Abstract
Purpose
Evaluation of long-term outcome and toxicity of moderately hypofractionated radiotherapy using intensity-modulated radiotherapy (IMRT) with simultaneous integrated boost treatment planning and cone beam CT-based image guidance for localized prostate cancer.
Methods
Between 2005 and 2015, 346 consecutive patients with localized prostate cancer received primary radiotherapy using cone beam CT-based image-guided intensity-modulated radiotherapy (IG-IMRT) and volumetric modulated arc therapy (IG-VMAT) with a simultaneous integrated boost (SIB). Total doses of 73.9 Gy (n = 44) and 76.2 Gy (n = 302) to the high-dose PTV were delivered in 32 and 33 fractions, respectively. The low-dose PTV received a dose (D95) of 60.06 Gy in single doses of 1.82 Gy. The pelvic lymph nodes were treated in 91 high-risk patients to 45.5 Gy (D95).
Results
Median follow-up was 61.8 months. The 5‑year biochemical relapse-free survival (bRFS) was 85.4% for all patients and 93.3, 87.4, and 79.4% for low-, intermediate-, and high-risk disease, respectively. The 5‑year prostate cancer-specific survival (PSS) was 94.8% for all patients and 98.7, 98.9, 89.3% for low-, intermediate-, and high-risk disease, respectively. The 5‑year and 10-year overall survival rates were 83.8 and 66.3% and the 5‑year and 10-year freedom from distant metastasis rates were 92.2 and 88.0%, respectively. Cumulative 5‑year late GU toxicity and late GI toxicity grade ≥2 was observed in 26.3 and 12.1% of the patients, respectively. Cumulative 5‑year late grade 3 GU/GI toxicity occurred in 4.0/1.2%.
Conclusion
Moderately hypofractionated radiotherapy using SIB treatment planning and cone beam CT image guidance resulted in high biochemical control and survival with low rates of late toxicity.
Keywords: Simultaneous integrated boost, Cone beam CT, Hypofractionation, Intensity-modulated radiation therapy, Image-guided radiation therapy
Introduction
Primary radiotherapy as an established curative treatment option for localized prostate cancer, one of the most common cancer types [1], has undergone substantial changes in clinical practice. Lately, hypofractionated [2–7] and dose-escalated [8–10] approaches are on the advance to improve the therapeutic ratio, reduce cost, and shorten the duration of prostate radiation therapy.
When on-board cone beam CT (CBCT) became available in 2004, highly conformal image-guided intensity-modulated radiation therapy (IG-IMRT) made dose-escalated radiotherapy with reduced target volume margins a viable option. As one of the first centers worldwide to implement CBCT-based IG-IMRT, we postulated that by adopting a combination of CBCT, IG-IMRT with simultaneous integrated boost (SIB), tight margins, and hypofractionated dose-escalated radiotherapy, high biochemical control with reduction of gastrointestinal toxicity would be achievable. In an earlier publication, the outcome and toxicity data of the first treated patients were reported [11] but limited long-term data are available for moderately hypofractionated dose-escalated CBCT-based image-guided IMRT with simultaneous integrated boost (SIB). In this publication, matured long-term outcome and toxicity data are presented.
Materials and methods
Patients and treatment
This updated analysis is based on 346 consecutive patients treated between 2005 and 2015 with moderately hypofractionated intensity-modulated CBCT-based image-guided radiotherapy for localized prostate cancer. All patients had pathologically confirmed prostate cancer with risk stratification according to D’Amico et al.
As published before, radiotherapy was delivered with IG-IMRT or IG-VMAT in 33 fractions with SIB and two dose levels of 1.82 and 2.31 Gy per fraction, resulting in a prescribed PTV dose of 60.06 Gy (D95) and a PTVBoost mean dose of 76.23 Gy. 32 fractions were applied only in patients with low-risk prostate cancer in 44 cases. For pelvic lymphatic radiation, the prescribed dose was 45.5 Gy (D95) with 1.82 Gy per fraction. A CTVP−SV was generated consisting of the prostate and the base of the seminal vesicles, whereas CTVP+SV included the prostate and the seminal vesicles. PTVBoost was defined by a 5-mm margin around CTVP−SV with avoidance of the rectum. The PTV was created by a 10-mm margin around CTVP+SV in all but the dorsal direction, where a 7-mm margin was used. Pinnacle3 (Philips Radiation Oncology Systems, Fitchburg, WI, USA) was used for treatment planning. More information on target volume definition, treatment planning, and treatment delivery has been described before [11, 12].
