Graphical abstract
Keywords: Dairy, Ultrasound, Properties, Non-thermal, Processing, Emerging technologies
Highlights
-
•
HIU is a potential technology for adoption in the dairy industry.
-
•
Ultrasound significantly modifies the components in milk.
-
•
Ultrasound improves gelation and syneresis during cheese production.
-
•
The texture of dairy products can be manipulated by ultrasound.
-
•
Ultrasound reduces fermentation time or dairy products.
-
•
Improvements depend on time, frequency and intensity of sonication.
Abstract
Alternative methods for improving traditional food processing have increased in the last decades. Additionally, the development of novel dairy products is gaining importance due to an increased consumer demand for palatable, healthy, and minimally processed products. Ultrasonic processing or sonication is a promising alternative technology in the food industry as it has potential to improve the technological and functional properties of milk and dairy products. This review presents a detailed summary of the latest research on the impact of high-intensity ultrasound techniques in dairy processing. It explores the ways in which ultrasound has been employed to enhance milk properties and processes of interest to the dairy industry, such as homogenization, emulsification, yogurt and fermented beverages production, and food safety. Special emphasis has been given to ultrasonic effects on milk components; fermentation and spoilage by microorganisms; and the technological, functional, and sensory properties of dairy foods. Several current and potential applications of ultrasound as a processing technique in milk applications are also discussed in this review.
1. Introduction
Emerging food processing technologies are focussed on the production of palatable, healthy, safe, nutritious and minimally-processed foods. The search for such alternative processes has driven attention to emerging food technologies such as non-thermal technologies, to avoid altering the flavour or nutritional content of foods during production. High-intensity ultrasound (HIU) is a promising emerging technology, especially designed for economy, simplicity, and energy-efficiency. HIU has attracted considerable interest in food science and technology due to its wide range of applications, either in processing or evaluation of products [1], [2]. HIU offers a great potential to control, improve, and accelerate processes without damaging the quality of food and other products. Hence, HIU applications in the food industry including the dairy industry continue to be subject of research.
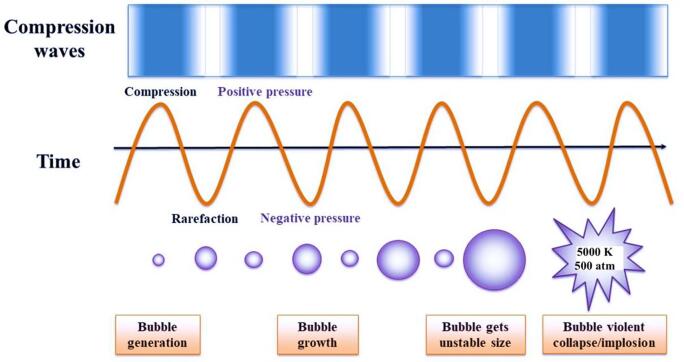
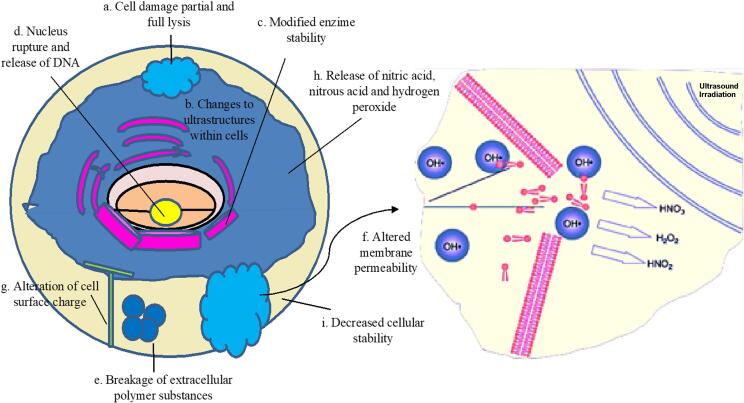
Ultrasound (US) is defined as sound waves of high frequency, above the threshold of human hearing (~20 kHz), ultrasound application can be divided in high intensity - low frequency (I = 10–1000 W/cm2 and F = 20–100 kHz) and low intensity - high frequency (I < 1 W/cm2 and F > 1 MHz) [3]. Ultrasound generates alternating high- and low-pressures, causing compression and expansion (rarefaction) cycles in the medium. Rarefaction leads to the formation of cavitation bubbles, which are tiny vacuum bubbles that occur when the negative pressure exerts. The vacuum bubbles grow over several compression/rarefaction cycles until they cannot absorb more energy and the cavitation bubble undergoes an implosive collapse releasing energy (Fig. 1). This process of bubble generation, growth and implosion is regarded as acoustic cavitation or implosion [4]. Cavitation bubbles generate extreme conditions of temperatures (5000 K) and pressures (500 atm) which can generate very high shear forces [5]. The violent collapse of a cavitation bubble gives rise to physical and chemical effects in the liquid such as microstreaming, agitation, turbulence, microjetting, shock waves, generation of radicals, sonoluminescence etc. [6]. Chemical reactions taking place within these environments are the formation of highly reactive radical species [7], [8], [9], [10]. When an argon-saturated water is sonicated, formation of H and OH radicals takes place as H radicals are reducing in nature, whereas OH radicals are oxidizing in nature. At present, it is known that the radical yield increases with an increase in frequency, it reaches a maximum value and decreases with further increase in the frequency. The highest sonochemical yield is obtained between 200 and 800 kHz [11]. In general, the release energy to the medium, causes structural damage in a nano- micro- or macro-scale [12].
Fig. 1.
Ultrasonic cavitation (Modified from Soria and Villamiel [22]).
The ultrasound-induced chemical and physical effects provoke changes in milk constituents that lead to significant effects in milk and dairy product properties. These effects are the main topic of this review. Ultrasound has been used in food and dairy processing, in applications such as, the enhancement of whey ultrafiltration, extraction of functional foods, reduction of product viscosity, homogenization of milk fat globules, crystallization of ice and lactose and for cutting of cheeseblocks [13]. In recent years, the research of HIU applied on dairy or dairy by-products has been aimed at reducing processing time or enhance the physicochemical quality of different foods. A large number of studies have been published since 2018. Most of them with very consistent results on the benefits of HIU for the quality parameters of fresh milk, cheese, butter, ice cream, fermented milk products, whey protein preparations, and other beverages.
However, application of ultrasound on the processing of foods have evidenced negative impact of heat on thermo-labile compounds such as, vitamins, and pigments [14]. But the application of ultrasound in beverages has also showed healthy benefits such as increase of levels of antioxidants and bioactive compounds [15]. The effect of HIU application in dairy systems has taken an important place in food science and technology. Its effects on microorganisms have been extensively investigated as a method for preservation [16], [17] due to its role improving safety and delaying food spoilage [18]. As well as in the inactivation of milk enzymes [19]. One of the most relevant uses of ultrasound is in fermentation processes [20] and in the production of functional fermented beverages, with considerable improvement in sensory properties [21]. The purpose of this review is to provide an overview of the latest research on the application of HIU in the processing and preservation of milk and dairy products and to highlight the technological improvements that could be obtained through its application. Particular attention is given to the effects on physicochemical, functional and sensory properties of dairy systems.
2. Physicochemical properties
The ultrasonication processing conditions in foods are highly dissimilar among studies. Variations on intensity, time, pulsations and probes are still wide and heterogeneous. However, promising results in physicochemical properties are found in all these studies for potential application in the dairy industry. Those benefits are discussed in this section and the summary of the results are listed in Table 1.
Table 1.
Recent research (2018-to date) about ultrasound effect in physicochemical properties of milk and dairy products.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Milk and milk-based beverages | |||
| Raw sheep milk | Ultrasonic probe.VC Vibra Cell Ultrasound, model VC 130 (Sonics Inc., USA), 20 kHz. Maximum power = 78 W (for 6 or 8 min) or 104 W (for 4 or 6 min). Pulse duration of 4 s. | HIU did not affected proximate composition, free amino acids, or amino acid profile. | [18] |
| Anhydrous milk fat and milk fat/rapeseed oil blends | A 110 mL flow cell system (sonolab SL10 ultrasonic, Syrris Ltd, UK). 20 kHz, 40 W at 28 °C. Recycling mode with a flow rate of 200 mL/min1 and a total volume of 500 mL samples collected at 0, 2.5, 5, 7, and 10 min of ultrasonication. | HIU had no effect on melting behaviour, but it reduced the fat crystal size. No effect on hardness evaluated by needle penetration. | [26] |
| Pasteurized full fat milk. | An 800 mL milk flow cell system (200 mL/min) was used for the study (Sonolab SL10 ultrasonic, Syrris Ltd, United Kingdom) 20 kHz, 10, 30 and 50 W at 27, 50, 30 min and 70 °C. Energy in the flow = 91, 273 and 454 W/L, respectively. | HIU accelerates the acid gel formation at 30 W and 50 °C. Protein denaturation is promoted by HIU increase of temperature. Sonication power higher than 30 W and higher temperature than 50 °C, reduced fat globule size from 3.39 to 3.89 μm to 0.37–1.9 μm. The specific surface area is increased by HIU (from 2.07 to 45.09 m2ag−1fat). | [25] |
| Raw bovine milk | DRC-8-DPP-FGS, Advanced Sonic Processing Systems, USA). Continuous ultrasonication (16 and 20 kHz, nominal 1200 W. 100 W/cm3 and delivered 1.36 kW/pass 14 to 18 min. | Droplets diameters are highly reduced as more passes and shorter exposure to ultrasonication. An increase of submicron droplets was observed at inlet temperature of 54 °C. US reduces gelling times and increases curd firmness at 42 °C. | [28] |
| Pasteurized fresh bovine milk | Ultrasonicator VCX500, Sonicsâ, Newtown, USA). 20 kHz frequency for 3 min with 100% power (500 W. | Ultrasonication does not modify pH, enzyme activity along 21 d, compared to control. US did show a dispersion of 1 µm particles, which could be by reduction of fat globules size. US have a reduced denaturation of a-lacto-albumin and k-casein. | [30] |
| Bovine milk | Ultrasonic processor (Sonics & Materials, Inc, VCX 1500 HV, USA), 20 kHz. 1500 W, Amplitude of 95% for 10 or 15 min. | HIU for 10 min reduced pH and increased specific gravity. Total solids were increased after 7 d of storage. Physical stability is improved by 15 min of HIU, after 7 d of storage. Increased percentage of settled solids. L* is increased with 10 min of HIU and C* is reduced by HIU, after 14 d of storage. a* and b* are not affected. |
[29] |
| Chocolate milk beverage | An ultrasonic device was used. Disruptor, 800 W, (Indaiatuba, Brazil), 19 kHz. Nominal power of 400 W. Energy density 0.3, 0.9, 1.8, 2.4, and 3.0 kJ/cm3. with 13 mm diameter probe. Maximum temperature of 42 °C. | Higher energy densities of HIU lead to higher homogenization of the beverage and smaller particle size distribution of fat globule. HIU affected the antioxidant activity, ACE inhibitory activity, fatty acid profile, and volatile profile. Ultrasonication also reduced the losses of nutrient compounds, and allows a better preservation of short chain fatty acids and medium chain fatty acids. | [27] |
| Cheeses and creams | |||
| Fresh raw milk | Ultrasonic processor Hielscher UP400s , 24 kHz, 400 W, amplitude of 100 and 50% for 0, 5, and 10 min. | HIU increased cheese yield (%), despite an increase of exudate. Yellow tones and coloration in cheese is promoted by HIU at 10 min. But not L*, a*, nor C* colour coordinates are affected. pH increased from 6.6 to 6.74 after 5 min of ultrasonication but reduced at 10 min. | [41] |
| Fresh cream | Ultrasound processor (APU400; Adeeco Co., Iran), 20 kHz, 100 and 300 W for 0, 5, 10 and 15 min. A pulse mode of on-time 2 s and off-time 4 s was used. A 12 mm in diameter ultrasonication probe was immersed to a depth 2 cm Temperature = 50 °C. | Cream particle diameter (0.296–5.867 µm) and particle size distribution were improved with HIU. HIU reduces yellowness. | [43] |
| Retentate of ultrafiltered milk | Ultrasonic Homogenizer (model no. HD2200, Bandelin, Germany), 20, 40, and 60 kHz for 20 min at intensity of 80%. | HIU reduced pH and improved titratable acidity at the end of storage period. However, there was not linear relationship between frequency and acidity. | [42] |
| Fermented milk products | |||
| Fermented milk products | A review of multiple ultrasound parameters. | US reduces processing time and improves quality, stability, and emulsification of products. It may be beneficial when is used as pre-treatment (before inoculation), but it can be detrimental when it is applied during fermentation. Main reported problems are formation of large protein aggregates, and reduction of firmness. | [44] |
| Yogurt and ice cream | A review of multiple ultrasound parameters applied in the production of yoghurt and ice cream | Ultrasound promotes fat drop reduction, increases viscosity, whey coagulation, and water-holding capacity. As well, Ultrasonication reduces ice crystal size and decreases freezing time. | [45] |
| High-protein fermented milk | An ultrasonic device (Sonopuls HD 2200; Bandelin, Germany), 30 W 22.5 W cm−2, energy input of 1765 J/kg1. Booster horn (SH 213 G), and a probe equipped with a titanium flat tip (TT13; d ¼ 13 mm, l ¼ 5 mm). | HIU reduced pH during 9 d of storage. Water-holding capacity was reduced by HIU when pH of 4.8 and 5.0 was reached. A slightly effect on particle size reduction was observed. HIU highly altered yogurt visual appearance. Sonicated yogurt had an increase of homogeneity. | [52] |
| Whey protein concentrates and other whey products | |||
| Chocolate whey beverage | An ultrasound processor with a 13-mm probe was used (Unique, Desruptor 800 W, Indaiatuba, Brazil), 19 kHz, 800 W, 5000 J/ml. Two different processes: A = 20% (160 W for 937 s un 30 mL, with 34 °C of final temperature). B = 90% (720 W for 208 s in 30 mL, with 71 °C of final temperature). | 720 W of HIU for 208 s highly reduced droplet size, contributing to kinetic stability. HIU increased lightness compared to 160 W for 937 s, but it did not modify Chroma. HIU application in any way increased the stability of the whey beverage and reduces hue angle. Zeta values were increased with 160 W for 937 s ultrasonication. | [31] |
| Whey protein concentrate | Ultrasonic processor (Sonics: VCX 750, Vibra Cell Sonics, Sonics & Materials Inc, USA). Maximum net power = 750 W and 220 V. 20 kHz, 20% amplitude for 19.75 min. Temperature = 185 °C inlet and 85 °C outlet. | HIU promotes narrowed size distribution and smaller particle size (0.68 ± 0.23 μm), than controls (2.453 ± 0.717 μm). HIU increased the heat stability from 178 to 1076, heat coagulation time, the solubility of protein from 72.22 to 79.21, and protein aggregation. | [46] |
| Prebiotic inulin-enriched whey beverage. | A 13 mm diameter probe was attached to an ultrasonic device (Unique, Desruptor, 800 W, Brazil), 19 kHz, 0, 200, 400 and 600 W for 3 min. Temperature < 56 °C. | Zeta potential was slightly reduced by the highest power of US (-19 ± 1 mV). US (400 W) promotes cell membrane rupture and increased homogeneity. Particle size distribution is also decreased (5 to 12 µm) by 600 W. Colour of ultrasonicated samples trended to be bluer, duller and with higher Hue values. | [48] |
Physicochemical parameters in dairy products are highly important for quality acceptance. Consumer preference is based on physicochemical quality characteristics, such as colour and texture. Furthermore, processors directly depend on these parameters to guarantee a longer shelf life of the product, proper technological characteristics for further processing, or nutritional benefits for the consumers [23]. Parameters such as pH, fat globule characteristics, oxidative stability or change of the composition are of importance for dairy processors. Hence, to improve those parameters have been always a common aim among researchers and processors. HIU has the potential and already provided positive effects on the physicochemical parameters. Nevertheless, comparisons and combinations with other techniques (i.e. micro-fluidisation or pasteurization), or application on new products, are gaps of knowledge that has been partially researched in the last years.
HIU application in milk and other milk-based beverages, have shown some advantages on quality physicochemical parameters. Lately, buffalo milk [24], sheep milk [18], and supplemented milk [25], [26], [27], in addition to bovine milk [28], [29], [30] have been evaluated after the application of HIU. In general, positive effects of HIU have been confirmed, mainly on size of fat globule or fat crystal size, fat droplets and other milk particles, and altered the curd matrix [24], [26], [30], [31]. The alteration on the size of milk components resulted in physical benefits for the food, for instance; gel strength increase, gel hardness, gel formation acceleration, specific surface area increase, curd firmness reduction, and small particle size distribution of fat [24], [26], [28], [30], [31]. More details on milk components, structural, and functional properties will be given in the next sections.
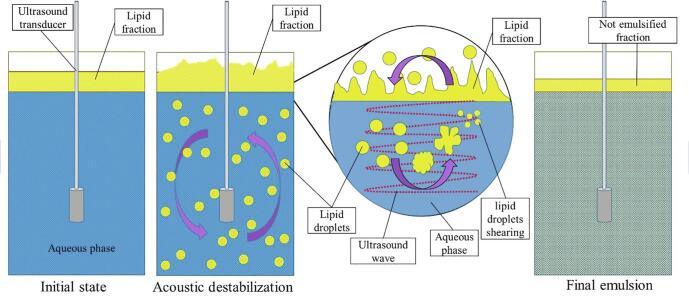
Particle fat size reduction by ultrasonication depends on the ultrasound power applied [32]. For dairy, reduction of droplet size is important in processes such as emulsification. This reduction of size is the first step in the process [33]. First, acoustic vibration lead to a dispersion of fat droplets into the aqueous fraction of the milk through turbulence. Later, cavitation promotes the breakup of fat droplets [34]. Ultrasonication has the capacity to disintegrate the milk fat globule membrane, leading to a reduction of fat globules, smaller than 1 µm, and a modification to a granular surface of the fat globule, due to casein micelles interacting with the disrupted membrane [35]. The mechanism has been proposed in a non-dairy matrix by Kaci et al. [36]. They propose that ultrasonication causes a splitting of big existing droplets to the aqueous phase. There, those droplets are reduced in size by cavitation, a collapse of micro-bubbles resulting from ultrasound waves. The collapse leads to highly localized turbulence that disperses all around the liquid (see Fig. 2).
Fig. 2.
Representation of droplet lipid size by high-intensity ultrasound during emulsification (Modified from Kaci et al. [36]).
Further benefits by HIU application in milk and milk compositions, may be classified as technological. HIU have shown to increase the zeta-potential in milk and the stability of the product. Some authors have found that HIU does not promote the nutrient composition change, the free amino acids, or the formation of products from lipid oxidation [18], [24], [26]. On the other hand, HIU has shown a reduced effect on protein denaturation of lacto-albumin [30] and a rising of casein association with fat globules [28], which is beneficial for gelling formation. More information about effects on chemical composition is offered in the Section 3. HIU also reduced pH of milk and increased total solids, without effect on yellowness and redness [27], [29]. From the nutritional point of view, HIU application has the capacity to allow better preservation of fatty acids in milk, compared with common processes such as pasteurization [27].
A factor that may be considered as important to achieve an effect of HIU on physicochemical characteristics of milk and dairy products is the time of HIU application. In Table 1, a trend can be observed when processing time is lower than 10 min. No effects are observed on milk pH or enzyme activity when ultrasonicating for 3 min [30]. In addition, no effect was observed on melting activity or hardness of anhydrous milk fat blends with rapeseed oil after 0 to 10 min [26]. As well, no effect was observed on proximate composition of raw sheep milk when HIU was applied for 6 min [18]. In heavy cream, a non-consistent effect was observed on characteristics such as droplet size and melting peak, and the effect on firmness was not consistent when ultrasonication was from 1 to 90 s [37].
A disadvantage reported from the HIU application in milk, could be that ultrasonication has the capacity to modify colour, an important reduction of lightness and chroma may be detrimental for appearance [29]. More importantly, there is a report of denaturation of major proteins in pasteurized milk, by HIU application [4], [25]. The colour of milk and dairy products depends on a variety of factors. The most relevant factors seem to be the diet, the breed, and the physiological state of the animal. From the diet, carotenoids and retinol deposited by the animal in the milk, seems to be the main factors responsible for milk colour. Other natural dietary components involved in colour of dairy are xanthophills, riboflavins, and tocopherols [38], [39]. Carotenoids and retinol are sensitive to physicochemical factors. After harvesting, milk colour may depend on factors such as fat concentration, photo-degradation, storage conditions, homogenisation or presence of additives. Casein micelles and fat globules are able to disperse light. Hence, milk shows high values of whiteness and lightness. However, any treatment affecting the physical structure of milk may affect its colour [40]. Colour in thermo-sonicated milk changes, having a higher L*, related to a higher homogenization and smaller size of fat globules. Furthermore, cavitation may also promote the release of encapsulated triacylglycerols, cholesterol, and phospholipids in the fat globule, which could change the light dispersion and the milk product [35].
HIU applied in solid or semi-solid dairy products have also shown benefits in the physicochemical parameters of the product, when the technology is applied in raw milk for producing cheese and cream production. An increase in yield was observed in the Mexican Panela cheese production when HIU was applied for 5 or 10 min to the raw material. This could be an interesting result from the economical point of view. However, other parameters such as syneresis or yellowness were increased, while pH was reduced only when 10 min of ultrasonication was applied to the milk. In cheese, similarly to milk and cream, duration seemed to have an interaction with the potency of sonication for affecting characteristics [41]. In feta-type cheese, HIU reduced the pH of the cheese after storage of 60 d. Furthermore, HIU improved the water-holding capacity and gumminess of the product. The authors reported an effect of ripening period and frequency of ultrasonication (variations of 20 to 60 kHz), but no significant interaction between both variables [42]. In cream and butter, the positive effects of HIU are similar. In both foods, HIU increase apparent viscosity, firmness and hardness. More relevant findings are that HIU reduced the churning time and melting peak [37], [43]. Which may also have an important impact on the economics of the production.
HIU application in fermented milk products is well documented. Two reviews have recently been published on the use of the HIU technology on fermented products and in yogurt and ice cream. HIU is an excellent technology for pre-treatment processing, but it does not offer positive effects in the product, when it is applied as post-processing treatment. When it is applied in post-fermentation, it may lead to formation of visible large particles, which affects the texture, reducing firmness [44]. HIU has the potential to increase homogeneity, viscosity, firmness, water-holding capacity, and strength of gel in yogurt. Furthermore, HIU has shown to reduce the crystal size and the freezing time in ice cream [45].
Whey proteins utilization has taken high relevance from different points of view. Whey has also a very high nutritional value. In Table 1, the effect of HIU on some beverage products from whey is provided. Whey proteins have been used in different formulations to produce new dairy products. The use of HIU to improve physicochemical properties on these kind of products is thoroughly documented. Similar to milk, HIU by cavitation effect, reduced droplet size, improved stability and heat stability of the whey beverages, promoted narrowed size distribution and increased homogeneity of the beverages [31], [46], [47], [48]. Ultrasound net power tested for effects on whey proteins in beverages or emulsions varied from 0 to 800 W [31], [46], [47], [48], [49]. Effects on protein internal structure were observed with a power density of 250 W/L (F = 20 or 28 kHz), which will be discussed in the next section [47]. With a net power of 400 W (F = 19 kHz), reductions of Zeta potential have been reported, together with kinetic stability and a decrease in particle size, leading to a higher homogenization in a prebiotic beverage enriched with whey [48]. Higher net power such as 720 and 750 W have resulted in the reduction of particle sizes, an increase of Zeta values, lightness, and kinetic stability [31], as well as an increase of heat stability and protein solubility [46]. High net power such as 500 and 800 W resulted in an increase of in the degree of hydrolysis [49], [50] A possible disadvantage of applying high net powers such as 600 W is that HIU may modify the colour of the product giving the beverages a blue colour with high hue tones compared to control samples [48].
Time of ultrasonication for whey protein beverages or emulsions is again a factor of importance. There is enough evidence that times longer than 15 min may increase the release of sulfhydryl groups, leading to an increase of free thiols in the solutions [26], [50], as well as the formation of protein crosslinking [26], [51].
The most frequently used ultrasound frequencies that have an effect on physicochemical properties of dairy, reported in this section, are around 20 kHz (19–24 kHz). Despite variations in the ultrasonication time or power, the size reduction of fat globules and a higher homogenization are common effects in liquid and semiliquid dairy by applying this range of ultrasonication frequencies. The change in colour parameters of milk and milk-based products is apparently promoted by variations in ultrasonication power. Yellowness and lightness are the coordinates more easily modified with ultrasonication powers greater than 400 W. Higher ultrasonication powers seem to promote changes in chroma or a* coordinates. A reduced number of studies applied a different range of frequencies (40 & 60 kHz) [42]. Nevertheless, no linear relationship between frequency and pH or acidity was found, which implies that ultrasonication may improve those variables, but the changes are not uniform. Furthermore, no improvement of protein or fat content in cheese can be observed by increasing the ultrasonication power [42].
3. Chemical properties
3.1. Whey proteins
The effect of ultrasound on milk proteins has been extensively documented in recent years (Table 2). Ultrasound effectiveness as a pretreatment tool to modify the molecular structure of food proteins is well known [53]. Abadía-García et al. [54] reported that the density of ultrasound in whey protein before hydrolysis with plant proteases exerts a significant effect on proteolysis, reducing hydrolysis time by up to 95% in bromelain hydrolysates. Hence, ultrasound increases the susceptibility to enzymatic hydrolysis, which could be used to produce bioactive peptides from whey proteins. In this regard, Uluko et al. [55] reported an increase in the number of peptides after pretreatment with ultrasound to improve the hydrolysis of the milk protein concentrate. All these effects are due to the changes ultrasound produced in the milk and dairy products’ proteins. On this topic, Wu et al. [50] observed an increase in the inhibitory and immunomodulatory activities of whey protein hydrolysates due to the effects of ultrasound-pretreated whey proteins. They suggested that ultrasound improves enzymolysis in whey proteins for the generation of new bioactive peptides, which can be used as a drug or a functional ingredient. Ahmadi et al. [56]reported that ultrasonication of whey protein concentrate partially replaces heat denaturation prior to transglutaminase treatment. This improves the rheological, functional, and textural properties of whey protein systems. Furthermore, the ultrasonication increased the stability of the foam and reduced the syneresis, obtaining uniform gels with higher hardness. Despite the differences in the characteristics of the equipment and experimental conditions, in all these studies the production of bioactive compounds from samples containing serum proteins was reported, except in the studies by Uluko et al. [55] and Ahmadi et al. [56], who used milk protein concentrate with caseins in its composition.
Table 2.
