Abstract
AIM
To assess the effectiveness, safety, and cost-effectiveness of the Argus II in treatment of the retinitis pigmentosa (RP) patients.
METHODS
The ProQuest, Web of Science, EMBASE, MEDLINE (via PubMed) were searched using combinations of the keywords of Argus, safety, effectiveness, bionic eye, retinal prosthesis, and RP through March 2018. The retrieved records were screened and then assessed for eligibility.
RESULTS
Totally 926 records were retrieved from the searched databases and finally 12 studies included. The RP patients showed improvements in visual function after receiving the prosthesis, compared to the time before the prosthesis or the time it was off. This was measured by square localization, direction of motion, and grating visual acuity tests. No major adverse effect was reported for the Argus II prosthesis itself and/or the surgery to implement it, but the most frequently reported items were hypotony, and conjunctival dehiscence. The incremental cost-effectiveness ratio (ICER) was calculated to be €14603 per quality-adjusted life year (QALY) in UK and $207 616 per QALY in Canada.
CONCLUSION
The available evidence shows that the Argus II prosthesis in RP patients is effective in improvement of their visual function. Some minor adverse effects are reported for the prosthesis. The cost-effectiveness studies show that the technology is cost-effective only at high levels of willingness-to-pay.
Keywords: retinitis pigmentosa, Argus II, retinal prosthesis, effectiveness, adverse, cost-effectiveness
INTRODUCTION
Retinitis pigmentosa (RP) is defined as a group of inherited retinal deterioration that result in blindness due to harm to the photoreceptors[1]. Yet, the inner retinal cells including the amacrine, horizontal, ganglion, and bipolar cells and the nerve fiber layer to a large extent remain preserved. Although it is a rare genetic disorder but about 100 000 people are affected by it in the United States[2]. Although patients have variable clinical symptoms of the RP, most of them lose the rod and cone photoreceptor cells around age 40. After all, there is no treatment for the RP[2].
Loss of the vision have some intense social and psychological disability[3]. Education about the genetic disorder, psychological consultation and rehabilitation can be helpful to the patients to cope with the social and psychological impacts of loss of vision. Economic and social disadvantages of the RP have significant effect on the patients, their families, and the society in general because they have more repeated medical visits, and most of them do not have the ability to do their daily tasks[4]–[5].
As mentioned above, there is no cure that can restitute the functional vision or ensure prevention of the visual loss. Over the past two decades, new retinal treatment methods were tried including gene therapy, stem cell transplantation, and the electronic neural prostheses[6]–[9]. Of all these methods, only retinal prostheses were commercialized for the restoration of some visual functions in the RP patients.
Argus II (Second Sight Medical Products, Inc, Sylmar, California, USA), is one of the surgical implantable devices currently available for the patients with RP. The prosthesis restores partial functional vision in the patients with bare to no light perception because of advanced RP. The Argus II system received the CE mark in Europe in 2011[6] and Humanitarian Device Exemption Approval from the United States Food and Drug Administration (FDA) in 2013[7], with funding available through the Centers for Medicare and Medicaid Services. The Argus II system works in this way that the visual images are taken by the camera and then are transformed to electrical stimulation pulses. Then the images are transmitted wirelessly to the implant using an antenna. After receiving the images, the implant produces small pulses of electricity and stimulates the inner retinal cells. Then the stimulated retina cells transfer the received visual data to the brain through the optic nerve, and the picture is perceived in the brain.
Clinical trial studies[10]–[12] show that Argus has been effective in improving visual function in patients with RP. Totally 60% of people did not experience any serious side effects due to the device or surgery[10]. Conjunctival erosion and hypotension were the most common side effects observed after 5y. In a health technology evaluation study conducted for ArgusII, the ICER obtained was €14 603 per quality-adjusted life year (QALY). The economic evaluation performed in this study showed that Argus II is a cost-effective intervention[4]. Current study sought to systematically assess published evidence about the clinical effectiveness, safety and cost-effectiveness of Argus II device in patients with RP, to inform country's reimbursement policy decisions and its implementation in routine practice.
