European Caucasian participants. Moreover, the increased level of Lp(a) was associated with the progression of aortic stenosis.
Madsen et al.62 have reported the results of a population-based study of 3,600 patients. The study found that the higher Lp(a) level was associated with recurrent cardiovascular disease. However, the reduction of Lp(a)<50 mg/dL within 5 years will reduce the 20% risk of cardiovascular disease recurrence. Xu et al.63 reviewed the results of a long-term cohort study of 6,175 patients. The study found that the incidence of serious adverse events was significantly higher in patients with both high Lp(a) levels and three-vessel coronary diseases. On the other hand, it showed that higher Lp(a) levels can be potential biomarkers for risk stratification and prognostic factors. Secondly, Liu et al.64 published a long-term prospective study of 4,078 patients. The study found that in patients with stable coronary heart disease after coronary intervention, plasma Lp(a) levels have a good predictive value for cardiovascular events, and that high Lp(a) levels (≥30 mg/dL) indicate a poor prognosis.
Lp(a) GUIDELINES AND CARDIOVASCULAR DISEASE
Numerous clinical studies in the past twenty years have confirmed the association between plasma Lp(a) and the risk of cardiovascular disease.10,65 Although Lp(a) was an independent risk factor for ASCVD, the latest international clinical guidelines do not recommend direct reduction of plasma Lp(a) concentrations. The main reasons have been that there were no good clinical medicine that directly lowered plasma Lp(a) concentrations, and that the data had not fully shown the prognostic benefit.
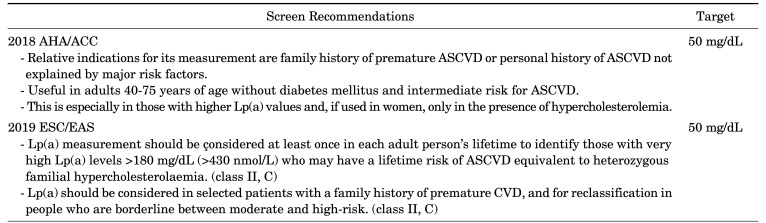
The 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) Guidelines for the management of dyslipidemia recommend that every adult have at least one Lp(a) assessment in his lifetime in order to determine the populations with extremely high levels of Lp(a) genetics (Lp(a)>180 mg/dL). This is because patients with hereditary high Lp(a) levels are likely to be at risk of ASCVD in their lifetime (Table 1, class IIa, C).66
TABLE 1. Guideline recommendations for screening of Lp(a).
2018 AHA/ACC: The 2018 American Heart Association/American College of Cardiology (AHA/ACC) Guideline on the Management of Blood Cholesterol, 2019 ESC/EAS: The 2019 European Society of Cardiology/European Atherosclerosis Society Guidelines for the management of dyslipidemia.
The 2018 American Heart Association/American College of Cardiology (AHA/ACC) Guidelines on the Management of Blood Cholesterol recommend Lp(a) as a primary risk prevention for adults aged 40–75.67 However, there is limited guidance on when Lp(a) should be measured.
TREATMENT OF INCREASED Lp(a)
1. Nicotinic acid
Current lipid-lowering therapies are unable to specifically reduce the concentration of Lp(a). However, niacin has been widely used to treat dyslipidemia, and especially in the case of using high doses, the Lp(a) concentration has been significantly reduced.29 High-dose niacin (2–4 g/day) can reduce Lp(a) by 25–40%.68 However, niacin did not show any ability to reduce the concentration of Lp(a) at low doses. The mechanism by which niacin reduces Lp(a) may be by reducing the synthesis of triglycerides required for lipoprotein lipidation69 and reducing the global liver cAMP levels which is required to stimulate apo(a) transcription.70 However, due to the potential adverse reactions, such as migraines, tachycardia, blushing, and liver toxicity, the recent European guideline do not recommend the use of niacin as a way to reduce Lp(a).66 In the AIM-HIGH study, a total of 3,414 patients were randomly assigned to receive niacin.71 They showed that adding niacin to statin therapy did not increase the clinical benefit during the 36-month follow-up period. In addition, poor tolerability and potential adverse events (skin, gastrointestinal system, bleeding, myopathy, infection and diabetes) were observed in the HPS2-THRIVE study.72
2. Antibody-based therapeutics
1) Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors
PCSK9 inhibitors such as evolocumab or alirocumab can decrease the degradation of LDL receptors.73 They can increase the number of hepatocyte LDL receptors and enhance the ability of LDL clearance from plasma. In the FOURIER study, evolocumab reduced the plasma Lp(a) concentration by 26.9% (6.2%–46.7%).74 The risk of Major cardiovascular events (death from CHD, myocardial infarction and acute revascularization) in patients with higher Lp(a) level was reduced by 23% (HR 0.77; 95% CI, 0.67–0.88). In the ODYSSEY OUTCOMES trial, it was shown that the Lp(a) reduction caused by alirocumab was related to the reduction of cardiovascular event risk and was not directly related to the simultaneous reduction of LDL.75 In addition, after the use of alirocumab, for every 5 mg/L reduction of Lp(a), the total number of cardiovascular events was relatively reduced by 2.5%.76 Although PCSK9 inhibitors can reduce plasma Lp(a) levels, the mechanism of action and clinical correlation remains to be explored.
