Abstract
The bone is among the most common sites of metastasis in patients with lung cancer. Over 30%–40% of lung cancers can develop bone metastasis, and no effective therapeutic methods exist in clinic cases. Wnt/β-catenin signaling and Dickkopf1 (DKK1) play important roles in the progression of lung cancer, which preferentially metastasizes to the skeleton. However, the role of DKK1 in osteotropism of small cell lung cancer (SCLC) remains to be elucidated. This study aimed to define the role of DKK1 in SCLC bone metastasis and investigate the underlying mechanisms. Our results demonstrated that the expression level of DKK1 was dramatically higher in bone metastatic SCLC cells (SBC-5 cell line) compared with that in cells without bone metastatic ability (SBC-3 cell line). Therefore, we hypothesized that DKK1 was involved in the bone metastasis of SCLC. We then suppressed the DKK1 expression in SBC-5 cells by RNAi and found that downregulation of DKK1 can inhibit cell proliferation, colony formation, cell migration, and invasion, but increase the apoptosis rate. Downregulation of DKK1 did not affect the cell cycle progression of SBC-5 cells in vitro. In vivo, downregulated DKK1 in SBC-5 cells resulted in attenuated bone metastasis. These results indicated that DKK1 may be an important regulator in bone metastases of SCLC, and targeting DKK1 may be an effective method to prevent and treat skeleton metastases in SCLC cases.
Key words: Dickkopf1 (DKK1), Bone metastasis, Small cell lung cancer (SCLC)
INTRODUCTION
It was reported that 14.1 million new cancer cases and 8.2 million cancer deaths occurred in 2012 worldwide. Lung cancer is the most common cancer in the world, in terms of both morbidity (1.8 million cases, 12.9% of total) and mortality (1.6 million deaths, 19.4%) because of high case fatality1.
The bone is among the most common sites of metastasis in patients with lung cancer and, in particular, bone metastasis is often associated with skeletal-related events (SREs) including bone pain, hypercalcemia, pathological fractures, spinal cord compression, and bone marrow infiltration, all of which may decrease the quality of life2–4. Therefore, effective therapeutic methods for preventing bone metastasis of lung cancer are urgently needed. However, the precise mechanisms of bone metastasis are still not completely understood.
Dickkopf1 (DKK1), a secreted protein, is known as a negative regulator of the Wnt signaling pathway5. The Wnt signaling pathway plays an essential role in determining cell fate and regulating cell proliferation. Dysregulation of this pathway can lead to some developmental defects and cancer6. Several studies suggested that DKK1 was involved in bone metastasis in cancer, such as breast cancer6 and prostate cancer (PC)7,8. Recently, a study has shown that the serum DKK1 level with bone metastasis was exclusively higher than the level without bone metastasis in non-small cell lung cancer (NSCLC) patients, and lung cancer cell-produced DKK1 may be related to NSCLC and its bone metastasis9. However, the autocrine function of DKK1, as well as the underlying mechanisms, is still unknown. Furthermore, no studies have elucidated the biological effect of DKK1 on the small cell lung cancer (SCLC) and its bone metastasis. In this study, we interfered the DKK1 expression by lentiviral vector-mediated RNAi and then detected changes regarding cell behavior in vitro, including invasion, migration, proliferation, apoptosis, and cell cycle. We then investigated the effect of downregulation of DKKI expression on bone metastasis in vivo based on a multiple-organ metastasis model of human SCLC cells, which is a classical model established previously in NK cell-depleted SCID mice by S. Yano. Our data demonstrated that DKKI was closely related to the osteotropism metastasis of SCLC and may be a critical contributor to tumors for the development of bone metastases. It provided a promising target for the prevention and effective treatment of bone metastases.
MATERIALS AND METHODS
Cell Culture
The human SCLC cell lines SBC-5 and SBC-3 were gifts from Professors Sone and Yano (Tokushima University, Japan). They were maintained at 37°C with 5% CO2 in RPMI-1640 (Gibco, Gaithersburg, MD, USA) supplemented with 10% (v/v) heated-inactivated fetal bovine serum (FBS; Bio International), 100 U/ml streptomycin, and 100 U/ml penicillin.