Effects of ultrasound on the components of dairy products and milk ingredients: proteins, enzymes, fat, and lactose.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Whey sweet, protein content on whey 8.13 g/L | Ultrasonic homogenizer (model not specified), 0.092, 0.151, and 0.220 W/mL, 20 kHz, 13 mm probe, 12, 52.5 and 32.25 °C; 5, 10 and 15 min. Papain and bromelain hydrolysis after ultrasound application. | Protein rearrangement and aggregate formation due to changes in denaturation enthalpy, reduction of reactive thiol groups, and changes in secondary structure. | [54] |
| Reconstituted buffalo’s milk and fresh buffalós milk | Ultrasonic horn (3000, Misonix Incorporated, USA); 44, 54 and 66 W, 20 kHz, 3 mm probe, 45 °C; 5, 7.5, 10, 12.5, 15, 17.5 and 20 min. | Reduction of fat globule diameter by up to 92% (sub-micron level) and increase in surface area. Decreased the size of milk particles. Improved the gel strength. Increased free saturated fatty acids and gel hardness. | [24] |
| Whey protein concentrate 10% w/w | Ultrasonic probe (XL2020, Misonix sonicator, USA), 550 W, 20 kHz, 10 mm probe, 25–30 °C; 2.5, 5 and 7.5 min. Transglutaminase hydrolysis after ultrasound application. | Modification of the protein structure to make it more susceptible to transglutaminase. | [56] |
| Buffalo’s milk | Ultrasonic homogenizer (Kirchfeld, Germany), 430 and 338 W, 28 kHz, 0.5 cm tip, 20 °C; 5, 10 and 15 min. | Smaller and more uniform globules of fat as the frequency and duration of the ultrasound increases. | [99] |
| Fresh cream, fat content 30% | Ultrasonic processor (APU400, Adeeco Co., Iran); 100 and 300 W, 20 kHz, 12 mm probe, pulse 2 s on and 4 s off, 50 °C; 5, 10 and 15 min. | Denaturation of protein chains, which were opened to cover the fat globules. Fragmentation of fat globules. | [43] |
| Whey protein concentrate, 10% w/w, pH 6.5–7.1 | Ultrasonic processor (VCX 750, Vibra Cell Sonics, USA), 750 W (amplitude 20%), 20 kHz, 13 mm probe, 49 °C, 20 min. | Decrease in the consistency index in the protein solution, greater elasticity, reduction in the size of aggregates, and greater hydrophobicity surface. | [57] |
| Semi-skimmed sheep milk | Ultrasonic equipment (VC 130, Sonics and Materials Inc., USA), 78 and 104 W, 20 kHz, probe 5 × 60 mm diameter; pulse 4, 6 and 8 s at 40–69 °C. Storage time (30, 90 and 180 days). | There was no release of free fatty acids or changes in the protein profile of fresh and frozen stored semi-skimmed sheep milk due to ultrasound. | [18] |
| Reconstituted whey powder, 6% (in water) | Ultrasonic processor (S-4000, QSonica LLC, USA), 20 kHz. Microbiological preservation of whey (480 W and 600 W, 45 and 55 °C; 6.5, 8 and 10 min) and dairy cultures activation (84 W and 102 W, 37 and 43 °C, 150 s). | There was no denaturation and precipitation of serum proteins compared to pasteurization; smaller diameter particle size. | [20] |
| Full cream UHT milk | Ultrasonic processor (VCX 750, Sonics and Materials, USA), 750 W, 20 kHz, 13 mm probe, 24–26 °C; 2.5, 5, 6, 7.5, and 10 min. | The protein, casein, and lactose contents were unchanged. The fat content increased due to the increase in the surface area of the globules. The lactoperoxidase and alkaline phosphatase enzymes were not inactivated. | [84] |
| Whey protein concentrate, 50 g/kg (w/w) | Ultrasonic horn (Branson Sonifier, USA), 450 W (amplitude 50%), 20 kHz, 19 mm probe, 6 ± 4 °C; 1, 5, 10, 20, 30 and 60 min. | Decrease in denaturation enthalpy with 5 min ultrasound. Prolonged sonication caused protein aggregation. Minor changes in secondary structure and hydrophobicity of proteins. | [58] |
| β-Lactoglobulin (β-LG) and α-Lactalbumin (α-LA), 1.5% w/w, pH 7.0 | Ultrasonic horn (Branson Sonifier, USA), 450 W (amplitude 50%), 20 kHz, < 10 °C; 1, 5, 10, 20, 30, and 60 min. | Increase of thiol content and superficial hydrophobicity of β-LG. Minor changes in secondary and tertiary structures. α-LA was the most affected. | [59] |
| Fresh pasteurized skim milk, reconstituted micellar casein powder and reconstituted casein powder, 50 and 150 g/kg solutions | Ultrasonic horn (Sonifier 450, Branson, USA), 450 W (amplitude 50%), 20 kHz, 10 °C; 1, 5, 20, 30, and 60 min. | In fresh milk, the fat globule size was reduced but the casein micelle size was unchanged. Soluble whey protein increased and there was no change in soluble calcium content. | [78] |
| Reconstituted micellar casein and tetrasodium pyrophosphate, 50 g/kg solutions | Ultrasonic sonifier (Branson Sonifier 450, USA), 450 W (amplitude 50%, actual power 31 W), 20 kHz, 19 mm horn, 6 ± 4 °C; 1, 5, 10 and 30 min. | Change in the surface hydrophobicity of proteins. Surface charge was inaltered. | [73] |
| Reconstituted calcium-enriched skim milk, 10% w/w solutions, pH 6.7 | Ultrasonic sonifier (Branson Sonifier 450), 450 W (actual power 31 W), 20 kHz, 19 mm horn; 1, 10 and 20 min, <10 °C. | Breakdown of whey/whey and whey/casein aggregates, making them heat stable. Decreased gelation and syneresis times and increased viscosity. | [74] |
| Raw milk, ultrafiltrate retentate, skim milk, skim milk concentrate and cream. | Ultrasonic horn (Branson Sonifier 450, US), 450 W (operated at 101 and 189 W), 20 kHz, 19 mm probe, 50 and < 10 °C; 30 s and 1, 10 and 30 min. | Decreased fat globule size in milk. Formation of flocculated fat particles in cream treated at < 10 °C. | [88] |
| Whey protein emulsion gels, 10% w/w, pH 7.0 | Mono-frequency, simultaneous dual frequency ultrasound (Meibo Biotechnology Co., China), 250 W/L; 20, 28 and 20/28 kHz; for 5, 10, 15 and 20 min. | Ultrasonication reduced alpha-helix, but increased beta-sheets, beta-turn and random coil, increase of random windings exposing tryptophan residues. Multi-frequency highly increased the secondary structure change. | [47] |
| Whey protein isolate, 15% w/w | Ultrasound equipment (no specified) at 300 W/cm2, 24 kHz, <30 °C. 30 min. | Aggregation and gelation due to a greater number of disulfide bonds. | [60] |
| Whole non-homogenized pasteurized milk | Ultrasonic flow cell (Sonolab SL10 ultrasonic, Syrris Ltd, United Kingdom); 91, 273 and 454 W/L; 20 kHz; 27, 50 and 70 °C; 30 min, average residence time in the flow cell 4.13 min. | Increase in gel strength due to denaturation of whey proteins. Changes in the fat globule membrane increased its association with caseins, facilitating the formation of highly functional fat globule/protein complexes. | [25] |
| Fresh cow milk | Ultrasonic processor (S-4000, Misonix, country not specified), 600 W (amplitude 25%, 50%, and 100%), 25 kHz, 19 mm sonotrode; 0.5, 1, 2 and 4 min. | Increase in the size, physical stability, and encapsulation capacity of micellar casein due to the electrostatic repulsion between casein molecules, which increased the availability of interior hydrophobic areas. | [75] |
| Anhydrous milk fat | Ultrasonic flow cell (Sonolab SL10 ultrasonic, Syrris Ltd, United Kingdom), 40 W, 20 kHz, 28 °C; 2.5, 5, 7, and 10 min. | Acceleration of the crystallization process in mixtures with a high content of rapeseed oil. Reduction in crystal size without effects on lipid oxidation. | [26] |
| Prebiotic whey beverages | Ultrasonic device (Unique, Disruptor, Brazil); 200, 400 and 600 W, 19 kHz, 13 mm probe, without controlled temperature (maximum temperature 53 °C), 3 min. | Improvement in the kinetic stability of beverages, avoiding phase separation due to the decrease in particle size, denaturation of whey proteins, and gelling of polysaccharides. | [48] |
| Commercial whey protein concentrate, 5% w/v | Cell disruptor (JY92-IIN, Ningbo Scientz, China), 20 kHz, 800 W, 13 mm probe, <50 °C, 1–10 min. Water bath sonication (70 °C). Hydrolysis with alcalase, papain and trypsin after ultrasound application. | Increase in the degree of hydrolysis of the enzymes. Decreased antigenicity of β-lactoglobulin from 50.81% to 48.82%, contrary to heat treatment. | [49] |
| Retentate of ultrafiltered milk | Ultrasonic homogenizer (HD2200, Sonopuls Bandelin Company, Germany), intensity of 80%; 20, 40 and 60 kHz, probe and temperature not specified, 20 min. | The fat and protein content in cheese was not affected during maturation (60 days). Proteolysis and lipolysis were accelerated. | [42] |
| Whey protein isolates (WPI) and whey protein concentrates, 10% (w/w) | Probe (V1A, Sonics and Materials Inc., USA), 43–48 W/cm2, 20 kHz, 1.2 cm probe, 15 and 30 min. Bath (SO375T, Sonomatic), 1 W/cm2, 40 kHz, 15 and 30 min. | Ultrasonic bath showed a reduction in the size of particles and changes in the composition of the molecular weight of protein fractions. Ultrasonic probe showed a decrease in molecular weight and protein fractionation. | [70] |
| Whey protein isolate (100 g/L) and calcium lactate (final concentration of 1.20 mmol/L), pH 7.0 | Ultrasound processor (JY92-2D, NingBo Scientz Biotechnology Co. Ltd, China); 0, 10.19, 12.74, 15.29 and 17.83 W/cm2 (0, 200, 400, 600 or 800 W), 20 kHz, 0.636 cm probe, 3 s on/1 s off, 25 ± 2 °C, 20 and 40 min. | Improvement in surface hydrophobicity and simple sulfhydryl groups, which led to microstructural changes. HIU (200 to 800 W for 20 and 40 min) reduces the particle size. | [61] |
| Whey protein isolate, pH 7.0, 50 mg/mL, heated at 75 °C for 15 min and cooled at 25 °C | Ultrasound processor (JY92-2D, Ningbo Scientz Biotechnology Co. Ltd, China), 600 W (41–45 W/cm), 20 kHz, pulse 1 s on and 2 s off, 25 °C; 20, 40 and 60 min. | Higher molecular size. Losses of free amino groups. Effects on the β-sheet, β-turn, and random coil of serum proteins. | [51] |
| Recombined milk emulsions | Turbiscan tube (Formulaction, France). One-transductor plate using 400 kHz, 2.45 W/cm2 (Submersible Transducers, Sonosys Ultraschallsysteme GmbH, Germany) and 1.6 MHz, 2.2 W/cm2 (Nebulizer, APC Inter- national Inc., USA), 5 min, 35 °C; and two-transducter plate (400 kHz). | The recombined emulsion and raw milk presented flocculation and coalescence in the cream layer of the emulsion. | [100] |
| Inhomogeneous goat milk | Ultrasonic processor (UP100H, Hielscher, Germany), 100 W (operated at amplitude 60 and 100%), 30 kHz, probe 7 and 10 mm, temperature not specified; 3, 6, and 9 min. | Higher heterogeneity of fat globules (between 0.3 and 4 μm) and increased total area; destruction of the fat globule membrane and formation of casein-fat globule complexes. | [89] |
| Fresh samples of paneer whey | US pretreatment: ultrasonic horn (Sonics and Materials, USA), 100 W (80% duty cycle), 20 kHz; 5, 10 and 15 min. Thermosonication pretreatment: 60 °C, 100–250 W; 5, 10, 15 and 25 min. | Lactose recovery up to 94.5% after ultrafiltration and sonocrystallization. | [97] |
| Whey protein concentrate, concentration not specified | Ultrasonic processor (VCX 750, Vibra Cell Sonics, USA), 750 W (amplitude 20%), 20 kHz, temperature not specified, 19.75 min. | Homogeneous distribution and decreased particle size, increased solubility, greater thermal stability, significant alteration in the secondary structure. | [46] |
| Yogurt, pH 5.7 to 5.1 | Electrodynamic vibration exciter-shaker (TV 51110, Tira GmbH, Schalkau, Germany), 25 to 1000 Hz, acceleration amplitudes 5, 10, 15, 20 and 25 m/s2; 200 s and 20 min. | Increase in the number of large particles (greater than0.9 mm) from 34 to 242 particles per 100 g of yogurt. Protein aggregation. | [80] |
| Pasteurized heavy whipping cream (40% fat) | Ultrasonic probe (Misonix Inc., USA), 85 W, 20 kHz, 1.27 cm probe; amplitude of 108 µ for 10, 30, 60, and 90 s. | Weakening of the fat globule membrane. Low melting point triacylglycerol crystallization during whipping and melting of high-melting-point triacylglycerols. Solid fat content was reduced when ultrasonication was applied for 90 s. | [37] |
| Whole raw bovine milk | Ultrasonic milk fractionation prototype device with fully-submersible plate transducers (Sonosys Germany), 283–625 W, 22.2–57.9 °C. Batch sonication: 2 MHz (330 W/L); 5, 10 and 20 min. Stage-based fractionation: 1 MHz and 2 MHz (single or dual), maximum power or 50%, 5 min. | Milk fractionation and formation of a vertically increasing fat concentration gradient, with larger fat globules on the surface. | [34] |
| Skim milk | Ultrasonic reactor, 20 kHz, 30 °C, pH 6.7 and 8.0; 20, 400, and 1600 kHz; 101, 8.6, and 11 kW/m2; < 30 °C, pH 6.7–8.0. | At low frequencies and high pH disruption of casein micelles and protein release from the micellar phase to the serum phase. The released protein formed aggregates with surface charge similar to micelles. | [79] |
| Homogenized pasteurized fresh milk | Ultrasonic processor, 24 kHz, 22 mm diameter probe, 5 min, 45 °C. | Microstructural changes in yogurt, obtaining a honeycomb network with a porous nature and smaller particle sizes (<1μm). | [101] |
| Raw milk | Ultrasonic processor, 400 W, 24 kHz, probe 22 mm, amplitude 70% and 100%; 50, 100, 200, and 300 s. | Significant increases in levels of free fatty acids and oxidation. Milk composition skewed by sonication. CO2 reduced pyrolytic processes and formation of oxidation products caused by ultrasound. | [102] |
| Cristallyzed anhydrous milk fat | Sonicator (S-3000, Misonix Inc., USA); 50, 30, 20 and 5 W; 20 kHz, pulse 10 s on and 5 s off, 10 s. | Decreased crystallization induction time and generation of smaller crystals. | [92] |
| Whey protein isolate | Ultrasonic probe, 130 W, 15 min, pulses of 10 s, < 65 °C. | Little effect on the emulsifying properties of whey proteins, unlike high pressures, which increased free SH groups and surface hydrophobicity and charge. | [103] |
| Whole milk powder, 13% w/w + 0.02% sodium azide | Ultrasonic homogenizer (XL200, Misonix, USA), 50 W, 22.5 kHz, 30 min (0–500 J/mL), without or with temperature control (20–80 °C). | Homogenization of fat globules, denaturation of serum proteins, aggregation of fat globules and proteins. | [62] |
| Sodium caseinate, whey protein isolate and milk protein isolate solutions at 0.5–5 wt%. | Ultrasonics processor (Viber Cell 750, Sonics, USA), 34 W/cm2, 20 kHz, 45 °C, 12 mm probe, 2 min. | Reduction in micelle size and hydrodynamic volume of proteins. MPI emulsions showed very small droplet sizes and stability against coalescence. | [104] |
| Reconstituted lactose solutions (lactose monohydrate 12–18% w/v and acetone 80% v/v). | Ultrasonic bath (Aqua Scientif Instruments, India), 120 W, surface area of 225 cm2, 30 °C, 2–8 min. | Lactose crystallization in 80%–92%, achieving recovery after 4 min of sonication. | [98] |
| Fresh pasteurized homogenized skim milk | Ultrasonic processor (VC750, Vibracell Sonics and Materials, US); 150, 262, 375, 562 and 750 W; 20 kHz, <87 °C, 10 min. | Reduction in fat globule size. Changes in viscosity of yogurt due to protein denaturation, decreasing the content and composition of soluble protein and forming insoluble high-molecular-weight co-aggregates. | [76] |
| Fresh pasteurized homogenized skim milk (93% v/v) and flaxseed oil (7% v/v) | Ultrasonic unit (102 CE, Branson Sonifier), 176 W, 20 kHz, 12 mm probe, water temperature at 22.5 °C, 1–8 min. | Finer sonoemulsified oil globules, stabilized by partially denatured serum proteins. | [87] |
| Fresh pasteurized homogenized skim milk | Ultrasonic horn (102 CE, Branson Sonifier), 90 and 180 W (applied powers), 20 kHz, 12 mm probe, 22–37 °C; 15, 30, 45 and 60 min. | Modification in the size of casein micelles, fat globules, and soluble particles depending on the ultrasonic power. Whey proteins were denatured and formed soluble serum-serum/serum-casein aggregates. | [53] |
| Whey protein isolate, 10% w/w, pH 7.0 | Ultrasonic processor (VCX800, Vibra cell Sonics, USA), 107 W/cm2 (40% amplitude), 20 kHz, 13 mm probe, 10 s on 5 s off, samples were preheated before ultrasonication; 5, 10, 20 and 40 min. | Particle size reduction and increase of free surface sulfhydryl groups in preheated whey protein solutions. Disulfide bond formation after gelation due to reduction in free sulfhydryl content. | [63] |
| β-lactoglobulin (BLG), 2 mg/L, pH 6.5 | Sonifier (150, Branson Ultrasonic Corp., USA), 135 W/cm2 (amplitude 120 μm), probe not specified, 20 kHz, with cooling (25–35 °C) and without cooling (25–65 °C); 5, 15, 30, and 60 min. | Modifications in the secondary structure of BLG. Formation of BLG dimers, trimers, and oligomers. More exposed hydrophobic surfaces. | [71] |
| β-lactoglobulin (BLG, 4 mg/mL) in the presence of D-arabinose, D-lactose, D-glucose, D-ribose, D-galactose and D-fructose monohydrates (217.5 mM) | Sonicator (Branson Sonifier 150, Branson Ultrasonic Corp., USA), 9.5 W (135 W/cm2), 20 kHz, 10–15 °C, 60 min. | Formation of products of the Maillard reaction (MRP). Ribose induced BLG modification in 76% and three bound anhydroribose units. Minor alterations in secondary and tertiary structures. The MRPs showed high antioxidant capacity. | [66] |
| α -Casein and whey powder, 2.0 mg/mL and 40 mg/mL, respectively | Ultrasonic processor (Sonics Vibracell VC 505, USA), 500 W, 20 kHz, 13 mm probe, 60 °C, 30 min. | No change in band intensity on SDS-PAGE gels, therefore there was no change in protein concentration. No change in IgE binding values (allergy). | [67] |
| Fresh Cheddar cheese whey (pasteurized) | Ultrasound reactor with submersible stainless-steel plate transducers (Sonosys Ultraschallsysteme GmbH, Germany) at 45, 66, 143, 46, 95.5 and 187.5 W; 400 and 1000 kHz at 37 °C. | Better fat separation from whey and greater oxidative stability of lyophilized fat-enriched layers. | [86] |
| Milk protein concentrate, 15.4 g/100 mL | Equipment not specified (JY92-IIN), 800 W, 13 mm probe, <50 °C; 1, 3, 5, and 8 min. Neutrase hydrolysis after ultrasound application. | Improvement in degree of hydrolysis during enzymatic hydrolysis. Production of new low-molecular-weight peptides. | [55] |
| Raw milk standardized to 3% fat | Continuous system, 16/20 kHz, 1.36 kW/paso; 0.15, 0.3, and 0.45 L/min; 14–18 min, 42 or 54 °C. | Sub-micron lipid droplets embedded in protein chains of the curd matrix. | [28] |
| Fresh skim milk and cream | Batch sonicator (Branson Ultrasonics 2000 series, USA); 77, 104 and 115 W, 20 kHz, 115 W, 1 and 3 min (two 30 s intervals with a 1 s-break), 72 °C. | Decreased total plasmin activity dependent on storage time in skim milk. Decrease in fat globule size. | [85] |
| Crystallized anhydrous milk fat with and without the use of US | Sonicator (S-3000, Misonix Inc., USA), 0.3175 cm tip, 10 and 15 min, stirring at 200 rpm. | Decrease in the induction time of crystallization and in the generation of more crystals and more viscous materials. | [93] |
| Milk inoculated with starter culture for yogurt production | Ultrasonic generator (CP502, Cole-Parmer Instrument Company, USA); 450, 225 and 90 W; 1, 6 and 10 min; 450, 90, and 225 W; 13 mm probe; 1, 6, and 10 min. US before inoculation: 450, 90, and 225 W; 6 min. US during inoculation: 450, 90, and 225 W, 8 min. | Homogenizing effect, producing fat globules of an average diameter equal to 2 µm or less. | [95] |
| Whey protein | Ultrasonic processor (JY92-II, Haishukesheng Ultrasonic Equipment Co., Ningbo, China); 100, 200, 300, 400, and 500 W; 20 kHz, 1.5 cm probe, 2 s on 2 s off, <25 °C, 15 min. Alcalase hydrolysis after ultrasound application. | Increased degree of hydrolysis in whey protein. Whey protein display, resulting in a 43.7% increase in free sulfhydryl content and a 62.6% increase in surface hydrophobicity. Decrease in the α-helix content and significant increase in β-sheets. | [50] |
| Reconstituted milk protein concentrate, ultrafiltered milk with 15% solid content | Ultrasonic horn (CTXNW-10B, Hongxiang Biotechnology Co., China), 600 W (50% amplitude), 20 kHz, 50 mm probe, 5 s on 3 s off, < 50 °C; 0.5, 1, 2, and 5 min. | Particle size reduction. Increase in solubility and emulsifying activity index. Increase in surface hydrophobicity without changes in the molecular weight of proteins. | [64] |
| α-lactalbumin (α-LA), 100 mg/mL, pH 6.0 | Ultrasonic processor (DY-1200Y, Deyangyibang Instruments Co. Ltd, China), 400 W (12.74 W/cm2), 20 kHz, 2 cm diameter probe, pulse 2 s on and 5 s off, 14–20 °C; 20, 40, 60, 80, or 100 min. α-LA was catalyzed by laccase in the presence of ferulic acid. | Improvement of laccase-catalyzed cross-linking of α-LA. Increase in the surface hydrophobility and strength of the gel without significant changes in the conformational structure of the α-LA conjugates catalyzed by laccase. | [105] |
| Micellar casein concentrate | Cell disruptor, 58 W/L, 20 kHz, pulse on 3 s and off 2 s; 0.5, 1, 2, and 5 min. | Increased surface hydrophobicity and reduced particle size. Changes in secondary structure, with an increase in β-sheets and random coils and a reduction in α-helix and β-turns. | [77] |
| Skimmed fresh goat’s milk | Cell disruptor (JY92-IIN, Ningbo Scientz, China), 800 W, 20 kHz, 13 mm probe for 0–20 min. | Smaller particle sizes. Increased denaturation of serum proteins and soluble Ca and P content. | [106] |
| Reconstituted whey protein-concentrate and isolate | Ultrasonic horn (Branson Sonifier, US), 450 W, 20 kHz, 19 mm probe, 6 ± 4 °C; 1, 5, 10, 20 and 60 min. | Reduction in size of suspended insoluble aggregates, compact network of densely packed whey protein aggregates. | [65] |
Arzeni et al. [57] found that ultrasound treatment modifies the functional properties of serum proteins, such as gelation, viscosity, and solubility. This is due to molecular modifications (increase in hydrophobicity and variation in particle size), which are dependent on the nature of the protein and the degree of denaturation and aggregation. On the contrary, Chandrapala et al. observed that ultrasound treatment in serum protein concentrate caused minor changes in the protein structure, preserving the functional properties [58]. Later, the same researchers also reported that the effect of ultrasonication on serum proteins differs substantially when mixtures and/or pure serum proteins are used. Under the same ultrasound conditions, α-lactalbumin is more affected than β-lactoglobulin, with significant increases in surface hydrophobicity. Meanwhile, in the mixture of the two proteins, it decreases surface hydrophobicity because the thiol exposed in β-lactoglobulin interacts with the disulfide bond of α-lactalbumin [59]. Another study with serum protein mixtures reported changes in the physical properties of gels containing large amounts of α-lactalbumin. This suggests that the combined effect of ultrasound- and heat-mediated protein deployment, followed by aggregation and gelation due to a greater number of disulfide bonds, led to the formation of a stronger network with greater water-holding capacity [60]. The effects of ultrasound treatment on the properties of whey proteins are dependent on the experimental conditions (see Table 2). This accounts for the changes in the functional properties of whey proteins observed by Arzeni et al. [57], with temperatures <49 °C during sonication at 750 W (amplitude 20%), while Chandrapala et al. [58], [59] kept the samples at <10 °C during processing at 450 W (50% amplitude).
Jiang et al. [61] reported that ultrasound treatment had a considerable impact on the physical, functional, and oxidative properties of serum proteins in the presence of calcium lactate. They found that ultrasound extensively modified the microstructure and increased the strength of the gel, emulsion capacity, and radical scavenging capacity 2,2-diphenyl-1-picrilhidrazil (DPPH). Ultrasound treatment modifies and restructures whey proteins, solving problems associated with their heat stability and solubility and facilitating their use as a functional ingredient in heat-processed products and protein-rich foods [46]. However, the temperature during the whole milk ultrasound process is important. In this regard, Nguyen and Anema [62] showed that controlled (60 °C) and uncontrolled (<90 °C) temperatures caused the denaturation of serum proteins and gels with greater firmness and shorter gelation time compared to untreated milk. When the ultrasonication temperature was kept below 60 °C, gels with very high firmness were produced without denaturation of the serum proteins. Similar results were obtained by Shen et al. [63], who reported that HIU modifies serum proteins and improves their gelation properties. The water-holding capacity, strength, and firmness of the gel were positively correlated with the content of free surface sulfhydryl (-SH) in preheated protein isolate (WPI) solutions. However, the correlation was negative with the size of the preheated WPI particles and the content of -SH free of acid-induced WPI gels.
Changes in protein functionality are closely related to surface hydrophobicity and reduction in particle size. It has been reported that power ultrasound (20 kHz, 600 W, 50% amplitude) can significantly improve the solubility, emulsification, and gelation of reconstituted and ultrasonicated milk protein concentrate [64]. Zisu et al. [65] also reported a reduction in gelation time and syneresis, as well as improvements in gel resistance in ultrasound-treated isolate and reconstituted whey protein concentrate. Shear forces generated a reduction in the size of the suspended insoluble aggregates, leading to reduced turbidity. They treated dairy ingredients (reconstituted whey protein concentrate, whey protein, and milk protein retainer) with ultrasound for at least 1 min and up to 2.4 min in pilot-scale ultrasonic reactors. They observed a decrease in viscosity and an improvement in heat stability in ingredients containing whey proteins due to the reduction in particle size. These properties were maintained after spray-drying.
Casein, β-lactoglobulin, and α-lactalbumin are the main allergens in milk proteins. Stanic-Vucinic et al. [71] found that ultrasound treatment at 20 kHz frequency, 9.5 W (135 W/cm2) for 5, 15, 30, and 60 min and raising the sample temperature from 25 to 65 °C did not decrease the allergenicity of β-lactoglobulin. They monitored allergenicity in individual patients’ sera, basophil activation test, and skin prick testing in 41 cow’s milk allergy patients. Ultrasound treatment induces changes in secondary structure and the formation of dimers, trimers, and oligomers and increases the exposition of hydrophobic surfaces. As well, makes the crosslinking of β-lactoglobulin more efficient by crosslinking with enzymes that require nucleophilic residues for its action to take place. Similarly, Tammineedi et al. [67] reported that HIU at 20 kHz frequency and 500 W power for up to 30 min at 60 °C did not reduce the allergenicity of α-casein or whey proteins, unlike other technologies such as UV-C light, which reduced the allergenicity of milk proteins 25%–27.7% after 15 min of treatment. Li et al. [68] stated that HIU treatment was effective in reducing the allergenicity of shrimp after 0, 2, 8, 10, and 30 min at 30 kHz, and 800 W at 0 °C and 50 °C. The allergenicity of the boiled shrimps treated at 0 °C y 50 °C decreased by nearly 50% and 40%, respectively, with 10 min of ultrasound treatment.
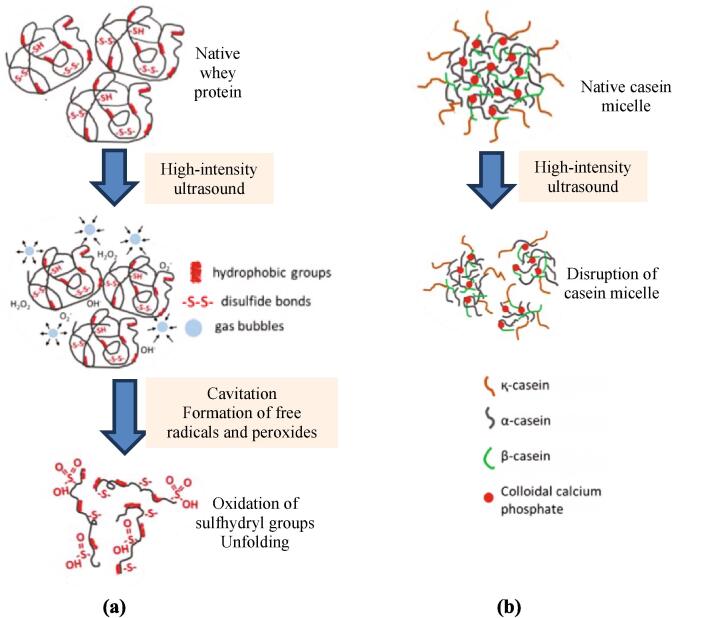
As mentioned in section 1, HIU has cavitation as the mechanism responsible for its effects. Cavitation generates hydrogen peroxide that induces protein oxidation. Free radicals and superoxides promote protein crosslinking and HIU also modifies the hydration status of proteins [13], [69]. Fig. 3 (a) depicts the effect of HIU on the structure of whey protein. Traditional heat treatments induce serum protein aggregation, which is dependent on the formation of disulfide bonds. However, HIU treatment induces structural changes. Jambrak et al. [70] reported that after treatment with an ultrasonic probe of 20 kHz, ultrasound caused a decrease in particle size, narrowed their distribution, and significantly increased the specific free surface, whereas ultrasonic bath treatment with 40 kHz ultrasound for 15 and 30 min showed significant changes in the composition of the molecular weight of whey protein fractions. The ultrasound-induced structural changes in proteins were associated with partial cleavage of intermolecular hydrophobic interactions, rather than peptide or disulphide bonds. Yanjun et al. [64] applied ultrasound (12.5 W and 50% amplitude for 0.5, 1, 2, and 5 min) to reconstituted milk protein concentrate and observed significant changes in physical and functional properties but no significant change in protein molecular weight as evaluated on sodium-dodecyl sulfate–polyacrylamide gel. Probably ultrasound duration treatment was not enough to appreciate changes in secondary and primary structure of proteins. Frydenberg et al. [60] studied the mechanisms mediating the effects of HIU (24 kHz, 300 W/cm2) on whey proteins on the molecular level. They observed no change in secondary structure by circular dichroism. However, these authors pointed out that other methods, such as IR spectroscopy, could be applied in order to further investigate the impact of ultrasonication on whey protein secondary structure. They suggest that HIU and heat combined mediates an unfolding of the proteins, making the intramolecular bonds more accessible to the forces of ultrasound. They concluded that the α-La:β-Lg ratio is also important for the inter- and intramolecular interactions formed subsequent to ultrasound-mediated unfolding of the proteins, where higher amounts of α-La appears associated with higher degree of disulfide bonding.
Fig. 3.
Effect of HIU on whey protein structure (a) and casein micelle structure (b) (modified from Nunes and Tavares [72]).
It is important to point out that at low temperatures HIU does not significantly modify the protein secondary and tertiary structures. Stanic-Vucinic et al. [66] investigated whether HIU can promote glycation of β-lactoglobulin by Maillard reaction to safely modify food proteins and their functions. They showed that glycoconjugation of β-lactoglobulin occurs efficiently in the presence of various sugars, especially ribose, and demonstrated improved functional properties of the obtained glycoconjugates with a minor influence on protein secondary and tertiary structures, with a significant increase in the content of early and intermediate Maillard products, fluorescence, darkening intensity and activity and antioxidant solutions. Here, the temperature of the reaction was 10–15 °C whereas in a previous work [71] was 25–35 °C and 25–65 °C. This difference could explain the different effects observed in the secondary structure of proteins.
3.2. Caseins
The effect of ultrasound on caseins is summarized in Table 2. Structural changes have been observed in size, physical stability, and encapsulation capacity of casein due to ultrasound [53], [62], [73], [74], [75], [76], [77]. Chandrapala et al. [78] reported no change in casein micelle size or soluble calcium concentration (mineral balance) of fresh skim milk treated with ultrasound at 450 W, 20 kHz, 10 °C, for 1–60 min). As well, soluble whey protein increased and viscosity decreased due to breakdown of whey-casein protein aggregates in reconstituted casein treated with ultrasound. Liu et al. [79] reported similar results when reconstituted skim milks (10% w/w total solids, pH 6.7–8.0) were ultrasonicated (20, 400 or 1600 kHz at a specific energy input of 286 kJ/kg) at a milk temperature of <30 °C. Application of ultrasound to milk at different pH altered the assembly of the casein micelle in milk, with greater effects at pH 8.0 and frequency of 20 kHz. Sonicated milk at 20 kHz caused greater disruption of casein micelles causing release of protein from the micellar to the serum phase than when sonicated at 400 and 1600 kHz. Regarding protein gels (casein), Chandrapala et al. [73] reported that ultrasonication before the addition of tetrasodium pyrophosphate (TSPP) formed a firm gel with a fine protein network and low syneresis. On the contrary, when ultrasound was applied after the addition of TSPP, it formed a weak and inconsistent gel with high syneresis. The advantages of ultrasound application to the breakdown of protein aggregates have been widely used in the development of calcium-fortified dairy products, including the acid gelation of these products [74]. The use of alkaline pH and ultrasonication in fresh milk have been broadly used to create natural casein micelle nanocapsules with high nanoencapsulation efficiency. This maintains their natural structure and morphological characteristics due to electrostatic repulsions that facilitate interior hydrophobic areas [75]. It can be beneficial in the encapsulation of unsaturated fatty acids, oils, and other hydrophobic compounds used to enrich and fortify food and pharmaceutical products.
