Abstract
Although osseointegration and clinical success of titanium (Ti)-implanted materials depend on neovascularization in the reactional peri-implant tissue, very little has been achieved considering the Ti-molecules release on the behavior of endothelial cells. To address this issue, we challenged endothelial cells (HUVECs) with Ti-enriched medium obtained from two types of commercial titanium surfaces [presenting or not dual-acid etching (DAE)] up to 72 h to allow molecular machinery analysis. Our data show that the Ti-enriched medium provokes significant stimulus of angiogenesis-related machinery in endothelial cells by upexpressing VEGFR1, VEGFR2, VEGF, eNOS, and iNOS genes, while the PI3K/Akt signaling pathway was also significantly enhanced. As PI3K/AKT signaling was related to angiogenesis in response to vascular endothelial growth factor (VEGF), we addressed the importance of PI3K/Akt upon Ti-enriched medium responses by concomitantly treating the cells with wortmannin, a well-known PI3K inhibitor. Wortmannin suppressed the angiogenic factors, because VEGF, VEGFR1, and eNOS genes were downregulated in those cells, highlighting the importance of PI3K/AKT signaling on driving angiogenic phenotype and angiogenesis performance within the peri-implant tissue reaction. In conjunction, these data reinforce that titanium-implantable devices modify the metabolism of surrounding cells, such as endothelial cells, probably coupling osteogenesis and angiogenesis processes in peri-implant tissue and then contributing to successfully osseointegration of biomedical titanium-based devices.

Introduction
Titanium-based implants are widely used in dentistry and medical fields contributing to therapies for edentulism and stabilization of bone fractures, respectively, mainly due to their properties including mechanical strength, high corrosion resistance, low toxicity, and adequate biocompatibility acceptance [1–3]. However, current studies have shown that titanium alloys are not inert as once believed, and they dynamically release particles and molecules to their embedding microenvironment affecting the surrounding cell behavior, as osteoblast-like cells, and the vascular endothelium that irrigates the injured tissue and then affects the endothelial cells (ECs) [4, 5]. Because of that and especially considering vascularization, which is an important step to regenerate the tissue surrounding the implants, studies are necessary to address the effect of titanium on ECs.
For the biocompatibility and performance of biomaterials, some properties of the biomaterial surfaces should be consider critical issues that trigger signals of host tissue interactions [6]. As previously mentioned, adequate vascularization is required for successful tissue regeneration. Osseointegration is an important process that occurs in the appositional interface between tissue and biomaterial, in which an orchestrated action of bone formation and bone resorption helps to maintain the health of neoformed bone.
To improve the osseointegration, reducing patient recuperation time and decreasing implant-associated complications, several modifications on biomaterial surfaces have been widely proposed, such as nanotopographical and acid etching [7, 8]. These physicochemical properties require more investigation about their effects on biological performance for full comprehension of the mechanism underlying wound healing after an implant is introduced [1]. To better address this issue, our group has investigated the interaction between the biomaterial and the host tissue in a direct [9] and indirect way [10], presenting specific signaling cascades triggered by this interface. Although titanium has been considered for decades a resistant and inert material, it can release molecules triggering dynamic responses in its microenvironment and surrounding tissues [9], and it is reasonable to hypothesize that it could compromise the role of blood vessels during bone growth by impairing EC phenotypes. Thus, better understanding the properties of titanium-enriched medium on angiogenesis in wound healing and osseointegration process is urgently required [11, 12].
As important members within the angiogenesis process, EC is housed in the lumen of the vessel, is constantly exposed to the blood flow forces, regulates the blood supplement to tissue, and is crucial in the angiogenesis process [13]. The formation of new blood vessel supports new bone formation by transporting fundamentals constituents, such as nutrients, regulatory factors, oxygen, and osteoprogenitor cells [12]. Some authors have already reported coupling angiogenesis with osteogenesis, which are both important and dynamic processes to drive bone growth and remodeling during wound healing [14].
In bone development and healing, the angiogenesis and osteogenesis are coupled processes, through complex intercellular signaling, that also exists in the osseointegration and biomedical devices, to ensure an appositional bone development on the implanted device [15]. First, some growth factors are released by extravasate platelets from injured vessels on the alveolar bone site, such as PDGF (platelet-derived growth factor), TGF-β (transforming growth factor beta), FGF (fibroblast growth factor), and VEGF (vascular endothelial growth factor). Among them, VEGF is one of the main factors responsible for angiogenesis promotion and is crucial to osteogenesis [16].
