Abstract
Objective
To assess the efficacy and safety of ribavirin and interferon-α (RBV/IFN-α) therapy in COVID-19 patients.
Methods
A multicenter, retrospective cohort study of COVID-19 patients admitted to 4 hospitals in Hubei Province, China, from 31 December 2019 to 31 March 2020. Patients were divided into 2 groups according to their exposure to RBV/IFN-α therapy within 48 h of admission. Mixed-effect Cox model and Logistic regression were used to explore the association between early treatments of RBV/IFN-α and primary outcomes.
Results
Of 2037 patients included, 1281 received RBV/IFN-α (RBV, IFN-α or RBV combined with IFN-α) treatments and 756 received none of these treatments. In a mixed effect model, RBV/IFN-α therapy was not associated with progression from non-severe into severe type (adjusted hazard ratio (aHR) = 1.09, 95% CI: 0.88−1.36) or with reduction in 30-day mortality (aHR = 0.89, 95% CI: 0.61−1.30). However, it was associated with a higher probability of hospital stay >15 days (adjusted odds ratio (aOR) = 2.11, 95% CI: 1.68−2.64) compared with no RBV/IFN-α therapy. The propensity score-matched cohort and subgroup analysis displayed similar results.
Conclusion
RBV/IFN-α therapy was not observed to improve clinical outcomes in COVID-19 patients suggesting that RBV/IFN-α therapy should be avoided in COVID-19 treatment.
Keywords: COVID-19, Antiviral therapy, Ribavirin, Interferon-α, Cohort studies
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected more than 83.3 million patients with more than 1,831,000 deaths worldwide (WHO, 2021a). This pandemic has brought a huge challenge to the global public health system (Wu and McGoogan, 2020, WHO, 2020a). Except for the experience of treatment of earlier strains of coronavirus, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), there was insufficient evidence to support a specific antiviral therapy (Sanders et al., 2020). In addition to the urgency of developing a vaccine looking for effective therapy for patients with COVID-19 is also an important issue.
Many repurposed drugs have been shown to have in vitro activity against the close relatives of SARS-CoV-2 which are all beta-coronaviruses (Liu et al., 2020, Sallard et al., 2020). Among these, antivirals such as ribavirin and interferon-α are provisionally recommended in the Chinese guidelines of treatments against COVID-19 and such as ribavirin and interferon-β are recommended in the Iranian treatment protocol (National Health Commission of the People’s Republic of China, 2020, Jamaati et al., 2020). However, the guidelines issued by the World Health Organization (WHO) and National Institutes of Health recommend that interferons (α, β) with or without ribavirin not be administered as treatment or prophylaxis for COVID-19 outside of the context of clinical trials (National Institutes of Health, 2020, WHO, 2021b). Ribavirin is a common antiviral drug that inhibits the replication and spread of multiple viruses. Interferon-α is an important type-I interferon with a broad antiviral activity in vitro. Reviews of the available scientific literature suggested that ribavirin and interferon-α might be used alone or in combination to treat coronavirus infections, including SARS and MERS, but conclusions about their clinical efficacy were inconsistent (Chen et al., 2004, Khalid et al., 2015, Omrani et al., 2014, Arabi et al., 2020, Falzarano et al., 2013, Arabi et al., 2017). For example, a retrospective cohort study found that ribavirin and interferon-α therapy was associated with significantly improved survival at 14 days in patients with severe MERS, but not at 28 days (Omrani et al., 2014). Another multicenter observational study showed that ribavirin and interferon-α therapy for critically ill patients with MERS was not associated with a reduction in 90-day mortality or faster MERS-CoV RNA clearance (Arabi et al., 2020). The combination of interferon-α with ribavirin gave excellent results in MERS-CoV infected rhesus macaques (Falzarano et al., 2013) but was inconclusive in humans (Arabi et al., 2017).
Although SARS-COV-2 presents similar properties with SARS-COV and MERS-COV, the viral load of SARS and MERS peaks at around day 7–10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza (Cheng et al., 2004, To et al., 2020). Some researchers think that interferon therapy should be limited to the early phases of the infection (Sallard et al., 2020, To et al., 2020, Siddiqi and Mehra, 2020). Reliable evidence on whether early therapy with ribavirin and interferon-α alone or in combination has an impact on progression, outcome and safety in admitted patients with COVID-19 is required. Large randomized controlled trials (RCTs) can provide the best evidence for drug efficacy evaluation. A randomized, open-label clinical trial in China in patients with mild to moderate COVID-19 evaluated the effectiveness of ribavirin plus interferon-α, lopinavir/ritonavir plus interferon-α, and ribavirin plus lopinavir/ritonavir plus interferon-α, and found that there were no significant differences among the 3 regimens in terms of antiviral effectiveness (Huang et al., 2020). Although clinical trials have provided some evidence of the efficacy of ribavirin and interferon-α therapy (Huang et al., 2020, Zhou et al., 2020) the sample size of these studies was small, therefore further research is required.
The present study used a retrospective cohort design to assess the impact of early treatment with ribavirin and/or interferon-α (RBV/IFN-α) on disease progression, mortality rate, length of hospital stay, and safety in hospitalized patients with COVID-19, to provide clinical evidence for screening effective antiviral drugs and optimizing treatment regimens for patients with COVID-19.
Methods
Ethical statement
This study was approved by the Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology [Number:2020IECA252]. The informed consent from patients was waived by the Ethics Committee because of the retrospective nature of the study.
Study design and patients
This multicenter, retrospective cohort study analyzed data on all eligible hospitalized patients with COVID-19 admitted to 4 hospitals in Hubei Province, China including the Central Hospital of Wuhan of Tongji Medical College, Huazhong University of Science and Technology; Wuhan Red Cross Hospital; the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture; and Lichuan People’s Hospital.