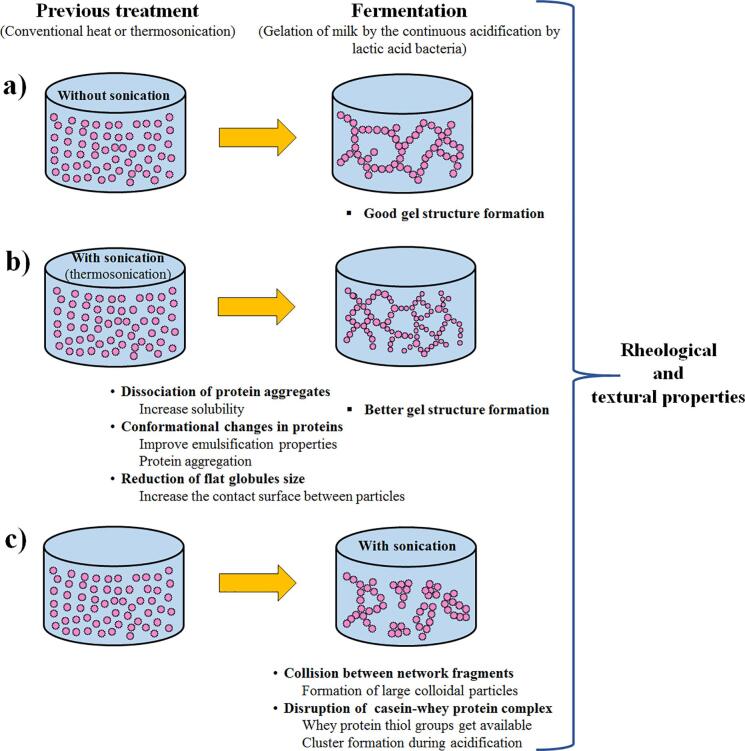
Regarding the benefits of HIU in the manufacture of yogurt, Sfakianakis et al. [76] reported a positive increase in viscosity, a decrease in the pH rate, and a decrease in the duration of pH lag phase of milk during yogurt production fermentation, compared with homogenized milk with conventional pressure. They attributed these positive effects in yogurt made with ultrasonicated milk to the formation of aggregates between whey (denatured whey proteins) and casein molecules. Shanmugam et al. [53]obtained similar results and reported the formation of soluble aggregates of serum-serum/serum-casein in skim milk homogenized, pasteurized, and treated for 30 min with ultrasound, reducing the turbidity of the milk without modifications. In studies with yogurt, Körzendörfer et al. [80] reported the formation of compact yogurts with large particles and low water retention and viscosity due to ultrasonication (30 kHz) of denatured (95 °C, 256 s) skim milk standardized in protein and inoculated with starter cultures (Lactobacillus bulgaricus and Streptococcus thermophilus) with a vibration exciter and shaker during the fermentation process. In another study, Körzendörfer and Hinrichs [52] reported the feasibility of applying ultrasound during the thermophilic fermentation of milk. This allowed them to obtain high-protein yogurt with reduced viscosity, which facilitates further processing. In the studies of Sfakianakis et al. [76] and Shanmugam et al. [53], the milk was previously treated with ultrasound before the yogurt fermentation process.
Zhang et al. [77] showed changes in the functional properties and structural characteristics of ultrasound-treated micellar protein concentrate before spray-drying. They observed a significant increase in conductivity, solubility, emulsification, and gelation as the ultrasonication time increased. They explained the changes by citing the exposure of hydrophobic regions from the interior to the surface of the molecules, as well as the reduction in particle size and changes in secondary structures.
Traditional heat treatments such as pasteurization do not alter the structure of the casein micelle due to its lack of tertiary structure, however heat treatments such as ultrapasteurization induce the precipitation of soluble calcium and the solubilization of colloidal calcium phosphate, dissociating κ-casein and some α-caseins from micelles, leading to aggregation and complexation with serum proteins and individual caseins [81], [82], [83]. In contrast, HIU does not produce changes in the native structure but rather with the reduction in particle size [77], [78]. Fig. 3 (b) depicts the effect that HIU has on the structure of the casein micelle.
3.3. Enzymes
In enzyme studies (Table 2), Jiang et al. [51] reported that ultrasonication and preheating applied to increase the crosslinking of transglutaminase catalyzed whey protein isolate and produced polymers with high molecular weights. This in turn produced an increase in emulsifying activity and foaming stability. Therefore, these products have potential as emulsifiers and foam stabilizers for processing and storing food products. In the case of ultrasonicated fresh milk, Balthazar et al. [18] found that, in sheep’s milk, there were no changes in the protein profile or free amino acids, maintaining the quality of the product and its suitability for the production of dairy products. Barukčić et al. [20] obtained interesting results in sweet whey, and reported an increase in stability due to the homogenization caused by the effect of ultrasound. Hence, HIU has the potential to replace conventional pasteurization because whey proteins, being heat-labile, do not precipitate. Cameron et al. [84] also suggested that ultrasonication is an effective means of pasteurization in raw milk; however, it should be combined with an adequate heat treatment to achieve inactivation of alkaline phosphatase and lactoperoxidase. Vijayakumar et al. [85] reported a decrease in total plasmin activity of up to 94% in skim milk and cream treated with HIU and heat (thermosonication). However, the enzymatic activity after 30 d increased up to 5–10 times in skim milk and remained unchanged in cream. This confirms that thermosonication helps to extend the shelf life of milk due to the inactivation of proteases.
3.4. Fat
Structural changes in fat globules of milk by HIU effect modify their integrity, reducing the size and diameter to submicron levels when compared to shear homogenization (Table 2). Abesinghe et al. [24] reported that ultrasonicating buffalo’s milk for 15 min significantly reduces the size of the fat globule compared to traditional homogenization. This led to the formation of better gelling properties for yogurt production while avoiding syneresis. However, lipolytic rancidity was significantly increased due to the increase in the content of free saturated fatty acids in milk. Torkamani et al. [86] reported a decrease in oxidative volatile compounds in skim milk whey powder obtained from pasteurized and ultrasound-treated liquid whey. The volatiles were below the detection threshold and showed oxidative stability during 14 d of storage.
Several authors have described the importance of milk proteins as natural emulsifiers that stabilize emulsions produced with ultrasound assistance [43], [87]. Shanmugam and Ashokkumar [87] reported that whey proteins stabilize sono-emulsified oil globules, which contributes to the production of gels with better textural attributes compared to those produced with non-ultrasound-treated pasteurized skim milk. Amiri et al. [43] reported similar results and observed that ultrasound treatment of whipped cream fragments reduces the size of fat globules. Consequently, denatured proteins open to cover the globules, confining air bubbles and improving the stability and firmness of the whipped cream.
Chandrapala et al. [78] found that the fat globule size was reduced to 10 nm in fresh skim milk treated with ultrasound for 60 min. The formation of flocculated fat particles in ultrasonicated cream at low temperatures (<10 °C) could reduce the energy demand compared to traditional homogenization, carried out at 50 °C [88]. Karlović et al. [89] also reported that milk proteins, especially caseins, adsorb to the membrane surface of fat globules, functioning as natural emulsifiers in ultrasound-treated goat’s milk. In this regard, O’Sullivan et al. [90] showed different emulsifying properties in ultrasound-treated milk proteins. Milk protein isolate produced emulsions with smaller droplet sizes and without coalescence after 28 d. Meanwhile, emulsions produced from whey protein isolate and sodium caseinate had the same droplet sizes as controls without ultrasound (120 nm), despite the reduction in protein size.
HIU application to milk cream modifies the physical properties of butter, depending on the ultrasonication duration. HIU changes the texture and melting behaviour when applied for 10 s. The hardness of the butter is increased due to crystallization of low-melting-point triacylglycerols. It is promoted by forming a narrow lattice. On the other side, the use of long ultrasonication times causes the lattice to melt [37]. Ultrasonication of raw whole cow's milk at 2 MHz, compared to 1 MHz, is more effective for manipulating smaller fat cells retained in the later stages of skimming, eliminating 59% of fat. At 1 MHz, it removes only 47% of the fat after three stages of ultrasound-assisted fractionation [34]. The temperature range of operation utilized in this study is within the ‘optimal’ range between 20 and 55 °C, previously reported by Leong et al. [91]. An aggregation of homogenized fat globules occurs when there is no control on milk temperature treated with ultrasound (>90 °C). Possibly, due to an incorporation of denatured serum proteins. This aggregation is detrimental to the acid gelation of milk [62].
Ultrasound constitutes a technology with potential for application to accelerate the crystallization of mixtures of anhydrous milk fat and vegetable oils, as it is used in dairy ingredients. Martini et al. [92] altered the crystallization behaviour of a lipid model system (anhydrous milk fat) by HIU application. They suggested that HIU may be used as an additional processing variable to improve the lipid crystal lattice and its low-lipid physicochemical characteristics. This would allow for obtaining more viscous materials in lipid-based foods such as mayonnaise, margarine, and spreads, which could be formulated with healthier lipids without affecting the physicochemical and sensory qualities. Wagh et al. [93] also reported that ultrasonication of anhydrous milk fat leads to a decrease in the induction time in crystallization, coupled with the generation of a higher number of crystals and greater viscosity.
In cheese, Torkamani et al. [94] reported different results when evaluating the effects of ultrasound treatment on lipid oxidation in cheddar cheese serum. They found no changes in the composition of phospholipids or in the FFA concentration. However, Jalildazeh et al. [42] reported that ultrasonicated, ultrafiltered milk retentate improves the organoleptic properties of feta-type white cheese, mainly due to lipolysis (content of free fatty acids [FFAs]) and proteolysis (nitrogen soluble in water) that occur during the maturation process (60 d). The differences in the results of these investigations could be due to the characteristics of the treated samples and the experimental and subsequent storage conditions. Torkamani et al. [94] used freshly pasteurized Cheddar cheese whey (fat content not measured), ultrasonicated with a sonotrode and a plate transducer system at 400, 1000 y 2000 kHz for 10 and 30 min at 37 °C. Besides, Jalilzadeh et al. [42] used retentate of ultrafiltered milk (containing 16% fat) using an ultrasonic homogenizer for 20 min at lower frequencies (20, 40 y 60 kHz). Hence, the phenomena of lipolysis and proteolysis occurred naturally during the ripening of the cheese.
In milk inoculated with a thermophilic culture for yogurt, ultrasonication before or after inoculation significantly reduces fat globule size and decreases fermentation time [95]. Regarding the quality of the yogurt, they reported an increase in water-holding capacity and viscosity and a decrease in the syneresis.
3.5. Lactose
Studies that address ultrasound and its effect on carbohydrate metabolism in milk are primarily focused on the hydrolysis of lactose during fermentation processes for the formation of organic acids (mainly lactic, acetic, and propionic acids). This part will be addressed in the “Fermented dairy products” section.
The benefits of ultrasound treatment in the fermentative activity of beneficial strains in milk have reduced the required fermentation times to lower the pH. This facilitates breakdown for the release of extracellular and intracellular enzymes that promote lactose hydrolysis [62], including changes in the number of viable cells [96].
Khaire and Gogate [97] reported a reduction in hydrodynamic size in lactose recovered from ultrasound-treated whey. The pretreatment whey thermosonication improved lactose sonocrystallization in terms of recovery and chemical characteristics so that time of 25 min and power of 250 W resulted in higher recovery (up to 94.5%). In another study by Patel and Murthy [98] on ultrasonicated reconstituted lactose, spontaneous nucleation and seed growth accelerated growth in length and thickness, resulting in rod-shaped seed crystals. Therefore, sonocrystallization using acetone as an antisolvent resulted in a novel process for the rapid recovery of lactose.
Regarding the ultrasound frequency applied, 91% of the consulted papers applied frequencies between 20 and 30 while only 7% of the studies applied frequencies between 0.025 and 1 kHz and 2% used frequencies higher than 1000 kHz. The application of frequencies between 20 and 60 kHz have resulted in modifications in the structure of proteins and formation of aggregates due to denaturation, reduction in particle size [24], [25], [28], [43], [46], [47], [48], [51], [54], [56], [58], [59], [61], [70], [79] and reduction in fat globule size [24], [43], [78], [88], [99]. Additional observations at these frequencies (20 to 60 kHz) were whey protein denaturation and casein-fat globule aggregation [62], [101], [102] as well as proteolysis during cheese maturation [42]. Contrarily other studies did not show any change in dairy protein properties [18], [20] probably due to other experimental parameters such as power, intensity, time or temperature of the ultrasonication treatment. At frequencies between 0.025 and 1 kHz an increase in large yogurt protein aggregates were observed [80] while the use of frequencies between 400 and 2000 promoted milk fractionation and formation of an increasing fat concentration gradient [34] as well as the separation of whey fat which contributes to improve the oxidative stability of cheese [86]. In conclusion, the use of frequencies around 20 kHz present the optimum results regarding the milk chemical components.
4. Rheological and textural properties
The success of any food in a market depends on factors such as its nutritional contribution, safety, shelf life, convenience, and mainly its sensory attributes. The rheological properties are useful for the development of new products, calculation of processes, transportation, and quality control of products [107]. Furthermore, these properties are influenced by the microstructure of the product. The effect of applying ultrasound on the rheological properties in milk and dairy products depends on factors such as the volume of the sample, the heat generated, and the density of the ultrasound [56]. Flow properties identify the type of fluid. Newtonian fluids are characterized by a flow index (n) equal to 1, and most dairy products behave like non-Newtonian fluids with n values less than 1.
These fluids are known as pseudoplastics or shear-thinning fluids since their apparent viscosity decreases with increasing shear rate (γ) [27]. Various mathematical models can describe the different flow patterns of fluids. The most commonly applied models for pseudoplastic fluids are the Power Law model (τ = kγn) and the Herschel-Bulkley model (τ = kγn + τ0), where n is the flow index (dimensionless), k is the consistency index (Pa.sn), and τ is the yield stress (Pa). In these models, k is taken as an analogy of viscosity. The effect of ultrasound treatments on the n and k indices has been evaluated by some authors of studies in milk and dairy products, which are discussed below.
Regarding the viscoelastic properties, these provide valuable information about solid or semi-solid dairy products, such as creams, cheeses, and fermented dairy products [101], [108]. Tests such as the deformation or frequency sweep can provide information about the behaviour of the storage modulus (G') and the loss modulus (G''), which define the elastic and viscous behaviour of the materials, and are also related to the softness and firmness of gel-like structures, as is the case with fermented products. In addition, these types of tests can also provide information about possible long-term flow behaviour, an important indicator for packaging selection and shelf-life determination [109].
Texture is an important attribute to consumers and it can determine the acceptance of a specific product [110]. This attribute depends on the microstructure of the product and is evaluated by consumers through the senses. However, one way to obtain more objective data is through a texture profile analysis (TPA). Here, the sample is subjected to two deformations to simulate chewing [109], [111]. Findings related to parameters such as firmness, adhesiveness, cohesiveness, and elasticity are obtained from the resulting graph [111]. Most research on the effect of ultrasound on the textural properties of dairy products has been done on fermented products. Table 3 summarizes the effects of applying ultrasound on the rheological and textural properties of milk and dairy products, which are discussed below.
Table 3.
Effect of ultrasound on the rheological and textural properties of milk and dairy products.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Dairy emulsions (7–15% olive oil, 14% sucrose, 11% skimmed milk powder). | Ultrasonic processor UP400S with a titanium probe (H22D, l = 22 cm). 24 kHz for 3 min, energy density and amplitude were 85 W/cm2 and 120 µm respectively. | Ultrasonication promoted lower values in apparent viscosity and k with weaker structures. | [107] |
| Yogurt (Raw cow milk with 3.02% fat, 3.01% protein). | Ultrasonic processor UW3200 with an ultrasonic probe (TT13, diameter = 13 mm). 24 kHz, 15 min, 100, 125 or 150 W, 70 °C. Conventional Process: 90 °C, 10 min. In both process, samples of 800 mL were transferred into beakers (1L). | Increases in HIU power promoted decrease in apparent viscosity. | [110] |
| Yogurt (Fat content: 0.1, 1.5 and 3.5%) | Ultrasonic processor (BerHielscher UP 400S) with an ultrasonic probe (Horn H22D, diameter dip = 22 mm). 200 mL milk samples in 250 mL beaker. 24 kHz, 400 W, 10 min, 45–72 °C. Conventional Process: 90 °C, 10 min. | Thermosonication increased the viscosity of the product and the storage modulus G'. | [101] |
| Soft cheese (Raw whole milk). | Ultrasonic processor (Hielscher UP400S) with a 22 mm diameter probe. 24 kHz, 400 W, 15 s; 1, 10, and 30 min; 63 or 72 °C. Sample of 500 mL. Conventional Process; 63 °C, 10 min or 72 °C, 15 s. | Thermosonication increased cheese yield (20.6%) and decreased hardness. The cheeses obtained from thermosonicated milk crumbled easier, which is desirable in this product. | [113] |
| Acid-induced gels from milk (Commercial full fat milk with 3.5% fat). | Ultrasonic flow cell (Sonolab SL10). Frecuency of 20 kHz; at 27, 50, and 70 °C wirth different power levels (10, 30, and 50 W); samples of 100 mL. | Gel strength increased with ultrasound treatment and varied with power and temperature. The firmness of the gel was a consequence of the denaturation of whey proteins and changes in the membrane of the fat globule. | [25] |
| Cream cheese (Raw milk with 3% fat). | Ultrasonic processor (Sonifier 450) with a probe of 12.7 mm diameter. 450 W), d = 12.7 mm. 24 kHz, 4–63 °C; 0, 20, 50, 80, and 100 W; 0–30 min. Sample of 500 g in glass vessels of 600 mL. | Reduction in the size of fat globules. The viscoelastic-elastic behaviour (G' > G'') was not affected by TS. Modulus G' and spreadability were better at ≤ 50 W. Higher power did not benefit spreadability and rheological properties due to particle coalescence. | [108] |
| Yogurt (Milks were sonicated during fermentation At pH 5.2 and 42 °C) | Ultrasonic water bath (300 W, V = 19 L; RK 1028/H; Bandelin, Germany) and sonicated at 35 kHz for 5 min at 42 °C. | Sonicated set gels exhibited syneresis and were softer than controls. Sonication increased particle numbers, but the effect was less pronounced when YF-L 901 was used, indicating EPS as a tool to reduce syneresis and particle formation due to vibrations. Rheological parameters and size of microgel particles were more influenced by starter cultures than by sonication. | [178] |
| Protein-enriched Greek yogurt (bovine raw milk standardized to 3.5 to 12.0% protein. Milks were sonicated during fermentation from pH 5.8–5.1). | Ultrasonic device (Sonopulus HD 2200) equipped with a titanium flat tip (TT13; diameter = 13 mm, length = 5 mm). 20 kHz, 20 W (Sonopuls), equipped with a titanium flat tip (d = 13 mm, length 5 mm). | The yogurt obtained by US during fermentation exhibited lower values in G', showing gels with weaker structure. HIU reduced the firmness of the gels by up to 80%. | [117] |
| Whipped cream (Fresh cream with a fat content of 30%) | Ultrasound processor (Adeeco APU400). Frecuency of 20 kHz at 100 and 300 W for 0, 5, 10, and 15 min at 50 °C. Samples of 200 mL. | Ultrasound changes depended on the intensity and duration of the ultrasonication. With increasing power, the size of the fat globules increased and the firmness was lower. In general, the modulus G' increased in the US samples, evidencing an improvement in the structure of the cream. | [43] |
| Chocolate milk beverage | Disruptor Indaiatuba (Indaiatuba 800 W) equipped with a 13 mm diameter probe. Frecuency of 19 kHz at 400 W with energy densities of 0.3–3.0 kJ/cm3. Conventional Process: high-temperature short-time (HTST) pasteurization (72 °C/15 s). | The consistency index k recorded its highest values at an energy density of 0.3 kJ/cm3 and 0.9 kJ/cm3. A higher energy density decreased k and increased the flow index (n), a consequence of a more intense destruction of the microstructure. | [27] |
| Yogurt (reconstituted buffalo's milk) | 10 mm ultrasonic horn (Misonix Inc, model 3000) with stainless steel micro tip (diameter = 3 mm). Frequency of 20 kHz at 66 W during 15 min at 45 °C. Shear homogenization: 1188 J/mL energy density. | Ultrasonication increased gel hardness by up to 98%, as well as adhesiveness, gumminess, elasticity and cohesiveness. | [24] |
4.1. Milk, reconstituted products, and emulsion
The changes in the rheological properties of dairy products after ultrasound application have been studied. Monteiro et al. [27] applied HIU (19 kHz and 400 W) at different energy densities (0.3 kJ/cm3, 0.9 kJ/cm3, 1.8 kJ/cm3, 2.4 kJ/cm3, and 3.0 kJ/cm3) to pasteurize chocolate-flavored milk (65 g whey powder, 35 mL whole milk, 3 g cocoa powder, 10 g crystal sugar and 1 g gelatin). In general, when the energy density increased, the milk presented lower values for consistency index (k) and higher values for flow index (n; more Newtonian fluids). The apparent viscosity of the samples was observed to decrease at the maximum energy density. On the other hand, the highest values for k and the lowest for n were recorded at energy densities of 0.3 and 0.9 kJ/cm3. With regard to reconstituted products, HIU application can help reconstitute powdered milk. Nguyen and Anema [62] applied US (22.5 kHz and 50 W) for reconstitute whole milk and evaluate their effect on gels formed by acidification with glucono-d-lactone (GDL, 2.2% w/w). The authors observed that the gels obtained by acidification from the milk reconstituted by the HIU showed superior firmness to the gels obtained from the control milk (80 °C, 30 min). This effect previously observed was attributed to a higher destruction of the structure. That is, the US broke the fat globules (see section 2) into smaller particles and promoted denaturation of the milk proteins (see section 3). It increased the pH for the start of gelation, thus decreasing the gelation time of milk to form stronger gels. The rheological and textural properties of gels obtained from reconstituted milks can improve if properly combined during the ultrasonication, considering time, intensity, and temperatures above 60 °C.
Gregersen et al. [25] evaluated the acid formation of milk gels subjected to different powers and temperatures (Table 3). The modulus G', interpreted as gel strength during acid gelation, increased with ultrasound treatment and its values were affected by the temperature and power of the treatment. For example, it was observed that ultrasound (20 kHz, 50 W at 50 °C) increased the modulus G' by up to 10 times. This phenomenon was attributed to the structural changes that the fatty cell membrane underwent and the denaturation of serum proteins (see section 3). These changes probably facilitate interactions between fatty cells and caseins, increasing the strength of the gels.
Aslan and Dogan [107] studied the effect of ultrasound on dairy emulsions (7, 10 and 15% olive oil, 14% sucrose, 11% skimmed milk powder, 0.2% xanthan gum and 0.3% mono-diglycerides as emulsifier) preparation. They compared the emulsification of olive oil in milk-based media by mechanical agitation and HIU application (Table 3). They observed that the HIU promoted a decrease in the apparent viscosity and the k index compared to emulsions obtained by mechanical agitation. The effect was a consequence of the decrease in the size of the oil droplets and a more effective distribution of the proteins in the system. Furthermore, it has been speculated that denaturation of proteins caused by HIU reduces the apparent viscosity and the k index. Cavitation generates shear forces that change the molecular structure of proteins, thus affecting their functional properties [47]. HIU has been reported to increase the solubility of whey proteins [46], improve emulsifying properties, and reduce its viscosity [112].
4.2. Cheese and cream
High-intensity ultrasound has been used to produce cheese with the aim of pasteurizing the milk. In addition, it has been observed that applying HIU to milk improves the milk’s curdling properties since HIU breaks the fat globules into smaller particles and alters their membrane [102], [108], [113]. These changes reduce curdling time [102] and increase the firmness of the curd [102], [106]. Zhao et al. [106] evaluated the effects of applying HIU (20 kHz, 800 W, 0–20 min) on the curd properties of goat’s milk with renin. With a 10-min ultrasonication, the curd presented a denser gel crosslinked network, resulting in a more homogeneous microstructure with abundant pores, but smaller than the milk curd without sonication. This suggests that the curd of goat's milk treated with HIU will show greater firmness, registering values of G'max (maximum value for the storage modulus) higher than 100 Pa, even higher than those reported in cow's milk. Similar effect was observed in the adhesiveness (the strength of the internal bonds of the sample). Hence, it can be assumed that HIU promotes strong interactions between the components of the milk, improving the setting properties.
Regarding studies carried out on cheese, Bermúdez-Aguirre and Barbosa-Cánovas [113] reported that fresh cheese obtained from milk treated with thermosonication (400 W, 24 kHz, 63 °C, 30 min) was softer and more brittle than cheese from the control milk (without thermosonication). Those characteristics resulted in an easier cheese to crumble, which is a desirable attribute of fresh cheese. These authors explained this behaviour by noting that the microstructure of thermosonicated milk cheese presented a more homogeneous structure compared to non-sonicated milk cheese. Moreover, they noted that thermosonication improved homogenization of proteins and fat and increased retention of water molecules in the matrix. Hence, it can be assumed that HIU promotes strong interactions between the components of the milk, improving the setting properties.
In a recent study, Almanza-Rubio et al. [108] applied HIU (20 kHz, 0–100 W at 4–63 °C for 3–30 min) to raw milk (3% fat) intended for the production of cream cheese. Regarding the texture, the spreadability of cream cheese (<100 N) obtained from thermosonicated milk at ≤50 W, 50 °C, and long times (≤30 min) was lower than that of cheese obtained from milk without thermosonication (209 N). These values were lower than those registered in commercial cheeses made from whole milk (127–20115 N). However, a power greater than 50 W increased the size of the fat globules as a result of their coalescence, and most likely promoted a greater denaturation of the proteins, thus altering the membranes of the fat globules. The results showed that the viscoelastic behaviour (G' > G'') of cream cheese was not affected by thermosonication. The increase in dissipation energy significantly reduced the G' and G'' modulus as well as their complex viscosity (η*), the reduction of these parameters was maximized at a power of 50 W, and higher values caused slight improvements in rheological properties. The thermosonication at ≤50 W reduced the size of the fat globules, increasing their retention in the cheese and promoting a decrease in spreadability and the G' modulus. In general, these results suggest that the thermosonication application allows for improving the textural and rheological properties of cream cheese under specific conditions.
On the other hand, Amiri et al. [43] evaluated the effect of HIU on the structural properties whipped cream a fat content of 30%, considering ultrasound intensity and time (20 kHz, 100 and 300 W, 0–15 min) at 50 °C. Compared to the control (sample without sonication), the HIU-treated cream increased the G' values, showing a more rigid structure. However, as the intensity of the process increased, the modulus G' decreased. The chains of the denatured proteins presented a more open conformation that allowed them to cover the new fat globules, resulting in a more elastic network. However, increasing the time and power of the HIU treatment promoted greater denaturation of proteins and fatty cells, originating a structure with weaker infrastructural bonds and thus decreasing G'. The firmness, consistency, and cohesiveness of the cream increased with HIU, but these properties decreased with increasing sonication time of up to 15 min. The above results suggest that HIU treatment can improve the textural and rheological properties of whipped cream. This effect was attributed to the fat globule disruption (see section 2) and the denaturation of the milk proteins (see section 3).
4.3. Fermented dairy products
Most studies of the effect of ultrasound on dairy foods have evaluated the production of fermented products such as yogurt [44]. Yogurt quality is associated with physical properties developed as a consequence of the formation of a homogeneous gel, which occurs due to destabilization and aggregation of protein micelles when pH drops during fermentation [114], [115]. The texture of the gel largely determines consumer preferences, and depends mainly on the viscosity, gel structure, and particle size distribution [44], [110]. In addition to the characteristics of the gel formed during the fermentation, the final structure will depend on the type of yogurt, since in the case of set-style yogurt, the fermentation takes place in the container, while in stirred yogurt, mechanical agitation is necessary after fermentation [114]. Set-style yogurt is intended to present a gel with a homogeneous structure, no syneresis, and a smooth and homogeneous consistency [114]. On the other hand, in the case of stirred yogurt, the gel structure is less homogeneous and compact, with a soft and creamy texture and low levels of syneresis [101], [114]. It has been reported that ultrasound applied before or during fermentation affects the gel formation (Fig. 4a), thus modifying the texture of the final product. The application of HIU can positively or negatively affect the texture of fermented products; HIU applied before fermentation improves the texture (Fig. 4b), while HIU applied during fermentation (Fig. 4c) negatively affects the texture [44].
Fig. 4.
Schematic representation of gel formation and microstructure in a) yogurt obtained in the traditional fermentation, b) yogurt obtained from ultrasound pre-treated milk and c) yogurt with sonication during fermentation. Adapted from Munir et al. [116] and Nöbel et al. [114].
Riener et al. [101] compared the rheological properties of yogurt obtained using thermosonication (24 kHz, 400 W, 45–72 °C, 10 min) and conventional heating (90 °C for 10 min) in full-fat (11.6% total solids, 3.0% protein, 3.5% fat, 0.8% ash and 0.14% acidity), semi-skim (9.5% total solids, 3.1% protein, 1.5% fat, 0.8% ash and 0.12% acidity), and skim milk (8% total solids, 3.2% protein, 0.1% fat, 0.8% ash and 0.12% acidity). The milk at 40 °C was inoculated with commercial yoghurt starter culture. Yogurt obtained from HIU-treated milk had a G' value up to 25% higher than its counterpart made from conventionally heated milk. As discussed in section 3, denaturation of whey proteins by conventional heating facilitates their interactions with other milk components. However, the level of protein denaturation was lower in the thermosonicated milks, so apparently, protein denaturation was not a determining factor in gel firmness in this research [101]. Apparently, protein denaturation was not a determining factor in gel firmness. Subsequently, Riener et al. [115] also observed similar effect related to the properties of textures and rheology under the same operating conditions previously described above [101]. The texture profile analysis (TPA) revealed that the firmness of the yogurt obtained from thermosonicated milk (1.8 N) was higher than that of its counterpart obtained from milk treated conventionally (0.90 N). The thermosonication also increased the chewiness and gumminess of the yogurt, while its adhesiveness and elasticity did not show significant differences from those of the control yogurt (milk without thermosonication). Regarding the flow properties, both samples maintained their pseudoplastic character; however, compared to the sample without TS, the consistency index, an analogy of viscosity, increased 2.4-fold when HIU was applied.