Considering the pivotal role of ECs in wound healing [17], their biological responses to different biomaterials must be investigated. Thus, this study aims to evaluate the behavior of human umbilical vein endothelial cell (HUVEC) exposed to a titanium-enriched medium by exploring molecular approaches focusing on comprehending intracellular pathways involved with angiogenesis and cell survival, and proliferation signaling. The cascade of events for wound healing is complex and involves highly regulated processes, such as angiogenesis. The comprehension of these processes at the cellular and molecular level is far from complete [18]. In this context, genes such as VEGF and its receptors, VEGFR1 and VEGFR2, are relevant as they are closely related to the angiogenesis process and are required for tissue wound healing [19]. Downstream to activate receptor tyrosine kinases, protein kinase B (PKB or Akt) is a multifaceted protein and plays an important role in cell metabolism, growth, proliferation, and survival. Its activation is controlled by a multi-step process, mainly involving phosphoinositide-3-kinase (PI3K) [20–22]. Specifically, we evaluated whether the Ti-enriched medium was able to modulate EC performance. Summarizing, our data show that the Ti-enriched medium requires an upmodulation of PI3K/AKT signaling in ECs to maintain their angiogenic phenotype.
Materials and methods
Reagents, TiO2 alloys, antibodies, and primers
Two different titanium surfaces (discs) were investigated in this study, distinguished by the surface properties, as follows: Machined (Wo/DAE), and Dual Acid-Etched (W/DAE). The metallic TiO2-based alloys were generously donated by the S.I.N. (São Paulo, SP, Brazil). The cell culture flasks were purchased from TPP (Trasadingen, Switzerland), and the cell culture reagents from Nutricell (Campinas, SP, Brazil). Ripa buffer (R0278), Phosphatase inhibitor cocktail 2 (P5726), and bovine serum albumin (A7906) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Gotaq qPCR master mix (A6002) was purchased from PROMEGA (Madison, WI, USA). The following antibodies were purchased from Cell Signaling (Danvers, MA, USA): Akt Antibody (4691P, 60 kDa); PhosphoAkt (S473) Antibody (4060P, 60 kDa); Anti-Rabbit IgG (7074).
Cell culture and PI3K inhibition
HUVECs were used in this study. ECs were cultivated in RPMI medium (Nutricell, Campinas, SP, Brazil) supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% fetal bovine serum (FBS), and maintained at 37 °C and 5% CO2. HUVECs were cultivated in culture flasks until proper amount and then were seeded in traditional culture dishes until adhesion, when the culture medium was exchanged for the different medium treatments as described below, for 72 h. To investigate the role of PI3K in the signaling behavior of ECs, we again carried out the treatment as described, but using a culture medium containing Wortmannin (5 μM), a PI3K inhibitor.
TiO2-enriched medium obtaining
To prepare the TiO2-enriched medium, dual-acid-etching (DAE) treating surface (named W/DAE) and the machined surfaces (named Wo/DAE), the experimental alloys (n = 6) were incubated in cell culture media (RPMI) without FBS up to 24 h at 37 °C, 5% CO2, and 95% humidity [0.2 g/mL (w/v); ISO 10993:2016]. The TiO2-enriched medium contains molecules released from those metallic alloys and might affect the biology of ECs. To test this hypothesis, the TiO2-enriched medium was further used to treat the ECs.
Total mRNA isolation and RT‐qPCR analysis
After treatment, the cells were harvested and total mRNA isolated using Ambion TRIzol Reagent (Life Sciences, Thermo Fisher Scientific Inc., Waltham, MA, USA) and treated with DNase I (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) synthesis was performed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Real‐Time qPCR was carried out in 10 μl, containing PowerUpTM SYBRTM Green Master Mix 2× (5 μl; Applied Biosystems, Foster City, CA, USA), 0.4 μM of each primer, 50 ng of cDNA, and nuclease-free H2O. Results were expressed as relative amounts of the transcripts using glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) and 18S as reference genes (housekeeping gene), using the cycle threshold (Ct) method. Primers and run details are described in Table 1.
