中性粒细胞缺乏(粒缺)伴发热患者是一组特殊的疾病人群。由于免疫功能低下,炎症相关临床症状和体征常不明显,病原菌及感染灶也不明确,发热可能是感染的唯一征象,如未及时给予恰当的抗菌药物治疗,感染相关死亡率高。因此,充分认识粒缺伴发热患者的相关风险、诊断方法以及如何合理使用抗菌药物,对于降低粒缺伴发热的发生率和死亡风险至关重要。
《中国中性粒细胞缺乏伴发热患者抗菌药物临床应用指南(2016年版)》发表至今4年,对临床诊疗发挥了很好的指导作用。期间国际及国内关于粒缺伴发热的诊疗理念发生了一些重要变化,我国在粒缺伴发热的病原学,尤其是耐药菌监测方面也积累了大量临床研究和流行病学数据,新型靶向药物及免疫治疗的应用带来新的危险因素。因此,参考欧洲白血病感染相关指南(ECIL指南)[1]–[3]、美国感染病学会(IDSA)肿瘤合并粒缺患者治疗指南(IDSA指南)[4]、2019年西班牙血液恶性肿瘤患者粒缺伴发热管理共识[5]等,结合我国当前细菌流行病学、耐药菌监测数据及抗菌药物临床应用经验总结,中华医学会血液学分会和中国医师协会血液科医师分会对2016年版指南进行修订。
一、定义
1. 粒缺:指外周血中性粒细胞绝对计数(ANC)<0.5×109/L,或预计48 h后ANC<0.5×109/L;严重粒缺指ANC<0.1×109/L。
2. 发热:指单次口腔温度≥38.3°C(腋温≥38.0°C),或口腔温度≥38.0°C(腋温≥37.7°C)持续超过1 h。粒缺期间应避免测定直肠温度,以防止定植于肠道的微生物侵入。
需要指出的是,发热是粒缺患者应用抗菌药物的指征,由于这群患者临床表现差异较大,临床医师的判断在决定是否需要给患者使用抗菌药物治疗时起着关键性作用。即使患者不能满足上述定义,也需要医师仔细甄别是否需要应用抗菌药物治疗,例如,对于全身状况不良的患者(尤其是老年患者)应警惕感染时可能无发热或表现为低体温。在考虑细菌感染同时也需要警惕其他病原菌感染和混合感染。
二、流行病学
1. 粒缺伴发热的流行病学:超过80%的血液肿瘤患者和10%~50%的实体肿瘤患者在≥1个疗程化疗后会发生与粒缺有关的发热。血液肿瘤患者粒缺伴发热常有较高的死亡率,其血流感染(BSI)的相关死亡率达7.1%~42%[6]–[8]。粒缺伴发热患者的临床表现常不典型,感染部位不明显或难以发现,病原菌培养阳性率低。能明确感染部位者占50%左右,最常见的感染部位是肺,其后依次为上呼吸道、肛周和BSI等[8]。
我国粒缺伴发热的病原流行病学资料大多来源于BSI数据,与国外调查结果基本一致。致病菌以革兰阴性杆菌为主,占50%以上。常见革兰阴性杆菌包括大肠埃希菌、肺炎克雷伯菌、铜绿假单胞菌、嗜麦芽窄食单胞菌和鲍曼不动杆菌;常见革兰阳性球菌包括肠球菌、链球菌属、金黄色葡萄球菌和凝固酶阴性葡萄球菌。病原谱因感染部位和危险因素不同存在差异[6],[9]–[12]。中国医学科学院血液病医院(中国医学科学院血液学研究所)和北京大学人民医院单中心粒缺伴发热BSI流行病学研究显示,成人BSI以革兰阴性杆菌为主,最常见的病原菌为大肠埃希菌、肺炎克雷伯菌、铜绿假单胞菌,革兰阳性球菌中最常见的是葡萄球菌属[6],[13]–[14]。上海和广东地区血液科数据显示,粒缺伴发热患者病原菌以大肠埃希菌、肺炎克雷伯菌、凝固酶阴性葡萄球菌、铜绿假单胞菌和草绿色链球菌群等为主[10],[15]。总体而言,我国不同区域粒缺伴发热BSI的病原谱基本相同,但与医院其他科室存在差异。值得一提的是,草绿色链球菌群从免疫功能正常群体血液分离出时可能被作为污染菌,但该菌在粒缺患者中可引起临床脓毒症的风险,因此不应被认为是一种污染菌,血液科不可忽视该菌群[16]。
2. 耐药菌感染的流行病学:粒缺伴发热患者超过半数的耐药菌从BSI中检出,而呼吸道感染的耐药菌检出率较低。近5年BSI患者产超广谱β内酰胺酶(ESBL)大肠埃希菌(产ESBL-EC)、产ESBL肺炎克雷伯菌(产ESBL-KP)、耐碳青霉烯肺炎克雷伯菌(CRKP)、耐碳青霉烯铜绿假单胞菌(CRPA)、耐碳青霉烯鲍曼不动杆菌(CRAB)发生率分别为39.1%~68.3%、7.3%~41.2%、0.5%~11.4%、0~3.2%、5.7%~7.8%[6],[8]–[13],[15]。与欧美国家相比,我国整体人群碳青霉烯类耐药的肠杆菌科细菌(CRE)感染的发生率相对高、且逐年增加,是粒缺伴发热目前面临的挑战。CHINET监测网资料显示,CRE检出率2014年为12.5%,2016年为22.9%,2019年则升至26.8%[17]–[19]。基于出院人口的CRE检出率,以江苏最高(14.38例/10万患者日),其次为上海(7.00例/10万患者日),青海最低(0.32例/10万患者日)[20]。在分离菌株中,最常见的为肺炎克雷伯菌,其次为大肠埃希菌、阴沟肠杆菌和产气肠杆菌[20]–[21]。国内血液病患者CRE流行病学数据较少,中国医学科学院血液病医院(中国医学科学院血液学研究所)数据显示0.72%患者存在CRE定植,其中25.5%在定植后发生了CRE感染[22];浙江大学附属第一医院2018—2019年的数据显示造血干细胞移植患者CRE定植率可能高达10.8%[23]。就临床分离株而言,广东地区50家医院血液内科血标本来源菌的CRE检出率低于重症监护治疗病房(ICU),高于呼吸内科[15]。
尽管相当一部分的粒缺伴发热患者最终无法明确致病原,考虑到这类患者病情严重、病死率较高,尽早开始抗菌药物治疗可显著改善粒缺伴发热患者的预后;同时,运用多种病原学检测方法明确病原菌,对目标性抗感染治疗至关重要。
三、诊断
1. 病史询问和体格检查:详细了解既往抗菌药物使用、耐药和定植情况,发现感染的高危和隐匿部位;但相当一部分患者无法明确感染部位。