Gursoy et al. [110] produced yogurt with thermosonicated raw cow milk (11.56% total solids, 3.02% fat, 3.01% protein, 4.74% lactose and pH was 6.47) inoculated with 2.5% of commercial starter cultures (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus). They observed that an increase in power (100–125 W) during thermosonication (24 kHz, 15 min, 70 °C) reduced syneresis and increased apparent viscosity. The milk subjected to thermosonication to form gels with a stronger and more viscous network [43], [44], [45], [101], [110], [115]. Furthermore, increased HIU potency promoted a more extensive dissociation of protein micelles into smaller particles, thus contributing to the formation of gels with a stronger network [110].
Frydenberg et al. [60] recently studied the role of protein interactions in gel network properties and reported the effect of HIU (24 kHz, 300 W/cm2, 2078 J/mL) on the properties of serum protein isolate (WPI) gels with different α-lactalbumin:β-lactoglobulin ratios. Sonication was observed to increase the hardness of the WPI gels as a consequence of the increase in disulfide bridges. Shen et al. [63] observed that the application of ultrasound at a frequency of 20 kHz for 20 min in glucono-δ-lactone-induced whey protein isolated gels (10% w/v) breaks non-covalent bonds, promoting a reduction in the particle size of serum proteins and an increase in free sulfhydryl (–SH) groups, allowing new particles to interact via disulfide bonds to form a denser and stronger internal network, and resulting in firmer and stronger elastic gels (G'). Furthermore, as mentioned at the beginning of this section, sonication can also be applied during the fermentation process, which is useful for semi-continuous or continuous yogurt production. Applying HIU during fermentation has been reported to reduce fermentation time [95], [114]. However, application of HIU during fermentation results in the formation of large particles that may not be desirable in yogurt, impoverishing its rheological properties [114]. These authors [114] applied HIU (45 kHz, 17 kW/m3, 42 °C, pH 4.6–5.7) during fermentation for yogurt production. The results showed that sonication during fermentation promoted the formation of large colloidal particles, whose size and stability was closely related with the pH reduction during fermentation. In a critical pH range of 5.1–5.4, sonication for 5 min was sufficient to promote the formation of large particles in the system. This effect may be the consequence of a kinematic mechanism, sonication during fermentation increased the shear force and promoted the collision of gel fragments in a system with low ζ-potential (pH 5.4), which facilitated the formation of bonds between particles. These groups are different from the homogeneous gel network; since they are unstable due to a higher presence of weaker bonds. The formation of large particles that may not be desirable in yogurt, which impoverishes its rheological properties.
As previously described, the firmness of the gels obtained during fermentation can be increased by applying a thermosonication pretreatment to the milk. Nguyen and Anema [62] reported that after application of HIU (22.5 kHz, 50 W, 3 min) to reconstitute whole milk at temperatures of 20 and 40 °C under these conditions, the denaturation of whey proteins was practically nil. However, when the HIU process temperature stabilized above 60 °C, the denaturation of the whey proteins was 80%. As previously mentioned, heating of the milk (~90 °C, 30 min) and thermosonication prior to fermentation denature the whey proteins for subsequent interactions in the gel network. However, it has been reported that the firmness of yogurt can be improved by homogenizing milk using HIU at temperatures below 60 °C.
In this regard, Abesinghe et al. [24] prepared yogurt from reconstituted buffalo’s milk (16.7% total solids; 7.76% fat and 4.83% protein) ultrasonicated (20 kHz, 66 W, 15 min). The sonicated milk was inoculated with commercial starter cultures (Lactobacillus bulgaricus, Streptococcus thermophilus, S. lactis, S. diacetylactis and S. cremoris) and incubated at 42 °C until the pH reached 4.5. TPA analysis revealed that compared to non-homogenized milk and homogenized milk, the hardness of yogurt from HIU-homogenized milk increased by up to 98% and 121% respectively. This phenomenon occurred because the sample size (50 mL) could allow a higher level of energy transfer during the process, which promoted the denaturation of the proteins, thus increasing the firmness of the gel. The other textural properties, adhesiveness, gumminess, and elasticity, were also improved by applying HIU. Except for gumminess, an increase in these properties is desirable in this type of product.
In general, the application of HIU during the fermentation process does not improve the textural and rheological properties of the yogurt. In this sense, Körsendörfer et al. [178] studied the impact of short-time sonication during fermentation on physical properties of set-style and stirred yogurt. They fermented skim milk with a low exopolysaccharide-producing starter (EPS; YC-471) and a high EPS (YF-L 901) and sonicated for 5 min at a pH of 5.2. Sonicated set gels exhibited syneresis and were softer than the controls. The authors proposed EPS as a tool to reduce syneresis and particle formation due to vibrations since the rheological parameters and the size of microgel particles were more influenced by starter cultures than by sonication. However, this may be advantageous in obtaining another type of yogurt, as reported by Körzendörfer et al. [117]. These authors applied HIU (20 kHz, 20 W) to produce Greek yogurt from bovine raw milk standardized to 3.5, 4.0, 6.0, 8.0, 10.0, and 12.0% (wt/wt) protein inoculated with mesophilic (Lactococcus lactis ssp. lactis and Lactococcus lactis ssp. cremosris) or thermophilic starter cultures (Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophiles) and fermented until pH 4.6. Samples were sonicated during fermentation from pH 5.8–5.1. The G' modulus decreased by 74% when going from 2489 Pa to 644 Pa in HIU-treated yogurt and the control sample, respectively. The phase angle experienced the opposite effect when increasing from 13.2° to 13.5°, evidencing a more viscous than elastic behaviour in the sonicated samples. These rheological characteristics were desirable because, due to the high protein content (10%) of the milk in the fermentation without HIU, mechanical agitation was required to achieve a smoother consistency.
As observed in Table 3, the most reported frequencies for different dairy products were 19, 20, 24 and 35 kHz showing similar effects on the rheological properties of milk and emulsion, such as a reduction of the parameter k and a decrease in the cheese softness. In fermented dairy products, the rheological properties depended on the stage of production when the sonication was applied. Sonication of milk at 24 kHz before fermentation offers higher yogurt firmness than when sonication was applied during fermentation.
5. Functional properties
Diverse studies have evaluated the influence of high-intensity ultrasound processing on bioactive or functional compounds as described in Table 4.
Table 4.
Influence of ultrasound treatments on milk bioactive compounds.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Milk (fat free, 1%, 2%, and whole) | Ultrasonic Probe (Hielscher USA Inc., USA, model UP400S) at 400 W, 24 kHz, 120 μm amplitude during 30 min at 63 °C. | Protein content varied from 3.10% to 3.17% and from 2.72% to 2.7% in fat-free and whole UHT milk respectively. | [122] |
| Milk | Ultrasonic Probe (Vibra-Cell High Intensity VCX 750. Sonics and Materials, Inc., USA) at 750 W, 20 kHz, 124 μm amplitude at 24–26 °C. | Protein content augmented from 3.03% to 3.25% in raw milk. Protein content in sonicated UHT milk did not change (3.11% to 3.12%). | [84] |
| Milk | Ultrasonic Probe (22 mm diameter, Hielscher USA, Inc., model UP400S) at 400 W, 24 kHz, 120 μm amplitude at 63 °C. | Protein content decreased from 3.28% to 3.0%. | [121] |
| Casein solutions with Aspergillus oryzea fungal protease nanoparticles | Ultrasonic disintegrator (Model MSE Soniprep 150) at amplitude 4 µm, 1.5 W. | Sonication improved the production of angiotensin-converting enzyme inhibitor peptides and shortened the bioprocessing time by six times | [131] |
| Milk | 450 Sonifier II (Branson Ultrasonic Corp.), at 150 W and 20 kHz, 120 μm amplitude at 30, 55 and 75.5 °C during 56, 70 and 102 s. | Synergistic effect between heat and ultrasound in the whey and casein protein denaturation. | [19] |
| Milk | Ultrasonic Probe) Hielscher®, USA model UP400S) at 400 W, 24 kHz, 120 μm amplitude. | Fat content increased from 4.04% to 4.24%. | [35] |
| Yogurt from sonicated milk | Ultrasonic probe (TT13, with a diameter of 13 mm, Bandelin Sonopuls UW3200, Germany) at 24 kHz, 100, 125 and 150 W at 70 °C. | Protein content was similar to yogurt from untreated milk. | [110] |
| Camel milk casein and whey proteins | Benchtop sonicator (Omni Sonic Ruptor 400 150 Ultrasonic Homogenizer, Kennesaw, USA) at 30 kHz and 400 W for 45 min. | Antioxidant activity, aromatic amino acid content, and angiotensin I-converting enzyme (ACE) inhibitory activity increased in whey proteins and caseins. | [123] |
| Human milk | Ultrasound bath (BRANSONIC, model CPX3800H) at 40 kHz and 100 W and Intensity of 1591 mW/cm2. | Higher antioxidant activity in thermosonicated human milk than in raw milk. Similar retinol content. | [126] |
| Chocolate milk beverage | Ultrasonic probe (13 mm), Disruptor, 800 W, (Indaiatuba, Brazil) at 19 kHz at 0.3, 0.9, 1.8, 2.4 and 3.0 kJ/cm3 | The greatest antioxidant activity (87.2%) and ACE-inhibitory activity (82.4%) was obtained at the highest energy density. | [27] |
| β-lactoglobulin solution | Ultrasonic probe (VCX 800, Vibra Cell, Sonics, USA (13-mm high grade titanium alloy) at 20 kHz, 800 W, 60 W/cm2, at 0, 45, 50 and 55 °C | Ultrasound improved the ABTS radical scavenging (60.8%) with respect to native β-lactoglobulin (19.5%). | [132] |
| Whey protein isolate and calcium lactate | Ultrasound processor (JY92-2D, NingBo Scientz Biotechnology Co. Ltd, China), 10.19, 12.74, 15.29 17.83 W/cm2 (0, 200, 400, 600 or 800 W), 20 kHz, 0.636 cm probe, 3 s on/1 s off, 25 ± 2 °C, 20 and 40 min. | Gel strength enhanced, solubility decreased, emulsification activity reduced and emulsion stability were enhanced by ultrasonic treatment combined with calcium lactate. Antioxidant activities of sonicated whey protein isolate dispersions mostly increased. | [61] |
| Prebiotic soursop whey beverage | Ultrasonic device (Unique, Disruptor, 800 W, Indaiatuba, Brazil) at 19 kHz, 3 min and 200, 400 and 600 W. | At 600 W the ascorbic acid content and antioxidant activity decreased. Total phenolic content and ACE inhibitory activity augmented. | [124] |
| Skim milk | Ultrasonic processor (UPI1000HD, Hielscher Ultrasonics GmbH, Germany), 20 kHz, cross section 3.1 cm2 (101 kW/m2) horn-transducer, <30 °C, 15 min. | Ultrasound significantly changed the renneting properties (rennetingtime, rennet curd firmness and rennet gel network) in milk at pH 8.0 and re-adjusted to pH 6.7. Gelation time was reduced from 40 to 35.3 min and curd firmness was augmented from 6.6 to 13.9 after sonication. | [129] |
| Raw milk | DRC-8-DPP-FGS (Advanced Sonic Processing Systems, Oxford, CT with a 2.4 kW dual-frequency reactor cell (parallel diaphragm plates)) at 16–20 kHz, 1.36 kW/pass at flow rates of 0.15, 0.30, and 0.45 L/min, 42 or 54 °C. | Sonicated milk at 42 °C gelled faster and formed firmer curds than the raw milk control. | [28] |
| Goat’s milk | 13 mm ultrasonic probe placed at the centre and to a depth of about 3 cm, at 20 kHz and 800 W during 0 – 20 min | Rennet coagulation time of sonicated goat’s milk (after 10–15 min) was similar to untreated milk. | [106] |
| Reconstituted skim milk | Horn-transducer ultrasound processing unit (UPI1000hd, Hielscher Ultrasonics, Germany), at 20, 400 and 1600 kHz, specific energy of 286 kJ/kg and < 30 °C. | Ultrasound caused disruption of casein micelles and re-assembly of proteins. | [79] |
| Yogurt from sonicated milk | Ultrasonic processor Bandelin (Sonopuls UW3200, Germany) at 24 kHz, 100, 125 and 150 W at 70 °C. | Thermosonication reduced the serum separation of yoghurt along storage. | [110] |
| Reconstituted milk protein concentrate | Ultrasonic horn (CTXNW-10B, Hongxianglong Bictechnology Co. Ltd., China) at 600 W, 20 kHz, during 0 to 5 min at < 50 °C | Solubility augmented from 35.78% to 88.30% after 5 min of HIU processing. The US-treated samples presented higher elasticity than the control. | [64] |
| Whey protein suspensions | Ultrasonic probe (Sonics and Materials Inc., USA, Model: V1A) power 600 W at 40 or 20 kHz, 600 W, during 15 and 30 min | Foam capacity and stability was improved. Solubility augmented | [133] |
| Milk for yogurt elaboration | Ultrasonic (UP 400S, Hielscher, Germany) fitted with an ultrasonic probe (Horn H22D, 22 mm diameter tip) at 24 kHz, 400 W and 45 °C for 10 min. | Gels made from thermosonicated milk showed lower syneresis (51% to 38%) than those pasteurized (62% to 40%). | [115] |
ACE = Angiotensin-converting enzyme; UHT = Ultra high temperature pasteurization
The effect of HIU treatment on protein content in milk has been widely studied. Milk proteins ensure the supply of essential amino acids in the diet, improve physical performance, and, in the case of immunoglobulins, show an immunoprotective effect. Other peptides possess antithrombotic, antimicrobial, antioxidant, and antihypertensive activity (through inhibition of the angiotensin-converting enzyme; Fox et al. [120]. Cameron et al. [84] reported that protein content in raw milk significantly increased from 3.03% to 3.25% after ultrasound processing (750 W, 20 kHz, and 124 µm amplitude) for 15 min at room temperature, whereas protein content remains unchanged (3.11% to 3.12%) in ultra-high temperature (UHT) milk after processing by ultrasound. On the contrary, Bermúdez-Aguirre et al. [121] reported that in thermosonicated (400 W, 24 kHz, 36 to 120 µm amplitude, 63 °C) raw whole milk, protein content diminished from 3.28% to 3.02%, a function of the intensity of treatment. These findings were attributed to protein denaturation by heat. Previously, the authors reported that thermosonication (400 W, 24 kHz, 120 μm amplitude) UHT milks with different fat contents (fat-free, 1%, 2%, 3.4%) did not have a significant influence on protein content [122]. However, Gammoh et al. [123] reported an increment of aromatic amino acid content in extracts of camel milk whey proteins and caseins after ultrasound processing. Villamiel and De Jong [19] evaluated the effects of continuous-flow high-intensity ultrasound processing (with and without heat) on whey proteins and caseins. The authors reported that ultrasound led to denaturation of both whey proteins, α-lactalbumin and β-lactoglobulin. This denaturation was higher in whole milk and ultrasound combined with heat, which was due to the modification of the tertiary and/or quaternary structures of casein, without altering the micelle as presented in section 3. Gursoy et al. [110] analyzed the effects of ultrasound treatment on protein content in yogurt produced with thermosonicated milk. The protein content varied from 1.20% at 150 W to 1.14% for control yogurt. In the case of lipids, it was reported that thermosonication of whole milk caused the disintegration of fat globule membranes and the release of triacylglycerides, showing a higher value of fat content after sonication (4.24%) compared to unprocessed milk (4.04%; [35]). In the industry, ascorbic acid is a heat-sensitive compound present in many foods. Both ascorbic acid and phenolic compounds have elevated antioxidant activity. Guimarães et al. [124] reported that ultrasonication reduced the ascorbic acid content (from 18 to 14 mg/100 mL), whereas the content of phenolic compounds (from 634 to 777 mg of equivalents to gallic acid/100 mL) increased when a prebiotic whey sour beverage was processed at 19 kHz and 600 W for 3 min.
Functional compounds are highlighted for their high potential to protect the human body against reactive oxygen species (ROS) and reduce oxidative stress [125]. Diverse studies have demonstrated an incremental increase in antioxidant activity in milk and dairy products subjected to sonication. For instance, Parreiras et al. [126] evaluated the influence of thermosonication of human milk (40 kHz, a power of 100 W and at 60 °C for 4 min) on antioxidant activity, expressed as ABTS free radical scavenging and DPPH inhibition (%) and retinol content. They reported that thermosonicated human milk exhibited higher antioxidant activity than raw and pasteurized human milk. In addition, its retinol content was similar to those of the pasteurized and raw samples. Similarly, ultrasonicated (30 kHz, 400 W, and room temperature) camel milk protein extracts showed higher antioxidant activity than unprocessed extracts [123]. Some studies analyzed the influence of different HIU energy densities (0.3–3.0 kJ/cm3) on antioxidant activity in a chocolate milk beverage. The ultrasonicated beverage at the highest energy density (3.0 kJ/cm3) showed superior antioxidant activity (87.2%) to that of pasteurized milk (76.7% [27]). Also Jiang et al. [61] observed an increase in DPPH radical scavenging activity and ferrous reducing power of sonicated (600 and 800 W for 40 min) whey protein isolate dispersions.
Milk and many dairy products have peptides with the capacity to inhibit the angiotensin-converting enzyme (ACE). ACE inhibition exerts an antihypertensive effect as a consequence of a decrease in angiotensin II [127]. Guimarães et al. [124] analyzed the influence of ultrasound on ACE inhibition in a prebiotic whey sour beverage; they reported that as sonication power augmented from 0 to 600 W, ACE inhibition increased from 18% to 23%.
Ultrasound processing is able to improve the functional properties of dairy systems. The bioactivity of functional compounds increased when ultrasound processing was applied [84], [123]. This fact is extremely important because an increase in these compounds may indicate a greater antioxidant and anticarcinogenic activity. In the last years, an increment in natural antioxidants has been increased. In the case of milk and dairy products antioxidant and anticarcinogenic activity could be due to sulfur containing amino acids, vitamins A, E, carotenoids, among others, which could provide greater benefits to the health of consumers [128].
5.1. Functional characteristics
HIU has been applied in the processing of milk and dairy products to improve their functional properties, such as gelation, solubility, and foaming [51], [106], [129]. Reconstituted skim milk was ultrasonicated at 20, 400, and 1600 kHz, leading to casein micelle disruption, which can influence the functional properties of milk products [79]. Zhao et al. [106] evaluated the influence of ultrasound (20 kHz, 800 W for 0–20 min) on goat’s milk and observed that the rennet coagulation time was similar to that of unprocessed milk. However, they also found an increase in coagulum strength, gel firmness, water-holding capacity and cross-linking of milk gels at the ultrasound times of 0–10 min. Similarly, the rennet gelation time and curd firmness were improved when skim milk was sonicated at 20 kHz [129].
Another important functional property of milk or dairy products is their solubility. In this case, Yanjun et al. [64] analyzed the effect of HIU pretreatment (20 kHz, 200 W) on solubility of reconstituted milk protein concentrate. Solubility increased significantly from 35.78% to 88.30% after 5 min of ultrasound pretreatment. The authors considered this effect a consequence of the changes in the three-dimensional structures of globular proteins.
Thermosonication (45 °C during 10 min at 24 kHz) of preheated milk at different fat concentrations (0.1%, 1.5%, and 3%) reduced the syneresis in yogurt. The syneresis ranged from 62% to 40% and from 51% to 38% in yogurt from conventionally heated and thermosonicated milks respectively [115]. Another potential application of HIU in the cheese industry is ultrasound-assisted cutting. Yildiz et al. [130] reported advantages such as high precision, low product loss, less deformation and friction, the ability to handle sticky or brittle food, and less degradation of lipids when obtaining low peroxide values in cuts of cheddar, mozzarella, and Swiss cheese.
6. Microbiological properties
6.1. Fermented dairy products
In recent years, several products have been proposed as carrier foods for probiotics to improve the health and nutrition of consumers. Probiotics are live microorganisms that, in adequate amounts, confer a health benefit on the host, [134], mainly through the process of replacing or including beneficial bacteria in the gastrointestinal tract. Lactic acid bacteria (LAB) have probiotic potential and are used as starter cultures in the production of fermented foods and dairy products. Lactose is hydrolyzed by β-galactosidase to produce galactose and glucose. These products are further metabolized by LAB and synthesize organic acid byproducts such as lactic acid during milk fermentation, decreasing the pH of milk (to approx. 4.0). This growth condition is unfavorable for pathogenic bacteria and most spoilage bacteria over the LAB. To exert any beneficial health effect, the concentration of probiotics in a product must be at least 107 cfu/mL [135]. Most commercial probiotics incorporated in dairy products are strains belonging to the genera Lactobacillus and Bifidobacterium. However, species from other genera, e.g., Bacillus and Saccharomyces, are also used as probiotics [136].
Three decades ago, Toba demonstrated that HIU processing results in a significant improvement in milk fermentation [137]. The interest in probiotics as healthy food additives, together with the impact of HIU on fermentation, has stimulated research in this area [50], [95], [138], [139], [140]. Nowadays it is known that HIU allows the release of β-galactosidase from yogurt starter cultures to improve viability [141]. Thus, HIU stimulates cellular growth and proliferation, and shortens the lactic fermentation time [138], [142]. Some reports about these effects are outlined in Table 5. An increase in metabolic activity resulting from HIU has also been observed in non-dairy fermentation [143], [144], [145], [146], [147].
Table 5.
Effect of ultrasound on microorganism performance in fermented dairy products.
| Sample | Experimental parameters | Starter culture | Effect of ultrasound | Reference |
|---|---|---|---|---|
| Reconstituted skim milk (100 g/liter) | Sonicator (Ohtake Works, Tokyo, 20 kHz, 60 W) for 20 min at 0 °C. Batch method. Inoculated reconstituted skim milk was sonicated at 41 °C for 8 h. The milk was then incubated at 41 °C for an additional 12 h. | 3% starter culture (Lactobacillus delbrueckii ssp. bulgaricus B-6, L. delbrueckii ssp. bulgaricus B-5b or L. helveticus LH-17). | Ultrasonic treatment of milk released ß-galactosidase from starter bacteria during fermentation and used for hydrolysis of lactose in situ. | [137] |
| Fresh milk | A fermenter and an ultrasonic generation system (model US150V, Cho-onpa Kogyo Co., Japan) at 200 kHz and 17.2 kW/m2 for 2, 4, 6, 8 and 12 h at 37 + 0.2 °C. | L. delbrueckii ssp. bulgaricus B-5b, L. helveticus LH-17, L. delbrueckii ssp. lactis SBT-2080 and L. acidophilus SBT-2068. | High viable cell count and high degree of lactose hydrolysis (70% vs. 40% in conventional fermentation). | [140] |
| Fresh milk | Ultrasound generator (20 kHz, 500 W, Cole Parmer) at power levels of 20, 50, and 100. 450 W and 225 W for 1, 6 and 10 min. | Streptococcus thermophilus, Lactobacillus bulgaricus, Bifidobacterium and Lactobacillus acidophilus. | Improved homogenization. Reduced total fermentation time by 0.5 h. Higher amplitude improved the water-holding capacity and viscosity and reduced syneresis. | [95] |
| Skim milk | Seed culture: 28, 33, 40 and 68 kHz, the pulse duration 30, 50, 100, 200 and 300 s, the ultrasonic treatment time 0.5, 1, 2, 3 and 4 h and the ultrasonic power 80, 100, 120, 140, 160 and 180 W/L. Fermented milk: 28 kHz, ultrasonic pulsed model of on-time 100 s and off-time 10 s and power 100 W/L for 30 min after the fermentation time of 9 h at 37 °C. | Lactobacillus paracasei. | Peptide content increased 64.23%. Peptide yield was 14.2%. Extracellular enzyme activities increased. The effect disappeared in the progress of fermentation after the ultrasound was removed. | [154] |
| Nonfat dry milk | Sonicator (type US-150 V, Cho-onpa Kogyo, Tachikawa, Japan) at 200 kHz and 12.9 kW/m2, 14.7 kW/m2 and 17,2 kW/m2. Ultrasonic irradiation was continuous for 12 h at 37 °C. | Lactobacillus delbrueckii ssp. bulgaricus B-5b | High ultrasonic intensity decreased the viable cell count, but the count increased again after sonication stopped.Lactose was hydrolized at 55% vs. 35.6% in control fermentation. | [152] |
| Nonfat dry milk | Sonicator (type US-150 V, Cho-onpa Kogyo, Tachikawa, Japan) at 200 kHz and 17.2 kW/m2 . Ultrasonic irradiation was continuous for 16 h or for 2, 4, 6 or 8 h at 37 °C. | Lactobacillus delbrueckii ssp. bulgaricus B-5b | High degrees of lactose hydrolysis and high cell viabilities were obtained with the combination of pH-controlled sonicated fermentation and static incubation. | [153] |
| Reconstituted skim milk | Ultrasound system with Sonotrode BS2d34 (Hielscher Ultrasonics GmbH, Germany) at an amplitude of 50% (aprox. 100 W) and 20 kHz for 7, 15, and 30 min at 30–40 °C. | Bifidobacterium infantis, Bifidobacterium animalisssp. lactis (BB-12), and Bifidobacterium longum (BB-46) | Ultrasound reduced the fermentation time required to reach a pH of 4.7, ruptured bacteria cells, and released intracellular enzyme β-galactosidase.The lower the concentration of lactose, the higher the amount of oligosaccharides in the fermented milk with ultrasound treatment. | [169] |
| Reconstituted skim milk | Ultrasound system with Sonotrode BS2d34 (Hielscher Ultrasonics GmbH, Teltow, Germany) at an amplitude of 30% (aprox. 100 W) and 20 kHz for 7, 15, and 30 min at 30–40 °C. | Bifidobacterium breve ATCC 15700, Bifidobacterium infantis, Bifidobacterium animalis ssp. lactis (BB-12), and Bifidobacterium longum (BB-46) | With ultrasound treatment, lactose consumption by bacteria increased. The production of major organic acid in the later stage of the milk fermentation was stimulated. | [170] |
| Pasteurized milk | Ultrasonic system (power sonic 405, Korea) at amplitude of 30% (approx. 116 W) and 40 kHz for 0, 5, 10, 15, and 20 min at 37 °C. | Lactobacillus acidophilus LA-5, Lactobacillus casei LC, Lactobacillus reuteri LR-MM53, Bifidobacterium bifidum (BB-12) and Bifidobacterium longum (BB-536) | The optimal time span of ultrasound treatment was 10 min for all fermented milk samples, which can be applied to increase the number of viable cells of probiotic bacteria and β-galactosidase enzyme. | [96] |
| Dried skim milk | Ultrasonic homogenizer SonoPuls mini20 (Bandelin, Germany) with 2.5 mm probe transducer operating at 30 kHz and 2 to 8 W, for 1–3 min. | Lactococcus mixed culture, Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus | Ultrasonication of fermenting milk allowed for acceleration of fermentative process by 10% and improved the quality of the final product. | [172] |
| Whey | Ultrasonic processor S-4000 (QSonica LLC, USA) at 20 kHz and 84 W over 150 s. First stage: at 480 W and 600 W were combined at 45 °C and 55 °C for 6.5, 8 or 10 min. Second stage: activation of dairy cultures at 84 W and 102 W for 75 s and 150 s respectively. | Lactobacillus acidophilus La-5 | Better microbiological quality and sensory properties of whey in comparison to pasteurization. With ultrasound, whey fermentation lasted 30 min less and resulted in a higher viable cell count. | [20] |
| Milk | UP100H ultrasound apparatus (Hielscher; Germany) at 100 W, 30 kHz and 25% amplitude for 5, 10, and 15 min. | Lactobacillus plantarum AF1 | Cell membrane permeability of sonicated samples was increased 88%–94%. Ultrasound improved metabolism of Lactobacillus, increased antioxidant activity and quality of fermented milk. | [175] |
| Feta-type cheese | Ultrasonic Homogenizer (model no. HD2200, Bandelin Company, Germany) at 20, 40, and 60 kHz for 20 min at an intensity of 80% | Lactobacillus bulgaricusand mesophilic Lactococcus lactis | Sonication at 60 kHz accelerated lipolysis and proteolysis. The pH values at the end of storage were lower than that of control. The highest acidity was found in the sample sonicated at 20 kHz. | [42] |
| Skim dry milk | Ultrasound device (Volna-M UZTA-0.4/22-OM) at 22 ± 1.65 kHz and 60 W/L, 90 W/L, 120 W/L intensity for 1, 3, 5 min. | Kefir containing Lactococcus lactis subsp. Lactis and cremoris, Lactobacillus kefir, Acetobacter subsp. aceti, Saccharomyces lactis. Yoghurt containing Streptococcus salivarius ssp. Thermophilus, Lactobacillus delbrueckii ssp. bulgaricus YF-L811, YF-L703, YF-L705 b YF-L706 and Kefir fungi | The use of ultrasound (120 W/L, 3 min) affected the composition of microflora. Ultrasound increased antioxidant activity and improved the accumulation of biologically active compounds. | [179] |
| Skim pasteurized milk | Ultrasonic water bath (300 W, 19 L; Bandelin, Germany) and sonicated at 35 kHz for 5 min at 42 °C. | Starter cultures YC-471 and YF-L 901 (Chr. Hansen, Hørsholm, Denmark) that consist of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophiles strains | Sonicated set gels exhibited syneresis and were softer than controls. Sonication increased particle numbers, but the effect was less pronounced when YF-L 901 was used, indicating EPS as a tool to reduce syneresis and particle formation due to vibrations. Rheological parameters and size of microgel particles were more influenced by starter cultures than by sonication. | [178] |
| Skim milk | Ultrasonic device (Sonopuls HD 2200; Bandelin, Germany) at 20 kHz with a nominal maximum ultrasonic output of 200 W and in pulse mode of 1 s (0.2 s on, 0.8 s off). The amplitude was set to 10%. | Lactococcus lactis ssp. lactis and Lactococcus lactis ssp. Cremoris. YC-471 (Chr. Hansen A/S, Hørsholm, Denmark) containing Lactobacillus delbrueckii ssp. bulgaricus and Str. thermophilus. | Ultrasound led to a softening of the set gel, facilitating subsequent stirring; improved the visually perceived coarseness; and resulted in reduced viscosity. | [117] |
When cells are subjected to low intensity and sub-lethal sonication, their plasma membrane permeability is increased and their morphology is altered [148], [149], [150] with the formation of temporary pores, also called sonoporation, on cells after treatment ([150]. These cell changes have been attributed to intense micro-convection induced by HIU and cavitation in the liquid medium as mentioned in Section 1. The propagation of HIU waves also causes oscillatory motion of the fluid elements of the liquid, which induces micro-mixing in the medium [151]. This effect was first investigated by Sakakibara et al. [152]. In their detailed study, they observed an increase in the hydrolysis of lactose during the process of milk fermentation with Lactobacillus delbrueckii under HIU treatment (200 kHz, 17.2 kW/m2). They found that cell viability decreased due to HIU. However, another relevant finding was that the viable cell count increased again when HIU was stopped because HIU did not destroy the cells’ propagation abilities. Subsequently, Wang et al. [153] reported that when HIU (200 kHz, 17.2 kW/m2) is used in milk fermentation, the release of ß-galactosidase from L. bulgaricus cells is more effective in pH-controlled fermentation and static incubation. Other authors have addressed the effects of HIU on the yield of yogurt peptides in the fermentation of skim milk by Lactobacillus paracasei with the aid of ultrasound (28 kHz, pulsed model, 100 W/L for 30 min). The peptide content increased by 49.5% and the number of viable cells increased by 43.5% compared with those in the untreated samples [154]. The authors showed that HIU increased the extracellular enzyme activities of the acid, neutral, and alkaline proteases in the fermented media. Other beneficial effects of HIU are the activation of enzymes in enzyme-modulated reactions [155]. If the right types of cavitation can be identified, optimized, and controlled, then combinations of these bio-effects could be successfully implemented in milk fermentation.