Table 1.
Expression primers sequences and qPCR cycle conditions
| Gene | Primer | 5′-3′ Sequence | Work condition |
|---|---|---|---|
| AKT | Forward 1 | CAGCGCGGCCCGAAGGAC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Forward 2 | GGACTCCCGTTTGCGCCAGT | ||
| Reverse | GACGCTCACGCGCTCCTCTC | ||
| VEGFR1 | Forward | CAGGCCCAGTTTCTGCCATT | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | TTCCAGCTCAGCGTGGTCGTA | ||
| VEGFR2 | Forward | CCAGCAAAAGCAGGGAGTCTGT | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | TGTCTGTGTCATCGGAGTGATATCC | ||
| VEGF | Forward | TGCAGATTATGCGGATCAAACC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | TGCATTCACATTTGTTGTGCTGTAG | ||
| INOS | Forward | TGGATGCAACCCCATTGTC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | CCCGCTGCCCCAGTTT | ||
| ENOS | Forward | TATTTGATGCTCGGGACTGC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | AAGATTGCCTCGGTTTGTTG | ||
| GAPDH | Forward | AGGCCGGTGCTGAGTATGTC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | TGCCTGCTTC ACCACCTTCT | ||
| 18S | Forward | CGGACAGGATTGACAGATTGATAGC | 95 °C—3 s; 55 °C—8 s; 72 °C—20 s |
| Reverse | TGCCAGAGTCTCGTTCGTTATCG |
Western blotting
After 72 h of treatment with TiO2-enriched medium, the challenged HUVECs were washed in ice-cold PBS and protein extracts were obtained using a RIPA lysis buffer (Sigma Aldrich, St. Louis, Missouri, USA) and supplemented with a cocktail of antiproteases and anti-phosphatases (Sigma Aldrich, St. Louis, MO, USA) up to 1 h on ice. Protein extracts were cleared by centrifugation 14,000 rpm for 15 min at 4 °C. The precipitate was then resuspended in 100 μL of RIPA lysis buffer (Sigma Aldrich, St. Louis, Missouri, USA). The protein extracts were clarified and the protein concentration determined by the Lowry method [23]. Protein extracts were resolved by SDS-PAGE and later transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). An equal volume of gel loading buffer [100 mmol L−1 Tris–HCl (pH 6.8), 200 mmol L−1 dithiothreitol, 4% SDS, 0.1% bromophenol blue, and 20% glycerol] was added to the samples and boiled for 5 min at 95 °C. Aliquots of the samples (75–100 μg/lane) were resolved into SDS-PAGE (8, 10, or 12% gels) and later transferred to PVDF membranes (Millipore, USA), which were blocked with 5% nonfat dry milk dissolved in tris-buffered saline (TBS)-Tween-20 (0.05%) and then incubated overnight with the appropriate primary antibody (1:1000) at 4 °C. After 1×-washing in TBS–Tween-20 (0.05%) and 2×-washing in TBS, the membranes were incubated with horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse IgGs antibodies (1:2000), diluted in blocking buffer for 1 h. Immunoreactive bands were detected using Enhance Chemiluminescence (ECL, Pierce, USA).
Statistical analyses
Results were represented as mean ± standard deviation (SD). The samples assumed a normal distribution with p < 0.05 considered statistically significant and p < 0.001 considered highly significant. In the experiment with >2 groups, we used one-way ANOVA with multiple comparisons, to compare all pairs of groups. In this case, the significance level was considered when alpha = 0.05 (95% confidence interval). The software used was GraphPad Prism 7 (GraphPad Software, USA).
Results and discussion
The biological responses of tissue surrounding the implanted metallic biomaterials require a well-coordinated cascade of events involving a coupled network between undifferentiated cells and angiocrine mediators released by ECs. It is known that the correct coupling between endothelium and bone guarantees proper cell differentiation and tissue regeneration, resulting in successful osseointegration. Although endothelium is not properly in close contact with the implanted materials, we have recently reported that titanium-based devices release a considerable amount of titanium and this interferes in cell metabolism [9, 10, 24–26], which could affect cells away. In this study, to evaluate whether the surface particles released from Ti affect the metabolism of ECs, we previously prepared a Ti-enriched medium and used it to further challenge HUVEC cells up to 72 h (Fig. 1). We suggest Ti-enriched medium mimics the Ti released when the devices are implanted in a host, covering an indirect and alternative model to study the effect of Ti on eukaryotic and adherent cells, as has been suggested previously. The performance of ECs was estimated by evaluating widely accepted angiogenic biomarkers at the mRNA levels, such as: VEGFR1, VEGFR2, VEGF, eNOS, and iNOS.