2. 实验室检查:全血细胞计数、肝肾功能和电解质检查,至少每3 d复查1次;降钙素原、C反应蛋白等感染相关指标的检查对感染诊断有提示意义。
3. 微生物学检查:至少同时行两套血培养检查,如果存在中心静脉导管(CVC),一套血标本从CVC的管腔采集,另一套从外周静脉采集。无CVC者,应采集不同部位静脉的两套血标本进行培养,采血量为每瓶10 ml。如果经验性抗菌药物治疗后患者仍持续发热,可以每隔2~3 d进行1次重复培养。同时根据临床表现,对可能出现感染的部位进行相应的微生物学检查。除培养外,根据疾病情况,也应当进行其他微生物学检测,包括:
(1)微生物涂片:采集组织分泌物如下呼吸道标本、肛周样本,伤口创面和脓肿分泌物等进行涂片检测,是经济快捷发现病原菌的方法。涂片阳性且与培养结果一致,对病原学诊断有一定参考价值,可作为初始经验性抗感染治疗的依据。
(2)血清学检测:急性期血清学IgM抗体阳性对诊断有指导价值,恢复期IgG抗体滴度呈4倍或4倍以上变化或IgM抗体由阴转阳具有回顾性确诊的价值。但粒缺患者由于免疫功能底下,急性期血清学阳性检出率低。血清1,3-β-D葡聚糖试验(G试验)、血清或分泌物半乳甘露聚糖抗原试验(GM试验)阳性对侵袭性真菌病诊断有辅助价值。
(3)聚合酶链反应(PCR)和宏基因组二代测序(mNGS):PCR和mNGS等分子生物学技术检测出病原微生物,可作为病原学诊断的参考,但需结合流行病学和临床特征综合评估是否为致病菌。PCR检测血液或组织中微生物DNA/RNA含量,对某些病毒性疾病如疱疹病毒感染的诊断具有确诊价值。基于mNGS通过分析临床标本中微生物的DNA/RNA含量与丰度来判断致病菌,有望提高病原检测的敏感性,缩短检测时间,对罕见病原菌感染的诊断具有优势。但该技术临床应用尚需解决许多问题,如标本中人类基因组的干扰、检验质量良莠不齐、结果解释缺乏规范,结论易失信等,目前尚不作为常规临床检测方法推荐。
4. 相关感染部位的评估和影像学检查:X线、CT、B超、PET-CT等。
四、患者危险分层和耐药评估
危险分层是粒缺伴发热患者治疗开始前的必要工作,对于后续经验性选择抗菌药物至关重要[1],[3]–[4](表1),危险分层包括高危和低危患者,高危患者必须住院治疗,不符合低危标准的患者均应按照高危患者进行处理。
表1. 中性粒细胞缺乏伴发热患者的危险分层[1],[3]–[4].
| 危险度 | 定义 | |
| 高危 | 符合以下任意一项: | |
| 1. | 预计严重中性粒细胞缺乏(<0.1×109/L)持续>7d | |
| 2. | 有以下任一种临床合并症(包括但不限于):①血流动力学不稳定;②口腔或胃肠道黏膜炎,吞咽困难;③胃肠道症状(腹痛、恶心、呕吐和腹泻);④新发的神经系统改变或精神症状;⑤血管内导管感染,尤其是导管腔道感染;⑥新发的肺部浸润或低氧血症,或有潜在的慢性肺部疾病 | |
| 3. | 肝功能不全(转氨酶水平>5倍正常上限)或肾功能不全(肌酐清除率<30ml/min) | |
| 4. | 合并免疫功能缺陷疾病 | |
| 5. | 接受分子靶向药物或免疫调节药物治疗 | |
| 低危 | 预计中性粒细胞缺乏时间≤7d,无活动性合并症,肝肾功能正常或损害较轻并且稳定 | |
随着抗菌药物耐药问题日趋严重,粒缺伴发热患者在经验性治疗前,还应进行耐药危险因素评估[2](表2)。
表2. 中性粒细胞缺乏伴发热患者耐药细菌感染的危险因素[2].
| 1. | 患者有耐药病原菌定植或感染病史、尤其是:①产超广谱β内酰胺酶(ESBL)或碳青霉烯酶的肠杆菌;②耐药非发酵菌:铜绿假单胞菌、鲍曼不动杆菌、嗜麦芽窄食单胞菌;③耐甲氧西林金黄色葡萄球菌(MRSA)、尤其是万古霉素最低抑菌浓度(MIC)≥2mg/L;④耐万古霉素肠球菌(VRE) |
| 2. | 接触过广谱抗菌药物(尤其是第三代头孢菌素类、喹诺酮类) |
| 3. | 重症疾病:如晚期肿瘤、脓毒血症、肺炎 |
| 4. | 院内感染 |
| 5. | 长期和(或)反复住院 |
| 6. | 留置导管 |
| 7. | 老年患者 |
| 8. | 重症监护病房患者 |
五、初始经验性抗菌药物治疗
在危险分层和耐药危险因素评估后,尽快使用抗菌药物初始经验性治疗,而不必等待微生物学的结果,其原则是覆盖可迅速引起严重并发症或威胁生命的最常见和毒力较强的病原菌,同时必须考虑本区域、本院及本科室感染的流行病学覆盖耐药菌,直至获得准确的病原学结果。
选择恰当的经验性抗菌药物治疗具有重要临床意义。接受不恰当的初始经验性抗菌药物治疗(IIAT:抗菌药物对致病病原体的体外药敏试验为耐药和/或中介)可导致感染相关病死率增高。近期两项中国血液病粒缺合并革兰阴性杆菌BSI病例研究均证实接受IIAT 7 d内病死率可高达29.9%~37.7%[24]–[25]。因此,制定合理的经验性抗菌药物治疗方案至关重要。制定经验性抗菌药物治疗方案需要综合评估患者(危险分层、感染部位、脏器功能、耐药危险因素)、细菌(当地及本单位、科室的流行病学和耐药监测数据)、抗菌药物(广谱、药物代谢/效应动力学、不良反应等)等多方面的因素,选择具有杀菌活性、抗假单胞菌活性和安全性良好的广谱抗菌药物,并需注意与治疗原发疾病的药物(化疗药物、免疫抑制剂等)之间是否存在毒副作用的叠加。
对于低危患者,初始治疗可以在门诊或住院接受口服或静脉注射经验性抗菌药物治疗[1]。对接受门诊治疗的患者需要保证密切的临床观察和恰当的医疗处理,如病情加重须尽快住院治疗。高危患者必须立即住院治疗,根据危险分层、耐药危险因素、当地病原菌和耐药流行病学数据及临床表现复杂性对患者进行个体化评估[1]。抗菌药物升阶梯和降阶梯策略的适应证及经验性抗菌药物选择的建议见表3[2]。
表3. 中性粒细胞缺乏(粒缺)伴发热患者升阶梯和降阶梯策略的适应证和经验性抗菌药物选择的建议.