Real-Time (RT)-PCR Assay
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA (500 ng) was subjected to reverse transcription. Real-time polymerase chain reaction (RT-PCR) was carried out using SYBR Premix Ex Taq II (Takara, Japan), and the results were analyzed using the comparative threshold cycle (Ct) method for relative quantification. The PCR primers used are shown in Table 1.
Table 1.
Primers for RT-PCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| DKK1 | TAGAGTCTAGAATGCAAGGATCTC | CAAAAACTATCACAGCCTAAAGGG |
| β-Actin | TGGCACCCAGCACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
Western Blot Analysis
After quantification using the BCA kit, 20 μg of boiled protein dissolved in loading buffer was separated on 8% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. It was then incubated overnight with mouse polyclonal antibodies against DKK1 (1:1,500; Bioworld) and β-actin (1:10,000; Sigma). Secondary antibodies (1:5,000; anti-mouse) were applied, and the relative content of the target proteins was detected by chemiluminescence.
Cells Stable Transfection
DKK1 RNAi plasmids (Table 2) were obtained from Genechem (Shanghai, P.R. China). The plasmids were transfected into SBC-5 cells using Lipofectamine 2000 following the manufacturer’s instructions. The transfected cells were selected with medium containing 1 μg/ml puromycin from the second day. After 1 month of transfection, puromycin-resistant colonies were isolated with limiting dilution and expanded. The stably transfected cells were designated as SBC-5-Ri-DKK1 and maintained in growth medium containing 0.5 μg/ml puromycin.
Table 2.
Information of shRNA Targeting the Human DKK1 Gene
| Symbol/ID | Target Sequence | Sequence (5′–3′) |
|---|---|---|
| Ri-DKK1-12 | GCCGGATACAGAAAGATCACCATCA | |
| DKK1-RNAi (31612-1)-a | CcggGCCGGATACAGAAAGATCACCATCACTCGAGTGATGGTGATCTTTCTGTATCCGGCTTTTTg | |
| DKK1-RNAi (31612-1)-b | aattcaaaaaGCCGGATACAGAAAGATCACCATCACTCGAGTGATGGTGATCTTTCTGTATCCGGC | |
| Ri-DKK1-13 | GTATCACACCAAAGGACAAGAAGGT | |
| DKK1-RNAi (31613-1)-a | CcggGTATCACACCAAAGGACAAGAAGGTCTCGAGACCTTCTTGTCCTTTGGTGTGATACTTTTTg | |
| DKK1-RNAi (31613-1)-b | aattcaaaaaGTATCACACCAAAGGACAAGAAGGTCTCGAGACCTTCTTGTCCTTTGGTGTGATAC | |
| Ri-DKK1-14 | AGAACGGAAGTGTGATATGTTTAAA | |
| DKK1-RNAi (31614-1)-a | CcggAGAACGGAAGTGTGATATGTTTAAACTCGAGTTTAAACATATCACACTTCCGTTCTTTTTTg | |
| DKK1-RNAi (31614-1)-b | aattcaaaaaAGAACGGAAGTGTGATATGTTTAAACTCGAGTTTAAACATATCACACTTCCGTTCT |
MTT Assay
Cells at 80% confluence were harvested and placed into 96-well plates (2,000 cells/well, five wells for each group). Every day one plate was taken for MTT analysis. MTT solution (20 μl) (5 mg/ml) was added into every well, and then the cells were incubated at 37°C for 3 h. The solution was removed, and 150 μl of DMSO was added to dissolve the formazine. Absorbance was detected at 490 nm with an ELISA plate reader (Thermo Forma MK3). This assay was performed three times.
Colony Formation Assay
A total of 200 cells/well were plated at equal density in a six-well plate. After incubation for 2 weeks, the cells were stained with 0.25% crystal violet, and the number of colonies was counted manually. Triplicate plates were set up for accuracy.