The diagnosis of COVID-19 was confirmed according to the WHO interim guidance and the Diagnosis and Treatment Protocol for Coronavirus Pneumonia (trial version 7) released by the National Health Commission of China (National Health Commission of the People’s Republic of China, 2020, WHO, 2020b). Based on the definition of the protocol, patients were divided into a non-severe and severe type according to respiratory rate, pulse oxygen saturation and acute organ failure. Patients were categorized as severe if they had any of the following criteria: respiratory rate (RR) ≥30 breaths/min, pulse oxygen saturation (SpO2) ≤93%, shock, or acute organ failure. The inclusion criteria were: (1) patients with COVID-19, aged ≥18 years, who were admitted to the above-mentioned hospitals from 31 December 2019 to 31 March 2020; (2) patients who received the treatments of interest or none of these treatments within 48 h of admission. The exclusion criteria contained: (1) patients with incomplete medical records (e.g., transfer to any other hospitals); (2) patients who were intubated, dead or discharged within 24 h of admission; (3) patients with pregnancy, acute lethal organ injury (e.g., acute myocardial infarction, acute pulmonary embolism, or acute stroke), acquired immune deficiency syndrome or leukemia. These specific criteria were established to avoid non-uniform enrolment of patients, which could skew the interpretation of the results.
Data collection
Patient data collected included demographic information, clinical characteristics, medical history, laboratory data, in-hospital medication, and clinical outcomes. The patient demographic information (age and gender), clinical characteristics (e.g., fever, cough, respiratory rate, and pulse oxygen saturation), medical history (e.g., length of hospital stay, and comorbidities), and in-hospital medication and clinical outcomes were obtained from the electronic medical system. Laboratory data (including blood counts, liver function, renal function, and cardiac function) were collected from the laboratory information system. The personal identification information including name and ID was anonymized and a new study ID was generated for each patient to protect patient privacy. The data were carefully reviewed and confirmed by experienced physicians to guarantee the accuracy of data extraction procedures. The full dataset and statistical code in this study are available from the corresponding author on reasonable request.
Exposure and outcomes
The early treatments of interest were defined as patients receiving therapy with ribavirin and interferon-α (RBV/IFN-α) within 48 h of admission. RBV/IFN-α therapy included 3 groups: RBV alone, IFN-α alone, and RBV combined with IFN-α (RBV&IFN-α). There were 3 different subtypes of IFN-α used in patients: IFN-α2a, IFN-α2b and FN-α1b. Patients who did not receive RBV/IFN-α were classified as the control group. The dosing protocol of RBV/IFN-α is shown in Table S1.
The primary outcomes considered in this study were: (1) progressing from non-severe COVID-19 into severe type; (2) all-cause mortality during 30 days; (3) length of hospital stay over 15 days. The secondary outcomes considered were indicators of drug safety including blood counts, liver function, renal function, and cardiac function.
Statistical analyses
Data are presented as the medians and interquartile ranges (IQRs), or numbers and percentages (%), as appropriate. Comparisons of parameters for continuous variables were conducted with the Wilcoxon-Mann–Whitney-Test. For categorical variables, Pearson’s χ2 test or Fisher’s exact tests were used. The risk of outcomes of interest was calculated by the Cox proportional hazard model if the hazard for the RBV/IFN-α and No RBV/IFN-α groups were proportional (through an evaluation of Schoenfeld residuals) or Logistic regression. Multivariate analyses for primary outcomes were adjusted for age, sex, comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease [COPD], chronic liver disease [CLD], and chronic kidney disease [CKD]), and drug therapy (antibiotics and corticosteroids). We also adjusted for the disease severity in 30-day mortality and length of hospital stay. Site was modeled as a random effect in the mixed-effect Cox model and Logistic regression model. To minimize the effect of potential confounding factors associated with exposure, we adjusted for the differences in baseline characteristics by establishing propensity score-matched cohorts, including age, symptoms (fever and shortness of breath), CKD and drug therapy (antibiotics and corticosteroid drugs). Finally, a matched cohort of RBV/IFN-α group and No RBV/IFN-α group was established with the pairing ratio at 1:1.
To identify the effect of different treatment regimens, we assessed the impact of the 3 treatments (RBV alone, IFN-α alone and RBV&IFN-α) on outcomes compared with No RBV/IFN-α and performed subgroup analyses examining the association of RBV alone compared to No RBV, IFN-α alone compared to No IFN-α, and RBV&IFN-α compared to No RBV&IFN-α. We further carried out sensitivity analyses to evaluate the effect of different subtypes of IFN-α (IFN-α2a, IFN-α2b, and IFN-α1b) on the outcomes.
We employed the difference-in-difference (DID) methodology to assess the impact of the treatments of interest on drug safety indicators while controlling for confounding factors in linear regression analysis (Wing et al., 2018, Pinheiro et al., 2020). The DID estimations from linear regression models were able to capture the net effects of the treatments of interest. In our study, a negative or positive estimate from the DID models would indicate that a measure of blood examination indicator decreased or increased more over time in patients receiving RBV/IFN-α than those receiving No RBV/IFN-α. All analyses were performed using SAS 9.4 (by SAS Institute Inc., Cary, NC, USA) and SPSS version 23. P < 0.05 was considered statistically significant.