| 治疗策略 | 适应证 | 初始抗菌药物选择 |
| 升阶梯策略 | 1. 无复杂临床表现a | 1. 抗假单胞菌头孢菌素(如头孢吡肟、头孢他啶) |
| 2. 不确定有无耐药菌定植 | 2. β内酰胺酶抑制剂复合制剂(如哌拉西林/他唑巴坦、头孢哌酮/舒巴坦) | |
| 3. 此前无耐药菌感染 | 3. 哌拉西林+阿米卡星 | |
| 4. 本中心粒缺伴发热因耐药菌导致感染罕见 | ||
| 降阶梯策略 | 1. 复杂临床表现a | 1. 碳青霉烯类单药 |
| 2. 存在耐药菌定植 | 2. 抗假单胞菌β内酰胺类联合氨基糖苷类或喹诺酮类(重症患者选择β内酰胺类中的碳青霉烯类) | |
| 3. 发生过耐药菌感染 | ||
| 4. 本中心粒缺伴发热常见因耐药菌导致感染 | 3. 早期覆盖革兰阳性耐药菌(如果存在革兰阳性球菌风险):糖肽类、利奈唑胺或新型抗菌药物 |
注:a复杂临床表现包括:血流动力学不稳定、局灶性感染(如肺炎、肠炎、中心静脉导管相关感染)、长期和严重营养不良、并发症(出血、脱水、器官衰竭、慢性病)、高龄(60岁以上)
高危患者静脉应用的抗菌药物必须是能覆盖铜绿假单胞菌和其他严重革兰阴性杆菌的广谱抗菌药物。鉴于目前国内流行病学数据,尤其是耐药菌比例和耐药谱的变化,经验性用药时,还应参照本地区、本院和本科室最新的耐药菌流行病数据、感染部位、药物在目标人群的药物代谢/效应动力学等,尽可能做到准确的经验用药。对于既往发生过广泛耐药(XDR)细菌定植或感染的患者,初始经验用药更应慎重。对于有产ESBL菌定植或感染病史及产ESBL菌感染高危患者,选择碳青霉烯类单药或β内酰胺类联合氟喹诺酮类或氨基糖苷类抗菌药物治疗;既往有CRE感染或定植患者初始抗菌药物选择可参考中国XDR共识[26]及CRE共识[27]。
在以下特定情形,初始经验性用药中需要同时覆盖严重的革兰阴性杆菌和革兰阳性球菌:
①血液动力学不稳定或有其他严重BSI证据;
②X线影像学确诊的肺炎;
③在最终鉴定结果及药敏试验结果报告前,血培养为革兰阳性球菌;
④临床疑有严重导管相关感染;
⑤任一部位的皮肤或软组织感染;
⑥MRSA、VRE或耐青霉素肺炎链球菌定植;
⑦严重黏膜炎且已接受氟喹诺酮类药物预防和头孢他啶经验性治疗。
六、抗菌药物的调整
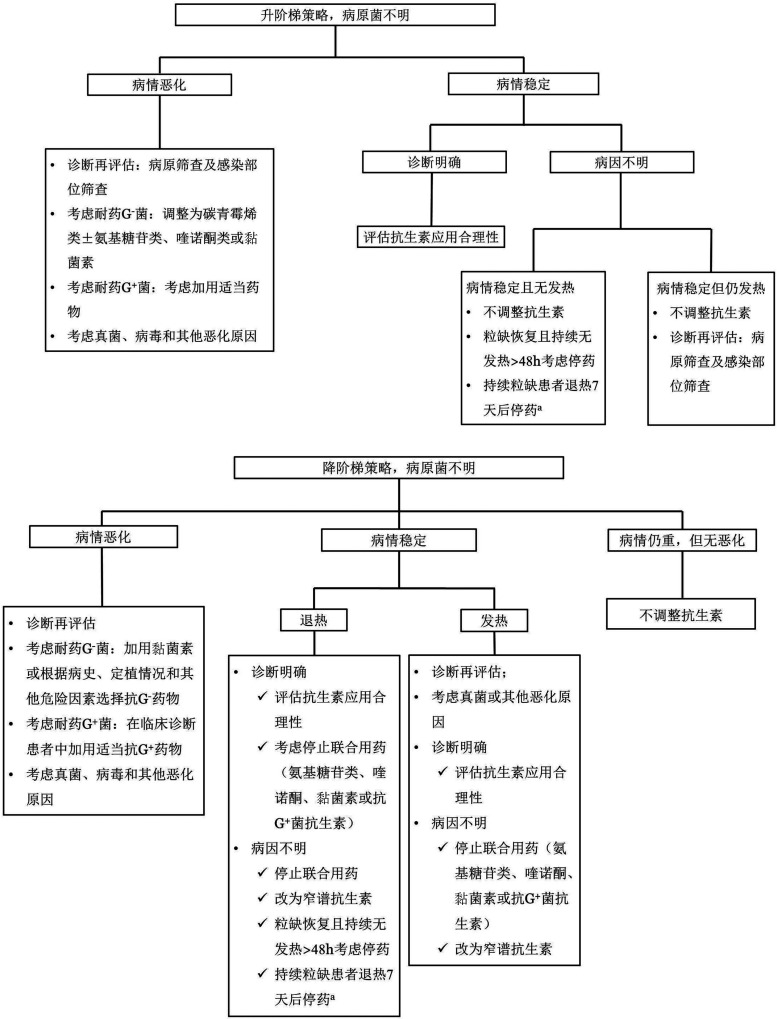
在接受经验性抗菌药物治疗后,应根据危险分层、确诊的病原菌和患者对初始治疗的反应等综合判断,决定后续如何调整抗菌治疗。临床上,在初始经验性抗菌药物应用,如果出现病情加重,如血流动力学不稳定,宜及时调整抗菌药物。对于明确病原菌的患者,可根据所识别细菌和药敏结果采用窄谱抗生素治疗,检出细菌如属于耐药菌,应根据病原体及其MIC选择针对性抗菌药物,有条件的医院可行耐药表型、耐药基因检测。一般推荐联合抗菌药物治疗耐药菌感染,具体药物选择详见表4[28]–[74]。对于接受抗感染治疗72~96 h后,未能明确病原菌的患者,抗菌药物的调整流程见图1。在抗菌药物治疗无效时,需考虑真菌、病毒和其他病原菌感染的可能,参照相关指南和共识尽早开始抗真菌和抗其他病原菌的治疗。
表4. 中性粒细胞缺乏伴发热患者抗菌药物调整的具体药物选择.