MATERIALS AND METHODS
Ethical Approval
The study protocol has been reviewed and approved at the National Institute for Health Research (NIHR) of Iran (code of ethics: IR.tums.NIHR.REC.1396.46).
Participant(s)
Patients with RP.
Intervention(s)
Receiving Argus II retinal prosthesis system.
Comparator(s)
RP patients that were not received the Argus II or when the Argus II is turned off.
Outcomes
Outcomes of interest were: visual function (e.g. object localization, motion detection, grating visual acuity), functional outcomes (e.g. orientation and mobility), quality of life, adverse events, and cost-effectiveness ratios.
Study Type
The eligible study designs were clinical trials, observational studies, health technology assessments (HTA) and economic evaluations.
Search Strategy
We performed a literature search in March 2018, using PubMed, EMBASE, Scopus, Cochrane Central Register of Clinical Trials and Web of Science, for studies published till March, 2018. The search strategy was formulated using following key terms and their combinations: Argus II, implant*, bionic eye, prosthesis, effectiveness, efficacy, cost, adverse, safety, utility, degenerat*, pigment*, blindness, discord, disability, economic analysis. Complementary search was performed through reference list and citations of the relevant articles in Google Scholar.
Study Selection
Studies, published in English which examined the effect of Argus II retinal prosthesis in patients with RP were eligible to be included. Two reviewers independently assessed the title and abstracts then full texts of the retrieved papers according to eligibility criteria. In case of disagreement, third reviewer was consulted. Studies were included if they reported quantitative effect sizes about clinical and economic outcomes. Studies that were not in English, commentaries, reviews or technical reports were excluded. If the full text of eligible papers were not available, we sent email to the authors for possibility of sharing the paper.
Quality Assessment and Data Extraction
Quality assessment and data extraction were conducted according to the Joanna Briggs Institute (JBI) critical appraisal tool[8] and data extraction form[8]. The selected articles were independently rated for quality by two investigators. In case of disagreement, a third investigator was consulted to resolve the disagreement. The check lists consisted of questions with possible Yes, No, Not applicable answers for each. Studies with No answers ≥5, were rated as poor quality and excluded from data extraction.
Data on study characteristics including study design, sample size, intervention and comparison options, follow-up duration, reported outcomes were extracted by two reviewers then summarized in tables and described in texts. Data extraction form was first piloted and then administered for data collection. All reviewers were blind to papers' identity. Authors were contacted for missing data or additional details.
Data Analysis and Synthesis
Because of the heterogeneity problem, we did not perform pooled estimation of results. Thus, the results were synthesized and summarized in a narrative way. Findings from eligible studies were reported as cost-effectiveness ratios [for example cost per life year gained (LYG) and cost per QALY].
RESULTS
Study Inclusion
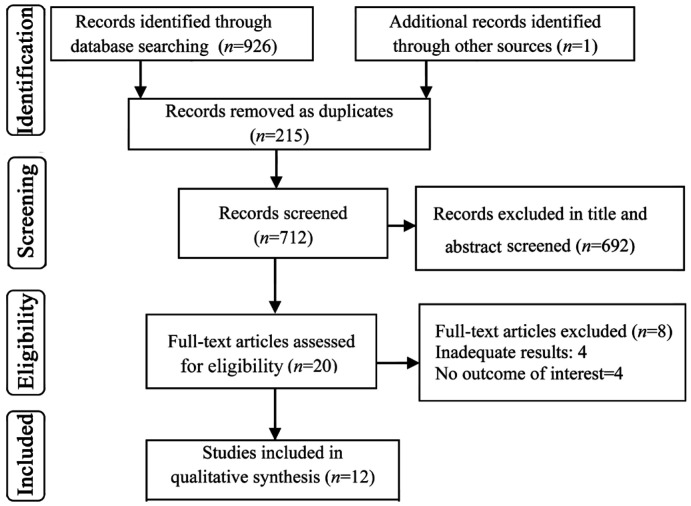
A total of 926 articles were initially recognized by the search strategy. Figure 1 presents the PRISMA flowchart for the study selection process. Twenty full-text studies were assessed for eligibility. Finally, 12 studies included in data extraction. The complementary search also resulted in one additional relevant study.