Physician-recorded toxicity and biochemical control were assessed prospectively. Biochemical failure was defined according the Phoenix definition as nadir plus an increase of ≥2 ng/ml in prostate-specific antigen (PSA). Androgen deprivation therapy was recommended for patients with intermediate- (6 months) and high-risk disease (24–36 months) and prescribed at the discretion of the treating urologist. Assessment of toxicity during radiotherapy was performed every 2 weeks until the end of treatment, 6 weeks after treatment, and in 6‑month intervals thereafter. Two years after treatment, follow-up continued yearly. Gastrointestinal (GI) and genitourinary (GU) toxicity was scored using Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Toxicity data of the earlier patient group from 2005 to 2010 was retrospectively reclassified from CTCAE v3.0 to v4.0 to achieve comparability. Acute toxicity was defined as occurring within 3 months after radiotherapy. Late toxicity assessment included the 6‑monthly and all later follow-ups.
Statistics
Biochemical relapse-free survival, overall survival, prostate cancer-specific survival, and freedom from distant metastasis were calculated using the Kaplan–Meier method and log-rank tests were applied for analysis. For the comparison of toxicity of prostate only versus prostate and pelvic lymph node radiotherapy one-sided Fisher’s exact tests were performed. Statistical analysis was conducted using IBM SPSS v.25.0 (IBM Corp., Armonk, NY, USA). Differences were considered statistically significant in case of p < 0.05.
Results
The reviewed 346 male patients had a median age of 73 (range 47–84) years and a median Karnofsky score of 90%. In reference to the risk classification of D’Amico 78, 122 and 142 patients had low-, intermediate-, and high- risk prostate cancer, respectively. Clinical characteristics are summarized in Table 1.
Table 1.
Patient and treatment characteristics
| Characteristic | All N = 346 (100%) |
Prostate only N = 255 (73.7%) |
Prostate + pelvic LN N = 91 (26.3%) |
|---|---|---|---|
| Age, median (range) in years | 73 (47–84) | 73 (47–83) | 72 (52–84) |
| KPS, median (range) in % | 90 (60–100) | 90 (70–100) | 90 (60–100) |
| iPSA, median (range) in ng/ml | 8.4 (0.1–434.8) | 6.7 (0.1–434.8) | 24.8 (3.2–334) |
| Gleason score | |||
| ≤6 | 120 (34.7%) | 114 (44.7%) | 6 (6.6%) |
| 7 | 142 (41.0%) | 111 (43.5%) | 31 (34.1%) |
| 8–10 | 80 (23.1%) | 27 (10.6%) | 53 (58.2%) |
| N/A | 4 (1.2%) | 3 (1.2%) | 1 (1.1%) |
| T stage | |||
| T1 | 228 (65.9%) | 180 (70.6%) | 48 (52.7%) |
| T2 | 78 (22.5%) | 57 (22.4%) | 21 (23.1%) |
| T3 | 32 (9.2%) | 15 (5.9%) | 17 (18.7%) |
| T4 | 6 (1.7%) | 2 (0.8%) | 4 (4.4%) |
| N/A | 2 (0.6%) | 1 (0.4%) | 1 (1.1%) |
| D’Amico risk group | |||
| Low risk | 78 (22.5%) | 78 (30.6%) | 0 (0.0%) |