| 病原菌 | 推荐药物 | 备注 |
| 肠杆菌科细菌 | ||
| 产ESBL肠杆菌科细菌 | 可供选择药物:头孢菌素类(头孢西丁、头孢美唑、头孢米诺);氧头孢烯类(拉氧头孢、氟氧头孢);β内酰胺酶抑制剂复合制剂(哌拉西林/他唑巴坦、头孢哌酮/舒巴坦);碳青霉烯类(亚胺培南/西司他丁、美罗培南、比阿培南) 联合治疗方案:碳青霉烯类+喹诺酮类或氨基糖苷类;β内酰胺酶抑制剂复合制剂+喹诺酮类或氨基糖苷类 |
①方案应结合药敏及个体因素选择[28]; ②大部分仅需单药治疗,仅少数严重感染需要联合用药[29] |
| 对碳青霉烯类耐药的肠杆菌科细菌 | 可供选择药物:替加环素;头孢他啶/阿维巴坦;多黏菌素类(多黏菌素B、多黏菌素E) 联合治疗药物:磷霉素钠;氨基糖苷类(阿米卡星、异帕米星);碳青霉烯类(亚胺培南、美罗培南、比阿培南) ①当碳青霉烯类MIC<16mg/L,须与其他药物联合使用[30]–[32],增加给药次数或剂量,延长滴注时间[33] ②当碳青霉烯类MIC>16mg/L,应避免使用[30] ③当多黏菌素B或EMIC≤2mg/L时可使用,XDR或PDR可同时辅助吸入多黏菌素E或B[34]–[36] ④当多黏菌素B或EMIC>2mg/L,联合使用敏感药物(如磷霉素钠、替加环素)的联合方案[37]–[38] 联合治疗方案: ①两药联合:头孢他啶/阿维巴坦+氨曲南;多黏菌素+替加环素;替加环素+氨基糖苷类;多黏菌素+碳青霉烯类;替加环素+碳青霉烯类;多黏菌素+磷霉素;替加环素+磷霉素;磷霉素+氨基糖苷类;美罗培南+厄他培南(双碳青霉烯联合方案) ②三药联合:多黏菌素+替加环素+碳青霉烯类;多黏菌素+磷霉素+碳青霉烯类;多黏菌素+替加环素+磷霉素;替加环素+氨基糖苷类+碳青霉烯类 |
①应以早期、足量、联合为原则[39]–[43]; ②针对我国流行的碳青霉烯酶,KPC:头孢他啶/阿维巴坦[44]–[46],IMP:氨曲南/阿维巴坦[47]; ③多黏菌素E剂量可增加至300 mg/d[48]–[49] ④美罗培南可用至2 g每8 h 1次,比阿培南可用至0.3~0.6 g每6~8 h 1次,均持续静脉输注3 h以上[50]–[51] ⑤两种碳青霉烯类联用:厄他培南+多利培南或亚胺培南或美罗培南[52]–[55],由于体内证据较少,需谨慎使用[52],[56]–[57] |
| 非发酵菌 | ||
| 铜绿假单胞菌 | 可供选择药物:头孢菌素类(头孢他啶、头孢吡肟、头孢噻利);碳青霉烯类(亚胺培南、美罗培南、比阿培南);β内酰胺酶抑制剂复合制剂(哌拉西林/他唑巴坦、头孢哌酮/舒巴坦);氟喹诺酮类(环丙沙星、左氧氟沙星);氨基糖苷类(阿米卡星、妥布霉素、异帕米星);氨曲南;多黏菌素类(多黏菌素B、多黏菌素E) 单药治疗:对于非MDR轻症患者,没有明显基础疾病时,可以用除氨基糖苷类外的具有抗铜绿假单胞菌活性的抗菌药物 联合治疗方案: ①MDR菌:抗铜绿假单胞菌β内酰胺类+氨基糖苷类或氟喹诺酮类或磷霉素;多黏菌素+β内酰胺类或环丙沙星或磷霉素;氨基糖苷类+环丙沙星或左氧氟沙星 ②XDR菌:多黏菌素+β内酰胺类+环丙沙星或磷霉素 ③XDR或PDR菌引起的肺炎:可在静脉用药的基础上,雾化吸入氨基糖苷类(如妥布霉素、阿米卡星)[58]–[59]或多黏菌素B或多黏菌素E[60]–[62] ④对碳青霉烯类耐药的铜绿假单胞菌:多黏菌素+头孢他啶/阿维巴坦;多黏菌素+β内酰胺类或环丙沙星或磷霉素或碳青霉烯类;β内酰胺类+氨基糖苷类或磷霉素;氨基糖苷类+环丙沙星或左氧氟沙星 |
①哌拉西林/他唑巴坦可用至4.5 g每6 h 1次,持续滴注3 h[63]; ②严重感染时,可增加剂量、延长滴注时间(2~3 h以上)[64]; ③双β内酰胺类联用可能有效,但需慎用,建议方案:头孢他啶或氨曲南+哌拉西林/他唑巴坦,头孢他啶+头孢哌酮/舒巴坦,头孢他啶或头孢吡肟+氨曲南[65]–[66] |
| 鲍曼不动杆菌 | 可供选择药物:舒巴坦及其复合制剂(头孢哌酮/舒巴坦、氨苄西林/舒巴坦);碳青霉烯类(亚胺培南/西司他丁、美罗培南、比阿培南);多黏菌素类(多黏菌素B、多黏菌素E);替加环素;四环素类(米诺环素、多西环素);氨基糖苷类(阿米卡星、异帕米星);喹诺酮类(环丙沙星、左氧氟沙星、莫西沙星) ①非MDR感染:根据药敏结果选用β内酰胺类抗菌药物 ②XDR或PDR:舒巴坦及其复合制剂+多黏菌素或替加环素或多西环素或碳青霉烯类;多黏菌素+碳青霉烯类;替加环素+碳青霉烯类或多黏菌素;舒巴坦及其复合制剂+多西环素+碳青霉烯类;舒巴坦及其复合制剂+替加环素+碳青霉烯类;亚胺培南/西司他丁+利福平+多黏菌素或妥布霉素 ③碳青霉烯类耐药鲍曼不动杆菌:多黏菌素+舒巴坦及其复合制剂或碳青霉烯类或利福平或氨基糖苷类或替加环素 |
①对于MDR感染,舒巴坦剂量可增至6~8 g/d[67]–[70]; ②碳青霉烯类可增加剂量、延长滴注时间[71]–[72] |
| 嗜麦芽窄食单胞菌 | 可供选择的药物:复方磺胺甲恶唑;β内酰胺酶抑制剂复合制剂(头孢哌酮/舒巴坦);氟喹诺酮类(左氧氟沙星、莫西沙星);替加环素;四环素类(米诺环素、多西环素);头孢菌素(头孢他啶) 联合治疗方案:复方磺胺甲恶唑+头孢哌酮/舒巴坦或氟喹诺酮类或四环素类或头孢他啶或多黏菌素;氟喹诺酮类或多黏菌素+头孢哌酮/舒巴坦或头孢他啶 |
①联合用药适用于严重感染、XDR或PDR菌株感染等[73]–[74]; ②嗜麦芽窄食单胞菌对碳青霉烯类天然耐药; ③替加环素的临床经验有限 |
| 肠球菌 | ||
| 耐万古霉素粪肠球菌 | 可供选择的药物:利奈唑胺、达托霉素、替加环素 | ①根据药敏结果及感染部位选择[2]; ②血流感染慎用替加环素 |
| 耐万古霉素屎肠球菌 | 可供选择的药物:利奈唑胺、替加环素 | |
| 葡萄球菌 | ||
| 万古霉素中介金黄色葡萄球菌 | 可供选择的药物:糖肽类、利奈唑胺、替加环素、达托霉素 | ①根据药敏结果及感染部位选择[2]; ②肺部感染宜选利奈唑胺 |
注:ESBL:超广谱β内酰胺酶;MIC:最低抑菌浓度;XDR:泛耐药;PDR:全耐药;MDR:多重耐药
图1. 经验性抗菌药物治疗后的中性粒细胞缺乏(粒缺)伴发热患者治疗调整流程.
a有研究报道经验性治疗后退热72 h,血流动力学稳定,感染的症状和体征消失,但ANC仍<0.5×109/L,可考虑停止抗菌药物经验治疗,但宜严密观察24~48 h
七、抗菌药物治疗的疗程
对于不明原因发热的粒缺患者抗菌药物经验性治疗后若ANC≥0.5×109/L、稳定退热48 h,可考虑停用抗菌药物;若ANC持续<0.5×109/L,抗菌药物可用至退热7 d后停药;此外,有研究报道经验性治疗后退热72 h,血流动力学稳定,感染的症状和体征消失,但ANC仍<0.5×109/L,可考虑停止抗菌药物经验治疗,但宜严密观察24~48 h,如果再出现发热尽早加用抗菌药物治疗[75]。ANC仍<0.5×109/L者如果已停用经验性抗菌药物,可考虑加用氟喹诺酮类药物预防治疗。微生物学证实及临床证实的感染治疗疗程取决于特定的微生物和感染部位[5],[76]–[80],详见表5。
表5. 中性粒细胞缺乏伴发热患者微生物证实及临床证实的感染治疗疗程.
| 感染类型 | 疗程 |
| 细菌性肺炎 | 7~14d |
| 细菌性鼻窦炎 | 7~14d |
| 皮肤软组织感染 | 7~14d |
| 腹部复杂感染 | 感染证据完全消失,ANC≥0.5×109/L |
| 存在深部组织感染、心内膜炎、化脓性血栓性静脉炎或接受适当抗菌药物治疗拔除导管后仍有持续性(>72h)血流感染 | >4周或至病灶愈合、症状消失 |
| 革兰阴性杆菌血症 | 10~14d |
| 革兰阳性球菌血症 | 7~14d(复杂感染及特殊病原菌需治疗较长时间) |
| 耐甲氧西林金黄色葡萄球菌血流感染 | 糖肽类药物、达托霉素等治疗至少14d,合并迁徙性病灶者适当延长 |
| 耐甲氧西林凝固酶阴性的葡萄球菌或肠球菌引起的血流感染 | 体温正常后持续治疗5~7d |
| 导管相关性血流感染 | 建议拔除导管,未拔除导管者适当延长疗程 |
八、抗菌药物预防给药的指征
对于高危粒缺患者,可以应用氟喹诺酮类药物预防,但氟喹诺酮类药物的预防仅可降低BSI发生率,对总体死亡率无影响[81]–[84],预防用药宜充分考虑本地区细菌耐药的流行病学特点及药物不良反应等。最佳的开始给药时间和给药持续时间尚无定论,推荐从粒缺开始应用至ANC>0.5×109/L或出现明显的血细胞恢复的证据[77],[85]。需要注意的是,长期使用喹诺酮类药物预防可能导致革兰阳性球菌感染,并可能导致多药耐药菌株的定植或感染增加及氟喹诺酮耐药菌血症菌株增加。对于低危患者及多药耐药菌定植的患者反对预防性应用抗菌药物。CRE定植患者不推荐预防用药。
References