6.1.1. Mechanism of ultrasound in fermentation processes
The mechanism of ultrasonic stimulation for the improvement of fermentation is largely attributed to the cavitation effects and sonoporation. During cavitation, the microjets formed increase the cell porosity and helps to transfer the oxygen to interior cells enhancing the mass transfer [156]. Under these conditions, ultrasound enhances the growth of microorganisms due to increased cell membrane permeability for substrates, gases, and other nutrient components [157]. The mass transfer effects generated due to acoustic cavitation could also be used to simply enhance the rates of chemical reactions or chemical processes such as dairy fermentation. The physical and chemical effects generated during acoustic cavitation have been used for a number of applications [158], [159]. Chemical reactions could be initiated by the acoustic cavitation process or may simply refer to increased reaction rates/efficiencies of reactions that would otherwise occur at a slower rate or lower efficiency [10]. Thermal, cavitation and shearing effects are the forces inducing the changes. Among the physical effects reported are: push and pull effect of micro-bubble, micro-streaming (liquid flow around micro bubbles) that tears the lipid membrane, penetration of micro bubbles into a cell x[44], cell damage due to temperature, changes to ultra-structures within cells, modified enzyme stability; nucleus rupture and release of DNA; breakage of extracellular polymer substances; altered membrane permeability (enhanced mass transport inside and outside the cell); and alteration of cell surface charge [160]. Whereas the main chemical changes include: the formation of free radicals and H2O2 [161], [162], membrane disruption through lipid peroxidation [163], [164]; conformational unfolding of proteins located on the surface of the cell membrane; membrane permeabilisation [163]; release of compounds (nitric acid, nitrous acid and hydrogen peroxide); and decreased cellular stability [160]. The molecular effects of ultrasound are illustrated in Fig. 5 (a to i).
Fig. 5.
Molecular effects of ultrasound. Modified from Rokhina et al. [160]. Physical changes: [thermal effects (a), and non-thermal effects (cavitation and shearing, b, c, d, e, f, g], and chemical changes: (h,i = cavitation-induced generation of radicals).
6.1.2. Effect of ultrasound on bacteria during fermentation
A low level of sonoporation improves the permeability of cell membranes, resulting in improved mass transfer of substrates across the microbial cell membrane and efficient removal of by-products of cellular metabolism, which eventually improves microbial growth [155]. The ultrasound effect in exposure duration less than 20 min, colonies of bacteria grew on the media, but their sizes are very small. For example, increasing the exposure duration causes the lactobacillus to adhere together and develop streptobacillus or filaments. This could be due the activation of some special proteins in the cell wall of the bacteria [165]. In comparison to lactococcus, lactobacillus is more affected by ultrasound shock, the reduction of lactobacillus population was obvious in different stages. Micro-cracks and ruptures are formed on the cell wall. In short exposure times, bacteria recover damages like micro-cracks and micro-voids, but in prolonged exposure times results in an excessive level of sonoporation that produces severe mechanical shear and cell disruption that induce the release of intracelular compounds, enzymes, polysaccharides and polymers to the environment and eventually lead to cell death [155], [166].
Many studies have been published regarding the use of low-frequency ultrasound (100 kHz) as a promising solution to improve microbial fermentative productivity [167], [168]. This application has mainly been focused on the metabolite production of probiotics [143], [146], [169], [170]. With the use of ultrasound, the increase in the viability of bacteria and the breakdown of complex compounds such as sugars and proteins offer the opportunity for the development of new functional and bioactive dairy products [50], [171]. Shershenkov and Suchkova [172] investigated the effects of ultrasound (30 kHz, 2–8 W, 1–3 min) on the metabolic activity of lactic acid bacteria (Lactococcus mixed culture, Streptococcus thermophilus, and Lactobacillus delbrueckii ssp. Bulgaricus) added to milk and observed that ultrasound allowed for the acceleration of the fermentative process by 10% and improved the quality of the final product. Lye et al. [173] showed that ultrasound increases cell membrane permeability. They also found that HIU enhances the cholesterol-lowering ability of Lactobacilli for the development of new functional and bioactive dairy products. Nguyen et al. [169] studied the effect of HIU processing (20 kHz) on the fermentative activities of four strains of Bifidobacterium in milk. They noted that HIU reduced the fermentation time required to reach a pH of 4.7. HIU released the intracellular enzyme β-galactosidase, and the lower the concentration of lactose, the higher the amount of oligosaccharides found in the fermented milk with HIU treatment. In an additional study, HIU also stimulated the production of major organic acids in the later stage of milk fermentation [170].
Milk-borne bioactive peptides can easily be released during enzymatic hydrolysis of casein [131]. Thermosonication promotes the disassociation of casein micelles into subunits that can form strong networks by re-aggregating during fermentation [101]. Barukčić et al. [20] noted the influence of HIU (480 W, 10 min, 55 °C) on fermentation of pasteurized or thermosonicated whey. HIU (84 W, 150 s) increased the number of viable Lactobacillus acidophilus La-5 during the activation process and the whey fermentation by ultrasonicated culture lasted 30 min less.
According to Huang et al. [174] HIU promotes microbial growth by loosening cell bunches, increasing membrane permeability, adjusting the culture medium, and providing effects on cellular components, cellular functions, and genetics. They concluded that ultrasound alters the characteristics of enzymes and substrates and the reactions between them, providing an optimal environment for the reactions at a molecular level. In the same way, Gholamhosseinpour and Hashemite [175] studied the impact of HIU (100 W, 30 kHz, 25% amplitude for 5, 10, and 15 min) on Lactobacillus plantarum AF1 during milk fermentation. They found that cell membrane permeability of sonicated samples was increased 88%–94% compared to the control. β-galactosidase activity and cell population increased with ultrasonication time. As well, glucose, galactose, and lactic acid increased significantly with fermentation and ultrasonication time. Antioxidant activity was enhanced significantly during a 24-h fermentation after 15 min of HIU application.
The effect of HIU on cheese production has also been addressed. Jalilzadeh et al. [42] studied the properties of Iranian ultrafiltered feta-type cheese after HIU application. Ultrasonication (20, 40, and 60 kHz for 20 min at an intensity of 80%) accelerated lipolysis and proteolysis. The highest rates on the lipolysis index were observed on day 60 of ripening for samples sonicated at 60 kHz. The highest acidity was found in the sample sonicated at 20 kHz. HIU may improve organoleptic properties due to higher lipolysis and proteolysis. Ojha et al. [176] investigated the influence of ultrasonication (20, 45, 130, and 950 kHz) on the growth kinetics of Lactobacillus sakei. The growth rate of L. sakei increased following ultrasonication at 20 kHz. The authors observed significant changes on the cell surface of L. sakei with the appearance of sonoporation. Their results revealed that HIU was influencing various metabolic pathways. The modification of bacterial activity by HIU could be attributed to both the mechanical effects, in which the shear forces cause the disruption of cell membranes, and the free radicals generated by cavitation. However, it is important to point out that HIU effects vary among bacteria types. Gao et al. [162] reported that HIU application resulted in lethal damage to E. aerogenes and B. subtilis (up to 4.5-log reduction), whereas Staphylococcus spp. was not affected noticeably. The main reason for bacterial resistance to ultrasonic deactivation was due to the properties of the bacterial capsule. Microbes with a thicker and “softer” capsule were highly resistant to the ultrasonic deactivation process. The same authors reported that E. aerogenes was more sensitive to ultrasound in water than in reconstituted skim milk with different protein concentrations. Furthermore, high-frequency US was not able to inactivate E. aerogenes in milk when applying a power of 50 W for 60 min [177].
Potoroko et al. [179] evaluated the effectiveness of low-frequency ultrasound (22 ± 1.65 kHz, 60–120 W/L) during the pre-fermentation stage, noting its influence on the accumulation of exopolysaccharides (EPS), vitamin C content, antioxidant activity, and the accumulation of biologically active compounds. Overall, their results showed that reconstitution of dry milk with ultrasound improves accumulation of biologically active compounds and raises the nutritional quality of the fermented product. Recently, Niamah [96] investigated the effects of ultrasound (40 kHz, 116 W for 0, 5, 10, 15, and 20 min) on the growth of five different strains of probiotic bacteria (L. acidophilus LA-5, Lactobacillus casei LC, Lactobacillus reuteri LR-MM53, Bifidobacterium bifidum BB-12, and Bifidobacterium longum BB-536) in fermented milk. Bacteria strains were ruptured by ultrasonication, causing an increase in the extracellular release of β-galactosidase enzyme. The optimal time span of ultrasound treatment was 10 min for all fermented milk samples, and it can be applied to increase the number of viable cells of probiotic bacteria and β-galactosidase enzyme. Körsendörfer et al. [117] revealed that HIU is a promising tool to weaken the gel and facilitate further processing. This enables new approaches to the manufacture of Greek yogurt, particularly to avoid production of acid whey and develop products with novel textures. The same authors pointed out that investigating ultrasonication parameters such as point of time, pH, duration, and amplitude will help optimize the treatment. However, the fundamental molecular mechanism influencing the behaviour of microorganisms subjected to ultrasonic waves remains to be fully explained.
Most studies that used ultrasound for fermentation purposes applied 20 to 30 kHz and their results were very effective. The fermentative process was accelerated, showing an increase cell membrane permeability, higher released of ß-galactosidase, higher degree of lactose hydrolysis, stimulation of the production of major organic acids and also positively affected the composition of microflora in fermented drinks [20], [42], [95], [137], [154], [169], [170], [172], [175], [179]. It is important to note that when frequency increased to 40 kHz [42], [96], 60 kHz [42] or 200 kHz [140], [152], [153] the effects of ultrasound on the fermentation process or product quality did not improve.
6.2. Spoilage and pathogenic microorganisms
Ultrasonic processing inactivates microorganisms that cause spoilage, making it a potential alternative to conventional technology [62]. The acoustic cavitation generated by applying low frequencies instantly forms microbubbles that collapse and generate high rates of micro shear in the milk, causing rupture and shear in the bacteria wall [177]. The cell wall of Gram-positive bacteria contains more peptidoglycan and is thicker than the wall of Gram-negative bacteria, making the former more resistant to physical and chemical treatments [180], [181]. Some reports about these effects are outlined in Table 6.
Table 6.
Recent studies of the effect of ultrasound on pathogenic bacteria and deterioration in milk and dairy products.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Buffalo’s milk | Ultrasonic homogenizer (Son prep 150 MSE, Korea), 430 and 338 W, 28 kHz, at 20 °C; 5, 10, and 15 min. | Significant decrease in counts of aerobic, coliform, and Staphylococcus bacteria as the power and time of exposure to ultrasound increased. | [99] |
| Semi-skim milk, fresh | Equipment (Sonics and Materials Inc., USA), 130 W (operated at 78 and 104 W), 20 kHz, between 40 and 69 °C; 4, 6, or 8 min. | Significant decrease in total aerobic mesophilic bacteria, total coliforms, and Staphylococcus spp., preserving some bacteria from lactic acid immediately after US and after 7 d storage at 4 °C. | [18] |
| Reconstituted and whey powder | Ultrasonic processor (S-4000, Q́Sonica LLC, USA), 480 and 600 W, 20 kHz, at 45 or 55 °C; 6.5, 8, or 10 min. | The 480 W and 55 °C combination was the best treatment for bacterial inactivation, making it an alternative to pasteurization. | [20] |
| Whole raw milk | Ultrasonic processor (UP400S, Hielscher USA), 400 W (operated to 0.86, 1.71, 2.57 and 2.85 W/cm2), 24 kHz, for 30 min at 63 °C. | After 10 min, thermal pasteurization was achieved (5 log reductions of L. innocua with intensities of 90% and 100%). Intensities of 60%, 90%, or 100% reduced the natural flora of raw milk by 3 log during the first 10 min, unlike pasteurization which reduced it by 1.89 log. | [185] |
| UHT milk inoculated with E. coli, L. monocytogenes and P. fluorescens of culture to yield 1 × 104 or 1x106 CFU/mL | Ultrasonic processor (VCX 750, Sonics and Materials, USA), 750 W (operated at 100% amplitude), 20 kHz, at 24–26 °C; 2.5, 5, 6, 7.5 and 10 min. | After 10 min of treatment with US, elimination of 100% of E. coli and 99% of L. monocytogenes. 6 min were sufficient to eliminate P. fluorescens in 100%. | [84] |
| Raw, thermized, and/or homogenized pasteurized milk | Ultrasonic processor (UP 2005, IKA, United Kingdom), 200 W, 24 kHz, at 15–25 °C depending on time sonication; 2, 4, 8 and 16 min. | The shelf life of milk cannot be extended by using ultrasound or the thermization/ultrasound combination (55 °C/15 s). The total viable count and the psychrotrophic count increased significantly during storage at 4° C. | [187] |
| Raw milk incubated and inoculated UHT milk | Sonifier probe (450, Branson Ultrasonics, USA), 150 W (118 W/cm2), 20 kHz, 20 and 57 °C; 1, 3, 4, or 6 min. | Ultrasound treatment with and without mild heating (57 °C) was effective for reducing total aerobic bacteria in raw milk and L. monocytogenes in inoculated UHT milk. | [186] |
| Bovine milk | Ultrasound system (Sonics and Materials Inc., USA), 500 W (operated at 75 W), 20 kHz, at 5 °C, 15 min. | US application was inefficient for microbial control in raw milk; significant reduction of only 0.61, 1.23, 1.15, and 0.77 log CFU/mL in the counts of mesophilic aerobes, total coliforms, E. coli, and S. aureus respectively. | [188] |
| Nonfat, low-fat and full-cream milk samples inoculated with L. monocytogenes. | Ultrasonic tank (W 338, Honda Electronics Co., Japan), 600 W; 28, 45, and 100 kHz (multi-frequency), temperature increase up to >60 °C, 50 min. | Biphasic inactivation of L. monocytogenes, with slow lag phase inactivation (sub-lethal cell damage) and log phase cell inactivation. Faster inactivation in whole milk. | [193] |
| Pasteurized skim milk and inoculated UHT milk | Ultrasonic processor (Sonicator 3000, Misonix Inc., USA), 500 W (amplitude 0–216 μm), 20 kHz at 10–84 °C for 0.17–5 min. | Reduction in at least 5 log for thermophilic bacteria (Pseudomonas, Brochothrix, and Enterobacteriaceae) and in 1–2 log for Bacillus atrophaeus spores at 84.8 °C, 216 μm, and 5.8 min. | [195] |
| 5%, 10%, and 15% reconstituted skim milk, inoculated with E. aerogenes (108 CFU/mL) | Ultrasonic homogenizer (Sonic Ruptor 250, Omni International, USA); 8.2, 8.5 and 9.2 W, 20 kHz at < 30 °C, 10–60 min, 15 mL. Ultrasound generator (K80, Meinhardt Ultraschalltechnik, Germany), 50 W, 850 kHz, <20 °C, 10–60 min, 5 mL. | Complete inactivation of E. aerogenes was not achieved. The higher the protein concentration in milk, the lower the inactivation of E. aerogenes. | [177] |
| UHT whole milk, UHT skim milk inoculated with E. coli and L. monocytogenes (mid-log or mid-stationary) | Ultrasonic processor (UP400S, Hielscher, USA), 85 W/cm2, 24 kHz, < 35 °C, 5–55 min. | Significant decrease in L. monocytogenes and E. coli counts as treatment time increased. High D-values indicate the protective effect of milk on these bacteria. Log phase is more sensitive to treatment than lag phase. | [182] |
| Prebiotic whey beverage | Ultrasonic device (Unique, Desruptor, Brazil), 800 W (operated at 200, 400 and 600 W), 19 kHz at maximum temperature 53 °C for 3 min. | Non-thermal processing (ultrasound) was comparable to HTST treatment (75 °C, 15 s) for the inactivation of aerobic mesophilic bacteria, molds, and yeasts. | [48] |
| Raw milk | Ultrasonic processor (S-4000, Misonix Sonicators, USA); 600 W (60, 90 and 120 μ m amplitude), 20 kHz at 20, 40 or 60 °C; 6, 9 for 12 min. | Gram-negative bacteria (E. coli) are more susceptible to ultrasound than Gram-positive bacteria (S. aureus). Greater inactivation in long periods and higher temperature and amplitude. | [183] |
| Raw coẃs milk | Ultrasonic processor (S-4000, Misonix Sonicators, USA); 600 W (60, 90 and 120 μ m amplitude), 20 kHz at 20, 40 or 60 °C; 6, 9 or 12 min. | Significant inactivation of mesophilic bacteria (3.68 log) was obtained with 120 μm, 9.84 min, and 45.34 °C. | [189] |
| Cow's milk | Ultrasonic processor (VCX 1500 HV., Sonics and Materials, USA); 1500 W (95% amplitude), 20 kHzat 48–55 °C for 10 or 15 min. | Decrease in counts of aerobic mesophilic bacteria and Enterobacteriaceae during storage. | [29] |
| Retentate of ultrafiltered milk inoculated with E. coli O157:H7, S. aureus, Cl. sporogenes and P. chrysogenum | Ultrasonic homogenizer (HD2200, Sonopuls Bandelin Company, Germany), intensity of 80%; 20, 40 and 60 kHz for 20 min. | The highest inactivation rate of E. coli O157:H7 and S. aureus was observed in samples sonicated at 60 kHz. There were no significant differences between different sonication frequencies for the deactivation of P. chrysogenum or Cl. sporogenes. | [42] |
| Fresh unpasteurized sweet whey | Ultrasonic processor (UP400S, (Hielscher Ultrasonics GmbH, Germany), 400 W (60, 80 and 100% of nominal output powder), 24 kHz at 55 °C; 5, 6.5, and 8 min. | US alone had no effect on microbial reduction. The combination of 400 W and 55 °C was more effective for microbial inactivation. | [21] |
| Raw cow's milk | Ultrasonic processor (S-4000, Misonix Sonicators, USA), 600 W (60, 90 and 120 μ m amplitude), 20 kHz at 20, 40, and 60 °C; 6, 9, and 12 min. | The lowest enterobacteria count (1.06 log) was obtained with 120 μm, 12 min, and 60 °C. | [190] |
| Raw skim milk inoculated with 106 CFU/mL Paenibacillus amylolyticus | Ultrasonic equipment (Branson 2000, Branson Ultrasonics, USA), 2200 W (21, 42, 63, 72 and 84% amplitude), 20 kHz for 10, 20, 30 and 60 min. | Cold sonication and thermosonication were not appropriate to reduce the total aerobic bacteria count during storage over 50 d at 4 °C; counts of spore-forming decomposing bacteria increased. | [184] |
| Raw whole milk inoculated to a final concentration of 105 CFU/mL (E. coli, S. aureus, P. fluorescens and D. hansenii var. hansenii) | Ultrasonic processor (UP400S, Hielscher Inc., USA), 400 W (70 and 100% amplitude), 24 kHz at 15–46 °C during sonication for 500, 100, 200, and 300 s. | Inactivation of E. coli, P. fluorescens, and D. hansenii at 100% intensity. S. aureus was resistant to the strongest treatment, with an irrelevant log reduction. | [192] |
| UHT milk | Ultrasonic processor (UP200H, Hielscher GmbH, Germany), 50 and 100% amplitude (62.5 and 125 μ m), 24 kHz, at 23–60 °C during sonication for 5, 10, and 15 min. | Gram-positive organisms S. aureus and L. monocytogenes were more resistant to ultrasound than L. plantarum and L. pentosus. Gram-negative organisms were more susceptible to ultrasound than Gram-positive organisms. | [180] |
| Standardized milk | Continuous ultrasound unit (DRC-8-DPP-FGS, Advanced Sonic Processing Systems, UK) with a 2.4 kW dual-frequency reactor with 2-low-frequency (16 and 20 kHz, 1200 W); inlet temperatures: 42 or 54 °C, outlet temperatures: 19–31 °C for 2, 3 and 6 min. | At 54 °C and with flow rates of 0.3 and 0.45 L/min, the total aerobic count was reduced by more than 1 log CFU/mL and the psychrophilic counts below the detection limit (10 CFU/mL). | [28] |
Gera and Doores [182] showed that the D values for the inactivation of Escherichia coli (Gram-negative) were significantly lower than for L. monocytogenes (Gram-positive), suggesting that E. coli is more sensitive to ultrasonication than L. monocytogenes. These researchers suggested that the presence of lactose exerts a protective effect on bacteria and E. coli exhibits nonlinear (tailed) inactivation kinetics. Meanwhile L. monocytogenes showed linear logarithmic inactivation kinetics. Herceg et al. [183] also confirmed a higher susceptibility of E. coli compared to S. aureus (Gram-positive) in ultrasound-treated cow's milk. On the other hand, Lim et al. [184] reported that thermosonication induces vegetative cells of spore-forming anaerobic bacteria that form heat-resistant spores, allowing higher rates of deterioration than traditional pasteurization. Cold sonication (ultrasound treated milk at 12.5 ± 5 °C with subsequent pasteurization treatment) might be an appropriate method but needs more research to optimize conditions and understand the effect. Lim et al. [184] reported significant increases in the total aerobic bacteria count of skim milk stored for up to 50 d at 4 °C, inoculated with Paenibacillus amylolyticus (a bacterium of psychophilic contamination, thermotolerant and spore-forming), ultrasonicated, and heated.
Basic tests of microbiological quality in milk include the total aerobic count (mesophilic, psychrophilic, and/or thermophilic bacteria, depending on the incubation temperature) and the total coliform bacteria count, indicators of product stability during storage and of inadequate hygienic practices respectively. In this regard, control of total aerobic mesophilic bacteria and total coliforms by ultrasonication has been reported in sheep's milk [18], buffalo’s milk x[99], and rennet cheese whey [21]. In these studies, the temperature of the milk was maintained at 40–69 °C, 20 °C, and 35–55 °C during ultrasonication. Therefore, in addition of heating, other variables such as the frequency, intensity and time of treatment must be considered in the effects that ultrasound has on the counts of mesophilic bacteria. In this regard, in sheep's milk [18] the experimental conditions were 78 and 134 W, 20 kHz and 4–8 min, while in buffalo milk [99] these conditions were 430 and 338 W, 28 kHz and 5–15 min. In both studies, a decrease in the counts of this group of bacteria was reported despite the fact that the ultrasound temperature in buffalo milk was maintained at 20 °C; so, it is likely that the effect of ultrasound on the bacterial flora is due to increase in potency and treatment time. In cow’s milk [185] a decrease in the counts of mesophilic bacteria was also observed at different intensities (power 400 W, intensities of 0.86–2.85 W/cm2, 24 kHz, 63 °C) and longer treatment times (30 min).
D’Amico et al. [186] also reported significant reductions of up to 5 log in total aerobic bacteria counts in ultrasonicated raw milk in combination with mild heat (57 °C). Likewise, Hernández-Falcón et al. [29] reported a significant decrease in the aerobic mesophilic counts during storage of homogenized and non-homogenized cow's milk treated with ultrasound and heat (thermosonication at <45 °C). Barukčić et al. [20] reported significant decreases in viable cell count in thermosonicated whey (45–55 °C). Conversely, Chouliara et al. [187] observed significant increases in total viable counts and in psychrotrophic counts from raw milk, thermized milk (55 °C by 15 s), pasteurized milk, and ultrasonicated (75 °C by 15 s) milk. At the end of storage, the lowest counts were obtained in pasteurized-ultrasonicated milk.
Ultrasonication alone seems to be ineffective for decontamination of milk. Engin and Yuceer [188] reported that ultrasonication of raw milk (5 °C) was ineffective for reducing mesophilic bacteria to acceptable levels. The counts for this group of bacteria decreased by only 0.61 log. Similarly, these authors reported a reduction of only 1.23 log for total coliform bacteria and 0.77 log for Staphylococcus aureus in raw milk treated with ultrasound at 5 °C, making the use of this technology by itself inefficient. On the other side, Herceg et al. [189] reduced the counts of mesophilic bacteria in raw cow's milk with thermosonication. The studies carried out by Hernández-Falcón et al. [29] demonstrate the efficacy of ultrasound and heat (thermosonication at < 45 °C) for a significant reduction in Enterobacteriaceae counts during storage of cow's milk at 8 °C over 1, 7, and 14 d. Similar results were reported by Juraga et. al. [190], who found that thermosonication treatment of raw milk was efficient to achieve significant reduction in counts of Enterobacteriaceae of up to 1.06 log CFU/mL when temperatures of 60 °C were used for 12 min.
Ultrasound treatment has been reported to significantly decrease Staphylococcus spp counts in sheep’s milk [18], buffalo’s milk [99], Iranian feta-type cheese stored (0–28 d) and made from thermized ultrafiltered milk inoculated with S. aureus [42]. The results obtained by these researchers are due to the use of heat in combination with ultrasound, a more efficient method of achieving a reduction in Staphylococcus spp to acceptable levels.
Listeria monocytogenes is a foodborne pathogenic microorganism that survives in a wide range of temperatures (1–45 °C) and pH levels (4.1–9.6). It competes with other microorganisms that grow in refrigeration [191]. Milk and dairy products are prone to its contamination due to production facility conditions, environments, and storage temperatures. The decrease of 5 log of Listeria innocua in raw cow's milk by thermosonication at 63 °C constitutes an alternative to conventional pasteurization technology [185].
Pathogens like E. coli can form biofilms within pasteurization equipment and their presence indicates post-pasteurization and fecal contamination. In this regard, Cameron et al. [84] suggested that E. coli and Pseudomonas fluorescens have been eliminated by up to 100% in raw milk after 10 and 6 min of ultrasound treatment, while L. monocytogenes was reduced by 99% within 10 min of treatment. However, Marchesini et al. [192] showed that treatment with HIU for more than 100 s leads to sensorial deterioration due to the formation of unpleasant flavors in the milk and inadequate disinfection for direct consumption. However, short ultrasonication preserves the structural and functional integrity of Debaryomyces hansenii, a yeast naturally present in milk that could be used as part of starter cultures to promote milk curdling. Similar results were obtained by D’Amico et al. [186] in UHT milk inoculated with L. monocytogenes. They observed 5 log reductions when the ultrasound treatment was carried out in continuous flow and combined with gentle heat (58 °C).
Gabriel [193] also reported the inactivation of L. monocytogenes in skim, whole, and low-fat milk treated with ultrasound in a multi-frequency modality. They achieved decimal reduction times of 24.81, 29.17, and 30.64 min respectively. Jalilzadeh et al. [42] also reported a significant reduction in E. coli O157:H7 counts in Iranian feta-type cheese made from thermized ultrafiltered milk during 0–28 d storage. Other pathogenic bacteria causing opportunistic and nosocomial infections, such as Enterobacter aerogenes, have been reduced in reconstituted skim milk by low and high frequency ultrasound, depending on the treatment time and the concentration of proteins in the milk [177]. Penicillium is the mold genus most frequently isolated from cheese samples whose strains with psychrotrophic characteristics can grow during cold storage. In this regard, Jalilzadeh et al. [42] found no significant differences between different ultrasonication frequencies for the deactivation of P. chrysogenum in Iranian feta-type cheese ultrafiltered and stored for 30 d. Jeličić et al. [21] also reported the inactivation of molds and yeasts in ultrasound- and heat-treated rennet cheese whey.
Ultrasound assisted by the active lactoperoxidase system or the addition of H2O2 is more efficient for reducing or eliminating microbial counts compared to ultrasound alone. In this regard, Shamila-Syuhada et al. [180] reported the inactivation of the pathogenic bacteria Staphylococcus aureus, L. monocytogenes, and Salmonella enterica ssp. enterica serovar Typhimurium, as well as inactivation of deterioration microbiota such as E. coli, P. fluorescens, L. plantarum and Lactobacillus pentosus. Ultrasonication, in the presence of H2O2 (from 0.01 to 0.1%), had a greater effect in reducing microbial counts at an amplitude of 62.5 μm and for 5 min compared to ultrasound alone. However, the addition of H2O2 to milk for cheese making could be considered as long as the H2O2 is eliminated at the end of the process [180].
Thermophilic bacteria and spores can survive pasteurization and reduce product quality and shelf life due to production of acids, lipases, proteases, and unpleasant flavors during storage [194]. Ganesan et al. [195] showed the effectiveness of ultrasonication and heating (72–85 °C) on pasteurized milk to reduce Bacillus spores by 1–2 log. They identified some bacteria and groups of thermophilic bacteria in pasteurized milk, such as Pseudomonas, Lactococcus, Leuconostoc, Brochothrix, and unknown members of Enterobacteriaceae, that can be efficiently inactivated at 84.8 °C, 216 μm, and 5.8 min of thermosonication. On the other hand, Jalilzadeh et al. [42] did not report significant differences between different frequencies of ultrasound for the deactivation of Clostridium sporogenes.
