Abstract
The consequences of SARS-CoV-2 infection in pregnancy have not been well defined. However, there have been a number of reports of poor maternal and fetal outcomes worldwide. This report presents a case of stillbirth with associated placental pathology during week 35 in an otherwise healthy pregnant woman with SARS-CoV-2 infection.
Placental findings in this case showed patchy acute chorionitis and diffuse infarction/villous necrosis of the placental parenchyma resulting in extensive vascular malperfusion. Fetal autopsy was most significant for placental findings and no congenital malformations were discovered. The findings in this case are consistent with reports in the literature of pathological placental changes associated with COVID-19.
This case of fetal demise in a woman with confirmed SARS-CoV-2 infection without any other medical or obstetric disorders and no alternate cause suggests that fetal death can be an outcome of COVID-19 during pregnancy. This outcome was supported by the histopathological findings in the placenta.
Continued research is imperative to confirm the findings in this case and multiple similar cases. Additionally, increased screening and collection of COVID-19 data specific to pregnant women and their fetuses and infants is needed to increase knowledge, support research efforts, and create guidelines for clinical practice that will prevent potential negative outcomes and loss of life.
Keywords: Placental pathology, Stillbirth, Pregnancy, COVID-19, SARS-CoV-2
Highlights
-
•
Describes a case of stillbirth during week 35 in an otherwise healthy pregnant woman with SARS-CoV-2 infection
-
•
Placental pathology associated with SARS-CoV-2 infection seen in the current literature is consistent in this case
-
•
This case suggests stillbirth is a potential outcome of COVID-19 infection during pregnancy
-
•
Findings warrant increased surveillance of and prevention of gaps in care for this vulnerable patient population
-
•
Findings warrant increased collection of COVID-19 data specific to pregnant women and continued placental pathology research research
1. Introduction
The global impact of the novel coronavirus (2019-nCoV, or SARS-CoV-2) has been well documented; however, studies and data to inform proper management of pregnant women and fetuses during the COVID-19 pandemic, though increasing recently, have been limited. There have been a number of reports of poor maternal and fetal outcomes worldwide, but few studies have directly linked these outcomes to COVID-19, likely due to poor data collection for pregnant women early on and the limited statistical power of smaller studies and observational reports available in the literature thus far.
Though limited data exists regarding the effects of COVID-19 on fetal health and development, concern for and clinical reports of increased rates of miscarriage and stillbirth, growth restriction, and preterm births have been emerging since early in the pandemic [[1], [2], [3]]. More recently, reports from the CDC have shown stillbirth rates of 2.2%–3% in multistate surveillance studies [4,5]. When compared to the average US stillbirth rate of <1% before the pandemic, this data trend is alarming [6].
Additionally, emerging evidence has suggested potential COVID-19-induced placental pathology with associated fetal death in some cases [1,2,7,8]. The placental pathology in COVID-19 observed and discussed in five existing studies are also present in this case and confirmed with placental pathology study and fetal autopsy [[7], [8], [9], [10], [11]]. Interestingly, similarities in the clinical course and features for patients described in case reports by Richtmann et al. and Baud et al. (nulliparous, otherwise healthy women with no pregnancy complications who were managed as outpatients with relatively mild forms of COVID-19) are also consistent in this case [1,2].
We present a case of stillbirth with associated placental pathology during week 35 in an otherwise healthy pregnant woman with SARS-CoV-2 infection.
2. Case Description
A 31-year-old asymptomatic, healthy, white, nulliparous woman was well until week 32 of her pregnancy. Of note, this patient is an essential healthcare worker. She was practicing as a primary care clinician and actively caring for patients during the COVID-19 pandemic in the midst of widespread community transmission. The patient then developed fatigue, rhinorrhea, body aches, mild headache, sore throat, nausea and vomiting, diarrhea, cough, chills, and anosmia during her illness. She reported no fever, shortness of breath, or respiratory distress. Her illness had not required hospitalization. Positive RT-PCR for SARS-CoV-2 results were received using nasopharyngeal swabs collected on day 3 and 14 of the illness.
Unfortunately, the patient was not evaluated during her illness and experienced a 27-day gap in prenatal care due to ill-advised hospital-specific clinical policies limiting care of pregnant women until complete resolution of symptoms and evidence of negative RT-PCR COVID-19 testing.
The patient was evaluated at an outpatient obstetric clinic at 35 4/7 weeks of gestation for routine prenatal care after recent COVID-19 infection. She was afebrile, HR 103, BP 103/62, BMI 27.81, and physical exam was unremarkable except for discovery of absent fetal heart rate (FHR). There was no history of abdominal pain, vaginal bleeding, uterine contractions, or poor fetal movement. There was no report of fever, new illness, or injury. There were no comorbidities or pregnancy complications. Routine laboratory tests, genetic screenings, and all anomaly scans were normal.
After diagnosis of intrauterine fetal demise via obstetric ultrasound the patient was admitted to labor and delivery. Continued positive RT-PCR results were received upon admission using a nasopharyngeal swab (COVID day 24).