| Intermediate risk | 122 (35.3%) | 117 (45.9%) | 5 (5.5%) |
| High risk | 142 (41.0%) | 57 (22.4%) | 85 (93.4%) |
| N/A | 4 (1.2%) | 3 (1.2%) | 1 (1.1%) |
| Androgen deprivation | |||
| Low risk | 13/78 (16.7%) | 13/78 (16.7%) | 0 (0.0%) |
| Intermediate risk | 34/122 (27.9%) | 32/117 (27.4%) | 2/5 (40.0%) |
| High risk | 104/142 (73.2%) | 33/57 (57.9%) | 71/85 (83.5%) |
| N/A | 4/346 (1.2%) | 3/255 (1.2%) | 1/91 (1.1%) |
KPS Karnofsky score; LN lymph nodes; N/A not available; iPSA initial prostate-specific antigen
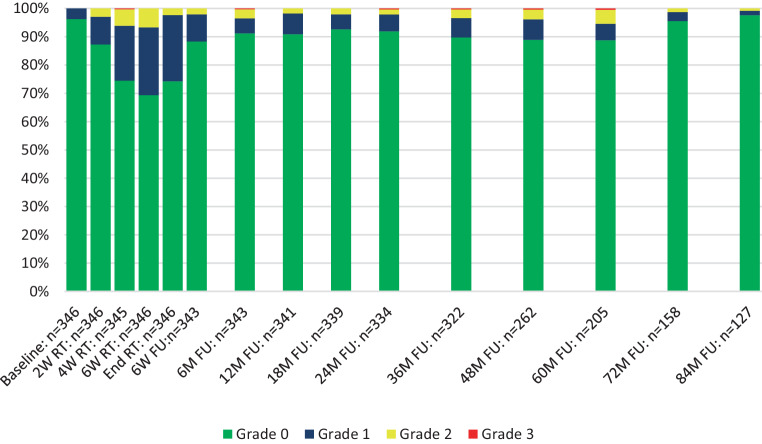
The time course of GI toxicity is shown in Fig. 1. No grade 4 toxicities were observed and 4 patients in total developed late grade 3 (1.2%) GI toxicity cumulated over 5‑year follow-up: 3 patients developed late rectal bleeding (mean 22.0 ± 15 months after radiotherapy, 10.0 ± 3.5 months duration) and 1 patient developed late fecal incontinence (48 months after radiotherapy, chronic); 1 patient with late rectal bleedings additionally suffered from proctitis grade 3. The maximum of acute GI toxicity occurred 6 weeks after the start of radiotherapy and decreased fast thereafter. Late grade 2 GI toxicity peaked at the 60-month follow-up and normalized thereafter. Overall, acute GI toxicity grade ≥2 was observed in 13.0% of patients and cumulative 5‑year late GI toxicity grade ≥2 in 12.1% of all patients.
Fig. 1.
Gastrointestinal toxicity. Shown is the time course of physician-recorded gastrointestinal toxicity according to CTCAE v4.0. RT radiotherapy, W weeks, M months, FU follow-up
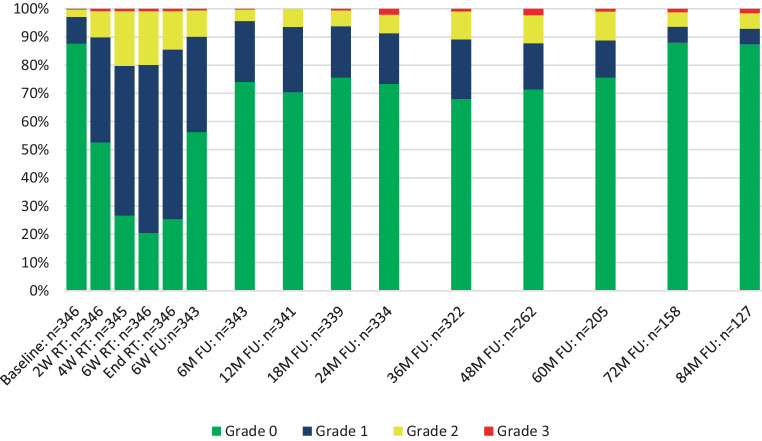
The time course of GU toxicity is presented in Fig. 2. No grade 4 toxicities were observed. After 5 years of follow-up, 14 patients (4.0%) developed late grade 3 GU toxicity: 5 patients developed late grade 3 macroscopic hematuria (mean 25.2 ± 14.9 months after radiotherapy, 12.0 ± 0.0 months duration; two chronic cases); 8 patients suffered from late grade 3 urinary incontinence (mean 31.5 ± 13.9 months after radiotherapy, 20.0 ± 15.0 months duration; two chronic cases); 1 patient developed late grade 3 non-infective cystitis (36 months after radiotherapy, chronic). The maximum of acute GU toxicity occurred 6 weeks after the start of radiotherapy and decreased significantly within 6 weeks. GU toxicity was increased in patients with pelvic node irradiation: cumulative 5‑year late grade ≥2 GU toxicity was observed in 23.5% of patients in the group of prostate only radiotherapy and 34.1% of the patients with prostate and pelvic lymph node irradiation (p = 0.036, one-sided Fisher’s exact test). Late grade 2 to 3 toxicity showed a peak at the 36-month follow-up and decreased after the 60-month follow-up. Overall acute GU toxicity grade ≥2 was observed in 30.1% and cumulative 5‑year late GU toxicity grade ≥2 in 26.3% of all patients.
Fig. 2.