Results
Patient characteristics
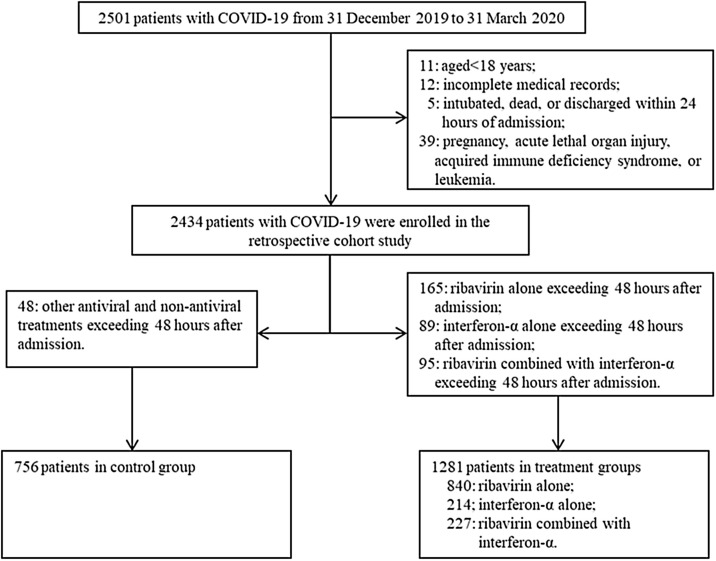
Overall, 2501 patients with COVID-19 were admitted to 4 hospitals in Hubei Province, China. According to the inclusion/exclusion criteria, after excluding 464 patients, eventually, 2037 patients were included in the analysis (Figure 1 ). Of these, 1281 patients received RBV/IFN-α (840 received RBV alone, 214 IFN-α alone and 227 RBV&IFN-α) treatments and 756 patients did not receive RBV/IFN-α treatments.
Figure 1.
The flowchart showing the strategy of patient enrollment.
Between the 2 groups, there were no significant differences in demographic characteristics, symptoms and comorbidities except for age, fever, shortness of breath, CLD, CKD, and antibiotic and corticosteroid therapies (Table 1 ). The RBV/IFN-α group had a higher percentage of severe patients (51.05% vs. 40.87%; P < 0.001), but had shorter median days from symptom onset to admission (8 days [IQRs: 5–14] vs. 11 days [IQRs 6–21]; P < 0.001) than the No RBV/IFN-α group. For the RBV/IFN-α group patient characteristics in the 3 treatment groups are shown in Table S2. Among subtypes of IFN-α, IFNα-2a was most frequently used, accounting for 89.25% in the IFN-α alone group and 60.79% in the RBV&IFN-α group. In the control group, 68.78% of patients received other antivirals, mainly abidor, oseltamivir or lopinavir-ritonavir, or a combination of these drugs.
Table 1.
Characteristics of patients in the RBV/IFN-α group and the No RBV/IFN-α group before and after propensity score matching.
| Parameters | Unmatched |
Matched (1:1) c |
|||||
|---|---|---|---|---|---|---|---|
| Total (n = 2037) |
RBV/IFN-α (n = 1281) |
No RBV/IFN-α (n = 756) |
P value |
RBV/IFN-α (n = 677) |
No RBV/IFN-α (n = 677) |
P value |
|
| Age (years) | 56[42−67] | 57[41−67] | 59[47−69] | <.001 | 58[42−68] | 58[46−68] | 0.211 |
| Gender | 0.983 | 0.663 | |||||
| Female | 1061(52.09) | 667(52.07) | 394(52.12) | 363(53.62) | 355(52.44) | ||
| Male | 976 (47.91) | 614(47.93) | 362(47.88) | 314(46.38) | 322(47.56) | ||
| Time from symptom onset to admission, days | 10[5−15] | 8[5−14] | 11[6−21] | <.001 | 10[5−16] | 11[6−21] | 0.001 |
| Symptoms and signs | |||||||
| Fever | 1358 (66.67) | 918(71.66) | 440(58.20) | <.001 | 361(53.32) | 411(60.71) | 0.006 |
| Cough | 1021(50.12) | 632(49.34) | 389(51.46) | 0.356 | 343(50.66) | 356(52.58) | 0.480 |
| Shortness of breath | 460(22.58) | 271(21.16) | 189(25.00) | 0.045 | 199(29.39) | 174(25.70) | 0.128 |
| Diarrhea | 95(4.66) | 65(5.07) | 30(3.97) | 0.253 | 32(4.73) | 27(3.99) | 0.506 |
| Nausea or vomiting | 39(1.91) | 21(1.64) | 18(2.38) | 0.238 | 14(2.07) | 17(2.51) | 0.586 |
| Comorbidity | |||||||
| Hypertension | 712(34.95) | 435(33.96) | 277(36.64) | 0.220 | 238(35.16) | 238(35.16) | 1.000 |
| Diabetes | 335(16.45) | 212(16.55) | 123(16.27) | 0.869 | 121(17.87) | 109(16.10) | 0.385 |
| Coronary artery disease | 171(8.48) | 100(7.86) | 71(9.54) | 0.191 | 56(8.32) | 59(8.87) | 0.719 |
| COPD | 133(6.60) | 77(6.05) | 56(7.53) | 0.198 | 46(6.84) | 53(7.97) | 0.428 |
| CLD | 113(5.61) | 90(7.08) | 23(3.09) | <.001 | 34(5.05) | 21(3.16) | 0.081 |
| CKD | 97(4.81) | 50(3.93) | 47(6.32) | 0.016 | 49(7.28) | 33(4.96) | 0.077 |
| Drug therapy | |||||||
| Antibiotic therapy | 1560(76.58) | 1082(84.47) | 478(63.23) | <.001 | 483(71.34) | 466(68.83) | 0.313 |
| Corticosteroid therapy | 836(41.04) | 676(52.77) | 160(21.16) | <.001 | 196(28.95) | 160(23.63) | 0.026 |
| Baseline disease severity | <.001 | 0.299 | |||||
| Non-severe type | 1074(52.72) | 627(48.95) | 447(59.13) | 367(54.21) | 386(57.02) | ||
| Severe type | 963(47.28) | 654(51.05) | 309(40.87) | 310(45.79) | 291(42.98) | ||
| Length of hospital stay, daysa | 16[10−24] | 18[12−27] | 12[9−18] | <.001 | 17[11−25] | 13[9−19] | <.001 |
| Progressing from non-severe to severe typeb | 413(27.61) | 261(29.23) | 152(25.21) | 0.088 | 137(27.02) | 139(26.28) | 0.786 |
| 30-day mortality | 138(6.77) | 90(7.03) | 48(6.35) | 0.557 | 38(5.61) | 46(6.79) | 0.367 |
COPD = chronic obstructive pulmonary disease; CLD = chronic liver disease; CKD = chronic kidney disease;
Data are presented as n (%) or median [Q1-Q3]. The P value was calculated by the Wilcoxon-Mann–Whitney-Test in continuous variables and Pearson’s χ2 test in categorical variables.