Physical exam was normal and fetal membranes were intact, but FHR was absent and repeat obstetric ultrasound confirmed fetal demise. Labor was induced with misoprostol and a female fetus was delivered 12 h later via vaginal delivery.
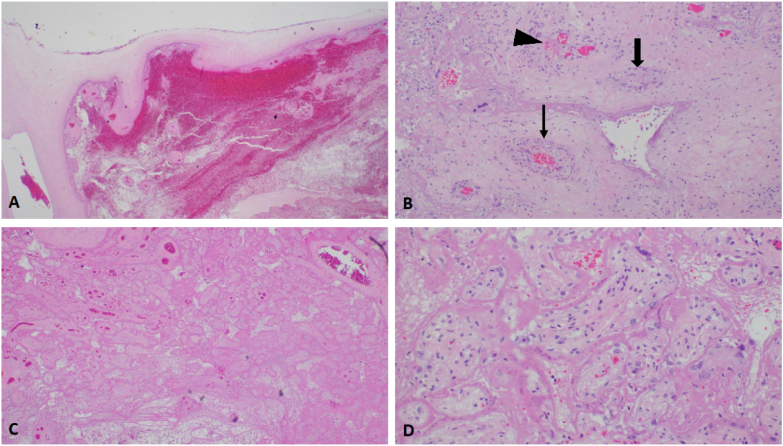
Histopathological examination of the placenta showed patchy acute chorionitis and diffuse infarction/villous necrosis of the placental parenchyma. No amnionitis or funisitis was identified. Fetal autopsy was most significant for placental findings with no congenital malformations found on thorough, unrestricted autopsy. The placenta showed extensive fetal vascular malperfusion and parenchymal infarct involving approximately 75% of the placenta (Fig. 1).
Fig. 1.
Placental histology.
A: Histologic section shows subchorial hematoma (hematoxylin and eosin, X20).
B. Stem villous showing complete occlusion of stem villous vessel (thick arrow), near total occlusion of another vessel (thin arrow) and thrombus/recanalization of a third (arrowhead) (H&E, X100).
C. Placenta infarct (H&E, X20).
D. Several avascular villi (H&E, X100).
At 1-week follow-up there were no physical complications. Psychiatric sequelae were as expected.
No alternate causes of fetal demise were identified. Tests for toxoplasma, HSV-2, rubella, and CMV, and urinalysis and urine drug screen were all negative. HSV-1 showed positive antibodies from past infection only. CBC, CMP, and coagulation studies were clinically acceptable, except for mild anemia (Hb 10.8 and Hct 32.2 post-delivery) and elevated WBC (12.3 pre-delivery, 20.1 post-delivery). Placental culture, maternal side, showed light E. coli growth, while culture of the fetal side of the placenta showed only normal flora. The patient was treated with a 7-day course of cephalexin. Full maternal fetal medicine lab work-up was unremarkable, including antiphospholipid antibody panel, phosphatidylserine AB (IgA,IgG,IgM), cardiolipin AB (IgA,IgG,IgM), and lupus anticoagulant evaluation with reflex.
3. Discussion
This case of fetal demise in a woman with confirmed COVID-19 with no significant preexisting conditions or pregnancy complications suggests that fetal death is a potential outcome of COVID-19 during pregnancy, supported further by the histopathological findings in the placenta.
The placental findings in this case indicate extensive fetal vascular malperfusion and parenchymal infarcts resulting in a severe loss of a significant percentage of chorionic villi. Similar findings were present in several studies. A case series described by Richtmann et al. found signs of significant placental inflammation in all patients and some cases of villitis and intervillitis with associated deposits of intervillous fibrin [2]. A report by Baud et al. described a single case of placental pathology involving mixed inflammatory infiltrates composed of neutrophils and monocytes in the subchorial space and unspecific increased intervillous fibrin deposition [1]. Lastly, a slightly larger study by Shanes et al. found increased rates of maternal vascular malperfusion features, intervillous thrombi, and increased incidence of chorangiosis [7].
Significant changes have been noted in numerous investigations of placental tissue secondary to maternal COVID-19. However, clarification of the direct and indirect ways SARS-CoV-2 may affect the placenta are still under investigation [7,[9], [10], [11]]. A recent study examining the physiologic interface between mother and fetus by Taglauer et al. found that SARS-CoV-2 spike glycoprotein (CoV2 SP) was present within the villious placenta of COVID-19-positive pregnant women with and without evidence of fetal transmission. Additionally, there was expression of viral entry proteins (ACE2 and TMPRSS2) located in an area of the placenta that is an important interface between mother and fetus [10]. These findings provide further insight into the potential underlying pathogenic mechanisms SARS-CoV-2 may utilize in pregnant women.
Strengths of this report include laboratory-confirmed infection using reliable testing methods and the completion of fetal autopsy with family consent by an experienced pediatric pathologist. A limitation of this study is the single case. Additionally, fetal and placental SARS-CoV-2 RT-PCR testing was not performed. During hospitalization, requests for these tests by the patient were denied, reportedly due to local health department restrictions in place at the time.