Genitourinary toxicity. Shown is the time course of physician-recorded genitourinary toxicity according to CTCAE v4.0. RT radiotherapy, W weeks, M months, FU follow-up
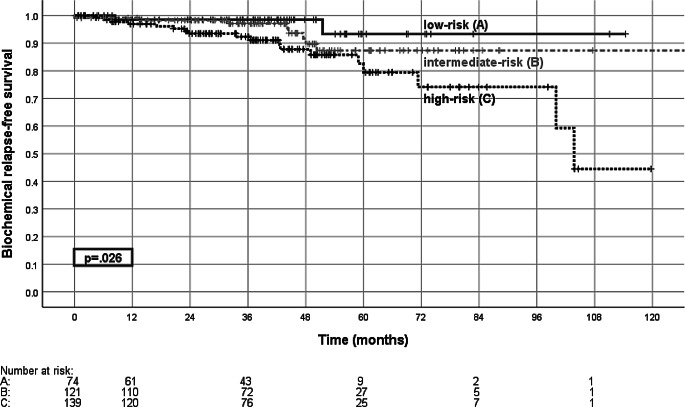
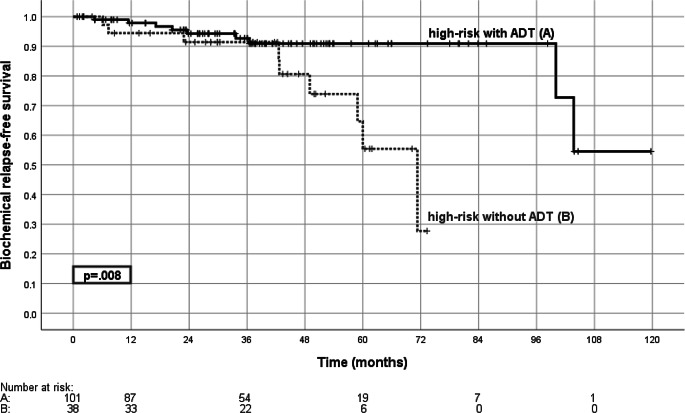
Median follow-up was 61.8 (range 0.5–147.3) months. The 5‑year biochemical relapse-free survival (bRFS) was 85.4% for all patients and 93.3, 87.4, and 79.4% for low-, intermediate-, and high-risk disease (Fig. 3). For high-risk patients 5‑year bRFS was 90.9% with androgen deprivation therapy and 55.4% without (p = 0.008, Fig. 4). For the cohort of intermediate-risk patients androgen deprivation therapy did not significantly influence bRFS.
Fig. 3.
Biochemical relapse-free survival. Shown is the biochemical relapse-free survival according to risk group for the low-risk group (A), intermediate-risk group (B), and high-risk group (C), with the comparison of low-risk versus high-risk (p = 0.026, log-rank test)
Fig. 4.
Biochemical relapse-free survival in high-risk patients with or without androgen deprivation therapy. Shown is the biochemical relapse-free survival with (A) and without (B) androgen deprivation therapy for the high-risk group (p = 0.008; log-rank test). ADT androgen deprivation therapy
The 5‑year prostate-specific survival (PSS) was 94.8% for all patients and 98.7, 98.9, and 89.3% for low-, intermediate-, and high-risk disease, respectively. 10-year prostate-specific survival was 92.4%. The 5‑ and 10-year overall survival rates were 83.8 and 66.3%, respectively.
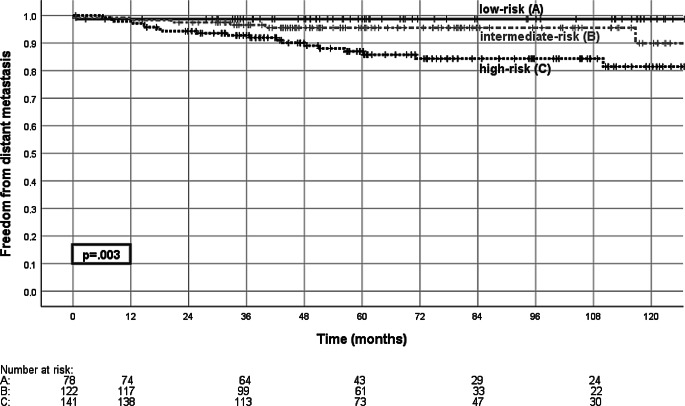
During follow-up 27 patients developed distant metastasis, resulting in 5‑ and 10-year freedom from distant metastasis rates of 92.2 and 88%, respectively. 5‑year freedom from distant metastasis was 98.7, 95.5, and 87.0% for low-, intermediate-, and high-risk disease, respectively (Fig. 5). Androgen deprivation therapy did not significantly influence freedom from distant metastasis in any risk group.