Fig. 1.
Experimental design of this study. To evaluate the biological response of endothelial cells to TiO2, the Ti-enriched medium was obtained by incubating Ti-based materials in the cell culture medium (FBS free), as recommended by ISO 10993:2016 (part 5). Thereafter, the Ti-enriched medium was used to challenge endothelial cells up to 72 h, when the samples were collected for the analysis. The main focus here was to evaluate whether there is some association of survival signaling an angiogenic stimulus of titanium, which could better support comprehension about their biocompatibility and dynamic relationship with the surrounding tissue
Within the repertoire of cascading events involved in the biological response to biomaterials, it is expected that viable cells interacting with biomaterials surfaces drive their biocompatibility [27, 28]. The surface modifications, such as DAE of titanium implants, have produced a positive effect on osseointegration in newly formed and native bone [29], triggering different signals in osteoblast-like cells [10], and our data show that these surfaces also trigger distinct responses in ECs. Experimentally, we proposed intracellular signaling pathways to evaluate cell viability mainly by considering PI3K/PKB/Akt upstream stimulus culminating in gene expression related to cell proliferation and survival [30, 31]. In addition, as these protein activities are regulated by phosphorylation, the effectivity of this signaling can be measured by the specific phosphorylation profile [32]. In turn, Akt is a serine/threonine protein kinase that is activated by a number of growth factors and cytokines in an upstream phosphatidylinositol-3 kinase-dependent manner.
Here, the PI3K/Akt signaling was evaluated by measuring the phosphorylation profile of Akt at S473. Phosphorylation of Akt at S473 guarantees its activity, leading to additional substrate-specific phosphorylation events in both the cytoplasm and nucleus, including inhibitory phosphorylation of the pro-apoptotic FOXO proteins [33, 34]. Figure 2 shows that the phosphorylation of PKB/Akt was significantly increased in response to Ti-enriched medium, in both evaluated surfaces: w/DAE and wo/DAE. Importantly, our data demonstrated an upexpression of mRNA of Akt in ECs responding to the Ti-WoDAE-enriched medium (Fig. 2a). Variations in titanium surfaces can modulate the PI3K/Akt pathway differently in osteoblast-like cells [35], and we observed that distinct titanium surfaces developed different modulations in this pathway in ECs, seen in the changes to the gene expression and protein AKT phosphorylation pattern. Altogether, these data strongly suggest the importance of this signaling in challenged ECs, maybe modulating their viability and phenotype, as fully active PKB/Akt mediates numerous cellular functions including angiogenesis, metabolism, growth, proliferation, survival, protein synthesis, and transcription [33, 36].
Fig. 2.
