Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a member of the coronavirus family, which causes coronavirus disease 2019 (COVID-19). The phenotype of the disease varies from asymptomatic, to a mild phenotype, through to the severe form of acute respiratory distress syndrome (ARDS), which often leads to death, especially in those with underlying diseases. It has been reported that those who suffer from cancer (especially lung cancer and hematological malignancies) are at higher risk of serious complications and death from COVID-19. Some cancer treatments such as CAR T cell therapy can produce a cytokine storm, which is also a hallmark of severe COVID-19. Therefore, patients receiving CAR T cells are at higher risk if they become infected with COVID-19, and could be treated with anti-cytokine approaches.
1. Introduction
In early December 2019, the coronavirus family again became a controversial and major public health issue all over the world, because of its newest member, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused coronavirus disease 2019 (COVID-19) [1,2]. Members of the coronaviridae family are large, enveloped, single-stranded RNA viruses. They have a spherical morphology, with numerous large glycoprotein spikes on their envelope [3,4]. As noted in earlier reports, COVID-19 originated in Wuhan, Hubei Province in China, where bats were considered to be the possible primary host. Studies are currently ongoing to shed light on this issue, and to find the intermediate host through which COVID-19 was transferred to humans. A high rate of human to human transmission was established at the beginning of the outbreak [5]. In humans, the respiratory tract is usually affected by Human Coronaviruses (HCoV), while the most common reported symptoms, are fever, cough, rhinorrhea, sore throat, and loss of sense of smell [6,7]. Patients with cancer are thought to be at higher risk of infection with SARS-CoV-2, and for developing more severe phenotypes of COVID-19 [8]. Therefore, patients with cancer need emergency treatment for COVID-19. Meanwhile, there are several ongoing clinical trials for the management of COVID-19 in cancer patients.
2. T cell count in patients with COVID-19
T lymphocytes play an essential role to attack and destroy tumor cells and virus infected normal cells [9,10]. Tumor-associated antigens (TAA) are expressed on tumor cells, and can be derived from any protein or glycoprotein, which T cells are able to recognize as foreign [11]. CD8+ cytotoxic T cells play a key role in antitumor immune response, while CD4+ T cells play a complementary role in the recognition of tumor cells [10]. The tumor microenvironment (TME) can suppress the immune system via a variety of mechanisms. For instance, alterations in the numbers of peripheral T cells are one consequence of signaling by the TME. As the cancer progresses, the percentage of exhausted T cells expands, and since they are no longer able to produce effector cytokines, the expression of inhibitory receptors is increased [12]. The interaction between inhibitory receptors such as PD-1/PD-L1 expressed on Treg cells within the TME, and CD8+ T cells inhibits the proliferation of cytotoxic T cells [13]. On the other side, recent evidence has shown critical alterations in T cell function and numbers during COVID-19 infection. It has been shown that patients with COVID-19 display lymphopenia, which is leads to a low total number of T cells, including CD8+ T cells and CD4+ T cells [14]. Furthermore, T cells with an exhausted phenotype have been observed during both acute and chronic viral infections, and the percentage of PD-1 and Tim-3 expressed on the T cell surface is increased in symptomatic stages of infection . Detailed studies have shown that >70% of patients in the initial stage of COVID-19 infection experienced a reduction in CD4+ and CD8+ T cell numbers, while patients in the severe stage of disease showed a decrease of up to 95% in total T cell counts [9] (Fig. 1 ).
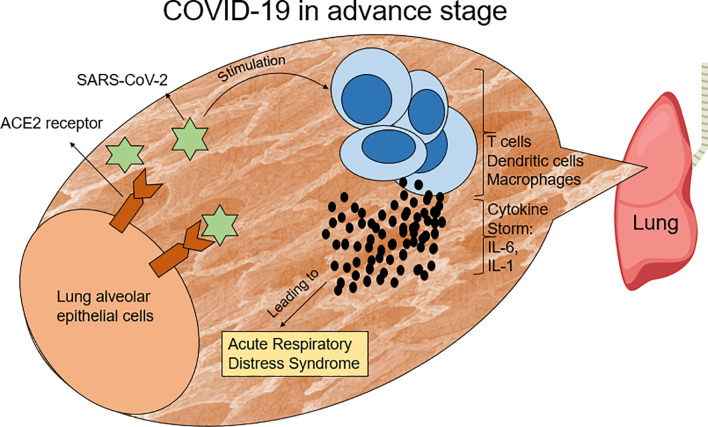
Fig. 1.