- 1.Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia[J] Haematologica. 2013;98(12):1826–1835. doi: 10.3324/haematol.2013.091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Averbuch D, Cordonnier C, Livermore DM, et al. Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4, 2011)[J] Haematologica. 2013;98(12):1836–1847. doi: 10.3324/haematol.2013.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maschmeyer G, De Greef J, Mellinghoff SC, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL)[J] Leukemia. 2019;33(4):844–862. doi: 10.1038/s41375-019-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america[J] Clin Infect Dis. 2011;52(4):e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 5.Gudiol C, Aguilar-Guisado M, Azanza JR, et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), the Spanish Network for Research in Infectious Diseases (REIPI) and the Spanish Society of Haematology and Haemotherapy (SEHH) on the management of febrile neutropenia in patients with hematological malignancies[J] Enferm Infecc Microbiol Clin. 2020;38(4):174–181. doi: 10.1016/j.eimc.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Yan CH, Wang Y, Mo XD, et al. Incidence, Risk Factors, Microbiology and Outcomes of Pre-engraftment Bloodstream Infection After Haploidentical Hematopoietic Stem Cell Transplantation and Comparison With HLA-identical Sibling Transplantation[J] Clin Infect Dis. 2018;67(suppl_2):S162–S173. doi: 10.1093/cid/ciy658. [DOI] [PubMed] [Google Scholar]
- 7.Zheng C, Tang B, Zhu X, et al. Pre-engraftment bloodstream infections in acute leukemia patients undergoing unrelated cord blood transplantation following intensified myeloablative conditioning without ATG[J] Ann Hematol. 2017;96(1):115–124. doi: 10.1007/s00277-016-2828-2. [DOI] [PubMed] [Google Scholar]
- 8.闫 晨华, 徐 婷, 郑 晓云, et al. 中国血液病患者中性粒细胞缺乏伴发热的多中心、前瞻性流行病学研究[J] 中华血液学杂志. 2016;37(3):177–182. doi: 10.3760/cma.j.issn.0253-2727.2016.03.001. [DOI] [Google Scholar]
- 9.朱 骏, 周 一飞, 白 海涛, et al. 中性粒细胞缺乏伴发热患者临床分离菌的分布及药敏分析[J] 中国感染与化疗杂志. 2016;16(3):241–246. doi: 10.16718/j.1009-7708.2016.03.001. [DOI] [Google Scholar]
- 10.朱 骏, 胡 炯, 毛 原飞, et al. 上海地区粒细胞缺乏伴发热血液病患者致病细菌的分布及耐药性分析的多中心、回顾性研究[J] 中华血液学杂志. 2017;38(11):945–950. doi: 10.3760/cma.j.issn.0253-2727.2017.11.009. [DOI] [Google Scholar]
- 11.Zhu J, Zhou K, Jiang Y, et al. Bacterial Pathogens Differed Between Neutropenic and Non-neutropenic Patients in the Same Hematological Ward: An 8-Year Survey[J] Clin Infect Dis. 2018;67(suppl_2):S174–S178. doi: 10.1093/cid/ciy643. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Feng S, Sun G, et al. Extensively drug-resistant Gram-negative bacterial bloodstream infection in hematological disease[J] Infect Drug Resist. 2019;12:481–491. doi: 10.2147/IDR.S191462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.徐 春晖, 朱 国庆, 林 青松, et al. 2014-2018年成人血液病患者血流感染病原菌分布及耐药性单中心结果分析[J] 中华血液学杂志. 2020;41(8):643–648. doi: 10.3760/cma.j.issn.0253-2727.2020.08.005. [DOI] [Google Scholar]
- 14.朱 国庆, 徐 春晖, 林 青松, et al. 2014-2018年儿童恶性血液病患者中性粒细胞缺乏期血流感染病原学和临床特征分析[J] 中华血液学杂志. 2020;41(8):655–660. doi: 10.3760/cma.j.issn.0253-2727.2020.08.007. [DOI] [Google Scholar]
- 15.卓 楚越, 郭 颖异, 刘 宁静, et al. 广东地区血液科血流感染的病原菌流行病学分析[J] 中华血液学杂志. 2020;41(12):996–1001. doi: 10.3760/cma.j.issn.0253-2727.2020.12.005. [DOI] [Google Scholar]
- 16.Shelburne SA, Sahasrabhojane P, Saldana M, et al. Streptococcus mitis strains causing severe clinical disease in cancer patients[J] Emerg Infect Dis. 2014;20(5):762–771. doi: 10.3201/eid2005.130953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.胡 付品, 朱 德妹, 汪 复, et al. 2014年CHINET中国细菌耐药性监测[J] 中国感染与化疗杂志. 2015;15(5):401–410. doi: 10.3969/j.issn.1009-7708.2015.05.001. [DOI] [Google Scholar]
- 18.胡 付品, 郭 燕, 朱 德妹, et al. 2016年中国CHINET细菌耐药性监测[J] 中国感染与化疗杂志. 2017;17(5):481–491. doi: 10.16718/j.1009-7708.2017.05.001. [DOI] [Google Scholar]
- 19.胡 付品, 郭 燕, 朱 德妹, et al. 2019年CHINET三级医院细菌耐药监测[J] 中国感染与化疗杂志. 2020;20(3):233–243. doi: 10.16718/j.1009-7708.2020.03.001. [DOI] [Google Scholar]
- 20.Zhang Y, Wang Q, Yin Y, et al. Epidemiology of Carbapenem-Resistant Enterobacteriaceae Infections: Report from the China CRE Network[J] Antimicrob Agents Chemother. 2018;62(2) doi: 10.1128/AAC.01882-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Sun QL, Shen YB, et al. Rapid increase in prevalence of Carbapenem-Resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China[J] J Clin Microbiol. 2018;56(4):e01932–17. doi: 10.1128/JCM.01932-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zhai W, Lin Q, et al. Carbapenem-resistant Enterobacteriaceae in hematological patients: Outcome of patients with Carbapenem-resistant Enterobacteriaceae infection and risk factors for progression to infection after rectal colonization[J] Int J Antimicrob Agents. 2019;54(4):527–529. doi: 10.1016/j.ijantimicag.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Yang TT, Luo XP, Yang Q, et al. Different screening frequencies of carbapenem-resistant Enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: which one is better?