Figure 1. PRISMA flow diagram for selection of studies on the safety, effectiveness and cost-effectiveness of the Argus II.
Characteristics of the Included Studies
Of the total 12 included studies, 7 were clinical trials[9]–[15], 3 were case series[16]–[18] and 2 were cost-effectiveness[4],[19]. All of the studies assessed Argus II in patients with lost vision due to advanced RP. All the studies were performed in the USA and the Europe. Main characteristics of the included studies are summarized in Tables 1 and 2.
Table 1. Results of safety, effectiveness of Argus II retinal prosthesis system in patients with retinitis pigmentosa.
| Author, year | Study design and country | Sample size (n) | Fallow up period | Outcomes | Resultsa | |
| Duncan et al, 2017[9] | Single-arm, prospective, unmasked clinical trial USA |
30 | 36mo | The Vision and Quality of Life Index (VisQoL) with six dimensions (injury, life, roles, friendship,assistance and activity) | VisQoL utility scores at follow-up (post-implant) indicated no statistically significant change from baseline; baseline utility scores ranged as 0.22-0.99 (normal distribution); mean VisQoL utility scores ranged between 0.63 to 0.67 throughout the follow-up period (36mo) | |
| da Cruz et al, 2016[10] | Prospective, multicenter, singe-arm clinical trial USA, Europe |
30 | 5y | Safety (the number, seriousness and relatedness of adverse events); visual function: as measured by 3 computers based (square localization, direction of motion and grating visual acuity); orientation and mobility tests | Visual function tests and functional vision task were significantly better when the Argus II was on. Square localization=80.9%; direction of motion=50%; grating visual acuity=38.1%; Adverse effect: conjunctiva erosion=4 (13.3%) 95%CI (3.1%-30.7%); Hypotony=4 (13.3%) 95%CI (3.1%-30.7%); Conjunctiva dehiscence=3 (10%) 95%CI (2.1%-26.5%); presumed endophthalmitis=3 (10%) 95%CI (2.1%-26.5%) | |
| Luo et al, 2015[16] | Single-center, prospective, internally-controlled case series UK |
5 | N/A | Localization and prehension of objects in 3-dimensional space | Percentage of successful prehension was: 71.3%±27.1% with prosthesis on and finger marker on; 77.5%±24.5% with prosthesis on and finger marker off; 0 with prosthesis off and finger marker on; 0 with prosthesis off and finger marker off | |
| Rizzo et al, 2014[17] | International case series UK |
6 | 12mo | Visual function (mobility, square localization, direction of motion, grating visual acuity, Goldmann visual field); safety | Mobility: all patients were able to use the device in everyday conditions and to locate a bright light on the ceiling. square localization=80%; direction of motion=60%; grating visual acuity=20%; Goldmann visual field results improved in all patients. Adverse effects: none of the patients had any adverse event. Only surgical complication occurred. | |
| Kotecha et al, 2014[18] | Hospital based case-series UK |
6 | N/A | Reaching and grasping tasks | successful grasps in patient with Argus II was more when the system was on (visit 1: median [IQR] percentage success: ‘Off’=0 [0 to 50]%, ‘On’=69 [67 to 95]%, ‘Scrambled’=59 [42 to 95]%; Friedman Chi-squared test statistic 6.5, P=0.04; visit 2 median [IQR] percentage success: ‘Off’=0 [0 to 25]%, ‘On’=69 [53 to 100]%, ‘Scrambled’=28 [13 to 63]%; Friedman Chi-squared test statistic 8.4, P=0.02) | |
| Dorn et al, 2013[13] | Clinical trial USA-Europe |
28 | 3-38mo | Detecting the direction of a moving object. | 15 patients with Argus II (54%) were significantly better in performing the task than patient with residual vision. 2 patients were significantly better in performing the task with their patients, 11 subjects had no difference in performing task with Argus and patients. | |
| da Cruz et al, 2013[14] | Prospective, internally controlled, multicenter trial USA-Europe |