Survival signaling was evaluated by measuring the phosphorylation profile of AKT. To understand whether survival signaling was required by endothelial cells responding to Ti-enriched medium (72 h), the cells were subjected to Ti-enriched medium obtained by incubating two different conditions of titanium discs [double acid-etching (DAE) treatment (W/DAE) and without DAE (Wo/DAE)] into cell culture medium up to 24 h. After subjecting the cells up to 72 h, the samples were obtained to allow the gene expression of AKT (a) and protein performance (b, c) by evaluating AKT phosphorylation. GADPH was considered a housekeeping gene. Statistics: the value obtained to Ctrl was considered 1, and the relative values obtained to W/DAE or Wo/DAE are shown in fold‐changes. One-way ANOVA with multiple comparisons were applied to compare all pairs of groups. Differences were considered significant when *p = 0.04 and ****p < 0.0001 when compared with the Ctrl; and ****p < 0.0001 when compared with W/DAE group
Later, the repertoire of genes involved with angiogenic phenotype was also investigated, and Fig. 3 provides a panorama where Ti medium enriched by Wo/DAE condition requires a significant activity of those genes, such as VEGFR1 (Fig. 3a), VEGFR2 (Fig. 3b), VEGF (Fig. 3c), iNOS (Fig. 3d), and eNOS (Fig. 3e). Importantly, VEGFR2 (Fig. 3b) and VEGF (Fig. 3c) genes are stimulated by both surfaces investigated here (W/DAE and Wo/DA). This different biological consequences between the surfaces can be explained by the concentration of titanium released into the medium. To date, VEGF has long been recognized as the key regulator of vascular development and function in health [37]. In addition, Kitamura et al. [38] demonstrated that the Akt/Girdin signaling pathway is essential in VEGF-mediated postneonatal angiogenesis. Nonetheless, they reveal that Girdin knockdown severely impairs cell migration and tube formation by HUVECs, and that Girdin−/− mice display significant defects in postnatal microvascular remodeling in the retina and the sprouting of vessels from aortae [38]. In conjunction, it seems acceptable to suggest that Ti released by implantable devices could also dynamically modulate the phenotype of ECs, which explains, in association with their effect on osteoblasts [9, 10, 25, 26, 39–42], the success of titanium in implantology applications, since angiogenic factors by ECs are required to drive angiogenesis that modulates the traffic of nutrients, growth factors, and undifferentiated cells to the site where the tissue is regenerate. We are convinced there is a coupling of mechanisms considering that cells respond to titanium directly (mainly osteogenic cells adhering on the surfaces directly) and indirectly (undifferentiated cells and ECs/angiogenesis), where these devices coordinate the surrounding microenvironment and drive the osseointegration-related mechanism.
Fig. 3.
Angiogenic stimulus of titanium (Ti) on endothelial cells. The samples were collected respecting the already described methodology to allow qPCR technology performance. To address the angiocrine effect of Ti on endothelial cells, we investigated VEGFR1 (a), VEGFR2 (b), VEGF (c), eNOS (d), and iNOS (e) genes. The GADPH gene was considered the housekeeping gene and used to normalize the values. Statistics: One-way ANOVA with multiple comparisons was used to compare all pairs of groups, and the differences were considered significant when a VEGFR1 ***p = 0.0009 and **p = 0.0019; b VEGFR2 **p = 0.0014 and **p = 0.0032; c VEGF **p = 0.004 and *p = 0.02; d eNOS ***p = 0.0006 and ***p = 0.0008; and e INOS ***p = 0.0002 and ****p < 0.0001. Black (*) when compared with Ctrl and red (*) when compared with W/DAE
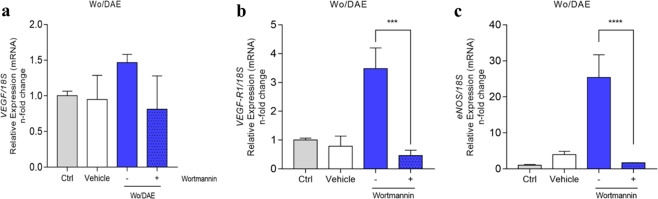
We noticed a potential involvement of survival signaling in modulating angiogenic stimulus in response to the Ti-enriched medium since there was a probable correlation between PKB/AKT performance and genes related to angiogenic signals, mainly in the Wo/DAE group (see Figs. 2 and 3). Finally, to test the hypothesis that PI3K/AKT drives angiogenic factors in response to the Ti-enriched medium, we subjected the cells to a chemical inhibition of PI3K using well-known Wortmannin, as it is widely proposed by others [43], and further investigated the behavior of VEGF (Fig. 4a), VEGFR1 (Fig. 4b), and eNOS (Fig. 4c) genes. Our data clearly shows that wortmannin suppresses the angiogenic factors because all of these three genes were significantly downregulated (Fig. 4). Altogether these data validate our hypothesis that PI3K/Akt signaling is involved in angiogenic signals triggered by tienriched medium. Mechanistically, VEGF induces nitric oxide production and release by nitric oxide synthase (NOS) in isolated vessels and in cultured ECs [44–46], which is attenuated by PI3K inhibitors [47]. Subsequently, it was demonstrated that VEGF stimulates PKB/Akt-mediated eNOS phosphorylation at Ser1177 [36, 48, 49].
Fig. 4.