Conditioned Medium Collection and Use
SBC-5 cells (1 × 105) were seeded to a six-well plate. After 24 h of incubation, the medium was replaced with serum-free medium after being washed twice with 1× PBS. The conditioned medium (CM) was collected after another 24 h of incubation and stored at −80°C. In the follow-up study, the CM was added to the bottom chamber of the culture plate at a ratio of 50%, and additional FBS was added to maintain the final concentration of 10%.
Cell Migration and Invasion Assay
Transwell invasion assays were performed in Transwell inserts (pore size, 8 μm thick) and 24-well companion plates (both from BD Corning). Inserts were coated with 70 μl of Matrigel basement membrane matrix (diluted 1:8; BD Corning). Cells were seeded in triplicate into the upper chambers in RPMI-1640 without FBS. Medium (500 μl) containing 10% FBS was added into the bottom chamber to serve as a chemoattractant. To test the specificity of DKK1, 10 μg/ml of anti-human DKK1-neutralizing antibody (R&D Systems, Minneapolis, MN, USA) or 16 μg/ml recombinant human DKK1 or the CM was collected before being added to the bottom chamber. After 24 h of incubation, cells that migrated through the inserts were fixed with 95% ethyl alcohol and then stained with 0.5% crystal violet. Invading cells on the bottom of the filters were imaged by microscopy (100×). However, only one step was different between the migration assay and the invasion assay, which was that the inserts in the migration assay need not be coated with Matrigel basement membrane matrix.
Flow Cytometry Analysis
Cell apoptosis was determined by flow cytometry. The Annexin-V-FITC-PI kit (Key GEN, Nanjing, P.R. China) was used to detect the apoptosis rate. The cells were collected and washed with cool PBS twice and resuspended in 200 μl of 1× binding buffer. Annexin V-FITC (10 μl) and PI (5 μl) were added, and the mixture was incubated at room temperature for 15 min in the dark. Finally, 300 μl of binding buffer was added into the mixture, which was analyzed with FACSCalibur system (BD).
In Vivo Metastasis Experiment
All animal experiments were performed according to the Guidelines on Animal Experimentation formulated by the Fourth Military Medical University, Xi’an, P.R. China. Four-week-old female NOD-SCID mice (Beijing HFK Bioscience Co. Ltd., Beijing, P.R. China) were divided into two groups, and each group consisted of five mice. SBC-5 cells and SBC-5-Ri-DKK1 cells were harvested, and only a single cell suspension of >90% cell viability was used. Cells (1 × 106) in 200 μl of PBS were injected into the mice via the tail vein. The mice were maintained under specific pathogen-free conditions. Five weeks later, the mice were anesthetized, and bone metastases were visualized by X-ray photographs. The number of osteolytic bone metastases on the X-ray photographs was evaluated independently by two investigators (S.H.C. and T.M.Z.).
Statistical Analysis
One-way ANOVA was used to determine the significant difference in cell migration and invasion assay, and Wilcoxon rank sum test was used for metastasis analysis in vivo. The other data were performed by t-test. Statistical tests were performed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). A value of p < 0.05 was considered statistically significant, and all statistical tests were performed two sided.
RESULTS
The Expression of DKK1 in SBC-5 Cells Was Higher Than That in SBC-3 Cells
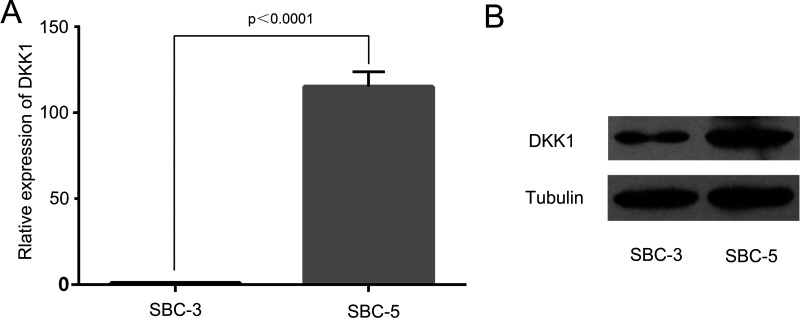
We detected the DKK1 expression level in SBC-5 cell and SBC-3 cell by RT-PCR and Western blot analysis. We found that the expression level of DKK1 in SBC-5 cell and SBC-3 cell was significantly different. RT-PCR assay indicated that DKK1 was highly expressed in SBC-5 cell compared with SBC-3 cell (p < 0.0001) (Fig. 1A), and Western blot assay confirmed this result (Fig. 1B).