21 | 19.9mo | Controlled, closed-group, forced-choice letter identification, and, open-choice two-, three- and four-letter word identification tests | Mean percentage correct for subjects tested were: Group A 72.3%±24.6% system on and 17.7%±12.9% system off; Group B 55.0%±27.4% system on and 11.8%±10.7% system off and Group C 51.7%±28.9% system on and 15.3%±7.4% system off. | |
| Ho et al, 2015[11] | Multicenter, single-arm, prospective clinical trial USA-Europe |
30 | 1-3y | Safety (the number, seriousness and relatedness of adverse events); Visual function: as measured by 3 computers based (square localization, direction of motion and grating visual acuity); orientation and mobility tests | First year | Third year |
| Square localization=93.8%; direction of motion=62.5%; grating visual acuity=48.2%; find the door=53%; gollow the line=72.8% | Square localization=89.3%; direction of motion=55.6%; grating visual acuity=33.3%; find the door=54.2%; follow the line=67.9% | |||||
| Adverse effects: Conjunctiva erosion=10.0%, 95%CI (2.1%-26.5%); Hypotony=6.7%, 95%CI (0.8%-22.1%); Conjunctiva dehiscence=10.0%, 95%CI (2.1%-26.5%); Presumed endophthalmitis=10.0%, 95%CI (2.1%-26.5%) | ||||||
| Humayun et al, 2012[12] | Single-arm, prospective, multicenter clinical trial USA- Europe |
30 | 36mo | Safety (the number, seriousness and relatedness of adverse events); visual function: as measured by 3 computers based (square localization, direction of motion and grating visual acuity); orientation and mobility tests | Square localization=96%; direction of motion=57%; grating visual acuity=23%; find the door=48%; follow the line=42%; Adverse effects: Conjunctiva erosion=2, 95%CI (0.8%-22.1%) Hypotony=3%; 95%CI (2.1%-26.5%); Conjunctiva dehiscence=3%; 95%CI (2.1%-26.5%); Presumed endophthalmitis=3%; 95%CI (2.1%-26.5%) |
|
| Barry et al, 2012[15] | Clinical trial USA |
21 | N/A | Hand-movement guidance and tracing sets of paths that were divided into three categories: right-angle/single-turn, mixed angle/single-turn, and mixed-angle/two-turn. | Right-angle/single turn sets, average tracing error was reduced by 63% and tracing time increased by 156% when using the prosthesis, relative to residual vision; with mixed angle/single turn sets, error was reduced by 53% and time to complete tracing increased by 184%; prosthesis use decreased error by 38% and increased tracing time by 252% for paths that incorporated two turns. | |
aThe control group was the same as the experimental group so that the patients were compared with themselves when the prosthesis is off or with the other healthy eye.
Table 2. Results of economic evaluation analysis on Argus II prosthesis.
| Author, year, location | Study design and perspective | Population | Interventions/ comparators | Health outcomes | Costs | Cost-effectiveness |
| Vaidya et al, 2014[4], United Kingdom | Cost-effectiveness analysis (utility measured as QALYs); Multi-state transition Markov model; Health care payer's perspective | n=1000 patients with retinitis pigmentosa; Mean age 46y and older | Argus II vs usual care | Expected QALYs: Base case analysis: Argus II: 7.34 usual care: 4.44. | Base case analysis: Argus II: €243 549; usual care: €201 094 | €14 603/QALY |
| Health Quality Ontario[19], 2016 Canada |
Cost-utility analysis (utility measured as QALYs); Markov cohort model; Perspective of the Ontario Ministry of Health and Long-Term Care. | n=1000 patients with retinitis pigmentosa; Men and/or women aged 50y and older | Argus II vs usual care | Utility: retinitis pigmentosa, no light perception=0.26; no grating visual acuity, light perception=0.35, grating visual acuity=0.52; average total effect: Argus II=3.21; standard care=2.08 | Average total cost Argus II=$361 034; Standard care=$126 428 |
$207 616/QALY |
QALY: Quality-adjusted life year.