SARS-CoV-2 stimulates lymphocytes and other immune cells to produce pro-inflammatory cytokines, such as IL-6 and IL-1, which produce a condition, named the cytokine storm. This situation leads to acute respiratory distress syndrome (ARDS) in COVID-19 patients.
According to the results of previous studies the major immunological abnormalities in patients with severe COVID-19, include depletion of CD4+, CD8+ T cells, and natural killer (NK) cells leading to lymphopenia [15]. Therefore poor outcomes such as mortality, ARDS, and ICU admission could be expected in severe COVID-19 patients with lymphopenia [16,17]. On the other hand, lymphopenia may increase the risk of hospitalization and emergency medicine [18]. Some therapeutic approaches have been recommended to prevent lymphopenia in COVID-19 patients. IL-7 therapy is one example, which could be used to restore the lymphocyte count (more than 2-fold) in COVID-19 patients [19]. Based on previous clinical trials, administration of recombinant human IL-7 (rhIL-7) is now available for patients with cancer, which leads to an increase in peripheral CD3+, CD4+, and CD8+ lymphocytes [20]. it is well known that some types of cancer treatment, like chemotherapy or radiation therapy may cause lymphopenia in cancer patients [[21], [22], [23]]. For example, severe treatment-related lymphopenia (TRL) has been reported in more than 40% of patients receiving combined chemoradiation treatment, which negatively influenced survival because of further tumor progression [15]. Hence, it is considered likely that the risk of COVID-19 infection may be higher in cancer patients.
3. COVID-19 in patients with cancer
Patients with cancer are considered to be more susceptible to COVID-19 infection, due to the disease itself or to the effects of anticancer therapy [8]. Of note, the symptoms of COVID-19 have been shown to be more severe among patients with cancer [24]. The following pathological features, including alveolar damage with fibrin rich hyaline membranes, the presence of multinucleated giant cells, chest radiographs showing patchy consolidation in the lungs with ground-glass opacity (GGO) have been reported in COVID-19 patients with cancer [8,25]. However, hyaline membrane formation was not observed in two COVID-19-infected lung cancer patients, which suggested they could be pre-symptomatic COVID-19 cases [26]. Despite the high susceptibility of patients with cancer to COVID-19, in a study conducted at the Institute of Cancer and Genomic Sciences, University of Birmingham, there was no difference between the high mortality rates of COVID-19 in patients who were receiving cancer therapy and those not on active treatment [27].
Furthermore, a cohort analysis was performed by the National Cancer Institute (NCI) to determine how COVID-19 affected the outcomes of patients undergoing cancer treatment, and whether being diagnosed with cancer affected COVID-19. Two thousand adult patients with cancer were included in this trial. The study gathered information and blood samples from patients with hematopoietic and lymphoid cell neoplasms, malignant solid neoplasms, and metastatic malignant solid neoplasms [28]. Another clinical study at the Peter MacCallum Cancer Centre contains 2282 cancer patients who are following a special protocol, In that study, three standard agents are being used, including Interferon-α (IFN-α), Selinexor, and Lenzilumab. The aim of that study was to provide practical guidelines for prevention and treatment of COVID-19 in patients with cancer [29].
4. Type of cancer and severity of COVID-19 symptoms
The progression or further deterioration of COVID-19 patients depends on the type of cancer they are suffering from. Hematologic cancers and lung cancer are considered the deadliest cancer types, with the highest severity and death rates in patients who suffer from both cancer and COVID-19 infection. Hematologic cancer is considered to be the worst cancer type for concomitant COVID-19 infection, because of the severe immunosuppression it can produce [30].