Fig. 5.
Freedom from distant metastasis. Shown is freedom from distant metastasis for the low-risk group (A), intermediate-risk group (B), and high-risk group (C), with the comparison of low-risk vs. high-risk group (p = 0.003, log-rank test)
Discussion
Recent meta-analyses indicate that hypofractionated radiotherapy is non-inferior to conventional radiotherapy in terms of tumor control [13–15], but may suffer from an increased risk of toxicity [14, 16]. We report a “real world” cohort from a single institution. Strengths are the uniform manner of target contouring, dose prescription, and IMRT realization, as well as strict CBCT-based image guidance. The variable use of additive androgen deprivation and pelvic node irradiation in about 25% of patients with an assumed higher risk of lymph node metastasis introduces some heterogeneity which is difficult to classify.
In our patient cohort the 5‑year prostate-specific survival/biochemical relapse-free survival of 94.8/85.4% for all patients and the 5‑year bRFS of 79.4% in the high-risk group support the high efficiency of dose-escalated hypofractionated primary radiotherapy with SIB for prostate cancer. Assuming a dose–effect relationship [17, 18], this result may in part be explained by the dose prescription of 33-times 1.82/2.31 Gy. With an assumed α/β ratio of 1.5 or 2.7 Gy, this corresponds to a mean PTVBoost EQD2 dose of 83 or 81 Gy, respectively. The EQD2 of 81 Gy2.7 in our study exceeded other recent trials of moderately hypofractionated radiotherapy like the CHHIP [2], PROFIT [19] (73 Gy2.7), and RTOG [4] (77 Gy2.7) trials, while the HYPRO [20] trial used a higher EQD2 (84 Gy2.7). Our outcome data are comparable to the trials mentioned above. For example, the CHHIP trial reported a 5-year bRFS of 90.6% and the PROFIT trial of 85%. While bRFS was superior in the CHHIP trial, the relative number of high-risk patients was lower than in our own patient cohort (12% vs. 41%) [2, 20]. Interestingly, for the subgroup of high-risk patients in our cohort, the 5‑year bRFS was significantly increased with androgen deprivation therapy (ADT; 5‑year bRFS 90.9% vs. 55.4%, p = 0.008), which clearly underlines the importance of ADT in the high-risk group [21, 22], despite treatment with escalated radiotherapy doses. In contrast, the sequence of ADT and radiotherapy appears to be less important [23]. Short-term androgen deprivation was part of the treatment protocol in the CHHIP trial; it was excluded in the PROFIT trial, which included only patients with intermediate risk. In our cohort no influence of androgen deprivation therapy on bRFS could be observed in the intermediate-risk group. Further improvements in biochemical control can be achieved by standardized androgen deprivation therapy at least in the high-risk group. Prescription of ADT in our patients was at the discretion of the treating urologist and only 73.2% of our high-risk cancer patients received ADT. Patient preference and comorbidities were considered in the decision-making process for or against ADT.
We electively irradiated the pelvic lymph nodes in patients with an estimated risk of lymph node involvement greater than 15% (according to the Roach formula) [24]. Even though evidence for prophylactic pelvic lymph node irradiation is sparse and the GETUG-01 study observed no improvement of event-free survival after a median follow-up of 11.4 years [25], the RTOG 9413 trial showed improved progression-free survival with pelvic lymph node radiation treatment [26]. Therefore, pelvic lymph node irradiation may have contributed to biochemical control in high-risk patients.
This report differs from other studies, as a simultaneous integrated boost was used and the high-dose PTV was restricted to the prostate +5-mm margin with avoidance of the rectum. The low-dose PTV (margin of 10 mm, 7 mm towards posterior) was covered by the 60 Gy isodose. To ensure precise application, an integrated offline/online protocol of volumetric cone beam CT-guided radiotherapy was strictly implemented at our institution [11, 27–30]. Accurate positioning with daily IGRT contributes to improved tumor control and minimizes dose deviations [31–33], which is especially important for small PTV margins in high-risk disease with possible microscopic extracapsular extension [34]. Our patient distribution is comparable to the Austrian-German dose-escalation trial (74 Gy for intermediate- and high-risk patients) published by Goldner et al. using 3D conformal radiotherapy [35]: while GU and GI toxicity (grade ≥2) were higher (34 and 30%), 5‑year bRFS seemed inferior (80% intermediate, 60% high risk) to our study. This underlines both the importance of technical advances (IMRT and IGRT) and of high effective doses.