Figure 1.
Differential expression of DKK1 in SBC-5 and SBC-3 cells. (A) mRNA levels of DKK1 in human lung cancer cell lines. (B) Protein levels of DKK1 in human lung cancer cell lines.
Expression of DKK1 in Transfected Cells Was Suppressed Successfully
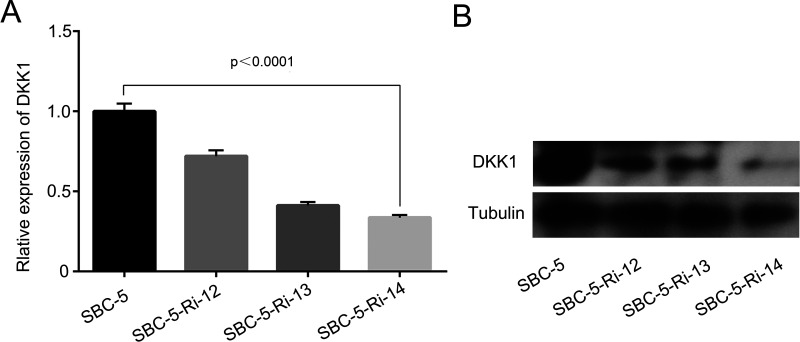
We checked the transfection efficiency in SBC-5 cells by RT-PCR and Western blot. Both of the mRNA level and protein level of DKK1 in SBC-5 cells were stably decreased by shRNA (Fig. 2A and B). Among these shRNAs targeting to three different sites, Ri-DKK1-14 was the most effective one, so we named it SBC-5-Ri-DKK1 for the following tests.
Figure 2.
Expression of DKK1 in parental cells and transfected cells. (A) The mRNA level of DKK1 was measured by RT-PCR. (B) The protein expression was measured by Western blot.
Cell Proliferation
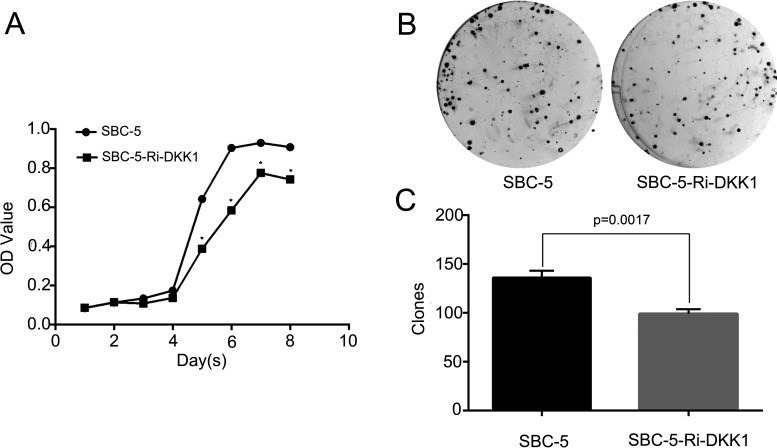
Next, the effect of decreased DKK1 expression on cell proliferation in SBC-5 cells was analyzed by MTT and colony formation assay. As shown in the cell proliferation curve, the down expression of DKK1 could inhibit the proliferation ability of SBC-5 cells (p < 0.0001) (Fig. 3A). Consistently, from the colony formation assay, we observed that the number of colonies formed by SBC-5-Ri-DKK1 cells was significantly less than that by the parental SBC-5 cells (p = 0.0017) (Fig. 3C), and the colony size was also much smaller in the former group (Fig. 3B). These results implied that DKK1 played a role in the proliferation of SBC-5 cells.
Figure 3.