Non-small-cell lung cancer (NSCLC) is the most prevalent form of lung cancer, and is associated with severe hypoxia [31]. Hypoxia is one of the prominent features of the TME in solid tumors. Cancer cells show increased consumption of oxygen due to their rapid proliferation, resulting in reduced oxygen levels in regions of solid tumors. In fact, hypoxic regions lead to necrosis and tumor progression, and can make the tumor cells resistant to radiotherapy and chemotherapy [31,32]. Hypoxia not only occurs during lung cancer, but it is also a consequence of the COVID-19 infection. Some symptoms of coronavirus infection like pneumonia, result in more pronounced respiratory failure [33]. Patients with COVID-19 infection, especially elderly individuals, can also experience the condition named “silent hypoxia” [34]. Unfortunately, silent hypoxia in the initial stages of COVID-19 pneumonia can be without any noticeable signs, since patients often have only mild difficulty in breathing. When the pneumonia is severe, patients find it difficult to breathe, and may need hospital admission. Unlike other types of pneumonia, the alveoli in COVID-19 patient lungs are deflated down to a very small volume and cannot absorb oxygen from the inhaled air. However, there is no fluid or pus in the lungs which still normally remove carbon dioxide. Rapid diagnosis of silent hypoxia is critical to prevent the pneumonia from progressing to a severe type [35]. According to a study by Xie et al., severe lymphopenia and high levels of C-reactive protein (CRP) are related to the severity of hypoxemia and the mortality rate in these patients [36].
However, there is no agreement about the course of COVID-19 infection in patients with lung cancer. Some studies asserted that it may be longer and more severe, while others held the opposite view [[37], [38], [39]]. Lung cancer could be more dangerous than other solid tumors in COVID-19 patients, because of the pre-existing lung dysfunction. Furthermore, primary or metastatic lung tumors could make patients more vulnerable to rapid decline and death [30].
5. CAR T cell cancer therapy in the COVID-19 setting
Chimeric antigen receptor (CAR) T cell is a promising new treatment for cancer. Unfortunately, the COVID-19 pandemic has had a negative impact on CAR T cells, as it has had on other cellular therapies [40]. Despite the impressive results of CAR T cell therapy on various types of cancer, it also has some serious adverse effects, such as cytokine release syndrome (CRS) and neurotoxicity [41]. CRS, also known as cytokine storm syndrome (CSS) or hypercytokinemia, is a systemic inflammatory response that must be managed in order to avoid tissue damage, and to allow the therapy to continue for a sufficient time [[42], [43], [44]]. CRS is caused by treatments like CAR T cell therapy and certain other drugs [43]. The cytokine storm is also able to stimulate the acute respiratory distress syndrome (ARDS), which is a leading cause of death in patients with COVID-19 [30]. CRS frequently reaches its peak level some days after the CAR T cell infusion, along with profound shock and multiple organ dysfunction syndrome (MODS), which can become a life-threatening issue [45]. High levels of IL-6, IL-10, and interferon (IFN)-Υ are detected in the serum of CRS patients, and IL-6 is the most important cytokine [43]. COVID-19 patients in dire need of ICU support have shown higher concentrations of IP10, MCP1, MIP1A, and TNFα. The cytokine storm would be expected to be correlated with disease severity [46]. Elevated IL-6 levels in COVID-19 can lead to respiratory failure, ARDS, and adverse clinical outcomes [47]. Some treatment approaches are now being considered for reduction of CNS toxicity in COVID-19 patients, including administration of tocilizumab or Ibrutinib [48,49]. Unfortunately, COVID-19 pneumonia is accompanied by mild or severe cytokine storms in late-stage disease, which may result in the death of the patient. IL-6 is important in CRS in COVID-19 patients, because the treatment outcome of severe COVID-19 patients can be predicted via the degree of inhibition of the IL-6 signal transduction pathway [50].
Patients treated with CAR T cells are priority candidates for allocation of healthcare resources, such as ICU beds, dialysis machines, and ventilators, if they have been exposed to COVID-19. Logistical and transportation resources are potential limitations in the delivery of CAR-T products, and will restrict the accessibility of CAR T cell therapy for COVID-19 patients. As a result, alternative cancer treatments should be suggested for patients who live in countries, regions, or communities with a high prevalence of COVID-19 [51].