Main Findings
Effectiveness
Three clinical trials[10]–[12] and one case series[17] assessed the effect of Argus II on improving the vision of patients with RP. Three main tests were conducted in all studies as follow: square localization test (participants should touch a white square on a black background of the monitor), direction of motion test (the patient should trace a white object that moves in the black background of the monitor), and grating visual acuity test (visual perception of participants are measured with square-wave gratings of various spatial frequencies presented on a computer monitor). All four studies concluded after 3mo, 1, 3, and 5y that the visual function of the RP patients that have received the Argus II prosthesis was improved by the prosthesis compared to the time prior to it or the time the system was off. The extent of improvement was: Square localization: 96%, 89%, and 80%; Direction of motion: 57%, 55.6%, 60%, and 50%; Grating visual acuity: 23%, 33.3%, 38.1%, and 20%.
Two other tests were used in the clinical trials[11]–[12] to assess the direction and movement: find the door and follow the line on monitor. Both studies reported that the visual function of the patients was significantly bettered compared to the time when the system was off. Find the door: 48%, 54%; and follow the line: 42%, 67.9% improved. Another clinical trial study[13] examined detecting the direction of a moving object on the monitor by the RP patients who received the Argus II and compared it with the time the prosthesis was off. The results showed that 54% of the patients had significantly better performance when the system was on. The reason that the Argus II system is compared with the time prior to it or with the time it was off, is that there is no other safe eye prosthesis similar to it as an alternative.
Two studies[16],[18] assessed the localization, reaching and grasping task of a little white square in 11 patients. The number of successful grasps was much higher when the prosthesis was on. This showed that the Argus II system facilitates the reaching and grasping task.
An internally controlled prospective study[14] with a sample of 28 RP patients used an Optotype in slides to identify the letters and words on screen. The study reached these findings in three steps: Average score for the letters L, T, E, J, F, H, I, U was 72.3±24.6 when the device was on and 17.7±12.9 when it was off; for the letters A, Z, Q, V, N, W, O, C, D, M was 55±27.4 when on and 11.8±10.7 when off; for the letters K, R, G, X, B, Y, S, P was 51.7±28.9 when on and 15.3±7.4 when off.
Multicenter Trial
The vision and quality of life (VisQol) index is used to measure the changes in the utility score and the quality of life. VisQol is a 6-dimension tool including: injury, life, roles, assistance, activities, and friendship. All 6 dimensions were assessed for the patients before and after implanting the Argus II. The utility score of the baseline was 0.22 to 0.99 and of the follow up period was 0.36 to 0.76. The utility score did not improved significantly compared to the baseline, but those patients who were not affected in the dimensions of injury, life, and role showed significant improvement after implanting the Argus II system[9].
Adverse Effect
Of the 12 studies, four had investigated the adverse effects of the prosthesis or the surgery of implanting it[10]–[12],[17]. According to the definition of the ISO 14155, serious adverse effect is a medical event that results in death, life threatening, or permanent damage to function or the structure of the body. Study of Ho et al[11] stated that after one year of implanting the prosthesis, 66.7% of the patients reported no serious adverse effect as a result of the Argus II prosthesis or the surgery of implanting it and after three years, 11 of them (37%) reported some serious adverse effects. The most frequent adverse effects were conjunctival erosion, conjunctival dehiscence and presumed endophthalmitis in the first year and conjunctival erosion and Hypotony in the third year.
Study of Humayun et al[12] reported that the patients who had the Argus II for 36mo reported no serious adverse effect. The most frequent adverse effects were: hypotony, conjunctival dehiscence, and presumed endophthalmitis. Ten cases (33%) reported the non-serious effect of macular edema.
The clinical trial by da Cruz et al[10] reported that 60% of the patients had no serious adverse effect due to the prosthesis or its surgery. Conjunctival erosion and the hypotony were the most frequent adverse effects after 5y. Thus, the most frequent adverse effects of the Argus II system in the patients were hypotony, conjunctival dehiscence, presumed endophthalmitis, and conjunctival erosion.
Cost-Effectiveness
Results of the literature search found one cost-effectiveness study[4] and one HTA study[19] on Argus II. The cost-effectiveness study[4] was conducted in 2014 in the United Kingdom with a cohort of 1000 patients with average age of 46y. The time horizon of the study was 25y. The control group was those patients of RP who received the routine care. The incremental cost-effectiveness ratio (ICER) was calculated to be €14 603 per QALY. The economic evaluation showed that the Argus II was cost-effective.