More than 50% of the articles cited here report the use of frequencies of 20 kHz, while the remaining 50% applied frequencies between 24 and 100 kHz. In all of them the main observations are reductions in the counts of total aerobic bacteria, mesophilic, psychrophilic and thermophilic bacteria and molds and yeasts [28], [29], [48], [186], [189], [190], [195], including a decrease in total coliforms and pathogenic bacteria such as S. aureus [18], [183], L. monocytogenes [84], [186], Pseudomonas fluorescens [84], E. coli [183], and Enterobacter aerogenes [177]. In contrast, other studies have reported that ultrasonication is an inappropriate technology for reducing the total aerobic count [187], [188], [189] and very low reductions in the counts of coliforms, E. coli, and S. aureus [188], and increases in the counts of spore-forming bacteria [184] have been found. This difference could be due to the effect of other variables in the application of ultrasound such as the temperature [188]. The use of frequencies greater than 20 kHz has also produced significant decreases in the counts of aerobic bacteria [99], [185] , coliforms [99], S. aureus [42], [99], [180] , Listeria inoccua [185], E. coli [42], [84], [182], L. monocytogenes [84], [180], [182], [193], and P. fluorescens [84].
7. Sensory properties
As mentioned in previous sections, HIU, as an emerging technology, has been designed to enhance quality including the sensory properties of food products. This section aimed to present up-to-date reports on the effects of HIU on the sensory properties of dairy foods and the role of volatile compounds in sensory quality. Most available articles on this topic have been collected and presented in Table 7.
Table 7.
High-intensity ultrasound studies of dairy foods with sensory assessment.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Milk | Ultrasonic processor (24 kHz, 200 W, model UP 2005, IKA, U.K.) at 24 kHz for 0, 2, 4, 8, and 16 min. The processor was fitted with an ultrasonic probe with a 22 mm diameter tip. During sonication sample was at 15–25 °C. | Thermized milk sonicated for 2 min retained an acceptable taste and odor after 4 days of storage while its untreated counterpart had deteriorated in flavor. | [187] |
| Milk | US system (Sonics and Materials Inc., USA) comprised of 20 kHz frequency acoustic power unit (VibraCell-500W), a transducer and a sonotrode. The treatment was applied at 5 °C. The sonotrode was applied at 75 W for 15 min. The power density of the US treatment was 150 W L−1.The US intensity was 135 J mL−1. | No major differences in aroma-active compounds in sonicated milk, but some volatiles were generated by HIU in the milk. | [188] |
| Milk | Ultrasonic processor (UP400S, Hielscher Inc., USA) at 400 W and 24 kH equipped with a 22 mm diameter horn. Samples were sonicated for for 50, 100, 200, and 300 s. | A significant increase in a burnt off-flavor with increasing intensity and 200 s of the ultrasound treatment. The rubbery aroma was less intense on reducing the sonication power from 400 to 100 W. | [102] |
| Milk | Ultrasonic processor (S-4000, Misonix Sonicators, USA) set at 600 W, frequency od 20 kHz. | HIU treatment longer than 6 min resulted in milk tasting like “foreign-metal,” “burnt,” and “rubbery.” | [202] |
| Milk | Ultrasonic processor (UP 100H; Hielscher, Germany) at 100 W, 30 kHz, amplitude of 60% for 5 and 10 min. | Milk beverages homogenized by ultrasound and containing mint and rosemary showed the highest acceptability. | [205] |
| Milk | Ultrasonic processor (Branson 2000, Branson Ultrasonics, USA) at 2,200 W max power and 20 kHz. | A rubbery aroma appeared in the ultrasonicated samples but dissipated over time. | [184] |
| Fermented milk drink | Ultrasonic bath (model RK103H, Banelin, Germany), samples were treated at 35 kHz and 60–80 °C for 1, 3, and 5 min. Samples were submerged in a 90 °C for thermal treatment for 1 min. | Sensory properties of the thermosonicated samples were better than those of the thermally treated samples after storage. | [222] |
| Milk and cream | A batch sonicator (Branson Ultrasonics 2000 series, USA), with a maximum output power of 2.2 kW was used. Ultrasound was generated at 20 kHz, and 77, 104 and 115 for 1 and 3 min. Sonication was conducted in 30-s intervals, with a 1-s break. | The extreme conditions of thermosonication used in the research experiments did not significantly increase the intensity of offensive rubbery and eggy odor attributes in skim milk and cream. | [85] |
| Yogurt | Ultrasonic processor (UP 400S, Hielscher, Germany), at 400 W and 24 kHz for 2.5, 5, 10, 15 and 20 min with a continuous application. Samples were equilibrated for 5 min at 45 °C. | Thermosonicated yogurts showed superior texture and color. Samples with a fat content of 0.1% scored best in overall acceptability. | [115] |
| Yogurt | Ultrasonic water bath (200 W, 300 × 240 × 200 mm, 12 L; USC1200TH, VWR International GmbH, Germany). The ultrasound input was equivalent to an average power density of 17 kW/m3. Both water baths were operated at 42 °C. | A short sonication of 5 min during a 360 min-fermentation induced the formation of large particles, which were perceived as grainy in stirred yogurt samples. | [114] |
| Yogurt | Ultrasonic processor (VC750Vibracell©; Sonics and Materials Inc., USA), 20 kHz at 150, 262, 375, 562, or 750 W (corresponding to 20%, 35%, 50%, 75% or 100% of the processor's total power) for 10 min. | Ultrasonication of milk led to yogurts with off-flavors and burned and pungent characteristics. Increasing US amplitude did further intensify the off-flavors and decrease the degree of likeness. | [207] |
| Whey | Ultrasonic device (UP400S, Hielscher Ultrasonics GmbH, Germany), 400 W, amplitude 20%–100% and 24 kHz for 5, 6.5 and 8 min. Samples (100 mL) were placed into 300 mL glasses. Power level of 100% , 80% and 60% corresponded to approximately 400, 320 and 240 W. | Ultrasonication and thermosonication treatments considerably improved sensory properties of rennet cheese whey in comparison with simulated pasteurization processes. | [21] |
| Whipped cream | Ultrasound processor (APU400, Adeeco Co., Iran) at 20 kHz and at 100 and 300 W for 0, 5, 10, and 15 min at 50 °C (an ultrasound pulse mode of on-time 2 s and off-time 4 s). | Ultrasound treatment for 5 min at 300 W recorded the best texture and body. The sonicated sample had the desirable color. Ultrasound improved the organoleptic properties. | [43] |
| Cheese | Sonopuls Ultrasonic Homogenizer (model HD2200, Bandelin Co., Germany) at 20, 40, and 60 kHz for 20 min at 80% intensity. | Ultrasound significantly improved the color and appearance of feta cheese, with no difference in texture. Improvement of organoleptic properties due to higher lipolysis and proteolysis. | [42] |
| Genoise, cake, and mousse | Ultrasonic bath (R.E.U.S., France) with 14 × 10 cm internal dimensions and maximal capacity of 1 L operating at 25 kHz with maximum power of 150 W. Ultrasound application time was 5, 5, and 2 min for Genoise, cake, and mousse, respectively. | Ultrasound-assisted preparations were considered superior according to the sensory analysis and physicochemical data. | [201] |
7.1. Dairy products
7.1.1. Milk
HIU minimizes the typical changes in sensory properties induced by the extreme heat of pasteurization [196], [197]. Nevertheless, this technology is not yet widely accepted in the dairy industry [198]. HIU is not currently being used in fluid milk for processing or preservation, in part because of limited knowledge about its effects on shelf-limiting enzymes and sensory and other quality parameters [167], [198]. More than 10 years ago, Chouliara et al. [187] reported that ultrasonication failed to extend the shelf life of milk products as documented by sensory evaluation. However, it is evident that not much has changed since then. A rubbery aroma and an off-taste that lies between “burnt” and “foreign” have been reported in milk after HIU (200 W) treatment for 2 min [187] or for<200 s [102]. Some authors have attributed these changes to denaturation of proteins [19], [62], [199]. Others agree that the undesirable flavor of sonicated milk is due to alterations in physical properties and degradation of components by the high heat of cavitation [115] and oxidation of PUFA hydroperoxides (Fig. 6). This oxidation generates volatile compounds in milk after HIU application [53], [101], [115], [187], [188], [192], [200], [201].
Fig. 6.
Ultrasound-induced modifications that lead to changes in sensory properties of dairy products.
The off-flavor of sonicated milk seems to increase with sonication time. Jurić et al. [202] ultrasonicated (24 kHz, 200 W) milk for longer than 6 min and it resulted in a sensory unacceptable product that tasted like “foreign-metal,” “burnt,” and “rubbery.” They observed a decrease in MUFA and PUFA with a simultaneous increase in saturated fatty acids, and the rubbery aroma was less intense after reducing the sonication power from 400 to 100 W. Lipid oxidation can be controlled by decreasing the sonication time and the temperature in the system to reduce the adverse sensory aroma formed in sonicated milk [85], [172], [193], [203]. As reported by Vijayakumar et al. [85], no significant differences were found between the intensities of offensive “eggy” and “rubbery” odor attributes after HIU for 1 to 3 min of skim milk, pasteurized commercial skim milk, and cream. Leong et al. [91] observed that, only in extended ultrasonication of 20 min using 1 MHz at elevated temperatures, HIU promoted the derivation of oxidative volatiles above the human sensory threshold. Paniwnyk [204] stated that the highest concentrations of deterioration products were seen at 1000 kHz and energies above 271 kJ/kg for raw milk and 102 kJ/kg for pasteurized skim milk. That author suggests that this information could be of interest when designing larger scale up reactors for use in the dairy industry to ensure milk sensory quality retention. Recently, Lim et al. [184] applied high-power (165 W, amplitude of 200 μmp-p/60 s), low-frequency sonication (20 kHz) to milk and suggested that the rubbery aroma in the ultrasonicated samples may originate from heat-induced oxidation of lipids into volatile compounds, instead of from a radical mechanism. They observed that the rubbery aroma dissipated very quickly. These authors pointed out that, at the frequency they used, the cavitation bubbles were larger and less numerous than what would be present at higher frequencies. Large bubbles collapse more violently than small bubbles, but the result is fewer free radicals [102], [203], so low frequencies could provide better results in terms of aroma of the treated milk.
Milk of other species has also been ultrasonicated, aiming to improve its sensory characteristics and enhance the bioactive composition. Komes et al. [205] applied HIU (100 W for 5 and 10 min) to goat’s milk and added plant extracts during the development of milk beverages. The product exhibited significantly improved sensory properties in comparison to plain goat’s milk, with the highest overall acceptability determined for samples enriched with mint and rosemary.
7.1.2. Yogurt
Texture, flavor, and aroma contribute largely to the sensory perception and consumer acceptability of yogurt. Flavor, mouthfeel, a smooth and thick consistency in the mouth during consumption are key aspects of quality in yogurt [73], [80], [208], [209], [210], [211], [212], [213]. HIU application prior to culturing allows yogurts with superior sensory properties compared to yogurts elaborated with untreated milk [211], [115]. For instance, HIU is able to improve yogurt texture [95], [211], [101] but not much research has been done on yogurt properties from the sensory standpoint. Flavor and aroma are highly dependent on the presence of volatile components. Acetic acid is an undesirable end-product in fermentation of milk by Bifidobacteria owing to its “vinegary” flavor and aroma. In contrast, lactic acid is a milder, sweeter acid that does not generate an objectionable odor [215]. Nguyen et al. [170] provided evidence that ultrasonication stimulates the production of major organic acids in the later stage of milk fermentation. Nonetheless, HIU decreased the ratio of acetic acid to lactic acid and the ratio of total acetic and propionic acids to lactic acid in yogurt fermented with different species of Bifidobacteria. A recent sensory study of the effects of HIU on yogurt by Sfakianakis and Tzia [207] showed that ultrasonication of milk led to yogurts with lower degrees of likeness and off-flavors (pungent and fatty flavors). These authors recommended that adequate descriptive sensory evaluation of ultrasonicated yogurt be carried out and that each individual sensory characteristic and its relation to the degree of likeness be taken into account. Furthermore, before HIU is considered a potential alternative to conventional processing to obtain good quality yogurt, sensory characteristics with the greatest impact on quality must be identified. More extensive studies, including sensory assessment of products, need to be done before declaring HIU acceptable in yogurt manufacturing.
7.1.3. Whey
Whey is a major by-product of the dairy industry originating from cheese and casein manufacture. It is widely used as an ingredient in foods due to its unique functional properties, i.e., emulsification, gelation, thickening, foaming [133], and fat- and flavor-binding capacity [216]. In addition, it offers numerous nutritional advantages to formulated products. Whey proteins create viscosity and facilitate whipping, foaming, and aeration. Elasticity and hardness of whey protein isolates are correlated to sensory perception of food texture [208]. Jeličic et al. [21] observed that rennet cheese whey, after ultrasonication (400 W and 320 W) and preheating (45 °C), considerably improved sensory properties in comparison with simulated thermal processes. Mouthfeel of whey samples was considerably better, there was no occurrence of sediment, and color remained unchanged in almost all samples. Barukčic et al. [20] obtained similar results and observed that thermosonication (480 W, 55 °C, 8 min) resulted in fermented whey with a noticeable increase in the viable cell count at the end of fermentation and an improvement in sensory properties. The rubbery flavor reported in milk after HIU treatment [101] was not present in whey suspensions sonicated at 20 kHz and 15 W for 15 min [217].
Torkamani et al. [94] mentioned that low amounts of aldehydes and a slight increase in concentration will be enough to change the flavor profile of whey once oxidation reactions are triggered. The most common whey protein-based ingredients are whey protein isolate and whey protein concentrate [218]. Whey proteins are hydrolyzed to produce bioactive peptides with improved sensorial properties such as color, flavor, and texture [219]. However, when proteins are hydrolyzed via enzymatic processes, they taste bitter and are not accepted by most consumers [220], [221]. Physical modification of whey proteins appears to have major advantages over other techniques. However, sensory and consumer properties remain to be evaluated in sonicated whey and whey products [39].
7.1.4. Other dairy foods
The use of HIU in the production of dairy products can affect proteolysis and the formation of compounds derived from lipid oxidation. Jalilzadeh et al. [42] applied HIU (20, 40, and 60 kHz for 20 min at 80% intensity) to feta cheese and observed a significant improvement in color and appearance, with no difference in texture. Further, they noted improvement in organoleptic properties due to higher lipolysis and proteolysis by HIU. Further studies are required to determine the impact of proteolysis and lipid oxidation due to HIU on the sensory quality of the processed product during storage, such as cheese [38].
Pingret et al. [201] compared sensory and physicochemical properties of three foam-type products prepared conventionally and with ultrasonic methods. Ultrasound used to assist foam-type food preparations (in this case a sponge cake, a chocolate Genoise, and a chocolate mousse) resulted in more homogeneous products that were not only well accepted by the panelists, but even preferred to conventionally prepared food preparations, as some organoleptic characteristics were improved.
Most sensory studies applied 20 kHz frequency obtaining favorable results. Few studies applied frequencies of 40 kHz or 200 kHz and the findings were similar than those with 20 kHz application.
7.2. Volatile compounds
The formation of volatile compounds by enzymatic and fermentation processes in foods plays an important role in the sensory properties of milk and dairy products. Ultrasound has been suggested for potential applications in the dairy industry [13] due to significant improvements it has demonstrated in the processing of dairy products. It has been well evidenced that HIU induces the formation of volatile compounds due to the oxidation of lipids. A complex mixture of secondary oxidation products such as volatile, non-volatile and polymeric compounds are formed through the decomposition reaction of hydroperoxides (Fig. 7). Only the secondary oxidation products are responsible for the undesirable changes in the aroma and flavour properties of foods [224]. This flavours are due to the formation of carbonyls and methional formed via photoxidative degradation of sulfur containing amino acids including methionine. Riboflavin, which is present in substantial amounts in milk, plays an important role as a photosensitizer in this process [224].
Fig. 7.
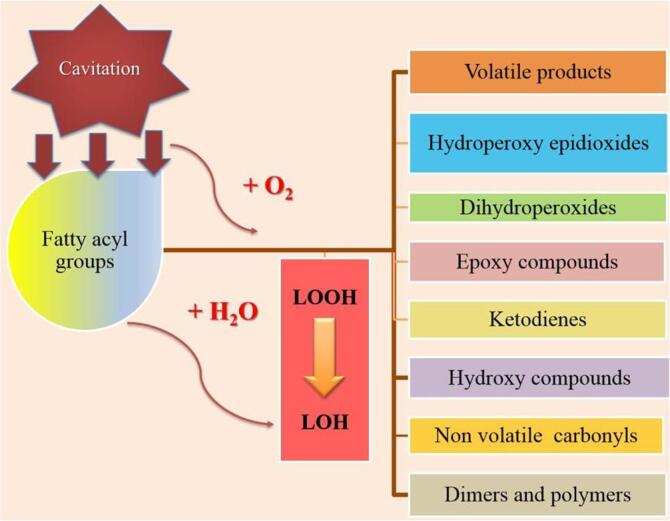
Types of compounds formed from hydroperoxides decomposition (Modified from [223]).
Ultrasound-induced cavitation produces free radicals and other reactive species that are susceptible to promoting oxidation of fat [101], induced by redox reactions in the medium [8]. In sonicated milk, such oxidation of lipids could induce the generation of volatile compounds, resulting in rapid deterioration of milk quality. As flavor profile is a function of the volatile components present in milk, ultrasonication could affect the flavor profile of milk and dairy products as mentioned in the section 7.1.
Studies of volatile formation in dairy products after ultrasound application are shown in Table 8. Chouliara et al. [187] applied HIU to milk and found an increase in volatile compounds (pentanal, hexanal, heptanal, and octanal) that were mainly products of lipid oxidation. These increased in concentration as sonication and storage time increased.
Table 8.
Volatile components and acid in dairy products assessed after high-intensity ultrasound.
| Sample | Experimental parameters | Effect of ultrasound | Reference |
|---|---|---|---|
| Cheese aroma | Sonifier (Bioblock Scientific model) at 20 kHz with a maximal power output of 600 W for 5 to 20 min. | In terms of flavor quality, the best system of cheese aroma encapsulation is obtained using ultrasound and maltodextrin as support. | [225] |
| Milk | Ultrasonic processor (UP 400S, Hielscher, Germany), at 24 kHz and 400 W for 2.5, 5, 10, 15 and 20 min with a continuous application at 45 °C. | Ultrasound generated volatiles considered to produce adverse sensory aspects of sonication of milk. | [214] |
| Raw, thermized, and pasteurized milk | Ultrasonic processor (24 kHz, 200 W, model UP 2005, IKA, U.K.) at 24 kHz for 0, 2, 4, 8, and 16 min at 15–25 °C. | Volatile compounds (pentanal, hexanal, heptanal, and octanal) identified in all samples were mainly products of lipid oxidation that increased in concentration with sonication and storage time. | [187] |
| Milk | Ultrasonic processor (UP400S, Hielscher Inc., USA) at 400 W and 24 kHz equipped with a 22 mm diameter horn. Samples were sonicated for for 50, 100, 200, and 300 s. | Significant increases in free fatty acid levels and oxidation in sonicated milk. | [102] |
| Milk | Transducers (Sonosys Ultraschallsysteme GmbH, Germany) of nominal frequency 1 MHz and 2 MHz applied in single- and multi-stage processing. 20, 400, 1000, 1600, and 2000 kHz; 4, 20, 45, and 63 °C. | At extended processing of 20 min using 400 and 1000 kHz ultrasound at elevated temperatures, it promoted the derivation of oxidative volatiles above the human sensory threshold. | [203] |
| Whey | 20 kHz sonotrode (Branson Digital Sonifier, Branson Ultrasonic Corporation, USA). Rectangular plate transducers at 400, 1000, and 2000 kHz and 8–390 kJ/kg | No changes in phospholipid composition or in lipid oxidation beyond the detectable odor thresholds for volatile compounds at any tested frequency. | [94] |
| Milk | 400-W ultrasonic processor (UP400S, Hielscher Inc., USA) for 50, 100, 200, and 300 s. The average acoustic power was 160.4 J s−1. | Possible markers (dodecanoic acid, octanoic acid, δ-dodecalactone, and decanoic acid methyl ester) of milk sensory degradation caused by US treatments. | [192] |
| Milk | A rectangular ultrasound reactor operating at 400 and 1,000 kHz (Sonosys Ultraschallsysteme GmbH, Germany) at 23–390 kJ/kg (70 W, 50 and 100%) and 37 °C. | Low-temperature sonication of milk better controlled the formation of oxidative volatile compounds below threshold concentrations. | [86] |
| Milk | A stainless steel vessel fitted with 100 mm × 100 mm plate transducers (Sonosys, Germany) at 1 MHz, 348 W; and 2 MHz, 280 W. | Lipid oxidation volatiles were below sensory detection levels, with no oxidation observed. | [227] |
| Yogurt | Ultrasound system (VC750Vibracell©; Sonics and Materials Inc, USA). Sample was sonicated at 20 kHz and 150, 262, 375, 562, or 750 W (corresponding to 20%, 35%, 50%, 75 or 100% of the processor’s total power) for 10 min. | Ultrasonication of milk led to yogurts with increased concentration of ketones, aldehydes, hydrocarbons, and dimethylsulfide. | [207] |
| Yogurt | Sonopuls Ultrasonic Homogenizer (model no. HD2200, Bandelin Company, Germany) at 20, 40, and 60 kHz for 20 min at an intensity of 80%. | Ultrasonication of milk led to yogurts with increased concentration of ketones, aldehydes, hydrocarbons, and dimethylsulfide. | [42] |
| Milkfat | Flow cell (Sonolab SL10 ultrasonic, Syrris Ltd, UK) at 40 W and 20 kHz in recycling mode with a flow rate of 200 mL/min at 28 °C and a total treatment volume of 500 mL. | Ultrasound had no effect on secondary oxidation products (heptanal and nonanal), suggesting no lipid oxidation promotion. | [25] |
| Chocolate milk beverage | An ultrasonic device (Disruptor, 800 W, Indaiatuba, Brazil) at 400 W of nominal power and energy densities of 0.3 kJ/cm3; 0.9 kJ/cm3; 1.8 kJ/cm3; 2.4 kJ/cm3, and 3.0 kJ/cm3. | Ultrasound allowed better preservation of volatile compounds compared with high-temperature short-time pasteurization. | [27] |
| Camel milk | Ultrasonic processor (Ultrasonic Processor FS-900N, Hanchen Instrument, China), set at 900 and 20 kHz. | Formation of volatile compounds in milk occurred, probably due to fatty acid oxidation, consistent with the observed reductions in some fatty acids (C18:1 trans, C18:1c9, C20:1n9, and C22:6n3). | [228] |
Riener et al. [101] and Chouliara et al. [187] reported comparable changes generated with a frequency of 24 kHz, showing that ultrasonicated pasteurized milk generates compounds that attribute a “rubbery” odor and a “burnt” and “strange” taste. However, Vercet et al. [211] previously pointed out that this offensive “cooked” flavor, which was distinguished during milk manothermosonization, was not detectable when milk was fermented in yogurts. This could be due to masking of the “cooked” flavor by flavor compounds generated through fermentation. Bermúdez-Aguirre et al. [35] also reported changes in ultrasonicated milk compounds. These ultrasound effects gained importance due to the impact of volatiles on dairy products’ acceptability.
Mongenot et al. [225] used ultrasound for emulsification of cheese aroma and found that it resulted in an unbalanced aroma in flavour of butyric acid. Their study of aroma profile showed that it was necessary to consider each fatty acid to estimate the efficiency of carriers and emulsification methods. Modification of milk compounds by HIU is mainly related to protein modifications. Villamiel and de Jong [19] observed increased denaturation of both α- lactalbumin and β- lactoglobulin in sonicated milk. Denaturation of these proteins resulted in exposure of the free sulfhydryl groups considered responsible for the “cooked” character of milk. Marchesini et al. [102] sonicated raw milk for cheese production and observed significant increases in free fatty acid levels and oxidation. The results of their sensory evaluations revealed a significant increase in a burnt off-flavor as intensity and duration of ultrasonication increased. They recommended using CO2 as a technical step to reduce pyrolytic processes and the consequent formation of oxidation products. In another study, Marchesini et al. [192] observed the production of volatile compounds dodecanoic acid, octanoic acid, δ-dodecalactone, and decanoic acid methyl ester in ultrasonicated milk (24 kHz, 160.4 J/s, 100 s). They suggested that these compounds were associated with the presence of metallic, burned, and sharp flavors and gummy textures in milk.
A study by Mortazavi and Tabatabai [226] showed that 20-min pulsed ultrasonication of ice creams resulted in the best sensory flavor, texture, and mouthfeel evaluations. Similarly, Chandrapala and Leong [196] emphasized that one possible concern when using HIU to separate milkfat is the potential for fat oxidation (i.e., lipolysis). They suggested that greater efforts should be made to limit excessive fat oxidation leading to rancid off-flavors in the final product.
Torkamani et al. [94] examined the effects of ultrasonication on the oxidation of lipids in cheddar cheese whey. The highest concentration of hydroxyl radical formation was in the sonicated whey (400 and 1000 kHz). No changes in phospholipid composition or lipid oxidation at any tested frequency or specific energy were detected. In another study, Torkamani et al. [86] reported the effect of ultrasound-enhanced fat separation on whey powder phospholipid composition and stability. Oxidative volatile compound content decreased in defatted whey powders below published odor detection threshold values for all cases. HIU enhanced fat separation from freshly pasteurized whey while improving whey powder oxidative stability. Contrarily, Johansson et al. [227] demonstrated that ultrasonication did not influence oxidative changes in milk. They showed that ultrasound operation under stable cavitating conditions (beyond 1 MHz) avoids exposure to cavitation-generated radicals that oxidize fat and eliminated the generation of off-flavors. Volatiles derived from lipid oxidation were below the sensorial human detection levels.
Sfakianakis and Tzia [207] reported that the volatile components present in yogurt made from conventionally homogenized milk resulted in the presence of off-flavors and led to a reduction of likeness of yogurt. Burned, pungent, or fatty characteristics may be the main characteristics responsible for rejection. Ultrasonication of milk led to an increase in ketones, aldehydes, hydrocarbons, and dimethylsulfide concentrations in yogurts. The authors proposed two statistical models to describe the degree of likeness by sensory characteristics or volatile components. In a recent study, Jalilzadeh et al. [42] reported that ultrasound accelerated lipolysis and proteolysis in Iranian ultrafiltered feta-type cheese; the highest rates of lipolysis and proteolysis were observed on day 60 of ripening for samples sonicated at 60 kHz. An interesting finding in this study was that ultrasound treatment improved the organoleptic properties of the cheese. The results showed that sonication can improve microbial, physicochemical, and sensorial properties of ultrafiltered white cheese. Therefore, ultrasound could also add sensory value to dairy products. Gregersen et al. [26] demonstrated that HIU avoids the formation of secondary oxidation products (heptanal and nonanal), in milk fat and rapeseed oil blends and emphasized that not only oxidation products can cause lipid-derived off-flavors. The heat and pressure generated locally by HIU may also result in self-accumulated volatiles that affect product quality. However, the use of ultrasonication processing by a large number of small transducers might be a gentler process, limiting lipid oxidation.
HIU as a non-thermal method for pasteurization of chocolate milk beverages also proved more effective than conventional pasteurization by HTST. Monteiro et al. [27] reported that the treatments subjected to the highest energy densities (2.4 kJ/cm and 3.0 kJ/cm) allowed for the identification of a higher number of volatile compounds. In particular, the compounds 8-nonen-2-one and octanoic acid were identified in all HIU treatments, which was not observed in the HTST-treated samples, whereas hydroxyacetone, dihydroxyacetone, 2-hydroxy-gamma-butyrolactone, 2-cyclopentene-1-one, 2-hydroxy-, and furfural were identified only after treatment with an energy density of 2.4 kJ/cm. These results suggest that heat degrades the majority of volatile compounds, while ultrasound better preserves these compounds in chocolate milk beverages than does HTST pasteurization. Recently, Dhahir et al. [228] investigated the inactivation of pathogenic bacteria in camel milk using ultrasound processing (900 W, 20 kHz, 100% power level). In their study, milk temperature was kept low (20 ± 3 °C) during ultrasound treatment and therefore any increase in volatile compounds would be attributed to the sonication reactions. The mechanisms by which sonication increases the formation of volatile compounds in milk are probably due to fatty acid oxidation. This is consistent with the observed reductions in some fatty acids (C18:1 trans, C18:1c9, C20:1n9, and C22:6n3).
At frequencies between 10 and 20 kHz, the oxidation of lipids in raw milk and dairy products remains below sensory detection levels [227], [25] and better preservation of the compounds is obtained than those generated by the conventional process [27]. The significant increase in the levels of free fatty acids and oxidation of raw milk by ultrasound effect can be observed at a frequency above 20 kHz with appearance of undesirable volatile compounds [187], [170], [214].
8. Summary and Future perspectives
It has been shown with extensive examples that the physicochemical and functional properties of milk and dairy products can be improved by the application of HIU. Acoustic cavitation-generated physical processes reduce the particle size and distribution of milk components, modify protein (casein, lactalbumin, and lactoglobulin) structures, and enhance enzyme activity, which lead to textural and shelf-life advantages benefiting both consumers and producers. HIU is a potential technology for adoption in the dairy industry, at least from the point of view of physicochemical and functional properties of dairy foods. Few disadvantages are observed in HIU application, except for some color variations and slight protein oxidation. Additionally, ultrasonication of milk can lead to products with lower degrees of likeness and off-flavors. More studies are needed that focus on proteolysis, lipolysis and fatty acid oxidation in order to identify the sensory characteristics with the greatest impact on quality. Ultrasonication alone seems to be ineffective for decontamination of milk, but efficient control of bacteria can be achieved by using ultrasound in combination with heat. Many studies have focused on the effects of ultrasound on whey protein. Structural changes in serum proteins induce susceptibility to enzymatic hydrolysis, facilitating the production of bioactive peptides and improving functional, rheological, and textural properties. A few studies of casein micelles show that ultrasound can alter the micelles and the distribution of P and Ca between the micellar and serum phases, improving gelation and syneresis. This could be an advantage in the cheese industry.