After excluding patients who died during hospitalization, the sample size was 1888.
After excluding severe patients on admission and prior to medication, the sample size was 1496.
The propensity score-matched (1:1) cohort was established with adjusted age, fever, shortness of breath, CKD, antibiotic and corticosteroid therapies.
Progression to severe type COVID-19
During the 30-day follow-up period, 498/1496 patients admitted with non-severe type COVID-19 progressed to severe type. There was no statistical difference between the percentage of patients who progressed into severe type between RBV/IFN-α group and No RBV/IFN-α group (29.23% [261/893] vs. 25.21% [152/603]; P = 0.088). In the mixed-effect Cox model treating site as a random effect, after adjusting for age, sex, comorbidities, and drug therapies, RBV/IFN-α therapy was not associated with changes in progressing to severe type (adjusted HR = 1.09, 95% CI: 0.88−1.36) (Table 2 ). Compared with No RBV/IFN-α therapy, none of the 3 treatment groups was associated with progression to severe type. Further analysis was done with propensity score-matched datasets, in which there were 677 patients in RBV/IFN-α group and 677 patients in No RBV/IFN-α group. The results were consistent, showing no association with the progression to severe type in RBV/IFN-α therapy (aHR = 1.03, 95% CI: 0.81−1.32) (Table 3 ).
Table 2.
Relative risk for outcomes in the RBV/IFN-α group and the No RBV/IFN-α group before propensity-score matching.
| Variable | Progression to severe type (30 days) |
All-cause mortality (30 days) |
Length of hospital stay>15 days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coxa |
Mixed-effect Modeld |
Coxb |
Mixed-effect Modeld |
Logisticc |
Mixed-effect Modeld |
|||||||
| aHR(95%CI) | P Value | aHR(95%CI) | P Value | aHR(95%CI) | P Value | aHR(95%CI) | P Value | aOR(95%CI) | P value | aOR(95%CI) | P value | |
| Overall analysis | ||||||||||||
| RBV/IFN-α vs no RBV/ IFN-α (ref) |
1.12(0.90−1.41) | 0.309 | 1.09(0.88−1.36) | 0.414 | 0.76(0.52−1.10) | 0.142 | 0.89(0.61−1.30) | 0.538 | 2.46(1.96−3.09) | <0.001 | 2.11(1.68−2.64) | <0.001 |
| RBV alone vs no RBV/IFN-α (ref) |
1.06(0.83−1.36) | 0.633 | 1.13(0.88−1.46) | 0.339 | 0.69(0.46−1.03) | 0.068 | 0.92(0.57−1.48) | 0.721 | 2.97(2.31−3.83) | <0.001 | 2.25(1.70−2.97) | <0.001 |
| IFNα alone vs no RBV/ IFN-α (ref) |
1.36(0.95−1.96) | 0.096 | 1.09(0.75−1.57) | 0.668 | 1.51(0.88−2.59) | 0.133 | 1.26(0.72−2.21) | 0.411 | 1.15(0.79−1.66) | 0.472 | 1.29(0.87−1.92) | 0.208 |
| RBV& IFN-α vs no RBV/IFN-α (ref) |
1.15(0.81−1.66) | 0.435 | 1.01(0.71−1.43) | 0.974 | 0.58(0.30−1.10) | 0.093 | 0.56(0.29−1.06) | 0.075 | 2.91(2.04−4.16) | <0.001 | 2.94(2.03−4.26) | <0.001 |
| Subgroup analysis | ||||||||||||
| RBV alone vs no RBV (ref) |
1.01(0.81−1.26) | 0.929 | 1.07(0.86−1.34) | 0.524 | 0.59(0.42−0.83) | 0.003 | 0.73(0.49−1.07) | 0.105 | 2.86(2.29−3.57) | <0.001 | 2.39(1.89−3.04) | <0.001 |
| IFN-α alone vs no IFN-α (ref) |
1.21(0.94−1.55) | 0.142 | 1.01(0.77−1.32) | 0.966 | 1.18(0.78−1.77) | 0.430 | 0.86(0.55−1.35) | 0.506 | 1.04(0.81−1.34) | 0.737 | 1.68(1.25−2.26) | 0.001 |
| RBV& IFN-α vs no RBV& IFN-α (ref) |
1.08(0.78−1.51) | 0.637 | 0.96(0.69−1.33) | 0.787 | 0.65(0.36−1.19) | 0.163 | 0.54(0.29−0.99) | 0.045 | 1.68(1.21−2.33) | <0.001 | 2.27(1.60−3.23) | <0.001 |
aHR = adjusted hazard ratio; aOR = adjusted odds ratio; COPD = chronic obstructive pulmonary disease; CLD = chronic liver disease; CKD = chronic kidney disease;
For the outcome of progression to severe type, Cox proportional hazards regression model was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, antibiotic therapy, and corticosteroid therapy.