The HTA study[19] examined the cost-utility of the Argus II system in comparison with the standard care by a Markov model. The time horizon was 10y. The results showed that the Argus II was a cost-effective intervention when the willingness to pay is above $207 616 per QALY. The study also reported that the technology cost is high.
Budget Impact
The only study[19] which assessed the budget effect of the Argus II prosthesis was the HTA study of the Ontario, Canada. The study was conducted with the perspective of the Ontario Ministry of Health and Long-term Care to estimate the costs of the Argus II in next five years (2015-2019). If in the next five years four prosthesis of Argus II be implanted each year, the budget effect of these processes would be $800 404, $813 979, $824 904, $832 688, and $837 956 for 2015 to 2019, respectively. Results of the sensitivity analysis showed that the decrease of the price of the Argus II in the future will lead to potential savings.
DISCUSSION
Argus II is a new technology to treat the patients suffering from the RP. It is currently the only commercialized available prosthesis for the patients with least or no vision. Improvement of the vision of these patients can lead to increased self-esteem, improved quality of life and decreased dependence on others.
An original study from America and Europe is taking place with 30 patients for 10y[12]. Three reports from this study have been published[10]–[12], and in the latest report[10], 5y of this study have been completed. This study seeks to collect long-term data on the safety and efficacy of the Argus II. Long-term data, according to them, is more important than the fact that the device is implanted in a large number of people worldwide. In the 5y that the study was performed, the visual function which was measured by 3 computers-based objective tests: square localization, direction of motion, and grating visual acuity were similar to two previous studies. The results of square localization test were over 80%, the direction of motion over 50%, and grating visual activity over 20%. This means a significant improvement in visual functions when the system was on compared to the time it is off. Another study from England with 6 patients[17], who received Argus II, showed that in addition to visual performance, the results of Goldman field trials have also improved in these patients after planting. He also stated that the perception of vision in these patients depends on the general state and mental health. Some RP patients may have some mental problems as a result of losing their vision. So, it is recommended for these patients to be in consultation with a psychiatrist and rehabilitation therapist.
Rizzo et al[17], who also performed visual function with these three tests in his study, stated that square localization and direction of motion tests are not always consistent with real visual performance because in the square localization test, the patient is likely to show the test margin instead of its center and these patients require a good hand-eye coordination, while some RP patients were deprived of vision for several years and this might affect their hand-eye coordination. While laboratory -based orientation and mobility tests (find the door and follow up the line) show more evidence of the long-term benefits of Argus II when the system was on than off.
A clinical trial[14] studied the patients who lost their vision due to the RP by three tests of letter identification, word recognition, and letter size reduction. The patients had the Argus II system averagely for 19.9mo. Study findings showed that the majority of the patients successfully detected the letters. This showed that the visual prosthesis can treat the deep blindness. The letters in this study were sized 0.9 to 18 cm and the smaller the size of the letters, the more errors in letter identification.
A cohort study[9] did not show significant changes in the utility score comparing the follow-up period after implanting and the baseline. However, a significant and sustained improvement in QOL was seen in the user's understanding of reducing the risk of injury, reducing the associated ability to perform in the post-implantation.
Receiving Argus II may facilitate patients' reach and grasp when the device is turned on versus when it's off, but the most important thing to consider is the delay in starting the movement, which is because when the object is located by the device the reach is based on the position of the head and the eye[18]. This suggests a special need for post-implantation rehabilitation in these patients.
Studies that evaluated safety of the Argus II -the prosthesis itself and/or the surgery of implanting- reported that the majority of the patients had no serious adverse effect (SAE). The longest follow- up period was 5y and the most frequent adverse effects were hypotony, conjunctival dehiscence, presumed endophthalmitis, and conjunctival erosion. these adverse effects are curable with standard ophthalmology care[10]–[11], yet have costs. In this study, out of 30 devices, 24 devices were planted and activated two devices were planted but inactivated, three cases were explanted and one patient death. One of the SAE that observed between 3 and 5y after implanting was rhegmatogenous retinal detachment that was resolved[10]. The incidence of endophthalmitis after 2mo of prosthetic implantation in this study was 10% (3 out of 30). The 10%, no definitive source of infection was found, due to potential factors such as a modest increase in surgical operation time in these 3 cases and the transport of people without a mask to the operating room. We should keep in mind that it is possible for the RP patients who receive the Argus II to show some adverse effects. Thus, these patients should be followed up and monitored for a long term after implanting the prosthesis.