Wortmannin targeting PI3K suppresses the angiogenic stimulus of titanium (Ti). The samples were collected respecting the already described methodology to allow qPCR technology performance. To address whether PI3K/AKT is involved in endothelial responding to the Ti-enriched medium, we reanalyzed the already known performance of genes, as was shown in Fig. 3, but now treated with wortmannin, a specific chemical inhibitor of PI3K (upstream to AKT). Thus, the suppression of the angiogenic stimulus by Ti is clear since VEGF (a), VEGFR1 (b), and eNOS (c) genes were significantly down expressed when PI3K was inhibited. The 18S gene was considered the housekeeping gene and used to normalize the values. Statistics: The statistical analysis test used was one-way ANOVA with multiple comparisons to compare all pairs of groups, and the figure specifies the differences between the absence (−) and the presence (+) of the Wortmannin. Differences were considered significant when b VEGFR1 ***p = 0.0002 and c eNOS ****p < 0.0001
Thus, we reasonably suggest that PI3K/AKT signaling participates in the angiogenic phenotype of ECs responding to the Ti-enriched medium. We previously reported that the major mechanotransduction pathway activates PI3K, which plays a pivotal role in guaranteeing EC phenotype and vascular homeostasis [43], and Ti-release displays a very similar effect, mainly in response to Wo/DAE.
Peri-implant healing seems to recapitulate conventional regenerative processes involving a coupling of biological systems, which requires a cascade of carefully and precisely regulated steps and events that correlate with the appearance of various cell types during distinct stages of healing [50–54]. Although titanium has been considered a physiologically inert material, we have demonstrated Ti develops dynamic interaction with the microenvironment by releasing particles and active molecules affecting osteoblast biology by positively coordinating proliferative and differentiation mechanisms, and the properties of the surfaces seems to influence this response [16]. These modifications on titanium surfaces aim to optimize the wound healing to reduce the clinical time for patient recovering and has been shown to trigger changes in osteoblast metabolism [24]. A previously published study indicated involvement of a set of genes related with osteogenesis in response to dual-acid-etched surface of titanium in human mesenchymal stromal cells [55]. The involvement of ECs has a pivotal role during crucial phases of wound healing. Their importance on osseointegration success has raised some questions mainly about the response to changes in titanium surfaces [6], and our data address this issue and provides further comprehension.
This study reinforces that the titanium-released particles modify differentially the EC behavior emphasizing the importance of survival and angiogenic related intracellular signaling in these responses by upactivating PI3K/AKT signaling, mainly in response to Wo/DAE. In conjunction, these data support that titanium-implantable devices interact dynamically with the host surrounding tissues modulating the activity of osteogenic and ECs, maybe coupling osteogenesis and angiogenesis processes in peri-implant tissue and then contributing with their successful osseointegration.
Acknowledgements
The authors would like to thank the FAPESP (2014/22689-3 and 2019/09140-6) and CNPq for financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NRF, Cardaropoli G, et al. Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 2009;88:579–96. doi: 10.1002/jbm.b.31264. [DOI] [PubMed] [Google Scholar]
- 2.Bonfante EA, Coelho PG. A critical perspective on mechanical testing of implants and prostheses. Adv Dent Res. 2016;28:18–27. doi: 10.1177/0022034515624445. [DOI] [PubMed] [Google Scholar]
- 3.Dohan Ehrenfest DM, Coelho PG, Kang B-S, Sul Y-T, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28:198–206. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Raines AL, Berger MB, Patel N, Hyzy SL, Boyan BD, Schwartz Z. VEGF-A regulates angiogenesis during osseointegration of Ti implants via paracrine/autocrine regulation of osteoblast response to hierarchical microstructure of the surface. J Biomed Mater Res A. 2019;107:423–33. doi: 10.1002/jbm.a.36559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Xu K, Tao B, Dai L, Yu Y, Mu C, et al. Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo. Acta Biomater. 2018;74:489–504. doi: 10.1016/j.actbio.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 6.An N, Schedle A, Wieland M, Andrukhov O, Matejka M, Rausch-Fan X. Proliferation, behavior, and cytokine gene expression of human umbilical vascular endothelial cells in response to different titanium surfaces. J Biomed Mater Res - Part A. 2010;93:364–72. doi: 10.1002/jbm.a.32539. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson SJ, Broggini N, Wieland M, de Wild M, Rupp F, Geis-Gerstorfer J, et al. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J Biomed Mater Res A. 2006;78:291–7. doi: 10.1002/jbm.a.30678. [DOI] [PubMed] [Google Scholar]