For the outcome of 30-day mortality, Cox proportional hazards regression model was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, disease severity, antibiotic therapy and corticosteroid therapy.
For the outcome of length of hospital stay>15 days, Logistic regression was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, disease severity, antibiotic therapy and corticosteroid therapy.
Site (hospital) was modeled as a random effect in the multivariate analyses of mixed-effect Cox model and Logistic regression.
Table 3.
Relative risk for outcomes in the RBV/IFN-α group and the No RBV/IFN-α group after propensity-score matching (1:1).
| Variable | Progression to severe type (30 days) |
All-cause mortality (30 days) |
Length of hospital stay>15 days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coxa |
Mixed-effect Modeld |
Coxb |
Mixed-effect Modeld |
Logisticc |
Mixed-effect Modeld |
|||||||
| aHR(95%CI) | P Value | aHR(95%CI) | P Value | aHR(95%CI) | P Value | aHR(95%CI) | P Value | aOR(95%CI) | P value | aOR(95%CI) | P value | |
| Overall analysis | ||||||||||||
| RBV/IFN-α vs no RBV/ IFN-α (ref) |
1.07(0.82−1.38) | 0.626 | 1.03(0.81−1.32) | 0.784 | 0.74(0.47−1.16) | 0.189 | 0.81(0.52−1.28) | 0.368 | 2.44(1.89−3.14) | <0.001 | 2.23(1.73−2.86) | <0.001 |
| RBV alone vs no RBV/IFN-α (ref) |
0.97(0.72−1.30) | 0.827 | 1.04(0.78−1.40) | 0.795 | 0.68(0.40−1.14) | 0.142 | 0.88(0.49−1.58) | 0.669 | 3.14(2.34−4.21) | <0.001 | 2.39(1.74−3.29) | <0.001 |
| IFNα alone vs no RBV/ IFN-α (ref) |
1.30(0.82−2.05) | 0.265 | 0.97(0.61−1.53) | 0.894 | 1.45(0.72−2.94) | 0.301 | 1.16(0.56−2.40) | 0.681 | 1.01(0.65−1.57) | 0.966 | 1.28(0.80−2.05) | 0.300 |
| RBV& IFN-α vs no RBV/IFN-α (ref) |
1.22(0.79−1.87) | 0.374 | 1.08(0.71−1.65) | 0.710 | 0.50(0.20−1.28) | 0.150 | 0.44(0.17−1.14) | 0.091 | 2.79(1.81−4.29) | <0.001 | 3.28(2.05−5.26) | <0.001 |
| Subgroup analysis | ||||||||||||
| RBV alone vs no RBV (ref) |
0.99(0.76−1.28) | 0.923 | 1.06(0.82−1.36) | 0.672 | 0.59(0.37−0.95) | 0.029 | 0.69(0.42−1.12) | 0.135 | 3.04(2.35−3.95) | <0.001 | 2.58(1.97−3.37) | <0.001 |
| IFN-α alone vs no IFN-α (ref) |
1.27(0.92−1.75) | 0.150 | 1.03(0.73−1.43) | 0.884 | 1.03(0.58−1.83) | 0.913 | 0.76(0.42−1.40) | 0.379 | 1.09(0.80−1.48) | 0.592 | 1.86(1.30−2.67) | 0.001 |
| RBV& IFN-α vs no RBV& IFN-α (ref) |
1.20(0.79−1.81) | 0.399 | 1.08(0.72−1.63) | 0.701 | 0.54(0.22−1.36) | 0.190 | 0.44(0.17−1.11) | 0.081 | 1.86(1.24−2.80) | 0.003 | 2.80(1.78−4.40) | <0.001 |
aHR = adjusted hazard ratio; aOR = adjusted odds ratio; COPD = chronic obstructive pulmonary disease; CLD = chronic liver disease; CKD = chronic kidney disease.
The propensity score-matched (1:1) cohort was established with adjusted age, fever, shortness of breath, CKD, antibiotic and corticosteroid therapies.
For the outcome of progression to severe type, Cox proportional hazards regression model was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, antibiotic therapy, and corticosteroid therapy.
For the outcome of 30-day mortality, Cox proportional hazards regression model was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, disease severity, antibiotic therapy and corticosteroid therapy.
For the outcome of length of hospital stay>15 days, Logistic regression was adjusted for the following variables: age, sex, hypertension, diabetes, COPD, CLD, CKD, disease severity, antibiotic therapy and corticosteroid therapy.
Site (hospital) was modeled as a random effect in the multivariate analyses of mixed-effect Cox model and Logistic regression.
All-cause mortality during 30 days
During the 30-day follow-up period, 138/2037 hospitalized patients with COVID-19 died. There was no significant difference in risk of 30-day mortality between the RBV/IFN-α group and the No RBV/IFN-α group (7.03% [90/1281] vs. 6.35% [48/756]; P = 0.557). In the mixed-effect Cox model, RBV/IFN-α therapy was not associated with a reduction in 30-day mortality (aHR = 0.89, 95% CI: 0.61−1.30) (Table 2). Compared with No RBV/IFN-α therapy, the 3 treatment groups were also not significantly associated with a reduction in 30-day mortality. The analysis with propensity score-matched cohort showed similar results (aHR = 0.81, 95% CI: 0.52−1.28) (Table 3).