The RP has vast social and economic costs. The Argus II has been assessed in only two economic evaluation studies[4],[19]. In the HTA study[19], Probabilistic Sensitivity Analysis indicated that when assuming a willingness-to-pay threshold of $50 000 per QALY, there was no chance for the Argus II system to be cost-effective. At the willingness-to-pay threshold of $100 000 per QALY, the chance of the Argus II system to be cost-effective was 21% and at a willingness-to-pay threshold of $200 000 per QALY, the chance increased to 45%.
The other study was conducted by Vaidya et al[4] and reported that the Argus II is totally cost effective at the willingness-to-pay (WTP) of 31 000 Euros. So, the system has high costs and is cost-effective only at the high levels of WTPs. The two studies shared the general limitations of economic modeling. Moreover, they were based on data from only 30 Argus II patients followed for 3 and 2y, respectively. The seven trials[9]–[15] included in this systematic review were prospective multicenter single arm studies to compare the patients with RP who were received the Argus II retinal prosthesis system versus those who were not received it. However, it must be considered that the issue of blinding was not discussed in these studies.
Quality of the included papers was moderate to high. On average, the interventional studies answered to 8 questions and the cost-effectiveness studies answered to all 9 questions of the quality appraisal tool. A bias of the semi-experimental studies was non-randomized control group which reduces internal validity of the studies. For example, due to limited number of participants one eye has been considered as experimental and the other eye as control. Some studies were conducted in only one center and there were differences in the participants of the studies which to some extent affect the generalizability if the findings. Sample sizes were limited[16]–[18] and this may reduce the power of the studies and prevents us from performing advanced statistical analyses. Authors of three studies[10],[16]–[17] have no financial disclosures; four studies[4],[10],[13]–[15] determined the funding situation was unclear and four studies had at least one author working at the second sight medical product. Most of the studies were performed in laboratory conditions with high contrasted objects and the results may be different in real-life situations.
In conclusion, this systematic review found that the available evidence supports the effectiveness of the Argus II retinal prosthesis in RP patients that have no or almost no vision use of Argus II in RP patients simplified and improved reach and grasp performance and detect the motion task compared with the original vision. The functional vision assessment showed better results when the prosthesis was switched on than when it was off. In terms of safety, it did not cause major adverse effects as a result of the surgery or the prosthesis itself. Yet, some minor problems, such as hypotony and conjunctival dehiscence, were reported by those who received it. The cost-effectiveness studies on Argus II showed that the technology is cost-effective only at high levels of WTP.
Acknowledgments
Foundation: Supported by the National Institute for Health Research (NIHR) of Iran. The founder is not involved in the design of the study or collection, analysis and interpretation of data, or in writing the manuscript.
Conflicts of Interest: Ostad-Ahmadi Z, None; Daemi A, None; Modabberi MR, None; Mostafaie A, None.