Effect of the downregulation of DKK1 by RNAi on the proliferation and colony formation in SBC-5 cells. (A) Cell proliferation was detected by MTT (*p < 0.0001) and (B) colony formation assay. (C) Statistical analysis of colony formation assay.
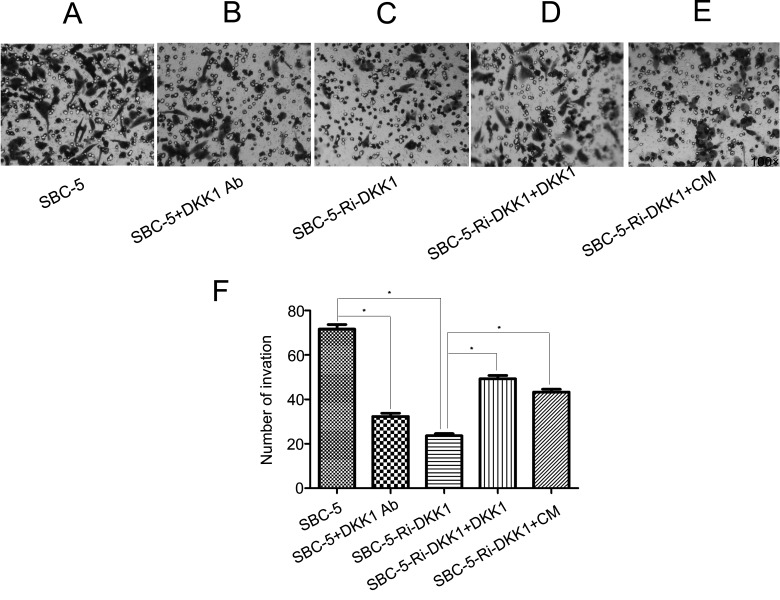
Reduced Expression of DKK1 Inhibits the Migration and Invasion in SBC-5 Cells
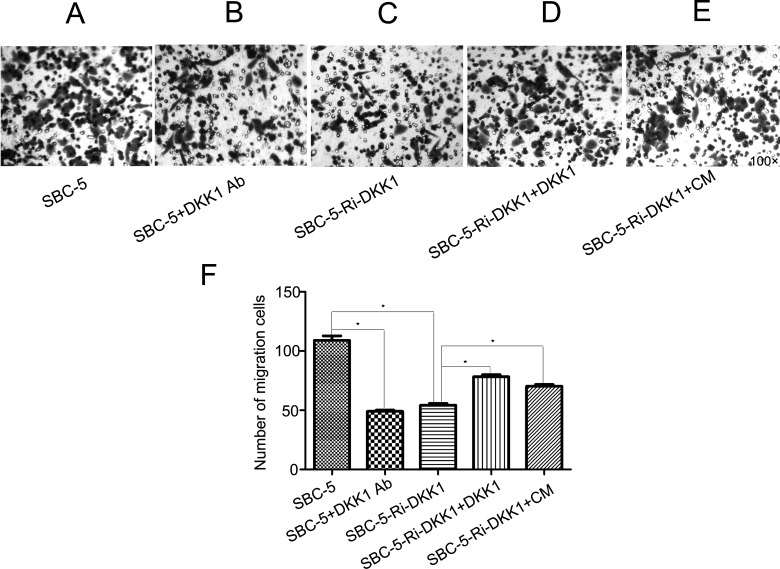
Through preliminary experiments, we found that the addition of a DKK1-neutralizing antibody and downregulation of DKK1 expression could dramatically suppress cell migration and invasion, while the recombinant DKK1 and CM of SBC-5 cells could reverse the effect of DKK1 RNAi (p < 0.0001). These results suggested that DKK1 was closely related to the capability of invasion and migration in SBC-5 cells (Figs. 4 and 5).
Figure 4.
Microscope photos of migration: (A) migration of SBC-5 cells, (B) migration of SBC-5 cells with 10 μg/ml of anti-human DKK1-neutralizing antibody, (C) migration of SBC-5-Ri-DKK1 cells, (D) migration of SBC-5-Ri-DKK1 cells with 16 ng/ml recombination human DKK1, and (E) migration of SBC-5-Ri-DKK1 cells with 50% conditioned medium (CM). (F) The statistical results of cell migration. Bars represent mean ± SD. *p < 0.0001.