Wortel et al. [36] compared the conventionally fractionated 78-Gy arms from two randomized consecutive Dutch trials using identical toxicity scoring. IG-IMRT compared to conventional 3D treatment led to a statistically significant reduction of GI toxicity ≥2 from 37.6 to 24.9%. There was at best a trend concerning GU toxicity ≥2 (36.4% vs. 46.2%). This is well in line with our results. Dose modulation using an integrated boost for the gross prostate with a tight margin may have further reduced relevant side effects.
Late GU and GI toxicity peaked between the 36-month to 60-month follow-up period consistent with earlier reports [11, 37]. We observed a late grade 3 GU toxicity rate of 4.0% and a cumulative GU toxicity ≥2 rate of 26.3% at 5 years after treatment, which seems promising considering a prescribed EQD2 > 80 Gy. Relevant structures at risk (urethra and bladder base) are always part of the anatomical CTV. Comparisons to the literature remain challenging, as particularly the reported GU toxicity rates differ considerably (grade ≥2, for example, 41.3% in the HYPRO trial [38], 29.7% RTOG 0415 trial [4], 22.2% PROFIT trial [19], 12.8% CHHIP trial [2], and 26.3% GETUG 06 trial (80 Gy) [39]). The prevalence of lower urinary tract symptoms increases in an ageing population and may influence reporting of long-term toxicity [40]. Prophylactic lymph node irradiation in 26.3% (n = 91) of our patients seemed to have an increased risk of GU toxicity in our series as well as in other trials [26, 41]. Overall, only 1.2% of the patients experienced late grade 3 GI toxicity and the cumulative late GI toxicity grade ≥2 rate of 12.1% remains favorable compared to randomized trials of hypofractionated radiotherapy (for example, Catton et al. 8.9% [19], Dearnarly et al. 14.7% [2], and Aluwini et al. 21.9% [38]). A low GI toxicity rate is likely attributed to our institutional policy to avoid an overlap between the organ at risk rectum and PTVBoost and the definition of strict constraints for the posterior rectal wall to further limit rectal toxicity. This zero margin for the high-dose volume approach theoretically bears the risk of underdosage of dominant index lesions in the peripheral zone, but adequate dose coverage was demonstrated in an earlier publication [12].
Although dose escalation above 80 Gy might not further increase biochemical control in moderately hypofractionated radiotherapy [42], new emerging techniques aim at improving prostate cancer treatment: the ASCENDE-RT trial impressively showed a high rate of biochemical control, despite a high proportion of high-risk patients, at the cost of increased toxicity by adding LDR brachytherapy to external beam radiotherapy [43]. Another recent approach is dose escalation to the dominant index lesion as examined in the FLAME-trial. Although outcome data are pending, the published toxicity rates are promising [10]. Furthermore, recent publications of phase 3 trials [5, 6] of ultrahypofractionated regimes [44] report encouraging outcome and toxicity data with application of precise position verification systems (fiducial markers and/or catheter demarcation of the urethra) and intrafractional motion monitoring. Randomized trials are needed for the comparison of moderately hypofractionated radiotherapy with the abovementioned techniques to find the best therapeutic ratio.
Conclusion
Moderately hypofractionated dose-escalated prostate radiotherapy using an integrated boost concept with no rectal margin for the high-dose PTV was safe and achieved high rates of long-term biochemical control and survival. Despite dose escalation, cone beam CT-based image-guided radiotherapy accomplished low rates of long-term toxicity, especially of gastrointestinal toxicity. Androgen deprivation in high-risk disease contributed to biochemical control.
Author Contribution
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Projekt DEAL.