[J] Antimicrob Resist Infect Control. 2020;9(1):49. doi: 10.1186/s13756-020-0706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Wu X, Cheng Q, et al. Inappropriate initial antimicrobial therapy for hematological malignancies patients with Gram-negative bloodstream infections[J] Infection. 2020;48(1):109–116. doi: 10.1007/s15010-019-01370-x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Zheng Y, Dong F, et al. Epidemiology of Febrile Neutropenia Episodes with Gram-Negative Bacteria Infection in Patients Who Have Undergone Chemotherapy for Hematologic Malignancies: A Retrospective Study of 10 Years' Data from a Single Center[J] Infect Drug Resist. 2020;13:903–910. doi: 10.2147/IDR.S241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.王 明贵. 广泛耐药革兰阴性菌感染的实验诊断、抗菌治疗及医院感染控制: 中国专家共识[J] 中国感染与化疗杂志. 2017;17(1):82–93. doi: 10.16718/j.1009-7708.2017.01.015. [DOI] [Google Scholar]
- 27.中华医学会血液学分会, 中国医师协会血液科医师分会. 血液肿瘤患者碳青霉烯类耐药的肠杆菌科细菌 (CRE) 感染的诊治与防控中国专家共识 (2020年版)[J] 中华血液学杂志. 2020;41(11):881–889. doi: 10.3760/cma.j.issn.0253-2727.2020.11.001. [DOI] [Google Scholar]
- 28.Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society[J] Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.周 华, 李 光辉, 陈 佰义, et al. 中国产超广谱β-内酰胺酶肠杆菌科细菌感染应对策略专家共识[J] 中华医学杂志. 2014;94(24):1847–1856. doi: 10.3760/cma.j.issn.0376-2491.2014.24.003. [DOI] [Google Scholar]
- 30.Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae[J] Curr Opin Infect Dis. 2016;29(6):583–594. doi: 10.1097/QCO.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 31.Rafailidis PI, Falagas ME. Options for treating carbapenem-resistant Enterobacteriaceae[J] Curr Opin Infect Dis. 2014;27(6):479–483. doi: 10.1097/QCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 32.Sharma R, Patel S, Abboud C, et al. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: pharmacodynamics and morphological changes[J] Int J Antimicrob Agents. 2017;49(2):224–232. doi: 10.1016/j.ijantimicag.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems?[J] Clin Microbiol Infect. 2011;17(8):1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 34.Giunta V, Ferrer M, Esperatti M, et al. ICU-acquired pneumonia with or without etiologic diagnosis: a comparison of outcomes[J] Crit Care Med. 2013;41(9):2133–2143. doi: 10.1097/CCM.0b013e31828a453b. [DOI] [PubMed] [Google Scholar]
- 35.Doshi NM, Cook CH, Mount KL, et al. Adjunctive aerosolized colistin for multi-drug resistant gram-negative pneumonia in the critically ill: a retrospective study[J] BMC Anesthesiol. 2013;13(1):45. doi: 10.1186/1471-2253-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumbarello M, De Pascale G, Trecarichi EM, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria[J] Chest. 2013;144(6):1768–1775. doi: 10.1378/chest.13-1018. [DOI] [PubMed] [Google Scholar]
- 37.Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options[J] Drug Resist Updat. 2010;13(4-5):132–138. doi: 10.1016/j.drup.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Morrill HJ, Pogue JM, Kaye KS, et al. Treatment Options for Carbapenem-Resistant Enterobacteriaceae Infections[J] Open Forum Infect Dis. 2015;2(2):ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akova M, Daikos GL, Tzouvelekis L, et al. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria[J] Clin Microbiol Infect. 2012;18(5):439–448. doi: 10.1111/j.1469-0691.2012.03823.x. [DOI] [PubMed] [Google Scholar]
- 40.Falagas ME, Lourida P, Poulikakos P, et al. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence[J] Antimicrob Agents Chemother. 2014;58(2):654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee GC, Burgess DS. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports[J] Ann Clin Microbiol Antimicrob. 2012;11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tascini C, Viaggi B, Menichetti F. Comment on: Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study[J] J Antimicrob Chemother. 2015;70(10):2921. doi: 10.1093/jac/dkv168. [DOI] [PubMed] [Google Scholar]