Ultrasound reduces the size of the fat globule, providing an alternative to traditional homogenization. The fat-protein interaction improves stability in cream and emulsions. As for lactose and enzymes, some studies have elucidated advantages in lactose crystallization and decreased activity of plasmin, lactoperoxidase, and alkaline phosphatase. Future research should examine the effects of ultrasound as a green technology in milk and genuine dairy products in order to scale this technology to the industry. Viscosity and elastic properties, which depend on the microstructure of the food, are affected by the application of ultrasound. Some of these changes may be desirable in some products, but undesirable in others. Ultrasound offers an excellent opportunity to manipulate the microstructure of dairy products because it makes it possible to enhance textures and obtain new textures that improve the quality of existing or new products. This represents a wide opportunity to continue evaluating the effect of the different conditions of the US process because the mechanisms by which the microstructure of dairy products is modified are unclear and in some cases contradictory. On the other hand, it is necessary to perform further studies of sensory evaluation to correlate the microstructural changes with the perception of the consumer. The effects of ultrasound on beneficial microorganisms used for fermentation applications have been presented. Up to now, the research has focused on the practical applications of US in the dairy industry rather than on the mechanisms of its effects on bacteria cells used for fermented dairy products. Ultrasonic processing of milk or dairy products has demonstrated efficient preservation or enhanced diverse functional properties, as well as improved process efficiency to develop value-added products. However, deeper knowledge is needed regarding the use of ultrasound as a tool for bioactive component production and enzymatic hydrolysis to increase the quantity and quality of functional dairy products. Specifically, more research is warranted to understand the interaction between ultrasonication parameters and sensory attributes of the final product. Finally, ultrasound’s effects on probiotics’ role in prolonging shelf life, health-promoting effects, and production for global consumers should also be considered.
Although ultrasound treatment alone has been efficient for controlling groups of microorganisms (mesophilic bacteria, psychrophilic bacteria, and total coliforms), the combination of heat and ultrasound is more appropriate for the control of pathogenic and spoilage bacteria. However, more studies are needed to evaluate the behaviour of microorganisms during cold storage and maturation (mainly cheeses). Future studies should focus on the use of other emerging technologies that help counteract the negative effects of heat application in milk and its products, particularly on the sensory qualities and nutritional value of foods. Despite the large number of publications regarding HIU treatment’s effects on quality properties of dairy products, only a few studies have assessed the effects of HIU on sensory characteristics. These studies mention that HIU preserves the sensory properties and quality of the final dairy product without causing the common side effects associated with conventional heat treatments. Ultrasonicated milk develops off-flavors and lipid oxidation, but these undesirable off-flavors seem to diminish with time after ultrasonication. Further research that focuses on sensory characterization of the product produced with ultrasonic application is needed to determine the ideal parameters for product quality. A balance between microbial, enzymatic, and sensory effects must be found before ultrasound technology is widely applied in the dairy industry. HIU induces chemical changes that, under certain conditions, could generate volatile compounds associated with modifications in flavor and general acceptability of milk and dairy products. These undesired reactions can be minimized by employing ultrasound at frequencies lower than 50 kHz where the number of free radicals formed is negligible. From the studies discussed in this review, it can be concluded that 20 kHz is an optimum frequency to obtain favourable changes in milk and dairy properties.
CRediT authorship contribution statement
Luis M. Carrillo-Lopez: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Visualization. Ivan A. Garcia-Galicia: Investigation, Writing - original draft, Writing - review & editing. Juan M. Tirado-Gallegos: Investigation, Writing - original draft, Writing - review & editing. Rogelio Sanchez-Vega: Investigation, Writing - original draft, Writing - review & editing. Mariana Huerta-Jimenez: Investigation, Writing - original draft, Writing - review & editing, Visualization. Muthupandian Ashokkumar: Writing - review & editing, Visualization. Alma D. Alarcon-Rojo: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Mariana Huerta-Jimenez, Email: mhuertaj@uach.mx.
Alma D. Alarcon-Rojo, Email: aalarcon@uach.mx.
References
- 1.Mason T.J., Paniwnyk L., Lorimer J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996;3 doi: 10.1016/S1350-4177(96)00034-X. [DOI] [Google Scholar]
- 2.Dolatowski Z.J., Stadnik J., Stasiak D. Applications of ultrasound in food technology, Acta Scientiarum Polonorum Technologia. Alimentaria. 2007;6:89–99. Available https://www.food.actapol.net/volume6/issue/8_3_2007.pdf. [Google Scholar]
- 3.B.K. Tiwari, T.J. Mason, Ultrasound Processing of Fluid Foods, in: Nov. Therm. Non-Thermal Technol. Fluid Foods, Elsevier Inc., 2012: pp. 135–165. 10.1016/B978-0-12-381470-8.00006-2.
- 4.BlakePerutz J.R., Suslick K.S., Didenko Y., Fang M.M., Hyeon T., Kolbeck K.J., McNamara W.B., III, Mdleleni M.M., Wong M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. London, Ser. A. 1999;357(1751):335–353. [Google Scholar]
- 5.B.G. Pollet, M. Ashokkumar, Fundamental and Applied Aspects of Ultrasonics and Sonochemistry, in: B.G. Pollet, M. Ashokkumar (Eds), Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry. SpringerBriefs in Molecular Science, pp. 1-20. (2019) htpps://doi.org/ 10.1007/978-3-030-25862-7.
- 6.M. Ashokkumar, T. Mason, Sonochemistry, in: A. Seidel, M. Bickford (Eds), Kirk–Othmer Encyclopedia of Chemical Technology, John Wiley and Sons, 2007, pp. 1–34. (2007) 10.1002/0471238961.1915141519211912.a01.pub2.
- 7.Suslick K.S., Hammerton D.A., Cline R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986;108:5641–5642. [Google Scholar]
- 8.RIESZ P., KONDO T. Free radical formation induced by ultrasound and its biological implications. Free Radical Biol. Med. 1992;13(3):247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 9.Henglein A. Chemical effects of continuous and pulsed ultrasound in aqueous solutions. Ultrason. Sonochem. 1995;2(2):S115–S121. [Google Scholar]
- 10.Ashokkumar M. Introductory text to sonochemistry. ChemTexts. 2018;4:1–9. doi: 10.1007/s40828-018-0061-4. [DOI] [Google Scholar]
- 11.Bhangu S.K., Ashokkumar M. Theory of Sonochemistry. Top Curr Chem (Z) 2016;374(4) doi: 10.1007/s41061-016-0054-y. [DOI] [PubMed] [Google Scholar]
- 12.Kehinde B.A., Sharma P., Kaur S. Recent nano-, micro- and macrotechnological applications of ultrasonication in food-based systems. Crit. Rev. Food Sci. Nutr. 2020:1–23. doi: 10.1080/10408398.2020.1740646. [DOI] [PubMed] [Google Scholar]
- 13.Ashokkumar M., Bhaskaracharya R., Kentish S., Lee J., Palmer M., Zisu B. The ultrasonic processing of dairy products – An overview. Dairy Sci. Technol. 2010;90(2-3):147–168. [Google Scholar]
- 14.Perez-Grijalva, J.C. Garcia-Zebada, V.M. Ruiz-Perez, D.I. Tellez-Medina, R.I. Guzman-Geronimo, R. Mora-Escobedo, Biofunctionality, colorimetric coefficients and microbiological stability of blackberry (Rubus fructicosus var. Himalaya) juice under microwave/ ultrasound processing, Rev. Mexic. Ingeniería Química, 17 (2017) 13-28. 10.24275/10.24275/uam/izt/dcbi/revmexingquim/2018v17n1/Perez.
- 15.Jiang B.o., Mantri N., Hu Y.a., Lu J., Jiang W.u., Lu H. Evaluation of bioactive compounds of black mulberry juice after thermal, microwave, ultrasonic processing, and storage at different temperatures. Food Sci. Technol. Int. 2015;21(5):392–399. doi: 10.1177/1082013214539153. [DOI] [PubMed] [Google Scholar]
- 16.Knorr D., Zenker M., Heinz V., Lee D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004;15(5):261–266. [Google Scholar]
- 17.Patist D. Bates, Ultrasonics innovations in the food industry: from the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008;9:147–154. doi: 10.1016/j.ifset.2007.07.004. [DOI] [Google Scholar]
- 18.Balthazar C.F., Santillo A., Guimarães J.T., Bevilacqua A., Corbo M.R., Caroprese M., Marino R., Esmerino E.A., Silva M.C., Raices R.S.L., Freitas M.Q., Cruz A.G., Albenzio M. Ultrasound processing of fresh and frozen semi-skimmed sheep milk and its effects on microbiological and physical-chemical quality. Ultrason. Sonochem. 2019;51:241–248. doi: 10.1016/j.ultsonch.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Villamiel M., de Jong P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000;48(2):472–478. doi: 10.1021/jf990181s. [DOI] [PubMed] [Google Scholar]
- 20.I. Barukčić, K. Lisak Jakopović, Z. Herceg, S. Karlović, R. Božanić, M., Influence of high intensity ultrasound on microbial reduction, physico-chemical characteristics and fermentation of sweet whey, Innov. Food Sci. Emerg. Technol. 27 (2015) 94–101. 10.1016/j.ifset.2014.10.013.
- 21.Jeličić I., Božanić R., Brnčić M., Tripalo B. Influence and comparison of thermal, ultrasonic and thermo-sonic treatments on microbiological quality and sensory properties of rennet cheese whey. Mljekarstvo. 2012;62:165–178. [Google Scholar]
- 22.Soria A.C., Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 2010;21(7):323–331. [Google Scholar]
- 23.M.J. Lewis, Physical Properties of Dairy Products, in: R.K. Robinson (Ed.), Mod. Dairy Technol., Springer US, Boston, MA., 1993: pp. 331–380. 10.1007/978-1-4684-8172-3_5.
- 24.Abesinghe A.M.N.L., Vidanarachchi J.K., Islam N., Prakash S., Silva K.F.S.T., Bhandari B., Karim M.A. Effects of ultrasonication on the physicochemical properties of milk fat globules of Bubalus bubalis (water buffalo) under processing conditions: a comparison with shear-homogenization. Innov. Food Sci. Emerg. Technol. 2020;59:102237. doi: 10.1016/j.ifset.2019.102237. [DOI] [Google Scholar]
- 25.Gregersen S.B., Wiking L., Hammershøj M. Acceleration of acid gel formation by high intensity ultrasound is linked to whey protein denaturation and formation of functional milk fat globule-protein complexes. J. Food Eng. 2019;254:17–24. [Google Scholar]
- 26.Gregersen S.B., Frydenberg R.P., Hammershøj M., Dalsgaard T.K., Andersen U., Wiking L. Application of high intensity ultrasound to accelerate crystallization of anhydrous milk fat and rapeseed oil blends. Eur. J. Lipid Sci. Technol. 2019;121 doi: 10.1002/ejlt.201800200. [DOI] [Google Scholar]
- 27.Monteiro S.H.M.C., Silva E.K., Alvarenga V.O., Moraes J., Freitas M.Q., Silva M.C., Raices R.S.L., Sant'Ana A.S., Meireles M.A.A., Cruz A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochem. 2018;42:1–10. doi: 10.1016/j.ultsonch.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Van Hekken D.L., Renye J., Jr., Bucci A.J., Tomasula P.M. Characterization of the physical, microbiological, and chemical properties of sonicated raw bovine milk. J. Dairy Sci. 2019;102(8):6928–6942. doi: 10.3168/jds.2018-15775. [DOI] [PubMed] [Google Scholar]
- 29.Hernández-Falcón T.A., Monter-Arciniega A., Cruz-Cansino N.del.S., Alanís-García E., Rodríguez-Serrano G.M., Castañeda-Ovando A., García-Garibay M., Ramírez-Moreno E., Jaimez-Ordaz J. Effect of thermoultrasound on aflatoxin M1 levels, physicochemical and microbiological properties of milk during storage. Ultrason. Sonochem. 2018;48:396–403. doi: 10.1016/j.ultsonch.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Jo Y.J., Choi M.J., Chun J.Y., Yeon-Ji J., Mi-Jung C., Ji-Yeon C. Effect of high-energy emulsification on properties of commercial low-temperature pasteurised milk. Int. J. Dairy Technol. 2019;72:357–363. doi: 10.1111/1471-0307.12596. [DOI] [Google Scholar]
- 31.Monteiro S.H.M.C., Silva E.K., Guimarães J.T., Freitas M.Q., Meireles M.A.A., Cruz A.G. High-intensity ultrasound energy density: how different modes of application influence the quality parameters of a dairy beverage. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104928. [DOI] [PubMed] [Google Scholar]
- 32.Freitas S., Hielscher G., Merkle H.P., Gander B. Continuous contact- and contamination-free ultrasonic emulsification—a useful tool for pharmaceutical development and production. Ultrason. Sonochem. 2006;13(1):76–85. doi: 10.1016/j.ultsonch.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.J.P. Canselier, H. Delmas, A.M. Wilhelm, B. Abismaïl, Ultrasound Emulsification—An Overview, J. Dispers. Sci. Technol. 23 (2002) 333–349. 10.1080/01932690208984209.
- 34.Leong T., Johansson L., Mawson R., McArthur S.L., Manasseh R., Juliano P. Ultrasonically enhanced fractionation of milk fat in a litre-scale prototype vessel. Ultrason. Sonochem. 2016;28:118–129. doi: 10.1016/j.ultsonch.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 35.D. Bermúdez-Aguirre, R. Mawson, G. V. Barbosa-Cánovas, Microstructure of fat globules in whole milk after thermosonication treatment, J. Food Sci. 73 (2008) 325–332. 10.1111/j.1750-3841.2008.00875.x. [DOI] [PubMed]
- 36.Kaci M., Meziani S., Arab-Tehrany E., Gillet G., Desjardins-Lavisse I., Desobry S. Emulsification by high frequency ultrasound using piezoelectric transducer: Formation and stability of emulsifier free emulsion. Ultrason. Sonochem. 2014;21(3):1010–1017. doi: 10.1016/j.ultsonch.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Lee J., Martini S. Modifying the physical properties of butter using high-intensity ultrasound. J. Dairy Sci. 2019;102(3):1918–1926. doi: 10.3168/jds.2018-15075. [DOI] [PubMed] [Google Scholar]
- 38.Nozière P., Grolier P., Durand D., Ferlay A., Pradel P., Martin B. Variations in carotenoids, fat-soluble micronutrients, and color in cows’ plasma and milk following changes in forage and feeding level. J. Dairy Sci. 2006;89(7):2634–2648. doi: 10.3168/jds.S0022-0302(06)72340-2. [DOI] [PubMed] [Google Scholar]
- 39.Havemose M.S., Weisbjerg M.R., Bredie W.L.P., Nielsen J.H. Influence of feeding different types of roughage on the oxidative stability of milk. Int. Dairy J. 2004;14(7):563–570. [Google Scholar]
- 40.Nozière P., Graulet B., Lucas A., Martin B., Grolier P., Doreau M. Carotenoids for ruminants: from forages to dairy products. Anim. Feed Sci. Technol. 2006;131(3-4):418–450. [Google Scholar]
- 41.Carrillo-Lopez L.M.L.M., Juarez-Morales M.G.M.G., Garcia-Galicia I.A., Alarcon-Rojo A.D.A.D., Huerta-Jimenez M. The effect of high-intensity ultrasound on the physicochemical and microbiological properties of Mexican panela cheese. Foods. 2020;9:1–14. doi: 10.3390/foods9030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jalilzadeh A., Hesari J., Peighambardoust S.H., Javidipour I. The effect of ultrasound treatment on microbial and physicochemical properties of Iranian ultrafiltered feta-type cheese. J. Dairy Sci. 2018;101(7):5809–5820. doi: 10.3168/jds.2017-14352. [DOI] [PubMed] [Google Scholar]
- 43.Amiri A., Mousakhani-Ganjeh A., Torbati S., Ghaffarinejhad G., Esmaeilzadeh Kenari R. Impact of high-intensity ultrasound duration and intensity on the structural properties of whipped cream. Int. Dairy J. 2018;78:152–158. [Google Scholar]
- 44.Abesinghe A.M.N.L., Islam N., Vidanarachchi J.K., Prakash S., Silva K.F.S.T., Karim M.A. Effects of ultrasound on the fermentation profile of fermented milk products incorporated with lactic acid bacteria. Int. Dairy J. 2019;90:1–14. [Google Scholar]
- 45.Akdeniz V., Akalın A.S. New approach for yoghurt and ice cream production: high-intensity ultrasound. Trends Food Sci. Technol. 2019;86:392–398. [Google Scholar]
- 46.Khatkar A.B., Kaur A., Khatkar S.K., Mehta N. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 2018;49:333–342. doi: 10.1016/j.ultsonch.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Y. Cheng, P.O. Donkor, X. Ren, J. Wu, K. Agyemang, I. Ayim, H. Ma, Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels, Food Hydrocoll. 89 (2019) 434–442. 10.1016/j.foodhyd.2018.11.007.
- 48.Guimarães J.T., Silva E.K., Alvarenga V.O., Costa A.L.R., Cunha R.L., Sant'Ana A.S., Freitas M.Q., Meireles M.A.A., Cruz A.G. Physicochemical changes and microbial inactivation after high-intensity ultrasound processing of prebiotic whey beverage applying different ultrasonic power levels. Ultrason. Sonochem. 2018;44:251–260. doi: 10.1016/j.ultsonch.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Hamdy A.M., Mohran M.A., Hassan A.I., Fahmy M.A. Effects of heat, ultrasound and microwave pretreatments on the antigenicity of whey protein concentrate (β-lactoglobulin), Assiut. J. Agric. Sci. 2018;49:75–87. doi: 10.21608/ajas.2018.28370. [DOI] [Google Scholar]
- 50.Wu Q., Zhang X., Jia J., Kuang C., Yang H. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: thermodynamic parameters, physicochemical properties and bioactivities. Process Biochem. 2018;67:46–54. doi: 10.1016/j.procbio.2018.02.007. [DOI] [Google Scholar]
- 51.Jiang Z., Wang C., Li T., Sun D., Gao H., Gao Z., Mu Z. Effect of ultrasound on the structure and functional properties of transglutaminase-crosslinked whey protein isolate exposed to prior heat treatment. Int. Dairy J. 2019;88:79–88. [Google Scholar]
- 52.Körzendörfer A., Hinrichs J. Manufacture of high-protein yogurt without generating acid whey – impact of the final pH and the application of power ultrasound on texture properties. Int. Dairy J. 2019;99 doi: 10.1016/j.idairyj.2019.104541. [DOI] [Google Scholar]
- 53.Shanmugam A., Chandrapala J., Ashokkumar M. The effect of ultrasound on the physical and functional properties of skim milk. Innovative Food Sci. Emerg. Technol. 2012;16:251–258. [Google Scholar]
- 54.Abadía-García L., Castaño-Tostado E., Ozimek L., Romero-Gómez S., Ozuna C., Amaya-Llano S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innovative Food Sci. Emerg. Technol. 2016;37:84–90. [Google Scholar]
- 55.Uluko H., Liu L., Li H., Cui W., Zhang S., Zhao L., Xue H., Lv J. Effect of power ultrasound pretreatment on peptidic profiles and angiotensin converting enzyme inhibition of milk protein concentrate hydrolysates: improving milk protein concentrate hydrolysates functionality using ultrasound. J. Sci. Food Agric. 2014;94(12):2420–2428. doi: 10.1002/jsfa.6572. [DOI] [PubMed] [Google Scholar]
- 56.Ahmadi Z., Razavi S.M.A., Varidi M. Sequential ultrasound and transglutaminase treatments improve functional, rheological, and textural properties of whey protein concentrate. Innovative Food Sci. Emerg. Technol. 2017;43:207–215. [Google Scholar]
- 57.Arzeni C., Martínez K., Zema P., Arias A., Pérez O.E., Pilosof A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012;108(3):463–472. [Google Scholar]
- 58.Chandrapala J., Zisu B., Palmer M., Kentish S., Ashokkumar M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011;18(5):951–957. doi: 10.1016/j.ultsonch.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Chandrapala J., Zisu B., Kentish S., Ashokkumar M. The effects of high-intensity ultrasound on the structural and functional properties of α-Lactalbumin, β-Lactoglobulin and their mixtures. Food Res. Int. 2012;48(2):940–943. [Google Scholar]
- 60.Frydenberg R.P., Hammershøj M., Andersen U., Wiking L. High intensity ultrasound effects on heat-induced whey protein isolate gels depend on α-lactalbumin: β-lactoglobulin ratio. Int. Dairy J. 2016;56:1–3. [Google Scholar]
- 61.Jiang Z., Yao K., Yuan X., Mu Z., Gao Z., Hou J., Jiang L. Effects of ultrasound treatment on physico-chemical, functional properties and antioxidant activity of whey protein isolate in the presence of calcium lactate: Effect of ultrasound on WPI. J. Sci. Food Agric. 2018;98(4):1522–1529. doi: 10.1002/jsfa.8623. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen N.H.A., Anema S.G. Ultrasonication of reconstituted whole milk and its effect on acid gelation. Food Chem. 2017;217:593–601. doi: 10.1016/j.foodchem.2016.08.117. [DOI] [PubMed] [Google Scholar]
- 63.X. Shen, C. Zhao, M. Guo, Effects of high intensity ultrasound on acid-induced gelation properties of whey protein gel, Ultrason. Sonochem. 39 (2017) 810–815. 10.1016/j.ultsonch.2017.05.039. [DOI] [PubMed]
- 64.Yanjun S., Jianhang C., Shuwen Z., Hongjuan L., Jing L., Lu L., Uluko H., Yanling S., Wenming C., Wupen G., Jiaping L. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014;124:11–18. doi: 10.1016/j.jfoodeng.2013.09.013. [DOI] [Google Scholar]
- 65.Zisu B., Lee J., Chandrapala J., Bhaskaracharya R., Palmer M., Kentish S., Ashokkumar M. Effect of ultrasound on the physical and functional properties of reconstituted whey protein powders. J. Dairy Res. 2011;78(2):226–232. doi: 10.1017/S0022029911000070. [DOI] [PubMed] [Google Scholar]
- 66.Stanic-Vucinic D., Prodic I., Apostolovic D., Nikolic M., Cirkovic Velickovic T. Structure and antioxidant activity of β-lactoglobulin-glycoconjugates obtained by high-intensity-ultrasound-induced Maillard reaction in aqueous model systems under neutral conditions. Food Chem. 2013;138(1):590–599. doi: 10.1016/j.foodchem.2012.10.087. [DOI] [PubMed] [Google Scholar]
- 67.Tammineedi C.V.R.K., Choudhary R., Perez-Alvarado G.C., Watson D.G. Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT – Food Sci. Technol. 2013;54(1):35–41. [Google Scholar]
- 68.X. Li, Z. Li, H. Lin, H. Samee, Effect of power ultrasound on the immunoactivity and texture changes of shrimp (Penaeus vannamei), Czech J. Food Sci. 29 (2011) 508–514. 10.17221/242/2009-cjfs.
- 69.Mirmoghtadaie L., Shojaee Aliabadi S., Hosseini S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016;199:619–627. doi: 10.1016/j.foodchem.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 70.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. [Google Scholar]
- 71.Stanic-Vucinic D., Stojadinovic M., Atanaskovic-Markovic M., Ognjenovic J., Grönlund H., van Hage M., Lantto R., Sancho A.I., Velickovic T.C. Structural changes and allergenic properties of β-lactoglobulin upon exposure to high-intensity ultrasound. Mol. Nutr. Food Res. 2012;56(12):1894–1905. doi: 10.1002/mnfr.201200179. [DOI] [PubMed] [Google Scholar]
- 72.Nunes L., Tavares G.M. Thermal treatments and emerging technologies: Impacts on the structure and techno-functional properties of milk proteins. Trends Food Sci. Technol. 2019;90:88–99. doi: 10.1016/j.tifs.2019.06.004. [DOI] [Google Scholar]
- 73.Chandrapala J., Zisu B., Kentish S., Ashokkumar M. Influence of ultrasound on chemically induced gelation of micellar casein systems. J. Dairy Res. 2013;80(2):138–143. doi: 10.1017/S0022029912000696. [DOI] [PubMed] [Google Scholar]
- 74.Chandrapala J., Bui D., Kentish S., Ashokkumar M. Heat stability and acid gelation properties of calcium-enriched reconstituted skim milk affected by ultrasonication. J. Dairy Res. 2014;81(2):238–244. doi: 10.1017/S0022029914000132. [DOI] [PubMed] [Google Scholar]
- 75.Ghasemi S., Abbasi S. Formation of natural casein micelle nanocapsule by means of pH changes and ultrasound. Food Hydrocolloids. 2014;42:42–47. [Google Scholar]
- 76.Sfakianakis P., Topakas E., Tzia C. Comparative study on high-intensity ultrasound and pressure milk homogenization: effect on the kinetics of yogurt fermentation process. Food Bioprocess Technol. 2014;8:548–557. doi: 10.1007/s11947-014-1412-9. [DOI] [Google Scholar]
- 77.Zhang R., Pang X., Lu J., Liu L., Zhang S., Lv J. Effect of high intensity ultrasound pretreatment on functional and structural properties of micellar casein concentrates. Ultrason. Sonochem. 2018;47:10–16. doi: 10.1016/j.ultsonch.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Chandrapala J., Martin G.J.O., Zisu B., Kentish S.E., Ashokkumar M. The effect of ultrasound on casein micelle integrity. J. Dairy Sci. 2012;95(12):6882–6890. doi: 10.3168/jds.2012-5318. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z., Juliano P., Williams R.PW., Niere J., Augustin M.A. Ultrasound effects on the assembly of casein micelles in reconstituted skim milk. J. Dairy Res. 2014;81(2):146–155. doi: 10.1017/S0022029913000721. [DOI] [PubMed] [Google Scholar]
- 80.Körzendörfer A., Temme P., Schlücker E., Hinrichs J., Nöbel S. Vibration-induced particle formation during yogurt fermentation—effect of frequency and amplitude. J. Dairy Sci. 2018;101(5):3866–3877. doi: 10.3168/jds.2017-13905. [DOI] [PubMed] [Google Scholar]
- 81.Bogahawaththa D., Buckow R., Chandrapala J., Vasiljevic T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov. Food Sci. Emerg. Technol. 2018;47:301–308. [Google Scholar]
- 82.Broyard C., Gaucheron F. Modifications of structures and functions of caseins: a scientific and technological challenge. Dairy Sci. Technol. 2015;95(6):831–862. [Google Scholar]
- 83.Dalgleish D.G., Corredig M. The Structure of the Casein Micelle of Milk and Its Changes During Processing. Annu. Rev. Food Sci. Technol. 2012;3(1):449–467. doi: 10.1146/annurev-food-022811-101214. [DOI] [PubMed] [Google Scholar]
- 84.Cameron M., McMaster L.D., Britz T.J. Impact of ultrasound on dairy spoilage microbes and milk components. Dairy Sci. Technol. 2009;89(1):83–98. [Google Scholar]
- 85.Vijayakumar S., Grewell D., Annandarajah C., Benner L., Clark S. Quality characteristics and plasmin activity of thermosonicated skim milk and cream. J. Dairy Sci. 2015;98(10):6678–6691. doi: 10.3168/jds.2015-9429. [DOI] [PubMed] [Google Scholar]
- 86.Torkamani A.E., Juliano P., Fagan P., Jiménez-Flores R., Ajlouni S., Singh T.K. Effect of ultrasound-enhanced fat separation on whey powder phospholipid composition and stability. J. Dairy Sci. 2016;99(6):4169–4177. doi: 10.3168/jds.2015-10422. [DOI] [PubMed] [Google Scholar]
- 87.Shanmugam A., Ashokkumar M. Functional properties of ultrasonically generated flaxseed oil-dairy emulsions. Ultrason. Sonochem. 2014;21(5):1649–1657. doi: 10.1016/j.ultsonch.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 88.Chandrapala J., Ong L., Zisu B., Gras S.L., Ashokkumar M., Kentish S.E. The effect of sonication and high pressure homogenisation on the properties of pure cream. Innovative Food Sci. Emerg. Technol. 2016;33:298–307. [Google Scholar]
- 89.Karlović S., Bosiljkov T., Brnčić M., Semenski D., Dujmić F., Tripalo B., Ježek D. Reducing fat globules particle-size in goat milk: ultrasound and high hydrostatic pressures approach. Chem. Biochem. Eng. Q. J. 2015;28:499–507. doi: 10.15255/cabeq.2014.19400. [DOI] [Google Scholar]
- 90.O’Sullivan J., Arellano M., Pichot R., Norton I. The effect of ultrasound treatment on the structural, physical andemulsifying properties of dairy proteins. Food Hydrocoll. 2014;42:386–396. doi: 10.1016/j.foodhyd.2014.05.011. [DOI] [Google Scholar]
- 91.Leong T., Juliano P., Johansson L., Mawson R., McArthur S.L., Manasseh R. Temperature effects on the ultrasonic separation of fat from natural whole milk. Ultrason. Sonochem. 2014;21(6):2092–2098. doi: 10.1016/j.ultsonch.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 92.S. Martini, A.H. Suzuki, R.W. Hartel, Effect of high intensity ultrasound on crystallization behavior of anhydrous milk fat, JAOCS, J. Am. Oil Chem. Soc. 85 (2008) 621–628. 10.1007/s11746-008-1247-5.
- 93.A. Wagh, M.K. Walsh, S. Martini, Effect of lactose monolaurate and high intensity ultrasound on crystallization behavior of anhydrous milk fat, JAOCS, J. Am. Oil Chem. Soc. 90 (2013) 977–987. 10.1007/s11746-013-2244-x.
- 94.Torkamani A.E., Juliano P., Ajlouni S., Singh T.K. Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason. Sonochem. 2014;21(3):951–957. doi: 10.1016/j.ultsonch.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 95.Wu H., Hulbert G.J., Mount J.R., Hongyu W., Hulbert G.J., Mount J.R. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov. Food Sci. Emerg. Technol. 2000;1:211–218. doi: 10.1016/S1466-8564(00)00020-5. [DOI] [Google Scholar]
- 96.A.K. Niamah, Ultrasound treatment (low frequency) effects on probiotic bacteria growth in fermented milk, Futur. Food J. Food, Agric. Soc. 7 (2019) 1–8. 10.17170/kobra-20190709592.
- 97.Khaire R.A., Gogate P.R. Intensified recovery of lactose from whey using thermal, ultrasonic and thermosonication pretreatments. J. Food Eng. 2018;237:240–248. [Google Scholar]
- 98.S.R. Patel, Z.V.P. Murthy, Ultrasound assisted crystallization for the recovery of lactose in an anti-solvent acetone, Cryst. Res. Technol. 44 (2009) 889–896. 10.1002/crat.200900227.