On the other hand, secondary hematophagocytic lymphohistiocytosis (HLH) is a condition that should be considered, because it may occur in patients with CRS due to COVID-19. An interleukin-1 (IL-1) receptor antagonist called anakinra, is under study to treat SARS-COV-2 with HLH [52]. Thus, patients who have been treated with CAR T cells can be faced with a serious hurdle, if they are infected with COVID-19 [53]. Furthermore, in immunotherapy, treatment-related toxicity is accompanied by a high release of cytokines that cause an inappropriate environment for lung epithelial cells. It is important to note that these findings are not yet conclusive, because larger sample sizes are needed to confirm the generalizability of the results [30]. There is an ongoing trial to test the efficacy and safety of NKG2D-ACE2 CAR-NK cells in COVID-19 patients. A phase I/II clinical trial is being conducted on a large group of patients (90 participants). Several features make NKG2D-ACE2 CAR-NK cells ideal for treating COVID-19 patients. This new CAR T cell preparation seems to be safe, with less severe side effects such as CRS, and shows increased activity, with improved pharmacokinetic properties. NKG2D-ACE2 CAR-NK cells show longer persistence and enhanced target cytotoxicity compared to other CAR-T cell types. NKG2D-ACE2 CAR-NK cells can be directed against the S protein of SARS-CoV-2, and NKG2DL on the surface of infected cells [54]. Efforts are being continued to find an improved solution for the treatment of COVID-19 in cancer patients.
Declaration of Competing Interest
None
References
- 1.Hanaei S., Rezaei N. COVID-19: developing from an outbreak to a pandemic. Arch Med Res. 2020;51(6):582–584. doi: 10.1016/j.arcmed.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabbari P., Jabbari F., Ebrahimi S., Rezaei N. COVID-19: a chimera of two pandemics. Disaster Med Public Health Prep. 2020:1–3. doi: 10.1017/dmp.2020.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne S.J.V. Vol. 149. 2017. Family Coronaviridae. [Google Scholar]
- 4.Lotfi M., Rezaei N. SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. 2020;92(10):1864–1874. doi: 10.1002/jmv.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique RJJoAR . 2020. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerna G., Percivalle E., Sarasini A., Campanini G., Piralla A., Rovida F., et al. Vol. 38. 2007. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients; pp. 244–250. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacs D., Flowers D., Clarke J., Valman H. MacNaughton MJAodic. Epidemiol. Coronavirus Respiratory Infections. 1983;58(7):500–503. doi: 10.1136/adc.58.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. 2020. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Vol. 11. 2020. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) p. 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Y.P., Jeng C.J., SCJ Chen. Vol. 2011. 2011. The roles of CD4+ T cells in tumor immunity. [Google Scholar]
- 11.McKee M.D., Roszkowski J.J., Nishimura MIJJotm . Vol. 3. 2005. T cell avidity and tumor recognition: implications and therapeutic strategies; p. 35. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y., Li Y., Zhu BJCd, disease . Vol. 6. 2015. T-cell exhaustion in the tumor microenvironment; p. e1792. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H.R., Park H.J., Son J., Lee J.G., Chung K.Y., Cho N.H., et al. Vol. 7. 2019. Tumor microenvironment dictates regulatory T cell phenotype: Upregulated immune checkpoints reinforce suppressive function; p. 339. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fathi N., Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafarzadeh A., Jafarzadeh S., Nozari P., Mokhtari P., Nemati MJSJoI . 2020. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. e12967. [DOI] [PubMed] [Google Scholar]
- 16.Huang I., Pranata RJJoIC . Vol. 8. 2020. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis; pp. 1–10. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W., Berube J., McNamara M., Saksena S., Hartman M., Arshad T., et al. Vol. 97. 2020. Lymphocyte subset counts in COVID-19 patients: a meta-analysis; pp. 772–776. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., et al. Vol. 5. 2020. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study; pp. 1–3. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laterre P.F., François B., Collienne C., Hantson P., Jeannet R., Remy K.E., et al. Vol. 3. 2020. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19) 7. e2016485-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sportès C., Babb R.R., Krumlauf M.C., Hakim F.T., Steinberg S.M., Chow C.K., et al. Vol. 16. 2010. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy; pp. 727–735. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson J.H., Aldape K.D., Archer G.E., Coan A., Desjardins A., Friedman A.H., et al. Vol. 13. 2011. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma; pp. 324–333. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg C., Ray-Coquard I., Philip I., Clapisson G., Bendriss-Vermare N., Menetrier-Caux C., et al. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. 2004;101:2675–2680. doi: 10.1002/cncr.20688. 11. [DOI] [PubMed] [Google Scholar]
- 23.Pike L.R., Bang A., Mahal B.A., Taylor A., Krishnan M., Spektor A., et al. Vol. 103. 2019. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors; pp. 142–151. 1. [DOI] [PubMed] [Google Scholar]
- 24.Sidaway PJNRCO . Vol. 1. 2020. COVID-19 and cancer: what we know so far. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respiratory Soc. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S-YJJoTO . 2020. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee L.Y., Cazier J.B., Starkey T., Turnbull C., Team UCCMP, Kerr R., et al. 2020. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.https://clinicaltrials.gov/ct2/show/NCT04387656. NCI COVID-19 in Cancer Patients, NCCAPS Study 2020 [updated December 24, 2020.