Figure 5.
Microscope photos of invasion: (A) invasion of SBC-5 cells, (B) invasion of SBC-5 cells with 10 μg/ml of anti-human DKK1-neutralizing antibody, (C) invasion of SBC-5-Ri-DKK1 cells, (D) invasion of SBC-5-Ri-DKK1 cells with 16 ng/ml recombination human DKK1, and (E) invasion of SBC-5-Ri-DKK1 cells with 50% conditioned medium (CM). (F) Statistical results of cell invasion. Bars represent mean ± SD. *p < 0.0001.
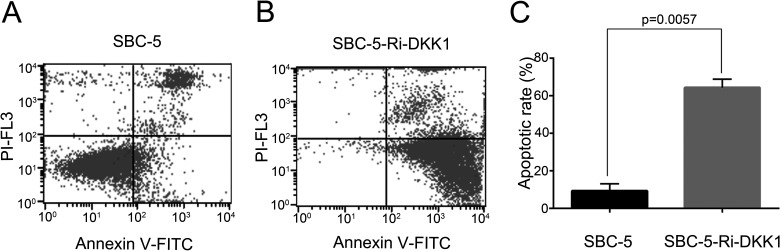
Downregulation of DKK1 Improves the Apoptosis of SBC-5 Cells
The cell apoptosis analysis showed that the apoptosis rates of SBC-5-Ri-DKK1 cells and SBC-5 cells were 64.34 ± 3.193% and 9.350 ± 2.680%, respectively (p = 0.0057) (Fig. 6), indicating that the downregulation of DKK1 could promote the apoptosis of SBC-5 cells.
Figure 6.
Cell apoptosis was analyzed by FACS analysis. (A) Representative image of cell apoptotic rate of SBC-5 cells and (B) SBC-5-Ri-DKK1 cells. (C) The statistical analysis of apoptosis rate in SBC-5 cells and SBC-5-Ri-DKK1 cells, presented as mean ± SD (p = 0.0057).
Downregulation of DKK1 Inhibited the Bone Metastasis In Vivo
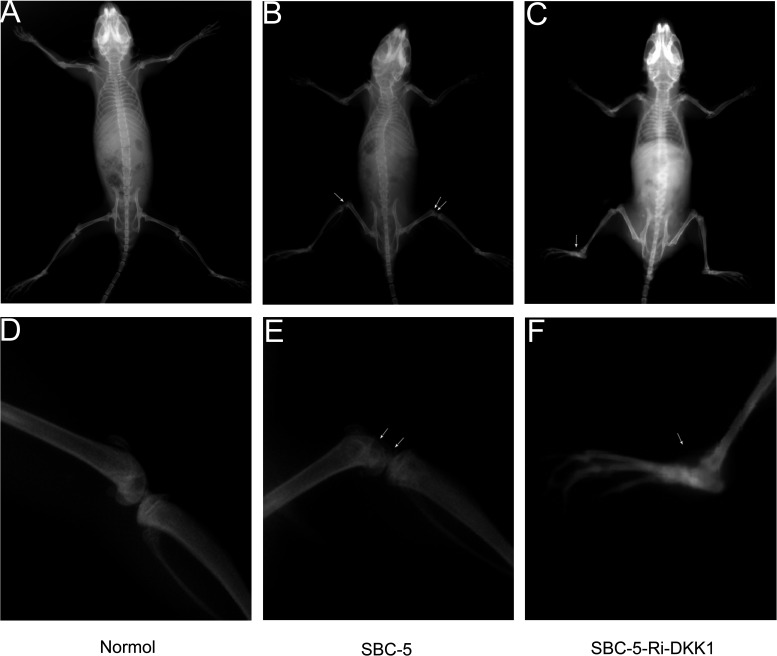
Last, we investigated the role that DKK1 played in vivo. Five weeks after inoculation of cancer cells, paralysis (probably caused by spinal cord compression and bone metastases in hindlimbs) occurred in one of five mice in the SBC-5-Ri-DKK1 group and in four of five mice in the SBC-5 group. Bone metastasis was found in two of five mice in the SBC-5-Ri-DKK1 group and in five of five mice in the SBC-5 group (Table 3). The total number of bone metastasis lesions that developed in the mice was also calculated, which is shown in Table 3. The results revealed that the downregulation of DKK1 could significantly inhibit specific metastasis to the bone. The image of bone metastasis lesions is shown in Figure 7.