Compliance with ethical guidelines
Conflict of interest
J. Tamihardja, M. Schortmann, I. Lawrenz, S. Weick, K. Bratengeier, M. Flentje, M. Guckenberger, and B. Polat declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries KC, Wortel RC, Oomen-de Hoop E, Heemsbergen WD, Pos FJ, Incrocci L. Hyprofractionated versus conventionally fractionated radiation therapy for patients with intermediate- or high-risk, localized, prostate cancer: 7-year outcomes from the randomized, multicenter, open-label, phase 3 HYPRO trial. Int J Radiat Oncol Biol Phys. 2020;106:108–115. doi: 10.1016/j.ijrobp.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 6.Brand DH, Tree AC, Ostler P, van der Voet H, Loblaw A, Chu W, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20:1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Pinitpatcharalert A, Kollmeier M, Goldman DA, McBride S, Gorovets D, et al. Early tolerance and tumor control outcomes with high-dose ultrahypofractionated radiation therapy for prostate cancer. Eur Urol Oncol. 2019 doi: 10.1016/j.euo.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray JR, Tree AC, Alexander E, Sohaib A, Hazell S, Thomas K, et al. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: efficacy and toxicity in the DELINEATE trial. Int J Radiat Oncol Biol Phys. 2019;106(4):715–724. doi: 10.1016/j.ijrobp.2019.11.402. [DOI] [PubMed] [Google Scholar]
- 9.Alexander EJ, Murray JR, Morgan VA, Giles SL, Riches SF, Hazell S, et al. Validation of T2- and diffusion-weighted magnetic resonance imaging for mapping intra-prostatic tumour prior to focal boost dose-escalation using intensity-modulated radiotherapy (IMRT) Radiother Oncol. 2019;141:181–187. doi: 10.1016/j.radonc.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monninkhof EM, van Loon JWL, van Vulpen M, Kerkmeijer LGW, Pos FJ, Haustermans K, et al. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: toxicity in the FLAME randomized controlled trial. Radiother Oncol. 2018;127:74–80. doi: 10.1016/j.radonc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Guckenberger M, Lawrenz I, Flentje M. Moderately hypofractionated radiotherapy for localized prostate cancer: long-term outcome using IMRT and volumetric IGRT. Strahlenther Onkol. 2014;190:48–53. doi: 10.1007/s00066-013-0443-x. [DOI] [PubMed] [Google Scholar]
- 12.Tamihardja J, Zenk M, Flentje M. MRI-guided localization of the dominant intraprostatic lesion and dose analysis of volumetric modulated arc therapy planning for prostate cancer. Strahlenther Onkol. 2019;195:145–152. doi: 10.1007/s00066-018-1364-5. [DOI] [PubMed] [Google Scholar]
- 13.Royce TJ, Lee DH, Keum N, Permpalung N, Chiew CJ, Epstein S, et al. Conventional versus hypofractionated radiation therapy for localized prostate cancer: a meta-analysis of randomized noninferiority trials. Eur Urol Focus. 2019;5:577–584. doi: 10.1016/j.euf.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Datta NR, Stutz E, Rogers S, Bodis S. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a systematic review and meta-analysis along with therapeutic implications. Int J Radiat Oncol Biol Phys. 2017;99:573–589. doi: 10.1016/j.ijrobp.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Hickey BE, James ML, Daly T, Soh FY, Jeffery M. Hypofractionation for clinically localized prostate cancer. Cochrane Database Syst Rev. 2019;9:Cd011462. doi: 10.1002/14651858.CD011462.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Franco R, Borzillo V, Ravo V, Ametrano G, Falivene S, Cammarota F, et al. Rectal/urinary toxicity after hypofractionated vs conventional radiotherapy in low/intermediate risk localized prostate cancer: systematic review and meta analysis. Oncotarget. 2017;8:17383–17395. doi: 10.18632/oncotarget.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelefsky MJ, Pei X, Chou JF, Schechter M, Kollmeier M, Cox B, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 19.Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 20.Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1061–1069. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- 21.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 22.Jackson WC, Hartman HE, Dess RT, Birer SR, Soni PD, Hearn JWD, et al. Addition of androgen-deprivation therapy or brachytherapy boost to external beam radiotherapy for localized prostate cancer: a network meta-analysis of randomized trials. J. Clin Oncol. 2020 doi: 10.1200/JCO.19.03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malone S, Roy S, Eapen L, E C, MacRae R, Perry G, et al. Sequencing of androgen-deprivation therapy with external-beam radiotherapy in localized prostate cancer: a phase III randomized controlled trial. J Clin Oncol. 2019 doi: 10.1200/jco.19.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roach M, 3rd, Marquez C, Yuo HS, Narayan P, Coleman L, Nseyo UO, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28:33–37. doi: 10.1016/0360-3016(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 25.Pommier P, Chabaud S, Lagrange JL, Richaud P, Le Prise E, Wagner JP, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the GETUG-01 randomized study. Int J Radiat Oncol Biol Phys. 2016;96:759–769. doi: 10.1016/j.ijrobp.2016.06.2455. [DOI] [PubMed] [Google Scholar]