Length of hospital stay
Of 2037 patients, 1888 were eventually discharged. The median length of hospital stay was 18 days in the RBV/IFN-α group and 12 days in the No RBV/IFN-α group (P < 0.001). The mixed-effects model (aOR = 2.11, 95% CI: 1.68−2.64) and further analysis with propensity score-matched cohort (aOR = 2.23, 95% CI: 1.73−2.86) showed that RBV/IFN-α use was associated with a higher probability of length of hospital stay >15 days (Table 2, Table 3). RBV alone and RBV&IFN-α groups were also associated with a higher probability of length of stay >15 days.
Subgroup and sensitivity analyses
Subgroup analyses of RBV alone vs. No RBV, IFN-α alone vs. No IFN-α, and RBV&IFN-α vs. No RBV&IFN-α were almost consistent with results of the overall analysis. In the mixed-effects model the RBV&IFN-α group was associated with a reduction in 30-day mortality (aHR = 0.54, 95% CI: 0.29−0.99) (Table 2) but no association was found after propensity score matching (aHR = 0.44, 95% CI: 0.17−1.11) (Table 3). The 3 treatment subgroups were associated with a higher probability of length of stay >15 days. In addition, the results of sensitivity analysis did not show differences in efficacy between different subtypes of IFN-α (IFN-α2a, IFN-α2b, and IFN-α1b) (Tables S3 and S4).
Safety evaluation
After adjusting for age, sex, comorbidities, disease severity, and drug therapies, the results of the DID model showed significant differences in some indicators of blood count, liver function, and renal function between the RBV/IFN-α group and No RBV/IFN-α group (Table S5). However, in further analysis with propensity score-matched cohort, DID estimators were only statistically significant at the 5% level in hemoglobin and uric acid. Compared with the No RBV/IFN-α group hemoglobin in RBV/IFN-α group decreased by 7.3 g/L and uric acid increased by 29.7 μmmol/L (Table 4 ).
Table 4.
Impact of the RBV/IFN-α therapy on blood examination indicators after propensity-score matching (standard error in parenthesis).
| Indicators | RBV/IFN-α |
No RBV/ IFN-α |
D-in-Db | P value | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 677) |
(n = 677) |
|||||||
| Before | After | Changea | Before | After | Changea | |||
| Blood count | ||||||||
| Hb (g/L) | 127.8(18.7) | 114.1(22.9) | −13.7 | 127.9(19.8) | 121.6(21.9) | −6.3 | −7.3(1.7) | <.001 |
| WBC (×10⁹/L) | 5.7(2.5) | 7.5(4.4) | 1.8 | 6.3(2.9) | 7.5(4.4) | 1.2 | 0.6(0.3) | 0.070 |
| Neut (×10⁹/L) | 4.0(2.5) | 5.5(4.5) | 1.5 | 4.4(2.8) | 5.4(4.5) | 1.0 | 0.5(0.3) | 0.082 |
| LY (×10⁹/L) | 1.3(0.6) | 1.6(0.6) | 0.3 | 1.3(0.6) | 1.6(0.6) | 0.3 | 0.0(0.0) | 0.610 |
| PLT (×10⁹/L) | 212.3(86.0) | 199.0(79.1) | −13.3 | 213.4(83.9) | 207.2(79.8) | −6.2 | −7.6(7.4) | 0.310 |
| Liver function | ||||||||
| AST (U/L) | 27.2(23.5) | 34.1(46.5) | 6.9 | 28.8(22.0) | 30.6(28.3) | 1.8 | 5.4(3.6) | 0.137 |
| ALT (U/L) | 32.0(50.2) | 46.9(79.1) | 14.9 | 30.3(30.5) | 51.4(155.5) | 21.1 | −6.2(8.6) | 0.466 |
| ALB (g/L) | 38.0(5.3) | 36.0(5.4) | −2.0 | 37.2(5.4) | 35.7(5.9) | −1.5 | −0.5(0.4) | 0.277 |
| A/G | 1.4(0.4) | 1.3(0.4) | −0.1 | 1.3(0.3) | 1.3(0.4) | 0 | −0.0(0.0) | 0.204 |
| Renal function | ||||||||
| Scre(μmmol/L) | 111.0(224.5) | 117.1(192.7) | 6.1 | 106.8(187.5) | 115.7(190.2) | 8.9 | −0.2(14.7) | 0.988 |
| BUN(mmmol/L) | 5.9(5.4) | 6.9(7.3) | 1.0 | 5.8(5.7) | 6.9(6.7) | 1.1 | −0.0(0.5) | 0.962 |
| UA(μmmol/L) | 290.5(118.8) | 324.7(123.1) | 34.2 | 281.9(117.8) | 288.1(114.2) | 6.2 | 29.7(10.0) | 0.003 |
| Other indicators | ||||||||
| D-Di(μg/mLF•EU) | 3.6(11.6) | 5.4(14.3) | 1.8 | 4.4(11.8) | 7.2(23.5) | 2.8 | −0.9(1.6) | 0.599 |
| IL-6 (log10 pg/mL) | 14.5(30.3) | 91(441.6) | 76.5 | 34.1(74.0) | 40.1(95.2) | 6.0 | 70.5(80.0) | 0.379 |
Hb= Haemoglobin; WBC = white blood cell count; Neut = neutrophil count; LY = lymphocytes count; PLT = platelet count; AST = aspartate transaminase; ALT = alanine transaminase; Alb = albumin; A/G = albumin/globulin ratio; Scre = serum creatinine; BUN = blood urea; UA = uric acid; IL-6= Interleukin-6; d-Di = D dimer.
The average change was computed from raw data.
The d-in-D result was adjusted using d-in-D estimation, the covariates include the age, sex, hypertension, diabetes, COPD, CLD, CKD, disease severity, antibiotic therapy and corticosteroid therapy.