3. Nucleic acid-based therapeutics
1) Mipomersen
Mipomersen is a 2′-O-methoxy ethyl (2′-MOE)-modified second-generation antisense oligonucleotide (ASO), which binds to the homologous apoB messenger ribonucleic acid (mRNA).77,78,79 Due to the inhibition of the synthesis of apolipoprotein B-100 (apoB-100), the plasma concentration of LDL, apoB and Lp(a) can be significantly reduced. In the existing phase III randomized trial, in patients with hypercholesterolemia of different causes, mipomersen continuously reduced the median plasma Lp(a) level by 26.4%.77 Mipomersen is approved by the FDA for lowering low-density lipoprotein cholesterol, apo(B) and other lipoprotein in homozygous familial hypercholesterolemia. A meta-analysis found that the level of Lp(a) was reduced 26% from baseline.11
2) ISIS-APO(a)Rx and AKCEA-APO(a)-LRx
ISIS-APO(a)Rx and AKCEA-APO(a)-LRx are 2′-O-methoxy ethyl (2′-MOE) modified antisense DNA oligonucleotides, targeting apolipoprotein (a) and Lp(a), by binding to the complementary apo(a) mRNA sequence to form a DNA double strand, thereby reducing the translation of apo(a).80 Both ISISAPO(a) Rx and AKCEA-APO(a)-LRx showed better tolerability and fewer adverse reactions in phase I of the trial.81 In both phase II trials, healthy participants with elevated Lp(a) significantly reduced plasma Lp(a) concentration in a dose-dependent manner.82 In the Phase II trial of AKCEA-APO(a)-LRx, the Lp(a) concentration was reduced by an average of 80% at dose of 20 mg per week, and 98% of patients reached the lipoprotein(a) level of 50 mg/dL or lower.83
CONCLUSIONS AND FUTURE PERSPECTIVES
In the past two decades, epidemiological studies have shown that Lp(a) is an independent causal risk factor for CVD and its clinical importance. However, the more direct mechanism between Lp(a) and atherosclerosis still needs clarification. On the other hand, specific therapies such as nucleic acid based medication for reducing Lp(a) will be important in clinical practice to decrease the residual risk of CHD.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Scandinavian Simvastatin Survival Study G. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 4.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 7.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg K. A new serum type system in man--the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 9.Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. 2013;61:1146–1156. doi: 10.1016/j.jacc.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Kassner U, Schlabs T, Rosada A, Steinhagen-Thiessen E. Lipoprotein(a)--an independent causal risk factor for cardiovascular disease and current therapeutic options. Atheroscler Suppl. 2015;18:263–267. doi: 10.1016/j.atherosclerosissup.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Jr, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaubatz JW, Chari MV, Nava ML, Guyton JR, Morrisett JD. Isolation and characterization of the two major apoproteins in human lipoprotein [a] J Lipid Res. 1987;28:69–79. [PubMed] [Google Scholar]
- 14.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 15.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 16.Emdin CA, Khera AV, Natarajan P, Klarin D, Won HH, Peloso GM, et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68:2761–2772. doi: 10.1016/j.jacc.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–137. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 18.Utermann G. The mysteries of lipoprotein(a) Science. 1989;246:904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- 19.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellis KL, Boffa MB, Sahebkar A, Koschinsky ML, Watts GF. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res. 2017;68:57–82. doi: 10.1016/j.plipres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Marcovina SM, Albers JJ, Gabel B, Koschinsky ML, Gaur VP. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a) Clin Chem. 1995;41:246–255. [PubMed] [Google Scholar]