This case and current evidence suggest that the adverse outcomes seen with other coronaviruses, including growth restriction, preterm delivery, miscarriage, and stillbirth are potential outcomes of COVID-19 infection during pregnancy [3,7,8]. As such, pregnant women with this novel coronavirus should be monitored closely and providers should act cautiously as they await further data and results of larger studies.
Over 50,000 cases of confirmed SARS-CoV-2 infection and 60 deaths have been reported in pregnant women in the US since January 2020 [12]. These values likely underestimate the reach of SARS-CoV-2 in pregnancy due to poor data collection and limited testing capacity early in the pandemic. Additionally, significant rates of asymptomatic women with SARS-CoV-2 infeciton have been discovered upon obstetric hospitalization [4,5]. Increased collection of COVID-19 data specific to pregnant women and their fetuses and infants is needed to increase knowledge and support research efforts, and can be utilized to create guidelines for clinical practice that will prevent potential negative outcomes and loss of life.
Universal testing for women admitted for delivery has been discussed since early on in the pandemic; however, as testing becomes more available and increased risk is continually uncovered for pregnant women and fetal health, universal screening procedures throughout pregnancy should be considered to increase surveillance, especially given the potential for poor fetal outcomes in asymptomatic and minimally symptomatic mothers.
More investigation and rigorous research are warranted to confirm placental pathology mechanisms in relation to COVID-19. Additionally, investigation of details like the effect of gestation at time of infection and the role of various potential therapies can help guide clinical practice.
Until more definitive answers are available, increased surveillance of pregnant women and fetuses is vital. Clinicians should remain vigilant, preventing gaps in care for this vulnerable population.
Acknowledgments
Contributors
Tiffany M. Poisson contributed to the writing, formatting, research, editing, and final review of the manuscript.
Gerald Pierone Jr. provided assistance with writing and editing at many draft phases, research, and final review of manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient Consent
Written consent was obtained from the patient
Provenance and Peer Review
This case report was peer reviewed
Acknowledgment
Dr. Ali G. Saad contributed creation of placental images and content for figures and reviewed the manuscript.
Contributor Information
Tiffany M. Poisson, Email: tpoisson@wfhcfl.org.
Gerald Pierone, Jr, Email: gpierone@wfhcfl.org.
References
- 1.Baud D., Greub G., Favre G. Second-Trimester miscarriage in a pregnant woman With SARS-CoV-2 infection [Internet] JAMA. 2020 Apr 30;323(21):2198–2200. doi: 10.1001/jama.2020.7233. cited 2020 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richtmann R., Torloni M.R., Oyamada Otani A.R., Levi J.E., Tobara M.C., de Almeida Silva C., Dias L., Miglioli-Galvão L., Silva P.M., Kondo M.M. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series [Internet] Case Rep. Womens Health. 2020 Jul 12;27 doi: 10.1016/j.crwh.2020.e00243. e00243. cited 2020 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections [Internet] Viruses. 2020 Feb 10;12(2):194. doi: 10.3390/v12020194. cited 2020 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahoy M.J., Whitaker M., O’Halloran A. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 — COVID-NET, 13 States, March 1–August 22, 2020 [Internet] MMWR Morb. Mortal. Wkly Rep. 2020 Sept;69:1347–1354. doi: 10.15585/mmwr.mm6938e1. cited Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panagiotakopoulos L., Myers T.R., Gee J. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics — eight U.S. Health Care Centers, March 1–May 30, 2020 [Internet] MMWR Morb. Mortal. Wkly Rep. 2020 Sept;69:1355–1359. doi: 10.15585/mmwr.mm6938e2. cited Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC Stillbirth Homepage. Data and Statistics. 2020 Sept 11. https://www.cdc.gov/ncbddd/stillbirth/facts.html [cited 2020 Nov 19] Available online.
- 7.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19 [Internet] Am. J. Clin. Pathol. 2020 Jul;154(1):23–32. doi: 10.1093/ajcp/aqaa089. cited 2020 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabary M., Khanmohammadi S., Araghi F., Dadkhahfar S., Tavangar S.M. Pathologic features of COVID-19: A concise review [Internet] Pathol. Res. Pract. 2020 Sep.;216(9):153097. doi: 10.1016/j.prp.2020.153097. Published online 2020 Jul 4 [cited 2020 Aug 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19 [Internet] Placenta. 2020 Sep 15;99:45–49. doi: 10.1016/j.placenta.2020.07.022. Published online 2020 Jul 25 [cited 2020 Aug 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taglauer E., Benarroch Y., Rop K., Barnett E., Sabharwal V., Yarrington C., Wachman E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads [Internet] Placenta. 2020 doi: 10.1016/j.placenta.2020.08.015. Published online 2020 Aug 25 [cited 2020 Aug 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey J.J., Magro C.M., Ma L.X., Nuovo G.J., Baergen R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients [Internet] Ann. Diagn. Pathol. 2020 Jun. 46:151530. doi: 10.1016/j.anndiagpath.2020.151530. Published online 2020 Apr 25 [cited 2020 Aug 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC Data on COVID-19 during Pregnancy: Severity of Maternal Illness [Internet] 2020 Dec 28. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19.html [cited 2020 Dec 31]. Available Online.