- 99.Rehman Saeed Al-Hilphy A., Kareem Niamah A., Al-Temimi A.B. Effect of ultrasonic treatment on buffalo milk homogenization and numbers of bacteria. Food. 2012;2(6):113–118. [Google Scholar]
- 100.Juliano P., Kutter A., Cheng L.J., Swiergon P., Mawson R., Augustin M.A. Enhanced creaming of milk fat globules in milk emulsions by the application of ultrasound and detection by means of optical methods. Ultrason. Sonochem. 2011;18(5):963–973. doi: 10.1016/j.ultsonch.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Riener J., Noci F., Cronin D.A., Morgan D.J., Lyng J.G. The effect of thermosonication of milk on selected physicochemical and microstructural properties of yoghurt gels during fermentation. Food Chem. 2009;114(3):905–911. [Google Scholar]
- 102.Marchesini G., Balzan S., Montemurro F., Fasolato L., Andrighetto I., Segato S., Novelli E. Effect of ultrasound alone or ultrasound coupled with CO2 on the chemical composition, cheese-making properties and sensory traits of raw milk. Innovative Food Sci. Emerg. Technol. 2012;16:391–397. [Google Scholar]
- 103.Moussier M., Bosc V., Michon C., Pistre V., Chaudemanche C., Huc-Mathis D. Multi-scale understanding of the effects of the solvent and process on whey protein emulsifying properties: application to dairy emulsion. Food Hydrocolloids. 2019;87:869–879. [Google Scholar]
- 104.O'Sullivan J., Murray B., Flynn C., Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids. 2016;53:141–154. [Google Scholar]
- 105.Yuan X., Li X., Zhang X., Mu Z., Gao Z., Jiang L., Jiang Z. Effect of ultrasound on structure and functional properties of laccase-catalyzed α-lactalbumin. J. Food Eng. 2018;223:116–123. [Google Scholar]
- 106.Zhao L., Zhang S., Uluko H., Liu L., Lu J., Xue H., Kong F., Lv J. Effect of ultrasound pretreatment on rennet-induced coagulation properties of goat’s milk. Food Chem. 2014;165:167–174. doi: 10.1016/j.foodchem.2014.05.081. [DOI] [PubMed] [Google Scholar]
- 107.Aslan D., Dogan M. The influence of ultrasound on the stability of dairy-based, emulsifier-free emulsions: rheological and morphological aspect. Eur Food Res Technol. 2018;244(3):409–421. [Google Scholar]
- 108.Almanza-Rubio J.L., Gutiérrez-Méndez N., Leal-Ramos M.Y., Sepulveda D., Salmeron I. Modification of the textural and rheological properties of cream cheese using thermosonicated milk. J. Food Eng. 2016;168:223–230. [Google Scholar]
- 109.Joyner (Melito) H.S. Explaining food texture through rheology. Curr. Opin. Food Sci. 2018;21:7–14. [Google Scholar]
- 110.Gursoy O., Yilmaz Y., Gokce O., Ertan K. Effect of ultrasound power on physicochemical and rheological properties of yoghurt drink produced with thermosonicated milk. Emir. J. Food Agric. 2016;28(4):235. [Google Scholar]
- 111.Peleg M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019;50(5):362–368. doi: 10.1111/jtxs.12392. [DOI] [PubMed] [Google Scholar]
- 112.Shen X., Fang T., Gao F., Guo M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocolloids. 2017;63:668–676. [Google Scholar]
- 113.D. Bermúdez-Aguirre, G. V Barbosa-Cánovas, Processing of Soft Hispanic Cheese (“Queso Fresco”) Using Thermo-Sonicated Milk: A Study of Physicochemical Characteristics and Storage Life, J. Food Sci. 75 (2010) S548–S558. 10.1111/j.1750-3841.2010.01850.x. [DOI] [PubMed]
- 114.Nöbel S., Ross N.-L., Protte K., Körzendörfer A., Hitzmann B., Hinrichs J. Microgel particle formation in yogurt as influenced by sonication during fermentation. J. Food Eng. 2016;180:29–38. [Google Scholar]
- 115.Riener J., Noci F., Cronin D.A., Morgan D.J., Lyng J.G. A comparison of selected quality characteristics of yoghurts prepared from thermosonicated and conventionally heated milks. Food Chem. 2010;119(3):1108–1113. [Google Scholar]
- 116.Munir M., Nadeem M., Qureshi T.M., Leong T.S.H., Gamlath C.J., Martin G.J.O., Ashokkumar M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems — a review. Innov. Food Sci. Emerg. Technol. 2019;57 doi: 10.1016/j.ifset.2019.102192. [DOI] [Google Scholar]
- 117.Körzendörfer A., Schäfer J., Hinrichs Jörg, Nöbel S. Power ultrasound as a tool to improve the processability of protein-enriched fermented milk gels for Greek yogurt manufacture. J. Dairy Sci. 2019;102(9):7826–7837. doi: 10.3168/jds.2019-16541. [DOI] [PubMed] [Google Scholar]
- 120.Fox P.F., Uniacke-Lowe T., McSweeney P.L.H., O'Mahony J.A., editors. Dairy Chemistry and Biochemistry. Springer International Publishing; Cham: 2015. [Google Scholar]
- 121.D. Bermúdez-Aguirre, R. Mawson, K. Versteeg, G. V. Barbosa-CÁnovas, Composition properties, physicochemical characteristics and shelf life of whole milk after thermal and thermo-sonication treatments, J. Food Qual. 32 (2009) 283–302. 10.1111/j.1745-4557.2009.00250.x.
- 122.Bermúdez-Aguirre D., Barbosa-Cánovas G.V. Study of butter fat content in milk on the inactivation of Listeria innocua ATCC 51742 by thermo-sonication. Innov. Food Sci. Emerg. Technol. 2008;9(2):176–185. [Google Scholar]
- 123.S. Gammoh, M.H. Alu’datt, C.C. Tranchant, D.G. Al-U’datt, M.N. Alhamad, T. Rababah, S. Kubow, M.S.Y. Haddadin, Z. Ammari, S. Maghaydah, H. Banat, Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus delbrueckii subsp. lactis, Lwt. 129 (2020) 109501. 10.1016/j.lwt.2020.109501.
- 124.Guimarães J.T., Silva E.K., Ranadheera C.S., Moraes J., Raices R.S.L., Silva M.C., Ferreira M.S., Freitas M.Q., Meireles M.A.A., Cruz A.G. Effect of high-intensity ultrasound on the nutritional profile and volatile compounds of a prebiotic soursop whey beverage. Ultrason. Sonochem. 2019;55:157–164. doi: 10.1016/j.ultsonch.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 125.Tan B.L., Norhaizan M.E., Liew W.P.P., Rahman H.S. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front. Pharmacol. 2018;9:1–28. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parreiras P.M., Vieira Nogueira J.A., Rodrigues da Cunha L., Passos M.C., Gomes N.R., Breguez G.S., Falco T.S., Bearzoti E., Menezes C.C. Effect of thermosonication on microorganisms, the antioxidant activity and the retinol level of human milk. Food Control. 2020;113 doi: 10.1016/j.foodcont.2020.107172. [DOI] [Google Scholar]
- 127.Ariyoshi Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993;4(5):139–144. [Google Scholar]
- 128.Khan I.T., Nadeem M., Imran M., Ullah R., Ajmal M., Jaspal M.H. Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 2019;18:1–13. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Z., Juliano P., Williams R.P.W., Niere J., Augustin M.A. Ultrasound improves the renneting properties of milk. Ultrason. Sonochem. 2014;21(6):2131–2137. doi: 10.1016/j.ultsonch.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 130.Yildiz G., Rababah T.M., Feng H. Ultrasound-assisted cutting of cheddar, mozzarella and Swiss cheeses – effects on quality attributes during storage. Innovative Food Sci. Emerg. Technol. 2016;37:1–9. [Google Scholar]
- 131.Madadlou A., Sheehan D., Emam-Djomeh Z., Mousavi M.E. Ultrasound-assisted generation of ACE-inhibitory peptides from casein hydrolyzed with nanoencapsulated protease. J. Sci. Food Agric. 2011;91(11):2112–2116. doi: 10.1002/jsfa.4438. [DOI] [PubMed] [Google Scholar]
- 132.Ma S., Wang C., Guo M. Changes in structure and antioxidant activity of β-lactoglobulin by ultrasound and enzymatic treatment. Ultrason. Sonochem. 2018;43:227–236. doi: 10.1016/j.ultsonch.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 133.Jambrak A.Režek., Mason T.J., Lelas V., Herceg Z., Herceg I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008;86(2):281–287. [Google Scholar]
- 134.G. Reid, M.E. Sanders, H.R. Gaskins, G.R. Gibson, A. Mercenier, R. Rastall, M. Roberfroid, I. Rowland, C. Cherbut, T.R. Klaenhammer, New scientific paradigms for probiotics and prebiotics, J. Clin. Gastroenterol. 37 (2003) 105–118. 10.1097/00004836-200308000-00004. [DOI] [PubMed]
- 135.Champagne C.P., Gardner N.J., Roy D. Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr. 2005;45(1):61–84. doi: 10.1080/10408690590900144. [DOI] [PubMed] [Google Scholar]
- 136.Fenster K., Freeburg B., Hollard C., Wong C., Laursen R.R., Ouwehand A.C. The production and delivery of probiotics: a review of a practical approach. Microorganisms. 2019;7:1–17. doi: 10.3390/microorganisms7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Toba T., Hayasaka I., Taguchi S., Adachi S. A new method for manufacture of lactose-hydrolysed fermented milk. J. Sci. Food Agric. 1990;52(3):403–407. [Google Scholar]
- 138.Masuzawa N., Ohdaira E. Attempts to shorten the time of lactic fermentation by ultrasonic irradiation. Jpn. J. Appl. Phys. 2002;41(Part 1, No. 5B):3277–3278. [Google Scholar]
- 139.Shimada T., Ohdaira E., Masuzawa N. Effect of ultrasonic frequency on lactic acid fermentation promotion by ultrasonic irradiation. Jpn. J. Appl. Phys. 2004;43:2831–2832. [Google Scholar]
- 140.Wang D., Sakakibara M. Lactose hydrolysis and β-galactosidase activity in sonicated fermentation with Lactobacillus strains. Ultrason. Sonochem. 1997;4(3):255–261. doi: 10.1016/s1350-4177(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 141.Shah N.P. Probiotic bacteria: selective enumeration and survival in dairy foods. J. Dairy Sci. 2000;83:894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- 142.Geciova J., Bury D., Jelen P. Methods for disruption of microbial cells for potential use in the dairy industry—a review. Int. Dairy J. 2002;12(6):541–553. [Google Scholar]
- 143.Chisti Y. Sonobioreactors: using ultrasound for enhanced microbial productivity. Trends Biotechnol. 2003;21:89–93. doi: 10.1016/S0167-7799(02)00033-1. [DOI] [PubMed] [Google Scholar]
- 144.Dahroud B.D., Mokarram R.R., Khiabani M.S., Hamishehkar H., Bialvaei A.Z., Yousefi M., Kafil H.S. Low intensity ultrasound increases the fermentation efficiency of Lactobacillus casei subsp.casei ATTC 39392. Int. J. Biol. Macromol. 2016;86:462–467. doi: 10.1016/j.ijbiomac.2016.01.103. [DOI] [PubMed] [Google Scholar]
- 145.Liong M.-T. Probiotics: a critical review of their potential role as antihypertensives, immune modulators, hypocholesterolemics, and perimenopausal treatments. Nutr. Rev. 2008;65:316–328. doi: 10.1111/j.1753-4887.2007.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 146.Runyan C.M., Carmen J.C., Beckstead B.L., Nelson J.L., Robison R.A., Pitt W.G. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 2006;52(5):295–301. doi: 10.2323/jgam.52.295. [DOI] [PubMed] [Google Scholar]
- 147.Shaheen M., Choi M., Ang W., Zhao Y., Xing J., Yang R., Xing J., Zhang J., Chen J. Application of low-intensity pulsed ultrasound to increase bio-ethanol production. Renewable Energy. 2013;57:462–468. [Google Scholar]
- 148.E. Ananta, D. Voigt, M. Zenker, V. Heinz, D. Knorr, Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosus to high-intensity ultrasound, J. Appl. Microbiol. 99 (2005) 271–278. 10.1111/j.1365-2672.2005.02619.x. [DOI] [PubMed]
- 149.Pitt W.G., Ross S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog. 2003;19(3):1038–1044. doi: 10.1021/bp0340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tachibana K., Uchida T., Ogawa K., Yamashita N., Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- 151.Singh S., Agarwal M., Bhatt A., Goyal A., Moholkar V.S. Ultrasound enhanced enzymatic hydrolysis of Parthenium hysterophorus: a mechanistic investigation. Bioresour. Technol. 2015;192:636–645. doi: 10.1016/j.biortech.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 152.Sakakibara M., Wang D., Ikeda K., Suzuki K. Effect of ultrasonic irradiation on production of fermented milk with Lactobacillus delbrueckii. Ultrason. Sonochem. 1994;1(2):S107–S110. [Google Scholar]
- 153.Wang D., Sakakibara M., Kondoh N., Suzuki K. Ultrasound-enhanced lactose hydrolysis in milk fermentation withLactobacillus bulgaricus. J. Chem. Technol. Biotechnol. 1996;65(1):86–92. [Google Scholar]
- 154.Huang G., Chen S., Tang Y., Dai C., Sun L., Ma H., He R. Stimulation of low intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019;51:315–324. doi: 10.1016/j.ultsonch.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 155.Ojha K.S., Mason T.J., O’Donnell C.P., Kerry J.P., Tiwari B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017;34:410–417. doi: 10.1016/j.ultsonch.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Avhad D.N., Rathod V.K. Ultrasound stimulated production of a fibrinolytic enzyme. Ultrason. Sonochem. 2014;21(1):182–188. doi: 10.1016/j.ultsonch.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 157.Pawar S.V., Rathod V.K. Role of ultrasound in assisted fermentation technologies for process enhancements. Prep. Biochem. Biotech. 2020;50(6):627–634. doi: 10.1080/10826068.2020.1725773. [DOI] [PubMed] [Google Scholar]
- 158.T.J. Mason, D. Peters, Practical Sonochemistry: Second Edition, Pract. Sonochemistry Second Ed. (2003) 1–155. 10.1533/9781782420620.
- 159.Ashokkumar M., Anandan S., Cavalieri F., Okitsu K., Yasui K., Chemat F. Springer; Berlin: 2016. Handbook on Ultrasonics and Sonochemistry. [Google Scholar]
- 160.Rokhina E.V., Lens P., Virkutyte J. Low-frequency ultrasound in biotechnology: state of the art. Trends Biotechnol. 2009;27(5):298–306. doi: 10.1016/j.tibtech.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 161.Gao S., Hemar Y., Ashokkumar M., Paturel S., Lewis G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014;60:93–104. doi: 10.1016/j.watres.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 162.S. Gao, G.D. Lewis, M. Ashokkumar, Y. Hemar, Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria, Ultrasonics Sonochemistry 21 (2014) 446-453. 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed]
- 163.Ewe J.-A., Wan-Abdullah W.-N., Alias A.K., Liong M.-T. Effects of ultrasound on growth, bioconversion of isoflavones and probiotic properties of parent and subsequent passages of Lactobacillus fermentum BT 8633 in biotin-supplemented soymilk. Ultrason. Sonochem. 2012;19(4):890–900. doi: 10.1016/j.ultsonch.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 164.Lentacker I., De Cock I., Deckers R., De Smedt S.C., Moonen C.T.W. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014;72:49–64. doi: 10.1016/j.addr.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 165.Tabatabaie F., Mortazavi A. Studying the effects of ultrasound shock on cell wall permeability and survival of some LAB in milk. World Appl. Sci. J. 2008;3:119–121. [Google Scholar]
- 166.Monsen T., Lovgren E., Widerstrom M., Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 2009;47(8):2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ashokkumar M. The characterization of acoustic cavitation bubbles – an overview. Ultrason. Sonochem. 2011;18(4):864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 168.Mason T.J., Riera E., Vercet A., Lopez-Buesa P. Application of ultrasound. Emerg. Technol. Food Process. 2005:323–351. doi: 10.1016/B978-012676757-5/50015-3. [DOI] [Google Scholar]
- 169.Nguyen T.M.P., Lee Y.K., Zhou W. Stimulating fermentative activities of bifidobacteria in milk by highintensity ultrasound. Int. Dairy J. 2009;19(6-7):410–416. [Google Scholar]
- 170.Nguyen T.M.P., Lee Y.K., Zhou W. Effect of high intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chem. 2012;130(4):866–874. [Google Scholar]
- 171.A.R.S. Al-Hilphy, D.K. Verma, A.K. Niamah, Technology for Treatment, 2016.
- 172.Shershenkov B., Suchkova E. Upgrading the technology of functional dairy products by means of fermentation process ultrasonic intensification. Agron. Res. 2015;13:1074–1085. [Google Scholar]
- 173.Lye H.-S., Alias K.A., Rusul G., Liong M.-T. Ultrasound treatment enhances cholesterol removal ability of lactobacilli. Ultrason. Sonochem. 2012;19(3):632–641. doi: 10.1016/j.ultsonch.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 174.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 175.Gholamhosseinpour A., Hashemi S.M.B. Ultrasound pretreatment of fermented milk containing probiotic Lactobacillus plantarum AF1: carbohydrate metabolism and antioxidant activity. J. Food Process Eng. 2019;42:1–9. doi: 10.1111/jfpe.12930. [DOI] [Google Scholar]
- 176.K.S. Ojha, C.M. Burgess, G. Duffy, J.P. Kerry, B.K. Tiwari, Integrated phenotypic-genotypic approach to understand the influence of ultrasound on metabolic response of Lactobacillus sakei, PLoS One. 13 (2018) 1–20. 10.1371/journal.pone.0191053. [DOI] [PMC free article] [PubMed]
- 177.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 2. A simple model for the inactivation mechanism. Ultrason. Sonochem. 2014;21(1):454–460. doi: 10.1016/j.ultsonch.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 178.Körzendörfer A., Nöbel S., Hinrichs J. Particle formation induced by sonication during yogurt fermentation – impact of exopolysaccharide-producing starter cultures on physical properties. Food Res. Int. 2017;97:170–177. doi: 10.1016/j.foodres.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 179.Potoroko I., Kalinina I., Botvinnikova V., Krasulya O., Fatkullin R., Bagale U., Sonawane S.H. Ultrasound effects based on simulation of milk processing properties. Ultrason. Sonochem. 2018;48:463–472. doi: 10.1016/j.ultsonch.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 180.Shamila-Syuhada A.K., Chuah L.-O., Wan-Nadiah W.A., Cheng L.H., Alkarkhi A.F.M., Effarizah M.E., Rusul G. Inactivation of microbiota and selected spoilage and pathogenic bacteria in milk by combinations of ultrasound, hydrogen peroxide, and active lactoperoxidase system. Int. Dairy J. 2016;61:120–125. [Google Scholar]
- 181.Ugarte-Romero E., Feng H., Martin S.E. Inactivation of Shigella boydii 18 IDPH and Listeria monocytogenes Scott A with power ultrasound at different acoustic energy densities and temperatures. J. Food Sci. 2007;72 doi: 10.1111/j.1750-3841.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 182.N. Gera, S. Doores, Kinetics and mechanism of bacterial inactivation by ultrasound waves and sonoprotective effect of milk components, J. Food Sci. 76 (2011) 111–119. 10.1111/j.1750-3841.2010.02007.x. [DOI] [PubMed]
- 183.Herceg Z., Jambrak A.R., Lelas V., Thagard S.M. The effect of high intensity ultrasound treatment on the amount of Staphylococcus aureus and Escherichia coli in milk. Food Technol. Biotechnol. 2012;50:46–52. [Google Scholar]
- 184.Lim S.-Y., Benner L.C., Clark S. Neither thermosonication nor cold sonication is better than pasteurization for milk shelf life. J. Dairy Sci. 2019;102(5):3965–3977. doi: 10.3168/jds.2018-15347. [DOI] [PubMed] [Google Scholar]
- 185.Bermúdez-Aguirre D., Corradini M.G., Mawson R., Barbosa-Cánovas G.V. Modeling the inactivation of Listeria innocua in raw whole milk treated under thermo-sonication. Innovative Food Sci. Emerg. Technol. 2009;10(2):172–178. [Google Scholar]
- 186.D.J. D’Amico, T.M. Silk, J. Wu, M. Guo, Inactivation of microorganisms in milk and apple cider treated with ultrasound, J. Food Prot. 69 (2006) 556–563. 10.4315/0362-028X-69.3.556. [DOI] [PubMed]
- 187.E. Chouliara, K.G. Georgogianni, N. Kanellopoulou, M.G. Kontominas, Effect of ultrasonication on microbiological, chemical and sensory properties of raw, thermized and pasteurized milk, Int. Dairy J. 20 (2010) 307–313. 10.1016/j.idairyj.2009.12.006.
- 188.Engin B., Karagul Yuceer Y. Effects of ultraviolet light and ultrasound on microbial quality and aroma-active components of milk: effects of ultraviolet light and ultrasound on milk. J. Sci. Food Agric. 2012;92(6):1245–1252. doi: 10.1002/jsfa.4689. [DOI] [PubMed] [Google Scholar]
- 189.Herceg Z., Juraga E., Sobota-Šalamon B., Režek-Jambrak A. Inactivation of mesophilic bacteria in milk by means of high intensity ultrasound using response surface methodologyInactivation of mesophilic bacteria in milk by means of high intensity ultrasound using response surface methodology. Czech J. Food Sci. 2012;30(No. 2):108–117. [Google Scholar]
- 190.Juraga E., Šalamon B.S., Herceg Z., Jambrak A.R. Application of high intensity ultrasound treatment on Enterobacteriae count in milk. Mljekarstvo. 2011;61:125–134. [Google Scholar]
- 191.Kornacki J.L. Controlling Listeria in the food processing environment. Food Technol. 2005;59:36–42. [Google Scholar]
- 192.Marchesini G., Fasolato L., Novelli E., Balzan S., Contiero B., Montemurro F., Andrighetto I., Segato S. Ultrasonic inactivation of microorganisms: a compromise between lethal capacity and sensory quality of milk. Innov. Food Sci. Emerg. Technol. 2015;29:215–221. doi: 10.1016/j.ifset.2015.03.015. [DOI] [Google Scholar]
- 193.Gabriel A.A. Inactivation of Listeria monocytogenes in Milk by Multifrequency Power Ultrasound : Ultrasound Inactivation of Listeria in Milk. J. Food Process. Preserv. 2015;39(6):846–853. [Google Scholar]
- 194.Burgess S.A., Lindsay D., Flint S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010;144(2):215–225. doi: 10.1016/j.ijfoodmicro.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 195.Ganesan B., Martini S., Solorio J., Walsh M.K. Determining the effects of high intensity ultrasound on the reduction of microbes in milk and orange juice using response surface methodology. Int. J. Food Sci. 2015;2015 doi: 10.1155/2015/350719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chandrapala J., Leong T. Ultrasonic processing for dairy applications: recent advances. Food Eng Rev. 2015;7(2):143–158. [Google Scholar]
- 197.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 198.B. Zisu, J. Chandrapala, High power ultrasound processing in milk and dairy products, in: Tomasula, N. Datta (Eds.), Emerging Dairy Processing Technologies: Opportunities for the Dairy Industry, Wiley Blackwell, 2015, pp. 149-179. 10.1002/9781118560471.ch6.
- 199.Villamiel M., de Jong P. Inactivation of Pseudomonas fluorescens and Streptococcus thermophilus in Trypticase® Soy Broth and total bacteria in milk by continuous-flow ultrasonic treatment and conventional heating. J. Food Eng. 2000;45(3):171–179. [Google Scholar]
- 200.Majid I., Nayik G.A., Nanda V. Ultrasonication and food technology: a review. Cogent Food Agric. 2015;1:1–11. doi: 10.1080/23311932.2015.1071022. [DOI] [Google Scholar]
- 201.Pingret D., Fabiano-Tixier A.S., Petitcolas E., Canselier J.P., Chemat F. First investigation on ultrasound-assisted preparation of food products: sensory and physicochemical characteristics. J. Food Sci. 2011;76:287–292. doi: 10.1111/j.1750-3841.2010.02019.x. [DOI] [PubMed] [Google Scholar]
- 202.Juric A., Delas I., Vukusic T., Milosevic S., Jambrak A.R., Herceg Z. Influence of gas phase plasma and high power ultrasound on fatty acids in goat milk. Am. J. Food Technol. 2016;11(4):125–133. [Google Scholar]
- 203.Juliano P., Torkamani A.E., Leong T., Kolb V., Watkins P., Ajlouni S., Singh T.K. Lipid oxidation volatiles absent in milk after selected ultrasound processing. Ultrason. Sonochem. 2014;21(6):2165–2175. doi: 10.1016/j.ultsonch.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 204.Paniwnyk L. Applications of ultrasound in processing of liquid foods: a review. Ultrason. Sonochem. 2017;38:794–806. doi: 10.1016/j.ultsonch.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 205.D. Komes, A. Bušić, A. Belščak-Cvitanovic, M. Brncić, T. Bosiljkov, A. Vojvodić, F. Dujmić, Novel approach to the development of functional goat’s milk-based beverages using medicinal plant extracts in combination with high intensity ultrasound treatment, Food Technol. Biotechnol. 55 (2017) 484–495. 10.17113/ftb.55.04.17.5123. [DOI] [PMC free article] [PubMed]
- 207.Sfakianakis P., Tzia C. Flavour profiling by gas chromatography–mass spectrometry and sensory analysis of yoghurt derived from ultrasonicated and homogenised milk. Int. Dairy J. 2017;75:120–128. [Google Scholar]
- 208.Foegeding E.A., Davis J.P. Food protein functionality: a comprehensive approach. Food Hydrocolloids. 2011;25(8):1853–1864. [Google Scholar]
- 209.Lucey J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002;85(2):281–294. doi: 10.3168/jds.s0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- 210.Pereira R.N., Vicente A.A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010;43(7):1936–1943. [Google Scholar]
- 211.Vercet A., Oria R., Marquina P., Crelier S., Lopez-Buesa P. Rheological Properties of Yoghurt Made with Milk Submitted to Manothermosonication. J. Agric. Food Chem. 2002;50(21):6165–6171. doi: 10.1021/jf0204654. [DOI] [PubMed] [Google Scholar]
- 212.Marafon A.P., Sumi A., Granato D., Alcântara M.R., Tamime A.Y., Nogueira de Oliveira M. Effects of partially replacing skimmed milk powder with dairy ingredients on rheology, sensory profiling, and microstructure of probiotic stirred-type yogurt during cold storage. J. Dairy Sci. 2011;94(11):5330–5340. doi: 10.3168/jds.2011-4366. [DOI] [PubMed] [Google Scholar]
- 213.Kalab M., Allan-Wojtas P., Phipps-Todd B. Development of microstructure in set-style yoghurt-A review. Food Microstructure. 1983;2:51–66. Available at: https://digitalcommons.usu.edu/foodmicrostructure/vol2/iss1/7. [Google Scholar]
- 214.Riener J., Noci F., Cronin D.A., Morgan D.J., Lyng J.G. Characterisation of volatile compounds generated in milk by high intensity ultrasound. Int. Dairy J. 2009;19(4):269–272. [Google Scholar]
- 215.Donkor O.N., Henriksson A., Vasiljevic T., Shah N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Lait. 2007;87(1):21–38. [Google Scholar]
- 216.Kühn J., Considine T., Singh H. Interactions of milk proteins and volatile flavor compounds: implications in the development of protein foods. J. Food Sci. 2006;71 doi: 10.1111/j.1750-3841.2006.00051.x. [DOI] [Google Scholar]
- 217.Martini S., Walsh M.K. Sensory characteristics and functionality of sonicated whey. Food Res. Int. 2012;49(2):694–701. [Google Scholar]
- 218.Krešić G., Lelas V., Jambrak A.Režek., Herceg Z., Brnčić S.R. Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J. Food Eng. 2008;87(1):64–73. [Google Scholar]
- 219.Jeewanthi R.K.C., Lee N.-K., Paik H.-D. Improved functional characteristics of whey protein hydrolysates in food industry. Korean J. Food Sci. Anim. Resour. 2015;35(3):350–359. doi: 10.5851/kosfa.2015.35.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yadav J.S.S., Yan S., Pilli S., Kumar L., Tyagi R.D., Surampalli R.Y. Cheese whey: a potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015;33:756–774. doi: 10.1016/j.biotechadv.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 221.Rani S., Pooja K., Pal G.K. Exploration of potential angiotensin converting enzyme inhibitory peptides generated from enzymatic hydrolysis of goat milk proteins. Biocatal. Agric. Biotechnol. 2017;11:83–88. [Google Scholar]
- 222.Erkaya T., Başlar M., Şengül M., Ertugay M.F. Effect of thermosonication on physicochemical, microbiological and sensorial characteristics of ayran during storage. Ultrason. Sonochem. 2015;23:406–412. doi: 10.1016/j.ultsonch.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 223.Pingret D., Fabiano-Tixier A.S., Chemat F. Degradation during application of ultrasound in food processing: a review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- 224.C. Jacobsen, 6 – Understanding and reducing oxidative flavour deterioration in foods, in: E.A.B.T.-O. in F. and B. and A.A. Decker (Ed.), Woodhead Publ. Ser. Food Sci. Technol. Nutr., Woodhead Publishing, 2010: pp. 122–142. 10.1533/9780857090447.1.122.
- 225.N. Mongenot, S. Charrier, P. Chalier, Effect of ultrasound emulsification on cheese aroma encapsulation by carbohydrates, J. Agric. Food Chem. 48 (2000) 861–867. 10.1021/jf990494n. [DOI] [PubMed]
- 226.Mortazavi A., Tabatabai F. Study of ice cream freezing process after treatment with ultrasound. World Appl. Sci. J. 2008;4:188–190. [Google Scholar]
- 227.Johansson L., Singh T., Leong T., Mawson R., McArthur S., Manasseh R., Juliano P. Cavitation and non-cavitation regime for large-scale ultrasonic standing wave particle separation systems – in situ gentle cavitation threshold determination and free radical related oxidation. Ultrason. Sonochem. 2016;28:346–356. doi: 10.1016/j.ultsonch.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 228.Dhahir N., Feugang J., Witrick K., Park S., AbuGhazaleh A. Impact of ultrasound processing on some milk-borne microorganisms and the components of camel milk. Emirates J. Food Agric. 2020;32:245–254. doi: 10.9755/ejfa.2020.v32.i4.2088. [DOI] [Google Scholar]