- 26.Roach M, Moughan J, Lawton CAF, Dicker AP, Zeitzer KL, Gore EM, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1504–1515. doi: 10.1016/S1470-2045(18)30528-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter A, Polat B, Lawrenz I, Weick S, Sauer O, Flentje M, et al. Initial results for patient setup verification using transperineal ultrasound and cone beam CT in external beam radiation therapy of prostate cancer. Radiat Oncol. 2016;11:147. doi: 10.1186/s13014-016-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guckenberger M, Meyer J, Vordermark D, Baier K, Wilbert J, Flentje M. Magnitude and clinical relevance of translational and rotational patient setup errors: a cone-beam CT study. Int J Radiat Oncol Biol Phys. 2006;65:934–942. doi: 10.1016/j.ijrobp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Guckenberger M, Meyer J, Wilbert J, Baier K, Sauer O, Flentje M. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther Onkol. 2007;183:307–313. doi: 10.1007/s00066-007-1695-0. [DOI] [PubMed] [Google Scholar]
- 30.Polat B, Guenther I, Wilbert J, Goebel J, Sweeney RA, Flentje M, et al. Intra-fractional uncertainties in image-guided intensity-modulated radiotherapy (IMRT) of prostate cancer. Strahlenther Onkol. 2008;184:668–673. doi: 10.1007/s00066-008-1875-6. [DOI] [PubMed] [Google Scholar]
- 31.Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 32.de Crevoisier R, Bayar MA, Pommier P, Muracciole X, Pene F, Dudouet P, et al. Daily versus weekly prostate cancer image guided radiation therapy: phase 3 multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2018;102:1420–1429. doi: 10.1016/j.ijrobp.2018.07.2006. [DOI] [PubMed] [Google Scholar]
- 33.Jereczek-Fossa BA, Maucieri A, Marvaso G, Gandini S, Fodor C, Zerini D, et al. Impact of image guidance on toxicity and tumour outcome in moderately hypofractionated external-beam radiotherapy for prostate cancer. Med Oncol. 2018;36:9. doi: 10.1007/s12032-018-1233-1. [DOI] [PubMed] [Google Scholar]
- 34.Teh BS, Bastasch MD, Wheeler TM, Mai WY, Frolov A, Uhl BM, et al. IMRT for prostate cancer: defining target volume based on correlated pathologic volume of disease. Int J Radiat Oncol Biol Phys. 2003;56:184–191. doi: 10.1016/s0360-3016(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 35.Goldner G, Bombosch V, Geinitz H, Becker G, Wachter S, Glocker S, et al. Moderate risk-adapted dose escalation with three-dimensional conformal radiotherapy of localized prostate cancer from 70 to 74 Gy. First report on 5-year morbidity and biochemical control from a prospective Austrian-German multicenter phase II trial. Strahlenther Onkol. 2009;185:94–100. doi: 10.1007/s00066-009-1970-3. [DOI] [PubMed] [Google Scholar]
- 36.Wortel RC, Incrocci L, Pos FJ, van der Heide UA, Lebesque JV, Aluwini S, et al. Late side effects after image guided intensity modulated radiation therapy compared to 3D-conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. Int J Radiat Oncol Biol Phys. 2016;95:680–689. doi: 10.1016/j.ijrobp.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Odrazka K, Dolezel M, Vanasek J, Vaculikova M, Zouhar M, Sefrova J, et al. Time course of late rectal toxicity after radiation therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:138–143. doi: 10.1038/pcan.2009.56. [DOI] [PubMed] [Google Scholar]
- 38.Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464–474. doi: 10.1016/S1470-2045(15)00567-7. [DOI] [PubMed] [Google Scholar]
- 39.Beckendorf V, Guerif S, Le Prise E, Cosset JM, Bougnoux A, Chauvet B, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 40.Rohrmann S, Katzke V, Kaaks R. Prevalence and progression of lower urinary tract symptoms in an aging population. Urology. 2016;95:158–163. doi: 10.1016/j.urology.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Murthy V, Maitre P, Bhatia J, Kannan S, Krishnatry R, Prakash G, et al. Late toxicity and quality of life with prostate only or whole pelvic radiation therapy in high risk prostate cancer (POP-RT): a randomised trial. Radiother Oncol. 2020;145:71–80. doi: 10.1016/j.radonc.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Vogelius IR, Bentzen SM. Dose response and fractionation sensitivity of prostate cancer after external beam radiation therapy: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2018;100:858–865. doi: 10.1016/j.ijrobp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104:778–789. doi: 10.1016/j.ijrobp.2019.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]