- 43.Tzouvelekis LS, Markogiannakis A, Piperaki E, et al. Treating infections caused by carbapenemase-producing Enterobacteriaceae[J] Clin Microbiol Infect. 2014;20(9):862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 44.Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination[J] Drugs. 2013;73(2):159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 45.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia[J] Antimicrob Agents Chemother. 2017;61(8):e00883–17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacVane SH, Crandon JL, Nichols WW, et al. In vivo efficacy of humanized exposures of Ceftazidime-Avibactam in comparison with Ceftazidime against contemporary Enterobacteriaceae isolates[J] Antimicrob Agents Chemother. 2014;58(11):6913–6919. doi: 10.1128/AAC.03267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R, Kim A, Tanudra MA, et al. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam[J] J Antimicrob Chemother. 2015;70(9):2618–2626. doi: 10.1093/jac/dkv132. [DOI] [PubMed] [Google Scholar]
- 48.Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients[J] Int J Antimicrob Agents. 2010;35(2):194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Dalfino L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study[J] Clin Infect Dis. 2012;54(12):1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falagas ME, Tansarli GS, Ikawa K, et al. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis[J] Clin Infect Dis. 2013;56(2):272–282. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]
- 51.Levy Hara G, Gould I, Endimiani A, et al. Detection, treatment, and prevention of carbapenemase-producing Enterobacteriaceae: recommendations from an International Working Group[J] J Chemother. 2013;25(3):129–140. doi: 10.1179/1973947812Y.0000000062. [DOI] [PubMed] [Google Scholar]
- 52.Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae[J] Antimicrob Agents Chemother. 2013;57(6):2900–2901. doi: 10.1128/AAC.00188-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 54.Wiskirchen DE, Crandon JL, Nicolau DP. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae[J] Int J Antimicrob Agents. 2013;41(6):582–585. doi: 10.1016/j.ijantimicag.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Fredborg M, Sondergaard TE, Wang M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae[J] Diagn Microbiol Infect Dis. 2017;88(4):355–360. doi: 10.1016/j.diagmicrobio.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Bulik CC, Tessier PR, Keel RA, et al. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse gram-negative organisms[J] Antimicrob Agents Chemother. 2012;56(1):544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae[J] Antimicrob Agents Chemother. 2013;57(5):2388–2390. doi: 10.1128/AAC.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hallal A, Cohn SM, Namias N, et al. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study[J] Surg Infect (Larchmt) 2007;8(1):73–82. doi: 10.1089/sur.2006.051. [DOI] [PubMed] [Google Scholar]
- 59.Arnold HM, Sawyer AM, Kollef MH. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia[J] Respir Care. 2012;57(8):1226–1233. doi: 10.4187/respcare.01556. [DOI] [PubMed] [Google Scholar]
- 60.Michalopoulos A, Fotakis D, Virtzili S, et al. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study[J] Respir Med. 2008;102(3):407–412. doi: 10.1016/j.rmed.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Ioannidou E, Siempos II, Falagas ME. Administration of antimicrobials via the respiratory tract for the treatment of patients with nosocomial pneumonia: a meta-analysis[J] J Antimicrob Chemother. 2007;60(6):1216–1226. doi: 10.1093/jac/dkm385. [DOI] [PubMed] [Google Scholar]
- 62.Pereira GH, Muller PR, Levin AS. Salvage treatment of pneumonia and initial treatment of tracheobronchitis caused by multidrug-resistant Gram-negative bacilli with inhaled polymyxin B[J] Diagn Microbiol Infect Dis. 2007;58(2):235–240. doi: 10.1016/j.diagmicrobio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 63.吕 扬, 闫 昭, 王 东浩, et al. 哌拉西林/他唑巴坦延长输注时间的优化给药方案与传统给药方式用于治疗医院获得性肺炎的研究[J] 中华危重病急救医学. 2013;25(8):479–483. doi: 10.3760/cma.j.issn.2095-4352.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 64.中华医学会呼吸病学分会感染学组. 铜绿假单胞菌下呼吸道感染诊治专家共识[J] 中华结核和呼吸杂志. 2014;37(1):9–15. doi: 10.3760/cma.j.issn.1001-0939.2014.01.005. [DOI] [Google Scholar]