Table 3.
Metastasis Lesions Formed in NOD-SCID Mice After Inoculation of SBC-5 and SBC-5-RI-DKK1 Cells for 5 Weeks
| Cell | Incidence | No. of Bone Metastasis |
|---|---|---|
| SBC-5 | 5/5 | 5 (4–6) |
| SBC-5-Ri-DKK1 | 2/5 | 0 (0–3)* |
The metastasis incidence in mice and the number of metastasis lesions in bone 5 weeks after inoculation of two groups of cells. Values are presented as median (range). *p = 0.008 compared with the control group (Wilcoxon rank sum test).
Figure 7.
The X-ray photographs of osteolytic bone metastases formed after inoculation of cancer cells for 5 weeks. (A, D) Bone image in normal mice. Images of bone metastasis lesions in (B, E) the SBC-5 group and (C, F) the SBC-5-Ri-DKK1 group.
DISCUSSION
The DKK family consists primarily of four subtypes of secreted protein in vertebrates (DKK1 to DKK4). Among this family, more research has focused on the DKK1 protein, which was discovered due to its ability to block Wnt signaling required for head induction during early Xenopus embryogenesis10. DKK1 is a downstream target of the Wnt/β-catenin signaling pathway, and activation of canonical Wnt signaling leads to an increase in transcription and translation levels of DKK1. Several studies stated that DKK1 is involved in the progression of cancer and predicted a poor prognosis. However, the role DKK1 played in cancer appears to be diverse. Serum concentration of DKK1 was decreased obviously in patients with colorectal cancer, gastric cancer, cervical adenocarcinoma, and ovarian cancer compared with healthy people11,12. Unlike samples from the patients mentioned above, patients with lung cancer11, multiple myeloma13, PC14, esophageal carcinomas15, hepatoblastoma, Wilms’ tumors16,17, and breast cancer6 showed a higher DKK1 concentration. These contradictory observations may be caused by the differences in tumor type, tumor stage, tissue origin (epithelial or mesenchymal), and cellular subtypes.
In our study, we confirmed that the expression level of DKK1 was much higher in SBC-5 cells with bone metastatic ability than the level in SBC-3 cells without bone metastatic ability. DKK1 may be an important factor in bone metastasis of SCLC. So we established a DKK1-RNAi-stable transfected cell line and found that downregulation of DKK1 expression could attenuate the cell proliferation, colony formation, cell migration, and invasion, but promote apoptosis. However, DKK1 did not change the cell cycle progression of SBC-5 cells in vitro (data not shown), suggesting that DKK1 accelerated the apoptosis through other pathways, but not the cell cycle, which needs further investigation. In vivo, downregulating DKK1 in SBC-5 cells could reduce the number and size of bone metastasis formed in NOD/SCID mice. These results indicated that DKK1 played an important role in the biological behavior of SCLC cells, which may be a potential mechanistic link between SCLC and bone metastases. Therefore, targeting DKK1 may be an effective method to prevent and treat skeleton metastases of SCLC.