Discussion
Considering differences in baseline, disease severity, site (hospital), and time, this retrospective study found that RBV/IFN-α therapy was not associated with progression from non-severe COVID-19 into the severe type or with decreased risk of 30-day mortality. Of note, RBV/IFN-α therapy might prolong the length of hospital stay in the final discharged patients with COVID-19.
Although preclinical studies showed that RBV/IFN-α therapy was beneficial (Chen et al., 2004, Falzarano et al., 2013) our study did not find a clinical benefit of RBV/IFN-α (including RBV alone, IFN-α alone, or RBV&IFN-α) therapy for patients with COVID-19. The possible explanations were as follows: first, the concentration and administration of RBV/IFN-α therapy differed between studies. In the MERS-CoV-infected rhesus macaques model with positive effect (Falzarano et al., 2013) the experimenter initiated a subcutaneous injection of 5 mega international units (MIU)/kg of IFN-α2b and intravenous ribavirin (30 mg kg body weight). Subsequently, the concentration of RBV/IFN-α therapy was adjusted according to the regimens. In a retrospective cohort study (Omrani et al., 2014) patients with severe MERS were given oral ribavirin (dose based on calculated creatinine clearance for 8–10 days) and subcutaneous pegylated interferon IFN-α2a (180 μg per week for 2 weeks), finding significant improvement in survival at 14 days, but not at 28 days. In our study, patients with COVID-19 were given intravenous ribavirin (500 mg twice a day or adjusted according to creatinine clearance) and vapor-inhaled IFN-α (5 million U or equivalent). Although the administration by vapor inhalation currently performed in China offered the advantage of targeting specifically the respiratory tract (Sallard et al., 2020, Dong et al., 2020) our results indicated that the pharmacodynamics of such an administration might not be ideal. Second, the type of IFN-I used differed between studies. One study examined the in vitro MERS-CoV susceptibility to different types of IFN (IFN-α2b, IFN-γ, IFN-α2a, IFN-β, et al) and found that IFN-β had the strongest MERS-CoV inhibition (Hart et al., 2014). It was repeatedly shown that IFN-β was a more potent inhibitor of coronaviruses than IFN-α (Clementi et al., 2020, Stockman et al., 2006, Hensley et al., 2004). Our patients with COVID-19 were treated based on IFN-α rather than IFN-β. The above differences in clinical concentration and administration and IFN-I type might account for the lack of clinical benefit of RBV/IFN-α therapy in our study. However, recently published clinical trials and updated treatment guidelines from the National Institutes of Health recommend against the use of ribavirin and interferon (α, β) in patients with COVID-19 (National Institutes of Health, 2020, Huang et al., 2020, Davoudi-Monfared et al., 2020). A randomized, open-label clinical trial in China showed no significant difference in antiviral effectiveness among ribavirin plus interferon-α and the other 2 groups for patients with mild and moderate COVID-19 (Huang et al., 2020). A randomized, open-label clinical trial in Iran evaluated subcutaneous interferon-β1a in patients with severe COVID-19, showing no difference between the interferon-β1a group and the control group in the length of hospital stay and duration of mechanical ventilation (Davoudi-Monfared et al., 2020). Although the reported 28-day mortality was lower in the interferon-β1a group, 4 patients in this group who died before receiving the fourth dose of interferon-β1a were excluded from the analysis, which makes it difficult to interpret these results. Furthermore, ribavirin alone or in combination with interferon (α-2a, α-2b, β-1a) in patients with SARS and MERS failed to show a significant positive effect on clinical outcomes (Arabi et al., 2020, Al-Tawfiq et al., 2014, Chu et al., 2004). The toxic side effects of interferon might outweigh the potential benefits. Therefore, we suggest that RBV/IFN-α therapy should be avoided in the clinical treatment of patients with COVID-19.
After removing deaths and controlling for potential confounders, we still found that the median length of hospital stay in the RBV/IFN-α group was longer than for the No RBV/IFN-α group. Studies suggested that the timing of IFN-I administration played a crucial role in therapeutic efficacy and IFN-I might fail to inhibit viral replication and had pro-inflammatory side-effects when administration was delayed (Hung et al., 2020, Channappanavar et al., 2019). In a recent study finding the advantages of early triple antiviral therapy (Hung et al., 2020) IFN-β1b injection was omitted to avoid its side-effects when patients with COVID-19 were recruited and treated between days 7 and 14 of symptom onset. Of note, the median number of days from symptom onset to admission was 10 days (IQR 5-15) for our patients recruited, but the timing of use of IFN-α was not restricted. Therefore, the inappropriate timing of IFN-α might lead to delayed rehabilitation of discharged patients and thus prolonged hospital stay.
In terms of drug safety, there was no difference in side-effects between the 2 groups except for decreased hemoglobin and increased uric acid. Anemia was a recognized complication of ribavirin therapy and was noted in previous studies investigating the role of ribavirin in the treatment of SARS coronavirus infection (Omrani et al., 2014, Knowles et al., 2003). Although RBV/IFN-α therapy had a significant effect on uric acid levels, the change did not result in serious renal dysfunction according to the data (uric acid = 324.7 μmmol/L) after treatment.