- 29.https://clinicaltrials.gov/ct2/show/NCT04534725. COVID-19 Prevention and Treatment in Cancer; a Sequential Multiple Assignment Randomised Trial; (C-SMART) 2020 [updated December 22, 2020.
- 30.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Vol. 10. 2020. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak; pp. 783–791. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dehdashti F., Mintun M.A., Lewis J.S., Bradley J., Govindan R., Laforest R., et al. Vol. 30. 2003. In vivo assessment of tumor hypoxia in lung cancer with 60 Cu-ATSM; pp. 844–850. 6. [DOI] [PubMed] [Google Scholar]
- 32.Karsch-Bluman A., Feiglin A., Arbib E., Stern T., Shoval H., Schwob O., et al. Vol. 38. 2019. Tissue necrosis and its role in cancer progression; pp. 1920–1935. 11. [DOI] [PubMed] [Google Scholar]
- 33.Ottestad W., Seim M., Mæhlen JOJTfDnl . 2020. COVID-19 with silent hypoxemia. [DOI] [PubMed] [Google Scholar]
- 34.Tobin M.J., Laghi F., Jubran AJAJoR, Medicine CC . 2020. Why COVID-19 silent hypoxemia is baffling to physicians. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teo JJJoms . Vol. 44. 2020. Early Detection of Silent Hypoxia in Covid-19 Pneumonia Using Smartphone Pulse Oximetry; pp. 1–2. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky ASJIcm . 2020. Critical care crisis and some recommendations during the COVID-19 epidemic in China; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo J., Rizvi H., Preeshagul I.R., Egger J.V., Hoyos D., Bandlamudi C., et al. 2020. COVID-19 in patients with lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewnard J.A., Liu V.X., Jackson M.L., Schmidt M.A., Jewell B.L., Flores J.P., et al. Vol. 369. 2020. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. 2020. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachanova V., Bishop M.R., Dahi P., Dholaria B., Grupp S.A., Hayes-Lattin B., et al. 2020. CAR T cell therapy during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., et al. Vol. 24. 2018. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells; pp. 739–748. 6. [DOI] [PubMed] [Google Scholar]
- 42.Riegler L.L., Jones G.P., Lee DWJT, management cr Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. 2019;15:323. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., et al. Vol. 6. 2018. Cytokine release syndrome; p. 56. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: Friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J., et al. Vol. 45. 2017. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia; p. e124. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Vol. 395. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China; pp. 497–506. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 48.Yáñez L., Sánchez-Escamilla M., MAJH Perales. Vol. 3. 2019. CAR T cell toxicity: current management and future directions. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahanshahlu L., Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother. 2020;129:110337. doi: 10.1016/j.biopha.2020.110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G-QJIjoaa . 2020. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y., Yin E.T.S., Yang Y., Wu H., Wei G., Su J., et al. 2020. CAR T-cell treatment during the COVID-19, Pandemic: Management Strategies and Challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., et al. 2020. Favorable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistiocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal S., CHJCD June. Vol. 10. 2020. Harnessing CAR T-cell Insights to Develop Treatments for Hyperinflammatory Responses in Patients with COVID-19; pp. 775–778. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.https://clinicaltrials.gov/ct2/show/NCT04324996. A Phase I/II Study of Universal Off-the-shelf NKG2D-ACE2 CAR-NK Cells for Therapy of COVID-19 2020 [updated November 17, 2020].