- 8.Cochran DL, Buser D, Ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, et al. The use of reduced healing times on ITI® implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI® SLA implants. Clin Oral Implants Res. 2002;13:144–53. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 9.Rossi MC, Bezerra FJB, Silva RA, Crulhas BP, Fernandes CJC, Nascimento AS, et al. Titanium-released from dental implant enhances pre-osteoblast adhesion by ROS modulating crucial intracellular pathways. J Biomed Mater Res A. 2017;105:2968–76. doi: 10.1002/jbm.a.36150. [DOI] [PubMed] [Google Scholar]
- 10.da Costa Fernandes CJ, Bezerra FJB, de Campos Souza B, Campos MA, Zambuzzi WF. Titanium-enriched medium drives low profile of ECM remodeling as a pre-requisite to pre-osteoblast viability and proliferative phenotype. J Trace Elem Med Biol. 2018;50:339–46. doi: 10.1016/j.jtemb.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Masaki C, Schneider GB, Zaharias R, Seabold D, Stanford C. Effects of implant surface microtopography on osteoblast gene expression. Clin Oral Implants Res. 2005;16:650–6. doi: 10.1111/j.1600-0501.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 12.Shi B, Andrukhov O, Berner S, Schedle A, Rausch-Fan X. The angiogenic behaviors of human umbilical vein endothelial cells (HUVEC) in co-culture with osteoblast-like cells (MG-63) on different titanium surfaces. Dent Mater. 2014;30:839–47. doi: 10.1016/j.dental.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011;15:1389–403. doi: 10.1089/ars.2010.3361. [DOI] [PubMed] [Google Scholar]
- 14.Ramasamy SK, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7:1–13. doi: 10.1038/ncomms13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Oliveira PGFP, Bonfante EA, Bergamo ETP, de Souza SLS, Riella L, Torroni A, et al. Obesity/metabolic syndrome and diabetes mellitus on peri-implantitis. Trends Endocrinol. Metab. (2020). 10.1016/j.tem.2020.05.005. [DOI] [PubMed]
- 16.Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, et al. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016;2016:6285620. doi: 10.1155/2016/6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 18.de AC, Gonzalez O, Costa TF, de Z, Andrade A, Medrado ARAP. Wound healing—a literature review. Bras Dermatol. 2016;91:614–20. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- 21.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 22.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 23.Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–7. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 24.Zambuzzi WF, Bonfante EA, Jimbo R, Hayashi M, Andersson M, Alves G, et al. Nanometer scale titanium surface texturing are detected by signaling pathways involving transient FAK and Src activations. PLoS ONE. 2014;9:e95662. doi: 10.1371/journal.pone.0095662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezerra F, Ferreira MR, Fontes GN, da Costa Fernandes CJ, Andia DC, Cruz NC, et al. Nano hydroxyapatite-blasted titanium surface affects pre-osteoblast morphology by modulating critical intracellular pathways. Biotechnol Bioeng. 2017;114:1888–98. doi: 10.1002/bit.26310. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes CJC, Bezerra F, Ferreira MR, Andrade AFC, Pinto TS, Zambuzzi WF. Nano hydroxyapatite-blasted titanium surface creates a biointerface able to govern Src-dependent osteoblast metabolism as prerequisite to ECM remodeling. Colloids Surf B Biointerfaces. 2018;163:321–8. doi: 10.1016/j.colsurfb.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Gemini-Piperni S, Takamori ER, Sartoretto SC, Paiva KBS, Granjeiro JM, de Oliveira RC, et al. Cellular behavior as a dynamic field for exploring bone bioengineering: a closer look at cell-biomaterial interface. Arch Biochem Biophys. 2014;561:88–98. doi: 10.1016/j.abb.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Zambuzzi WF, Coelho PG, Alves GG, Granjeiro JM. Intracellular signal transduction as a factor in the development of “smart” biomaterials for bone tissue engineering. Biotechnol Bioeng. 2011;108:1246–50. doi: 10.1002/bit.23117. [DOI] [PubMed] [Google Scholar]