The serum from patients with high DKK1 levels could inhibit osteoblast differentiation and OPG expression in vitro18. In myeloma, DKK1 plays a positive role in the development of osteolytic lesions16,19. DKK1 inhibits osteoblast differentiation and high circulating level of DKK1 in patients with multiple myeloma and is correlated with osteolytic lesions20,21. New research reports that DKK1 promotes PC elongation and filopodia formation, morphological changes characteristic of an invasive phenotype to bone22. Other studies have found that DKK1 was highly expressed in breast cancer cell lines, which preferentially metastasize to the bone and serum of breast cancer patients with osteolytic bone metastases23,24. However, further understanding of the mechanistic role that DKK1 plays in primary small lung cancer as well as its secondary osteolytic bone metastases should be facilitated. We need many more studies to reveal the exact mechanisms behind these effects of DKK1 on tumor cell growth, apoptosis, migration, invasion, and bone metastasis.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Nos. 81172011, 81572814, and 81272345) and the Natural Science Foundation of Shanxi, China (No. 2016JM8036).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K, Richhariya A, Dodge T, Wolff GG, Balakumaran A, Edelsberg J. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: A 15-year study in two large US health systems. Support Care Cancer 2013;21:3279–86. [DOI] [PubMed] [Google Scholar]
- 3. Silva SC, Wilson C, Woll PJ. Bone-targeted agents in the treatment of lung cancer. Ther Adv Med Oncol. 2015;7:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roato I. Bone metastases: When and how lung cancer interacts with bone. World J Clin Oncol. 2014;5:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menezes ME, Devine DJ, Shevde LA, Samant RS. Dickkopf1: A tumor suppressor or metastasis promoter? Int J Cancer 2012;130:1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, Keller ET, Li Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int J Cancer 2008;123:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 2008;68:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 2011;71:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu T, Teng J, Jiang L, Zhong H, Han B. Lung cancer-derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem Biophys Res Commun. 2014;443:962–8. [DOI] [PubMed] [Google Scholar]
- 10. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998;391:357–62. [DOI] [PubMed] [Google Scholar]
- 11. Sheng SL, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656–64. [DOI] [PubMed] [Google Scholar]
- 12. Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M, Sonoda T, Mori M, Imai K, Tokino T, Shinomura Y. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis 2007;28:2459–66. [DOI] [PubMed] [Google Scholar]
- 13. Xu Y, Chen B, George SK, Liu B. Downregulation of MicroRNA-152 contributes to high expression of DKK1 in multiple myeloma. RNA Biol. 2015;12:1314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 2008;68:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, Fujita M, Hosokawa M, Tsuchiya E, Kohno N, Kondo S, Nakamura Y, Daigo Y. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67:2517–25. [DOI] [PubMed] [Google Scholar]
- 16. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JJ. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. [DOI] [PubMed] [Google Scholar]
- 17. Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms’ tumors. Lab Invest. 2003;83:429–34. [DOI] [PubMed] [Google Scholar]
- 18. Brunetti G, Papadia F, Tummolo A, Fischetto R, Nicastro F, Piacente L, Ventura A, Mori G, Oranger A, Gigante I, Colucci S, Ciccarelli M, Grano M, Cavallo L, Delvecchio M, Faienza MF. Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: The role of DKK1, RANKL, and TNF-alpha. Osteoporos Int. 2016;27:2355–65. [DOI] [PubMed] [Google Scholar]
- 19. Politou MC, Heath DJ, Rahemtulla A, Szydlo R, Anagnostopoulos A, Dimopoulos MA, Croucher PI, Terpos E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 2006;119:1728–31. [DOI] [PubMed] [Google Scholar]
- 20. Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JJ. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009;113:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giuliani N, Rizzoli V. Myeloma cells and bone marrow osteoblast interactions: Role in the development of osteolytic lesions in multiple myeloma. Leuk Lymphoma 2007;48:2323–9. [DOI] [PubMed] [Google Scholar]
- 22. Hudson BD, Hum NR, Thomas CB, Kohlgruber A, Sebastian A, Collette NM, Coleman MA, Christiansen BA, Loots GG. SOST inhibits prostate cancer invasion. PLoS One 2015;10:e142058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P, Garnero P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer 2007;97:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mariz K, Ingolf JB, Daniel H, Teresa NJ, Erich-Franz S. The Wnt inhibitor dickkopf-1: A link between breast cancer and bone metastases. Clin Exp Metastasis 2015;32:857–66. [DOI] [PubMed] [Google Scholar]