- 23.Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57:526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronenberg F. Human genetics and the causal role of lipoprotein(a) for various diseases. Cardiovasc Drugs Ther. 2016;30:87–100. doi: 10.1007/s10557-016-6648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabel BR, Koschinsky MI. Analysis of the proteolytic activity of a recombinant form of apolipoprotein(a) Biochemistry. 1995;34:15777–15784. doi: 10.1021/bi00048a023. [DOI] [PubMed] [Google Scholar]
- 26.Austin MA, Sandholzer C, Selby JV, Newman B, Krauss RM, Utermann G. Lipoprotein(a) in women twins: heritability and relationship to apolipoprotein(a) phenotypes. Am J Hum Genet. 1992;51:829–840. [PMC free article] [PubMed] [Google Scholar]
- 27.Lamon-Fava S, Jimenez D, Christian JC, Fabsitz RR, Reed T, Carmelli D, et al. The NHLBI Twin Study: heritability of apolipoprotein A-I, B, and low density lipoprotein subclasses and concordance for lipoprotein(a) Atherosclerosis. 1991;91:97–106. doi: 10.1016/0021-9150(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–192. doi: 10.1016/j.jacc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 31.Cho T, Jung Y, Koschinsky ML. Apolipoprotein(a), through its strong lysine-binding site in KIV(10'), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. J Biol Chem. 2008;283:30503–30512. doi: 10.1074/jbc.M802648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riches K, Franklin L, Maqbool A, Peckham M, Adams M, Bond J, et al. Apolipoprotein(a) acts as a chemorepellent to human vascular smooth muscle cells via integrin αVβ3 and RhoA/ROCK-mediated mechanisms. Int J Biochem Cell Biol. 2013;45:1776–1783. doi: 10.1016/j.biocel.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zioncheck TF, Powell LM, Rice GC, Eaton DL, Lawn RM. Interaction of recombinant apolipoprotein(a) and lipoprotein(a) with macrophages. J Clin Invest. 1991;87:767–771. doi: 10.1172/JCI115079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argraves KM, Kozarsky KF, Fallon JT, Harpel PC, Strickland DK. The atherogenic lipoprotein Lp(a) is internalized and degraded in a process mediated by the VLDL receptor. J Clin Invest. 1997;100:2170–2181. doi: 10.1172/JCI119753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loscalzo J, Weinfeld M, Fless GM, Scanu AM. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis. 1990;10:240–245. doi: 10.1161/01.atv.10.2.240. [DOI] [PubMed] [Google Scholar]
- 36.Rouy D, Grailhe P, Nigon F, Chapman J, Anglés-Cano E. Lipoprotein(a) impairs generation of plasmin by fibrin-bound tissue-type plasminogen activator. In vitro studies in a plasma milieu. Arterioscler Thromb. 1991;11:629–638. doi: 10.1161/01.atv.11.3.629. [DOI] [PubMed] [Google Scholar]
- 37.Palabrica TM, Liu AC, Aronovitz MJ, Furie B, Lawn RM, Furie BC. Antifibrinolytic activity of apolipoprotein(a) in vivo: human apolipoprotein(a) transgenic mice are resistant to tissue plasminogen activator-mediated thrombolysis. Nat Med. 1995;1:256–259. doi: 10.1038/nm0395-256. [DOI] [PubMed] [Google Scholar]
- 38.Barre DE. Apoprotein (A) antagonises THE GPIIB/IIIA receptor on collagen and adp-stimulated human platelets. Front Biosci. 2004;9:404–410. doi: 10.2741/1194. [DOI] [PubMed] [Google Scholar]
- 39.Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32:1550–1551. doi: 10.1161/ATVBAHA.112.251306. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Boffa MB, Koschinsky ML. Apolipoprotein(a) inhibits in vitro tube formation in endothelial cells: identification of roles for Kringle V and the plasminogen activation system. PLoS One. 2013;8:e52287. doi: 10.1371/journal.pone.0052287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scipione CA, Sayegh SE, Romagnuolo R, Tsimikas S, Marcovina SM, Boffa MB, et al. Mechanistic insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a) J Lipid Res. 2015;56:2273–2285. doi: 10.1194/jlr.M060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Wiesner P, Tafelmeier M, Chittka D, Choi SH, Zhang L, Byun YS, et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J Lipid Res. 2013;54:1877–1883. doi: 10.1194/jlr.M036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haque NS, Zhang X, French DL, Li J, Poon M, Fallon JT, et al. CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein(a) in human vascular endothelial cells. Circulation. 2000;102:786–792. doi: 10.1161/01.cir.102.7.786. [DOI] [PubMed] [Google Scholar]