This study comprehensively evaluated the clinical efficacy and safety of RBV/IFN-α therapy in patients with COVID-19. The consistency between overall analysis and subgroup analysis suggested the robustness of the conclusions. However, our study had several limitations. Firstly, due to the retrospective cohort design, we could not exclude the influence of unmeasured confounders on the results, although we have carried on the statistical analysis of multiple factors and multiple models. Secondly, there was a lack of outcome measures that directly reflect the efficacy of antiviral drugs, for example, time to achieve a negative RT-PCR result for SARS-CoV-2 or time to resolution of symptoms. Because of the emergency situation and the lack of medical personnel, we were unable to record relevant data in a timely way. Hence we selected disease progression and 30-day mortality to reflect drug efficacy as far as possible. Thirdly, part of the combination of RBV and IFN-α might be due to chance. Since this study was retrospective, some combinations might be not planned a priori but at the physician’s discretion, although we had defined the combination therapy. Our data also do not apply to the use of any treatment regimen in outpatient or out-of-hospital settings. However, our study still provided referable clinical evidence for efficacy evaluations of the RBV/IFN-α therapy in patients with COVID-19.
Conclusion
This study found that RBV/IFN-α therapy was not associated with progression from non-severe COVID-19 into the severe type or with a reduction in 30-day mortality. By contrast, the inappropriate timing of IFN-α might prolong the patients’ hospital stay. Based on the above results and relevant guidelines, we suggested that RBV/IFN-α therapy should be avoided in patients with COVID-19. Under the current pandemic, looking for other specific antiviral therapy and promoting effective vaccines remain the priorities in responding to COVID-19.
Funding
This study was funded by the Fundamental Research Funds for the Central Universities, H uazhong University of Science and Technology [Grant number: 2020kfyXGYJ073]. The funder had no role in study design, the collection, analysis and interpretation of data, the writing, or the decision to submit the article for publication.
Author contributions
Hui Li, Nian Xiong, Changjun Li, and Xiaoxv Yin conceived the early study idea. Li Liu, Heping Yang and Xiangping Tan extracted the data from electronic case records. Yanhong Gong, Nan Jiang, Qiao Zong, and Jing Wang performed preliminary data analysis and the final data analysis. Zuxun Lu advised on and helped shape the research. Hui Li, Nian Xiong, and Changjun Li wrote the first draft of the manuscript. All authors contributed to interpreting the data and writing the final paper. All authors read and approved the final manuscript.
Conflicts of interest
None declare.
Acknowledgments
We thank all the staff of the Central Hospital and Red Cross Hospital of Wuhan, the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, and Lichuan People’s Hospital for supporting. We also appreciate all study participants who have contributed to the procedure of data collection.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.01.055.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Shalhoub S., Al Omari A., Mandourah Y., Al-Hameed F., Sindi A. Effect of ribavirin and interferon on the outcome of critically ill patients with MERS. Am J Respir Crit Care Med. 2017:A6067. [Google Scholar]
- Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Qasim E.A. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C., Tang B.S., Wu A.K., Chu C.M., Yuen K.Y. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J Infect. 2004;49:262–273. doi: 10.1016/j.jinf.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;49:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi N., Ferrarese R., Criscuolo E., Diotti R.A., Castelli M., Scagnolari C. Interferon-β-1a inhibition of severe acute respiratory syndrome-coronavirus 2 in vitro when administered after virus infection. J Infect Dis. 2020;222:722–725. doi: 10.1093/infdis/jiaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi-Monfared E., Rahmani H., Khalili H., Hajiabdolbaghi M., Mohamadreza S., Abbasian L. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. e01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P. Treatment interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol. 2014;95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley L.E., Fritz L.E., Jahrling P.B., Karp C.L., Huggins J.W., Geisbert T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Q., Tang S.Q., Xu X.L., Zeng Y.M., He X.Q., Li Y. No statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate coronavirus disease 2019: results of a randomized, open-labeled prospective study. Front Pharmacol. 2020;11:1071. doi: 10.3389/fphar.2020.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaati H., Dastan F., Tabarsi P., Marjani M., Saffaei A., Hashemian S.M. A fourteen-day experience with coronavirus disease 2019 (COVID-19) induced acute respiratory distress syndrome (ARDS): an Iranian treatment protocol. Iran J Pharm Res. 2020;19:31–36. doi: 10.22037/ijpr.2020.113337.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M., Al R.F., Khan B., Al M.A., Butt T.S., Al M.E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. 2015;20:87–91. doi: 10.3851/IMP2792. [DOI] [PubMed] [Google Scholar]
- Knowles S.R., Phillips E.J., Dresser L., Matukas L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhou P., Chen K., Ye Z., Liu F., Li X. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ. 2020;192:E734–E744. doi: 10.1503/cmaj.200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the People’s Republic of China . 2020. Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7)https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf [Accessed 2 January 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . 2020. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf [Accessed 5 January 2021] [PubMed] [Google Scholar]
- Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro L.C., Soroka O., Kern L.M., Leonard J.P., Safford M.M. Diabetes care management patterns before and after a cancer diagnosis: a SEER-Medicare matched cohort study. Cancer. 2020;126:1727–1735. doi: 10.1002/cncr.32728. [DOI] [PubMed] [Google Scholar]
- Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. Plos Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. COVID-19 Weekly Epidemiological Update.https://www.who.int/publications/m/item/weekly-epidemiological-update---29-december-2020 [Accessed 30 December 2020] [Google Scholar]
- WHO . 2020. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases Interim Guidance.https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 [Accessed 1 June 2020] [Google Scholar]
- WHO . 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Accessed 3 January 2021] [Google Scholar]
- WHO . 2021. Clinical Management of COVID-19.https://www.who.int/publications/i/item/clinical-management-of-covid-19 [Accessed 5 January 2021] [Google Scholar]
- Wing C., Simon K., Bello-Gomez R.A. Designing difference in difference Studies: best practices for public health policy research. Annu Rev Public Health. 2018;39:453–469. doi: 10.1146/annurev-publhealth-040617-013507. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X. Interferon-alpha2b treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.