- 65.Chinese XDR Consensus Working Group. Guan X, He L, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement[J] Clin Microbiol Infect. 2016;22 Suppl 1:S15–25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Dupont H, Marciniak S, Zogheib E, et al. Use of aztreonam in association with cefepime for the treatment of nosocomial infections due to multidrug-resistant strains of Pseudomonas aeruginosa to β-lactams in ICU patients: A pilot study[J] Anaesth Crit Care Pain Med. 2015;34(3):141–144. doi: 10.1016/j.accpm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Munoz-Price LS, Weinstein RA. Acinetobacter infection[J] N Engl J Med. 2008;358(12):1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 68.Betrosian AP, Frantzeskaki F, Xanthaki A, et al. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia[J] J Infect. 2008;56(6):432–436. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Fishbain J, Peleg AY. Treatment of Acinetobacter infections[J] Clin Infect Dis. 2010;51(1):79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 70.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management[J] Curr Opin Infect Dis. 2010;23(4):332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 71.陈 佰义, 何 礼贤, 胡 必杰, et al. 中国鲍曼不动杆菌感染诊治与防控专家共识[J] 中国医药科学. 2012;2(8):3–8. [Google Scholar]
- 72.Neonakis IK, Spandidos DA, Petinaki E. Confronting multidrug-resistant Acinetobacter baumannii: a review[J] Int J Antimicrob Agents. 2011;37(2):102–109. doi: 10.1016/j.ijantimicag.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 73.周 华, 李 光辉, 卓 超, et al. 中国嗜麦芽窄食单胞菌感染诊治和防控专家共识[J] 中华医学杂志. 2013;93(16):1203–1213. doi: 10.3760/cma.j.issn.0376-2491.2013.16.002. [DOI] [Google Scholar]
- 74.Abbott IJ, Slavin MA, Turnidge JD, et al. Stenotrophomonas maltophilia: emerging disease patterns and challenges for treatment[J] Expert Rev Anti Infect Ther. 2011;9(4):471–488. doi: 10.1586/eri.11.24. [DOI] [PubMed] [Google Scholar]
- 75.Aguilar-Guisado M, Espigado I, Martín-Peña A, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial[J] Lancet Haematol. 2017;4(12):e573–e583. doi: 10.1016/S2352-3026(17)30211-9. [DOI] [PubMed] [Google Scholar]
- 76.Heinz WJ, Buchheidt D, Christopeit M, et al. Diagnosis and empirical treatment of fever of unknown origin (FUO) in adult neutropenic patients: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO)[J] Ann Hematol. 2017;96(11):1775–1792. doi: 10.1007/s00277-017-3098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt-Hieber M, Teschner D, Maschmeyer G, et al. Management of febrile neutropenia in the perspective of antimicrobial de-escalation and discontinuation[J] Expert Rev Anti Infect Ther. 2019;17(12):983–995. doi: 10.1080/14787210.2019.1573670. [DOI] [PubMed] [Google Scholar]
- 78.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America[J] Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung N, Rieg S. Essentials in the management of S. aureus bloodstream infection[J] Infection. 2018;46(4):441–442. doi: 10.1007/s15010-018-1130-8. [DOI] [PubMed] [Google Scholar]
- 80.Chaves F, Garnacho-Montero J, Del Pozo JL, et al. Diagnosis and treatment of catheter-related bloodstream infection: Clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC)[J] Med Intensiva. 2018;42(1):5–36. doi: 10.1016/j.medin.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Mikulska M, Averbuch D, Tissot F, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines[J] J Infect. 2018;76(1):20–37. doi: 10.1016/j.jinf.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Alexander S, Fisher BT, Gaur AH, et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children With Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial[J] JAMA. 2018;320(10):995–1004. doi: 10.1001/jama.2018.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owattanapanich W, Chayakulkeeree M. Efficacy of levofloxacin as an antibacterial prophylaxis for acute leukemia patients receiving intensive chemotherapy: a systematic review and meta-analysis[J] Hematology. 2019;24(1):362–368. doi: 10.1080/16078454.2019.1589706. [DOI] [PubMed] [Google Scholar]
- 84.Egan G, Robinson PD, Martinez J, et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials[J] Cancer Med. 2019;8(10):4536–4546. doi: 10.1002/cam4.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lehrnbecher T, Fisher BT, Phillips B, et al. Guideline for Antibacterial Prophylaxis Administration in Pediatric Cancer and Hematopoietic Stem Cell Transplantation[J] Clin Infect Dis. 2020;71(1):226–236. doi: 10.1093/cid/ciz1082. [DOI] [PMC free article] [PubMed] [Google Scholar]