- 48.Syrovets T, Thillet J, Chapman MJ, Simmet T. Lipoprotein(a) is a potent chemoattractant for human peripheral monocytes. Blood. 1997;90:2027–2036. [PubMed] [Google Scholar]
- 49.Trégouët DA, König IR, Erdmann J, Munteanu A, Braund PS, Hall AS, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- 50.Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur Heart J. 2012;33:325–334. doi: 10.1093/eurheartj/ehr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ober C, Nord AS, Thompson EE, Pan L, Tan Z, Cusanovich D, et al. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J Lipid Res. 2009;50:798–806. doi: 10.1194/jlr.M800515-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta A, Virani SS, Ayers CR, Sun W, Hoogeveen RC, Rohatgi A, et al. Lipoprotein(a) and family history predict cardiovascular disease risk. J Am Coll Cardiol. 2020;76:781–793. doi: 10.1016/j.jacc.2020.06.040. [DOI] [PubMed] [Google Scholar]
- 54.Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee D, Wong EC, Shin J, Fortmann SP, Palaniappan L. Racial and ethnic variation in lipoprotein (a) levels among Asian Indian and Chinese patients. J Lipids. 2011;2011:291954. doi: 10.1155/2011/291954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019;139:1472–1482. doi: 10.1161/CIRCULATIONAHA.118.034311. [DOI] [PubMed] [Google Scholar]
- 58.Kwon SW, Lee BK, Hong BK, Kim JY, Choi EY, Sung JM, et al. Prognostic significance of elevated lipoprotein(a) in coronary artery revascularization patients. Int J Cardiol. 2013;167:1990–1994. doi: 10.1016/j.ijcard.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Lee SR, Prasad A, Choi YS, Xing C, Clopton P, Witztum JL, et al. LPA gene, ethnicity, and cardiovascular events. Circulation. 2017;135:251–263. doi: 10.1161/CIRCULATIONAHA.116.024611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 61.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40:255–266. doi: 10.1161/ATVBAHA.119.312951. [DOI] [PubMed] [Google Scholar]
- 63.Xu N, Jiang L, Xu L, Tian J, Zhang C, Zhao X, et al. Impact of lipoprotein(a) on long-term (mean 6.2 years) outcomes in patients with three-vessel coronary artery disease. Am J Cardiol. 2020;125:528–533. doi: 10.1016/j.amjcard.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 64.Liu HH, Cao YX, Jin JL, Zhang HW, Hua Q, Li YF, et al. Predicting cardiovascular outcomes by baseline lipoprotein(a) concentrations: a large cohort and long-term follow-up study on real-world patients receiving percutaneous coronary intervention. J Am Heart Assoc. 2020;9:e014581. doi: 10.1161/JAHA.119.014581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 66.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 67.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–276. doi: 10.1111/j.1365-2796.1989.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 69.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 70.Chennamsetty I, Kostner KM, Claudel T, Vinod M, Frank S, Weiss TS, et al. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J Lipid Res. 2012;53:2405–2412. doi: 10.1194/jlr.M029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 72.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Man LC, Kelly E, Duffy D. Targeting lipoprotein (a): an evolving therapeutic landscape. Curr Atheroscler Rep. 2015;17:502. doi: 10.1007/s11883-015-0502-0. [DOI] [PubMed] [Google Scholar]
- 74.O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 75.Szarek M, Bittner VA, Aylward P, Baccara-Dinet M, Bhatt DL, Diaz R, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–4255. doi: 10.1093/eurheartj/ehaa649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 77.Santos RD, Raal FJ, Catapano AL, Witztum JL, SteinhagenThiessen E, Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol. 2015;35:689–699. doi: 10.1161/ATVBAHA.114.304549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013;62:2178–2184. doi: 10.1016/j.jacc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 79.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 80.Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57:340–351. doi: 10.1194/jlr.R052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 82.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 83.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]