- 29.Qahash M, Hardwick WR, Rohrer MD, Wozney JM, Wikesjö UME. Surface-etching enhances titanium implant osseointegration in newly formed (rhBMP-2-induced) and native bone. Int J Oral Maxillofac Implants. 2007;22:472–7. [PubMed] [Google Scholar]
- 30.Sewduth R, Santoro MM. “Decoding” angiogenesis: new facets controlling endothelial cell behavior. Front Physiol. 2016;7:306. doi: 10.3389/fphys.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–93. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yudushkin I. Control of Akt activity and substrate phosphorylation in cells. IUBMB Life. 2020 doi: 10.1002/iub.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BA Hemmings, DF Restuccia, The PI3K-PKB/Akt pathway, Cold Spring Harb. Perspect. Biol. 2015;7. 10.1101/cshperspect.a026609. [DOI] [PMC free article] [PubMed]
- 34.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y-X, Du J, Si M-S, Mo J-J, Qiao S-C, Lai H-C. The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1 preosteoblast proliferation and differentiation on SLA and SLActive titanium surfaces. J Biomed Mater Res A. 2013;101:748–54. doi: 10.1002/jbm.a.34377. [DOI] [PubMed] [Google Scholar]
- 36.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–50. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 37.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10:329–37. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 39.Gomes OP, Feltran GS, Ferreira MR, Albano CS, Zambuzzi WF, Lisboa-Filho PN. A novel BSA immobilizing manner on modified titanium surface ameliorates osteoblast performance. Colloids Surf B Biointerfaces. 2020;190:110888. doi: 10.1016/j.colsurfb.2020.110888. [DOI] [PubMed] [Google Scholar]
- 40.da G, Feltran S, Bezerra F, da Costa Fernandes CJ, Ferreira MR, Zambuzzi WF. Differential inflammatory landscape stimulus during titanium surfaces obtained osteogenic phenotype. J Biomed Mater Res A. 2019;107:1597–604. doi: 10.1002/jbm.a.36673. [DOI] [PubMed] [Google Scholar]
- 41.Baroncelli M, Fuhler GM, van de Peppel J, Zambuzzi WF, van Leeuwen JP, van der Eerden BCJ, et al. Human mesenchymal stromal cells in adhesion to cell-derived extracellular matrix and titanium: Comparative kinome profile analysis. J Cell Physiol. 2019;234:2984–96. doi: 10.1002/jcp.27116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro AR, Oliveira F, Boldrini LC, Leite PE, Falagan-Lotsch P, Linhares ABR, et al. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater Sci Eng C Mater Biol Appl. 2015;54:196–206. doi: 10.1016/j.msec.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Gomes AM, Pinto TS, da Costa Fernandes CJ, da Silva RA, Zambuzzi WF. Wortmannin targeting phosphatidylinositol 3-kinase suppresses angiogenic factors in shear-stressed endothelial cells. J Cell Physiol. 2020;235:5256–69. doi: 10.1002/jcp.29412. [DOI] [PubMed] [Google Scholar]
- 44.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–92. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 45.Yang R, Thomas GR, Bunting S, Ko A, Ferrara N, Keyt B, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–44. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 46.van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–7. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 47.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Investig. 1997;100:3131–9. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 50.Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Ski Wound Care. 2007;20:35–39. doi: 10.1097/00129334-200701000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Strecker-McGraw MK, Jones TR, Baer DG. Soft tissue wounds and principles of healing. Emerg Med Clin North Am. 2007;25:1–22. doi: 10.1016/j.emc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117:72S–109S. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- 53.Broughton G, II, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 54.Rice J. Secondary intention wound healing–pathphysiology and management. Collegian. 2000;7:40–41. doi: 10.1016/s1322-7696(08)60377-7. [DOI] [PubMed] [Google Scholar]
- 55.Mamalis A, Silvestros S. Modified titanium surfaces alter osteogenic differentiation: a comparative microarray-based analysis of human mesenchymal cell response to commercial titanium surfaces. J Oral Implantol. 2013;39:591–601. doi: 10.1563/AAID-JOI-D-10-00209. [DOI] [PubMed] [Google Scholar]