REFERENCES
- 1.Kang K, Tarchick MJ, Yu XS, Beight C, Bu P, Yu MZ. Carnosic acid slows photoreceptor degeneration in the Pde6brd10 mouse model of retinitis pigmentosa. Sci Rep. 2016;6:22632. doi: 10.1038/srep22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Assistive Technology for Blindness and Low Vision. CRC Press; 2018. Low vision: types of vision loss and common effects on activities of daily life; pp. 77–98. [Google Scholar]
- 4.Vaidya A, Borgonovi E, Taylor RS, Sahel JA, Rizzo S, Stanga PE, Kukreja A, Walter P. The cost-effectiveness of the Argus II retinal prosthesis in Retinitis Pigmentosa patients. BMC Ophthalmol. 2014;14:49. doi: 10.1186/1471-2415-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahm BJ, Shin YW, Shim EJ, Jeon HJ, Seo JM, Chung H, Yu HG. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol. 2008;92(5):650–654. doi: 10.1136/bjo.2007.127092. [DOI] [PubMed] [Google Scholar]
- 6.Arevalo JF. Message from the president. Vis Pan-Am. 2018;17(4):95–96. [Google Scholar]
- 7.Greenemeier L. FDA approves first retinal implant. Nature. 2013 [Google Scholar]
- 8.The Joanna Briggs Institute. Joanna Briggs Institute Reviewers' Manual: 2014. edition. The Joanna Briggs Institute; 2014. [Google Scholar]
- 9.Duncan JL, Richards TP, Arditi A, da Cruz L, Dagnelie G, Dorn JD, Ho AC, Olmos de Koo LC, Barale PO, Stanga PE, Thumann G, Wang YZ, Greenberg RJ. Improvements in vision-related quality of life in blind patients implanted with the Argus II Epiretinal Prosthesis. Clin Exp Optom. 2017;100(2):144–150. doi: 10.1111/cxo.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale PO, Sahel JA, Stanga PE, Hafezi F, Safran AB, Salzmann J, Santos A, Birch D, Spencer R, Cideciyan AV, de Juan E, Duncan JL, Eliott D, Fawzi A, Olmos de Koo LC, Ho AC, Brown G, Haller J, Regillo C, del Priore LV, Arditi A, Greenberg RJ. Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology. 2016;123(10):2248–2254. doi: 10.1016/j.ophtha.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho AC, Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Handa J, Barale PO, Sahel JA, Stanga PE, Hafezi F, Safran AB, Salzmann J, Santos A, Birch D, Spencer R, Cideciyan AV, de Juan E, Duncan JL, Eliott D, Fawzi A, Olmos de Koo LC, Brown GC, Haller JA, Regillo CD, del Priore LV, Arditi A, Geruschat DR, Greenberg RJ. Long-term results from an epiretinal prosthesis to restore sight to the blind. Ophthalmology. 2015;122(8):1547–1554. doi: 10.1016/j.ophtha.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, Cideciyan AV, Duncan JL, Eliott D, Filley E, Ho AC, Santos A, Safran AB, Arditi A, del Priore LV, Greenberg RJ. Interim results from the international trial of second sight's visual prosthesis. Ophthalmology. 2012;119(4):779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn JD, Ahuja AK, Caspi A, da Cruz L, Dagnelie G, Sahel JA, Greenberg RJ, McMahon MJ, Argus II Study Group FT The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol. 2013;131(2):183. doi: 10.1001/2013.jamaophthalmol.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Cruz L, Coley BF, Dorn J, Merlini F, Filley E, Christopher P, Chen FK, Wuyyuru V, Sahel J, Stanga P, Humayun M, Greenberg RJ, Dagnelie G, for the Argus II Study Group The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol. 2013;97(5):632–636. doi: 10.1136/bjophthalmol-2012-301525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry MP, Dagnelie G. Use of the Argus II retinal prosthesis to improve visual guidance of fine hand movements. Invest Ophthalmol Vis Sci. 2012;53(9):5095. doi: 10.1167/iovs.12-9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo YHL, Zhong JJ, da Cruz L. The use of Argus® II retinal prosthesis by blind subjects to achieve localisation and prehension of objects in 3-dimensional space. Graefes Arch Clin Exp Ophthalmol. 2015;253(11):1907–1914. doi: 10.1007/s00417-014-2912-z. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo S, Belting C, Cinelli L, Allegrini L, Genovesi-Ebert F, Barca F, di Bartolo E. The Argus II retinal prosthesis: 12-month outcomes from a single-study center. Am J Ophthalmol. 2014;157(6):1282–1290. doi: 10.1016/j.ajo.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 18.Kotecha A, Zhong J, Stewart D, da Cruz L. The Argus II prosthesis facilitates reaching and grasping tasks: a case series. BMC Ophthalmol. 2014;14:71. doi: 10.1186/1471-2415-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Quality Ontario. Retinal Prosthesis System for Advanced Retinitis Pigmentosa: A Health Technology Assessment. Ont Health Technol Assess Ser. 2016;16(14):1–63. [PMC free article] [PubMed] [Google Scholar]